Abstract
Anaerobic digestion (AD) is an environmentally sustainable approach for managing invasive plants species, mitigating pollution, and generating renewable energy. However, the complex structure of these biomasses limits their biodegradability and necessitates pretreatment to enhance methane production. This study explored the biotransformation of two invasive species, Reynoutria japonica and Phragmites australis, harvested across diverse phenological stages. Bioprocess intensification was achieved through a single-stage process using a hydrolytic–methanogenic consortium under thermophilic conditions (55 °C, 25 days). The impact of harvest timing distinct plant fractions (shoot vs. root) on biogas production was meticulously evaluated. Results revealed progressive biogas production. Notably, winter-harvested shoot fractions exhibited the highest methane-rich biogas, achieving 551.12 ± 33.07 mL/g VS for Reynoutria and 401.42 ± 24.09 mL/g VS for Phragmites. The resulting digestate demonstrates a rich composition of essential macronutrients (N-P-K) vital for plant growth, highlighting its potential as a valuable biofertilizer. Significantly, complete inhibition of seed germination was observed, confirming the process’s efficacy in preventing the further propagation of invasive species. This research underscores that thermophilic anaerobic digestion, coupled with hydrolytic treatment, is a significant advancement in the valorization of invasive biomasses, contributing to both renewable energy production and ecological recovery.
1. Introduction
The global energy crisis threatens the sustainable development of human society and increases dependence on fossil fuels, whose use for heating, electricity, and transportation significantly contributes to greenhouse gas emissions, leading to climate change and global warming [1,2]. In response to these challenges, transitioning to renewable energy sources is essential to ensure a stable energy supply and reduce environmental impact [3]. Bioenergy presents a promising alternative, offering a cost-effective alternative and an environmentally sustainable solution to fossil fuels [1]. Various policies have been implemented to promote biofuels and biomass valorization, particularly in Europe and the United States [4].
One of the most promising technologies in this field is anaerobic digestion (AD), which has emerged as a reliable and cost-effective technique and a promising process for circular economy models to produce renewable energy [5,6]. AD is a biological process that converts organic matter into biogas in the absence of oxygen, while generating nutrient-rich digestate that can be used as fertilizer [7]. AD has been extensively studied for the management and valorization of various organic residues, including invasive plants, which pose a significant threat to ecosystems and economies [8,9]. Currently, numerous private and public stakeholders are engaged in the management and elimination of these invasive species. However, their management remains costly and inefficient due to their high dispersal capacity and the damage they cause [10]. Among existing control methods, incineration is a viable option, but is restricted for certain plant species due to the release of harmful volatile compounds [11]. Additionally, the use of herbicides poses risks to human health and the environment, limiting their effectiveness and acceptability [12].
A safe and sustainable management strategy for these aggressive invasive species is urgently required. In this context, AD emerges as a promising solution that simultaneously addresses biological invasion control and renewable energy [13]. This process has already proven effective in converting complex biomass into biogas and integrating waste valorization into circular economy models as a sustainable bioenergy technology [5,7,14]. Notably, Reynoutria japonica (Japanese knotweed) and Phragmites australis (common reed) are among the most problematic invasive species in the worldwide [15,16]. Reynoutria japonica is listed among the 100 most invasive species globally by the Global Invasive Species Database, due to its ecological impact and its ability to damage urban infrastructure through its aggressive and extensive root network [17]. Phragmites australis, a highly competitive perennial grass, rapidly colonizes wetlands and riparian zones, outcompeting native vegetation for water and nutrients, thereby reducing biodiversity and altering ecosystem functions [11,12,13]. AD’s ability to treat a wide range of substrates makes it an attractive choice for bioenergy production, fulfilling both the needs of sustainable renewable energy and alien weed management [8,13].
In Quebec and globally, both species are classified as priority invasive plants requiring coordinated monitoring and control efforts by federal, provincial, and municipal authorities. Current eradication methods, like mechanical removal, herbicide application, or burning, are often costly, labor-intensive, and environmentally unsustainable [18]. This study contributes to ongoing efforts to develop sustainable strategies for the management of these invasive species by exploring their valorization through AD. We propose integrating these biomass streams into existing biomethanization facilities, using laboratory-scale experiments that mimic full-scale operational conditions to assess their methanogenic potential under thermophilic conditions with hydrolytic pretreatment. Since digestate is typically applied to agricultural land, ensuring the complete inactivation of regenerative plant parts such as rhizomes or seeds is essential to prevent further spread. Our work integrates energy recovery with biosecurity, showing both methane production and complete suppression of germination and sprouting after AD. This dual outcome sets it apart from conventional studies limited to waste-to-energy conversion. By converting invasive plant biomass into renewable energy while preventing further propagation, the research advances an integrated strategy for regional invasive species management within a circular bioeconomy framework.
While the results of this study are promising, it is important to acknowledge the challenges associated with scaling up the process. Thermophilic anaerobic digestion requires higher thermal energy input compared to mesophilic operations, which may increase operational costs, particularly if external heating sources are needed [19]. This contrasts with mesophilic AD, which requires less energy for heating and is more resilient to temperature changes. While the higher biogas yield of thermophilic AD can potentially offset this energy cost, a careful and comprehensive energy balance is essential to prove economic viability. However, these issues can be mitigated by integrating the process into existing anaerobic digestion facilities, which already possess the necessary infrastructure and waste heat. Additionally, co-digesting with traditional feedstocks can reduce supply fluctuations.
Sourcing and transporting large volumes of invasive plant biomass like Reynoutria and Phragmites is a major logistical challenge. The collection is often difficult and expensive due to their remote locations. Once harvested, their low bulk density makes long-distance transportation costly. Additionally, a consistent, year-round supply is not guaranteed, which would require either an effective storage system or a co-digestion strategy with another available biomass.
The valorization of these invasive plants through AD could address a dual challenge: their safe disposal without the risk of further spread, and the production of clean, renewable energy. This research is distinguished by its dual objectives of producing renewable energy and directly controlling undesirable, non-native plant species, with a focus on biogas yields and the quality of the resulting digestate.
2. Materials and Methods
2.1. Inoculum Sampling
The inoculum used in this study was sourced from a municipal solid waste treatment, SEMER, located in Rivière-du-Loup, Quebec. Three distinct inoculants were collected to ensure a robust microbial community for AD. The inoculum used for each assay was composed of both hydrolytic and methanogenic consortia, mixed in appropriate volumetric ratios (v/v). The hydrolytic microbial community was obtained from a thermophilic hydrolyser operating at 55 °C, ensuring the efficient breakdown of complex organic matter. Meanwhile, the methanogenic consortium was obtained from a thermophilic anaerobic digestor, also maintaining a temperature of 55 °C, responsible for biogas production. The use of this consortium of hydrolytic and methanogenic bacteria for AD is an innovative method compared to most of the research, which only uses methanogenic bacteria. Table 1 provides an overview of the physicochemical characteristics of the mixed inoculants used in the assays.

Table 1.
Physicochemical properties of inoculum samples, consisting of a mixture of hydrolytic and methanogenic bacteria (V/V), used in anaerobic digestion (AD) assays and provided by a local biomethanization plant.
2.2. Plants Sampling
To encompass the various development stages of plants of Reynoutria and Phragmites, multiple harvests were conducted during three distinct periods of the year: winter, summer, and autumn. These samples were collected between December 2021 and October 2022 at the roadside stop in La Pocatière, Quebec, Canada. To collect plant root samples, the team employed equipment provided by the Ministry of Transport and Sustainable Mobility of Quebec. To prevent the spread of invasive plant material into the environment during collection and transportation, samples were securely sealed in plastic bags. Each sealed bag was labeled with pertinent information, such as species, location, and date of collection. Samples harvested in December 2021, July 2022, and October 2022 were used for BMP assays 1, 2, and 3, respectively. Assays 1 and 2 were conducted by using the shoots of both plants, while assay 3 was carried out by using the root portions of both species and the shoots of phragmites. The reason behind this disparity is related to the different stages of plant development during the distinct harvest periods. The substrates were first ground and homogenized to a particle size of less than 2.5 cm, and then they were characterized before the BMP assays to guarantee the homogeneity and repeatability of the tests [20].
2.3. Biotransformation Process
2.3.1. Experimental Set up for Batch AD System
BMP assays were conducted using the MEDUSA Anaero Technology system, comprising 15 automated 1 L reactors. Prior to each assay, the gas tightness of all bioreactors was tested by applying slight overpressure to detect any pressure drop. All seals, including septum, caps, and connectors were inspected for moisture or leakage. Following this preliminary testing, a mixture of inoculum (I) and substrate (S) (Reynoutria/Phragmites) was added to each reactor at an SIR ratio of 1:1, based on volatile solids content. In accordance with Holliger et al. (2021), an I/S ratio of 1 is recommended for lignocellulosic substrates with low biodegradability [20]. Reactors, with a working volume of 900 mL, were maintained at a temperature of 55 °C and stirred at 100 rpm for a duration of 25 days. To confirm the effectiveness of the experimental system, blank controls (inoculum only) and positive controls (crystalline cellulose) were run concurrently [21].
To assess the potential of AD to inhibit the germination and regeneration of invasive plants, a parallel experiment was conducted on a larger scale using 10 L reactors (Ray I, Anaero Technology) under the same conditions as the smaller reactors. This approach provides enough digestate for further fertilizing potential and germination tests. Both reactor sizes were subjected to continuous agitation and maintained at 55 °C for 25 days. The resulting digestates were stored for future analyses of fertilizing potential and germination tests.
2.3.2. Operational Conditions
The harvested plants were ground and characterized. The dried matter (DM) and volatile solids (VS) were determined according to APHA (2005) [22]. Total nitrogen (TN), was evaluated using the Hach method [23]. Mineral salts and metal contents were analyzed using a microwave plasma atomic emission spectrometry (MP-AES, Agilent technology), after nitric-chlorohydric acid digestion, according to plasma spectrometric method procedures [24].
At the experiment’s onset, 200 mL of the substrate/inoculum mixture were used for physicochemical characterization, including analyses of DM, VS, and TN. Ammonium nitrogen (N–NH4+) and volatile fatty acids (VFA) were assessed using Hach methods [23]. Alkalinity (Alk) was determined using an automated titrimetric method [24].
Determination analyses were performed using a DR 3800™ spectrophotometer procedure (Hach Lange AB, Skondal, Sweden). The volume of biogas generated was measured using a continuous automated flow meter. These volumes were then systematically adjusted to standard conditions (STP: 0 °C, 1 atm, dry gas). Prior to each test series, the flow meter was calibrated in compliance with VDI 4630 guidelines [21]. The net volume of biogas was calculated by subtracting the volume produced by the inoculum-only blank control. The amount of methane was measured using a Multitec 540 gas analyzer. The specific biochemical methane potential was determined by measuring the cumulative volume of methane (mL) produced per gram of volatile solids added. The test period was determined based on the stabilization criterion, which states that daily methane production must be less than 1% of the cumulative volume for three consecutive days [21]. Each data point represented the average of the measurements from three independent reactors.
After the AD step, the physicochemical characteristics of the resulting digestate were evaluated. The removal efficiencies of DM and VS were calculated using Equations (1) and (2). The increased ammonium was calculated according to Equation (3). And free NH3 content was calculated according to Equation (4).
where FAN is the free ammonia concentration (NH3) in the digester in g/L; TAN is the total ammonia concentration (NH4 + NH3) in the digester in g/L; and T is the temperature of the digester in °C [25].
DM removal (%) = (DM (initial) − DM (final))/DM (initial) × 100
VS removal (%) = (VS (initial) − VS (final))/VS (initial) × 100
N–NH4 increase (%) = (N–NH4 (initial) − N–NH4 (final))/N–NH4 (initial) × 100
2.3.3. Biogas Production Kinetics and Simulation
To assess the biogas accumulation and performance during AD, a modified Gompertz model (Equation (5)) was employed for nonlinear regressions analysis, enabling the generation of representative simulations and predictions.
where P denotes the cumulative biogas production (mL/g VS added) with respect to time t (days), P0 represents the maximum biogas potential (mL/g VS added), Rm signifies the maximal biogas generation rate (mL/g VS added.Day), L indicates the lag time (days), t represents the time of the experiment (days), and e refers to exponential function, which uses Euler’s number equal to 2.7183 [26].
The Gompertz model effectively captures the characteristic S-shaped curve of biogas production, with the parameters P0, Rm, and L determined through fitting the model to experimental data. The kinetic estimations were conducted using the IBM SPSS v30.
2.3.4. Digestate Valorization: Fertilizing Potential and Innocuity Assays
Fertilizing Potential Evaluation
Samples from the resulting digestates were analyzed to evaluate their fertilizing potential. The total acid digestion colorimetric method and plasma spectrophotometry (no. CPVQ-MI-1-NPK) were used to determine the NPK rates [27]. The remaining digestates were then used for germination tests.
Digestates Innocuity Test
To assess the innocuity of Reynoutria and Phragmite digestates from three distinct harvest periods, greenhouse germination trials were conducted. The germination experiment involved a series of containers, each containing 1 L of substrate (a 1-inch layer) mixed with either 300 mL of a digestate or a control. The control consisted of 300 mL of shredded biomass that had not undergone AD. Each treatment was repeated five times. The soil was kept at a consistent moisture level, and the greenhouse was kept at an average temperature of 20 °C, with a 14 h photoperiod [28].
Validation of Seed Germination Inhibition Using a Thermal Treatment Assay
This test was designed to determine if thermal treatment alone could inhibit the seeds and rhizomes germination of Reynoutria and Phragmite. Thermal treatment was carried out in a 55 °C water bath for 21 days to mimic AD conditions. Two additional incubation periods were tested: 1 day and 7 days for a total of three incubation periods.
For the seed water bath, 200 Phragmite seeds and 75 Reynoutria seeds were placed in 15 mL vials filled with distilled water. There were 5 vials for each species, repeated three times for each incubation period, for a total of 15 vials per species.
For the rhizome bath experiment, three rhizomes (measuring between 2 and 3 inches in length) of three different diameters with at least two buds were selected. The process was repeated nine times for each species to have three repetitions for each incubation period. The roots/rhizomes were placed in two different plastic containers, filled with distilled water, one for each species, for a total of 27 rhizome pieces per species. For each set up, samples were taken at the end of the incubation period. These samples comprised five replicates per species for the seeds and three replicates per species for the rhizomes, which were then tested for germination.
The seeds were meticulously placed on moistened filter paper in a parafilm-sealed Petri dish. Rhizomes were planted in 3.5-inch containers, each holding three rhizomes of three different diameters within a horticultural growing mix. Seeds and rhizomes were placed in a growth chamber at 22 °C with a 14 h photoperiod, as described by [28]. Controls for the seeds and rhizomes of each species were placed in the growth chamber on day 0.
2.4. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 30 software, with a significance level set at p < 0.05. Analysis of variance (ANOVA) and Games–Howell post hoc tests were used to group the levels of the variables exhibiting significant effects at a 95% confidence level. GraphPad Prism 2024 software was used to generate the figures and fit the modified Gompertz model.
3. Results
3.1. Physicochemical and Biochemical Properties of Raw Substrates
The harvested plants exhibited distinct morphological characteristics depending on the season. During the first harvest (winter), only the upper portion, comprising stems and leaves, were collected. The parts were dry, and notably, unlike Reynoutria, the Phragmites leaves were seed-filled. In the second harvest (summer), the shoots of both plants lacked flowers or seeds. During the third harvest (autumn), only the root part was harvested for Reynoutria. For Phragmites, both roots and stem and leaves were harvested, with aerial parts still bearing seeds.
The physicochemical properties of these plant samples across three harvest periods, winter (Harvest 1), summer (Harvest 2), and autumn (Harvest 3) are summarized in Table 2. Reynoutria showed lower DM in shoots during winter (28.75 ± 1.48%) and summer (25.97 ± 0.73%), while Phragmites exhibited a progressive decrease in DM from 77.88 in winter to 54.36% in autumn for stem and leaf parts. In contrast, DM in Reynoutria root parts (40.39 ± 0.02%) was higher compared to the Phragmites root parts (23.76 ± 0.23%), but despite this variability in DM content, both species consistently maintained high volatile solids percentages across all harvests and plant parts. Reynoutria VS ranged from roughly 84% to 98%, while Phragmites VS varied from approximately 84% to 92%. This indicates substantial organic matter content suitable for AD processes. Reynoutria’s apparent density varied between 0.38 and 0.52 g/cm3 during different harvest periods, while Phragmites’s density varied between 0.12 and 0.21 g/cm3 for shoots from different harvests, and 0.50 g/cm3 for roots from the third harvest.

Table 2.
Physicochemical characteristics of Reynoutria and Phragmites used in this study across seasonal harvests. Physicochemical characteristics of Reynoutria and Phragmites during various harvest seasons, including dried matter, volatile solids, and apparent density.
The salts and metal contents of Reynoutria and Phragmites were analyzed to evaluate their suitability as substrates for the methanogenic bacteria in AD processes. Nutrient availability significantly influences microbial performance, as methanogens require not only carbon (C) and nitrogen (N) for growth but also trace elements for enzymatic functions. Zhang and Loh (2019) emphasized that methanogenic processes are more stable and efficient in the presence of trace elements [29]. Furthermore, Wintsche et al. (2016) suggested that the absence of these elements can destabilize the process [30].
Table 3 details the macro, micro, and trace mineral contents of these invasive plants. Significant variations were observed between the species. Reynoutria exhibited concentrations of Ca (6.27–7.12 g/kg) and K (7.46–10.31 g/kg), with P (0.69–2.83 g/kg), Mg (1.07–3.13 g/kg), and Na (0.14–0.88 g/kg) levels varying within similar ranges. Meanwhile, Phragmites contained 1.19–3.29 g/kg of Ca, 0.51–2.96 g/kg of P, 1.46–12.13 g/kg of K, 0.43–2.18 g/kg of Mg, and 1.06–5.08 g/kg of Na. Regarding trace elements, Reynoutria contained 43.83–98.01 mg/kg of Zn and 40.41–203.58 mg/kg of Mn, whereas Phragmites exhibited Zn and Mn concentrations of 54.74–115.47 mg/kg and 35.75–93.57 mg/kg, respectively. Ni and Co contents were 1.1–2.0 mg/kg and 1.6–3.75 mg/kg in Reynoutria, and 0.9–1.47 mg/kg and 1.73–1.9 mg/kg in Phragmites, respectively. Furthermore, low concentrations of heavy metals (Cd, Pb, Cr) were found in both plants, making their valorization safe.

Table 3.
Evaluation of the salt and metal contents of Reynoutria and Phragmites plant samples.
The mineral richness of these invasive plants provides a promising substrate for methanogenic processes. Furthermore, the resulting digestate from AD can serve as a valuable biofertilizer, providing essential nutrients for plant growth [31].
The biochemical analysis of Reynoutria revealed a high lignin content, ranging from 52.94 ± 0.15% to 54.91 ± 0.30% across different harvests. Cellulose content varied between 28.44 ± 0.49% and 33.31 ± 1.66%, while hemicellulose ranged from 13.75 ± 1.51% to 16.76 ± 0.19%. Extractives were around 15.38 ± 1.12%, 8.56 ± 0.05%, and 8.07 ± 0.00% for plants from the first, second, and third harvests, respectively. Similar findings were observed in Phragmites, with lignin ranging from 50.03 ± 0.66% to 54.55 ± 0.27%, cellulose from 27.60 ± 0.20% to 34.45 ± 0.41%, hemicellulose from 11.26 ± 0.64% to 17.84 ± 0.43%, and extractives were around 15.92 ± 0.74%, 15.45 ± 1.20%, 16.75 ± 1.23%, and 12.86 ± 0.91% across the first, second, and third harvest shoots and root parts, respectively (Figure 1).
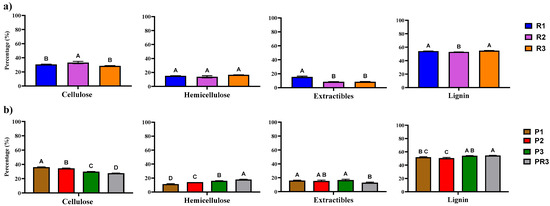
Figure 1.
Anaerobic digestion process of lignocellulosic materials. Compositional analysis of Reynoutria (a) and Phragmites (b). Legend: Percentages of extractives, cellulose, hemicellulose, and lignin in Reynoutria plants from first harvest (R1), second harvest (R2), and third harvest (R3) and in Phragmites (b) plants from three harvests (P1. P2. P3) and root parts (PR3). Values represent mean percentages ± standard deviation. The different letters indicate that results are statistically significantly different (p < 0.05).
The observed high lignin content in both Reynoutria and Phragmites presents a significant challenge for efficient AD. Lignin, a complex phenolic polymer, forms a recalcitrant structural component of plant cell walls, creating a physical barrier that hinders microbial access to fermentable polysaccharides like cellulose and hemicellulose [32]. This structural complexity and the aromatic nature of lignin impede the enzymatic hydrolysis process, a critical initial step in AD, where cellulolytic and hemicellulolytic enzymes are required to break down complex polymers into simpler sugars [33]. Specifically, the cross-linking of lignin with cellulose and hemicellulose creates a matrix that is resistant to enzymatic attack, necessitating pretreatment strategies to enhance the accessibility of these polysaccharides. Consequently, pretreatment strategies are essential to enhance substrate digestibility.
In this study, both plant materials were subjected to physical pretreatment via grinding to particles smaller than 2.5 cm prior to hydrolysis and AD. This physical pretreatment increases the surface area and reduces cross-links between cellulose, hemicellulose, and lignin components. Decreased cellulose improves access for hydrolytic enzymes, enhancing digestion and gas production [33,34].
Hydrolysis is the first step of the AD process and involves the enzymatic break down of complex molecules into simpler ones. During the subsequent phase of the process, these molecules are then converted into organic acids and volatile fatty acids, which are then used by methanogens to generate biogas [35]. Therefore, the hydrolysis step is a key factor determinant of feedstock biodegradation rate and AD kinetics [36]. Moreover, the biotransformation efficiency of lignocellulosic biomass using AD could be improved by co-digestion with a nitrogen rich feedstock by balancing the C:N ratio and enhancing biogas production [8]. In this study, the nitrogen-rich inoculum obtained from the municipal solid waste treatment plant was used to balance the C/N ratio, which is crucial for optimal AD.
3.2. Assessing the Progression of the Biotransformation Process
3.2.1. Physicochemical Characterization of Initial and Post Anaerobic Digestion Mixtures
Table 4 details the physicochemical characteristics of initial and post-AD Reynoutria and Phragmites. Results showed that the initial pH of the plants ranged from 6.63 to 7.17. After AD, the pH increased slightly to reach pH values of (8.02–8.29), suggesting the production of alkaline compounds, potentially due to ammonia release during protein degradation. pH is a critical parameter in AD, with an optimal range of 6.8 to 7.2, as within this range, methanogenic activity is maximized. However, the process can tolerate a pH of 6.5 to 8.0 [37,38]. The hydrolytic and acidogenic phases, involving the breakdown of complex organic matter into volatile fatty acids (VFAs), are less pH-sensitive, operating effectively between 5.5 and 6.5 [39]. In contrast, acetogenesis and methanogenesis, the final stages of AD, prefer neutral to basic pH levels, with methanogens exhibiting optimal performance around pH 7 [40]. In the present study, the initial pH was within an acceptable range, minimizing any adverse impact on the process.

Table 4.
Physical and chemical changes in Reynoutria and Phragmites during anaerobic digestion (AD) at various harvest times, exhibiting variances in concentrations of pH, alkalinity (ALK), and volatile fatty acids (VFAs).
Alkalinity prior to AD varied from 3445 to 5705.64 mg/L and increased to 8437.9–9947 mg/L (Table 4). This increase signifies the production of buffering compounds, such as bicarbonates, which are crucial for maintaining pH stability. Suitable alkalinity is essential for process stability and methane production, as it buffers pH fluctuations and prevents inhibition of the process due to VFA accumulation [41]. Initial VFA concentrations, indicators of intermediate organic acid production, ranged from 2786 to 5853 mg/L and decreased significantly to 250–938 mg/L post digestion (Table 4). This substantial reduction reflects the efficient conversion of VFAs into methane, a key objective of AD. Volatile fatty acids (VFAs) are important intermediates produced during the acidogenesis and acetogenesis steps [42]. The observed VFAs reduction indicates successful AD, while accumulation leads to microbial stress, resulting in acidification and process failure [43,44]. The significant VFAs reduction and alkalinity increase observed across all experiments indicate that the system maintained an adequate buffering capacity, preventing acidification. Research indicates that a system with a good buffering capacity can maintain stable operation despite elevated VFAs concentrations [45].
3.2.2. Carbon Removal and Ammonium Release Efficiency
The AD of invasive plants from different harvest periods was assessed for dry matter (DM) and organic matter removal efficiencies (Table 5). The results revealed that dry matter degradation ranged from 8 to 61% for Reynoutria and from 29% to 63% for Phragmites, while VS removal efficiencies were 27 to 41% and 12 to 30%, respectively. These results confirm the efficiency of organic matter degradation using the AD process and align with previous studies showing 50 and 70% volatile solids removal in agricultural and forest residues without pretreatment [46]. Notably, ammonium nitrogen levels increased significantly during AD, with increases ranging from 19 to 31% for Reynoutria and 22 to 40% for Phragmite, indicating organic nitrogen mineralization [31]. The resulting digestate, rich in ammonium nitrogen, possesses agronomic value and holds potential as a replacement for chemical fertilizers when managed effectively [47]. Nevertheless, a significant level of free ammonia (FAN) must be considered, because it raises the possibility of phytotoxicity during direct applications. In the present study, the free ammonia concentration calculations showed a range of 356.46 mg/L to 621.6 mg/L. Prior research indicates that biomethanization can be inhibited at higher concentrations of FAN [48]. Nonetheless, no inhibition has been seen in our bacteria, which originate from a biomethanization plant exposed to high ammonium concentrations. This could be explained by the bacteria’s adaptation. In line with our results, Kalamaras demonstrated that microbial consortia could adapt to extremely high total ammonia nitrogen (TAN) concentrations (up to 9000 mg TAN/L) by gradually and successively incubating microorganisms. His investigation indicates that methanogenesis remains effective at elevated TAN levels, particularly due to the predominance of hydrogenotrophic archaea and methanosarcina [49].

Table 5.
Dry and organic matter degradation, ammonium, and free ammonia release during AD of Reynoutria and Phragmites.
3.3. Biogas Production Performance
3.3.1. Daily and Cumulative and Biogas Yields
The daily rate of biogas production (mL/g VS added. day) from the AD of Reynoutria over 25 days, shown by (Figure 2a), revealed that all samples exhibited peak production within the first three days. Following this initial burst, production declined, except for the root parts from the third harvest, which showed other peaks after day 15. These results align with the predicted lag phase data (Table 6), representing the time required for the microbial consortia to adapt to the new substrate and achieve exponential growth [50]. The registered lag phase was consistently shorter for Reynoutria, with plants from the first and second harvests showing a duration of 0 days (Table 6). The maximum lag phase was observed for Reynoutria root parts from the third harvest, at 16 days. These findings suggest that assays with Reynoutria stem and leaves reached their peak production more rapidly. The delayed production observed in the third harvest root samples, compared to the other samples, is consistent with previous findings. In accordance with our results, Pan et al. (2021), in a study with Reynoutria under thermophilic conditions, also reported initial peak production within the first three days, followed by a decline until day 15 [51].
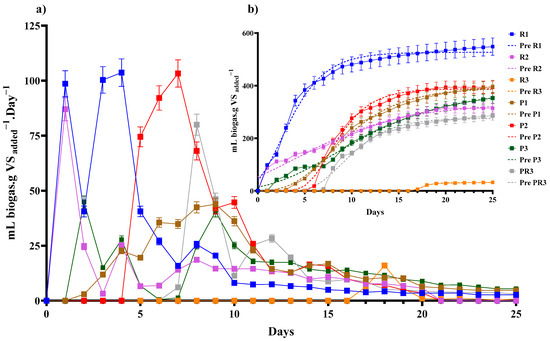
Figure 2.
Kinetics of daily (a) cumulative and predicted biogas (b) production modeled by the modified Gompertz plots of Reynoutria and Phragmites over different harvesting periods. Each data point represents the mean value, with error bars indicating the standard deviation. Legend: Reynoutria plants from first harvest (R1), second harvest (R2), and third harvest (R3) and Phragmites (b) plants from the three harvests (P1. P2. P3), and the root parts (PR3). Values represent mean percentages ± standard deviation.

Table 6.
Kinetics parameters of the modified Gompertz model for various assays from Reynoutria and Phragmites.
Cumulative biogas production from AD of Reynoutria from different harvest periods, shown in Figure 2b, revealed that biogas production gradually increased over time, reaching a peak value for each test. However, the assay conducted on the root parts (R3) exhibited a noticeable lag phase, with delayed biogas production until 15 days later. Notably, all digestion test groups demonstrated a strong fit to the modified Gompertz growth curve, with R2 ranging from 0.975 to 0.998 (Table 6), indicating a reliable model fit.
The assays demonstrated that the maximum yield was attained with Reynoutria from the first harvest: 551.12 ± 33.07 mL/g VS added. The second harvest yielded 314.18 ± 18.36 mL/g VS added, while the root parts from the third harvest (R3) produced 32.95 ± 1.93 mL/g VS added (Figure 3a). In comparison to the present findings, previous research has reported that mono-digestion of Reynoutria produced a methane yield of 104 (mL/g VS added, while the highest production of 232 mL/g VS added was achieved when co-digested with dairy manure at a ratio of 10/90 [51].
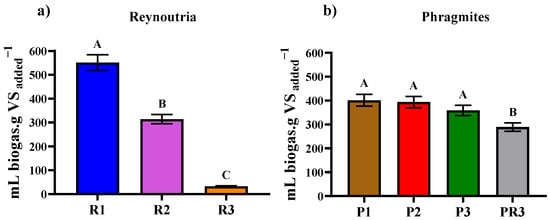
Figure 3.
Cumulative biogas production from (a) Reynoutria (R) and (b) Phragmites (P) (b) over different harvest periods. Each data point represents the mean value, with error bars indicating the standard deviation. Legend: Reynoutria plants from first harvest (R1), second harvest (R2), and third harvest (R3) and in Phragmites (b) plants from three harvests (P1. P2. P3) and root parts (PR3). Values represent mean percentages ± standard deviation. The different letters indicate that results are statistically significantly different (p < 0.05).
The daily biogas production from Phragmites is illustrated in Figure 2a. Notably, peak production occurred within the first 10 days, followed by a decline. By the 15th day, biogas production had nearly ceased across all assays.
The predicted lag phase presented in Table 6 reveals a variability among the different samples. The lowest lag phases (1.33 days) were registered with phragmites stems and leaves from the third harvest. Conversely, the highest lag phase (6.06 days) was observed in the root parts of Phragmites from the third harvest. The initial increase in biogas production during the first five days likely resulted from the fast conversion of readily digestible fractions. Subsequent peaks may be attributed to the degradation of more recalcitrant lignocellulosic [52]. The lag phase’s variability can be influenced by multiple factors. As Bertrand (2019) noted, the lag phase is a dynamic, adaptative, and evolutionary parameter [53]. Furthermore, biogas production can occur prior to the predicted lag phase being reached [54]. Shorter lag phases suggest that hydrolytic bacteria can rapidly respond to environmental changes, facilitating the rapid start of methanogenesis [55]. In the contrast, López-Aguilar (2023) reported higher methane production in the anaerobic co-digestion of food waste and Sargassum spp., despite a slower lag phase, by acclimatizing the inoculum to the substrate [56]. Various parameters, including substrate concentration, initial VFAs/VS ratio, and VFAs/ALK ratio, can influence the lag phase. Maintaining a VFAs/Alk ratio of 0.4 and an initial VFAS/VS ratio below 10% is crucial for minimizing the lag phase and improving biogas production performance [57].
The cumulative biogas production from Phragmites presented by Figure 2b mirrors the findings observed with Reynoutria. Detailed values for both species are provided in Tables S1 and S2 (Supplementary Materials). The curves for all assays revealed an increase in biogas production. The predictive cumulative biogas plots generated by the modified Gompertz model exhibited excellent agreement with the experimental data, as evidenced by R2 values ranging from 0.991 to 0.997 (Table 6). This strong correlation indicates a good fit between the mathematical model and the experimental results. Biogas production gradually increased, reaching its peak around day 15. Detailed values for both species are provided in Tables S5–S11 (Supplementary Materials).
In fact, the rapid biogas yield during the initial days is characteristic of thermophilic AD. This could be attributed to the high metabolic rate and efficient enzymatic system of thermophilic microbial communities, which contribute to the effective degradation of lignocellulosic biomass [58]. The sustained increase in biogas production across most reactor assays can be attributed to the optimized SIR, which ensured that the acid production and consumption rates were balanced [59,60]. Furthermore, the complex lignocellulosic composition’s complexity may have contributed to a gradual degradation rate, which allows methanogenic microorganisms to effectively consume VFAs produced in the system and prevent their accumulation, thereby preserving system stability [61].
The average cumulative biogas production from all the assay conditions, arranged in descending order, revealed the highest yield with Phragmites shoot parts from the first harvest, averaging 401.42 ± 24.09 mL/g VS added. Shoot parts from the second harvest produced 393.93 ± 23.48 mL/g VS added and shoot parts from the third harvest produced an average biogas production of 358.58 ± 21.06 mL/g VS added. The lowest average biogas production, 289.23 ± 17.48 mL/g VS added, was recorded with the root parts from the third harvest (Figure 3b).
According to previous studies, the AD of Phragmites revealed a highest average biogas of 76.1 ± 2.62 mL/g VS added after a 4% NaOH pretreatment, under mesophilic conditions [62]. In a separate study, Al-Iraqi et al. (2023) reported a peak biogas production of 82.17 ± 0.62 mL/g VS added through the co-digestion of Phragmites (25%) and food waste (75%) at an SIR of 1:2 under mesophilic conditions [59]. Wang (2015) observed a biogas yield of 308.2 mL/g VS under mesophilic conditions by the co-digestion of Phragmites (18.52% w/w) with feces (59.26% w/w) and kitchen waste (22.22% w/w), with the addition of clinoptilolite [63].
In contrast, the present study demonstrated significantly higher biogas production. This yield is likely attributed to the use of thermophilic conditions, which accelerate organic matter degradation and improve the kinetics. In fact, the elevated metabolic rate and efficient enzymatic systems of thermophilic microbial communities enable them to effectively degrade lignocellulosic biomass, thereby boosting biomethane production [64].
The variability in biogas production observed among different sample groups for each invasive plant could be attributed to the harvest time, which significantly influenced biomass yield. Variation in tissue quality through different harvest periods also affects decomposition and biogas production [65,66]. Moreover, various studies have illustrated the importance of choosing the right SIR. The SIR of 1/1 used in the present study resulted in efficient biogas production for both invasive plant species, except for Reynoutria root parts. In this regard, Li (2022) suggested that high S/I ratios are susceptible to irreversible acidification, whereas low S/I ratios favor methanogenesis [50]. Similarly, Al-Iraqi (2023) achieved more stability with an SIR of 1/1 [59].
3.3.2. Methane Content
Methane production serves as a key indicator for evaluating AD. The biogas produced from the AD of Reynoutria harvest 1 had a methane concentration ranging from 53% to 69%, with an average yield of 64.17. The second assay revealed a methane content ranging from 60 to 65%, with an average of 63.75%. In contrast, root parts from the third assay showed greater variation, with methane content ranging from 26% to 70% (average 52.33%) (Figure 4a). Pan (2021) reported that mono-digestion of Reynoutria yielded an average methane content of 46.4% and a methane yield of 104 mL/g VS [51]. However, co-digestion with dairy manure at various mixing ratios improved the methane content to 50.2–57.3% and increased the methane yield by 47.1% to 123.1% under thermophilic conditions.
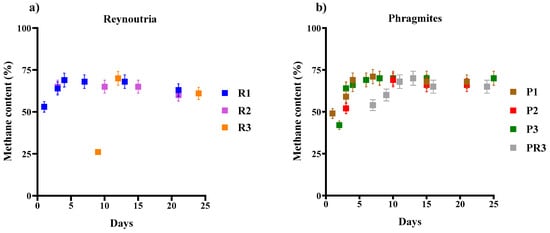
Figure 4.
Methane content of biogas generated in thermophilic anaerobic digestion of (a) Reynoutria and (b) Phragmites. Legend: Reynoutria plants from first harvest (R1), second harvest (R2) and third harvest (R3) and in Phragmites (b) plants from three harvests (P1. P2. P3) and root parts (PR3). Values represent mean percentages ± standard deviation.
The AD of Phragmites revealed the following methane content: for stems and leaves from the first harvest, methane content ranged from 49% to 71%, with an average of 64.00%. For the second harvest, biogas methane content ranged from 52% to 69% (average 63.25%). Shoots from the third harvest exhibited methane content ranging from 42% to 70%, with an average of 65.13%. Root parts produced biogas with a methane content ranging from 54% to 70% (average 63.67%) (Figure 4b). Detailed values for both species are provided in Tables S3 and S4 (Supplementary Materials).
The variability across different harvests may be attributed to the impact of harvesting time and vegetative period on biomass biochemical composition, which influences both biogas quality and the specific methane yield. In this context, Kandel et al. (2013) noted that young biomass, rich in non-structural and soluble sugars (extractables), leads to a high biogas production with a lower methane percentage at the onset of AD [67]. Another study presented by Kakuk et al. (2021) highlighted that green biomass is more suitable as a methane source than woody tissues, emphasizing that low lignin and high soluble content are essential for efficient AD [68]. Moreover, Baute et al. (2018) demonstrated that Phragmites exhibited a higher content of proteins and hemicellulose during the July harvest. However, the highest methane yield per area harvested was recorded in October, which coincides with the peak of their biomass yield [65].
The cumulative methane yields corresponding to Reynoutria were between 17.245 m3/t VS and 353.65 m3/t VS, and for Phragmites, the yields were between 184.15 VS and 256.90 m3/t VS. The amount of energy recovered from the biomass was determined by carefully measuring the amount of methane produced. Based on the lower heating value of methane ~10 kWh/m3), the energy potential output reaches 172.45 kwh to 3536.5 kwh and 1841.5 kwh to 2569 kwh for Reynoutria and phragmites, respectively [69]. While the present study did not provide a definitive energy balance, the existing literature on thermophilic anaerobic digestion frequently demonstrates that the methane energy output is greater than the energy input. The process allows the system to be energy self-sufficient and potentially generate a net energy surplus [70], A follow-up pilot-scale study will be conducted in a future publication to address this crucial point. This larger experiment will gather robust data on energy inputs and outputs under near-commercial conditions. This will allow us to perform a comprehensive techno-economic assessment and provide a complete picture of the process’s viability, from a small-scale proof-of-concept to a commercially relevant application.
When compared to previous studies, our yields are notably higher. For instance, anaerobic digestion of Reynoutria japonica using biological pretreatment via ensiling with lactic acid bacteria yielded 104 L CH4/kg VS. Although this method improved digestibility, a significant boost was only observed upon co-digestion with dairy manure, underscoring the limitations of single-substrate treatments without a more effective pretreatment [51].
Similarly, studies on Phragmites australis under mesophilic conditions report lower methane potentials. Mechanical pretreatment (chopping or grinding) followed by mesophilic digestion resulted in average yields of 226 ± 19 L of CH4 per kg of VS. Notably, even among different genotypes, biomethane production remained consistent, suggesting that the pretreatment type of operational conditions has greater influence than genetic variability [61]. Additional research demonstrated that the mono-digestion of common reed silage under mesophilic conditions, following a mechanical treatment, yielded 160.40 ± 4.09 NL CH4/kg VS [71].
Our thermophilic process with hydrolytic bacterial pretreatment consistently outperforms these mesophilic systems. We achieved a methane content of up to 70% higher than values reported for mechanically treated reed biomass. This significant improvement can be attributed to the synergistic effect of enhanced hydrolysis at elevated temperatures and the action of specialized hydrolytic enzymes, which accelerate the breakdown of lignocellulosic structures.
Furthermore, compared to other lignocellulosic feedstocks, our approach enabled faster and more efficient conversion, with high methane recovery within a short retention time, which is a key advantage for industrial application.
The exception was Reynoutria roots from the third harvest, which showed lower biodegradability, likely due to increased lignin content (Figure 5) [72], highlighting the importance of harvest timing.
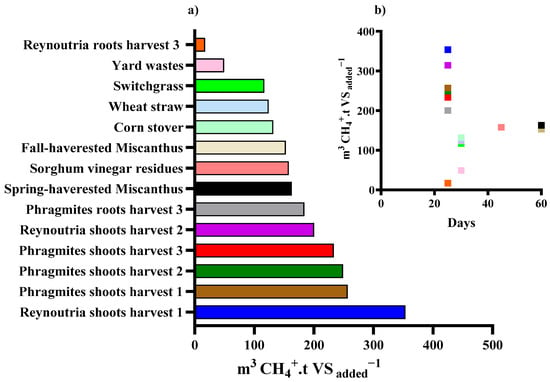
Figure 5.
An overview of the cumulative methane yields from various digestion tests using Reynoutria and Phragmites, in comparison to benchmark values for other lignocellulosic substrates documented in the literature (a). Plotting of substrates based on the amount of time it takes for them to reach their methane potential (b).
Overall, these comparisons demonstrate that integrating biological pretreatment with thermophilic AD offers a competitive advantage for recalcitrant invasive species. While chemical pretreatments may achieve similar or higher yields, they often involve high costs, corrosion risks, and inhibitor formation.
Our method, based on a consortium of hydrolytic–methanogenic bacteria, succeeded in degrading invasive plants and producing green energy within only 25 days under thermophilic conditions, and provides an environmentally friendly alternative ensuring rapid and complete degradation.
3.4. Digestates Valorization
3.4.1. Fertilizing Potential
The fertilizing capacity of digestates from Reynoutria and Phragmites, harvested in the second and third periods, was evaluated. The results presented in Table 7 demonstrate a richness of nitrogen (N), phosphorus (P2O5), and potassium (K2O) in all digestates, with comparable nutrient levels between the two plant species. This nutrient abundance underscores the potential of these digestates as valuable soil fertilizers. The digestates that are generated here have lower nutrient concentrations than those that are typically documented in the literature [73]. Nevertheless, their composition remains appropriate for use in fertilizer formulation, particularly as a source of organic matter and complementary nutrients.

Table 7.
Fertilizing potential of digestates from different assays.
Producing nutrient-rich digestates through the anaerobic digestion (AD) of invasive plants is a promising way to recycle nutrients in agriculture, aligning with the principles of the circular economy. However, concerns have been raised about the environmental safety of these digestates. Given the capacity of invasive plants to accumulate metals, it is imperative that the digestate be leached and analyzed for heavy metals before being applied [74,75]. Microbial assessment is also required to evaluate the effectiveness of the fertilizer and its impact on soil and crops [76]. The characterization of different harvested invasive plants shows that the salts and heavy metal contents were well below the established regulatory limits. Similarly, analyses of the specific digestate used in our experiments, performed by the supplying facility, confirmed that it contained a low concentration of heavy metal and was free of pathogens. This indicates that the thermophilic anaerobic digestion process, combined with a clean feedstock, effectively yields a safe, high-quality biofertilizer, suitable for agricultural use.
The composition and properties of digestates can vary significantly depending on several factors including the specific digested substrates, the operational parameters (e.g., temperature, retention time), and the digestion process [77]. In accordance with our findings, Slepetiene et al. (2020) demonstrated that the applied digestate positively impacted soil quality, fertility, mobile humic acid content, soil durability, and sustainability [78]. Moreover, research by Czatzkowska et al. (2024) has shown that digestates can enhance soil microbial diversity and activity, contributing to improved nutrient cycling and soil health [79]. Additionally, Li et al. (2024) highlight the potential of digestates as slow-release fertilizers, offering a more environmentally friendly alternative to conventional mineral fertilizers by reducing nutrient leaching and improving long-term soil fertility [80]. Furthermore, Alhammad & Seleiman (2023) provide evidence that digestate application can significantly enhance plant growth and yield, demonstrating its efficacy as a valuable agricultural resource [81].
3.4.2. Germination Assays
Following AD, the resulting digestates were used to investigate the impact of the process on invasive plants regeneration. Innocuity assays revealed complete inhibition (0.00 ± 0.00%) of germination for both Reynoutria and Phragmites digestates from different harvest periods for the five repetitions applied.
Additional experiments were performed using thermal treatment to confirm the inhibition of plant regeneration using AD. For each species and type of biomass (seed or rhizome), germination tests showed that only the control samples germinated in all three repetitions of each assay. A one-day incubation at 55 °C in a water bath affectively eliminated seed and rhizome viability. Overall, these trials demonstrate that the AD process successfully inhibits the growth of all parts of Reynoutria and Phragmites (Figure 6).
Figure 6.
Rate of rhizome germination for Reynoutria and Phragmites after three incubation periods (1, 7, and 21 days) at 55 °C following thermal treatment.
The study’s innovative approach, integrating thermophilic hydrolysis and AD, has been successfully implemented to prevent further dispersion of invasive plants, while simultaneously generating a new bioenergy source. Our results align with a previous study of Abbas et al. (2023), which reported complete inhibition of seed germination from invasive weeds and undesirable crop plants in mesophilic conditions [82]. However, as noted in Erraji et al. (2019) [83], some viability of undesirable seeds was occasionally observed, likely due to the lower temperatures used. In contrast, thermophilic conditions (55 °C) have been reported to ensure complete mortality across various weed species [84]. Moreover, our study extends beyond seed viability to demonstrate the complete inactivation of rhizomes, a critical aspect for controlling persistent invasive species, which is not always addressed in mesophilic studies.
4. Conclusions
Invasive plants, Reynoutria and Phragmites, exhibited substantial organic matter content, presenting a valuable opportunity for resource recovery. This study develops an innovative, integrated, single-stage bioprocess employing a tailored hydrolytic–methanogenic consortium to effectively degrade this organic matter and biotransform these problematic weeds into valuable resources. Notably, a significant production of methane-rich biogas was achieved from both of the invasive plant species, which were examined using an SIR of 1. Furthermore, the resulting digestates demonstrated a rich composition of essential macronutrients (N, P, and K), crucial for plant growth. Critically, innocuity assays revealed complete inhibition of seed germination after 25 days of AD, signifying the effective elimination of regenerative potential. This demonstrates a key advantage of our thermophilic process. However, more research is needed to compare its performance with that of mesophilic digestion and structured two-stage systems.
The biotransformation of invasive plants through thermophilic AD using a hydrolytic–methanogenic consortium represents a cost-effective and environmentally sound biotechnology. This approach enables the dual recovery of residual organic matter for renewable energy production and the safe disposal of invasive biomass, yielding a nutrient-rich digestate suitable for direct agricultural application or further processing into biochar for soil amendment. While the present study was conducted at laboratory and on a small pilot-scale (1 and 10 L reactors), it provides critical baseline data for process optimization and scalability assessment.
A follow-up pilot-scale study is currently in progress under diverse environment and operational conditions to validate the technical performance and real applicability of the process.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/en18195151/s1, Table S1: Cumulative biogas production from Reynoutria; Table S2: Cumulative biogas production from Phragmites; Table S3: Methane content of biogas generated from Reynoutria in thermophilic anaerobic digestion; Table S4: Methane content of biogas generated from Phragmites in thermophilic anaerobic digestion; Tables S5–S11: Gompertz model fitting outputs, including exported tables of parameter estimates, predicted values, R2, and screenshots of SPSS outputs illustrating the nonlinear regression process.
Author Contributions
Conceptualization, Investigation and Methodology: Z.D., J.C., P.F. and F.B.; Original draft preparation: Z.D.; Supervision and Validation: H.H., S.T. and S.L.; Project management: H.H.; Writing—review and editing: H.H., S.T., and S.L.; Funding acquisition: H.H. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) (Grant number CARD2 566803–21), the Ministry of Agriculture, Fisheries and Food (MAPAQ), the Ministry of Transport and Sustainable Mobility, and the Ministry of the Environment, the Fight Against Climate Change, Wildlife and Parks.
Data Availability Statement
The data presented in this study is available on request from the corresponding author.
Acknowledgments
Thanks to Ecological communities Bas-Saint-Laurent (CoÉco), who was involved in every stage of the project and ensured all coordination between the researchers and the ministries. The Ministry of Transport and Sustainable Mobility is acknowledged for the transport provided during the harvest.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Anaerobic digestion |
| ALK | Alkalinity |
| BMP | Biochemical Methane Potential |
| DM | Dried matter |
| I | Inoculum |
| N–NH4+ | Ammonium |
| Ntot | Total Nitrogen |
| P | Phragmites |
| P.1 | Phragmites from the first harvest |
| P.2 | Phragmites from the second harvest |
| P.3 | Phragmites from the third harvest |
| PR.3 | Phragmites from the third harvest of root parts |
| R | Reynoutria |
| R.1 | Reynoutria from the first harvest |
| R.2 | Reynoutria from the second harvest |
| R.3 | Reynoutria from the third harvest |
| S | Substrate |
| SIR | Substrate inoculum ratio |
| VFA | Volatile Fatty acids |
| VS | Volatile solids |
References
- Reid, W.V.; Ali, M.K.; Field, C.B. The Future of Bioenergy. Glob. Change Biol. 2020, 26, 274–286. [Google Scholar] [CrossRef]
- Tran, G.V.; Unpaprom, Y.; Ramaraj, R. Methane Productivity Evaluation of an Invasive Wetland Plant, Common Reed. Biomass Convers. Biorefin. 2020, 10, 689–695. [Google Scholar] [CrossRef]
- Gernaat, D.E.H.J.; de Boer, H.S.; Daioglou, V.; Yalew, S.G.; Müller, C.; van Vuuren, D.P. Climate Change Impacts on Renewable Energy Supply. Nat. Clim. Change 2021, 11, 119–125. [Google Scholar] [CrossRef]
- Ebadian, M.; van Dyk, S.; McMillan, J.D.; Saddler, J. Biofuels Policies that Have Encouraged Their Production and Use: An International Perspective. Energy Policy 2020, 147, 111906. [Google Scholar] [CrossRef]
- Zamri, M.F.M.A.; Hasmady, S.; Akhiar, A.; Ideris, F.; Shamsuddin, A.H.; Mofijur, M.; Fattah, I.M.R.; Mahlia, T.M.I. A Comprehensive Review on Anaerobic Digestion of Organic Fraction of Municipal Solid Waste. Renew. Sustain. Energy Rev. 2021, 137, 110637. [Google Scholar] [CrossRef]
- Qayyum, S.; Tahir, A.; Mian, A.H.; Zeb, S.; Siddiqui, M.F.; Rehman, B. Optimizing Biogas Production through Anaerobic Digestion: Transforming Food Waste and Agricultural Residues into Renewable Energy within a Circular Economy Paradigm. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]
- Zhou, L.; Hülsemann, B.; Merkle, W.; Guo, J.; Dong, R.; Piepho, H.P.; Gerhards, R.; Müller, J.; Oechsner, H. Influence of Anaerobic Digestion Processes on the Germination of Weed Seeds. Gesunde Pflanz. 2020, 72, 181–194. [Google Scholar] [CrossRef]
- Meerbeek, K.V.; Muys, B.; Hermy, M. Lignocellulosic Biomass for Bioenergy beyond Intensive Cropland and Forests. Renew. Sustain. Energy Rev. 2019, 102, 139–149. [Google Scholar] [CrossRef]
- de la Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Ballesteros, M.; Ruiz-Salvador, Á.R.; Raposo, F.; García-Gómez, J.C.; Borja, R. Turning an Invasive Alien Species into a Valuable Biomass: Anaerobic Digestion of Rugulopteryx Okamurae after Thermal and New Developed Low-Cost Mechanical Pretreatments. Sci. Total Environ. 2023, 856, 158914. [Google Scholar] [CrossRef] [PubMed]
- Hanley, N.; Roberts, M. The Economic Benefits of Invasive Species Management. People Nat. 2019, 1, 124–137. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Rodrigues, A.M.; Loureiro, L.M.E.F.; Sá, L.C.R.; Matias, J.C.O. Energy Recovery from Invasive Species: Creation of Value Chains to Promote Control and Eradication. Recycling 2021, 6, 21. [Google Scholar] [CrossRef]
- Lim, X. Herbicides─Unexpected Allies against Invasive Plants. ACS Cent. Sci. 2022, 8, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Singh, J.S. Invasive Alien Plant Species: Their Impact on Environment, Ecosystem Services and Human Health. Ecol. Indic. 2020, 111, 106020. [Google Scholar] [CrossRef]
- Baute, K.A.; Robinson, D.E.; Eerd, L.L.V.; Edson, M.; Sikkema, P.H.; Gilroyed, B.H. Survival of Seeds from Perennial Biomass Species during Commercial-Scale Anaerobic Digestion. Weed Res. 2016, 56, 258–266. [Google Scholar] [CrossRef]
- Martin, F.M.; Dommanget, F.; Lavallée, F.; Evette, A. Clonal Growth Strategies of Reynoutria japonica in Response to Light, Shade, and Mowing, and Perspectives for Management. NeoBiota 2020, 56, 89–110. [Google Scholar] [CrossRef]
- VanWallendael, A.; Hamann, E.; Franks, S.J. Evidence for Plasticity, but Not Local Adaptation, in Invasive Japanese Knotweed (Reynoutria japonica) in North America. Evol. Ecol. 2018, 32, 395–410. [Google Scholar] [CrossRef]
- Murrell, C.; Gerber, E.; Krebs, C.; Parepa, M.; Schaffner, U.; Bossdorf, O. Invasive Knotweed Affects Native Plants through Allelopathy. Am. J. Bot. 2011, 98, 38–43. [Google Scholar] [CrossRef]
- Cote, S. Analyse Socio-Économique de La Gestion Des Espèces Floristiques Exotiques Envahissantes Au Québec Avec Le Roseau Commun (Phragmites australis) En Exemple; Université de Sherbrooke: Sherbrooke, QC, Canada, 2023. [Google Scholar]
- Eftaxias, A.; Kolokotroni, I.; Michailidis, C.; Charitidis, P.; Diamantis, V. Techno-Economic Assessment of Anaerobic Digestion Technology for Small- and Medium-Sized Animal Husbandry Enterprises. Appl. Sci. 2024, 14, 4957. [Google Scholar] [CrossRef]
- Holliger, C.; Astals, S.; De Laclos, H.F.; Hafner, S.D.; Koch, K.; Weinrich, S. Towards a Standardization of Biomethane Potential Tests: A Commentary. Water Sci. Technol. 2021, 83, 247–250. [Google Scholar] [CrossRef]
- VDI 4630—Fermentation of Organic Materials—Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests|GlobalSpec. Available online: https://standards.globalspec.com/std/10052171/vdi-4630 (accessed on 4 September 2025).
- APHA. Standard Methods for the Examination of Water and Wastewater 5, 21st ed.; American Public Health Association/AmericanWater Works Association/Water Environment Federation: Alexandria, VA, USA, 2005. [Google Scholar]
- Water Analysis Handbook|Hach. Available online: https://www.hach.com/resources/water-analysis-handbook (accessed on 9 June 2025).
- Centre D’expertise en Analyse Environnementale du Québec. Available online: https://www.ceaeq.gouv.qc.ca/index.asp (accessed on 9 June 2025).
- Lymperatou, A.; Rasmussen, N.B.; Gavala, H.N.; Skiadas, I.V. Improving the Anaerobic Digestion of Swine Manure through an Optimized Ammonia Treatment: Process Performance, Digestate and Techno-Economic Aspects. Energies 2021, 14, 787. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Porhemmat, M.; Pramanik, B.K. Performance and Kinetic Model of a Single-Stage Anaerobic Digestion System Operated at Different Successive Operating Stages for the Treatment of Food Waste. Processes 2019, 7, 600. [Google Scholar] [CrossRef]
- Méthode D’analyse des sols, des Fumiers et des Tissus Végétaux—AGDEX 533—Mai 1988 Agri-Réseau|Documents. Agri-Réseau. Available online: https://www.agrireseau.net/documents/96351/methode-d_analyse-des-sols-des-fumiers-et-des-tissus-vegetaux-agdex-533-mai-1988 (accessed on 10 June 2025).
- Mal, T.K.; Narine, L. The Biology of Canadian Weeds. 129. Phragmites australis (Cav.) Trin. Ex Steud. Can. J. Plant Sci. 2003, 84, 365–396. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.C. Synergistic Effect of Activated Carbon and Encapsulated Trace Element Additive on Methane Production from Anaerobic Digestion of Food Wastes—Enhanced Operation Stability and Balanced Trace Nutrition. Bioresour. Technol. 2019, 278, 108–115. [Google Scholar] [CrossRef]
- Wintsche, B.; Glaser, K.; Sträuber, H.; Centler, F.; Liebetrau, J.; Harms, H.; Kleinsteuber, S. Trace Elements Induce Predominance among Methanogenic Activity in Anaerobic Digestion. Front. Microbiol. 2016, 7, 2034. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of Anaerobic Digestion on Digestate Nutrient Availability and Crop Growth: A Review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Bozieva, A.M.; Zharmukhamedov, S.K.; Leong, Y.K.; Lan, J.C.-W.; Veziroglu, A.; Veziroglu, T.N.; Tomo, T.; Chang, J.S.; Allakhverdiev, S.I. A Comprehensive Review on Lignocellulosic Biomass Biorefinery for Sustainable Biofuel Production. Int. J. Hydrogen Energy 2022, 47, 1481–1498. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Kasulla, S.; Zafar, S. Role of Microbial Hydrolysis in Anaerobic Digestion: Enhancing Biogas Production Efficiency. Partn. Univers. Innov. Res. Publ. 2024, 2, 43–50. [Google Scholar] [CrossRef]
- Nayeri, D.; Mohammadi, P.; Bashardoust, P.; Eshtiaghi, N. A Comprehensive Review on the Recent Development of Anaerobic Sludge Digestions: Performance, Mechanism, Operational Factors, and Future Challenges. Results Eng. 2024, 22, 102292. [Google Scholar] [CrossRef]
- Cioabla, A.E.; Ionel, I.; Dumitrel, G.A.; Popescu, F. Comparative Study on Factors Affecting Anaerobic Digestion of Agricultural Vegetal Residues. Biotechnol. Biofuels 2012, 5, 39. [Google Scholar] [CrossRef]
- Hossain, M.S.; ul Karim, T.; Onik, M.H.; Kumar, D.; Rahman, M.A.; Yousuf, A.; Uddin, M.R. Impact of Temperature, Inoculum Flow Pattern, Inoculum Type, and Their Ratio on Dry Anaerobic Digestion for Biogas Production. Sci. Rep. 2022, 12, 6162. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, G.; Pourcher, A.M.; Duedal, A.L.; Picard, S.; Roux, S.L.; Peu, P. Impact of pH in the First-Stage of a Two-Stage Anaerobic Digestion on Metabolic Pathways and Methane Production. Bioresour. Technol. Rep. 2022, 20, 101256. [Google Scholar] [CrossRef]
- Kinnunen, M.; Hilderbrandt, D.; Grimberg, S.; Rogers, S.; Mondal, S. Comparative Study of Methanogens in One- and Two-Stage Anaerobic Digester Treating Food Waste. Renew. Agric. Food Syst. 2015, 30, 515–523. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Wang, X. Effects of Alkalinity Sources on the Stability of Anaerobic Digestion from Food Waste. Waste Manag. Res. 2015, 33, 1033–1040. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; El-Mashad, H.M.; Chen, C.; Liu, G.; Zhang, R. Functions of Bacteria and Archaea Participating in the Bioconversion of Organic Waste for Methane Production. Sci. Total Environ. 2021, 763, 143007. [Google Scholar] [CrossRef]
- Chaher, N.E.H.; Nassour, A.; Hamdi, M.; Nelles, M. Monitoring of Food Waste Anaerobic Digestion Performance: Conventional Co-Substrates VS Unmarketable Biochar Additions. Foods 2021, 10, 2353. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Zhen, F.; Liu, H.; Xiao, F.; Sun, Y.; Peng, X.; Li, Y.; Wang, X. Thermodynamics of Volatile Fatty Acid Degradation during Anaerobic Digestion under Organic Overload Stress: The Potential to Better Identify Process Stability. Water Res. 2022, 214, 118187. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the Effect of High Concentrations of Volatile Fatty Acids in Anaerobic Digestion on Methanogenic Communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef]
- Subbarao, P.M.V.; Silva, T.C.D.; Adlak, K.; Kumar, S.; Chandra, R.; Vijay, V.K. Anaerobic Digestion as a Sustainable Technology for Efficiently Utilizing Biomass in the Context of Carbon Neutrality and Circular Economy. Environ. Res. 2023, 234, 116286. [Google Scholar] [CrossRef]
- Bareha, Y.; Girault, R.; Jimenez, J.; Trémier, A. Characterization and Prediction of Organic Nitrogen Biodegradability during Anaerobic Digestion: A Bioaccessibility Approach. Bioresour. Technol. 2018, 263, 425–436. [Google Scholar] [CrossRef]
- Li, B.; Ladipo-Obasa, M.; Romero, A.; Wadhawan, T.; Tobin, M.; Manning, E.; Higgins, M.; Al-Omari, A.; Murthy, S.; Novak, J.T.; et al. The Inhibitory Impact of Ammonia on Thermally Hydrolyzed Sludge Fed Anaerobic Digestion. Water Environ. Res. 2021, 93, 1263–1275. [Google Scholar] [CrossRef]
- Kalamaras, S.D.; Vasileiadis, S.; Karas, P.; Angelidaki, I.; Kotsopoulos, T.A. Microbial Adaptation to High Ammonia Concentrations during Anaerobic Digestion of Manure-Based Feedstock: Biomethanation and 16S rRNA Gene Sequencing. J. Chem. Technol. Biotechnol. 2020, 95, 1970–1979. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Peng, Y.; Huang, W.; Liu, J.; Mironov, V.; Zhang, S. Deeper Insights into the Effects of Substrate to Inoculum Ratio Selection on the Relationship of Kinetic Parameters, Microbial Communities, and Key Metabolic Pathways during the Anaerobic Digestion of Food Waste. Water Res. 2022, 217, 118440. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Qi, G.; Andriamanohiarisoamanana, F.J.; Yamashiro, T.; Iwasaki, M.; Umetsu, K. Anaerobic Co-Digestion of Dairy Manure and Japanese Knotweed (Fallopia japonica) under Thermophilic Condition: Optimal Ratio for Biochemical Methane Production. Anim. Sci. J. 2021, 92, e13523. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, J. Influence of Feed/Inoculum Ratios and Waste Cooking Oil Content on the Mesophilic Anaerobic Digestion of Food Waste. Waste Manag. 2018, 73, 156–164. [Google Scholar] [CrossRef]
- Bertrand, R.L. Lag Phase Is a Dynamic, Organized, Adaptive, and Evolvable Period that Prepares Bacteria for Cell Division. J. Bacteriol. 2019, 201, e00697-18. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Cayetano, R.D.A.; Mehrez, I.; Kumar, G.; Kim, S.H. Evaluation of the Biochemical Methane Potential of Different Sorts of Algerian Date Biomass. Environ. Technol. Innov. 2020, 20, 101180. [Google Scholar] [CrossRef]
- Mao, C.; Wang, X.; Xi, J.; Feng, Y.; Ren, G. Linkage of Kinetic Parameters with Process Parameters and Operational Conditions during Anaerobic Digestion. Energy 2017, 135, 352–360. [Google Scholar] [CrossRef]
- López-Aguilar, H.A.; Morales-Durán, B.; Quiroz-Cardoza, D.; Pérez-Hernández, A. Lag Phase in the Anaerobic Co-Digestion of Sargassum spp. and Organic Domestic Waste. Energies 2023, 16, 5462. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.H. Conditions of Lag-Phase Reduction during Anaerobic Digestion of Protein for High-Efficiency Biogas Production. Biomass Bioenergy 2020, 143, 105813. [Google Scholar] [CrossRef]
- Chachkhiani, M.; Dabert, P.; Abzianidze, T.; Partskhaladze, G.; Tsiklauri, L.; Dudauri, T.; Godon, J.J. 16S rDNA Characterisation of Bacterial and Archaeal Communities during Start-up of Anaerobic Thermophilic Digestion of Cattle Manure. Bioresour. Technol. 2004, 93, 227–232. [Google Scholar] [CrossRef]
- Al-Iraqi, A.R.; Gandhi, B.P.; Folkard, A.M.; Barker, P.A.; Semple, K.T. Influence of Inoculum to Substrate Ratio and Substrates Mixing Ratio on Biogas Production from the Anaerobic Co-Digestion of Phragmites australis and Food Waste. Bioenergy Res. 2023, 17, 1277–1287. [Google Scholar] [CrossRef]
- Ye, J.; Li, D.; Sun, Y.; Wang, G.; Yuan, Z.; Zhen, F.; Wang, Y. Improved Biogas Production from Rice Straw by Co-Digestion with Kitchen Waste and Pig Manure. Waste Manag. 2013, 33, 2653–2658. [Google Scholar] [CrossRef]
- Owamah, H.I.; Ikpeseni, S.C.; Alfa, M.I.; Oyebisi, S.O.; Gopikumar, S.; Samuel, O.D.; Ilabor, S.C. Influence of Inoculum/Substrate Ratio on Biogas Yield and Kinetics from the Anaerobic Co-Digestion of Food Waste and Maize Husk. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100558. [Google Scholar] [CrossRef]
- Al-Iraqi, A.R.; Gandhi, B.P.; Folkard, A.M.; Barker, P.A.; Semple, K.T. Determine the Optimal Parameters for Biogas Production from Common Reed (Phragmites australis). Bioenergy Res. 2023, 17, 1302–1314. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Xi, B.; Sun, W.; Xia, X.; Zhu, C.; He, X.; Li, M.; Yang, T.; Wang, P.; et al. Biogas Production Improvement and C/N Control by Natural Clinoptilolite Addition into Anaerobic Co-Digestion of Phragmites australis, Feces and Kitchen Waste. Bioresour. Technol. 2015, 180, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Hans, M.; Kumar, S.; Yadav, Y.K. Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass. Sustainability 2023, 15, 1859. [Google Scholar] [CrossRef]
- Baute, K.; Eerd, L.L.V.; Robinson, D.E.; Sikkema, P.H.; Mushtaq, M.; Gilroyed, B.H. Comparing the Biomass Yield and Biogas Potential of Phragmites australis with Miscanthus x giganteus and Panicum virgatum Grown in Canada. Energies 2018, 11, 2198. [Google Scholar] [CrossRef]
- Eller, F.; Ehde, P.M.; Oehmke, C.; Ren, L.; Brix, H.; Sorrell, B.K.; Weisner, S.E.B. Biomethane Yield from Different European Phragmites australis Genotypes, Compared with Other Herbaceous Wetland Species Grown at Different Fertilization Regimes. Resources 2020, 9, 57. [Google Scholar] [CrossRef]
- Kandel, T.P.; Sutaryo, S.; Møller, H.B.; Jørgensen, U.; Lærke, P.E. Chemical Composition and Methane Yield of Reed Canary Grass as Influenced by Harvesting Time and Harvest Frequency. Bioresour. Technol. 2013, 130, 659–666. [Google Scholar] [CrossRef]
- Kakuk, B.; Bagi, Z.; Rákhely, G.; Maróti, G.; Dudits, D.; Kovács, K.L. Methane Production from Green and Woody Biomass Using Short Rotation Willow Genotypes for Bioenergy Generation. Bioresour. Technol. 2021, 333, 125223. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, M.E.; Yangin Gomec, C.; Dereli, R.K.; Arikan, O.; Ozturk, I. Biomethane Production as an Alternative Bioenergy Source from Codigesters Treating Municipal Sludge and Organic Fraction of Municipal Solid Wastes. BioMed Res. Int. 2011, 2011, 953065. [Google Scholar] [CrossRef]
- Wu, L.-J.; Ye, F.; Yang, F.; Lyu, Y.-K. Applicability of Temperature-Phased Anaerobic Digestion in Enhancing Methanation of High-Solid Sludge: Process Performance, Microbial Community Analysis and Energy Balance Assessment. Bioresour. Technol. 2025, 431, 132614. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A. Anaerobic Co-Digestion of Common Reed and Plant-Based Biowaste from Households. Energies 2025, 18, 2178. [Google Scholar] [CrossRef]
- Dhaouefi, Z.; Lecoublet, M.; Taktek, S.; Lafontaine, S.; LeBihan, Y.; Braghiroli, F.; Horchani, H.; Koubaa, A. A Review of Operational Conditions of the Agroforestry Residues Biomethanization for Bioenergy Production Through Solid-State Anaerobic Digestion (SS-AD). Energies 2025, 18, 1397. [Google Scholar] [CrossRef]
- Elalami, D.; Oukarroum, A.; Barakat, A. Anaerobic Digestion and Agronomic Applications of Microalgae for Its Sustainable Valorization. RSC Adv. 2021, 11, 26444–26462. [Google Scholar] [CrossRef]
- Vidican, R.; Mihăiescu, T.; Pleșa, A.; Mălinaș, A.; Pop, B.-A. Investigations Concerning Heavy Metals Dynamics in Reynoutria japonica Houtt.-Soil Interactions. Toxics 2023, 11, 323. [Google Scholar] [CrossRef]
- Samadi, M.T.; Leili, M.; Asgari, G.; Chavoshi, S. The Potential of Phragmites australis to Bioaccumulation and Translocate Heavy Metals from Landfill Leachate. J. Water Process Eng. 2024, 64, 105657. [Google Scholar] [CrossRef]
- Nag, R.; Auer, A.; Nolan, S.; Russell, L.; Markey, B.K.; Whyte, P.; O’Flaherty, V.; Bolton, D.; Fenton, O.; Richards, K.G.; et al. Evaluation of Pathogen Concentration in Anaerobic Digestate Using a Predictive Modelling Approach (ADRISK). Sci. Total Environ. 2021, 800, 149574. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.; Roß, C.L.; Hoffmann, M.; Muskolus, A.; Ellmer, F.; Kautz, T. The Chemical Composition of Biogas Digestates Determines Their Effect on Soil Microbial Activity. Agriculture 2020, 10, 244. [Google Scholar] [CrossRef]
- Slepetiene, A.; Volungevicius, J.; Jurgutis, L.; Liaudanskiene, I.; Amaleviciute-Volunge, K.; Slepetys, J.; Ceseviciene, J. The Potential of Digestate as a Biofertilizer in Eroded Soils of Lithuania. Waste Manag. 2020, 102, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Czatzkowska, M.; Wolak, I.; Harnisz, M.; Korzeniewska, E. Microbial Diversity and Biosafety Judgment of Digestates Derived from Different Biogas Plants for Agricultural Applications. J. Environ. Manag. 2024, 371, 123329. [Google Scholar] [CrossRef]
- Li, B.; Zhao, N.; Ran, X.; Zheng, Y.; Sobhi, M.; Dong, R.; Guo, J. The Effect of Slow-Release Phosphate Fertilizers from Digestates on Maize Rhizosphere Soil Microbial Community and Nutrient Cycling: Response and Activation Mechanism. Appl. Soil Ecol. 2024, 201, 105528. [Google Scholar] [CrossRef]
- Alhammad, B.A.; Seleiman, M.F. Improving Plant Growth, Seed Yield, and Quality of Faba Bean by Integration of Bio-Fertilizers with Biogas Digestate. Agronomy 2023, 13, 744. [Google Scholar] [CrossRef]
- Abbas, A.M.; Abdelazeem, M.; Novak, S.J. Anaerobic Digestion Reduces Seed Germination and Viability of Six Plant Species from the Upper Nile Valley, Egypt. Agronomy 2023, 13, 396. [Google Scholar] [CrossRef]
- Erraji, H.; Mohamed, A.; Mzabri, B.; Charif, K.; Afilal, M.E.; Mzabri, I.; Charif, K. The Survival of Moroccan Invasive Weed Seeds during Anaerobic Digestion. Environ. Water Sci. Public Health Territ. Intell. 2019, 3, 141–146. [Google Scholar]
- Johansen, A.; Nielsen, H.B.; Hansen, C.M.; Andreasen, C.; Carlsgart, J.; Hauggard-Nielsen, H.; Roepstorff, A. Survival of Weed Seeds and Animal Parasites as Affected by Anaerobic Digestion at Meso- and Thermophilic Conditions. Waste Manag. 2013, 33, 807–812. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).