Development of a Spark-Ignited Combustion Strategy for 100% Ammonia (NH3) Operation in Internal Combustion Engines
Abstract
1. Introduction
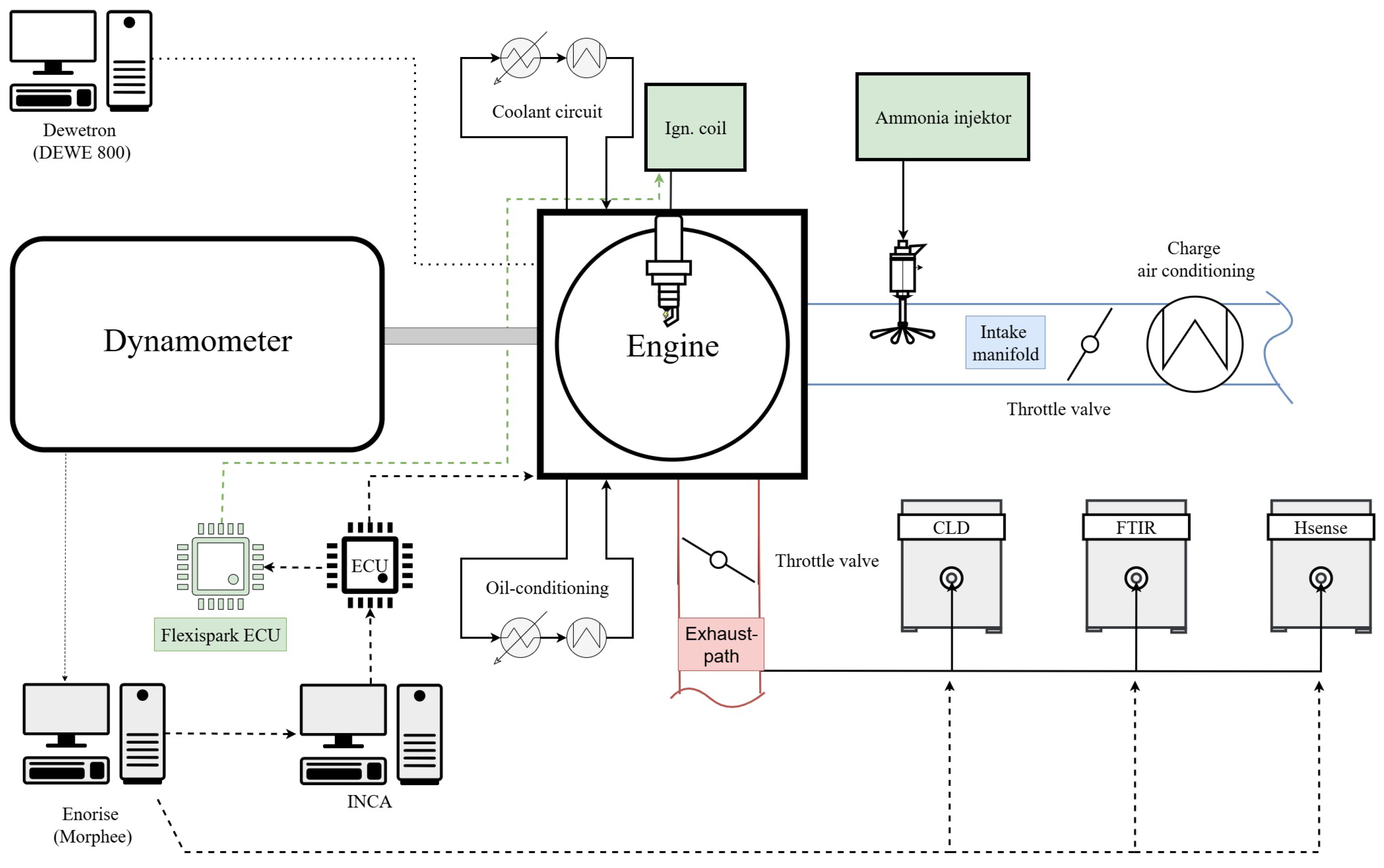
2. Experimental Setup
2.1. Engine Configuration
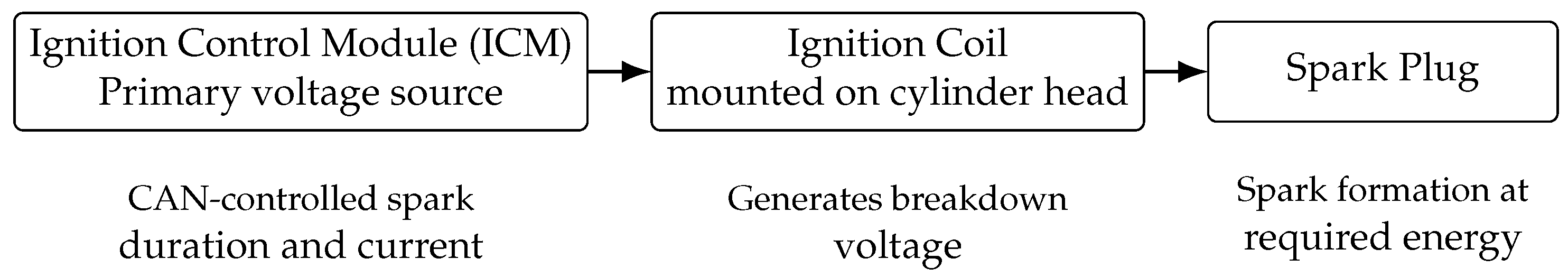
2.2. Ignition System Configuration
2.3. Instrumentation and Data Acquisition
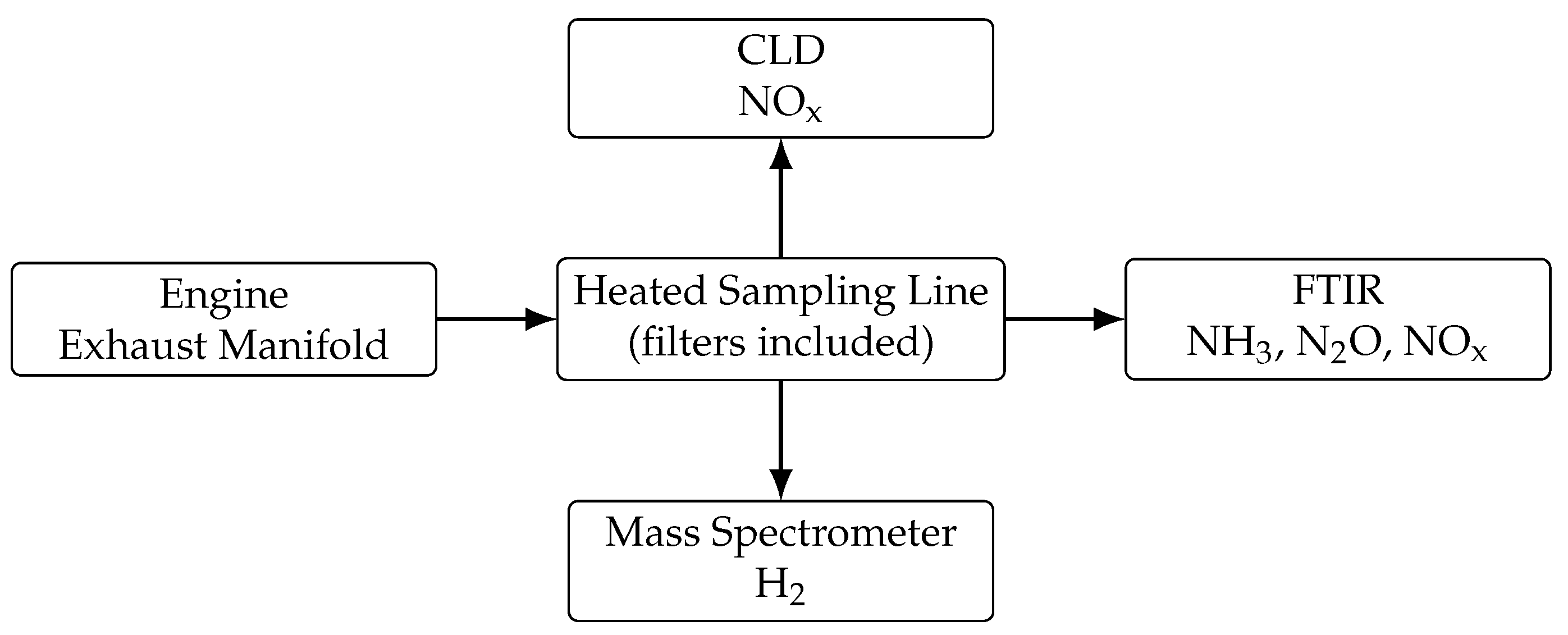
2.4. Emission Measurements
3. Ignition Characteristics of Pure NH3
4. Results and Discussion
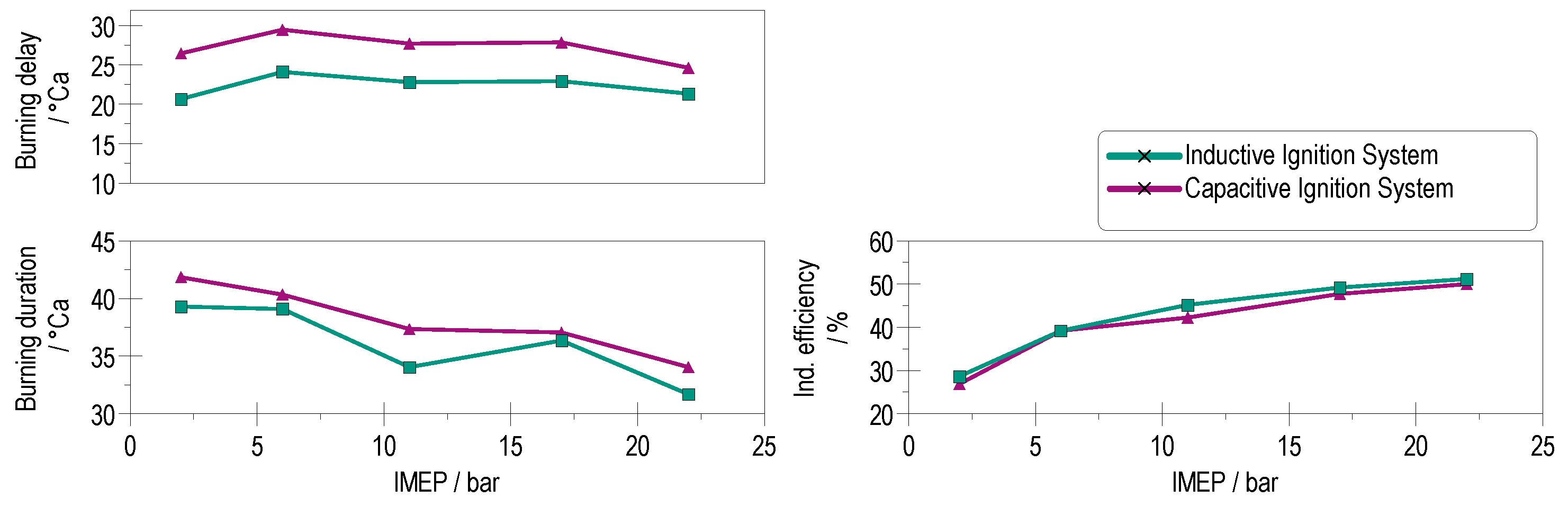
4.1. Combustion Stability and Burning Delay
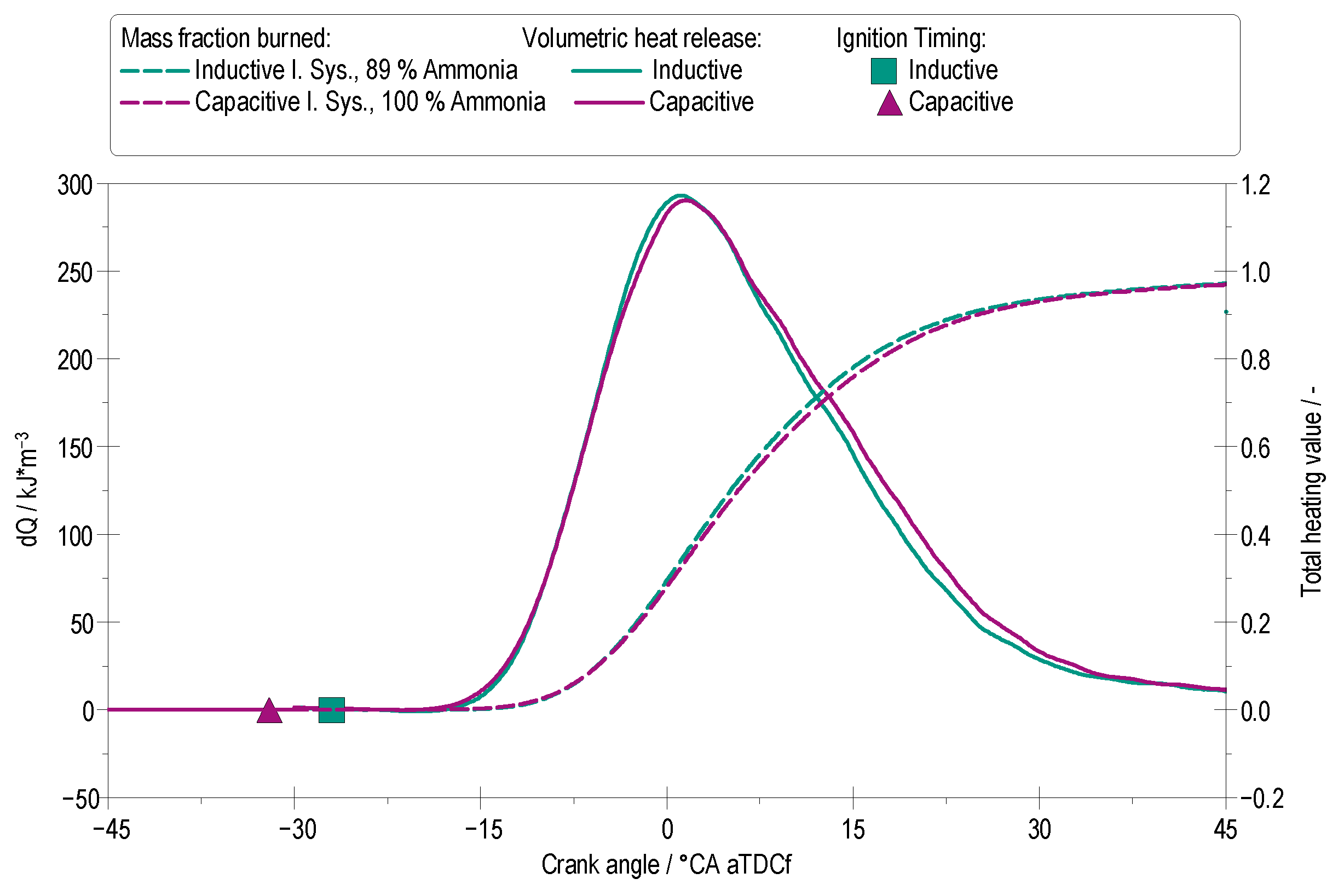
4.2. Heat Release
4.3. Effect of Spark Energy and Duration
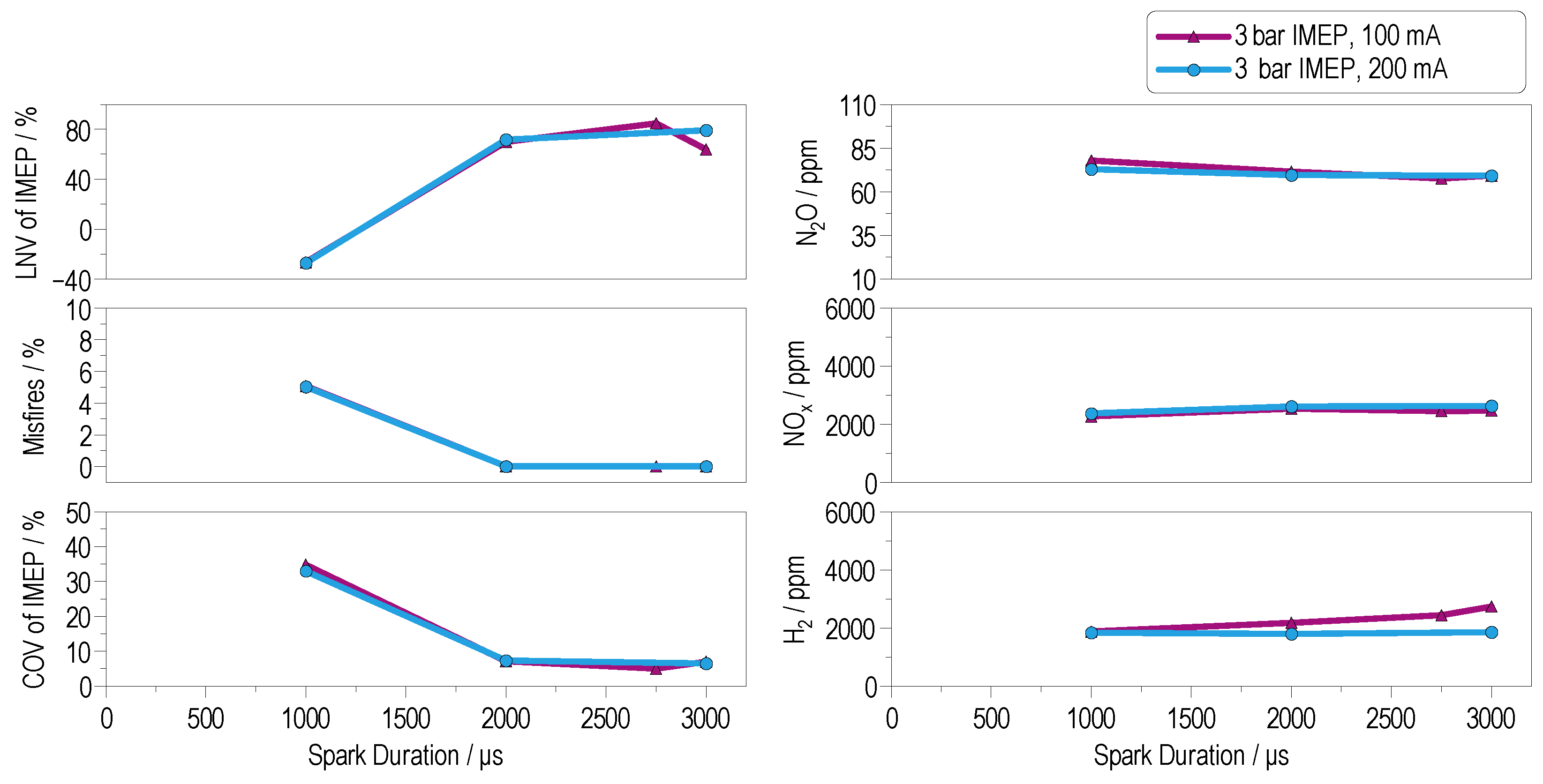
4.3.1. Low-Load Operation
4.3.2. Partial-Load Operation
4.3.3. High-Load Operation
5. Conclusions and Outlook
Summary of Key Findings
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| λ | Air–Fuel Ratio |
| AFR | Air–Fuel Ratio |
| CA | Crank angle |
| CAaTDCf | Crank angle after Top Dead Center firing |
| CAbTDCf | Crank angle before Top Dead Center firing |
| CLD | Chemiluminescence detector |
| CO2 | Carbon dioxide |
| COV | Coefficient of Variation |
| EAS | Energetic Ammonia Share |
| EGR | Exhaust Gas Recirculation |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GHG | Greenhouse Gas |
| H2 | Hydrogen |
| IMEP | Indicated Mean Effective Pressure |
| IMO | International Maritime Organization |
| LNV | Lowest Nominal Value |
| MFB | Mass Fraction Burnt |
| N2O | Nitrous oxide |
| NH3 | Ammonia |
| NOx | Nitrogen oxides |
| SI | Spark Ignition |
| TDC | Top Dead Center |
References
- International Maritime Organization (IMO). International Maritime Organization (IMO) Adopts Revised Strategy to Reduce Greenhouse Gas Emissions from International Shipping. Available online: https://www.imo.org/en/MediaCentre/PressBriefings/pages/Revised-GHG-strategy.aspx (accessed on 19 September 2025).
- Mei, B.; Zhang, J.; Shi, X.; Xi, Z.; Li, Y. Enhancement of ammonia combustion with partial fuel cracking strategy: Laminar flame propagation and kinetic modeling investigation of NH3/H2/N2/air mixtures up to 10 atm. Combust. Flame 2021, 231, 111472. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Demonstration of compression-ignition engine combustion using ammoniain reducing greenhouse gas emissions. Energy Fuels 2008, 22, 1234–1242. [Google Scholar] [CrossRef]
- Di Matteo, A.; Bracho, G.; Pinilla, D.; Somers, L.M.T. Numerical investigation of direct ammonia injection and combustion. Fuel 2025, 398, 135485. [Google Scholar] [CrossRef]
- Hayakawa, A.; Arakawa, Y.; Mimoto, R.; Somarathne, K.D.K.A.; Kudo, T.; Kobayashi, H. Experimental investigation of stabilization and emission characteristics of ammonia/air premixed flames in a swirl combustor. Int. J. Hydrogen Energy 2017, 42, 14010–14018. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Combustion Characteristics of Ammonia in a Modern Spark-Ignition Engine; SAE Technical Paper 2019-24-0237; SAE International: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Zander, M.; Thomas, W. Some thermodynamic properties of liquid ammonia: PVT data, vapor pressure, and critical temperature. J. Chem. Eng. Data 1979, 24, 1–2. [Google Scholar] [CrossRef]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/air premixed flames at various pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]
- Miller, J.A.; Melius, C.F.; Glarborg, P. The CH3+NO rate coefficient at high temperatures: Theoretical analysis and comparison with experiment. Int. J. Chem. Kinet. 1998, 30, 223–232. [Google Scholar] [CrossRef]
- Naruse, I.; Yamamoto, Y.; Itoh, Y.; Ohtake, K. Fundamental study on N2O formation/decomposition characteristics by means of low-temperature pulverized coal combustion. Symp. (Int.) Combust. 1996, 26, 3213–3221. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental study on ammonia/hydrogen/air combustion in spark ignition engine conditions. Fuel 2020, 276, 118–126. [Google Scholar] [CrossRef]
- Park, C.; Jang, Y.; Min, C.; Kim, Y.; Choi, Y. Experimental investigation of operating conditions on ammonia combustion in a direct-injection engine with hydrogen addition. Renew. Sustain. Energy Rev. 2024, 171, 113229. [Google Scholar] [CrossRef]
- Herwig, H. Wärmeübertragung A-Z: Systematische und Ausführliche Erläuterungen Wichtiger Größen und Konzepte; VDI-Buch; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar] [CrossRef]
- Čaika, V.; Dörr, N. Thermal and Transport Properties of Diesel Fuel and Their Effect on Injection Modelling; Consiglio Nazionale delle Ricerche: Rome, Italy, 2005. [Google Scholar] [CrossRef]
- Park, C.; Choi, Y.; Park, G.; Jang, I.; Kim, M.; Kim, Y.; Choi, Y. Investigation on the reduction in unburned ammonia and nitrogen oxide emissions from ammonia direct injection SI engine by using SCR after-treatment system. Heliyon 2024, 10, e37684. [Google Scholar] [CrossRef] [PubMed]
- Essmann, S.; Dymke, J.; Höltkemeier-Horstmann, J.; Möckel, D.; Schierding, C.; Hilbert, M.; Yu, C.; Maas, U.; Markus, D. Ignition characteristics of hydrogen-enriched ammonia/air mixtures. Appl. Energy Combust. Sci. 2024, 17, 100254. [Google Scholar] [CrossRef]
- Braun, A.; Baufeld, T.; Bernhardt, S.; Kubach, H.; Mohr, H.; Prehn, S. Combustion concept for ammonia-fuelled cracker-engine-unit as propulsion system for inland waterway vessels. In Proceedings of the 8th Rostock Large Engine Symposium, Rostock, Germany, 12–13 September 2024. [Google Scholar] [CrossRef]
- Braun, A.; Kubach, H.; Braun, S.; Reinbold, M.; Bernhardt, S.; Gierenz, N.; Buchholz, B.; Engelmeier, L.; Fehlemann, L.; Steffen, M.; et al. Entwicklung einer Ammoniak-betriebenen Cracker- Motor-Einheit als Antriebssystem für Binnenschiffe. In Proceedings of the Dessau Gas Engine Conference, Dessau, Germany, 15–16 May 2024. [Google Scholar] [CrossRef]
- Ängeby, J.; Gustafsson, B.; Johnsson, A. Zündsteuerungsmodul für Wasserstoffverbrennungsmotoren. Mtz—Mot. Z. 2023, 84, 48–53. [Google Scholar] [CrossRef]
- V&F Analyse- und Messtechnik GmbH. Produktflyer V&F HSense: Prozess-Massenspektrometer HSense; V&F Analyse- und Messtechnik GmbH: Absam, Österreich, 2024. [Google Scholar]
- Braun, A.; Baufeld, T.; Engelmeier, L.; Gierenz, N.; Kubach, H.; Mohr, H.; Prehn, S.; Silvestrini, S. Development of an ammonia-fueled cracker-engine-unit as propulsion system for inland waterway vessel. In Proceedings of the CIMAC Conference, Zurich, Switzerland, 19–22 May 2025. [Google Scholar] [CrossRef]
- Nakai, M.; Nakagawa, Y.; Hamai, K.; Sone, M. Stabilized Combustion in a Spark Ignited Engine through a Long Spark Duration; SAE International: Warrendale, PA, USA, 1985. [Google Scholar] [CrossRef]
- Sandhu, N.S.; Zhu, H.; Leblanc, S.; Yu, X.; Yang, H.; Ting, D.; Zheng, M. Effect of Spark Discharge Duration and Timing on the Combustion Initiation in a Lean Burn SI Engine; SAE International: Warrendale, PA, USA, 2021. [Google Scholar] [CrossRef]
- Yin, X.; Sun, N.; Sun, T.; Shen, H.; Mehra, R.K.; Liu, J.; Wang, Y.; Yang, B.; Zeng, K. Experimental investigation the effects of spark discharge characteristics on the heavy-duty spark Ignition natural gas engine at low load condition. Energy 2022, 239, 122244. [Google Scholar] [CrossRef]
- Han, S.B. Cycle-to-cycle variations under cylinder-pressure-based combustion analysis in spark ignition engines: Department of Mechanical Engineering, Induk Institute of Technology. KSME Int. J. 2000, 14, 1151–1158. [Google Scholar] [CrossRef]
- Stagni, A.; Arunthanayothin, S.; Dehue, M.; Herbinet, O.; Battin-Leclerc, F.; Brequigny, P.; Mounaim-Rousselle, C.; Favarelli, T. Low- and intermediate-temperature ammonia/hydrogen oxidation in a flow reactor: Experiments and wide-range kinetic modeling. Chem. Eng. J. 2023, 481, 144577. [Google Scholar] [CrossRef]
- Liu, W.; Qi, Y.; Zhang, R.; Zhang, Q.; Wang, Z. Hydrogen production from ammonia-rich combustion for fuel reforming under high temperature and high pressure conditions. Fuel 2022, 327, 124830. [Google Scholar] [CrossRef]
- Linde Gas GmbH. Data Sheet Ammonia: Ammonia, Anhydrous: Version 2.0. Linde Gas GmbH: Pullach, Germany, 2013. Available online: https://produkte.linde-gas.at/sdb_konform/NH3_10021772EN.pdf (accessed on 19 September 2025).
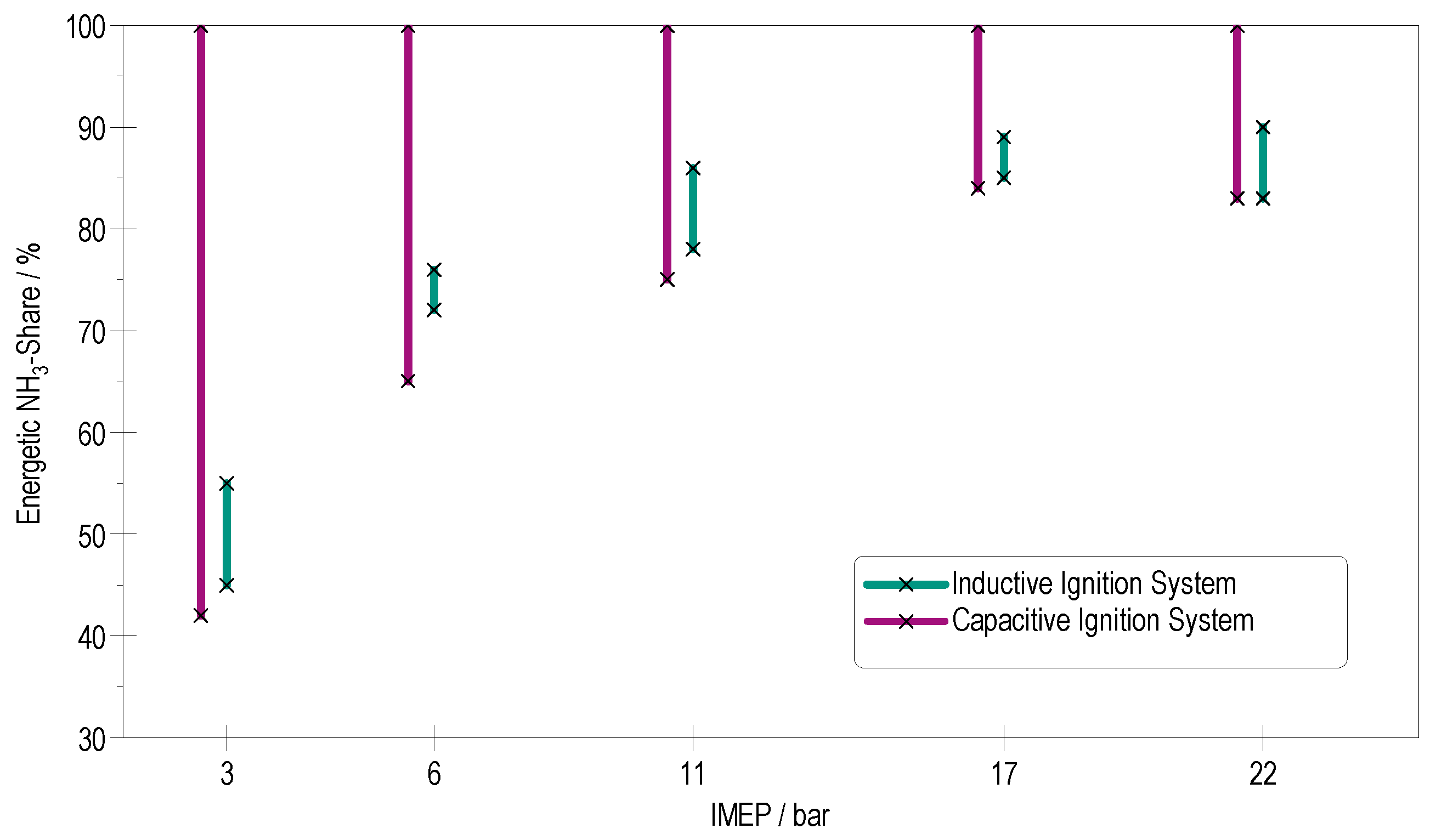

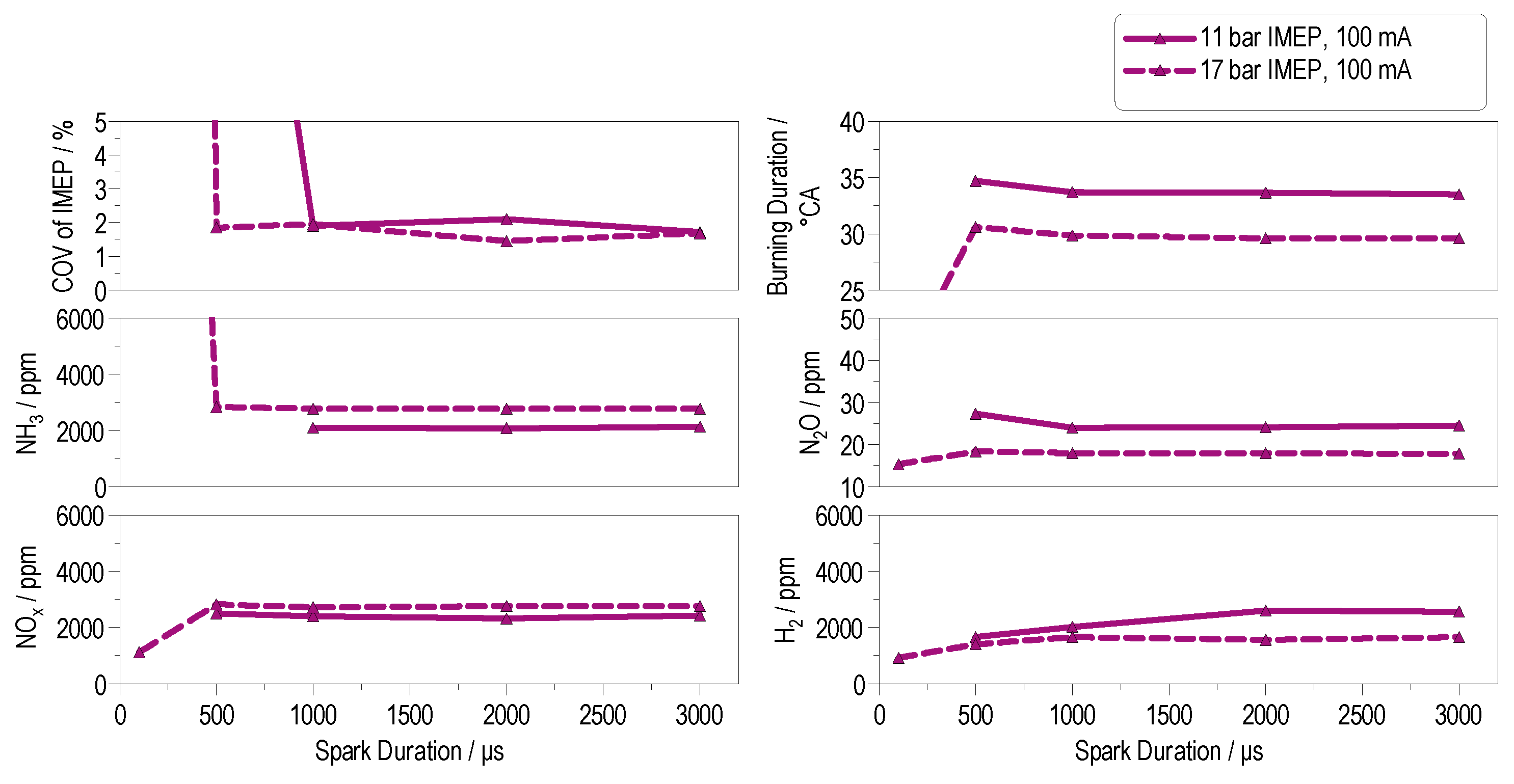

| Property | Unit | NH3 | H2 | Diesel Fuel | CH4 (LNG) |
|---|---|---|---|---|---|
| Volumetric Calorific Value | MJ/L at bar | 12.68 9 | 2.85 350 | 35.15 1 | 9.07 250 |
| Max. Laminar Flame Speed | m/s | 0.07 | 2.91 | 0.4 | 0.37 |
| Adiabatic Flame Temperature | K | 2073 | 2383 | 2303 | 2273 |
| Auto-ignition Temperature | K | 924 | 833 | >498 | 743 |
| Ignition Energy (1 bar and 298 K) | mJ | 18 a | 0.02 | 10–30 | 0.28 |
| Evaporation Energy | kJ/kg | 1368 | 223 | 200–300 | 511 |
| Tank Volume (rel. to diesel) | - | 2.8 | 4.2 | 1.0 | 1.3 |
| Engine Detail | Specification |
|---|---|
| Stroke | 157 mm |
| Bore | 135 mm |
| Max. Speed | 1900 rpm |
| Operation Principle | 4-stroke spark ignition (SI) |
| Displacement | 2.24 L |
| Engine Type | Modified Liebherr diesel-fuel engine |
| Ignition System Detail | Specification |
|---|---|
| Spark Current | Adjustable in the range of 50–300 mA |
| Spark Duration | Adjustable in the range of 40–3000 µs |
| Available Voltage | >40 kV for initial breakdown |
| Spark Energy | Configurable from 5 mJ up to 330 mJ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, A.; Grüninger, M.; Bäck, D.; Carlsson, T.; Ängeby, J.; Toedter, O.; Koch, T. Development of a Spark-Ignited Combustion Strategy for 100% Ammonia (NH3) Operation in Internal Combustion Engines. Energies 2025, 18, 5051. https://doi.org/10.3390/en18195051
Braun A, Grüninger M, Bäck D, Carlsson T, Ängeby J, Toedter O, Koch T. Development of a Spark-Ignited Combustion Strategy for 100% Ammonia (NH3) Operation in Internal Combustion Engines. Energies. 2025; 18(19):5051. https://doi.org/10.3390/en18195051
Chicago/Turabian StyleBraun, Annalena, Moritz Grüninger, Daniel Bäck, Tomas Carlsson, Jakob Ängeby, Olaf Toedter, and Thomas Koch. 2025. "Development of a Spark-Ignited Combustion Strategy for 100% Ammonia (NH3) Operation in Internal Combustion Engines" Energies 18, no. 19: 5051. https://doi.org/10.3390/en18195051
APA StyleBraun, A., Grüninger, M., Bäck, D., Carlsson, T., Ängeby, J., Toedter, O., & Koch, T. (2025). Development of a Spark-Ignited Combustion Strategy for 100% Ammonia (NH3) Operation in Internal Combustion Engines. Energies, 18(19), 5051. https://doi.org/10.3390/en18195051







