Experimental Studies on Diesel Deterioration: Accelerated Oxidation in a Reaction Vessel and Thermogravimetric Analysis
Abstract
1. Introduction
- (1)
- Real-time deterioration studies require year-scale monitoring, hindering rapid assessment. However, the studies on the storage and oxidative stability of diesel fuel are still lacking. The optional range for the accelerated oxidation experiment of diesel fuel, including temperature, oxygen exposure, and agitation-related parameters, is still undetermined. In particular, the temperature conditions lower than D2275 (heating bath at 95 °C) for oxidative stability tests should be further explored;
- (2)
- Existing accelerated tests fail to simulate synergistic property evolution;
- (3)
- The experimental data of the processes of diesel deterioration, which are affected by the reactor material, heating rate, bath gas, and reactive gas, is still lacking. The vaporization effect during the deterioration was rarely reported.
2. Experimental Methods
2.1. Apparatus Design
2.2. Accelerated Oxidation Experiments
2.3. Oxidation Stability Tests
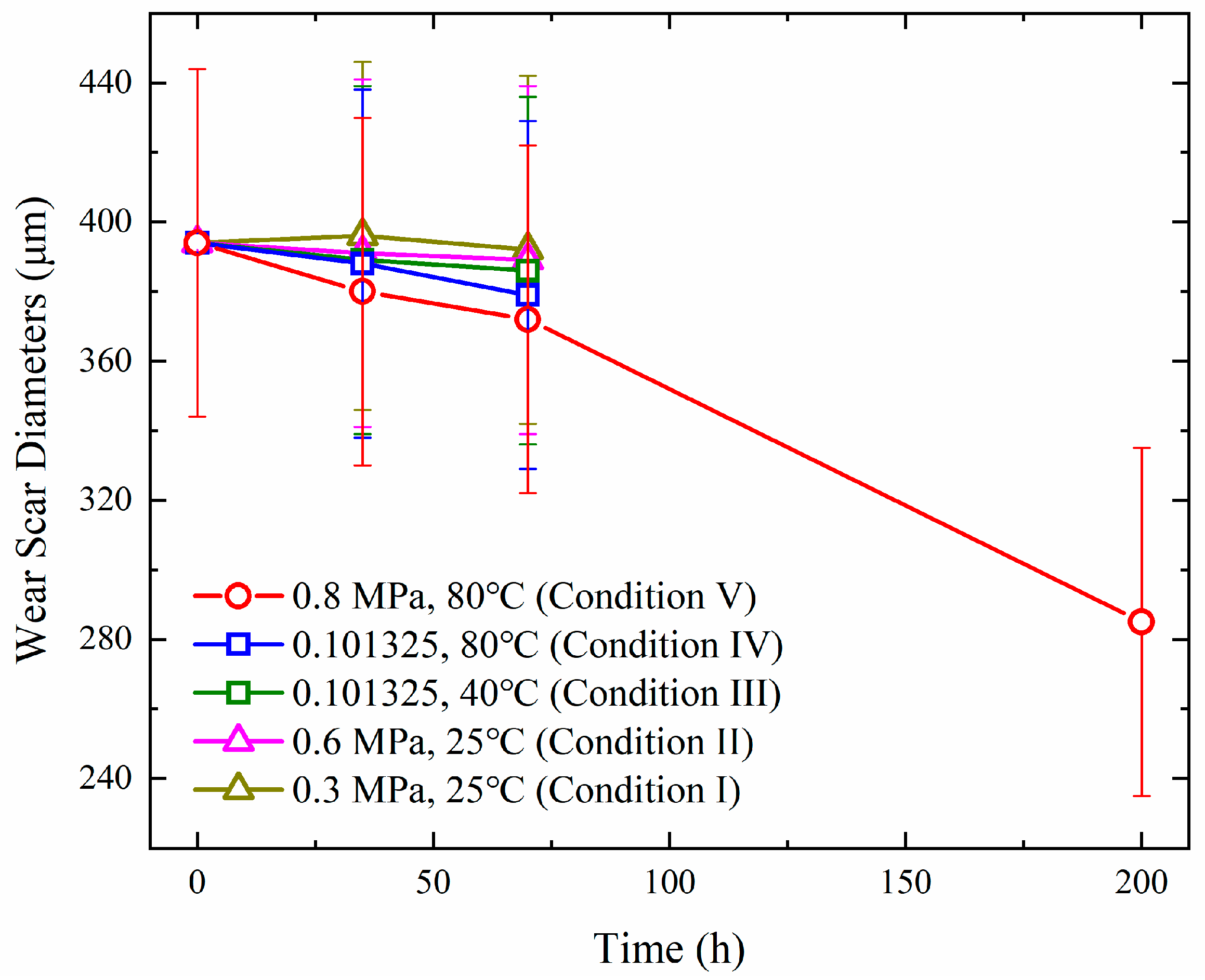
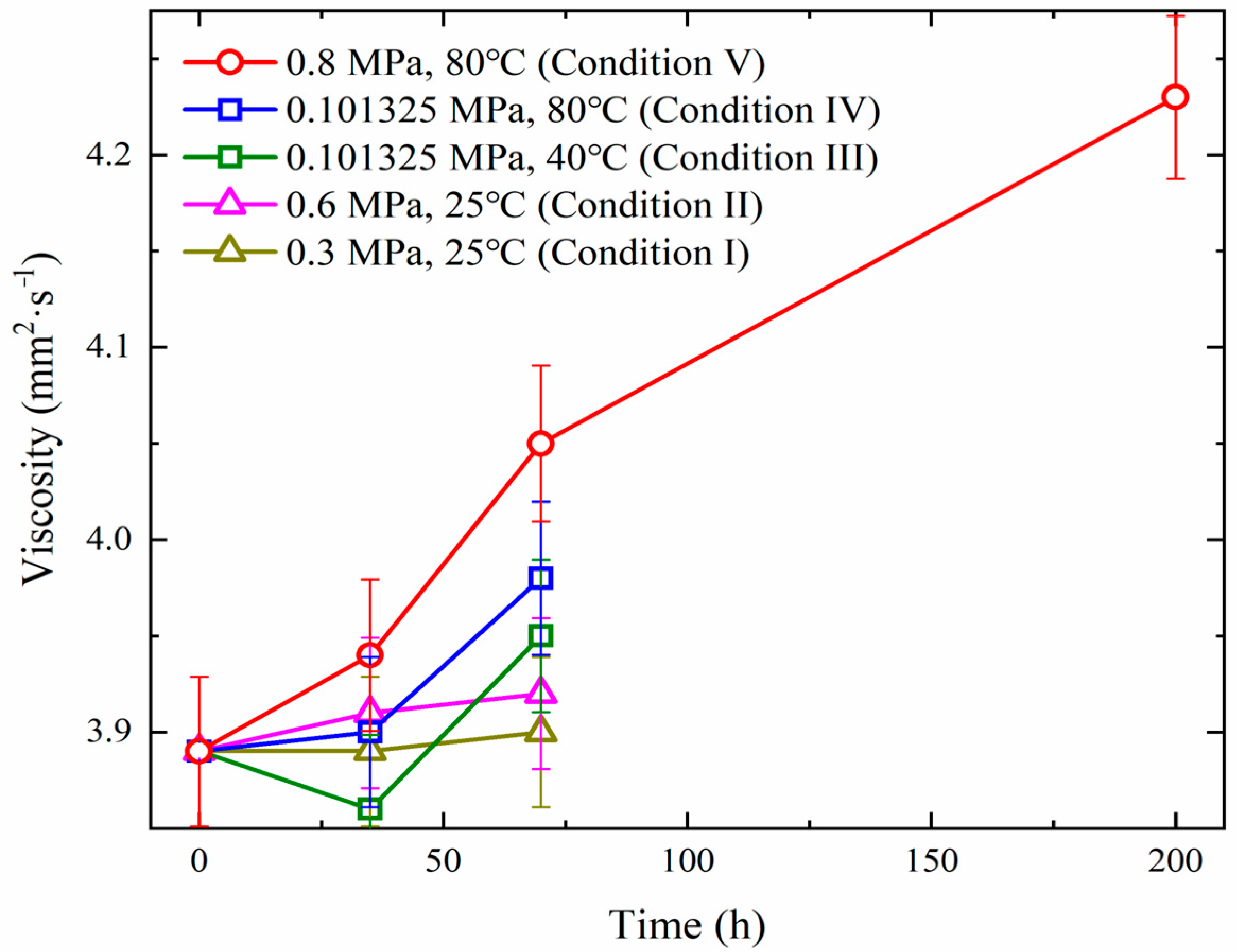
2.4. Wear Scar Diameter and Viscosity
2.5. Thermogravimetric Analysis (TGA)
2.6. Actual Diesel Engine Operating and Sample Collection
3. Results and Discussion
3.1. Experimental Investigations in the Reaction Vessel
- (1)
- The explosion potential at a total pressure higher than 0.3 MPa—the pressurization should not be performed before reaching the target temperatures, and the pressure ramp rate should be limited according to engineering practice;
- (2)
- The autoignition risk of diesel vapor (higher than their flash points);
- (3)
- The fire hazard in the absence of an active quench system and continuous monitoring;
- (4)
- Oxygen and other forms of corrosion at high temperatures, including oxygen permeation and PAH adsorption.
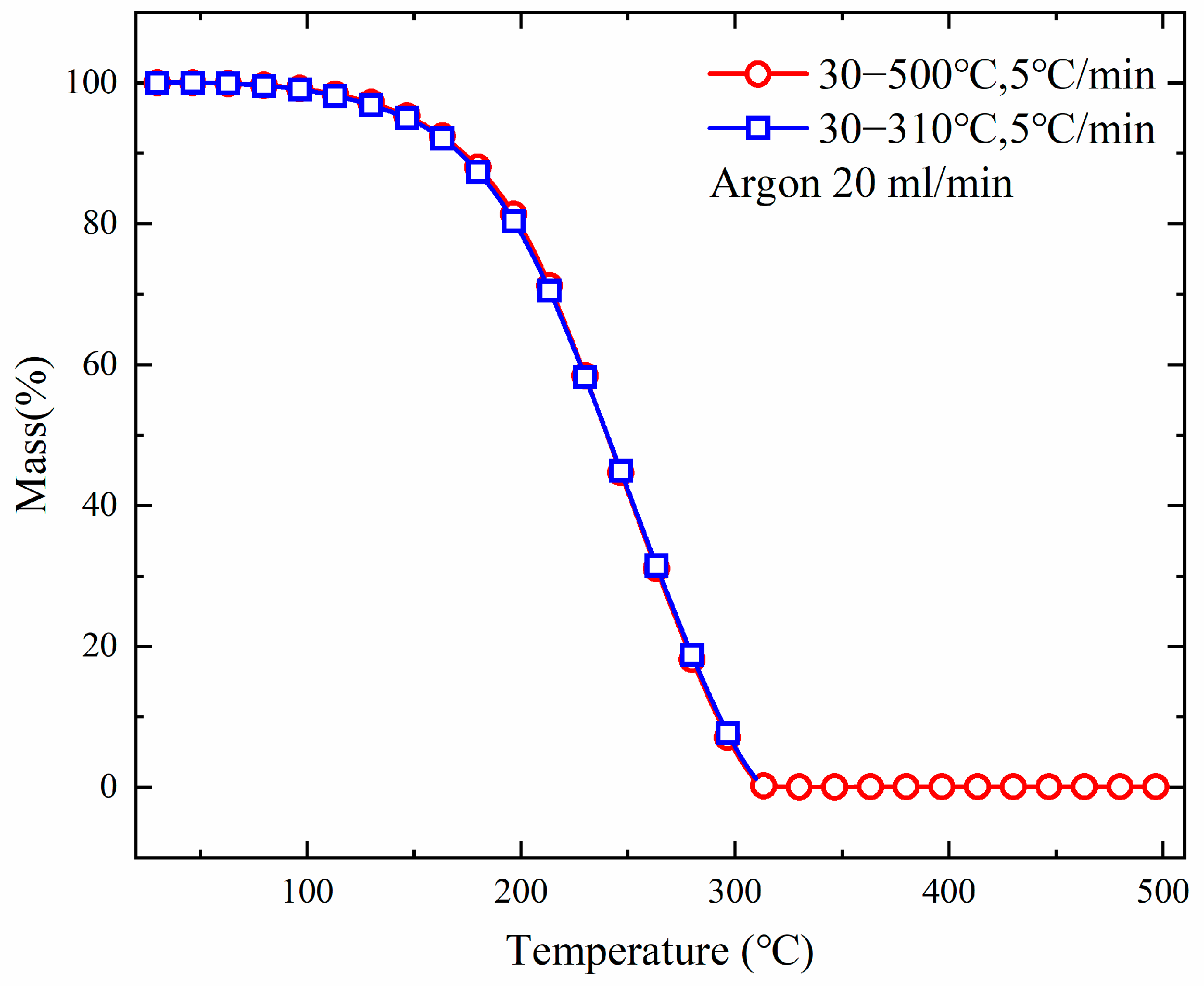
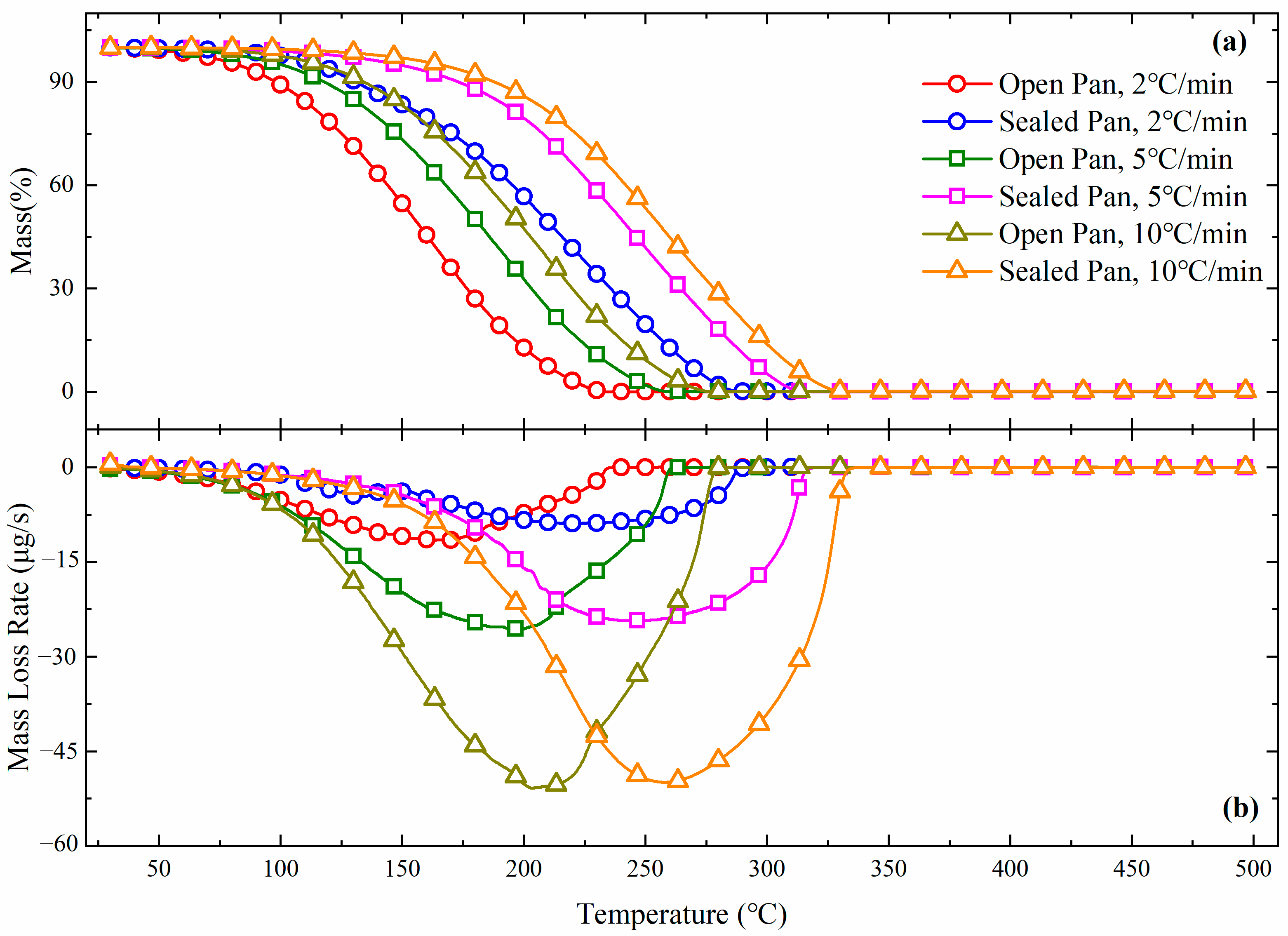
3.2. TGA
3.3. Actual Diesel Engine Operating
4. Conclusions
- (1)
- A temperature increase exhibits significantly stronger promotion effects on the deterioration of diesel fuel than an oxygen partial pressure increase. High temperatures promote diesel deterioration by activating radical-chain reactions, while the promotion effects of elevated oxygen partial pressure are attributed to the enhancement in oxygen mass transfer.
- (2)
- Non-temporal factors dominate actual diesel deterioration for EDG oil depot storage. Weak linear correlations between the time and viscosity/wear scar diameter were discovered.
- (3)
- A test method of accelerated oxidation under conditions of static 0.8 MPa and 80 °C was proposed, which could effectively compress long-term storage simulation (200 h lab aging equals three years of actual storage, summarized based on the comparison of the data of the wear scar diameter and viscosity obtained from accelerated oxidation and several representative oil depots). The optional temperature and pressure windows for acceleration oxidation were confirmed (40–80 °C/0.3–0.8 MPa).
- (4)
- The database of the deterioration and vaporization of diesel fuel was extended. TGA tests revealed critical vaporization controls, including the methods for suppressing carbon residues and lowering vaporization thresholds.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Wang, Z.; Liang, Y.; Liu, C.; Lu, X. Validation of a large-molecular weight five-component diesel surrogate: Emphasizing on NTC behavior. Proc. Combust. Inst. 2024, 40, 105686. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y. Petroleum Chemistry and Application; China PetroChemical Press Co., Ltd.: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Kančev, D.; Duchac, A.; Zerger, B.; Maqua, M.; Wattrelos, D. Statistical analysis of events related to emergency diesel generators failures in the nuclear industry. Nucl. Eng. Des. 2014, 273, 321–331. [Google Scholar] [CrossRef]
- Kančev, D.; Duchac, A.; Zerger, B.; Maqua, M.; Wattrelos, D. Events related to emergency diesel generators in the nuclear industry: Analysis of lessons learned from the operating experience. Prog. Nucl. Energy 2014, 75, 192–197. [Google Scholar] [CrossRef]
- Du Plessis, L.M.; De Villiers, J.B.M.; Van Der Walt, W.H. Stability studies on methyl and ethyl fatty acid esters of sunflowerseed oil. J. Am. Oil Chem. Soc. 1985, 62, 748–752. [Google Scholar] [CrossRef]
- Bondioli, P.; Gasparoli, A.; Lanzani, A.; Fedeli, E.; Veronese, S.; Sala, M. Storage stability of biodiesel. J. Am. Oil Chem. Soc. 1995, 72, 699–702. [Google Scholar] [CrossRef]
- D4625; Standard Test Method for Middle Distillate Fuel Storage Stability at 43 °C (110 °F). ASTM International: West Conshohocken, PA, USA, 2021.
- D2275; Standard Test Method for Oxidation Stability of Distillate Fuel Oil (Accelerated Method). ASTM International: West Conshohocken, PA, USA, 2014.
- Bondioli, P.; Gasparoli, A.; Bella, L.D.; Tagliabue, S. Evaluation of biodiesel storage stability using reference methods. Eur. J. Lipid Sci. Technol. 2002, 104, 777–784. [Google Scholar] [CrossRef]
- Bondioli, P.; Gasparoli, A.; Bella, L.D.; Toso, G. Biodiesel stability under commercial storage conditions over one year. Eur. J. Lipid Sci. Technol. 2003, 105, 735–741. [Google Scholar] [CrossRef]
- Middlebach, M.; Shcober, S. The influence of antioxidants on the oxidation stability of biodiesel. J. Am. Oil Chem. Soc. 2003, 80, 817–823. [Google Scholar] [CrossRef]
- Serrano, M.; Bouaid, A.; Martinez, M.; Aracil, J. Oxidation stability of biodiesel from different feedstocks: Influence of commercial additives and purification step. Fuel 2013, 113, 50–58. [Google Scholar] [CrossRef]
- Christensen, E.; McCormick, R.L. Long-term storage stability of biodiesel and biodiesel blends. Fuel Process. Technol. 2014, 128, 339–348. [Google Scholar] [CrossRef]
- Bezergianni, S.; Chrysikou, L.P. Oxidative stability of waste cooking oil and white diesel upon storage at room temperature. Bioresour. Technol. 2012, 126, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. Study and prediction of diesel deterioration characteristics of diesel engine. Guangdong Chem. Ind. 2024, 51, 44–47. (In Chinese) [Google Scholar]
- Hu, X.; Sun, J.; Jiang, B.; Wang, J.; Yang, Y. Liquid containing particle polymerization: Ethylene/α-olefin reaction in a new environment. Acta Polym. Sin. 2021, 52, 1138–1147. [Google Scholar]
- GB 19147-2016; Automobile Diesel Fuels. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and National Standardization Administration (People’s Republic of China): Beijing, China, 2016.
- Guan, Y.M.; Guan, D.; Zhang, C.; Yuan, S.H.; Cai, G.Q.; Zhang, L.Z. Diesel molecular composition and blending modeling based on SU-BEM framework. Pet. Sci. 2022, 19, 839–847. [Google Scholar] [CrossRef]
- Zhu, W.; Lei, X.; Wang, H.; Lin, H.; Han, S. Influence of natural aromatic ether extracts (estragole, methyleugenol and α-asarone) on the depressive effects of polymethacrylate copolymers as additives for diesel fuel. Fuel 2025, 388, 134546. [Google Scholar] [CrossRef]
- D5304-20; Standard Test Method for Assessing Middle Distillate Fuel Storage Stability by Oxygen Overpressure. ASTM International: West Conshohocken, PA, USA, 2020.
- SH/T 0175-2004; Standard Test Method for Oxidation Stability of Distillate Fuel Oil (Accelerated Method). National Development and Reform Commission (People’s Republic of China): Beijing, China, 2004.
- SH/T 0690-2013; Standard Test Method for Middle Distillate Fuel Storage Stability at 43 °C. National Energy Administration (People’s Republic of China): Beijing, China, 2013.
- NB/SH/T 0765-2021; Diesel Fuel—Assessment of Lubricity Using The High-Frequency Reciprocating Rig (HFRR). National Energy Administration (People’s Republic of China): Beijing, China, 2021.
- GB/T 265-1988; Petroleum products—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 1988.
- Wu, D.; Liang, X.; Wen, X.; Meng, Z.; Yu, Z.; He, Y.; Dai, P.; Yu, Y.; Li, N. Experimental Study of Diesel Engine Deposits on Failure of Honed Cylinder Surface Texture. Eng. Fail. Anal. 2025, 174, 109476. [Google Scholar] [CrossRef]
- Lyu, X.; Wang, K.; Liang, X.; Cui, L.; Wang, Y. Analysis of the Correlation Between Mechanical and physicochemical Properties of Particles Based on a Diesel Oxidation Catalytic System. Sci. Total Environ. 2024, 926, 171898. [Google Scholar] [CrossRef]
- GB/T 258-2016; Standard Test Method for Determination of Acidity of Light Petroleum Products. Inspection and Quarantine of the People’s Republic of China and National Standardization Administration (People’s Republic of China): Beijing, China, 2016.
- GB/T 1884-2000; Crude Petroleum and Liquid Petroleum Products—Laboratory Determination of Density—Hydrometer Method. The State Bureau of Quality and Technical Supervision (People’s Republic of China): Beijing, China, 2000.
- ISO 3675: 1998; Crude Petroleum and Liquid Petroleum Products—Laboratory Determination of Density—Hydrometer Method. International Organization for Standardization: Geneva, Switzerland, 1998.
- GB/T 1885-1998; Petroleum Measurement Tables. The State Bureau of Quality and Technical Supervision (People’s Republic of China): Beijing, China, 1998.
- GB/T 386-2021; Standard Test Method for Cetane Number of Diesel Oil. State Administration for Market Regulation (People’s Republic of China) and National Standardization Administration (People’s Republic of China): Beijing, China, 2021.
- Pichugin, V.F.; Lvanova, L.V.; Burov, E.A. Improving the tribotechnical characteristics of metallic pairs in diesel fuel by using additives. Chem. Technol. Fuels Oils 2013, 49, 309–312. [Google Scholar] [CrossRef]
- Barbour, R.; Rickeard, D.; Elliott, N. Understanding Diesel Lubricity; SAE Technical Paper 2000-01-1918; SAE International: Warrendale, PA, USA, 2000. [Google Scholar] [CrossRef]
- Leonardo, R.S.; Murta Valle, M.L.; Dweck, J. Thermovolumetric and thermogravimetric analysis of diesel S10. J. Therm. Anal. Calorim. 2020, 139, 1507–1514. [Google Scholar] [CrossRef]
- Batts, B.D.; Fathoni, A.Z. A literature review on fuel stability studies with particular emphasis on diesel fuel. Energy Fuels 1991, 5, 2–21. [Google Scholar] [CrossRef]
- Bergeron, I.; Charland, J.P.; Ternan, M. Color degradation of hydrocracked diesel fuel. Energy Fuels 1999, 13, 686–693. [Google Scholar] [CrossRef]
- Kalitchin, Z.D.; Ivanov, S.K.; Tanielyan, S.K.; Boneva, M.I.; Georgiev, P.T.; Ivanov, A.; Kanariev, K. Chemical stability of diesel fuels and sediment formation therein: 1. Evaluation of the chemical stability of diesel fuels by following the kinetics of sediment formation. Fuel 1992, 71, 437–442. [Google Scholar] [CrossRef]
- Ivanova, L.V.; Koshelev, V.N.; Burov, E.A. Influence of the hydrocarbon composition of diesel fuels on their performance characteristics. Pet. Chem. 2014, 54, 466–472. [Google Scholar] [CrossRef]






| No. | Gauge Pressure (MPa) | Total Pressure (MPa) | Temperature (°C) | Time (h) |
|---|---|---|---|---|
| TEST 1 | 0 | 0.101325 | 40 | 0 |
| TEST 2 | 0 | 0.101325 | 40 | 35 |
| TEST 3 | 0 | 0.101325 | 40 | 70 |
| TEST 4 | 0 | 0.101325 | 80 | 0 |
| TEST 5 | 0 | 0.101325 | 80 | 35 |
| TEST 6 | 0 | 0.101325 | 80 | 70 |
| No. | Gauge Pressure (MPa) | Total Pressure (MPa) | Temperature (°C) | Time (h) |
|---|---|---|---|---|
| TEST 7 | 0.199 | 0.3 | 25 | 0 |
| TEST 8 | 0.199 | 0.3 | 25 | 35 |
| TEST 9 | 0.199 | 0.3 | 25 | 70 |
| TEST 10 | 0.499 | 0.6 | 25 | 0 |
| TEST 11 | 0.499 | 0.6 | 25 | 35 |
| TEST 12 | 0.499 | 0.6 | 25 | 70 |
| No. | Gauge Pressure (MPa) | Total Pressure (MPa) | Temperature (°C) | Time (h) |
|---|---|---|---|---|
| TEST 13 | 0.699 | 0.8 | 80 | 0 |
| TEST 14 | 0.699 | 0.8 | 80 | 35 |
| TEST 15 | 0.699 | 0.8 | 80 | 70 |
| TEST 16 | 0.699 | 0.8 | 80 | 200 |
| No. | Focus | Reactor | Method | Heating Rate | Bath Gas |
|---|---|---|---|---|---|
| Test 1 | Reactor material | Pt-Rh crucible | Open | 2 °C/min | Ar 20 mL/min |
| Test 2 | Pt-Rh crucible | Sealed | 2 °C/min | Ar 20 mL/min | |
| Test 3 | Sapphire crucible | Open | 2 °C/min | Ar 20 mL/min | |
| Test 4 | Sapphire crucible | Sealed | 2 °C/min | Ar 20 mL/min | |
| Test 5 | Heating rate | Sapphire crucible | Open | 5 °C/min | Ar 20 mL/min |
| Test 6 | Sapphire crucible | Sealed | 5 °C/min | Ar 20 mL/min | |
| Test 7 | Sapphire crucible | Open | 10 °C/min | Ar 20 mL/min | |
| Test 8 | Sapphire crucible | Sealed | 10 °C/min | Ar 20 mL/min | |
| Test 9 | Bath gas | Sapphire crucible | Open | 5 °C/min | Ar 0 mL/min |
| Test 10 | Sapphire crucible | Open | 5 °C/min | Ar 40 mL/min | |
| Test 11 | Sapphire crucible | Sealed | 5 °C/min | Ar 40 mL/min | |
| Test 12 | Reactive gas | Sapphire crucible | Open | 5 °C/min | Air 20 mL/min |
| Test 13 | Sapphire crucible | Sealed | 5 °C/min | Air 20 mL/min |
| No. | Focus | Reactor | Method | T10 (°C) | T50 (°C) | T90 (°C) |
|---|---|---|---|---|---|---|
| Test 1 | Reactor material | Pt-Rh crucible | Open | 98.73 | 158.33 | 208.40 |
| Test 2 | Pt-Rh crucible | Sealed | 113.27 | 168.43 | 231.27 | |
| Test 3 | Sapphire crucible | Open | 98.13 | 155.20 | 204.93 | |
| Test 4 | Sapphire crucible | Sealed | 131.30 | 209.20 | 264.50 | |
| Test 5 | Heating rate | Sapphire crucible | Open | 118.17 | 180.17 | 231.50 |
| Test 6 | Sapphire crucible | Sealed | 173.33 | 240.25 | 291.92 | |
| Test 7 | Sapphire crucible | Open | 134.50 | 197.17 | 248.83 | |
| Test 8 | Sapphire crucible | Sealed | 188.17 | 254.17 | 306.17 | |
| Test 9 | Bath gas | Sapphire crucible | Open | 122.08 | 184.75 | 235.33 |
| Test 10 | Sapphire crucible | Open | 119.33 | 182.67 | 234.75 | |
| Test 11 | Sapphire crucible | Sealed | 122.58 | 200.25 | 280.92 | |
| Test 12 | Reactive gas | Sapphire crucible | Open | 116.67 | 178.67 | 229.17 |
| Test 13 | Sapphire crucible | Sealed | 171.50 | 238.25 | 293.42 |
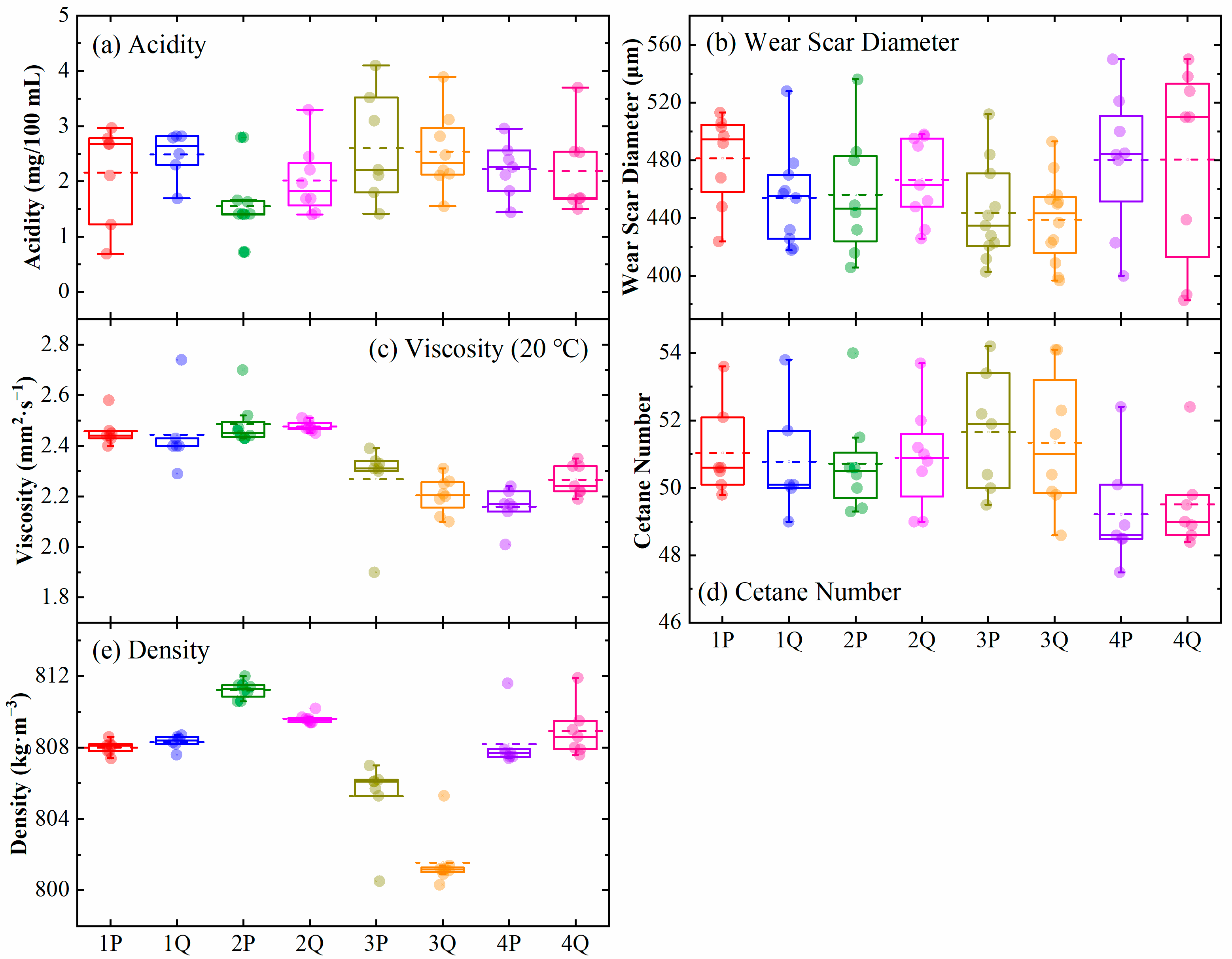
| Depot No. | Storage Days | Linear Fitting (Wear Scar Diameter) | Linear Fitting (Viscosity) | ||
|---|---|---|---|---|---|
| k | R2 | k | R2 | ||
| 1P | 1149 | 0.02372 | 0.10 | 3.27 × 10−5 | 0.050 |
| 1Q | 967 | 0.01861 | 0.029 | 1.63 × 10−4 | 0.13 |
| 2P | 1181 | −0.08585 | 0.70 | −5.15 × 10−5 | 0.055 |
| 2Q | 1241 | 0.02422 | 0.14 | 1.90 × 10−5 | 0.16 |
| 3P | 1065 | −0.01155 | 0.011 | 2.89 × 10−4 | 0.45 |
| 3Q | 1247 | 0.00249 | 0.0011 | 8.76 × 10−5 | 0.30 |
| 4P | 1148 | −0.04004 | 0.12 | 1.42 × 10−4 | 0.57 |
| 4Q | 1135 | −0.08995 | 0.31 | 1.43 × 10−4 | 0.81 |
| 5P | 351 | −0.01997 | 0.022 | 2.62 × 10−4 | 0.54 |
| 5Q | 351 | −0.00619 | 0.0031 | 2.58 × 10−4 | 0.92 |
| 6P | 258 | 0.03393 | 0.72 | 5.43 × 10−5 | 0.22 |
| 6Q | 258 | −0.15976 | 0.79 | 2.31 × 10−4 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Wang, M.; Li, P.; Che, S.; Liang, X.; Che, Y.; Yan, J.; He, Y. Experimental Studies on Diesel Deterioration: Accelerated Oxidation in a Reaction Vessel and Thermogravimetric Analysis. Energies 2025, 18, 5365. https://doi.org/10.3390/en18205365
Li N, Wang M, Li P, Che S, Liang X, Che Y, Yan J, He Y. Experimental Studies on Diesel Deterioration: Accelerated Oxidation in a Reaction Vessel and Thermogravimetric Analysis. Energies. 2025; 18(20):5365. https://doi.org/10.3390/en18205365
Chicago/Turabian StyleLi, Nan, Mingchang Wang, Pengpeng Li, Shuping Che, Xingyu Liang, Yinhui Che, Jia Yan, and Yongdi He. 2025. "Experimental Studies on Diesel Deterioration: Accelerated Oxidation in a Reaction Vessel and Thermogravimetric Analysis" Energies 18, no. 20: 5365. https://doi.org/10.3390/en18205365
APA StyleLi, N., Wang, M., Li, P., Che, S., Liang, X., Che, Y., Yan, J., & He, Y. (2025). Experimental Studies on Diesel Deterioration: Accelerated Oxidation in a Reaction Vessel and Thermogravimetric Analysis. Energies, 18(20), 5365. https://doi.org/10.3390/en18205365







