Thermal Performance Study of a Novel Double-Phase Cooling Strategy in Electric Vehicle Battery Systems
Abstract
1. Introduction
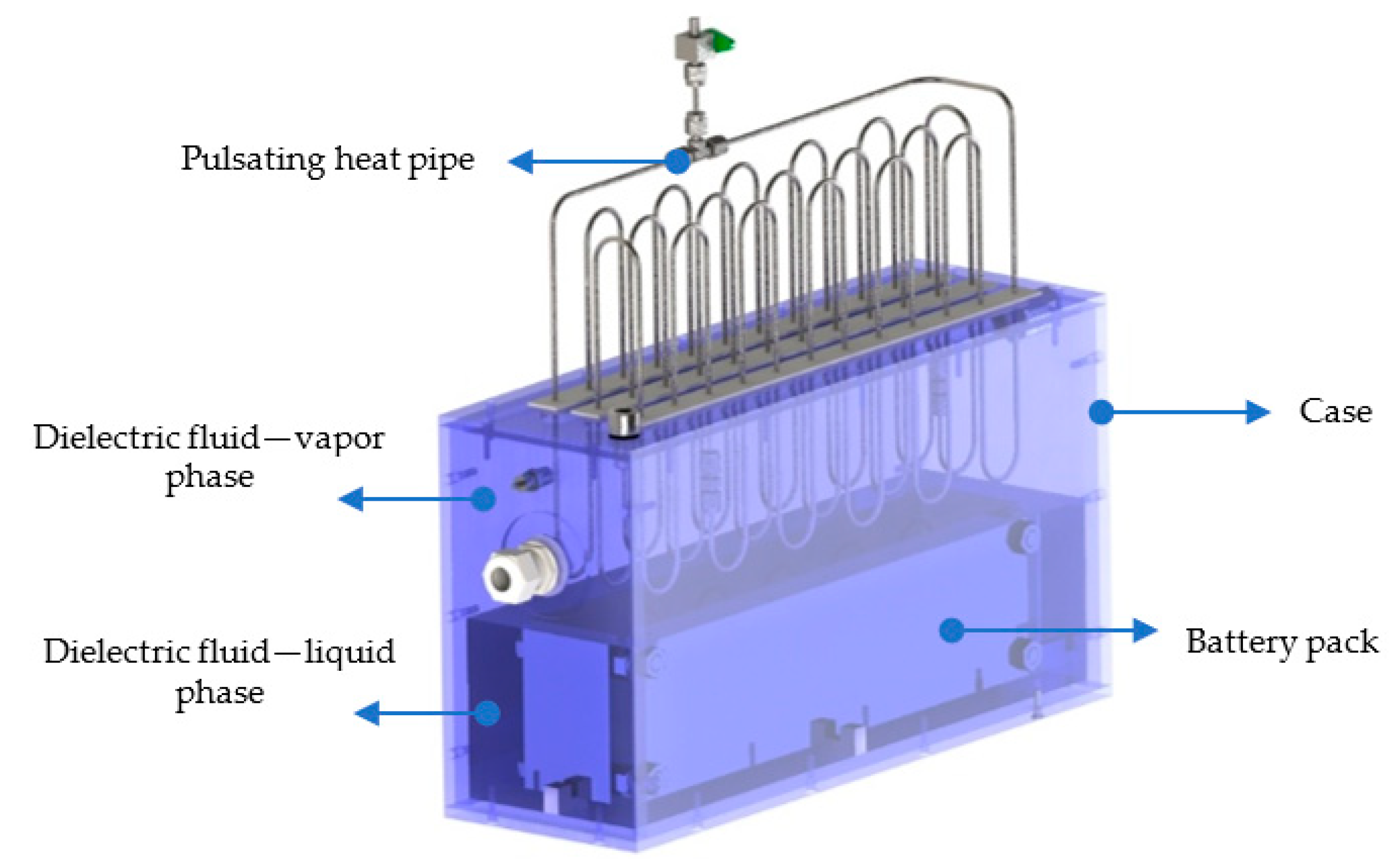
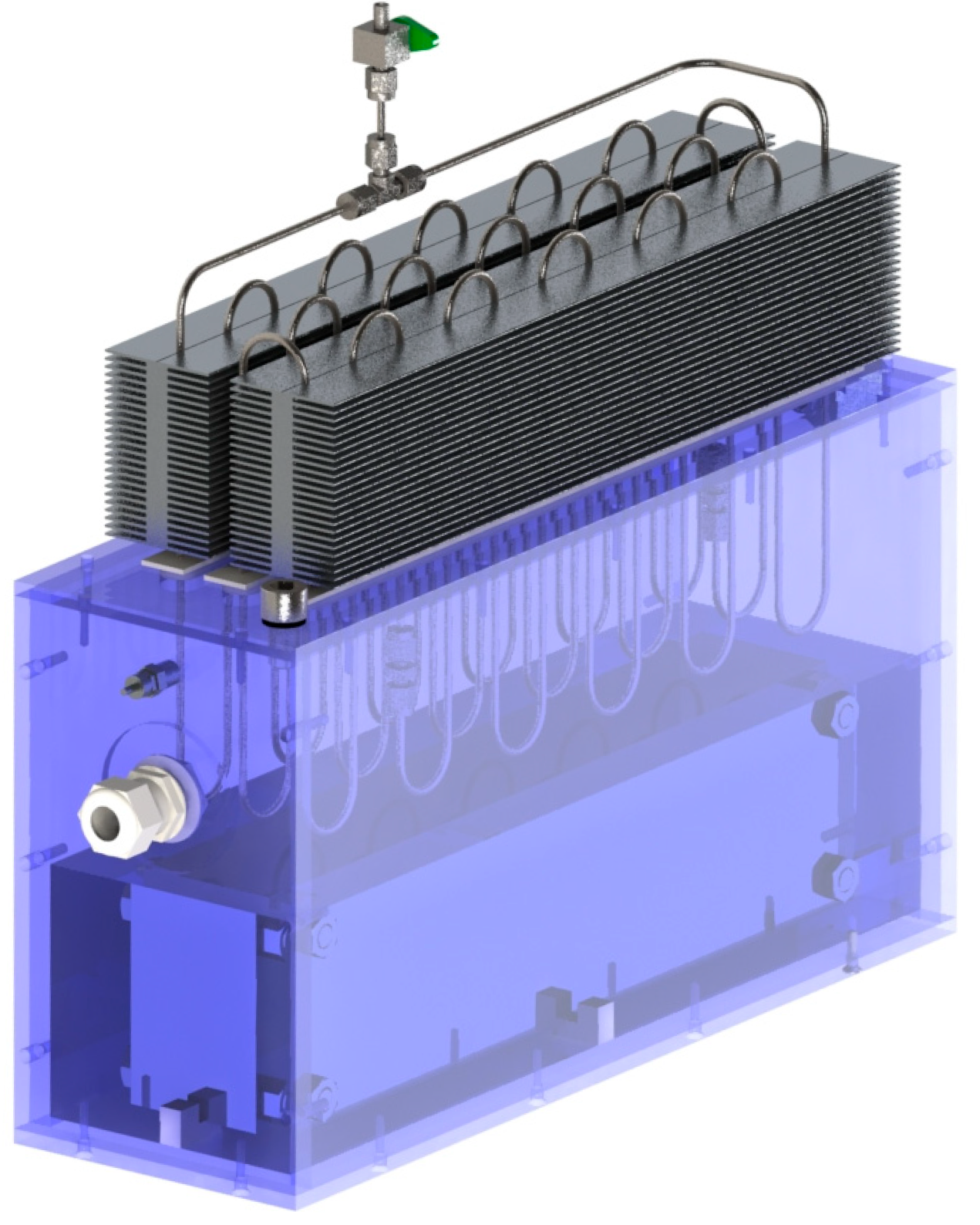
2. Experimental Setup
- (i)
- Natural convection refers to the battery pack being exposed directly to ambient air without any forced cooling or insulation.
- (ii)
- Direct immersion cooling means placing the battery pack inside a low-boiling-point fluid held in a tank.
- (iii)
- The full hybrid setup combines this immersion cooling with a PHP, which helps transfer heat from the fluid to the surrounding air.
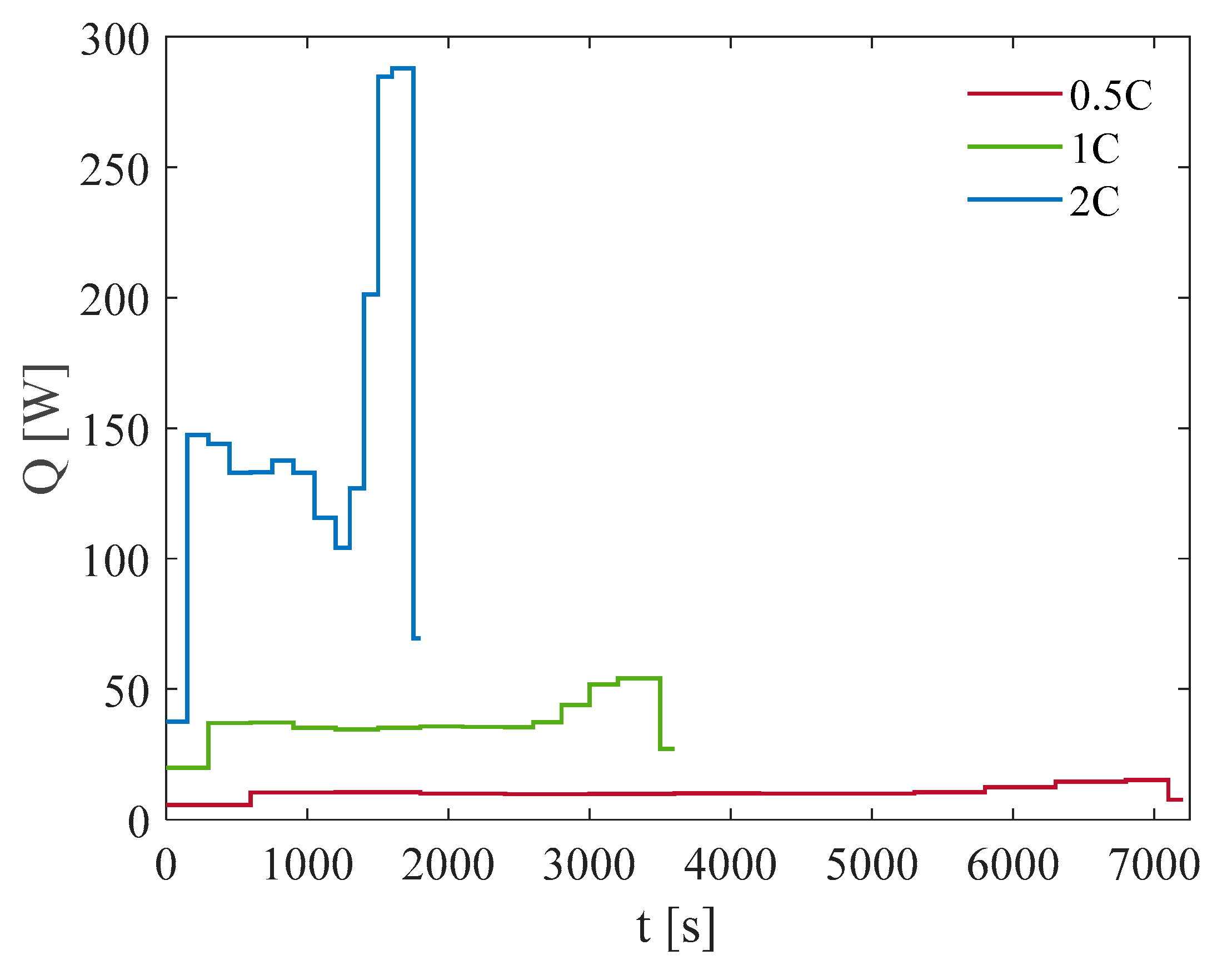
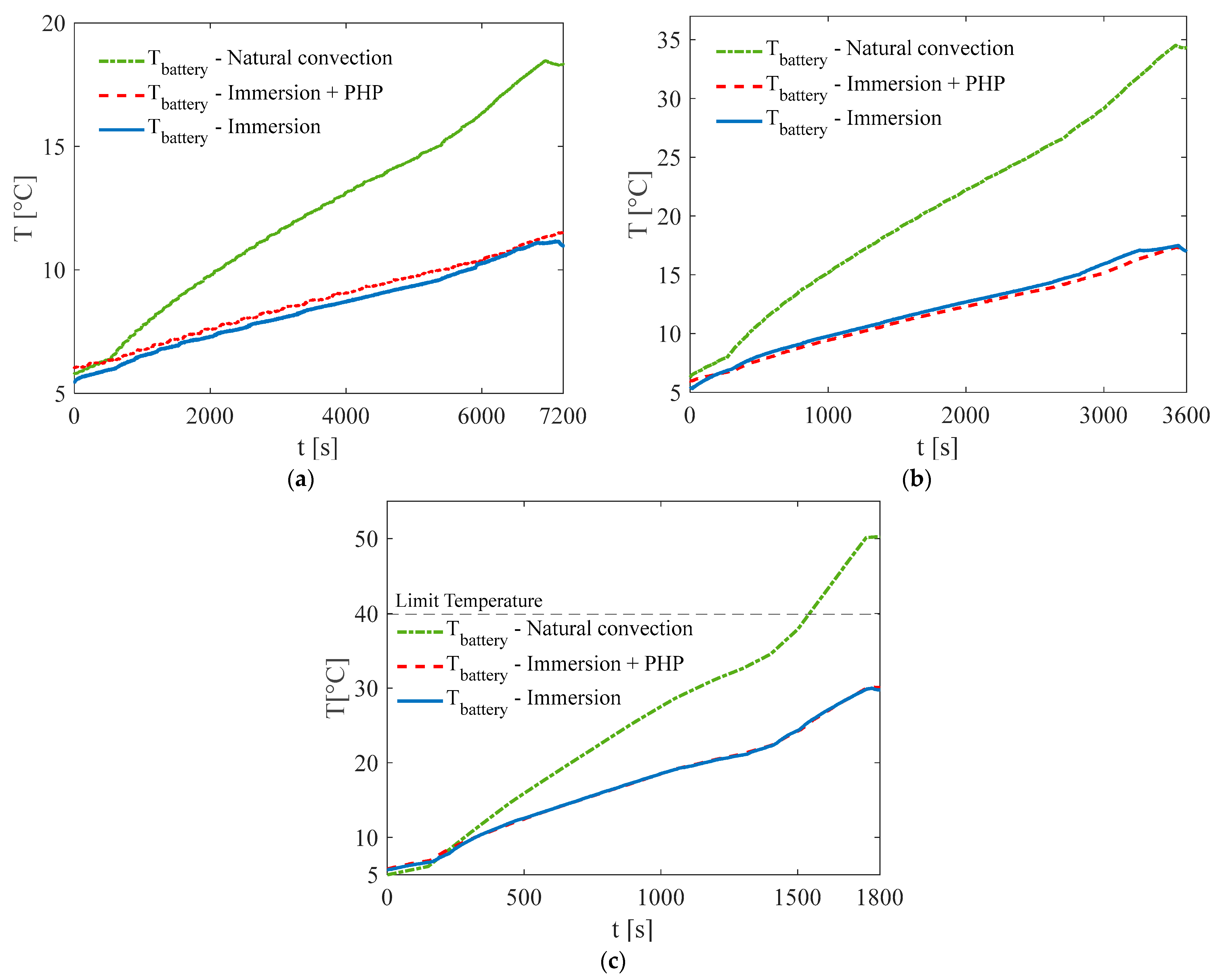
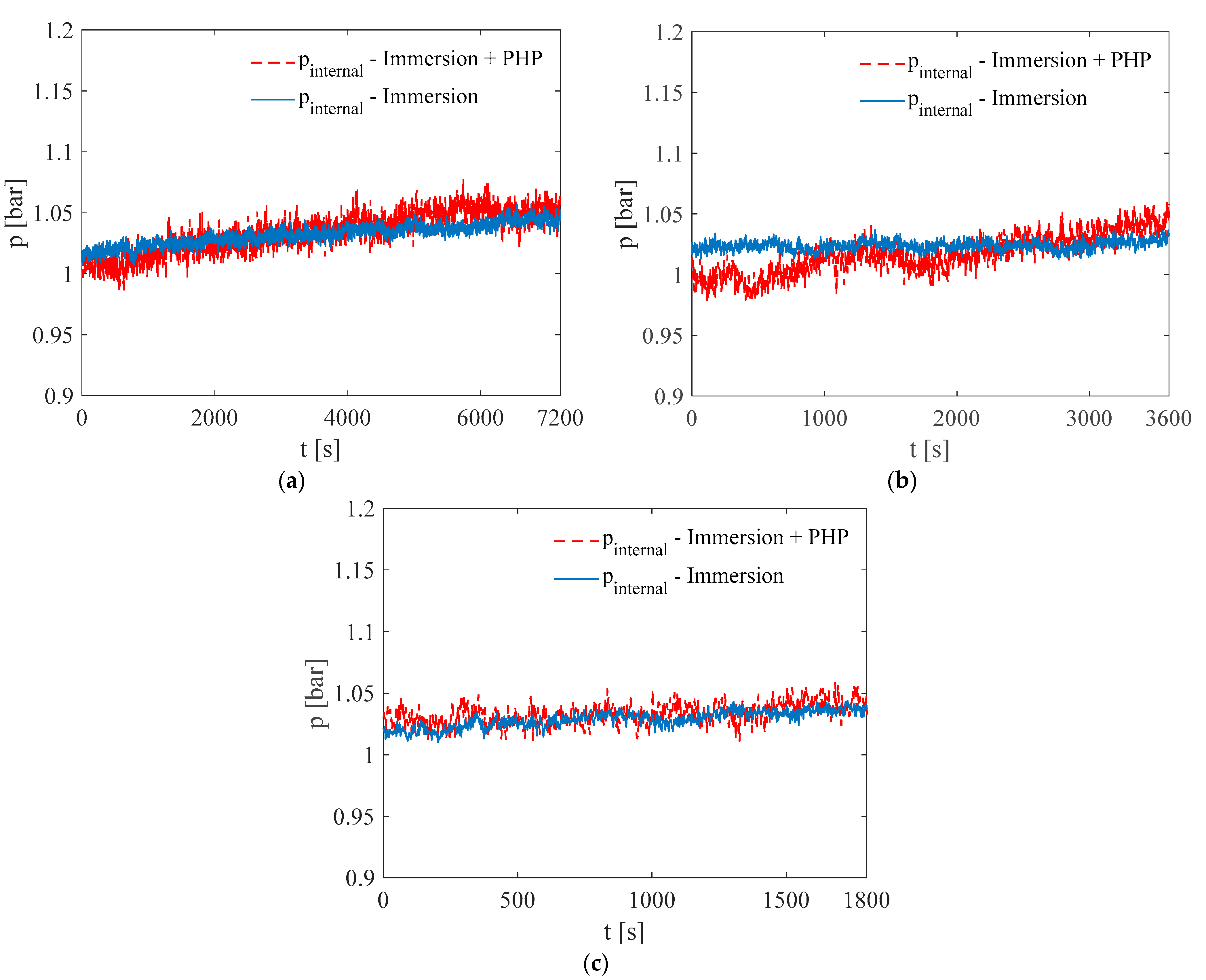
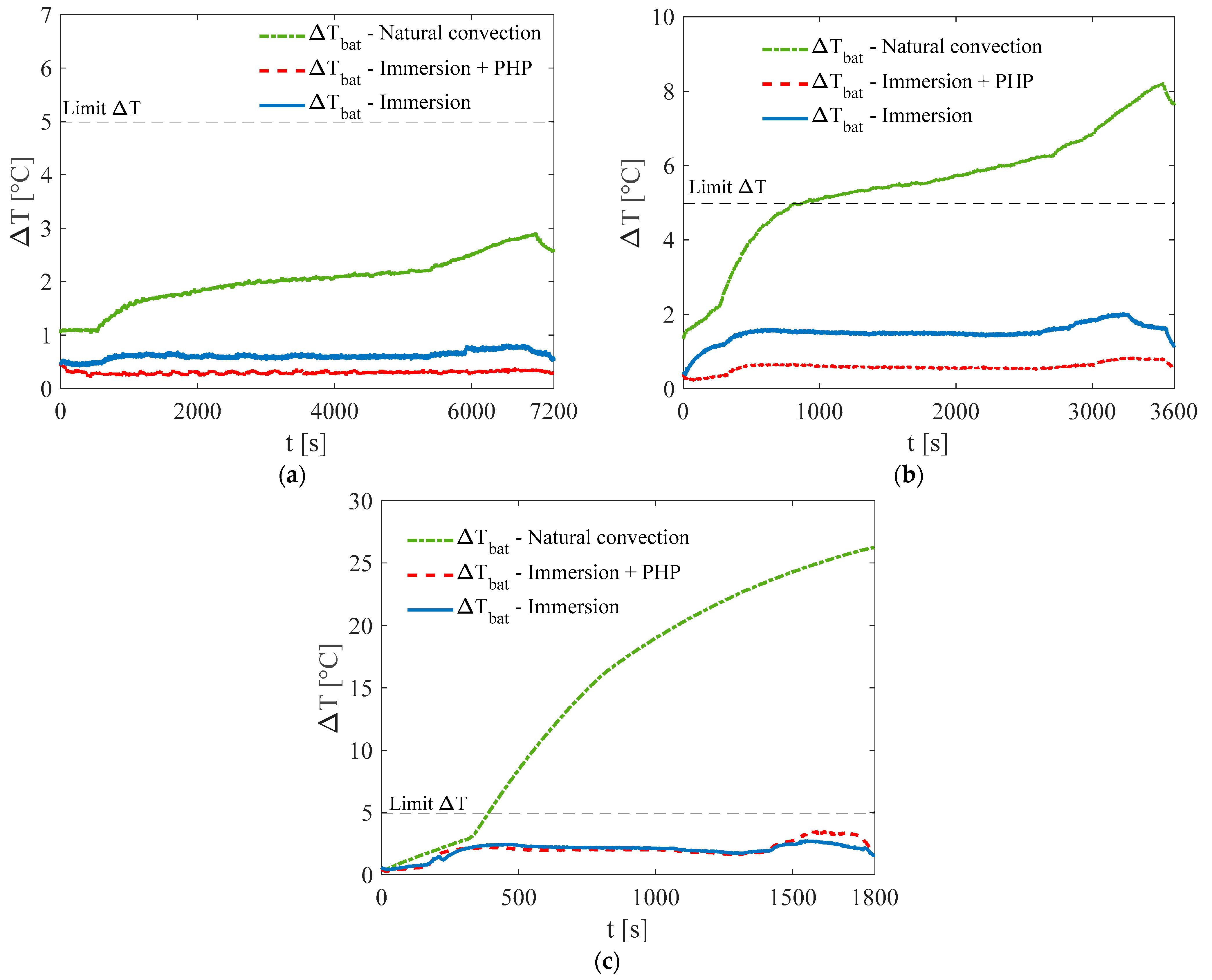
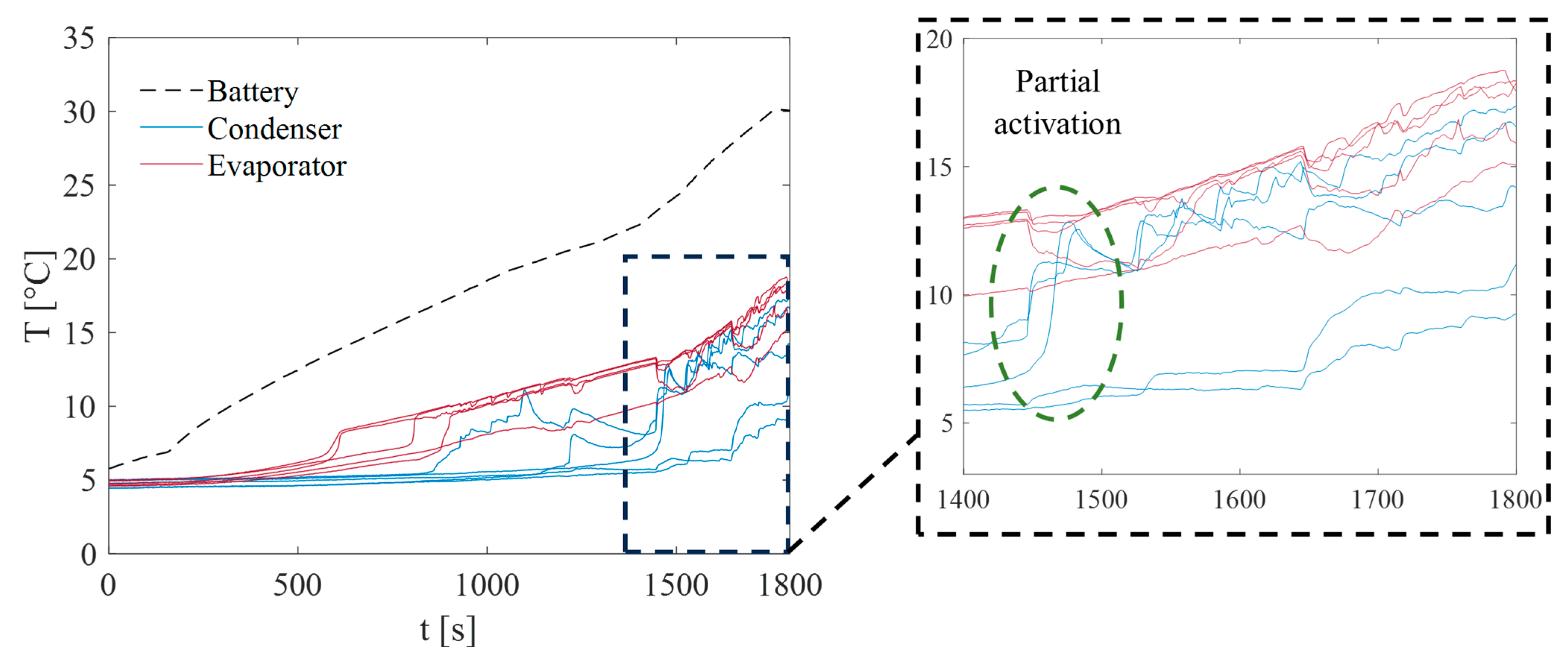
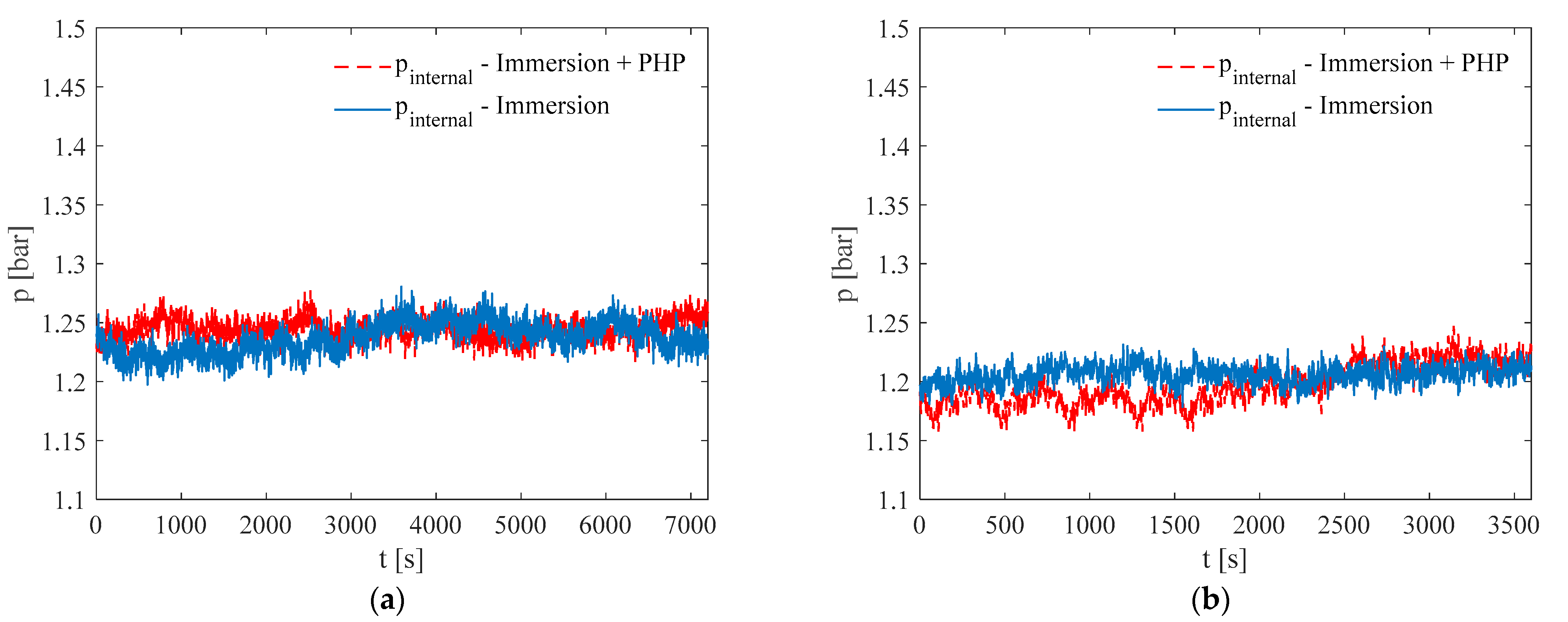
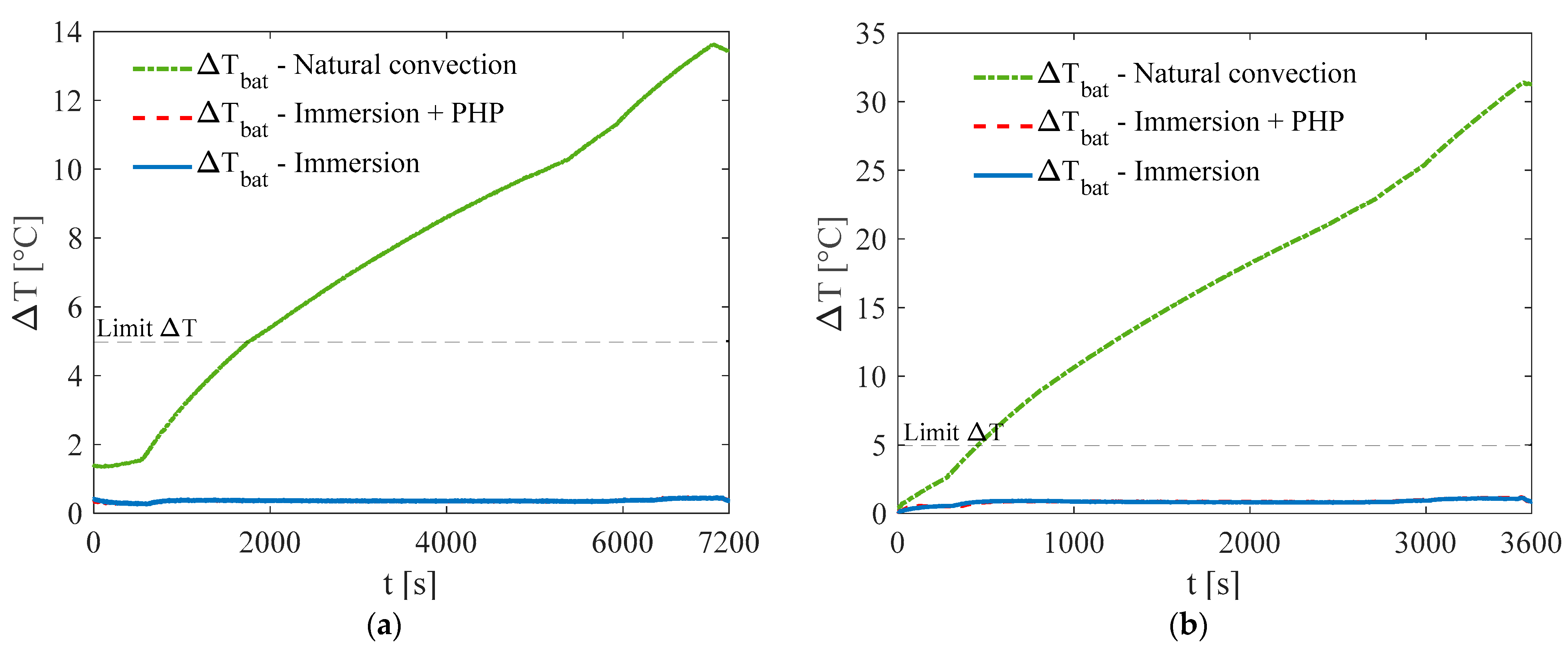
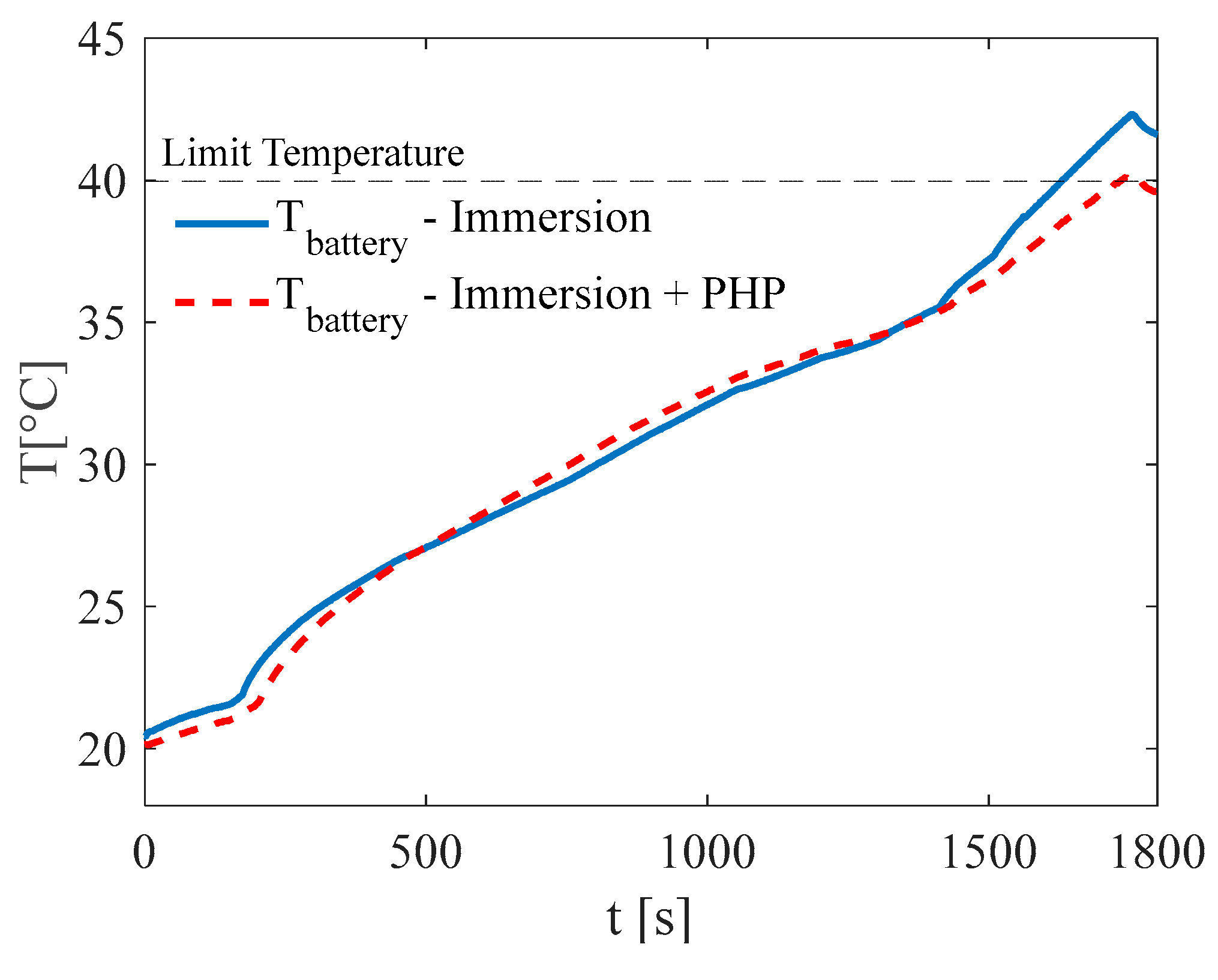
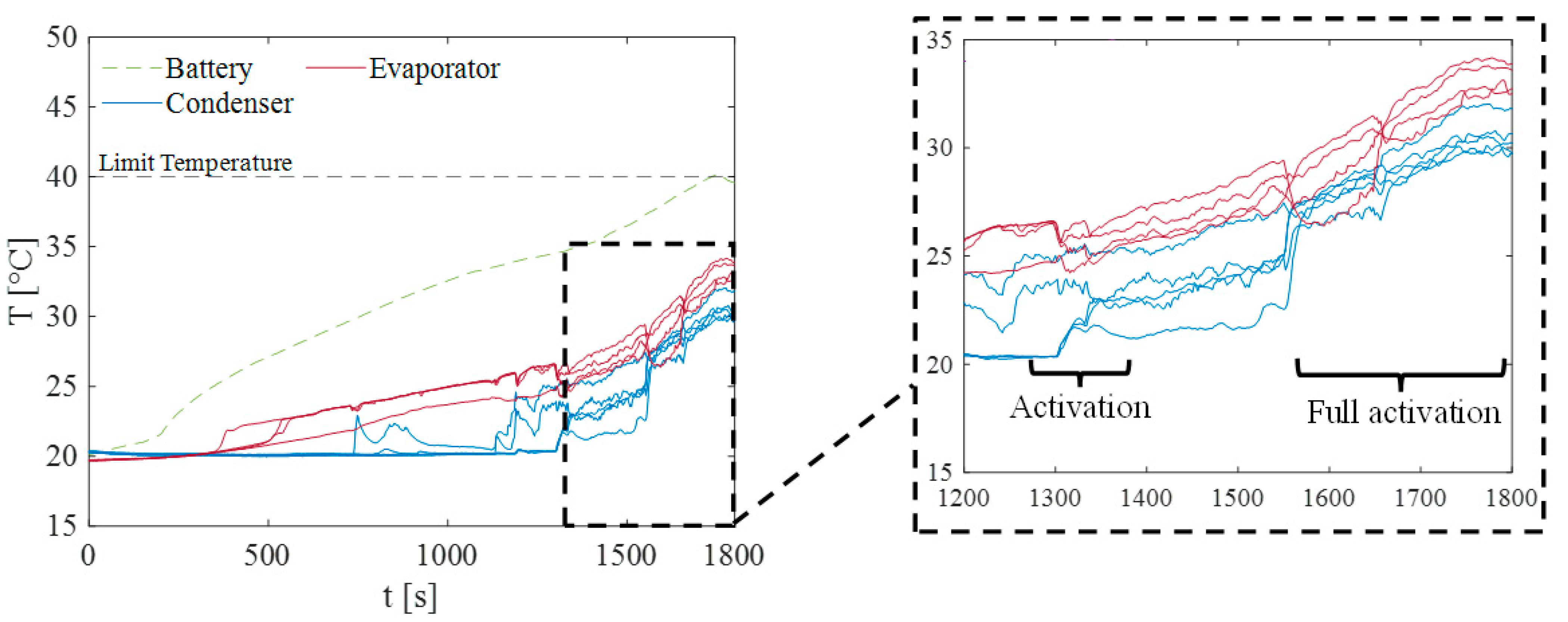
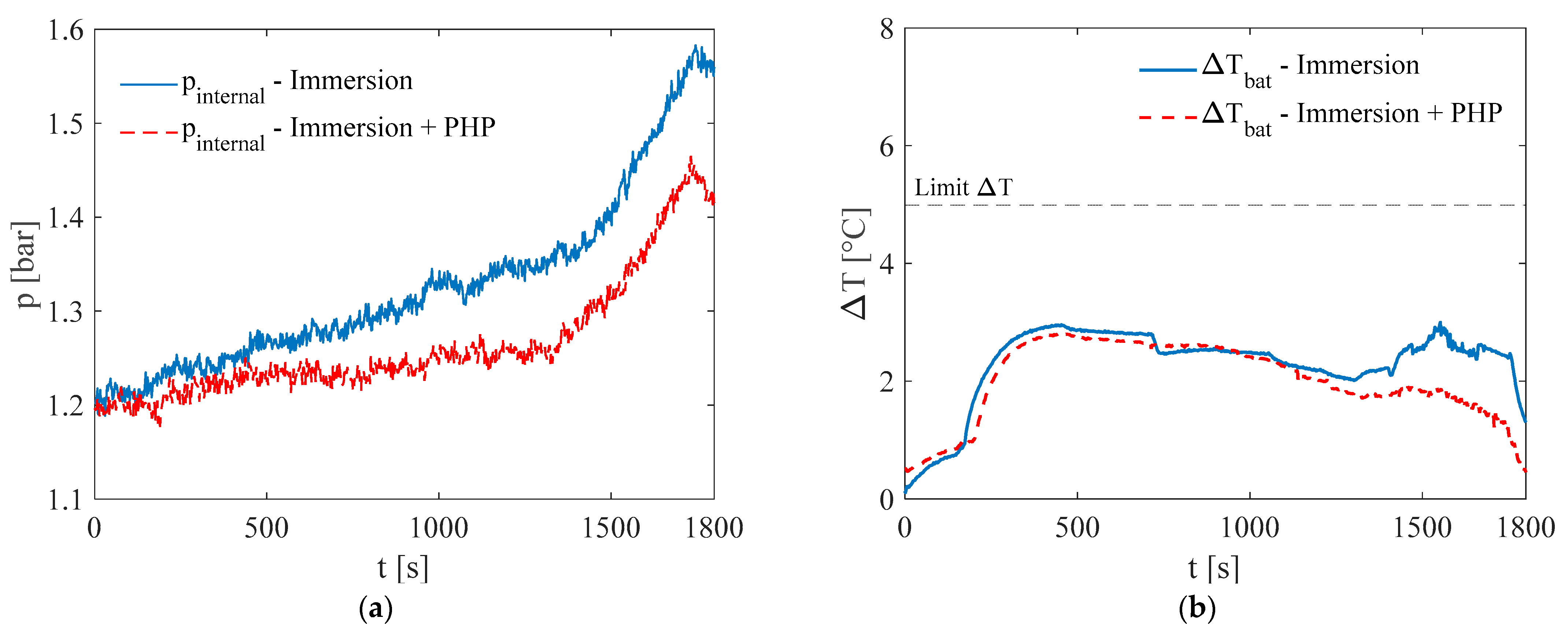
3. Results and Discussion
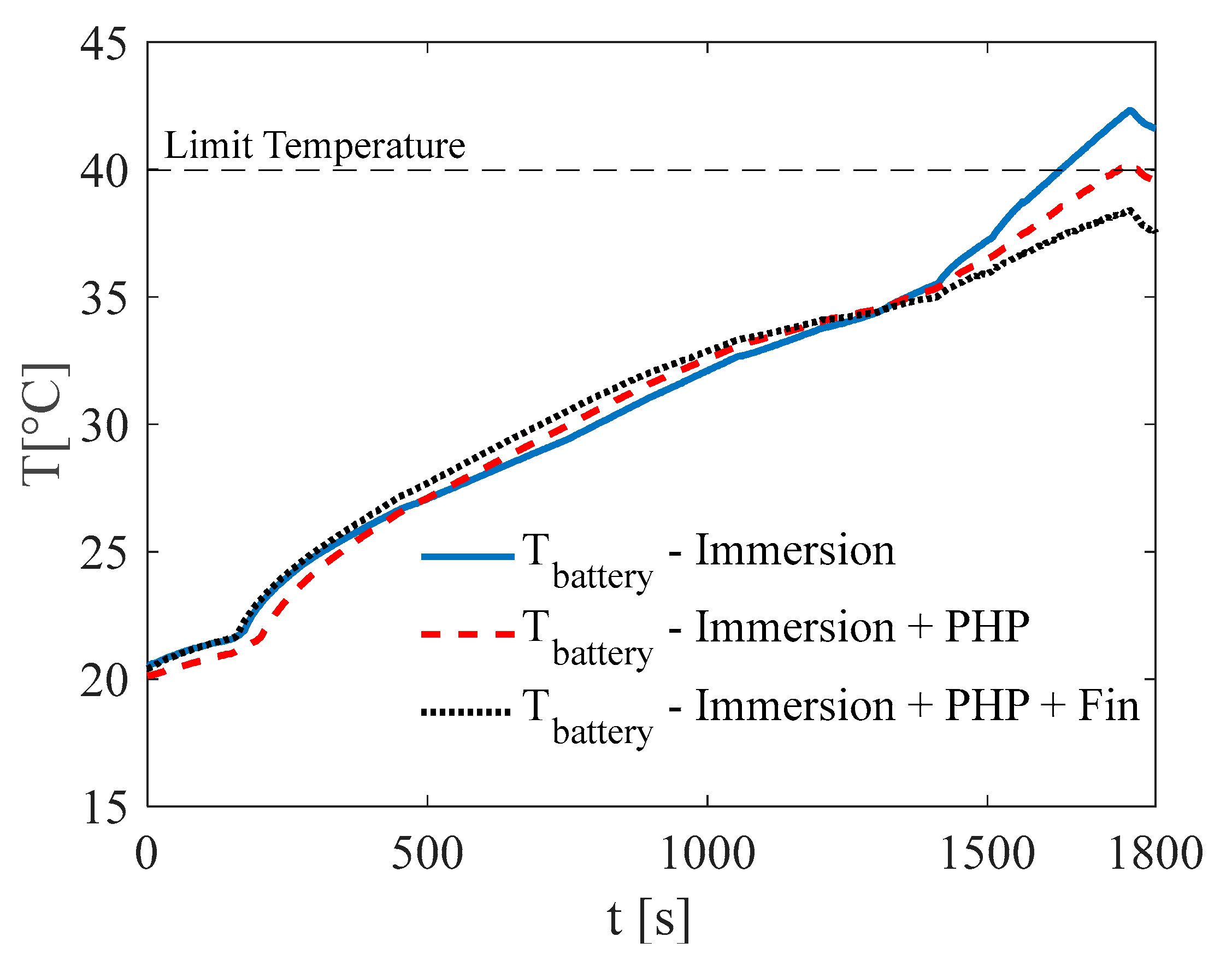
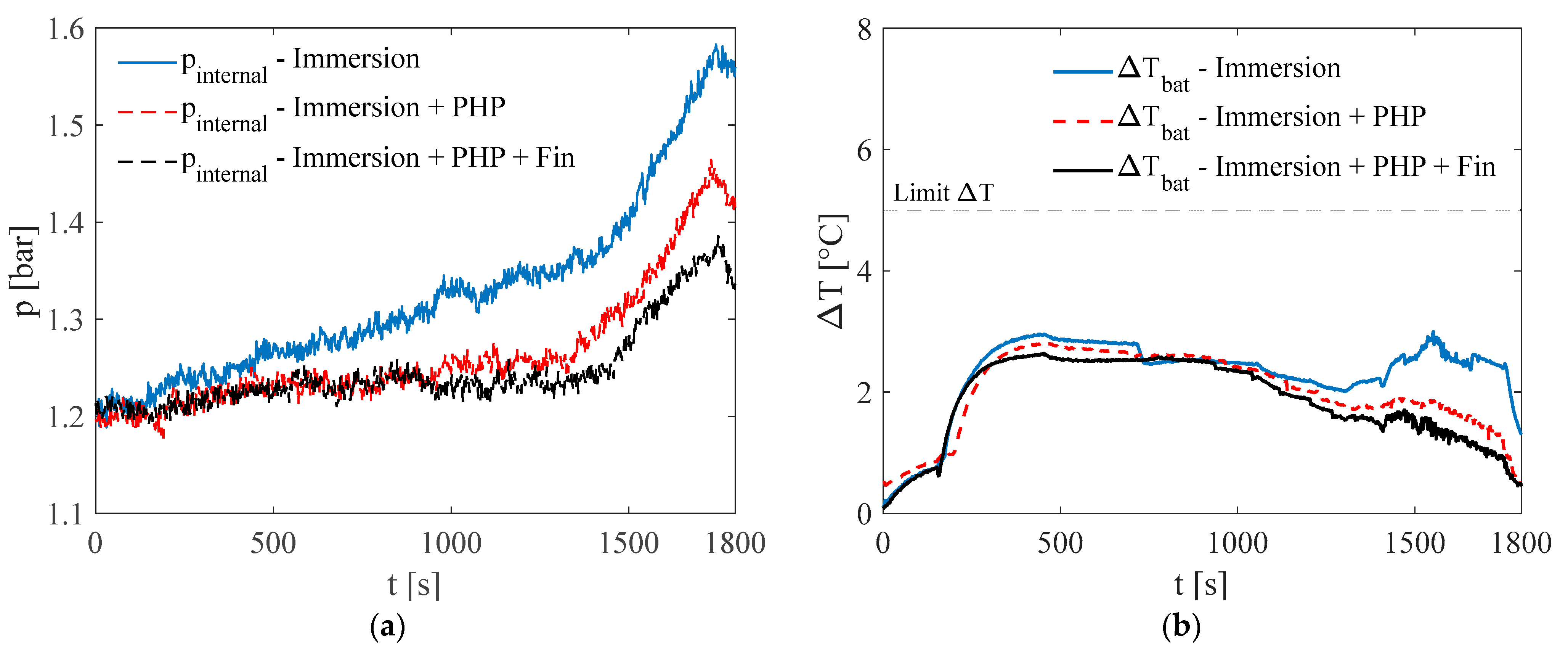
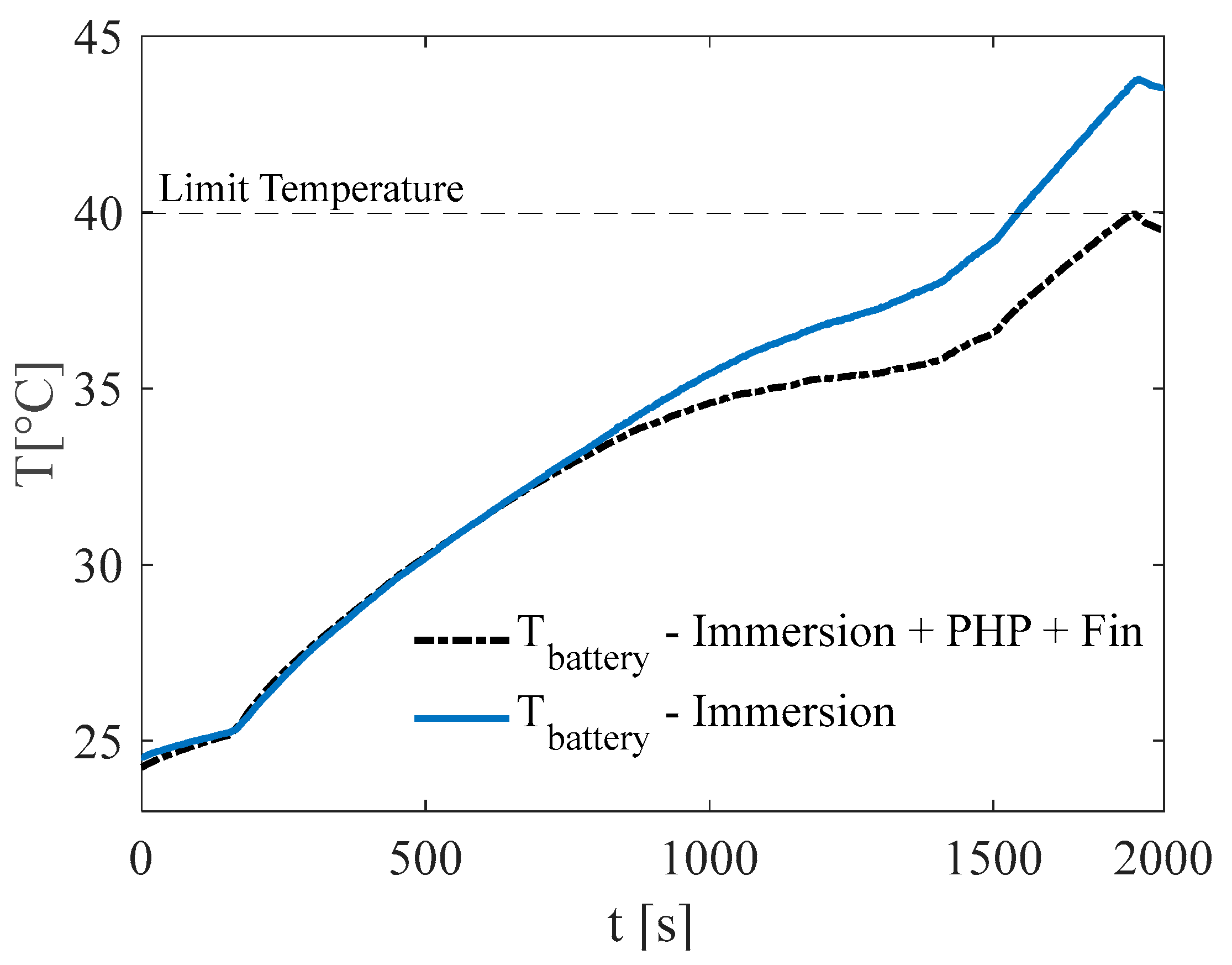
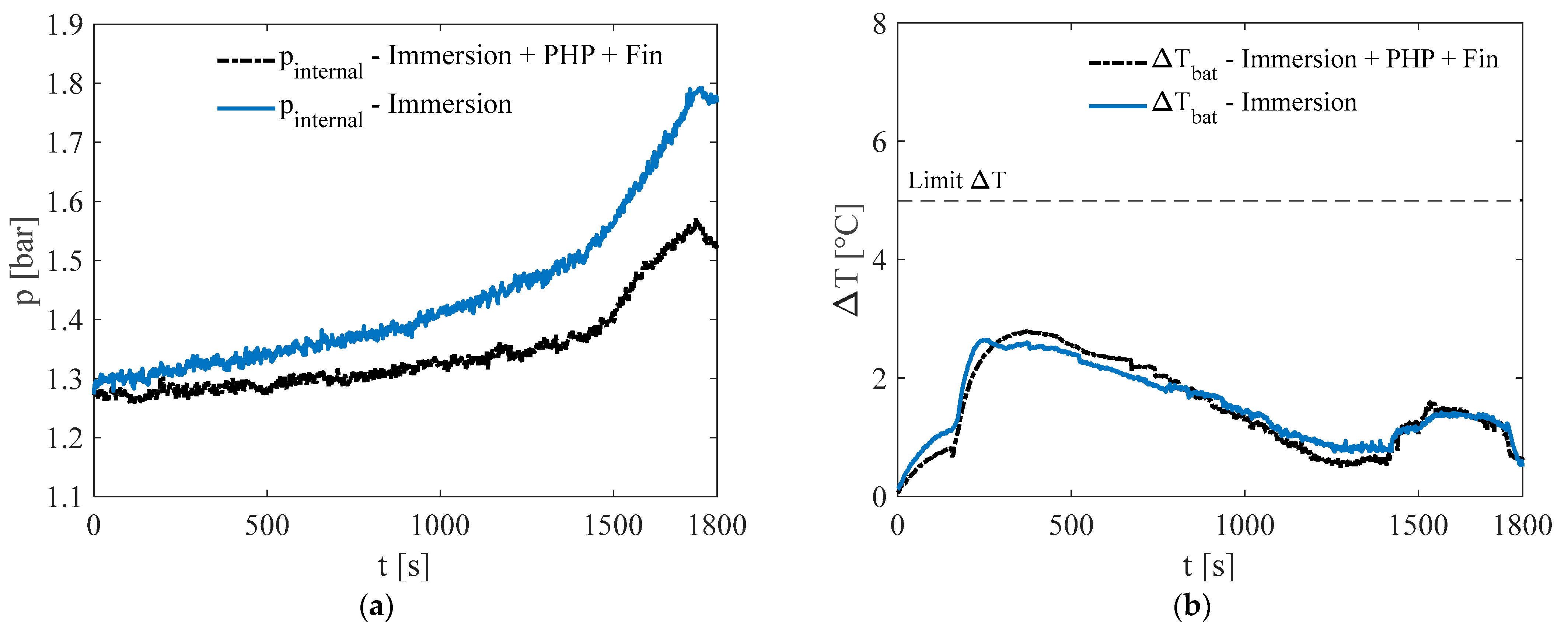
3.1. Test at Ambient Temperature of 5 °C
3.2. Test at Ambient Temperature of 20 °C
4. Conclusions
- -
- Performance optimization: The PHP design will be refined, with particular attention to increasing the heat exchange area in the evaporator and condenser sections to improve handling of higher discharge rates and operation in warmer environments.
- -
- System integration: Efforts will focus on making the system more compact and efficient by reducing the size of enclosure and optimizing fluid volume. This step is especially important for space-constrained applications such as electric vehicles.
- -
- Hybridization for extreme conditions: In the engineering phase of product development, active elements can be incorporated into the condenser section of the PHP. This hybrid approach would extend the applicability of the system to rare but extreme high-temperature scenarios. Since such conditions are expected to occur only occasionally and for limited durations, the required active components would remain simplified and significantly downsized compared to conventional cooling systems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Keiner, D.; Ram, M.; Barbosa, L.D.S.N.S.; Bogdanov, D.; Breyer, C. Cost Optimal Self-Consumption of PV Prosumers with Stationary Batteries, Heat Pumps, Thermal Energy Storage and Electric Vehicles across theWorld up to 2050. Sol. Energy 2019, 185, 406–423. [Google Scholar] [CrossRef]
- Mohammadi, F.; Saif, M. A comprehensive overview of electric vehicle batteries market. E-Prime Adv. Electr. Eng. Electron. Energy 2023, 3, 100127. [Google Scholar] [CrossRef]
- Shi, C.; Wang, T.; Liao, X.; Qie, B.; Yang, P.; Chen, M.; Wang, X.; Srinivasan, A.; Cheng, Q.; Ye, Q.; et al. Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater. 2019, 17, 136–142. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Arun, V.; Kannan, R.; Ramesh, S.; Vijayakumar, M.; Raghavendran, P.S.; Siva Ramkumar, M.; Anbarasu, P.; Sundramurthy, V.P. Review on Li-Ion Battery vs Nickel Metal Hydride Battery in EV. Adv. Mater. Sci. Eng. 2022, 2022, 7910072. [Google Scholar] [CrossRef]
- Gayathri, A.; Manimegalai, V.; Krishnakumar, P. Challenging Issues and Solutions on Battery Thermal Management for Electric Vehicles. In Electrical and Electronic Devices, Circuits, and Materials: Technological Challenges and Solutions; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 535–553. [Google Scholar]
- Lyu, Y.; Siddique, A.R.M.; Majid, S.H.; Biglarbegian, M.; Gadsden, S.A.; Mahmud, S. Electric vehicle battery thermal management system with thermoelectric cooling. Energy Rep. 2019, 5, 822–827. [Google Scholar] [CrossRef]
- Al-Zareer, M.; Dincer, I.; Rosen, M.A. A novel phase change based cooling system for prismatic lithium-ion batteries. Int. J. Refrig. 2018, 86, 203–217. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Luo, L.; Fan, Y.; Du, Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2022, 238, 121652. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, X.L.; Wei, L.C.; Cao, F.; Zhang, L.Y.; Meng, X.Z.; Jin, L.W. A comprehensive experimental study on temperature-dependent performance of lithium-ion battery. Appl. Therm. Eng. 2019, 158, 113800. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, C.; E, J.; Zhu, H.; Chen, J.; Wen, M.; Yin, H. Effects of different coolants and cooling strategies on the cooling performance of the power lithium-ion battery system: A review. Appl. Therm. Eng. 2018, 142, 10–29. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Habibi, S. Review of the Li-Ion Battery, Thermal Management, and AI-Based Battery Management System for EV Application. Energies 2023, 16, 185. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Feng, X.; Xu, C.; He, X.; Wang, L.; Zhang, G.; Ouyang, M. Mechanisms for the evolution of cell variations within a LiNixCoyMnzO2/graphite lithium-ion battery pack caused by temperature non-uniformity. J. Clean. Prod. 2018, 205, 447–462. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Wu, W.; Chen, K.; Hong, S.; Lai, Y. A critical review of battery thermal performance and liquid based battery thermal management. Energy Convers. Manag. 2019, 182, 262–281. [Google Scholar] [CrossRef]
- Akinlabi, A.A.H.; Solyali, D. Configuration, design, and optimization of air-cooled battery thermal management system for electric vehicles: A review. Renew. Sustain. Energy Rev. 2020, 125, 109815. [Google Scholar] [CrossRef]
- Wang, M.; Teng, S.; Xi, H.; Li, Y. Cooling performance optimization of air-cooled battery thermal management system. Appl. Therm. Eng. 2021, 195, 117242. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Zhang, Y.; Lv, S.; Ni, H.; Deng, Y.; Yuan, Y. A review of the power battery thermal management system with different cooling, heating and coupling system. Energies 2022, 15, 1963. [Google Scholar] [CrossRef]
- An, Z.; Jia, L.; Li, X.; Ding, Y. Experimental investigation on lithium-ion battery thermal management based on flow boiling in minichannel. Appl. Therm. Eng. 2017, 117, 534–543. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wen, X.; Cai, W.; Jiang, Y.; Wen, C.; Wang, Y.; Hu, L.; Yu, H.; Zhu, H.; et al. Experimental studies on two-phase immersion liquid cooling for Li-ion battery thermal management. J. Energy Storage 2023, 72, 108748. [Google Scholar] [CrossRef]
- Hirano, H.; Tajima, T.; Hasegawa, T.; Sekiguchi, T.; Uchino, M. Boiling liquid battery cooling for electric vehicle. In Proceedings of the 14th ITEC Asia-Pacific, Chiang Mai, Thailand, 28 November–1 December 2023. [Google Scholar]
- Roe, C.; Feng, X.; White, G.; Li, R.; Wang, H.; Rui, X.; Li, C.; Zhang, F.; Null, V.; Parkes, M.; et al. Immersion cooling for lithium-ion batteries—A review. J. Power Sources 2022, 525, 231094. [Google Scholar] [CrossRef]
- Saleem, A.; Iqbal, R.; Hussain, A.; Javed, M.S.; Ashfaq, M.Z.; Imran, M.; Hussain, M.M.; Akbar, A.R.; Shen, J.; Majeed, M.K. Recent advances and perspectives in carbon-based fillers reinforced Si3N4 composite for high power electronic devices. Ceram. Int. 2022, 48, 13401–13419. [Google Scholar] [CrossRef]
- Jalees, S.; Hussain, A.; Iqbal, R.; Raza, W.; Ahmad, A.; Saleem, A.; Majeed, M.K.; Faheem, M.; Ahmad, N.; Rehman, L.N.U.; et al. Functional PBI membrane based on polyimide covalent organic framework for durable lithium metal battery. J. Energy Storage 2024, 101, 113985. [Google Scholar] [CrossRef]
- Luo, J.; Zou, D.; Wang, Y.; Wang, S.; Huang, L. Battery thermal management systems (BTMs) based on phase change material (PCM): A comprehensive review. Chem. Eng. J. 2022, 430, 132741. [Google Scholar] [CrossRef]
- Idi, E.; Moussa, M.; Karkri, M.; Tankari, M.A. A passive thermal management system of Li-ion batteries using PCM composites: Experimental and numerical investigations. Int. J. Heat Mass Transf. 2021, 169, 120894. [Google Scholar] [CrossRef]
- Wang, J.; Gan, Y.; Liang, J.; Tan, M.; Li, Y. Sensitivity analysis of factors influencing a heat pipe-based thermal management system for a battery module with cylindrical cells. Appl. Therm. Eng. 2019, 151, 475–485. [Google Scholar] [CrossRef]
- Cattani, L.; Malavasi, M.; Bozzoli, F.; D’Alessandro, V.; Giammichele, L. Experimental analysis of an innovative electrical battery thermal management system. Energies 2023, 16, 5071. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Maghrabie, H.M.; Abo-Khalil, A.G.; Adhari, O.H.K.; Sayed, E.T.; Radwan, A.M.; Rezk, H.; Jouhara, H.; Olabi, A.G. Thermal management systems based on heat pipes for batteries in EVs/HEVs. J. Energy Storage 2022, 51, 104384. [Google Scholar] [CrossRef]
- Patel, J.R.; Rathod, M.K. Recent developments in the passive and hybrid thermal management techniques of lithium-ion batteries. J. Power Sources 2020, 480, 228820. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Lin, X. Recent developments of thermal management strategies for lithium-ion batteries: A state-of-the-art review. Energy Technol. 2022, 10, 2101135. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.-J.; Han, W.-S.; Rhi, S.-H. Anti-Gravity 3D Pulsating Heat Pipe for Cooling Electric Vehicle Batteries. Energies 2024, 17, 2283. [Google Scholar] [CrossRef]
- Khandekar, S.; Groll, M. On the definition of pulsating heat pipes: An overview. In Proceedings of the 5th International Conference (Heat Pipes, Heat Pumps and Refrigerators), Minsk, Belarus, 8–11 September 2003. [Google Scholar]
- Li, J.; Qiao, L.; Lv, W.; Zeng, X.; Chen, M. Performance response analysis of battery module with nanofluids pulsating heat pipes under normal and high-temperature charging scenarios. Appl. Therm. Eng. 2024, 257, 124339. [Google Scholar] [CrossRef]
- Cattani, L.; Sacchelli, F.; Bozzoli, F. Enhanced Passive Thermal Management for Electric Vehicle Batteries Using a 3D Pulsating Heat Pipe. Energies 2025, 18, 2306. [Google Scholar] [CrossRef]
- Mohseni, P.; Husev, O.; Vinnikov, D.; Strzelecki, R.; Romero-Cadaval, E.; Tokarski, I. Battery Technologies in Electric Vehicles: Improvements in Electric Battery Packs. IEEE Ind. Electron. Mag. 2023, 17, 55–65. [Google Scholar] [CrossRef]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A General Energy Balance for Battery Systems. Electrochem. Soc. 1985, 132, 5–12. [Google Scholar]
- Jindal, P.; Katiyar, R.; Bhattacharya, J. Evaluation of accuracy for Bernardi equation in estimating heat generation rate for continuous and pulse-discharge protocols in LFP and NMC based Li-ion batteries. Appl. Therm. Eng. 2022, 201, 117794. [Google Scholar] [CrossRef]


| Natural Convection | Immersion Cooling | Immersion Cooling + PHP | Immersion Cooling + PHP + Ext. Surface | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tamb | C-Rates | Tmax < 40 °C | ΔT < 5 °C | Tmax < 40 °C | ΔT < 5 °C | Tmax < 40 °C | ΔT < 5 °C | Tmax < 40 °C | ΔT < 5 °C |
| 5 °C | 0.5C | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | - | - |
| 1C | ✓ | X | ✓ | ✓ | ✓ | ✓ | - | - | |
| 2C | X | X | ✓ | ✓ | ✓ | ✓ | - | - | |
| 20 °C | 0.5C | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | - | - |
| 1C | X | X | ✓ | ✓ | ✓ | ✓ | - | - | |
| 2C | - | - | X | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 25 °C | 0.5C | - | - | - | - | - | - | - | - |
| 1C | - | - | - | - | - | - | - | - | |
| 2C | - | - | X | ✓ | - | - | ✓ | ✓ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacchelli, F.; Cattani, L.; Bozzoli, F. Thermal Performance Study of a Novel Double-Phase Cooling Strategy in Electric Vehicle Battery Systems. Energies 2025, 18, 4937. https://doi.org/10.3390/en18184937
Sacchelli F, Cattani L, Bozzoli F. Thermal Performance Study of a Novel Double-Phase Cooling Strategy in Electric Vehicle Battery Systems. Energies. 2025; 18(18):4937. https://doi.org/10.3390/en18184937
Chicago/Turabian StyleSacchelli, Federico, Luca Cattani, and Fabio Bozzoli. 2025. "Thermal Performance Study of a Novel Double-Phase Cooling Strategy in Electric Vehicle Battery Systems" Energies 18, no. 18: 4937. https://doi.org/10.3390/en18184937
APA StyleSacchelli, F., Cattani, L., & Bozzoli, F. (2025). Thermal Performance Study of a Novel Double-Phase Cooling Strategy in Electric Vehicle Battery Systems. Energies, 18(18), 4937. https://doi.org/10.3390/en18184937








