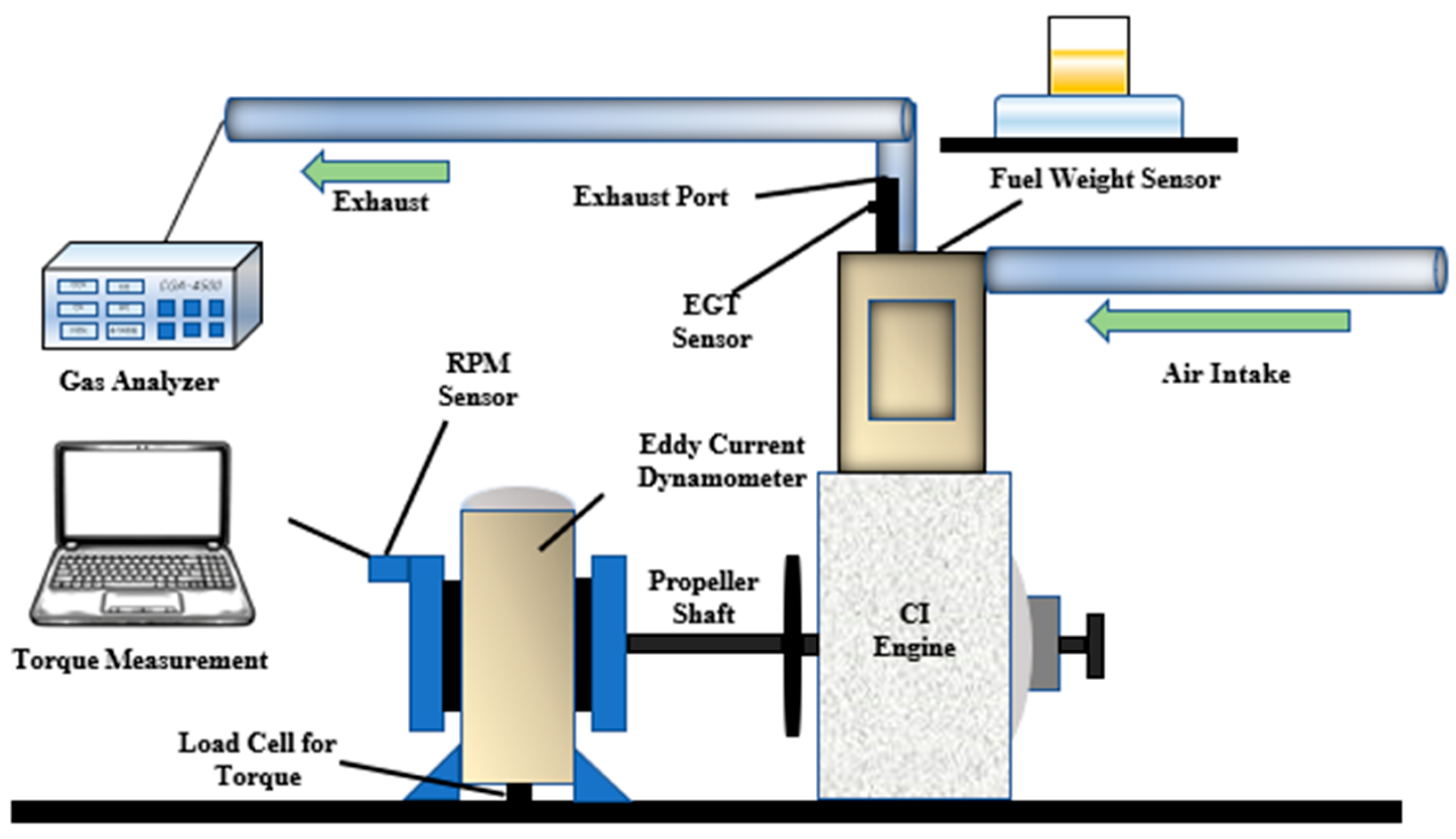
1. Introduction
In today’s society, compression ignition (CI) engines play a core role in multiple important fields such as commercial transportation, heavy machinery, and power generation due to their excellent efficiency, high power output, excellent fuel economy, long-lasting durability, and wide adaptability [
1,
2,
3]. Fossil fuels, as the main source of power for CI engines, occupy an important position in the global energy structure [
4]. According to a report by the International Energy Agency (IEA), although the use of renewable energy is gradually increasing, fossil fuels, including diesel, still account for over 80% of global energy consumption, and this proportion has not changed much in the past few decades [
5]. However, this high dependence on fossil fuels, especially the widespread use of diesel in CI engines, has caused a series of serious social and environmental problems.
Firstly, diesel, as a non-renewable resource, has limited reserves. With the development of the global economy and the growth of energy demand, the consumption rate of fossil fuels such as diesel is accelerating. This not only leads to the gradual depletion of resources but also increases the economic and environmental costs of obtaining these resources [
6,
7]. Although technological progress has improved the ability to extract resources that are difficult to access, it is also accompanied by higher extraction costs and increased environmental risks, such as ecological damage and pollution caused by deep-sea drilling and oil sand development. Secondly, the combustion of fossil fuels is the main source of global greenhouse gas emissions. According to the United Nations Framework Convention on Climate Change (UNFCCC), approximately 75% of greenhouse gas emissions originate from the combustion of fossil fuels. These emissions, especially carbon dioxide, play an important role in global climate change [
8,
9,
10].
Among numerous renewable energy sources, biofuels have received special attention due to their unique sustainability and environmental friendliness [
11,
12]. The production of biofuels utilizes biomass resources such as crops, agricultural residues, and food waste, converting these materials into energy. This process not only provides an effective alternative to fossil fuels but also helps address the disposal challenges of agricultural and food waste, thereby promoting the development of a sustainable ecological economy. In addition, the technological development of biofuels is constantly advancing, making them more feasible at both commercial and environmental levels. Currently, second-generation and third-generation biofuel technologies are being developed, and these focus on the use of more efficient and environmentally friendly raw materials such as non-food crops and microalgae, reducing competition with food crops while improving the yield and quality of biofuels [
13].
Cotton is a widely cultivated crop worldwide, with particular importance in regions such as the European Union, China, the United States, and India [
14]. Beyond its central role in the textile industry, cotton also occupies a critical position in the broader agricultural value chain. More importantly, cotton holds considerable potential for contributing to carbon reduction. Unlike fossil fuels, the carbon emissions of biofuels follow a “closed-loop” mechanism: carbon dioxide absorbed during plant growth through photosynthesis is nearly equivalent to the amount released during combustion, thereby achieving carbon neutrality [
15,
16]. Notably, life cycle assessment (LCA) studies have shown that ozone layer depletion, abiotic depletion, and fossil fuel consumption indicators can be reduced by approximately 16.09–44.09% compared with mineral diesel, with fossil fuel depletion substantially minimized. These results account for emissions from all stages, including feedstock cultivation, harvesting, oil extraction, conversion, and transportation [
17]. From a macro perspective, replacing conventional diesel with cottonseed biodiesel can significantly reduce net greenhouse gas emissions, offering meaningful benefits for mitigating global climate change. In addition, cottonseed, a by-product of the cotton industry, typically contains 20–25% oil [
18]. However, the presence of gossypol limits its use in the food industry. Converting this non-edible cottonseed oil into biodiesel not only avoids competition with food crops but also enhances the added value of the cotton industry chain, providing farmers and producers with an additional income stream. Ultimately, this approach delivers both environmental and economic benefits.
Similarly to cottonseed oil, this article believes that using waste cooking oil to produce biodiesel is also a good solution. The high price of biodiesel is an important factor hindering the development of the biodiesel industry. As a low-cost raw material, waste edible oil can not only reduce costs but also protect the environment. The rational and effective use of waste lard as a biofuel raw material means reducing waste and effectively preventing waste oil from being improperly treated or entering landfills [
19]. Abandoned restaurant lard is a good source of biodiesel raw materials, and it is usually a by-product of canteens, restaurants, and food processing industries. According to surveys, in countries such as China, South Korea, the United States, and Europe, pork is the main source of meat, and the amount of discarded restaurant lard produced every day is huge. It is an inexhaustible and excellent raw material. Reusing this waste instead of discharging or burying it can help reduce environmental pollution and waste disposal problems. In addition, the use of discarded restaurant lard to produce biodiesel effectively achieves the original intention of not competing with food crops for land.
Compared with diesel, biodiesel has a higher oxygen content, which helps improve the combustion process and reduce emissions such as carbon monoxide, hydrocarbons, and particulate matter. This characteristic gives biodiesel an advantage in reducing environmental pollution [
20]. Moreover, the carbon content of biodiesel is lower than that of diesel fuel, giving biodiesel significant advantages in reducing air pollution and environmental impact [
21].
Overall, biofuels represent a vital component of alternative energy sources, offering an important pathway for the global energy transition. Among various blending ratios, B20 is widely recognized as the highest biodiesel–diesel mixture that can be safely used in existing diesel engines without modification. It complies with international standards such as ASTM D7467 and presents relatively low risks in terms of material compatibility. Furthermore, compared with higher blends such as B30 or B50, B20 exhibits cold flow properties and storage stability closer to conventional diesel, reducing the likelihood of solidification in low-temperature environments or sediment formation during storage, thereby ensuring reliable fuel use. In this study, biodiesel was produced from cottonseed oil and waste lard through a transesterification process, and its blends with conventional diesel (B10 and B20) were systematically evaluated at different engine speeds using a four-cylinder, four-stroke, electronically controlled compression ignition (CI) engine. The objective of this work is to comprehensively assess the practicality of cottonseed oil and waste lard biodiesel as substitutes for diesel fuel and to explore their potential environmental and economic benefits. This study is expected to provide robust theoretical and practical support for reducing dependence on fossil fuels and mitigating harmful gas emissions.
3. Results and Analysis
3.1. Carbon Monoxide (CO)
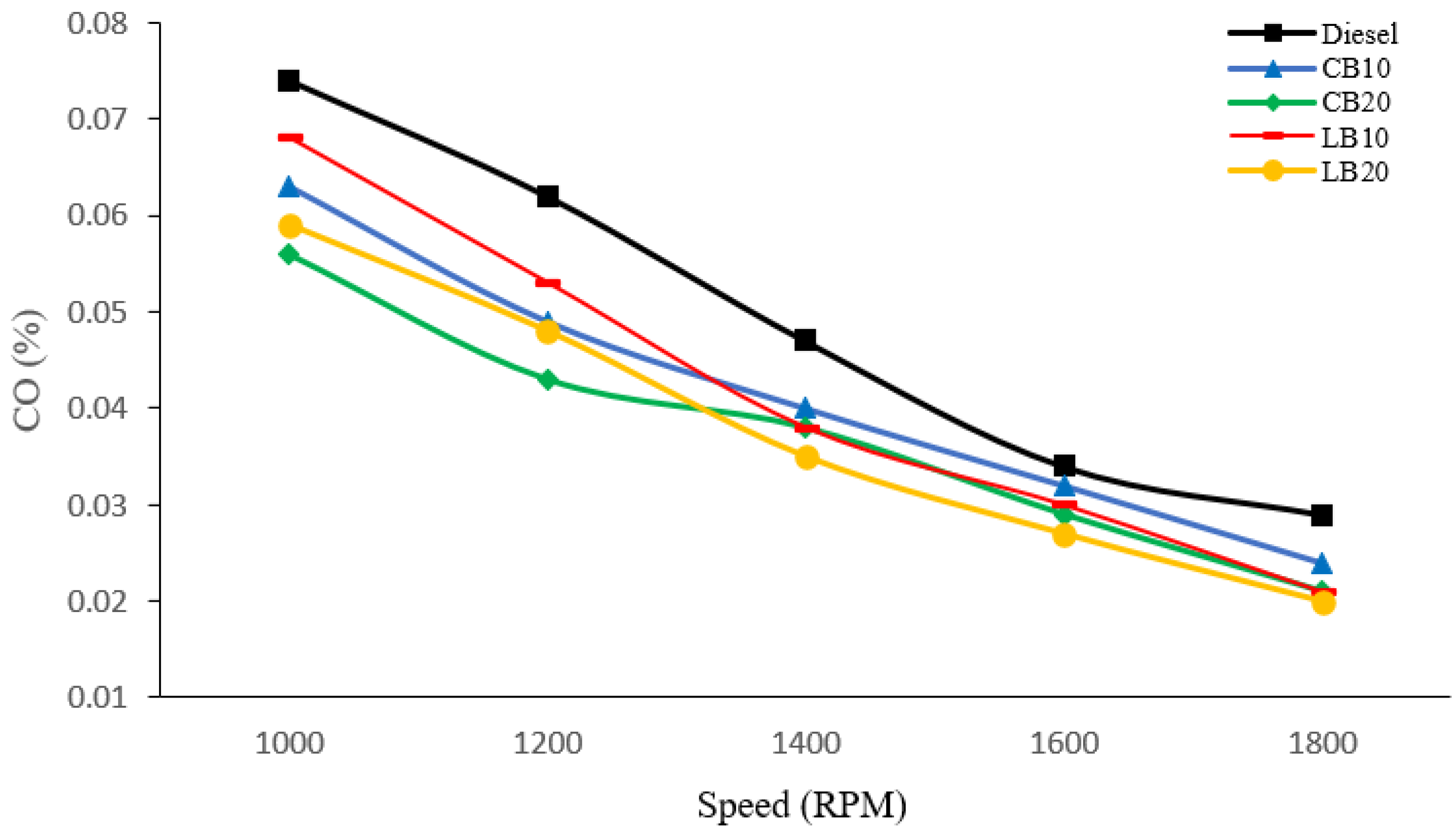
As shown in
Figure 2, the CO emissions of the five experimental fuels were compared at engine speeds of 1000, 1200, 1400, 1600, and 1800 rpm. It is evident that diesel fuel consistently exhibited the highest CO emissions across all speeds, with values of 0.074%, 0.062%, 0.047%, 0.034%, and 0.029%, respectively. In contrast, the CO emissions of biodiesel blends were lower than those of diesel, and further decreased with increasing biodiesel content. This reduction is mainly because CO formation originates from incomplete combustion caused by locally low temperatures and oxygen deficiency inside the cylinder [
22]. Biodiesel, as an oxygenated fuel, releases additional oxygen during combustion, which improves local oxygen concentration, increases combustion temperature, and promotes more complete fuel oxidation, thereby reducing CO emissions.
It is noteworthy that when the engine speed exceeded 1400 rpm, the CO emission trends of the fuels changed. At 1200 and 1400 rpm, CB20 exhibited the lowest CO emissions, whereas at higher speeds (1600 and 1800 rpm), LB20 produced lower CO emissions than the other fuels, with values of 0.035%, 0.027%, and 0.020%, respectively. This difference can be attributed to the distinct fatty acid compositions of the biodiesels. Waste cottonseed biodiesel (CB) contains a higher proportion of unsaturated fatty acids, whose molecular structures include double bonds and relatively shorter, less branched carbon chains. These characteristics result in lower boiling points and higher volatility, which facilitate rapid evaporation and the formation of a homogeneous combustible mixture at low-to-medium engine speeds, thereby enhancing combustion reactivity and reducing CO emissions [
23,
24]. In contrast, waste lard biodiesel (LB) is richer in saturated fatty acids, which have more stable molecular structures and higher boiling points. Although these properties hinder atomization and evaporation at lower speeds, they help maintain higher local combustion temperatures and more stable burning characteristics at elevated engine speeds, thus promoting more complete oxidation of hydrocarbons [
25].
3.2. Hydrocarbon (HC)
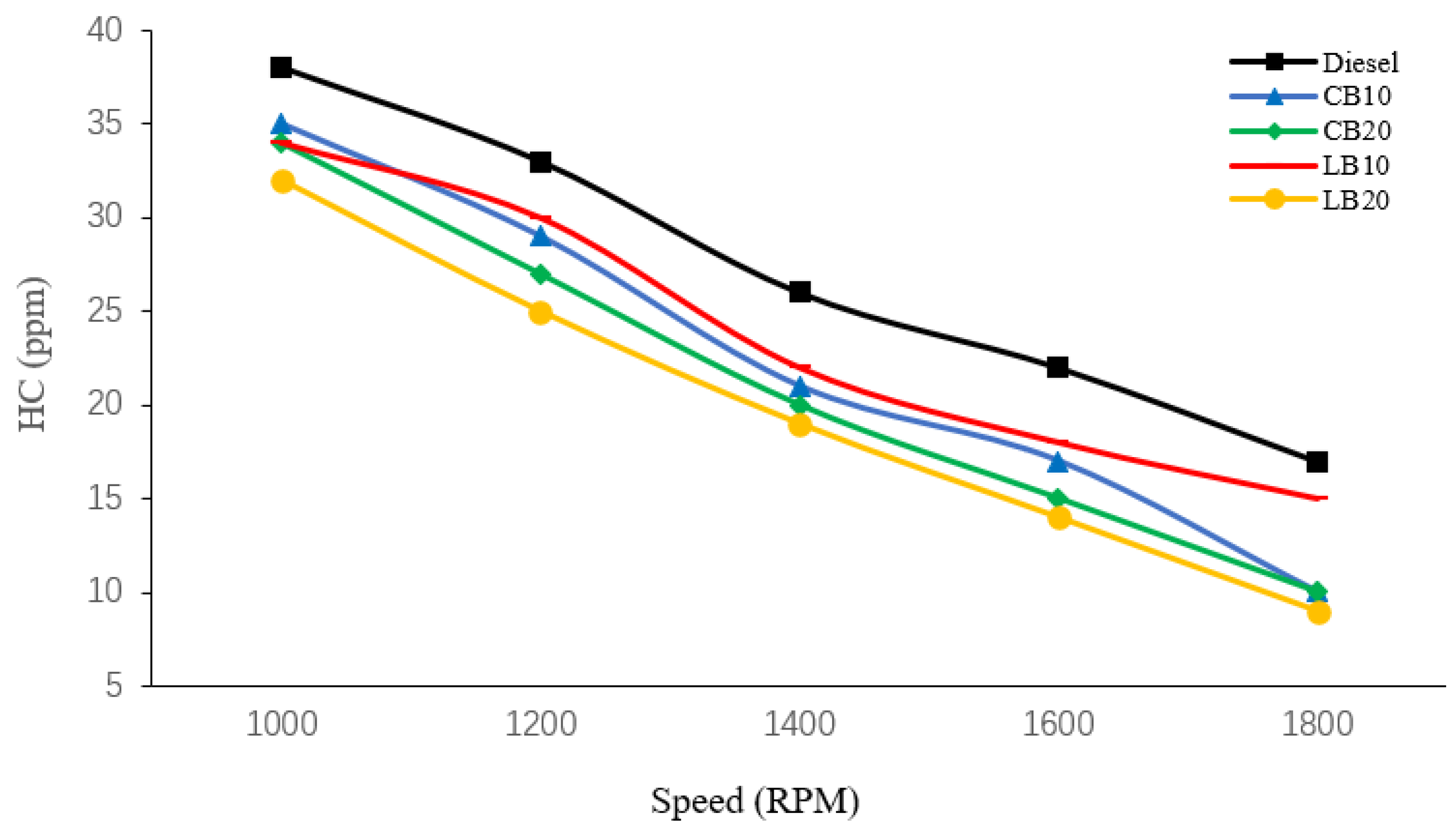
As shown in
Figure 3, the HC emissions of diesel and biodiesel blends at different engine speeds were compared. Diesel consistently exhibited the highest HC emissions at all speeds, with values of 38, 33, 26, 22, and 17 ppm, respectively. In contrast, the LB20 blend produced lower HC emissions of 32, 25, 19, 14, and 9 ppm at the corresponding speeds.
The HC emissions of biodiesel blends are lower than those of diesel for two main reasons. First, biodiesel contains inherent oxygen, which is released during combustion and promotes oxidation. At elevated temperatures, more hydrogen atoms combine with oxygen atoms, thereby reducing HC formation. Hoekman et al. [
23]. and Knothe [
25] reported that the higher oxygen content of biodiesel facilitates the cleavage of C–H bonds and enhances oxidation reactions, which significantly suppresses HC emissions. Second, the carbon-to-hydrogen ratio of biodiesel is lower than that of diesel, which reduces the number of carbon and hydrogen atoms available for HC formation and further decreases emissions. In comparison with other studies, Demirbas [
26] and Ravishankar Sathyamurthy et al. [
27]. also demonstrated that almost all types of biodiesels including those derived from waste oils, cottonseed oil, and soybean oil consistently produce lower HC emissions than diesel under a wide range of engine operating conditions.
From a more fundamental perspective, HC emissions mainly originate from unburned mixtures inside the combustion chamber. In particular, low-temperature regions near the cylinder walls hinder flame propagation, leaving portions of the mixture unburned. In addition, some unburned mixture may be compressed into crevice volumes and subsequently released when the exhaust valve opens, contributing to HC emissions. Therefore, even under overall efficient combustion, a certain level of HC formation is unavoidable. Meanwhile, LB20 consistently exhibited the lowest HC emissions across all speeds, which can be attributed to its lower carbon-to-hydrogen ratio.
Moreover, HC emissions of all fuels decreased with increasing engine speed, showing a trend similar to that of CO emissions. This is mainly because higher speeds lead to elevated combustion temperatures, more complete combustion, and thus reduced HC formation.
3.3. Carbon Dioxide (CO2)
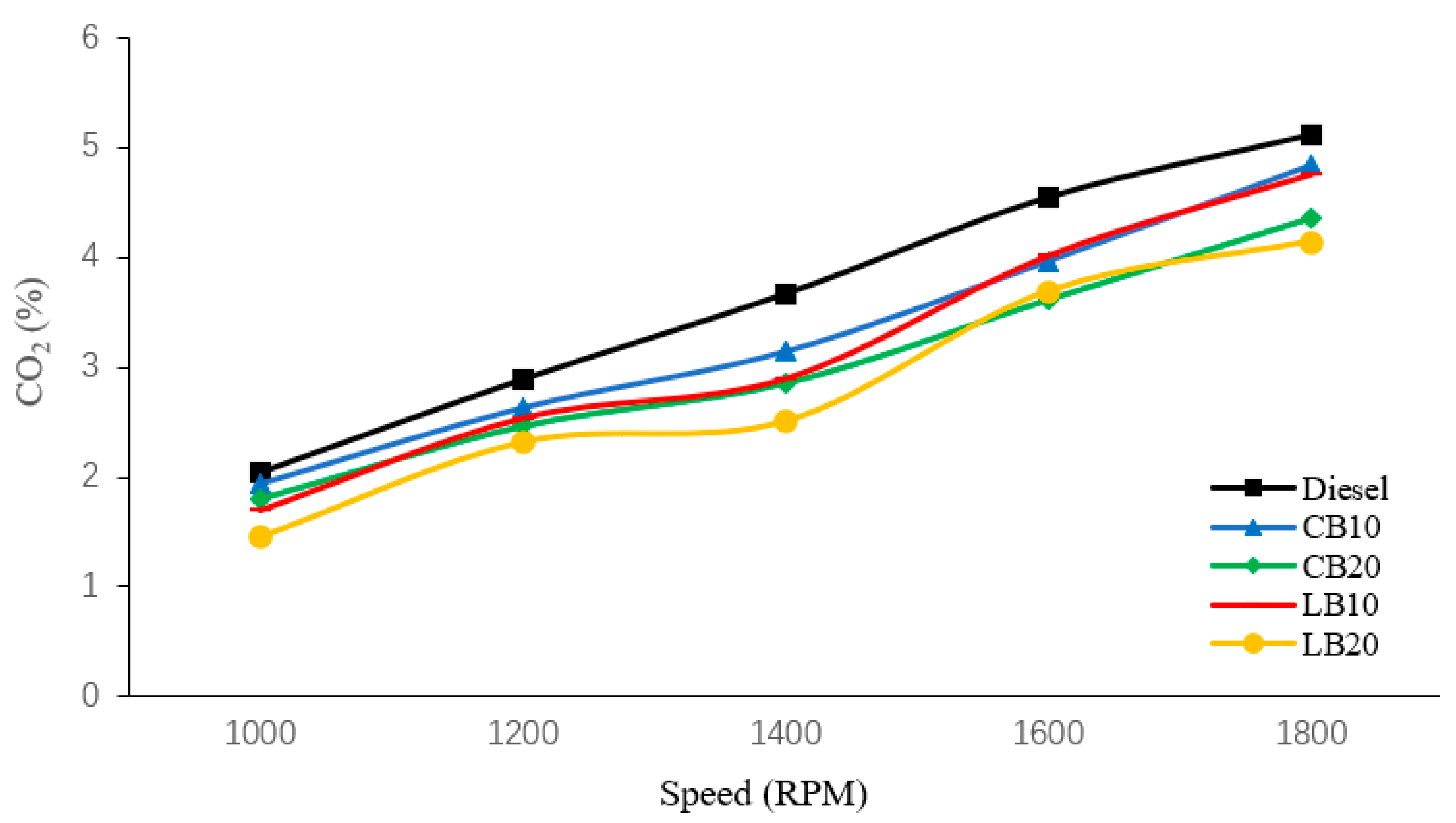
Figure 4 is a schematic diagram of the variation of CO
2 emissions from all experimental fuels with rotational speed. As shown in the figure, the CO
2 emissions from diesel fuel are higher than those of the other four blends, with 2.04%, 2.89%, 3.67%, 4.55%, and 5.12% at 1000 rpm, 1200 rpm, 1400 rpm, 1600 rpm, and 1800 rpm, respectively.
Compared with diesel, the CO
2 emissions of biodiesel blends are significantly reduced. Among all blends, the CO
2 emissions of CB20 and LB20 blends are lower. This is because the carbon atom content of biodiesel is much lower than that of diesel fuel, resulting in lower CO
2 emissions from the blends. A. Pugazhendhi et al. [
28] and Yohannes Kefale Mangesha et al. [
29] also observed a decreasing trend in CO
2 emissions with increasing biodiesel content in blended fuels when studying other types of biodiesels. They attributed this to the lower carbon-to-hydrogen ratio of biodiesel compared with diesel fuel, as well as the higher proportion of oxygen atoms in its molecular structure, which results in less carbon available per unit of energy to be converted into CO
2.
As the rotational speed increases, the CO2 emissions of diesel, CB10, CB20, LB10, and LB20 increase from 2.04%, 1.94%, 1.81%, 1.69%, and 1.46% at 1000 rpm to 5.12%, 4.83%, 4.36%, 4.75%, and 4.14% at 1800 rpm. On the one hand, fuel combustion becomes more complete and oxidation rate increases; On the other hand, at high speeds, fuel consumption increases, and more fuel participates in combustion, resulting in an increase in CO2 emissions.
3.4. Nitrogen Oxide Compound (NOx)
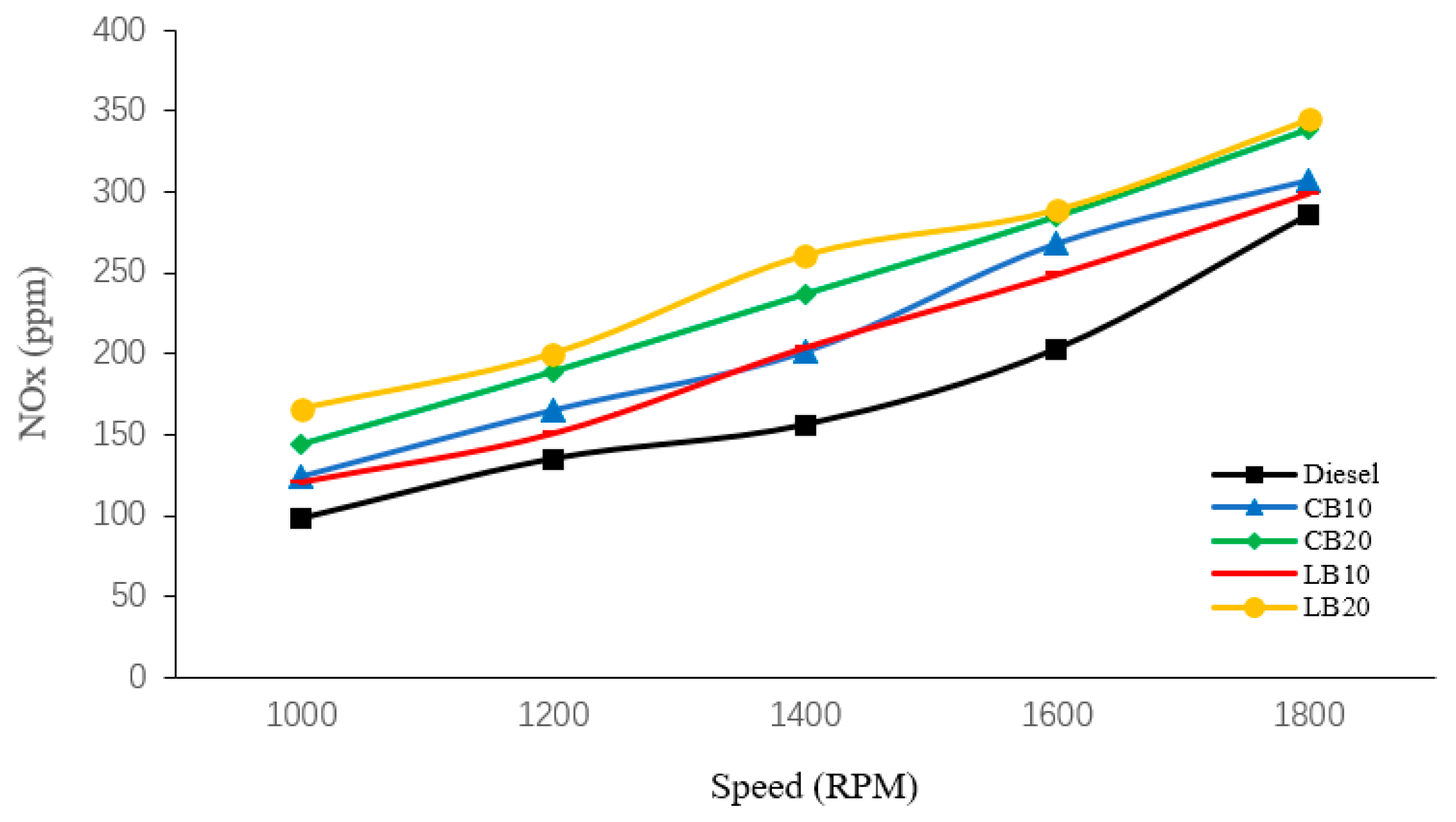
As shown in
Figure 5, diesel fuel exhibits the lowest NOx emissions among all tested fuels, while biodiesel blends consistently produce higher NOx levels than diesel. With an increasing biodiesel proportion, NOx emissions rise significantly. At all engine speeds, the NOx emissions of B20 blends are higher than those of B10 blends. Specifically, at 1000, 1200, and 1400 rpm, LB20 shows slightly higher NOx emissions than CB20, whereas at 1600 and 1800 rpm, the two are nearly identical.
The formation of NOx is primarily governed by in-cylinder oxygen concentration, combustion temperature, and reaction residence time. As an oxygenated fuel, biodiesel enhances the reaction rate and flame propagation. This leads to higher peak temperatures and prolonged high-temperature duration, thereby increasing NOx formation. Furthermore, waste lard biodiesel, with its relatively higher cetane number, tends to ignite earlier and produce higher peak temperatures, which further contributes to NOx formation. These differences explain the variations in NOx emissions between the two biodiesels under different engine speeds.
With increasing engine speed, NOx emissions from all fuels show an upward trend. This is because higher speeds intensify fuel injection and combustion, raising the in-cylinder temperature. At the same time, the oxygenated nature of biodiesel promotes more complete combustion, further increasing NOx generation. On the other hand, the shorter residence time at high speeds may limit the conversion of nitrogen oxides back to nitrogen, thereby maintaining elevated NOx levels. Yadelew Likina Alehegn [
30] reported similar findings in a study on Millettia ferruginea biodiesel, where an increasing trend of oxygen content in biodiesel was found to promote more complete combustion. This, in turn, led to higher combustion temperatures and consequently increased NOx emissions. The studies of Edgar N. Tec-Caamal et al. [
31] and Gopinath Dhamodaran et al. [
32] further corroborate the conclusions of the present work.
Although biodiesel demonstrates clear advantages in reducing CO and HC emissions, it is often associated with increased NOx, which remains a major obstacle to its broader application. Several strategies have been proposed to mitigate this issue. For example, optimizing the exhaust gas recirculation (EGR) rate can effectively lower combustion temperatures and suppress the formation of thermal NOx [
33]. In addition, alcohol-based fuels and water-emulsified fuels, due to their high latent heat of vaporization, can further reduce combustion temperatures and significantly decrease NOx emissions [
34,
35]. Therefore, future applications and research should focus on integrating engine structural optimization with fuel modification strategies, as effective NOx control will be essential for advancing the clean utilization of biodiesel.
3.5. Exhaust Gas Temperature (EGT)
The EGT changes at different engine speeds using diesel and several biodiesel blends as fuel are shown in
Figure 6. Under all engine operating conditions, the EGT of diesel fuel remained the lowest, at 89.3 °C, 113.5 °C, 127.3 °C, 138.7 °C, and 154.7 °C, respectively. At 1000 rpm, 1200 rpm, and 1400 rpm, the EGT of the CB20 blend was the highest, at 114.5 °C, 131 °C, and 149.1 °C, respectively. At 1600 rpm and 1800 rpm, LB20 blend exceeded CB20 blend, reaching the highest recorded EGT, at 171.4 °C and 189.1 °C, respectively.
The cetane number and oxygen content of biodiesel blends are higher than those of pure diesel fuel. The ignition delay during combustion is shortened, and the fuel is fully burned in the combustion chamber. The release of oxygen further promotes combustion, increases combustion temperature, and increases EGT.
As the engine speed increases, the EGT of all fuels shows an increasing trend. At high speeds, the combustion rate of the engine accelerates, and the amount of fuel burned per minute increases. This leads to more fuel being burned in a short period of time, releasing more heat into the exhaust gas and increasing its temperature.
3.6. Brake Thermal Efficiency (BTE)
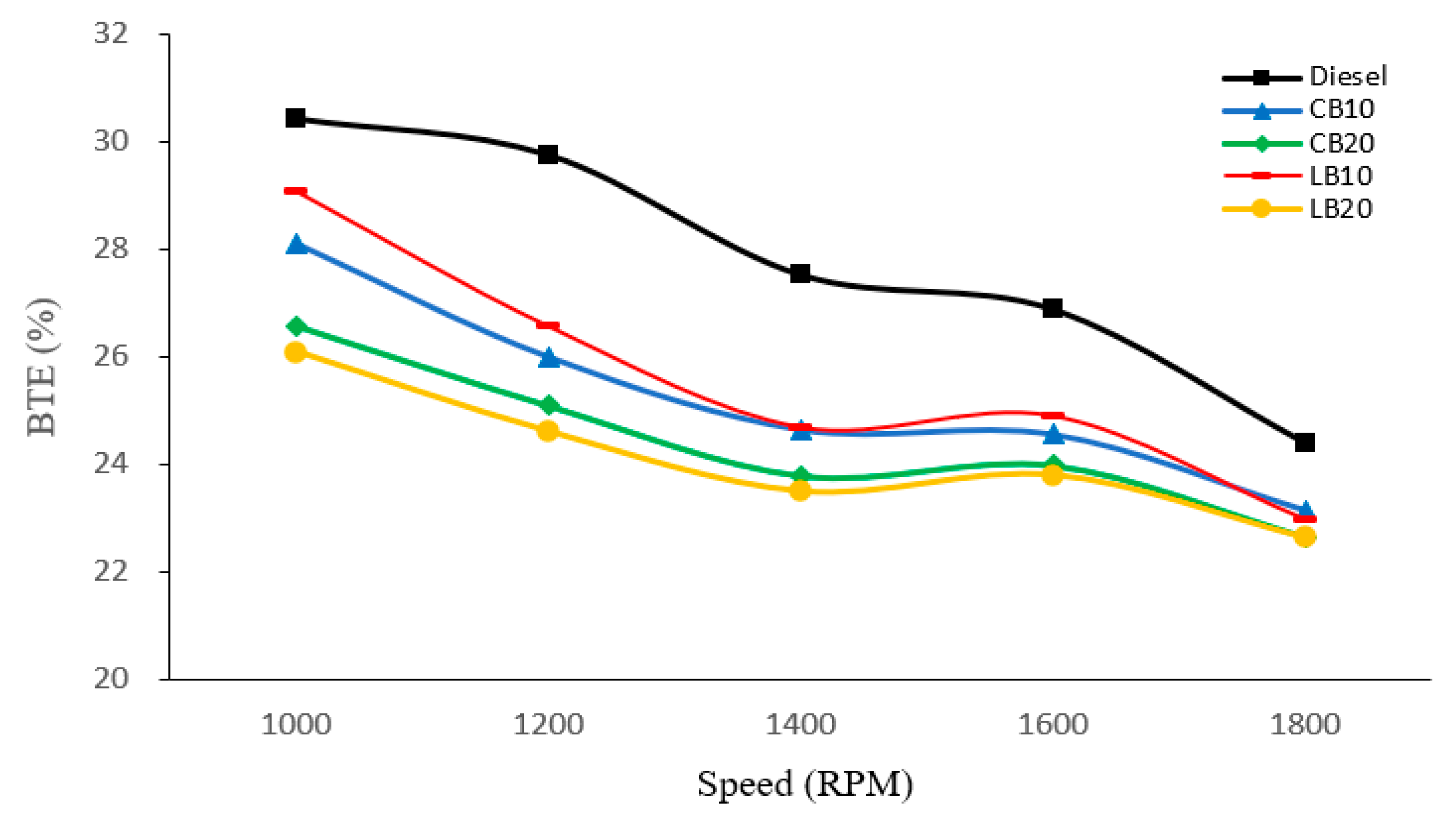
As shown in
Figure 7, the BTE of different fuels was compared across various engine speeds. Diesel consistently exhibited the highest BTE, with a value of 30.43% at 1000 rpm, decreasing to 24.37% at 1800 rpm. In contrast, the biodiesel blends showed lower overall BTE, with B10 blends performing slightly better than B20 blends. Compared with CB10, LB10 achieved a higher BTE, while both CB20 and LB20 recorded the lowest overall BTE values.
The lower BTE of biodiesel blends compared to diesel can be attributed to two main factors. First, biodiesel has a lower calorific value, meaning that less energy is released per unit mass of fuel, which reduces the effective power output. Second, the higher viscosity of biodiesel limits atomization and combustion characteristics, leading to slower combustion and reduced thermal efficiency. A. Madhan Kuma et al. [
36] reported a similar trend, attributing the reduction in BTE primarily to the lower calorific value of biodiesel. In contrast, Usame Demird et al. [
37] argued that, in addition to the lower calorific value, the higher viscosity of biodiesel is also a significant contributing factor. These findings are consistent with the conclusions of the present study.
In addition, the variation in BTE is not only influenced by fuel heating value and combustion properties but also by heat transfer losses within the combustion chamber. The oxygenated nature of biodiesel elevates the peak combustion temperature, which intensifies heat transfer losses and further reduces effective power.
With increasing engine speed, the BTE of all fuels shows a downward trend. This is because, at higher speeds, the combustion duration becomes shorter, preventing part of the fuel from burning at the optimal crank angle, thereby lowering the efficiency of energy conversion. At the same time, frictional and pumping losses increase with speed, which further contributes to the overall decline in BTE.
3.7. Brake Specific Fuel Consumption (BSFC)
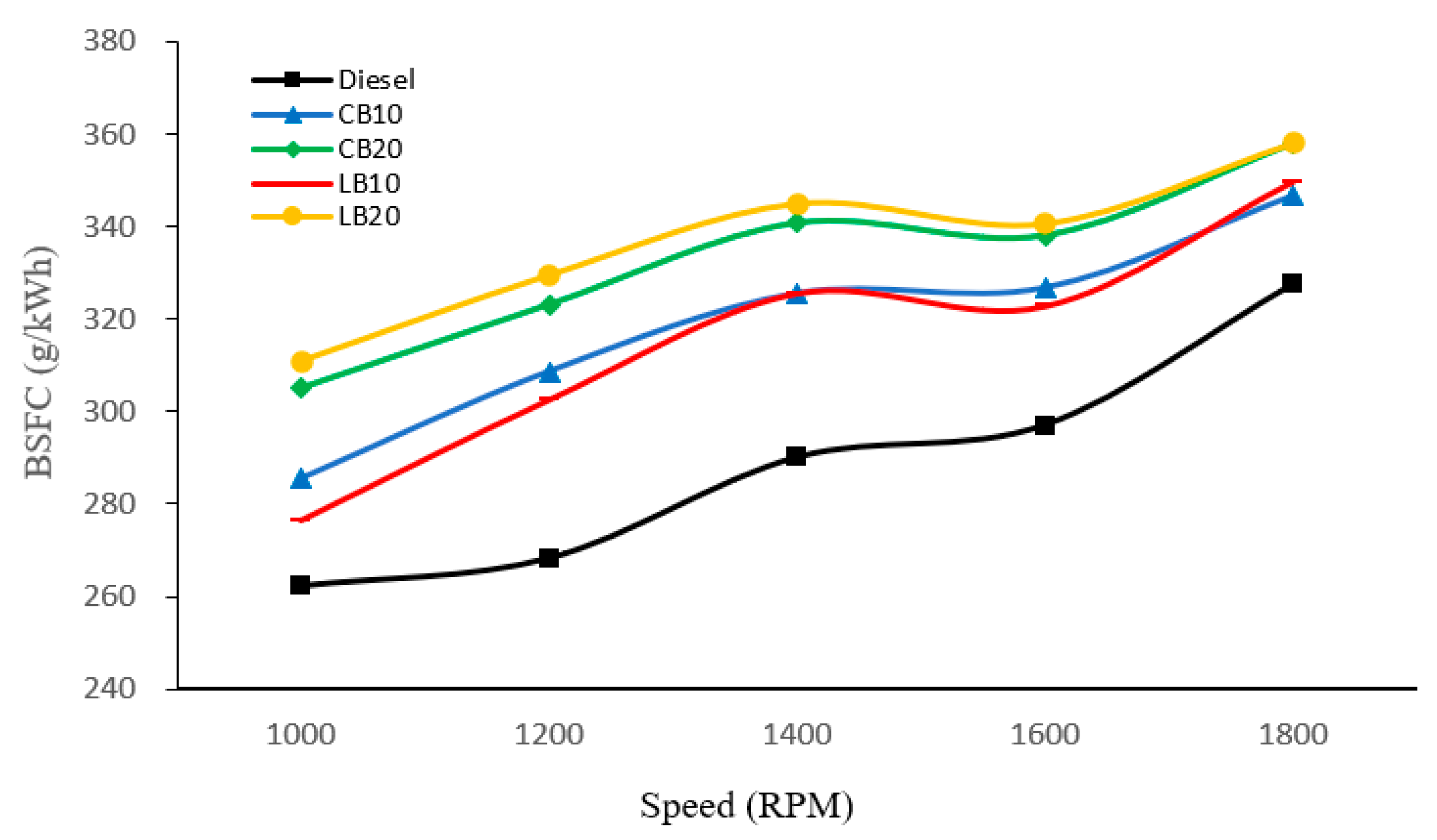
As shown in
Figure 8, the BSFC of different fuels was compared across various engine speeds. It is evident that diesel consistently exhibits the lowest BSFC at all speeds, with a value of approximately 262.21 g/kWh at 1000 rpm, gradually increasing to about 327.5 g/kWh at 1800 rpm. In contrast, biodiesel blends show significantly higher BSFC values than diesel, and BSFC further increases with higher blending ratios. Among all fuels, LB20 consistently records the highest BSFC, reaching nearly 358.11 g/kWh at 1800 rpm.
The higher BSFC of biodiesel blends compared to diesel can be explained by two main factors. First, the lower calorific value of biodiesel means that more fuel is required to produce the same power output, which directly increases BSFC. Second, the higher viscosity of biodiesel limits spray atomization and mixing, leading to slower combustion rates and reduced thermal efficiency, thereby further elevating BSFC.
From the perspective of fuel composition, differences also exist between waste cottonseed biodiesel and waste lard biodiesel. LB20 consistently exhibits higher BSFC than the other fuels, primarily because waste lard biodiesel has a lower calorific value, which increases fuel consumption to achieve the same power output.
In addition, the BSFC of all fuels shows a gradual upward trend with increasing engine speed. At higher speeds, the combustion duration becomes shorter, preventing some of the fuel from burning at the optimal crank angle, which lowers energy conversion efficiency. At the same time, frictional and pumping losses rise with increasing speed, further contributing to the overall increase in BSFC.
4. Conclusions
In this study, cottonseed oil biodiesel and waste lard biodiesel were blended with diesel at different ratios (B10 and B20). Their performance and emission characteristics were systematically evaluated at various engine speeds under a constant load of 50% using a four-cylinder, four-stroke, electronically controlled compression ignition engine.
The results showed that, compared with pure diesel, biodiesel blends significantly reduced CO, HC, and CO2 emissions. At 1800 rpm, the LB20 blend achieved reductions of 31.03% in CO, 47.06% in HC, and 19.14% in CO2. These improvements were mainly attributed to the higher oxygen content and lower hydrogen-to-carbon ratio of biodiesel, which promoted more complete combustion. However, all biodiesel blends exhibited higher NOx emissions than diesel, with the increase becoming more pronounced at higher blending ratios. At 1800 rpm, LB20 recorded the highest NOx emissions, 20.63% higher than those of diesel under the same condition.
In terms of performance, biodiesel blends showed higher BSFC and lower BTE compared with diesel, primarily due to their lower calorific value and higher viscosity. Notably, LB20 exhibited the lowest BTE (22.64%) and the highest BSFC (358.11 g/kWh) at 1800 rpm.
Overall, cottonseed oil and waste lard biodiesel demonstrate clear advantages in reducing certain emissions, but they also face challenges such as increased NOx emissions and reduced fuel economy. Therefore, future research should focus on fuel modification strategies (such as the addition of nanoparticles, alcohols, or water emulsification), engine optimization techniques (including injection timing adjustment and exhaust gas recirculation), multi-physics modeling, and reaction kinetics analysis, as well as life cycle and economic assessments. These efforts will help enhance fuel economy while maintaining environmental benefits, thereby promoting the sustainable application of biodiesel in the transportation and energy sectors.