Abstract
Ammonia, as a promising carbon-neutral fuel, has attracted growing attention for blended combustion applications from academia to industry. Low-NOx-combustion strategies such as staged combustion, oxygen-enriched combustion, and exhaust gas recirculation may lead to ammonia combustion in CO2-rich and NO-rich environments. In this work, the laminar burning velocities (SL) in NH3/CH4/O2/NO/CO2 flames with various ammonia blended ratios under atmospheric pressure were investigated using the heat flux method. The addition of NO to the oxidizer significantly enhances SL, with the enhancement factor ξ proportional to the NO fraction in the oxidizer and strongly dependent on the fuel composition. Chemical effects rather than thermal-diffusion effects dominate the enhancement of SL. Kinetic analysis shows that NO actively participates in the reaction network during the early flame stage, promoting the formation of key radicals such as H and OH through pathways like NH2 + NO = NNH + OH and NNH = N2 + H, thereby accelerating chain-branching and sustaining flame propagation.
1. Introduction
In recent years, ammonia (NH3) has attracted growing attention as a promising carbon-neutral fuel for blended combustion applications. However, one of the major challenges associated with its utilization is the formation of nitrogen oxides (NOx) during combustion, which poses a significant barrier to its practical deployment. To address this issue, various combustion strategies aimed at reducing NOx emissions have been extensively investigated, including staged combustion [1], oxygen-enriched combustion [2,3], and exhaust gas recirculation (EGR) [4,5]. These techniques often lead to ammonia combustion occurring in flue gas environment rich in CO2 and NOx, resulting in flame propagation behaviors that differ significantly from those in conventional fuel-air combustion systems. To better exploit these low-NOx ammonia combustion technologies and develop reliable chemical kinetic models, it is essential to understand the flame propagation characteristics of ammonia under CO2-diluted conditions and to evaluate the influence of NO addition on the combustion process.
The effect of CO2 dilution on hydrocarbon flame propagation characteristics has been extensively studied in the context of carbon capture and storage (CCS) research [6,7,8,9]. Studies under both atmospheric and high-pressure conditions have shown that CO2 dilution and N2 dilution both reduce combustion rates. However, CO2 exhibits a more pronounced inhibitory effect on laminar burning velocity (SL) due to its higher specific heat capacity and its participation in chemical reactions such as the reverse of CO + OH = CO2 + H, which competes with the chain-branching reaction H + O2 = O + OH for H radicals. In contrast, investigations into the impact of CO2 dilution on ammonia flame propagation characteristics remain relatively limited. Shi et al. [10] conducted experimental and kinetic modeling studies on the laminar burning velocities of pure ammonia and NH3/DME mixtures in O2/CO2 atmospheres. Zhu et al. [11] further investigated the synergistic effects of CO2 dilution and H2 addition on the laminar combustion characteristics of NH3/CH4 mixtures under high-temperature and high-pressure conditions. Their results revealed that CO2-involved reduction reactions, such as NH + CO2 = HNO + CO and the reverse of CO + OH = CO2 + H, significantly suppress the SL by interfering with key chain-branching pathways involving H and NH radicals.
The effect of NO addition in the oxidizer on the laminar burning velocity of ammonia fuels has been less extensively studied compared to other aspects of ammonia combustion. Mei et al. [12] first investigated the propagation characteristics of NH3/NO/N2 laminar flames at ambient temperature and pressure. Subsequently, Monnier et al. [13] and Liu et al. [14] extended these measurements to elevated temperatures and pressures, enabling a more realistic assessment of the kinetic interactions between NH3 and NO under combustion-relevant conditions. Their results indicate that NH3 primarily undergoes oxidation through the NH3-NH2-NNH-N2 pathway in NO/N2 atmospheres, with the reaction NH2 + NO = NNH + OH playing a crucial role. However, these studies all treated NO as the sole oxidant, which significantly deviates from practical application scenarios, and no work has previously been reported on laminar burning velocities under conditions involving both of CO2 dilution and NO addition.
Building upon the aforementioned background, the laminar flame propagation of NH3/CH4 in an O2/CO2 atmosphere without and with NO addition was investigated in this work using the heat flux method. The specific objectives of the present study are as follows: (1) to extend the laminar burning velocity dataset of NH3/CH4 flames under O2/CO2 conditions, thereby providing essential data for the validation of chemical kinetic models; (2) to quantify the thermal-diffusive, dilution, and chemical effects induced by CO2 dilution and NO addition; and (3) to investigate the influence mechanism of CO2 dilution and NO addition on NH3/CH4 flame propagation through sensitivity analysis.
2. Methodology
2.1. Heat Flux Method and Test Conditions
The heat flux method, originally proposed by Bosschaart and de Goey [15], has been widely adopted for measuring laminar burning velocities under a wide range of temperature and pressure conditions. Figure 1 presents the schematic diagram of the heat flux measurement setup. A porous plate with a diameter of 30 mm is installed at the center of the top burner disk, featuring hole diameters and center-to-center spacing of 0.3 mm and 0.4 mm, respectively. This optimized configuration effectively reduces local flame stretch and suppresses cellular flame instability, thereby promoting the formation of a smooth and spatially uniform flame front. In the heat flux method, the heat loss from the flame to the porous plate is counterbalanced by the convective heat gain of the incoming unburnt premixed gas flowing through the plate. When thermal equilibrium is achieved, indicated by a zero radial temperature gradient across the plate, an adiabatic, one-dimensional, stretch-free flat flame is established, and the laminar burning velocity can be determined from the incoming unburnt gas flow rate. Given that the flames stabilized on the heat flux burner propagate downwardly, the denser, unburned mixture is located below the lighter, burned flue gas. This stratification is inherently gravitationally stable, meaning that buoyancy forces act to suppress hydrodynamic instabilities rather than promote them. Consequently, the influence of buoyancy on the measured burning velocities is negligible.
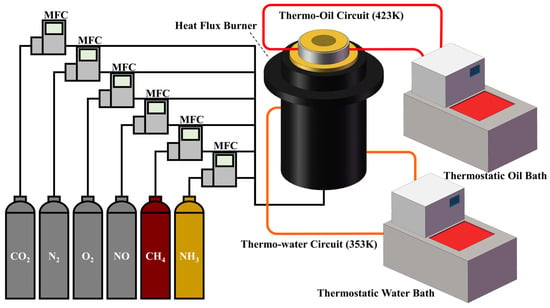
Figure 1.
Schematic diagram of the experimental setup of heat flux system.
Detailed operational conditions of the present laminar burning velocity measurements are summarized in Table 1. To prevent the formation of ammonium salts via the reactions NH3 + CO2 + H2O = NH4HCO3 and 2NH3 + CO2 + H2O = (NH4)2CO3 from affecting the composition of the gas mixture, all measurements were conducted at atmospheric pressure and an unburned mixture temperature (Tu) of 353 K, under which NH4HCO3 is nearly completely decomposed. No powder deposits were observed in the gas delivery lines before or after the experiments, confirming the absence of solid-phase formation. Furthermore, to mitigate the flame edge wrinkling and tilting commonly observed in ammonia-containing flames, which may affect the stability of the planar flame front, the burner plate was heated to 443 K using dimethicone as the heating medium. The gas flow rates were precisely controlled by mass flow controllers (Alicat, Tucson, AZ, USA). High-purity gases (NH3, CH4, CO2, NO, O2, and N2: 99.999%) were used in this study.

Table 1.
Experimental conditions of NH3/CH4 at Tu = 353 K.
2.2. Assessment of the Measurement Uncertainties
The uncertainty of SL () in this study was evaluated following the methodology established in previous investigations [16]. Two primary contributors to the overall uncertainty were considered: (1) , originating from burner plate temperature measurements using thermocouples, and (2) , arising from gas flow controller. The total measurement uncertainty is, therefore, expressed as follows:
where is the measurement sensitivity defined as , with being the parabolic coefficient that was interpolated to infer SL; is the uncertainty of the th mass flow controller; is the total flow rate of all gases; is the mean standard deviation of the thermocouple temperature, which has a specific constant value for a given porous plate, and can be calculated as the following expression:
2.3. Kinetic Simulation
Numerical simulations were conducted using the PREMIX module of Chemkin-Pro, which is specifically designed to model the propagation characteristics of one-dimensional, steady-state, adiabatic premixed flames. The chemical kinetic mechanisms employed in the simulations are the CEU-NH3-Mech 1.1 mechanism [17] (CEU-2022), and the mechanisms from Okafor et al. [18] (Okafor-2019), Shrestha et al. [19] (Shrestha-2021), Zhang et al. [20] (Zhang-2023), and Konnov et al. [21,22] (Konnov-2023). These parenthetical serve as abbreviated names for the mechanisms and will be used consistently throughout the subsequent analysis. Among them, the CEU-2022 mechanism developed by our group is an updated version of the original CEU-NH3-Mech mechanism, which has been widely used in studies [23,24] on laminar flame propagation of ammonia-hydrocarbon blended fuels and has demonstrated accurate predictive capability for burning velocities. And the updated mechanism was validated according to the comparison with experimental data [17] obtained under elevated pressure conditions. The Okaafor-2019, owing to its highly reduced reaction network and high computational efficiency, has been widely adopted in CFD simulations of ammonia-fueled combustion. In addition, mechanisms such as the Shrestha-2021, the Zhang-2023, and the Konnov-2023, have been developed through iterative refinement based on extensive validation against experimental laminar burning velocity data for NH3, NH3/H2, and NH3/C1 fuel systems. These mechanisms exhibit robust predictive performance across a range of operating conditions and are now recognized as representative chemical kinetic models in the field of ammonia combustion research.
For each case studied, a minimum of 800 grid points was utilized to ensure sufficient spatial resolution, and the adaptive grid refinement strategy was applied with the values of GRAD and CURV set to 0.01 to ensure grid independence. The thermal diffusion (Soret effect) and multicomponent transportation were considered in all simulation cases. Additionally, the effect of radiative heat loss was also considered as suggested by Nakamura and Shindo [25].
3. Results and Discussion
3.1. Effects of CO2 Dilution on SL of NH3/CH4 Flames
Figure 2 presents the measured and predicted laminar burning velocities of NH3/CH4 mixtures as a function of equivalence ratio for various ammonia blended ratios under different oxidizer compositions. It should be pointed out that the laminar burning velocities obtained in this work agree well with those under similar CO2-diluted conditions in the literature [26,27]. A significant reduction in laminar burning velocity (SL) is observed when the diluent in the oxidizer is changed from N2 to CO2, and the SL only exceeds that under air atmosphere when the oxygen concentration in the O2 + CO2 mixture is increased to 40%. It is primarily attributed to the higher specific heat capacity of CO2 and its role as a combustion product of CH4 oxidation, both of which effectively reduce the flame temperature and inhibit the forward progress of the methane oxidation reaction. Consistent with the trends observed in CH4 flames, the equivalence ratio corresponding to the peak SL of NH3/CH4 flames shifts significantly toward the fuel-lean side when the oxidizer is changed from O2/N2 to CO2-diluted conditions. Specifically, the peak SL occurs at an equivalence ratio of 1.05–1.10 under air atmosphere, whereas it shifts to 0.95–1.05 under CO2 dilution. Moreover, ammonia blending further accentuates this shift in the peak burning velocity toward the fuel-lean side.
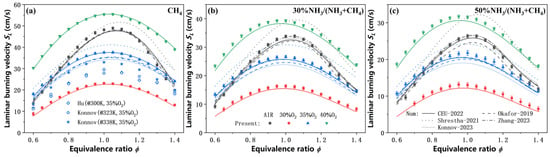
Figure 2.
Measured and predicted laminar burning velocities of NH3/CH4 flames as a function of the equivalence ratio. (a) Pure CH4 flames; (b) 30% NH3/70% CH4 mixture; (c) 50% NH3/50% CH4 mixture. (AIR: 21% O2/(O2 + N2), 30% O2: 30% O2/(O2 + CO2), 35% O2: 35% O2/(O2 + CO2), 40% O2: 40% O2/(O2 + CO2)).
The Shrestha-2021 significantly overpredicts the laminar burning velocities across all tested conditions, while the Okafor-2019 tends to underpredict them. Although the Konnov-2023 accurately captures the burning velocities of NH3/CH4 flames under air-fired conditions, it exhibits a stronger underprediction of burning velocities under CO2-diluted atmospheres, yielding simulation results even lower than those obtained with the Okafor-2019. In contrast, the simulations using the CEU-2022 and Zhang-2023 mechanisms produce nearly identical predictions and show excellent agreement with the experimental data in NH3/CH4 flames: only the SL near stoichiometric equivalence ratio is slightly underpredicted, but all other simulated values fall within the experimental uncertainty bounds.
Nonetheless, the overall agreement confirms that the heat flux method employed in the present study enables accurate measurement of laminar burning velocities for ammonia-containing fuels with an unburned gas temperature of 353 K under CO2 dilution. The CEU-2022 and Zhang-2023 provide quantitatively reliable predictions of SL of NH3/CH4 flames under both air and CO2-diluted conditions, with minor deviations observed only under specific blending and dilution combinations. However, the CEU-2022 consists of only 91 species and 444 elementary reactions, whereas the Zhang-2023 includes 152 species and 1388 reactions. Given its significantly higher computational efficiency and improved convergence behavior, subsequent numerical investigations in this work are based on the CEU-2022 mechanism.
3.2. Effects of NO Addition on SL of NH3/CH4/O2/NO/CO2 Flames
Given that ammonia combustion inherently promotes the formation of fuel-NOx, the exhaust gas from ammonia-containing fuels typically contains a non-negligible concentration of NO, in addition to CO2. This residual NO can be recirculated in systems employing exhaust gas recirculation (EGR) or oxy-fuel combustion with CO2 recycle, thereby reintroducing NO into the fresh mixture. The presence of NO in the reactants can significantly influence flame chemistry through redox interactions with NH3, which play a crucial role in the combustion characteristics of ammonia-containing fuels and may alter the reaction pathways, flame stability, and overall burning velocity.
As shown in Figure 3, the addition of NO in the oxidizer leads to a significant enhancement in SL across all equivalence ratios and oxygen concentrations tested. For example, at ϕ = 1.0 and 35% O2, the introduction of 2% NO increases SL by up to 15.4%/18.0% to the NO-free case for 30% and 50% NH3 blended, respectively. Despite this enhancement, the overall trend of SL versus equivalence ratio remains unchanged, and the peak burning velocity continues to occur within the ϕ = 0.95–1.0 range, consistent with the lean-shifted behavior induced by CO2 dilution. The CEU-2022 mechanism captures the general trend of SL increase with NO addition and reproduces the location of the peak velocity. However, it systematically underpredicts the magnitude of SL, particularly in the equivalence ratio range of 0.95–1.0, where the burning velocity reaches its maximum, and the deviation exceeds the experimental uncertainty bounds. While on the fuel-lean and fuel-rich sides of the equivalence ratio (ϕ < 0.85, ϕ > 1.15), the deviations between simulation and experiment generally fall within the measurement uncertainty.
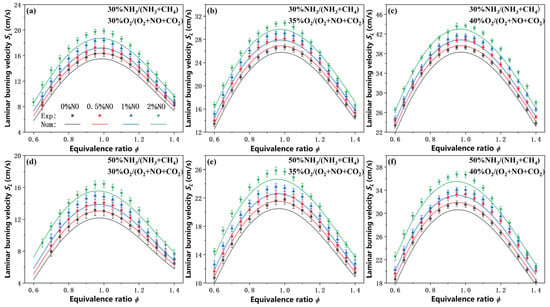
Figure 3.
Measured and predicted laminar burning velocities of NH3/CH4/O2/CO2 flames with various NO concentrations in the oxidizer under CO2 dilution. (a–c) 30% NH3/70% CH4 flames under three oxygen concentration conditions; (d–f) 50% NH3/50% CH4 flames under three oxygen concentration conditions.
To quantify the effect of NO addition on the laminar burning velocity of NH3/CH4/O2/CO2 flames, a normalized enhancement parameter defined in previous work [28] was employed:
where is the SL with NO addition and represents the corresponding value without NO addition under identical conditions.
Figure 4 presents the experimental and simulation results of SL enhancement at 353 K and 1 atm, as a function of equivalence ratio for various ammonia blended ratios, oxygen concentrations, and NO contents. Despite significant scatter in the measured data points, particularly under off-stoichiometric conditions, the enhancement factor still provides a more intuitive representation of the promoting effect of NO on the laminar burning velocity, which is positively corrected with both the ammonia blended ratio and the NO concentration, with exhibiting an approximately linear dependence on the NO content. A clear trend is observed that the maximum enhancement occurs at fuel-lean conditions, and the enhancement effect weakens with increasing equivalence ratio, reaches a minimum near stoichiometric conditions, and then increases again under fuel-rich conditions. Such non-monotonic trend may be attributed to the lower reaction rates and flame temperatures in fuel-lean and fuel-rich conditions compared to stoichiometric conditions. Additionally, as the oxygen concentration increases from 30% to 40%, the under 2% NO condition at the stoichiometric equivalence ratio decreases from 25.88% and 21.39% to 16.36% and 15.43% for 50% and 30% NH3 blended, respectively. This reduction in enhancement at higher O2 levels is attributed to the elevated baseline reactivity and flame temperature, which diminishes the relative promoting effect of NO. In other words, as the intrinsic combustion intensity increases with O2, the incremental benefit of NO addition becomes less pronounced.
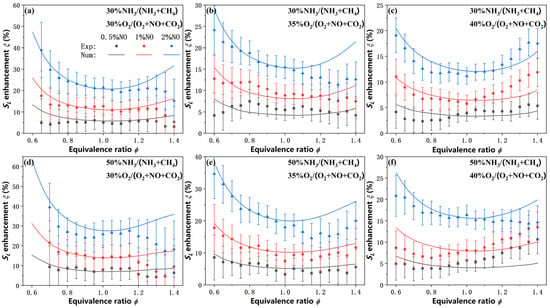
Figure 4.
Measured and predicted SL enhancement for NH3/CH4/O2/CO2 flames with NO addition. (a–c) 30% NH3/70% CH4 flames under three oxygen concentration conditions; (d–f) 50% NH3/50% CH4 flames under three oxygen concentration conditions.
Considering the experimental uncertainties, the CEU-2022 mechanism shows excellent agreement with the measured enhancement factors on the fuel-lean side and near the stoichiometric condition. However, noticeable discrepancies are observed on the fuel-rich side (ϕ > 1.20), where the model tends to underpredict or overpredict depending on oxygen concentration. These discrepancies may be partially attributed to experimental uncertainties associated with flame instability caused by flame front wrinkling or cellular flame formation during the measurement of burning velocities under fuel-rich conditions. Nevertheless, the overall trend of , including the U-shaped dependence on equivalence ratio and its positive correlation with NO concentration and ammonia blending ratio, is well captured by the CEU-2022 mechanism.
To further evaluate and compare the influence of NO addition versus direct oxygen enrichment on the laminar burning velocity of NH3/CH4/O2/CO2 flames, two additional experimental cases were designed, and the results are presented in Figure 5. In these cases, the oxidizer compositions were designed to maintain either a constant oxygen mole fraction (36% O2 vs. 35% O2 + 2% NO) or a constant dilution level (37% O2 vs. 35% O2 + 2% NO). As shown in Figure 5, the addition of NO results in a significantly higher SL compared to the 36% O2 condition, with the enhancement becoming increasingly pronounced as the ammonia blended ratio increases from 30% to 50%. For the 30% NH3 blend, the values of are comparable to those under the 37% O2 condition, and for the 50% NH3 blend, the enhancement even surpasses that achieved under the 37% O2 condition. This indicates that NO is not merely a passive diluent or minor contaminant, but an active chemical promoter that can outperform additional oxygen in enhancing flame propagation, particularly in ammonia-rich conditions. The superior promoting effect of NO can be attributed to the high reactivity of NO in the reaction of NH3/CH4 oxidation. Unlike O2, which primarily acts as an oxidizing agent and typically requires reactions such as R1 to generate reactive species like O and OH radicals before participating in the oxidation process, NO can directly participate in chain-branching and radical-propagating reactions (e.g., R2–R7) and promote the formation of key radicals such as OH, NH2, HNO, and HONO. The introduction of NO effectively activates alternative reaction pathways that sustain the radical pool and accelerate the overall oxidation process, particularly under fuel-lean and fuel-rich conditions where flame temperatures are relatively low and radical chain reactions are inherently weaker. This mechanistic behavior explains the previously observed trend that the enhancement factor is higher under off-stoichiometric conditions compared to stoichiometric mixtures.
H + O2 = O + OH
NH3 + NO = NH2 + HNO
NH2 + NO = NNH + OH
NH + NO = N2 + OH
CH3 + HNO = NO + CH4
NO + H = HNO
NO + OH = HONO
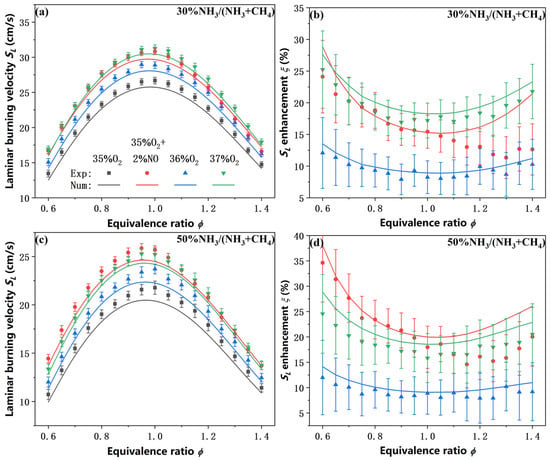
Figure 5.
Comparison of SL and between NO addition and oxygen-enriched conditions under constant oxygen mole fraction and constant dilution level (CO2 content). (a,c) SL of 30% NH3/70% CH4 and 50% NH3/50% CH4 flames; (b,d) for 30% NH3/70% CH4 and 50% NH3/50% CH4 flames.
3.3. Contribution of Thermal-Diffusion and Chemical Effects on SL of NH3/CH4 Flames
For flames in the O2/CO2 atmosphere, the impact of CO2 dilution is typically investigated considering three distinct effects: the dilution effect, the thermal-diffusion effect, and the chemical effect. Similarly, the influence of NO addition can also be decomposed into these three perspectives. Following the methodology used in previous studies on hydrocarbon fuels [29], the conventional fictitious diluent gas method was employed in the numerical simulations using the CEU-2022 mechanism to isolate and quantify each individual effect. Specifically, two hypothetical inert diluent gases, FCO2 and FNO, whose thermodynamic and transport properties are identical to those of CO2 and NO, respectively, were introduced in the laminar flame propagation simulations. Among all the simulated cases involving NO or FNO addition, the NO or FNO concentration in the oxidizer was consistently fixed at 2%.
As shown in Figure 6a,b, the laminar burning velocities simulated using different diluent gases are compared, enabling a clear distinction between the three contributions of CO2 dilution and NO addition. Since the concentrations of N2, FCO2, and CO2 are identical across the relevant cases, the dilution effect of CO2 can be considered negligible. Consequently, the difference between simulated SL of N2 dilution and FCO2 dilution reflects the thermal-diffusion effect. Similarly, the chemical effect can be derived from the difference between the simulated SL of FCO2-diluted and CO2-diluted conditions. These contributions are quantified using the following normalized contribution factors:
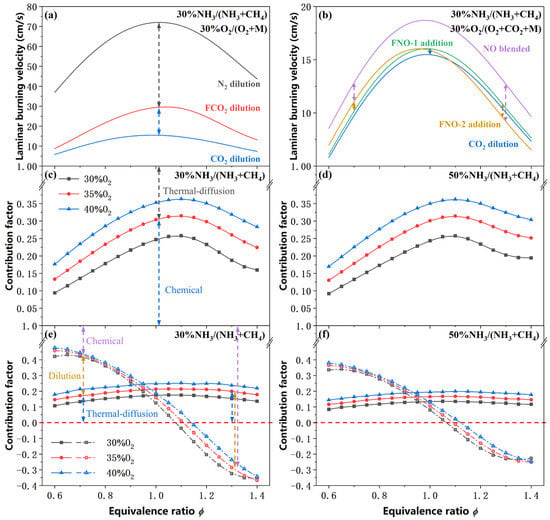
Figure 6.
Contribution of the dilution, thermal-diffusion and chemical effects of the CO2 dilution and NO addition (2%). (a,b) Comparison of SL under different dilution conditions; (c,d) Contribution factor of CO2 dilution; (e,f) Contribution factor of NO addition (2%), solid lines represent the contribution factor of the thermal-diffusion effect, while the dash-dot lines represent the sum of the thermal-diffusion and dilution effects.
It should be noted that, unlike CO2, NO itself acts as an oxidizing agent. Whether FNO is treated as a reactive oxidant in the flame simulations significantly affects the resulting burning velocity. To account for this, two distinct inert reference cases are defined. When FNO is not considered an oxidizing agent, referred to as FNO-1 addition, the difference between FNO-1 addition and CO2-diluted condition arises solely from the differences in thermodynamic and transport properties between FNO and CO2. And the corresponding differences between simulated SL can be attributed to the thermal-diffusion effect. In contrast, when FNO is treated as an oxidizing agent, referred to as FNO-2 addition, the nominal equivalence ratio is calculated based on the assumption that FNO contributes to oxidation, leading to a lower nominal ϕ than the actual value, which results in an apparent shift in the SL—ϕ curve toward the fuel-lean side. Therefore, the difference in simulated SL between the FNO-2 and FNO-1 conditions reflects the dilution effect associated with NO addition, specifically, its influence on the effective oxygen concentration in the premixed gas. Finally, the chemical effect is isolated by comparing the simulated SL of real NO cases with that of the FNO-2 cases, ensuring that the chemical contribution is evaluated under consistent stoichiometric definitions. These three contributions are quantified using the following normalized contribution factors:
As shown in Figure 6c,d, the thermal-diffusion effect dominates the reduction of SL in CO2-diluted atmosphere by contributing to over 60% of the total effect due to the low thermal conductivity and high specific heat of CO2. The contribution factor (CF) of chemical effect increases significantly with rising O2 concentration and reaches its maximum at an equivalence ratio of approximately 1.10. However, as illustrated in Figure 6e,f, the is markedly reduced, accounting for less than 25% of the total effect under NO addition conditions, where NO constitutes only 2% of the oxidizer. Instead, the dilution effect plays a more prominent role because of the reduction in O2 concentration and the corresponding shift in the nominal equivalence ratio caused by the inert dilution of FNO. The decreases monotonically with increasing ϕ, transitioning from a promoting effect on SL under fuel-lean conditions to an inhibiting effect under fuel-rich conditions. This trend arises because the inclusion of FNO as an oxidizer overestimates the effective oxygen availability, resulting in the nominal ϕ being lower than the actual value. This assumption shifts the fuel-lean side closer to stoichiometric conditions while pushing the fuel-rich side further away, thereby inducing the opposite trend in the contribution factor. Moreover, the combined contribution of the dilution and thermal-diffusion effects also exhibits a monotonic decreasing trend with increasing ϕ and reaches zero in the range of ϕ = 1.05–1.15, except for a minor non-monotonic behavior is observed under 30% O2 conditions in the range of ϕ = 0.6–0.7, attributed to the initial increase in .
Critically, this phenomenon indicates that the chemical effect becomes the dominant mechanism for flame propagation enhancement under NO addition. Moreover, all three positive contribution factors under fuel-lean conditions further explain the reason why the most significant enhancement in laminar burning velocity always occurs in the fuel-lean regime.
3.4. Sensitivity and Kinetic Analyses
To further illustrate the influence mechanism of CO2 dilution and NO addition on laminar burning velocities of NH3/CH4 flames, a sensitivity analysis was conducted for stoichiometric conditions under three different atmospheres with comparable laminar burning velocities, as shown in Figure 7. The variation in reaction sensitivity coefficients from AIR to CO2 conditions, particularly the significant increase in the sensitivity of (R8), is primarily attributed to the elevated oxygen mole fraction, which enhances chain-branching pathways and promotes radical generation. In contrast, the addition of 2% NO does not markedly alter the sensitivity weights of most reactions. However, it strongly promotes the forward direction of (R9), leading to accelerated OH radical accumulation. Concurrently, the substantial consumption of NH2 radicals intensifies the reverse reaction of (R10), increasing the competition for H atoms. These effects suppress the forward rate of (R8), thereby modulating the overall flame propagation behavior.
H + O2 = O + OH
NH2 + NO = NNH + OH
NH2 + O = HNO + H
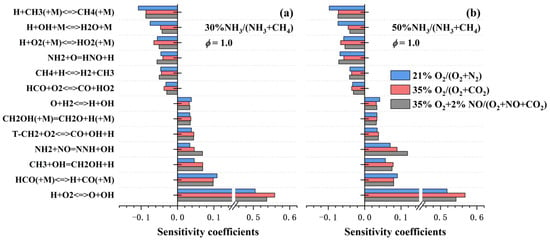
Figure 7.
Sensitivity coefficients of the laminar burning velocity of NH3/CH4 flames for ϕ = 1.0 under different atmosphere conditions, calculated by CEU-2022. (a) 30% NH3/70% CH4 flames; (b) 50% NH3/50% CH4 flames.
Crucially, the sensitivity analysis results in Figure 7 support the earlier discussion on the relative contributions of thermal-diffusion, dilution, and chemical effects: under CO2 conditions, the chemical effect does not dominate, as no CO2-involving reactions appear among the top-ranked sensitivity coefficients. In contrast, under NO condition, the chemical effect of NO addition becomes overwhelmingly dominant, which is clearly reflected in the sensitivity analysis, where the reaction (R9) exhibits a significantly enhanced sensitivity coefficient despite NO constituting only 2% of the oxidizer mixture. This highlights the exceptionally high reactivity of NO in nitrogen-containing fuel chemistry.
Figure 8 illustrates the reaction pathways for stoichiometric NH3/CH4 flames under CO2-diluted conditions, both without and with NO addition. The overall reaction network remains largely similar before and after NO addition. However, the flux contributions of reactions involving NO as a reactant, such as NH2 + NO = NNH + OH and NH + NO = N2 + OH, show a noticeable increase, while the relative fluxes of competing reactions are reduced. Moreover, the fraction of NO converted to N2 increases from 22.1% to 23.6%, indicating that NO addition promotes, to some extent, the conversion of fuel-Nitrogen toward N2, thereby potentially reducing the formation of nitrogenous pollutants.
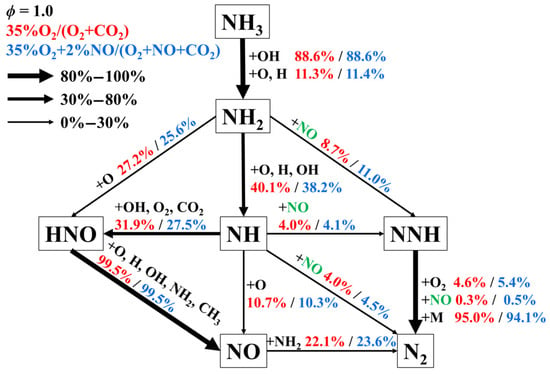
Figure 8.
Reaction pathway analysis for stoichiometric NH3/CH4 flames under CO2-diluted conditions without and with NO addition.
The pathway analysis underscores not only the transformation of nitrogen species but also the central role of key radicals in flame propagation. Specifically, active radicals such as H and OH drive chain-propagation reactions that sustain the combustion process, while NH2 serves as a critical indicator for NH3 oxidation. Additionally, NO is the critical emission species that warrants close attention in ammonia combustion research. Therefore, Figure 9 presents the temperature and mole fraction profiles of H, OH, and NH2, along with NO formation, for stoichiometric NH3/CH4 flames under different atmosphere conditions, further elucidating the coupling between radical dynamics and flame propagation. Although the 21% O2/(O2 + N2) condition exhibits the highest SL and a higher mole fraction of H radicals, its OH mole fraction is significantly lower than those under the two CO2-diluted conditions, primarily due to the substantially lower oxygen concentration. In contrast, the addition of NO in the oxidizer simultaneously increases the flame temperature and the concentrations of H, OH, and NH2 radicals. And the NH2 concentration exhibits a strong positive correlation with flame temperature, which can be attributed to the highly temperature-dependent NH3 decomposition reactions (e.g., NH3 + OH = NH2 + H2O).
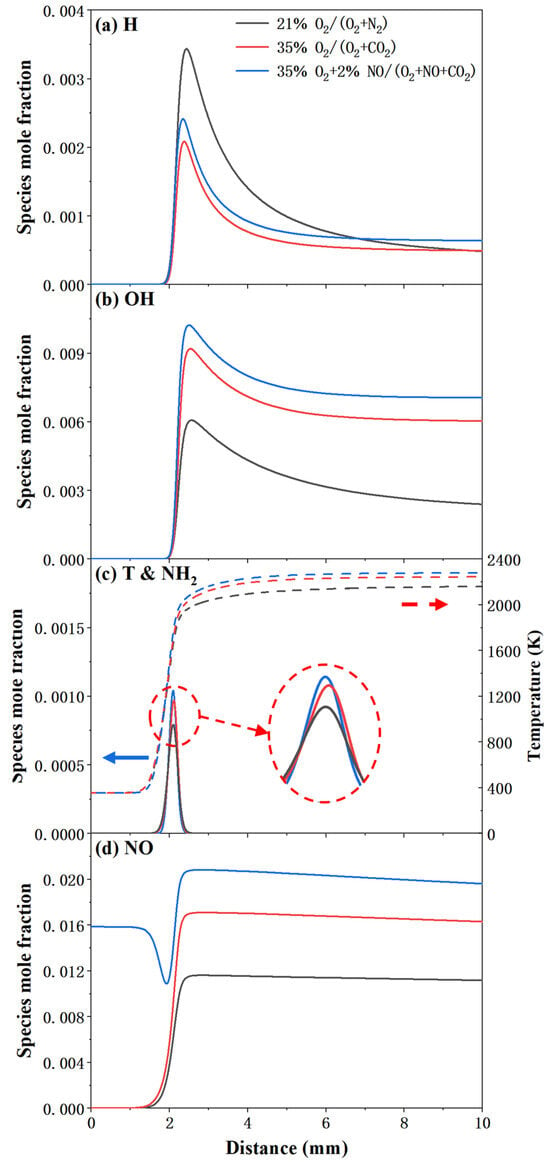
Figure 9.
Temperature and mole fraction profiles of H, OH, NH2, and NO for stoichiometric NH3/CH4 flames under different atmosphere conditions. (a) H radical; (b) OH radical; (c) T and NH2 radical, solid lines represents the profiles of NH2 radical and dashed lines represents the distribution of temperature; (d) NO.
Notably, the non-monotonic variation in NO mole fraction further indicates that NO is not merely a combustion product, but actively participates in reactions at the upstream edge of the flame front during the early reaction stage when the temperature rises rapidly. Although the addition of NO partially suppresses the H + O2 = O + OH reaction, the primary source of OH radicals in conventional hydrocarbon combustion, it still promotes the generation and accumulation of H and OH radicals through alternative pathways. Specifically, the reaction NH2 + NO = NNH + OH directly produces OH radicals, while the subsequent decomposition of NNH (e.g., NNH = N2 + H) contributes to H radical formation. These NO-involved reactions collectively enhance the radical pool, thereby sustaining and even accelerating flame propagation in NH3/CH4 systems despite the inhibition of classical chain-branching routes.
However, it should not be overlooked that despite the generally good agreement between simulation results and experimental measurements, certain discrepancies still exist. The underlying mechanisms of CO2 dilution and NO addition, particularly the dual role of NO as both a combustion intermediate and a reaction promoter, require further experimental investigation. This underscores the urgent need for accurate, spatially resolved measurements of key parameters beyond laminar burning velocity, including concentration profiles of radicals (e.g., H, OH, NH2) and major species (e.g., NO), as well as flame temperature. Such comprehensive experimental data are essential for placing stronger constraints on kinetic mechanism development and for achieving a deeper understanding of the coupling between thermal and chemical effects in NH3/CH4 combustion systems under CO2-rich and NO-doped conditions.
4. Conclusions
This work investigated the laminar burning velocities of NH3/CH4 flames under CO2-diluted conditions both without and with NO addition using the heat flux method, providing a comprehensive dataset for validating chemical kinetic mechanisms. The influence mechanisms of CO2 dilution and NO addition in the oxidizer were further explored through numerical simulations based on the CEU-2022 mechanism. The new findings are summarized below:
- (1)
- The addition of NO to the mixture stream significantly enhances the laminar burning velocity of NH3/CH4 premixed flames, with the promoting effect becoming increasingly pronounced as both the NO concentration and the ammonia blended ratio rise.
- (2)
- Thermal-diffusion effects dominate the reduction in laminar burning velocity (SL) of NH3/CH4 premixed flames under CO2 dilution. In contrast, the enhancement in SL induced by NO addition is almost entirely attributed to the chemical effects and the dilution effects, which exhibit a transition from promoting to inhibiting as the equivalence ratio increases, and are nonnegligible.
- (3)
- Kinetic analysis indicates that NO deeply participates in NHX chemistry during the early reaction stage and exerts a significant promoting effect on the generation and accumulation of H and OH radicals through key reaction pathways.
Author Contributions
Conceptualization, Z.Y., Y.H., W.W., S.L., S.W. and Z.W.; investigation, Z.Y. and J.J.; data curation, J.J.; writing—original draft, Z.Y.; writing—review & editing, Y.H., W.W., S.W. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (U24B2068, 52125605).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and Technology of Ammonia Combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, H. Kinetics Modeling of NO Emission of Oxygen-Enriched and Rich-Lean-Staged Ammonia Combustion under Gas Turbine Conditions. Fuel 2024, 355, 129509. [Google Scholar] [CrossRef]
- Ilbas, M.; Kekul, O.; Bektas, A.; Karyeyen, S. Oxidizer Effects on Ammonia Combustion Using a Generated Non-Premixed Burner. Int. J. Hydrog. Energy 2022, 47, 12317–12337. [Google Scholar] [CrossRef]
- Pandey, J.K.; Dinesh, M.H.; Kumar, G.N. A Comparative Study of NOx Mitigating Techniques EGR and Spark Delay on Combustion and NOx Emission of Ammonia/Hydrogen and Hydrogen Fuelled SI Engine. Energy 2023, 276, 127611. [Google Scholar] [CrossRef]
- Yang, C.; Wang, B.; Wang, H.; Hu, D.; Duan, B.; Wang, Y. The Effect of Changing EGR Rate on Engine Performance under Different Ammonia/Methanol Ratios. J. Energy Inst. 2024, 113, 101546. [Google Scholar] [CrossRef]
- De Persis, S.; Foucher, F.; Pillier, L.; Osorio, V.; Gökalp, I. Effects of O2 Enrichment and CO2 Dilution on Laminar Methane Flames. Energy 2013, 55, 1055–1066. [Google Scholar] [CrossRef]
- Glarborg, P.; Bentzen, L.L.B. Chemical Effects of a High CO2 Concentration in Oxy-Fuel Combustion of Methane. Energy Fuels 2008, 22, 291–296. [Google Scholar] [CrossRef]
- Xie, M.; Fu, J.; Zhang, Y.; Shu, J.; Ma, Y.; Liu, J.; Zeng, D. Numerical Analysis on the Effects of CO2 Dilution on the Laminar Burning Velocity of Premixed Methane/Air Flame with Elevated Initial Temperature and Pressure. Fuel 2020, 264, 116858. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Zhang, M.; Gong, J.; Jin, W.; Huang, Z. Experimental and Numerical Study on Laminar Flame Characteristics of Methane Oxy-Fuel Mixtures Highly Diluted with CO2. Energy Fuels 2013, 27, 6231–6237. [Google Scholar] [CrossRef]
- Shi, X.; Li, W.; Zhang, J.; Fang, Q.; Zhang, Y.; Xi, Z.; Li, Y. Exploration of NH3 and NH3/DME Laminar Flame Propagation in O2/CO2 Atmosphere: Insights into NH3/CO2 Interactions. Combust. Flame 2024, 260, 113245. [Google Scholar] [CrossRef]
- Zhu, W.; Meng, X.; Zhang, M.; Zhang, X.; Cui, Z.; Tian, J.; Long, W.; Bi, M. Synergistic Effects of CO2 Dilution and H2 Addition on the Laminar Combustion Characteristics of NH3/CH4 Blends at High Temperature and Pressure. Energy 2025, 320, 135267. [Google Scholar] [CrossRef]
- Mei, B.; Ma, S.; Zhang, X.; Li, Y. Characterizing Ammonia and Nitric Oxide Interaction with Outwardly Propagating Spherical Flame Method. Proc. Combust. Inst. 2021, 38, 2477–2485. [Google Scholar] [CrossRef]
- Monnier, N.; Lamoureux, N.; Zitouni, S.; Brequigny, P.; Mounaïm-Rousselle, C. Laminar Burning Velocity of NH3 /NO/N2 Mixtures: An Experimental and Numerical Study. Proc. Combust. Inst. 2024, 40, 105266. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Zhou, M.; Li, G. Laminar Flames and Chemical Kinetics Analysis of NH3/NO and NH3/N2O Mixtures in the Absence of Oxygen. Combust. Flame 2025, 279, 114341. [Google Scholar] [CrossRef]
- Bosschaart, K.J.; de Goey, L.P.H. Detailed Analysis of the Heat Flux Method for Measuring Burning Velocities. Combust. Flame 2003, 132, 170–180. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Wang, S.; Whiddon, R.; He, Y.; Lv, Y.; Konnov, A.A. Parametrization of the Temperature Dependence of Laminar Burning Velocity for Methane and Ethane Flames. Fuel 2019, 239, 1028–1037. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Chen, C.; Elbaz, A.M.; Sun, Z.; Roberts, W.L. Applying Heat Flux Method to Laminar Burning Velocity Measurements of NH3/CH4/Air at Elevated Pressures and Kinetic Modeling Study. Combust. Flame 2022, 236, 111788. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Measurement and Modelling of the Laminar Burning Velocity of Methane-Ammonia-Air Flames at High Pressures Using a Reduced Reaction Mechanism. Combust. Flame 2019, 204, 162–175. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Lhuillier, C.; Barbosa, A.A.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C.; Seidel, L.; Mauss, F. An Experimental and Modeling Study of Ammonia with Enriched Oxygen Content and Ammonia/Hydrogen Laminar Flame Speed at Elevated Pressure and Temperature. Proc. Combust. Inst. 2021, 38, 2163–2174. [Google Scholar] [CrossRef]
- Zhang, X. Combustion Chemistry of Ammonia/C1 Fuels: A Comprehensive Kinetic Modeling Study. Fuel 2023, 341, 127676. [Google Scholar] [CrossRef]
- Konnov, A.A. An Exploratory Modelling Study of Chemiluminescence in Ammonia-Fuelled Flames. Part 1. Combust. Flame 2023, 253, 112788. [Google Scholar] [CrossRef]
- Konnov, A.A. An Exploratory Modelling Study of Chemiluminescence in Ammonia-Fuelled Flames. Part 2. Combust. Flame 2023, 253, 112789. [Google Scholar] [CrossRef]
- Szanthoffer, A.G. Identification of Well-Parameterised Reaction Steps in Detailed Combustion Mechanisms—A Case Study of Ammonia/Air Flames. Fuel 2025, 380, 132938. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, T.; Zhai, Y.; Hou, R.; Tian, Z.-Y.; Ji, C. A Comparative Study on the Laminar C1–C4 n-Alkane/NH3 Premixed Flame. Fuel 2022, 324, 124732. [Google Scholar] [CrossRef]
- Nakamura, H.; Shindo, M. Effects of Radiation Heat Loss on Laminar Premixed Ammonia/Air Flames. Proc. Combust. Inst. 2019, 37, 1741–1748. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Liu, J.; Sun, N. Investigation of Laminar Flame Speeds of CH4/O2/CO2 Mixtures at Ordinary Pressure and Kinetic Simulation. Energy 2014, 70, 626–634. [Google Scholar] [CrossRef]
- Konnov, A.A.; Dyakov, I.V. Measurement of Propagation Speeds in Adiabatic Cellular Premixed Flames of CH4+O2+CO2. Exp. Therm. Fluid Sci. 2005, 29, 901–907. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Yu, Z.; Han, X.; He, Y.; Zhu, Y.; Konnov, A.A. Experimental and Kinetic Modeling Study of Laminar Burning Velocity Enhancement by Ozone Additive in NH3+O2+N2 and NH3+CH4/C2H6/C3H8+air Flames. Proc. Combust. Inst. 2023, 39, 4237–4246. [Google Scholar] [CrossRef]
- Halter, F.; Foucher, F.; Landry, L.; Mounaïm-Rousselle, C. Effect of Dilution by Nitrogen and/or Carbon Dioxide on Methane and Iso-Octane Air Flames. Combust. Sci. Technol. 2009, 181, 813–827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).