Long-Term Evaluation of CNT-Clad Stainless-Steel Cathodes in Multi-Channel Microbial Electrolysis Cells Under Variable Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Fabrication of a Multi-Channel Electrode Test Cassette
2.2. MEC Operation and Cathode Evaluation
2.3. Fabrication and Preparation of CNT and Carbon Cloth Cathode Material
2.4. Acid Whey Feedstock Characterization
2.5. Data Analysis and Calculations
3. Results and Discussion
3.1. Characterization of CNT Materials
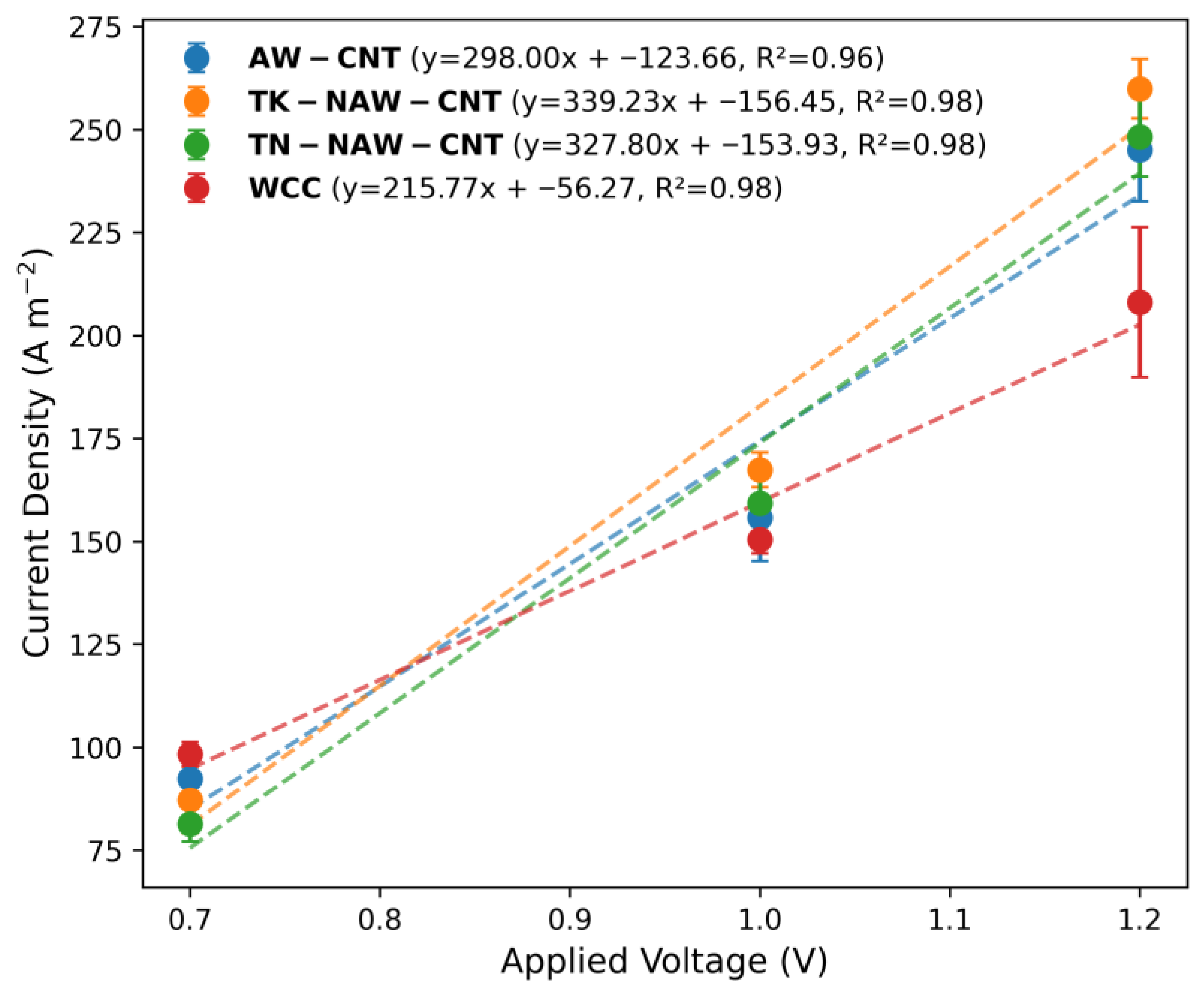
3.2. Effect of Applied Voltage on MEC Cathode Performance
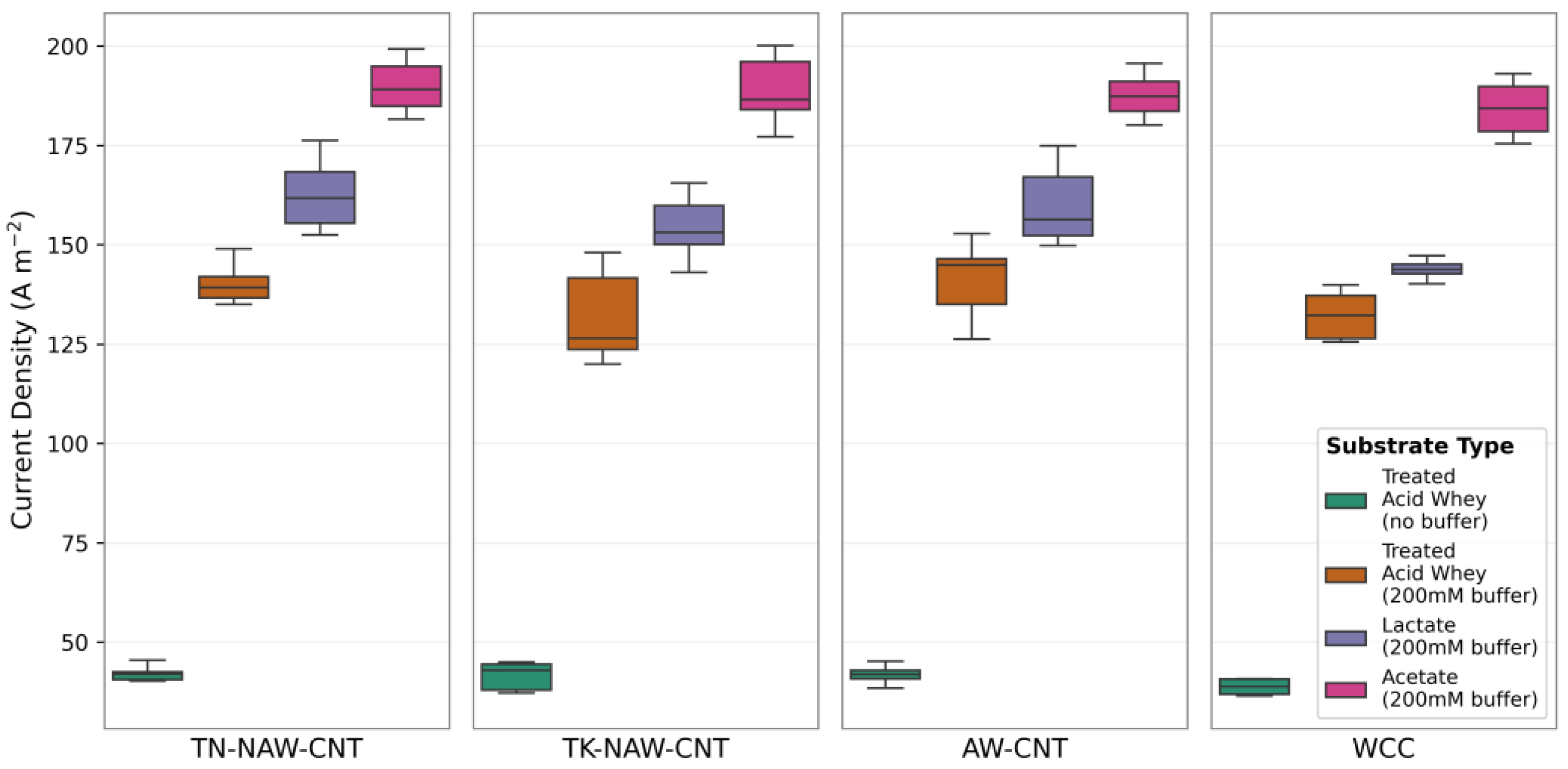
3.3. Effect of Nutritional Substrate on MEC Cathode Performance
3.4. Effect of Buffer Type and Concentration on MEC Performance
3.5. Long-Term Stability of Cathode Material
3.6. Hydrogen Production Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MEC | Microbial electrolysis cells |
| CNT | Carbon nanotube |
| AW-CNT | Acid-washed carbon nanotube |
| TN-NAW-CNT | Thin layer non-acid-washed carbon nanotube |
| TK-NAW-CNT | Thick layer non-acid-washed carbon nanotube |
| MoP | Molybdenum phosphide |
| WCC | Woven carbon cloth |
| BES | Bio-electrochemical system |
| COD | Chemical oxygen demand |
| BOD | Biological oxygen demand |
| EAM | Electrochemically active microorganisms |
| VFA | Volatile fatty acids |
| R | Cell resistance |
| I | Electrical current |
| V | Voltage |
| HER | Hydrogen evolution reaction |
| HPR | Hydrogen production rate |
| SS | Stainless steel |
| SSA | Specific surface area |
| Pt | Platinum |
| PBS | Phosphate buffer system |
| BBS | Bicarbonate buffer system |
| NaOH | Sodium hydroxide |
| HCl | Hydrochloric acid |
| CVD | Chemical vapor deposition |
| CV | Cyclic voltammetry |
| ASR | Area-specific resistance |
| LME | Linear mixed effects model |
| EMM | Estimated marginal means |
| HCO3− | Bicarbonate ion |
| H2CO3 | Carbonic acid |
| CO2 | Carbon dioxide |
| CO32− | Carbonate ion |
| H3PO4 | Phosphoric acid |
| H2PO4− | Dihydrogen phosphate |
| HPO42− | Hydrogen phosphate |
| PO43− | Phosphate |
References
- Cipriani, G.; Dio, V.D.; Genduso, F.; Cascia, D.L.; Liga, R.; Miceli, R.; Galluzzo, G.R. Perspective on Hydrogen Energy Carrier and Its Automotive Applications. Int. J. Hydrogen Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- Call, D.; Logan, B.E. Hydrogen Production in a Single Chamber Microbial Electrolysis Cell Lacking a Membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive Microorganisms in Bioelectrochemical Systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically Assisted Microbial Production of Hydrogen from Acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. Sustainable and Efficient Biohydrogen Production via Electrohydrogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18871–18873. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. High Hydrogen Production Rate of Microbial Electrolysis Cell (MEC) with Reduced Electrode Spacing. Bioresour. Technol. 2011, 102, 3571–3574. [Google Scholar] [CrossRef] [PubMed]
- Satinover, S.J.; Schell, D.; Borole, A.P. Achieving High Hydrogen Productivities of 20 L/L-Day via Microbial Electrolysis of Corn Stover Fermentation Products. Appl. Energy 2020, 259, 114126. [Google Scholar] [CrossRef]
- Tartakovsky, B.; Manuel, M.-F.; Wang, H.; Guiot, S.R. High Rate Membrane-Less Microbial Electrolysis Cell for Continuous Hydrogen Production. Int. J. Hydrogen Energy 2008, 34, 672–677. [Google Scholar] [CrossRef]
- Singh, L.; Miller, A.G.; Wang, L.; Liu, H. Scaling-up up-Flow Microbial Electrolysis Cells with a Compact Electrode Configuration for Continuous Hydrogen Production. Bioresour. Technol. 2021, 331, 125030. [Google Scholar] [CrossRef]
- Guerrero-Sodric, O.; Baeza, J.A.; Guisasola, A. Exploring Key Operational Factors for Improving Hydrogen Production in a Pilot-Scale Microbial Electrolysis Cell Treating Urban Wastewater. Chem. Eng. J. 2023, 469, 144001. [Google Scholar] [CrossRef]
- Park, S.G.; Rajesh, P.P.; Sim, Y.U.; Jadhav, D.A.; Noori, M.T.; Kim, D.H.; Al-Qaradawi, S.Y.; Yang, E.; Jang, J.K.; Chae, K.J. Addressing Scale-up Challenges and Enhancement in Performance of Hydrogen-Producing Microbial Electrolysis Cell through Electrode Modifications. Energy Rep. 2022, 8, 2726–2746. [Google Scholar] [CrossRef]
- Mccrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhao, S.; Zhao, K.; Muqsit, A.; Tang, H.; Chang, L.; Zhao, H.; Gao, Y.; Tang, Z. Ultrathin Platinum Nanowires Grown on Single-Layered Nickel Hydroxide with High Hydrogen Evolution Activity. Nat. Commun. 2015, 6, 6430. [Google Scholar] [CrossRef] [PubMed]
- Suharto, T.E.; Satar, I.; Daud, W.R.W.; Somalu, M.R.; Hong, K.B. Recent Advancement of Nickel Based-Cathode for The Microbial Electrolysis Cell (MEC) and Its Future Prospect. J. Eng. Sci. Technol. Rev. 2022, 15, 191–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Merrill, M.D.; Logan, B.E. The Use and Optimization of Stainless Steel Mesh Cathodes in Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2010, 35, 12020–12028. [Google Scholar] [CrossRef]
- Farhangi, S.; Ebrahimi, S.; Niasar, M.S. Commercial Materials as Cathode for Hydrogen Production in Microbial Electrolysis Cell. Biotechnol. Lett. 2014, 36, 1987–1992. [Google Scholar] [CrossRef]
- Satar, I.; Abu Bakar, M.H.; Wan Daud, W.R.; Mohd Yasin, N.H.; Somalu, M.R.; Kim, B.H. Feasibility of Ni/Ti and Ni/GF Cathodes in Microbial Electrolysis Cells for Hydrogen Production from Fermentation Effluent: A Step toward Real Application. Int. J. Energy Res. 2020, 44, 7464–7476. [Google Scholar] [CrossRef]
- Lei, G.; Wang, Y.; Xiao, G.; Su, H. CoMo/SS Cathode Catalyst for Enhanced Hydrogen Production in Microbial Electrolysis Cells. Catalysts 2025, 15, 439. [Google Scholar] [CrossRef]
- Jayabalan, T.; Manickam, M.; Naina Mohamed, S. NiCo2O4-Graphene Nanocomposites in Sugar Industry Wastewater Fed Microbial Electrolysis Cell for Enhanced Biohydrogen Production. Renew. Energy 2020, 154, 1144–1152. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Chandrasekhar, K.; Ismail, M.; Kalil, M.S. Hydrogen Gas Production with an Electroformed Ni Mesh Cathode Catalysts in a Single-Chamber Microbial Electrolysis Cell (MEC). Int. J. Hydrogen Energy 2015, 40, 14095–14103. [Google Scholar] [CrossRef]
- Jayabalan, T.; Matheswaran, M.; Preethi, V.; Naina Mohamed, S. Enhancing Biohydrogen Production from Sugar Industry Wastewater Using Metal Oxide/Graphene Nanocomposite Catalysts in Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2020, 45, 7647–7655. [Google Scholar] [CrossRef]
- Xie, X.; Song, M.; Wang, L.; Engelhard, M.H.; Luo, L.; Miller, A.; Zhang, Y.; Du, L.; Pan, H.; Nie, Z.; et al. Electrocatalytic Hydrogen Evolution in Neutral pH Solutions: Dual-Phase Synergy. ACS Catal. 2019, 9, 8712–8718. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Liu, X.-W.; Sun, X.-F.; Sheng, G.-P.; Zhang, Y.-Y.; Yan, G.-M.; Wang, S.-G.; Xu, A.-W.; Yu, H.-Q. A New Cathodic Electrode Deposit with Palladium Nanoparticles for Cost-Effective Hydrogen Production in a Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2011, 36, 2773–2776. [Google Scholar] [CrossRef]
- Kundu, A.; Sahu, J.N.; Redzwan, G.; Hashim, M.A. An Overview of Cathode Material and Catalysts Suitable for Generating Hydrogen in Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2013, 38, 1745–1757. [Google Scholar] [CrossRef]
- Chae, K.-J.; Choi, M.-J.; Kim, K.-Y.; Ajayi, F.F.; Chang, I.-S.; Kim, I.S. A Solar-Powered Microbial Electrolysis Cell with a Platinum Catalyst-Free Cathode To Produce Hydrogen. Environ. Sci. Technol. 2009, 43, 9525–9530. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jimenez, D.A.; Kim, K.-Y. Enhanced Wettability Improves Catalytic Activity of Nickel-Functionalized Activated Carbon Cathode for Hydrogen Production in Microbial Electrolysis Cells. Bioresour. Technol. 2022, 350, 126881. [Google Scholar] [CrossRef]
- Crandall, B.S.; Naughton, M.; Park, S.; Yu, J.; Zhang, C.; Mahtabian, S.; Wang, K.; Liang, X.; Fu, K.; Jiao, F. Transforming CO2 into Advanced 3D Printed Carbon Nanocomposites. Nat. Commun. 2024, 15, 10568. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Huang, Q.; Feng, Y.; Zhu, S.; Shen, S. Hydrogen Production with Carbon Nanotubes Based Cathode Catalysts in Microbial Electrolysis Cells. J. Chem. Technol. Biotechnol. 2012, 87, 1150–1156. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Chen, L.; Li, P.; Zhu, S.; Shen, S. Microbial Electrolysis Cells with Polyaniline/Multi-Walled Carbon Nanotube-Modified Biocathodes. Energy 2015, 88, 377–384. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Y.; Xu, Y.; Qiu, Y.; Chen, Y.; Zhu, S.; Shen, S. Hydrogen Production with Polyaniline/Multi-Walled Carbon Nanotube Cathode Catalysts in Microbial Electrolysis Cells. J. Chem. Technol. Biotechnol. 2015, 90, 1263–1269. [Google Scholar] [CrossRef]
- Wang, L.; Linowski, K.; Islam, M.Z.; Harrison, H.; Yu, C.; Liu, H. Efficient Hydrogen Production from Low-Conductivity High-Strength Wastewater without Buffer Addition Using Compact Electrode Assemblies in Membraneless Microbial Electrolysis Cells. Chem. Eng. J. 2025, 519, 165062. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, G.J. Electrical Conductivity of Carbon Nanotube- and Graphene-Based Nanocomposites. In Micromechanics Nanomechanics Composite Solids; Springer International Publishing: Cham, Switzerland, 2018; pp. 123–156. [Google Scholar] [CrossRef]
- Braunovic, M. Electrical Contacts: Fundamentals, Applications and Technology; Electrical and Computer Engineering; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-57444-727-9. [Google Scholar]
- Liu, H.; Logan, B.E. Electricity Generation Using an Air-Cathode Single Chamber Microbial Fuel Cell in the Presence and Absence of a Proton Exchange Membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Tan, J.; Jin, H.; Kim, Y.H.; Yang, X.; Son, D.H.; Ahn, S.; Zhou, H.; Yu, C. Creating Effective Nanoreactors on Carbon Nanotubes with Mechanochemical Treatments for High-Areal-Capacity Sulfur Cathodes and Lithium Anodes. Adv. Funct. Mater. 2018, 28, 1800595. [Google Scholar] [CrossRef]
- Wang, L.; Long, F.; Liang, D.; Xiao, X.; Liu, H. Hydrogen Production from Lignocellulosic Hydrolysate in an Up-Scaled Microbial Electrolysis Cell with Stacked Bio-Electrodes. Bioresour. Technol. 2021, 320, 124314. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Lme4: Linear Mixed-Effects Models Using “Eigen” and S4; 1.1-37; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, 2017; 1.11.2-8; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar] [CrossRef]
- Kane, S.; Ulrich, R.; Harrington, A.; Stadie, N.P.; Ryan, C. Physical and Chemical Mechanisms That Influence the Electrical Conductivity of Lignin-Derived Biochar. Carbon Trends 2021, 5, 100088. [Google Scholar] [CrossRef]
- Ouyang, L.; Thamdrup, B.; Trimmer, M. Coupled Nitrification and N2 Gas Production as a Cryptic Process in Oxic Riverbeds. Nat. Commun. 2021, 12, 1217. [Google Scholar] [CrossRef]
- Boldini, A.; Rosen, M.; Cha, Y.; Porfiri, M. Contactless Actuation of Perfluorinated Ionomer Membranes in Salt Solution: An Experimental Investigation. Sci. Rep. 2019, 9, 11989. [Google Scholar] [CrossRef]
- Lee, B.; Kim, C. Innovative Membrane Technology for Water Treatment Solutions: Current Status and Future Prospects of Carbon Nanotube Membranes. Environ. Eng. Res. 2024, 29, 240104. [Google Scholar] [CrossRef]
- Rousseau, R.; Ketep, S.F.; Etcheverry, L.; Délia, M.-L.; Bergel, A. Microbial Electrolysis Cell (MEC): A Step Ahead towards Hydrogen-Evolving Cathode Operated at High Current Density. Bioresour. Technol. Rep. 2020, 9, 100399. [Google Scholar] [CrossRef]
- Rossi, R.; Baek, G.; Logan, B.E. Vapor-Fed Cathode Microbial Electrolysis Cells with Closely Spaced Electrodes Enables Greatly Improved Performance. Environ. Sci. Technol. 2022, 56, 1211–1220. [Google Scholar] [CrossRef]
- Rossi, R.; Nicolas, J.; Logan, B.E. Using Nickel-Molybdenum Cathode Catalysts for Efficient Hydrogen Gas Production in Microbial Electrolysis Cells. J. Power Sources 2023, 560, 232594. [Google Scholar] [CrossRef]
- Erbay, C.; Pu, X.; Choi, W.; Choi, M.-J.; Ryu, Y.; Hou, H.; Lin, F.; De Figueiredo, P.; Yu, C.; Han, A. Control of Geometrical Properties of Carbon Nanotube Electrodes towards High-Performance Microbial Fuel Cells. J. Power Sources 2015, 280, 347–354. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; Zeng, Y.K.; An, L.; Wei, L. A Highly Permeable and Enhanced Surface Area Carbon-Cloth Electrode for Vanadium Redox Flow Batteries. J. Power Sources 2016, 329, 247–254. [Google Scholar] [CrossRef]
- Speers, A.M.; Reguera, G. Electron Donors Supporting Growth and Electroactivity of Geobacter Sulfurreducens Anode Biofilms. Appl. Environ. Microbiol. 2012, 78, 437–444. [Google Scholar] [CrossRef]
- Regueira-Marcos, L.; Muñoz, R.; García-Depraect, O. Biogenic Hydrogen Production from Household Food Waste via Lactate-Driven Dark Fermentation: A Comparative Study of Single-Stage and Two-Stage Configurations. J. Environ. Chem. Eng. 2025, 13, 117672. [Google Scholar] [CrossRef]
- Fan, Y.; Han, S.-K.; Liu, H. Improved Performance of CEA Microbial Fuel Cells with Increased Reactor Size. Energy Environ. Sci. 2012, 5, 8273. [Google Scholar] [CrossRef]
- Brownlie, W.J.; Sutton, M.A.; Cordell, D.; Reay, D.S.; Heal, K.V.; Withers, P.J.A.; Vanderbeck, I.; Spears, B.M. Phosphorus Price Spikes: A Wake-up Call for Phosphorus Resilience. Front. Sustain. Food Syst. 2023, 7, 1088776. [Google Scholar] [CrossRef]
- Ambler, J.R.; Logan, B.E. Evaluation of Stainless Steel Cathodes and a Bicarbonate Buffer for Hydrogen Production in Microbial Electrolysis Cells Using a New Method for Measuring Gas Production. Int. J. Hydrogen Energy 2011, 36, 160–166. [Google Scholar] [CrossRef]
- Liang, D.; Xu, W.; Liu, Y.; Peng, S.; Xie, B.; Lu, S.; Xiang, Y.; Liu, H. Can Bicarbonate Replace Phosphate to Improve the Sustainability of Bioelectrochemical Systems for H2 Production? RSC Adv. 2015, 5, 27082–27086. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. Handbook of Water and Wastewater Treatment Technologies, 1st ed.; Butterworth-Heinemann: Boston, MA, USA, 2002; ISBN 9786611071462. [Google Scholar]
- Sun, H.; Li, J.; Yang, M.; Shao, Q. Influence of Initial pH on Anodic BiofilmFormation in Single-Chambered MicrobialElectrolysis Cells. Pol. J. Environ. Stud. 2019, 28, 1377–1384. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, H.; Liu, H. Sustainable Power Generation in Microbial Fuel Cells Using Bicarbonate Buffer and Proton Transfer Mechanisms. Environ. Sci. Technol. 2007, 41, 8154–8158. [Google Scholar] [CrossRef]
- Marcus, Y. Ions in Water and Biophysical Implications; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-94-007-4646-6. [Google Scholar]
- Kilicaslan, A.F.; Dincer, I.; Khalvati, A. Microbial Electrolysis Cells Designed for BioH2 Production: Potential Improvements with Carbon Dioxide and Nitrogen. Int. J. Hydrogen Energy 2024, 62, 794–802. [Google Scholar] [CrossRef]
- Sikarwar, D.; Das, I.; Ganta, A.; Nambi, I.M.; Erable, B.; Das, S. Microbial Electrolysis Cells: Fuelling the Future with Biohydrogen—A Review. Sustain. Chem. Environ. 2025, 9, 100224. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Long, F.; Singh, L.; Trujillo, S.; Xiao, X.; Liu, H. Breaking the Loop: Tackling Homoacetogenesis by Chloroform to Halt Hydrogen Production-Consumption Loop in Single Chamber Microbial Electrolysis Cells. Chem. Eng. J. 2020, 389, 124436. [Google Scholar] [CrossRef]




| Process | Step | Variables | Setting |
|---|---|---|---|
| Ni Plating | Bath | Composition | NiSO4·6H2O (300 g L−1), NiCl2·6H2O (35 g L−1), H3BO3 (30 g L−1) |
| Plating | Current density/time | 2 mA cm−2/2 min | |
| CVD | Purge | Gas (flow rate)/time | Ar (600 sccm)/15 min |
| Ramp | Temperature (Zone 1/Zone 3) | 120 °C/650 °C under H2 400 sccm | |
| Growth | Gas (flow rates in sccm) | C2H4 (120 sccm); Ar (120 sccm); H2 (400 sccm) | |
| Duration | 15 min | ||
| Catalyst | 200 mg ferrocene (solid in zone 1) | ||
| Cooling | Gas (flow rates) | Ar (20 sccm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linowski, K.; Islam, M.Z.; Wang, L.; Long, F.; Yu, C.; Liu, H. Long-Term Evaluation of CNT-Clad Stainless-Steel Cathodes in Multi-Channel Microbial Electrolysis Cells Under Variable Conditions. Energies 2025, 18, 5241. https://doi.org/10.3390/en18195241
Linowski K, Islam MZ, Wang L, Long F, Yu C, Liu H. Long-Term Evaluation of CNT-Clad Stainless-Steel Cathodes in Multi-Channel Microbial Electrolysis Cells Under Variable Conditions. Energies. 2025; 18(19):5241. https://doi.org/10.3390/en18195241
Chicago/Turabian StyleLinowski, Kevin, Md Zahidul Islam, Luguang Wang, Fei Long, Choongho Yu, and Hong Liu. 2025. "Long-Term Evaluation of CNT-Clad Stainless-Steel Cathodes in Multi-Channel Microbial Electrolysis Cells Under Variable Conditions" Energies 18, no. 19: 5241. https://doi.org/10.3390/en18195241
APA StyleLinowski, K., Islam, M. Z., Wang, L., Long, F., Yu, C., & Liu, H. (2025). Long-Term Evaluation of CNT-Clad Stainless-Steel Cathodes in Multi-Channel Microbial Electrolysis Cells Under Variable Conditions. Energies, 18(19), 5241. https://doi.org/10.3390/en18195241






