Development of Low-Emission Cooking Device Based on Catalytic Hydrogen Combustion Technology
Abstract
1. Introduction
2. Experimental
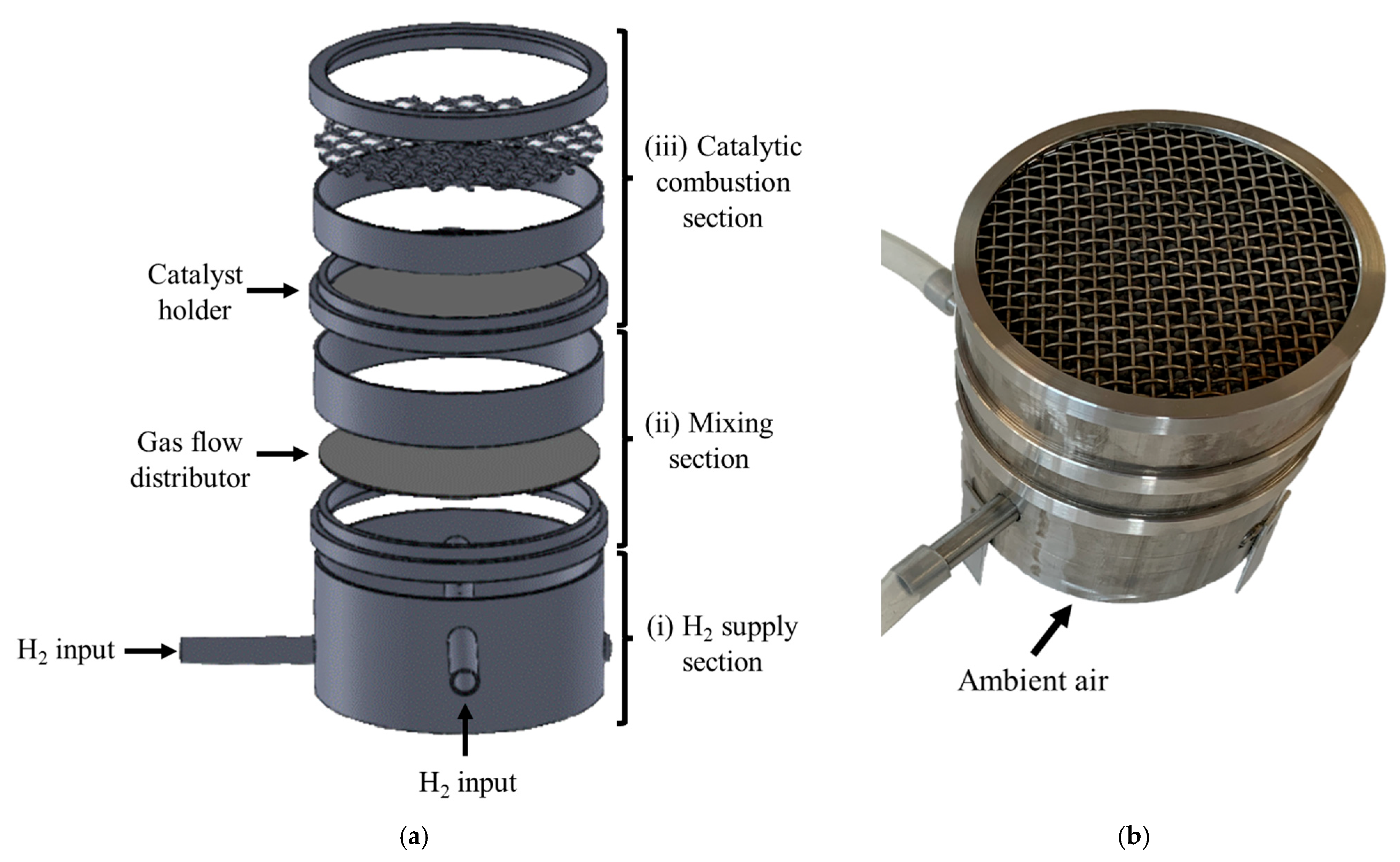
2.1. Cooking Device Design
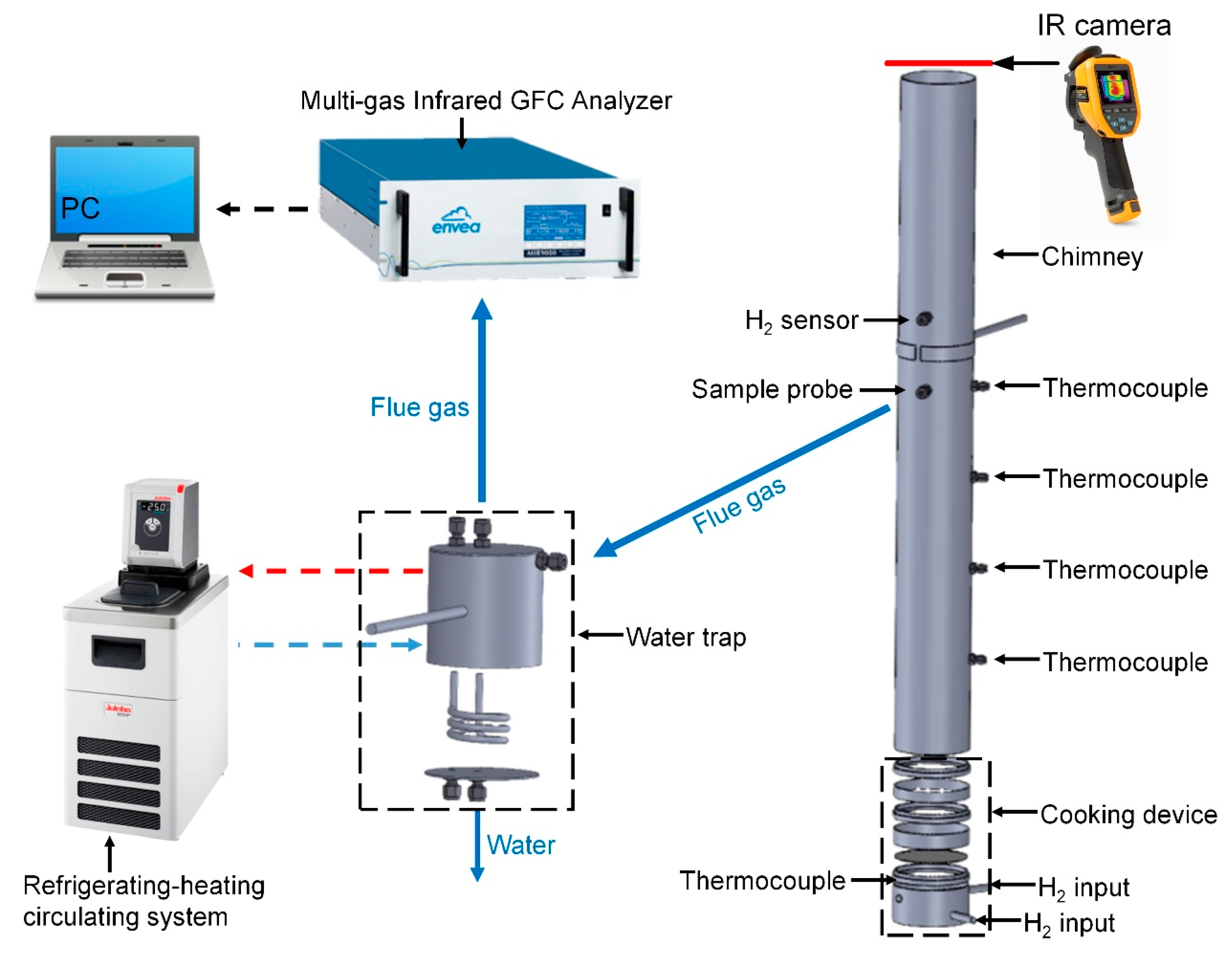
2.2. Experimental Setup
2.3. Evaluation Procedure
3. Results and Discussion
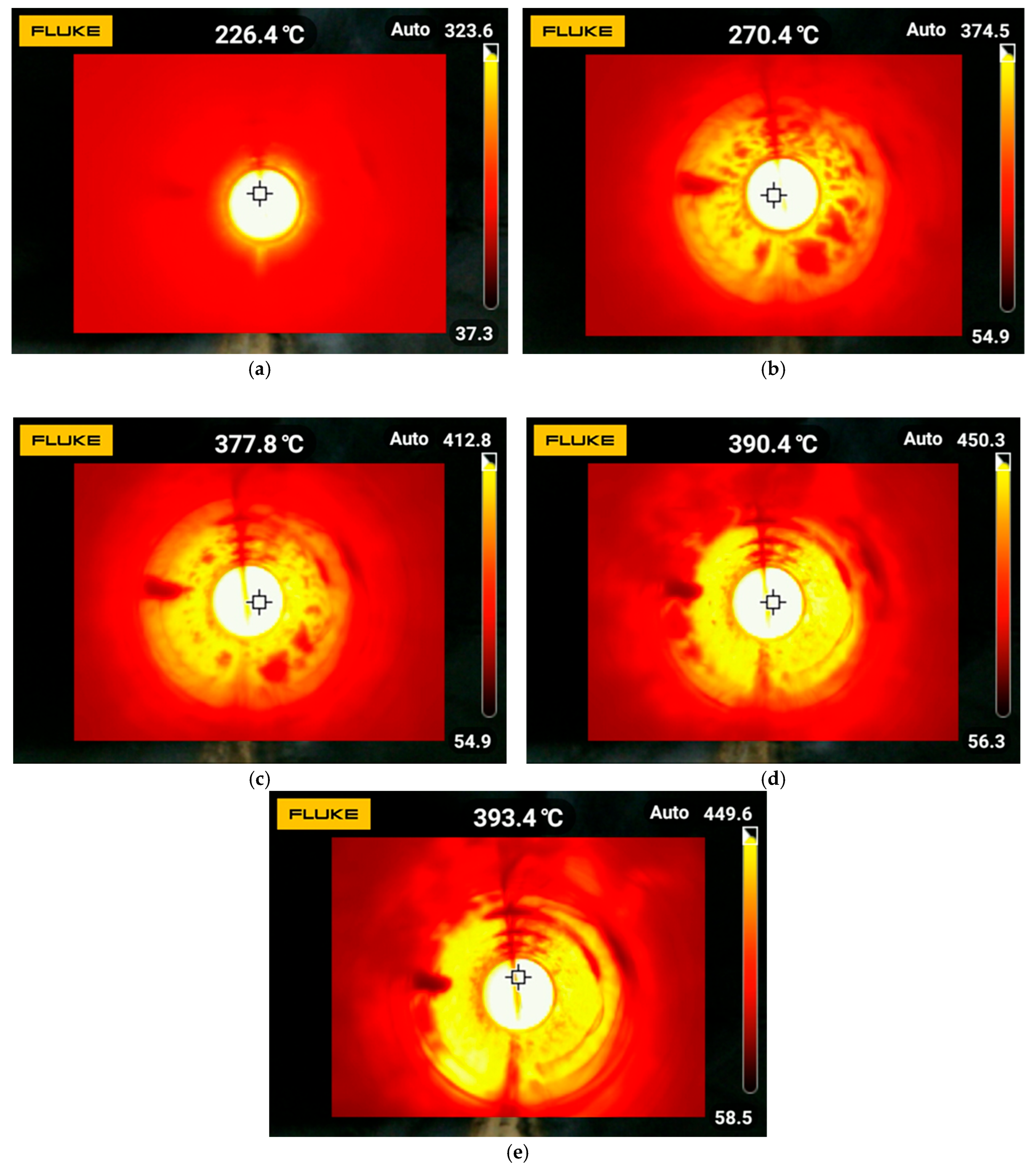
3.1. Combustion Temperature
3.2. H2 Slip and O2 Concentration
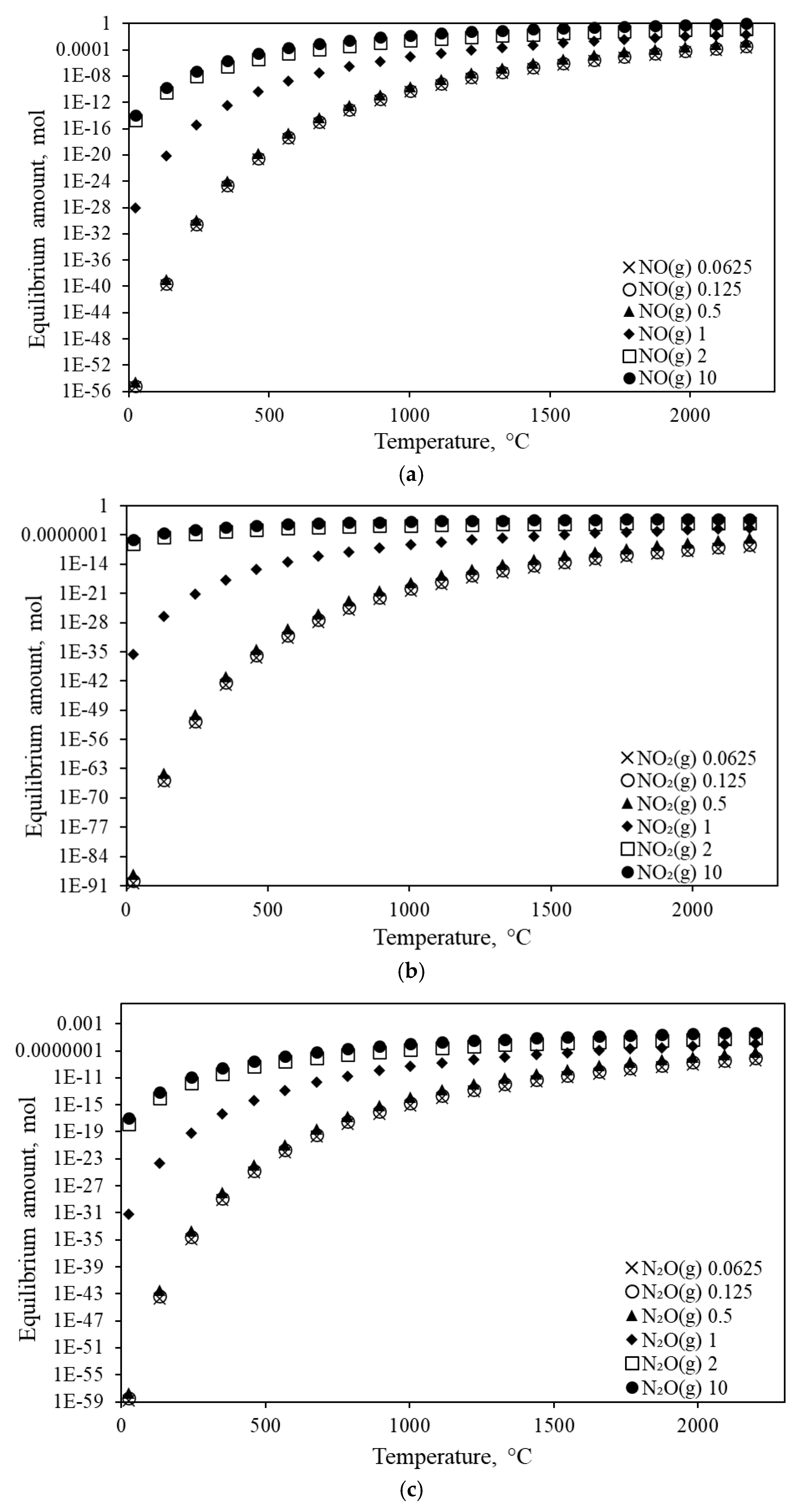
3.3. NOx Analysis
3.4. Effect of the Chimney and O2 Starvation
3.5. Efficiency Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tracking SDG 7: The Energy Progress Report. 2022. Available online: https://trackingsdg7.esmap.org/sites/default/files/download-documents/sdg7-report2022-full_report.pdf (accessed on 20 June 2025).
- du Preez, S.P.; Kozhukhova, A.E.; Bessarabov, D.G. Catalytic hydrogen combustion using platinum supported on anodized aluminium oxide adhered to metallic aluminium. S. Afr. J. Chem. 2022, 76, 31–37. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D.G. Catalytic hydrogen combustion for domestic and safety applications: A critical review of catalyst materials and technologies. Energies 2021, 14, 4897. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [PubMed]
- Bailis, R.; Ezzati, M.; Kammen, D.M. Mortality and greenhouse gas impacts of biomass and petroleum energy futures in Africa. Science 2005, 308, 98. [Google Scholar] [CrossRef]
- Rudel, T.K. The national determinants of deforestation in sub-Saharan Africa. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120405. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Khandelwal, A.; Leavey, A.; Biswas, P. A model for cost-benefit analysis of cooking fuel alternatives from a rural Indian household perspective. Renew. Sust. Energy Rev. 2016, 56, 291–302. [Google Scholar] [CrossRef]
- Yasmin, N.; Grundmann, P. Adoption and diffusion of renewable energy–The case of biogas as alternative fuel for cooking in Pakistan. Renew. Sust. Energy Rev. 2019, 101, 255–264. [Google Scholar] [CrossRef]
- Nwaokocha, C.N.; Giwa, S.O. Investigation of bio-waste as alternative fuel for cooking. In Proceedings of the 3rd International Conference On African Development Issues (CU-ICADI), Ota, Nigeria, 9–11 May 2016; pp. 1–5. [Google Scholar]
- Mishra, N.K.; Biswas, P.; Patel, S. Future of clean energy for cooking in India: A comprehensive analysis of fuel alternatives. Energy Sustain. Dev. 2024, 81, 101500. [Google Scholar] [CrossRef]
- Hurtado Pérez, E.; Mulumba Ilunga, O.; Alfonso Solar, D.; Moros Gómez, M.C.; Bastida-Molina, P. Sustainable Cooking Based on a 3 kW Air-Forced Multifuel Gasification Stove Using Alternative Fuels Obtained from Agricultural Wastes. Sustainability 2020, 12, 7723. [Google Scholar] [CrossRef]
- Kurchania, A.K.; Panwar, N.L.; Pagar, S.D. Design and performance evaluation of biogas stove for community cooking application. Int. J. Sustain. Energy. 2010, 29, 116–123. [Google Scholar] [CrossRef]
- Khandelwal, M.; Hill, M.E.; Greenough, P.; Anthony, J.; Quill, M.; Linderman, M.; Udaykumar, H.S. Why Have Improved Cook-Stove Initiatives in India Failed? World Dev. 2017, 92, 13–27. [Google Scholar] [CrossRef]
- Sambandam, S.; Balakrishnan, K.; Ghosh, S.; Sadasivam, A.; Madhav, S.; Ramasamy, R.; Samanta, M.; Mukhopadhyay, K.; Rehman, H.; Ramanathan, V. Can Currently Available Advanced Combustion Biomass Cook-Stoves Provide Health Relevant Exposure Reductions? Results from Initial Assessment of Select Commercial Models in India. EcoHealth 2015, 12, 25–41. [Google Scholar] [CrossRef]
- Pope, D.; Bruce, N.; Dherani, M.; Jagoe, K.; Rehfuess, E. Real-life effectiveness of ‘improved’ stoves and clean fuels in reducing PM2.5 and CO: Systematic review and meta-analysis. Environ. Int. 2017, 101, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Sun, L.; Ren, J.; Dong, L. Chapter 17-Opportunities and future challenges in hydrogen economy for sustainable development. In Hydrogen Economy, 2nd ed.; Scipioni, A., Manzardo, A., Ren, J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 537–569. [Google Scholar]
- Sarker, A.K.; Azad, A.K.; Rasul, M.G.; Doppalapudi, A.T. Prospect of Green Hydrogen Generation from Hybrid Renewable Energy Sources: A Review. Energies 2023, 16, 1556. [Google Scholar] [CrossRef]
- Kaleem, A.; Zaman, A.; Rajakaruna, S. Hydrogen at home: The current and future landscape of green hydrogen in residential settings. Sustain. Energy Technol. Assess. 2024, 72, 104058. [Google Scholar] [CrossRef]
- Varlamov, G.B.; Glazyrin, S.A.; Khamzina, A.; Bimurzina, Z.A.; Zhakiyev, N. Hydrogen energy for advancing energy efficiency and environmental friendliness of the heat supply system of residential house. Sustain. Energy Technol. Assess. 2024, 64, 103689. [Google Scholar] [CrossRef]
- Skottene, M.; Rian, K.E. A study of NOx formation in hydrogen flames. Int. J. Hydrogen Energy 2007, 32, 3572–3585. [Google Scholar] [CrossRef]
- Frassoldati, A.; Faravelli, T.; Ranzi, E. A wide range modeling study of NOx formation and nitrogen chemistry in hydrogen combustion. Int. J. Hydrogen Energy 2006, 31, 2310–2328. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Control of Nitrogen Oxides. In Gas Purification; Kohl, A.L., Nielsen, R.B., Eds.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 866–945. [Google Scholar]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion: Concepts and Applications, 3rd ed.; McGraw Hill Education: New York, NY, USA, 2011. [Google Scholar]
- Kim, J.; Yu, J.; Lee, S.; Tahmasebi, A.; Jeon, C.-H.; Lucas, J. Advances in catalytic hydrogen combustion research: Catalysts, mechanism, kinetics, and reactor designs. Int. J. Hydrogen Energy 2021, 46, 40073–40104. [Google Scholar] [CrossRef]
- Fumey, B.; Buetler, T.; Vogt, U.F. Ultra-low NOx emissions from catalytic hydrogen combustion. Appl. Energy 2018, 213, 334–342. [Google Scholar] [CrossRef]
- Basavaraju, A.; Ramesh, A.B.; Jajcevic, D.; Heitmeir, F. Experimental parametric investigation of platinum catalysts using hydrogen fuel. Int. J. Hydrogen Energy 2018, 43, 21307–21321. [Google Scholar] [CrossRef]
- du Toit, M.H.; Avdeenkov, A.V.; Bessarabov, D. Reviewing H2 combustion: A case study for non-fuel-cell power systems and safety in passive autocatalytic recombiners. Energ. Fuel. 2018, 32, 6401–6422. [Google Scholar] [CrossRef]
- du Toit, M.; Engelbrecht, N.; Oelofse, S.P.; Bessarabov, D. Performance evaluation and emissions reduction of a micro gas turbine via the co-combustion of H2/CH4/CO2 fuel blends. Sustain. Energy Technol. Assess. 2020, 39, 100718. [Google Scholar] [CrossRef]
- Arsalis, A. Thermodynamic modeling and parametric study of a small-scale natural gas/hydrogen-fueled gas turbine system for decentralized applications. Sustain. Energy Technol. Assess. 2019, 36, 100560. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Yang, W.; Liu, J.; Wang, Z.; Cen, K. Combustion of hydrogen–air in catalytic micro-combustors made of different material. Int. J. Hydrogen Energy 2009, 34, 3535–3545. [Google Scholar] [CrossRef]
- Sui, R.; Prasianakis, N.I.; Mantzaras, J.; Mallya, N.; Theile, J.; Lagrange, D.; Friess, M. An experimental and numerical investigation of the combustion and heat transfer characteristics of hydrogen-fueled catalytic microreactors. Chem. Eng. Sci. 2016, 141, 214–230. [Google Scholar] [CrossRef]
- Großmann, U.P.; Lehmann, J.; Menzl, F. A non-stationary hydrogen cooker with portable hydride storage and catalytic hydrogen burner. Int. J. Hydrogen Energy 2000, 25, 87–90. [Google Scholar] [CrossRef]
- Fumey, B.; Stoller, S.; Fricker, R.; Weber, R.; Dorer, V.; Vogt, U.F. Development of a novel cooking stove based on catalytic hydrogen combustion. Int. J. Hydrogen Energy 2016, 41, 7494–7499. [Google Scholar] [CrossRef]
- Vogt, U.; Fumey, B.; Bielmann, M.; Siong, V.; Gallandat, N.; Züttel, A. Catalytic hydrogen combustion on porous SiC ceramics. In Proceedings of the 15th European Fuel Cell Forum 2011, Luzern, Switzerland, 28 June–1 July 2011; pp. 45–54. [Google Scholar]
- Pangborn, J.B. Catalytic Combustion of Hydrogen in Model Appliances. J. Energy 1981, 5, 285–288. [Google Scholar] [CrossRef]
- Arrieta, C.E.; Amell, A.A. Combustion analysis of an equimolar mixture of methane and syngas in a surface-stabilized combustion burner for household appliances. Fuel 2014, 137, 11–20. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Jones, D.R.; Bessarabov, D.G.; Falch, A.; Mota das Neves Quaresma, C.; Dunnill, C.W. Development of a Pt/stainless steel mesh catalyst and its application in catalytic hydrogen combustion. Int. J. Hydrogen Energy 2019, 44, 27094–27106. [Google Scholar] [CrossRef]
- du Preez, S.P.; Jones, D.R.; Warwick, M.E.A.; Falch, A.; Sekoai, P.T.; Mota das Neves Quaresma, C.; Bessarabov, D.G.; Dunnill, C.W. Thermally stable Pt/Ti mesh catalyst for catalytic hydrogen combustion. Int. J. Hydrogen Energy 2020, 45, 16851–16864. [Google Scholar] [CrossRef]
- Mordarski, G.; Skowron, K.; Duraczyńska, D.; Drabczyk, A.; Socha, R.P. Development of a Multi-Bed Catalytic Heat Generator Utilizing a Palladium-Based Hydrogen Combustion System. Energies 2025, 18, 1348. [Google Scholar] [CrossRef]
- Choi, W.; Kwon, S.; Dong Shin, H. Combustion characteristics of hydrogen–air premixed gas in a sub-millimeter scale catalytic combustor. Int. J. Hydrogen Energy 2008, 33, 2400–2408. [Google Scholar] [CrossRef]
- Mori, Y.; Miyauchi, T.; Hirano, M. Catalytic combustion of premixed hydrogen-air. Combust. Flame 1977, 30, 193–205. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Shuro, I.; Bessarabov, D.G. Development of a low purity aluminum alloy (Al6082) anodization process and its application as a platinum-based catalyst in catalytic hydrogen combustion. Surf. Coat. Technol. 2020, 404, 126483. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Malakhov, A.A.; Bessarabov, D.G. A thermally conductive Pt/AAO catalyst for hydrogen passive autocatalytic recombination. Catalysts 2021, 11, 491. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D.G. Preparation of Pt/Ce–Zr–Y mixed oxide/anodized aluminium oxide catalysts for hydrogen passive autocatalytic recombination. Int. J. Hydrogen Energy 2022, 47, 12726–12738. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D. Preparation of highly active and thermally conductive Platinum nanoparticle/Ce–Zr–Y mixed oxide/AAO washcoat catalyst for catalytic hydrogen combustion technologies. ACS Appl. Nano Mater. 2022, 5, 8161–8174. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Shuro, I.; Bessarabov, D.G. Preparation of a cartridge-type Pt/Al2O3 catalyst using a sputter deposition method for catalytic hydrogen combustion. Energ Fuel 2022, 36, 13911–13923. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D.G. Development of Pt–Co/Al2O3 bimetallic catalyst and its evaluation in catalytic hydrogen combustion reaction. Int. J. Hydrogen Energy 2023, 51, 1079–1096. [Google Scholar] [CrossRef]
- Roine, A. HSC Chemistry [Software], Version 10; Metso: Pori, Finland, 2024. Available online: https://www.metso.com/portfolio/hsc-chemistry/ (accessed on 16 February 2024).
- Ridwan, M.K.; Saptoadi, H.; Afifah, N.A.; Akbar, R.A.; Asy-Syahrani, M.A. Analysis of the Effect of More Fuel and Air Variations Excess Air on Combustion Process Simulation at a 10 kW Biomass Power Plant Using ASPEN PLUS. J. Phys. Conf. Ser. 2024, 2828, 012032. [Google Scholar] [CrossRef]
- Appel, C.; Mantzaras, J.; Schaeren, R.; Bombach, R.; Inauen, A.; Kaeppeli, B.; Hemmerling, B.; Stampanoni, A. An experimental and numerical investigation of homogeneous ignition in catalytically stabilized combustion of hydrogen/air mixtures over platinum. Combust. Flame 2002, 128, 340–368. [Google Scholar] [CrossRef]
- Pizza, G.; Frouzakis, C.E.; Mantzaras, J.; Tomboulides, A.G.; Boulouchos, K. Dynamics of premixed hydrogen/air flames in microchannels. Combust. Flame 2008, 152, 433–450. [Google Scholar] [CrossRef]
- Mantzaras, J.; Bombach, R.; Schaeren, R. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum at pressures up to 10bar. Proc. Combust. Inst. 2009, 32, 1937–1945. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EU) No 813/2013 of 2 August 2013 Implementing Directive 2009/125/EC of the European Parliament and of the Council with Regard to Ecodesign Requirements for Space Heaters and Combination Heaters. 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013R0813&rid=1 (accessed on 25 June 2025).
- Schmidt, N.; Müller, M.; Preuster, P.; Zigan, L.; Wasserscheid, P.; Will, S. Development and characterization of a low-NOx partially premixed hydrogen burner using numerical simulation and flame diagnostics. Int. J. Hydrogen Energy 2023, 48, 15709–15721. [Google Scholar] [CrossRef]
- Tanno, S.; Ito, Y.; Michikawauchi, R.; Nakamura, M.; Tomita, H. High-Efficiency and Low-NOx Hydrogen Combustion by High Pressure Direct Injection. SAE Int. J. Engines 2010, 3, 259–268. [Google Scholar] [CrossRef]
- Barreiro, P.; Alava, I.; Blanco, J.M.; Lopez-Ruiz, G. An assessment of the operating conditions of the micromix combustion principle for low NOx industrial hydrogen burners: Numerical and experimental approach. Int. J. Hydrogen Energy 2024, 66, 208–222. [Google Scholar] [CrossRef]
- EN 30-2-1:2015; Domestic Cooking Appliances Burning Gas—Part 2-1: Rational Use of Energy—General. Slovenski Inštitut za Standardizacijo: Ljubljana, Slovenia, 2015.

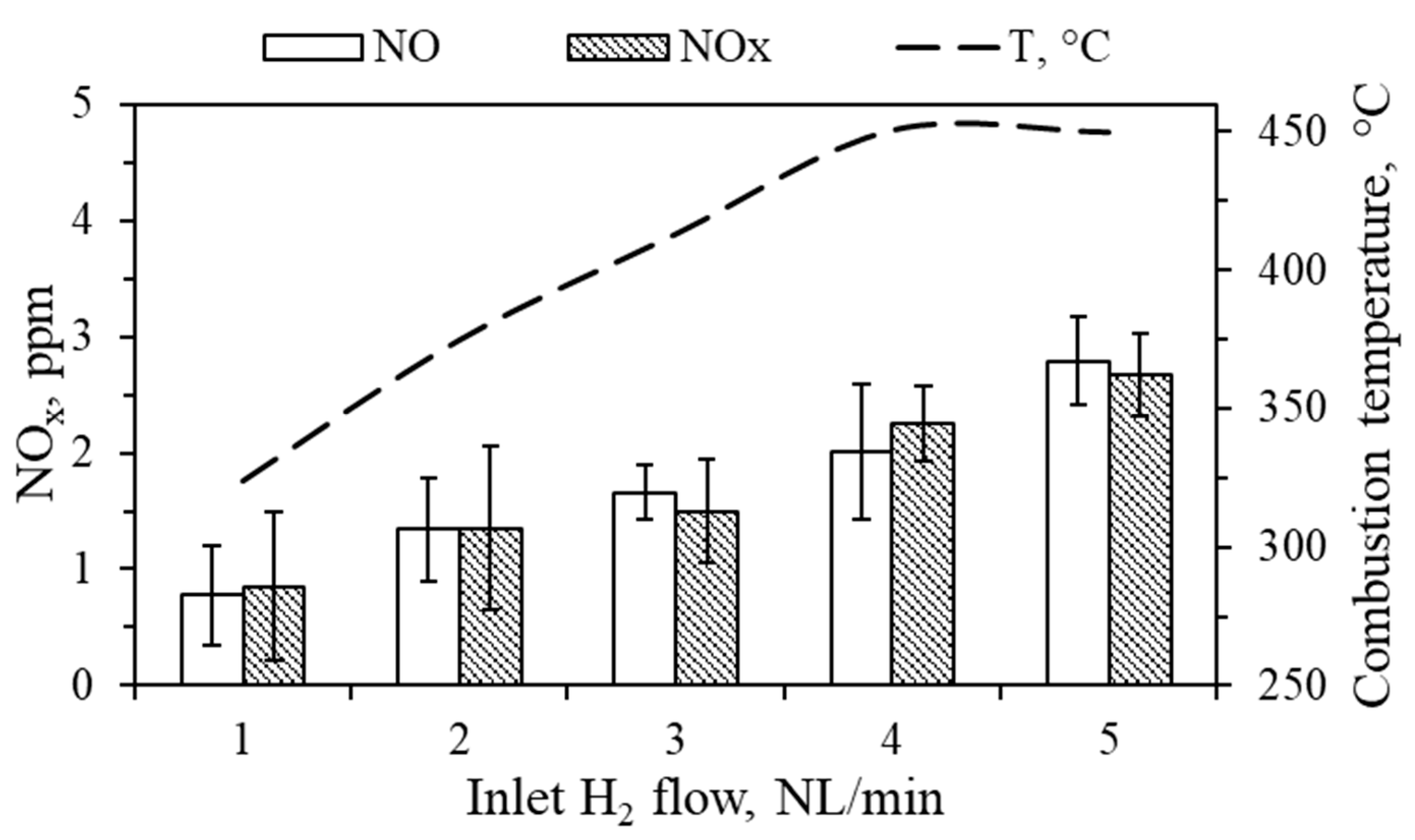



| H2 Flow, NL/min | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| H2 inlet, vol% | 12.40 ± 1.33 | 25.79 ± 1.74 | 34.80 ± 2.82 | 41.92 ± 2.08 | 47.87 ± 2.11 |
| H2 slip, vol% | 0.04 ± 0.02 | 1.04 ± 0.70 | 3.58 ± 1.62 | 8.05 ± 1.98 | 12.17 ± 2.31 |
| O2, % | 14.5 ± 0.3 | 10.2 ± 0.1 | 9.3 ± 0.3 | 7.8 ± 0.1 | 6.3 ± 0.8 |
| H2 Flow, NL/min | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| T, °C | 324.5 ± 6.5 | 450.1 ± 9.0 | 559.7 ± 11.2 | 587.0 ± 11.7 | 611.2 ± 12.2 |
| H2 slip, vol% | 0.09 ± 0.04 | 0.10 ± 0.07 | 0.59 ± 0.21 | 1.58 ± 0.56 | 1.75 ± 0.35 |
| Flue gas analysis | |||||
| NO, ppm | 2.5 ± 0.4 | 3.3 ± 0.5 | 3.7 ± 0.4 | 3.9 ± 0.5 | 4.1 ± 0.4 |
| NOx, ppm | 2.8 ± 0.4 | 3.4 ± 0.4 | 4.0 ± 0.4 | 4.2 ± 0.2 | 4.4 ± 0.4 |
| O2, % | 19.1 ± 0.2 | 18.7 ± 0.1 | 18.6 ± 0.1 | 18.8 ± 0.1 | 18.7 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhukhova, A.E.; du Preez, S.P.; Martinson, C.; Bessarabov, D.G. Development of Low-Emission Cooking Device Based on Catalytic Hydrogen Combustion Technology. Energies 2025, 18, 5074. https://doi.org/10.3390/en18195074
Kozhukhova AE, du Preez SP, Martinson C, Bessarabov DG. Development of Low-Emission Cooking Device Based on Catalytic Hydrogen Combustion Technology. Energies. 2025; 18(19):5074. https://doi.org/10.3390/en18195074
Chicago/Turabian StyleKozhukhova, Alina E., Stephanus P. du Preez, Christiaan Martinson, and Dmitri G. Bessarabov. 2025. "Development of Low-Emission Cooking Device Based on Catalytic Hydrogen Combustion Technology" Energies 18, no. 19: 5074. https://doi.org/10.3390/en18195074
APA StyleKozhukhova, A. E., du Preez, S. P., Martinson, C., & Bessarabov, D. G. (2025). Development of Low-Emission Cooking Device Based on Catalytic Hydrogen Combustion Technology. Energies, 18(19), 5074. https://doi.org/10.3390/en18195074







