Hydrogen Production from Biowaste: A Systematic Review of Conversion Technologies, Environmental Impacts, and Future Perspectives
Abstract
1. Introduction
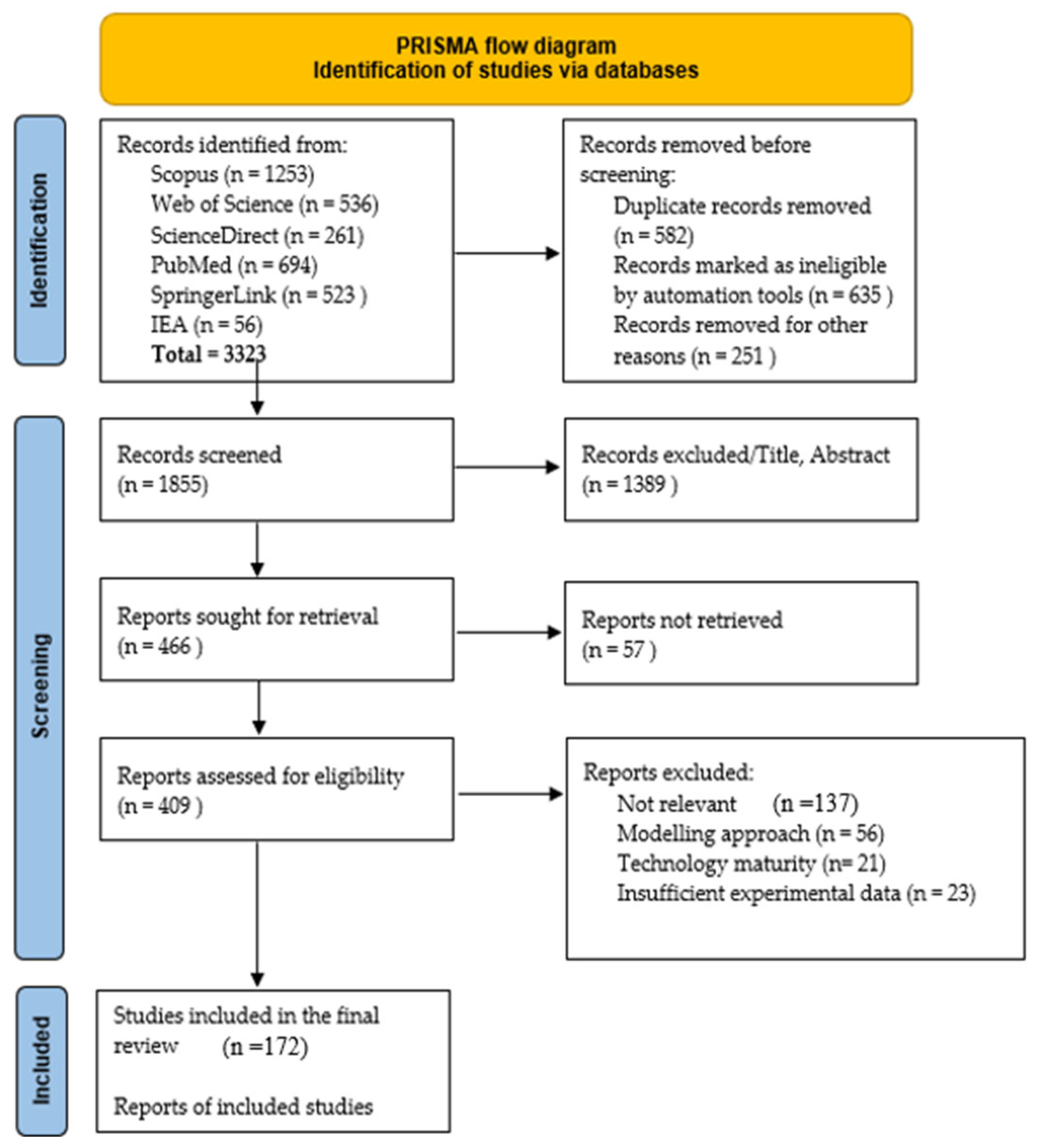
2. Review Methodology
3. Current Biowaste-to-Hydrogen Conversion Technologies
3.1. Thermochemical Conversion
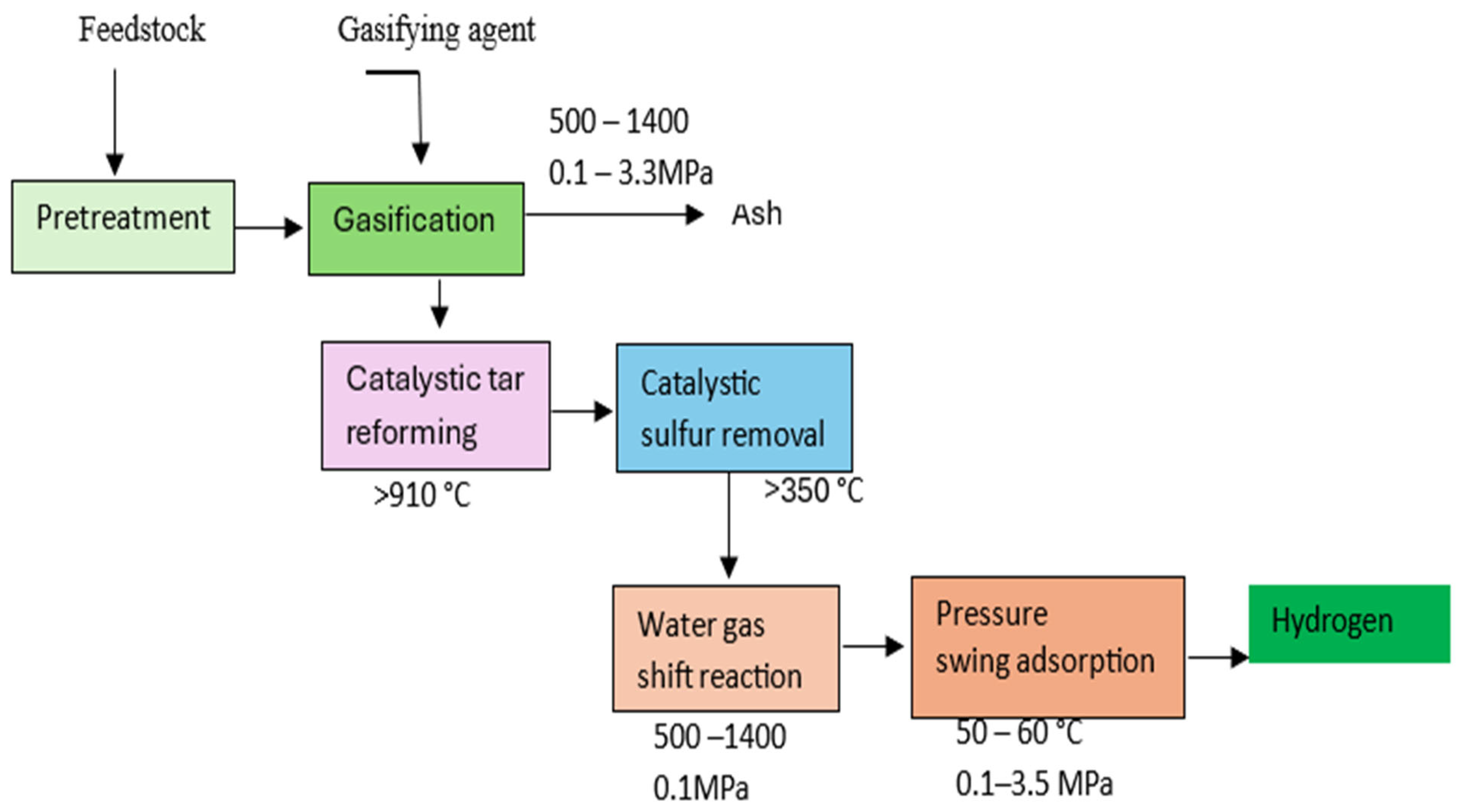
3.1.1. Gasification
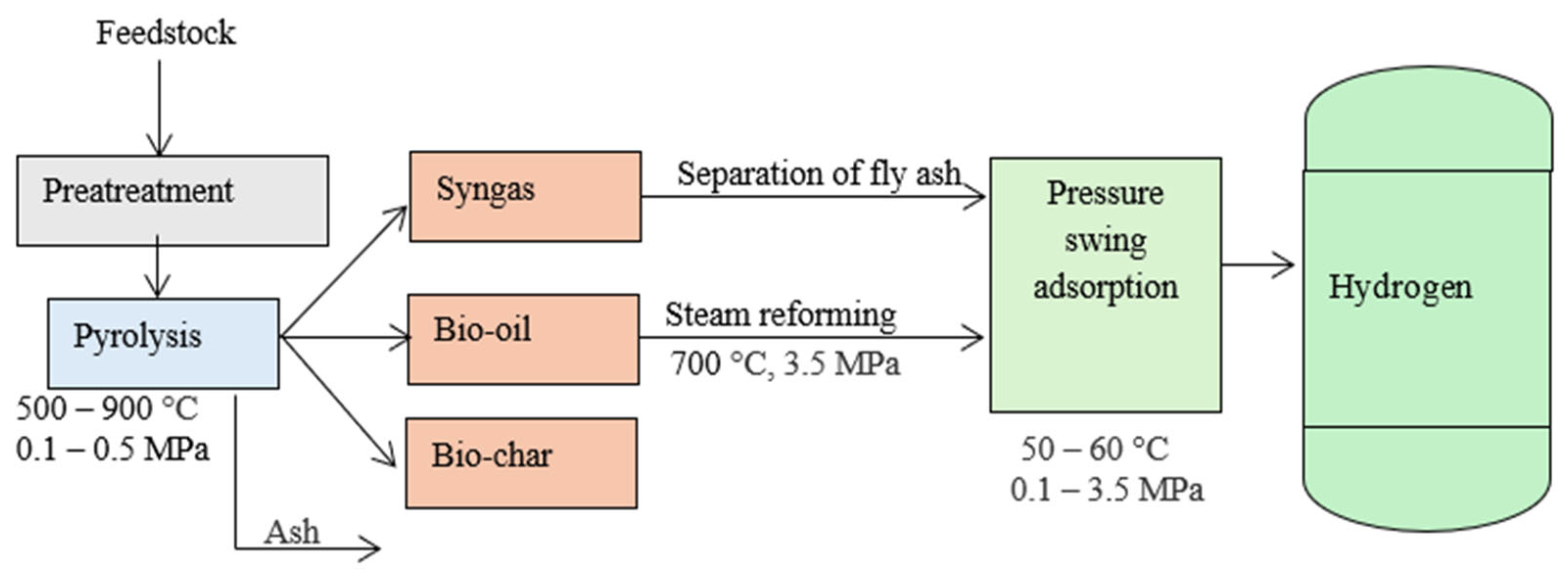
3.1.2. Pyrolysis
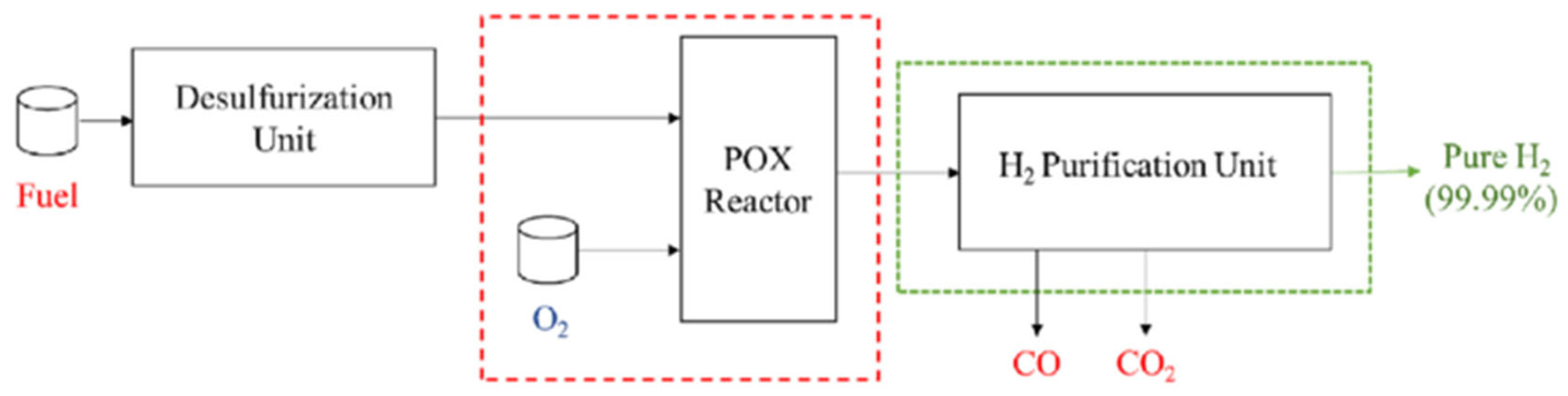
3.1.3. Partial Oxidation
3.1.4. Hydrothermal Gasification
3.1.5. Biogas Reforming
3.2. Biological Fermentation
3.2.1. Dark Fermentation
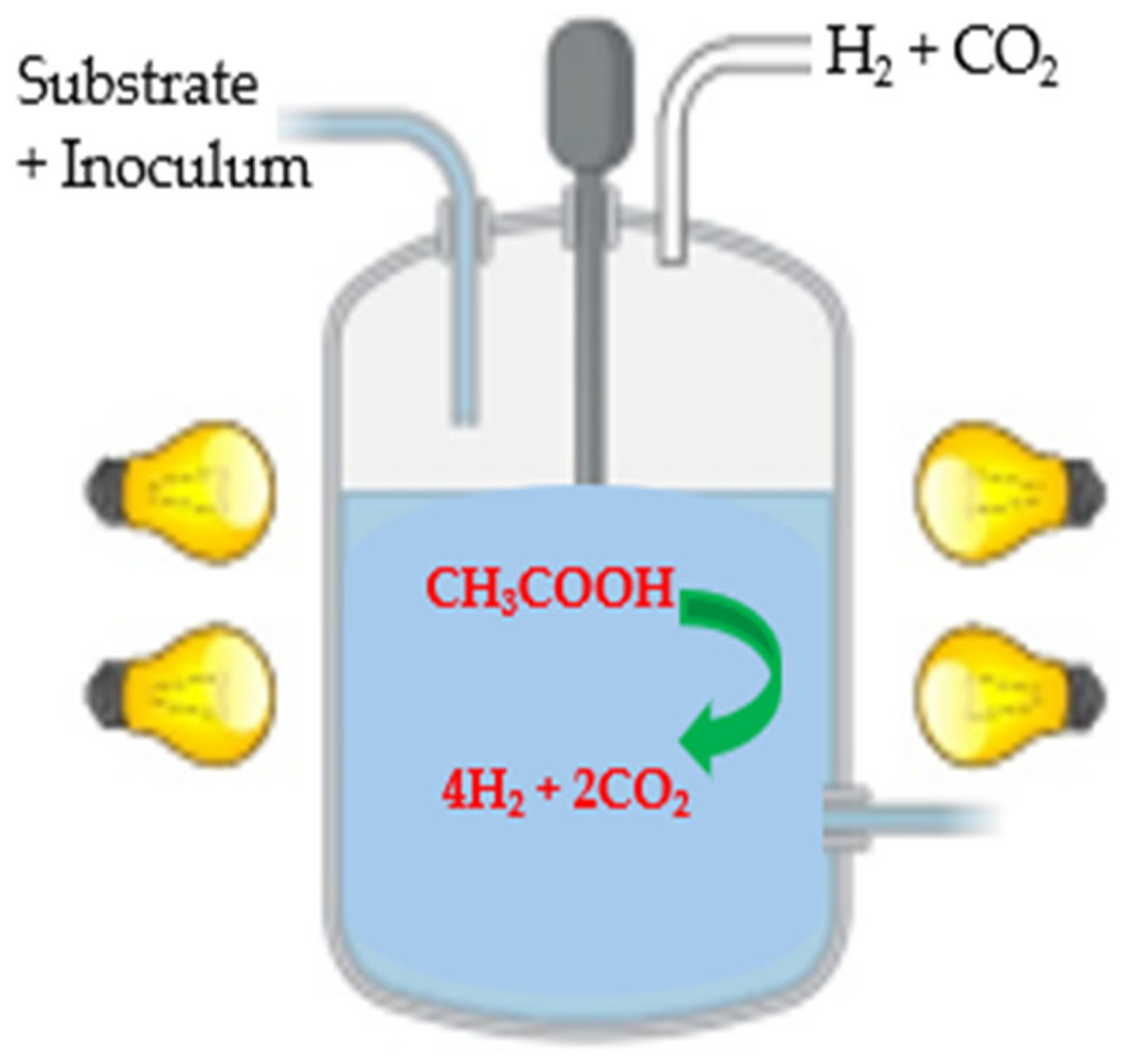
3.2.2. Photofermentation
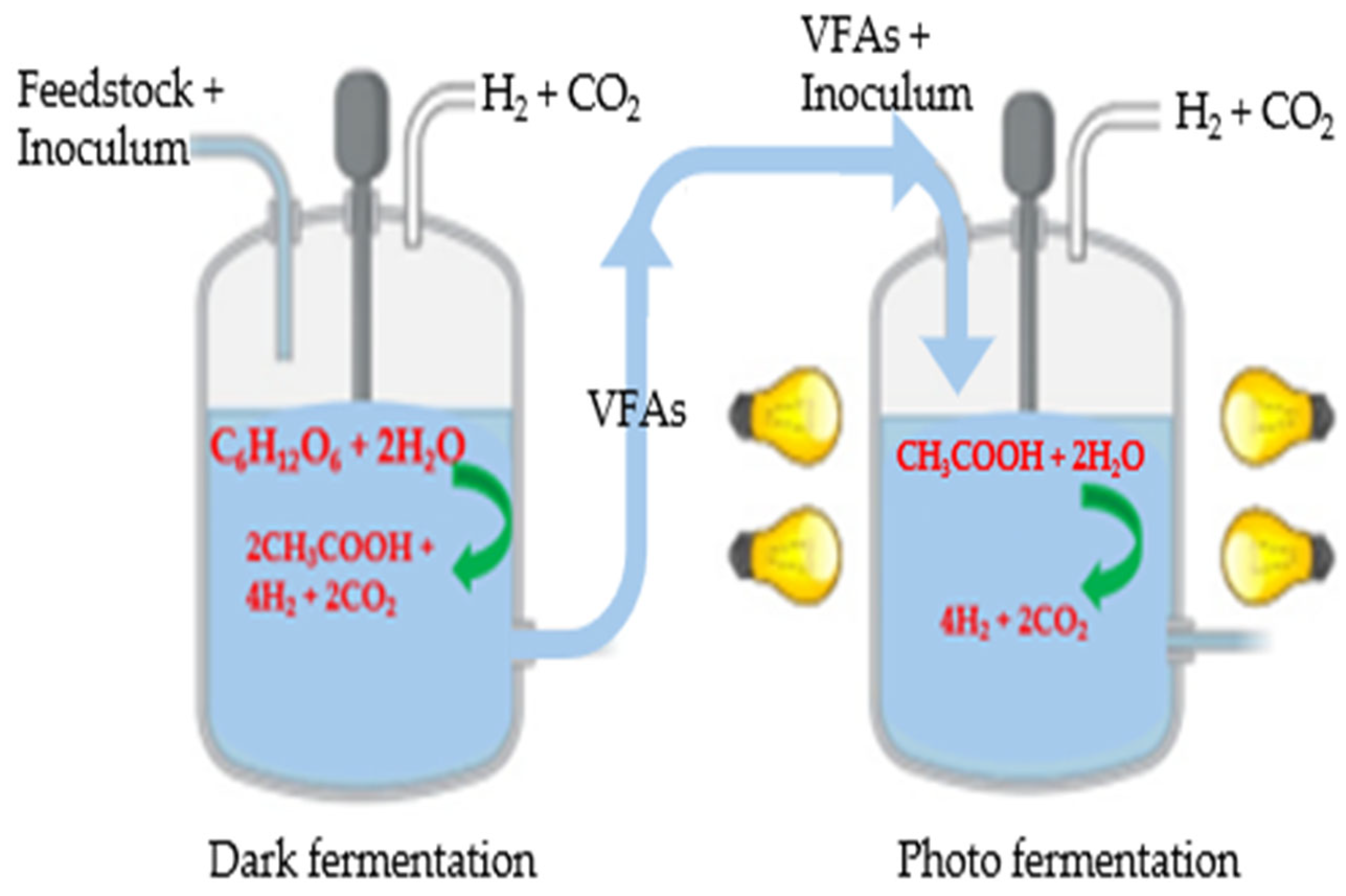
3.2.3. Sequential Fermentation
3.3. Microbial Electrolysis Cells (MECs)
4. Comparative Analysis of Technologies
- (i)
- Only peer-reviewed studies or institutional reports were included;
- (ii)
- Studies without experimental data or with technology-readiness levels of below 3 were excluded;
- (iii)
- Model-only studies were analyzed separately and not included in the quantitative comparisons. Future meta-analytical studies may benefit from subgroup-based or scenario-based sensitivity assessments to better evaluate the robustness of synthesis findings.
5. Environmental Impact Assessment of Hydrogen Production Technologies
5.1. Greenhouse Gas Emissions
5.2. Energy Use
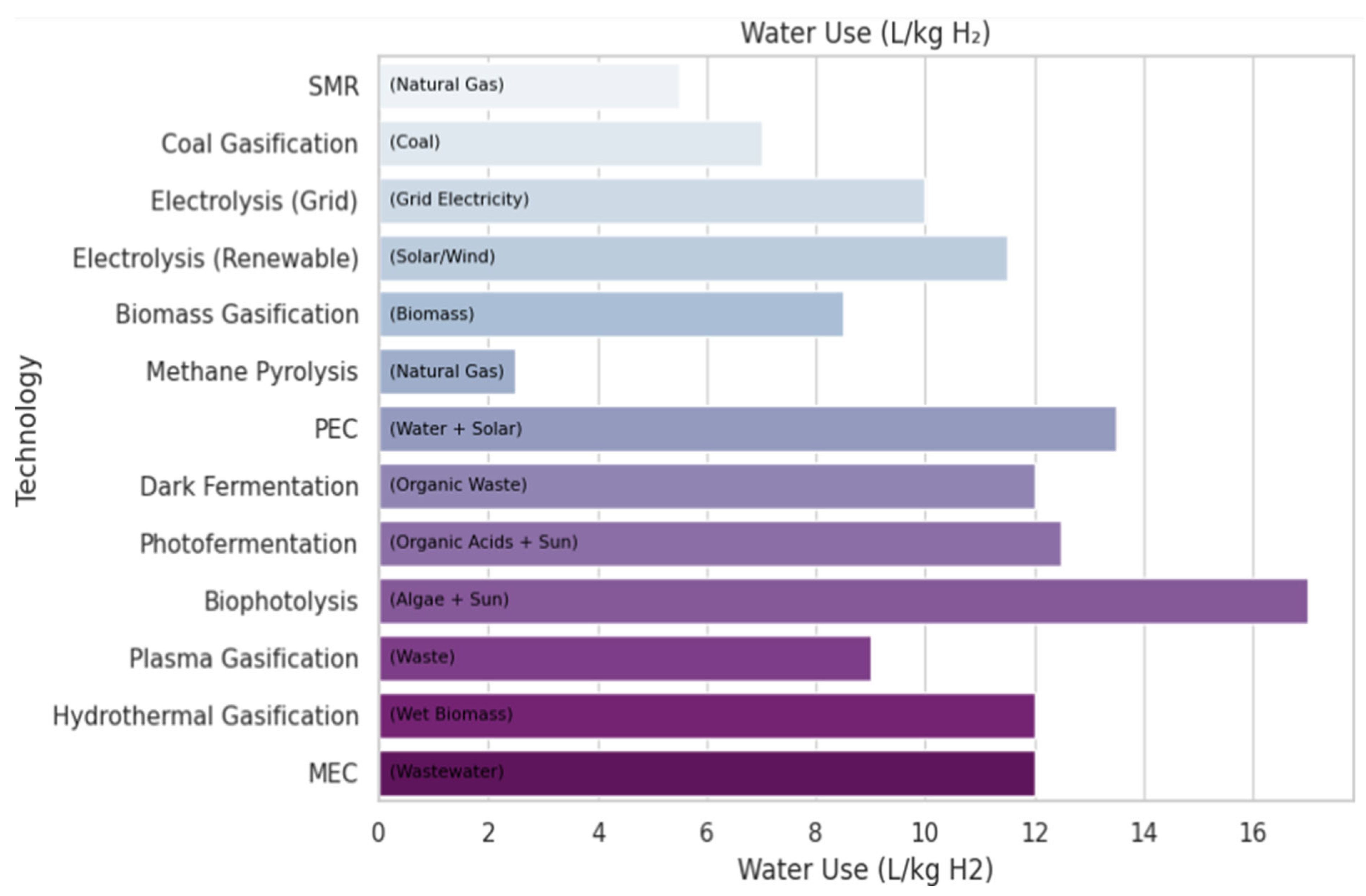
5.3. Water Use in Hydrogen Production Technologies
5.4. Air-Pollutant Emissions from Hydrogen Production Technologies
6. Socioeconomic and Regulatory Considerations for WtH Deployment
Technoeconomic Analysis (TEA)
7. Global Hydrogen Production Policies: Current Landscape and Future Directions
7.1. Global Policy Landscape and Strategic Frameworks
7.2. National and Regional Policy Mechanisms
7.3. Policy Support for Biowaste-to-Hydrogen Technologies
8. Challenges and Future Research Directions
8.1. Technological Prospects
8.1.1. Thermochemical Routes
8.1.2. Biochemical Routes
8.2. Environmental Prospects
8.3. Social Acceptance
8.4. Regulatory Barriers
8.5. Future Policymaking Recommendations
- Carbon pricing and credit mechanisms that reward low-carbon and circular hydrogen production, including from biogenic sources;
- Dedicated investment support for demonstration and scalable projects using biomass gasification, pyrolysis, and fermentation routes;
- Standardized certification systems to track origin and lifecycle emissions of hydrogen, enabling international trade in “green” and “bio-based” hydrogen;
- Inclusion of biowaste-to-hydrogen pathways in national waste management policies, linking clean energy goals with circular economy mandates.
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| °C | Degrees Celsius |
| MPa | Megapascals |
| CO2 | Carbon Dioxide |
| CH4 | Methane |
| N2O | Nitrous Oxide |
| CO | Carbon Monoxide |
| H2 | Hydrogen |
| CO2eq | Carbon Dioxide Equivalent |
| K2CO3 | Potassium Carbonate |
| KOH | Potassium Hydroxide |
| NaOH | Sodium Hydroxide |
| Na2CO3 | Sodium Carbonate |
| Ni-5132P | Nickel Catalyst |
| H+ | Proton |
| e− | Electron |
| WtH | Waste-to-Hydrogen |
| GHG | Greenhouse Gas |
| HRTs | Hydraulic Retention Times |
| TPOX | Thermal Partial Oxidation |
| CPOX | Catalytic Partial Oxidation |
| MSW | Municipal Solid Waste |
| WGSR | Water–Gas Shift Reaction |
| WGS | Water–Gas Shift |
| PSA | Pressure Swing Adsorption |
| DF | Dark Fermentation |
| FP | Photofermentation |
| SR | Steam Reforming |
| POX | Partial Oxidation |
| HTG | Hydrothermal Gasification |
| SCWG | Supercritical Water Gasification |
| MEC | Microbial Electrolysis Cell |
| PEC | Photoelectrochemical Cell |
| WTE | Waste-to-Energy |
| ADP | Adenosine Diphosphate |
| AD | Anerobic Digestion |
| ATP | Adenosine Triphosphate |
| VFAs | Volatile Fatty Acids |
| CCS | Carbon Capture and Storage |
| LCA | Lifecycle Assessment |
| R&D | Research and Development |
| PNSBs | Purple Non-Sulfur Bacteria |
| EROI | Energy Return On Investment |
| BRICS | Brazil, Russia, India, China, and South Africa |
| VOCs | Volatile Organic Compounds |
| UASB | Anerobic Sludge Blanket |
| CSTRs | Continuous Stirred-Tank Reactors |
| CRISPR/Cas9 | Clustered Regularly Interspaced Short Palindromic Repeat-Associated Protein 9 |
References
- Warming Projections Global Update. September 2020. Available online: https://climateactiontracker.org/documents/790/CAT_2020-09-23_Briefing_GlobalUpdate_Sept2020.pdf (accessed on 9 May 2025).
- Fasullo, J.T.; Otto-Bliesner, B.L.; Stevenson, S. ENSO’s Changing Influence on Temperature, Precipitation, and Wildfire in a Warming Climate. Geophys. Res. Lett. 2018, 45, 9216–9225. [Google Scholar] [CrossRef]
- A Study on the Effects of Global Warming in Bangladesh. Available online: https://www.researchgate.net/publication/282524296 (accessed on 9 May 2025).
- Abawalo, M.; Pikoń, K.; Landrat, M. Comparative Life Cycle Assessment of Hydrogen Production via Biogas Reforming and Agricultural Residue Gasification. Appl. Sci. 2025, 15, 5029. [Google Scholar] [CrossRef]
- European Commission Joint Research Centre. GHG Emissions of All World Countries: 2023; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- European Commission Joint Research Centre; IEA. GHG Emissions of All World Countries; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- Zheng, X.; Streimikiene, D.; Balezentis, T.; Mardani, A.; Cavallaro, F.; Liao, H. A Review of Greenhouse Gas Emission Profiles, Dynamics, and Climate Change Mitigation Efforts Across the Key Climate Change Players. J. Clean. Prod. 2019, 234, 1113–1133. [Google Scholar] [CrossRef]
- Wijayasekera, S.C.N.; Cooray, B.Y.; Premaratne, M.; Ariyadasa, T.U. Assessment of the Potential of CO2 Sequestration from Cement Flue Gas Using Locally Isolated Microalgae. In Proceedings of the 2020 Moratuwa Engineering Research Conference (MERCon), Online, 28–30 July 2020; pp. 124–129. [Google Scholar]
- Wijayasekera, S.C.; Hewage, K.; Hettiaratchi, P.; Siddiqui, O.; Razi, F.; Pokhrel, D.; Sadiq, R. Sustainability of Waste-to-Hydrogen Conversion Pathways: A Life Cycle Thinking-Based Assessment. Energy Convers. Manag. 2022, 270, 116218. [Google Scholar] [CrossRef]
- Cornelsen, N. LIFEBIOBEST Blog: Bio-Waste Separate Collection Takes Off; Zero Waste Europe: Brussels, Belgium, 2024. [Google Scholar]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018; ISBN 978-1-4648-1329-0. [Google Scholar]
- Meegoda, J.N.; Chande, C.; Bakshi, I. Biodigesters for Sustainable Food Waste Management. Int. J. Environ. Res. Public Health 2025, 22, 382. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen Production from Biomasses and Wastes: A Technological Review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Tian, H.; Li, J.; Yan, M.; Tong, Y.W.; Wang, C.-H.; Wang, X. Organic Waste to Biohydrogen: A Critical Review from Technological Development and Environmental Impact Analysis Perspective. Appl. Energy 2019, 256, 113961. [Google Scholar] [CrossRef]
- UN Environment Programme. UNEP Food Waste Index Report 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 7 May 2025).
- FAO. The State of Food and Agriculture 2022; FAO: Rome, Italy, 2022; ISBN 978-92-5-136043-9. [Google Scholar]
- Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics (accessed on 7 May 2025).
- National Food Waste Strategy—DCCEEW. Available online: https://www.dcceew.gov.au/environment/protection/waste/publications/national-food-waste-strategy (accessed on 7 May 2025).
- World Resources Report. Creating a Sustainable Food Future. Available online: https://research.wri.org/wrr-food (accessed on 12 May 2025).
- WWF. Driven to Waste: The Global Impact of Food Loss and Waste on Farms. Available online: https://www.worldwildlife.org/publications/driven-to-waste-the-global-impact-of-food-loss-and-waste-on-farms (accessed on 12 May 2025).
- Jin, X.-Y.; Jin, H.-J.; Iwahana, G.; Marchenko, S.S.; Luo, D.-L.; Li, X.-Y.; Liang, S.-H. Impacts of Climate-Induced Permafrost Degradation on Vegetation: A Review. Adv. Clim. Change Res. 2021, 12, 29–47. [Google Scholar] [CrossRef]
- Kazmi, B.; Sadiq, T.; Taqvi, S.A.A.; Nasir, S.; Khan, M.M.; Naqvi, S.R.; Al Mohamadi, H. Towards a Sustainable Future: Bio-Hydrogen Production from Food Waste for Clean Energy Generation. Process Saf. Environ. Prot. 2024, 183, 555–567. [Google Scholar] [CrossRef]
- ScienceDirect. Bio-Waste to Hydrogen Production Technologies. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780323884273000209?via%3Dihub (accessed on 12 May 2025).
- Mehanovic, D.; Al-Haiek, A.; Leclerc, P.; Rancourt, D.; Fréchette, L.; Picard, M. Energetic, GHG, and Economic Analyses of Electrified Steam Methane Reforming Using Conventional Reformer Tubes. Energy Convers. Manag. 2023, 276, 116549. [Google Scholar] [CrossRef]
- Rojas, J.; Zhai, S.; Sun, E.; Haribal, V.; Marin-Quiros, S.; Sarkar, A.; Gupta, R.; Cargnello, M.; Chueh, W.; Majumdar, A. Technoeconomics and Carbon Footprint of Hydrogen Production. Int. J. Hydrogen Energy 2024, 49, 59–74. [Google Scholar] [CrossRef]
- Eloffy, M.G.; Elgarahy, A.M.; Saber, A.N.; Hammad, A.; El-Sherif, D.M.; Shehata, M.; Mohsen, A.; Elwakeel, K.Z. Biomass-to-Sustainable Biohydrogen: Insights into the Production Routes, and Technical Challenges. Chem. Eng. J. Adv. 2022, 12, 100410. [Google Scholar] [CrossRef]
- Hassan, H.; Tian, S.; Safi, A.; Umar, M. Climate Commitments and Financial Moderation: A Deep Dive into Renewable Energy’s Influence on OECD Carbon Footprints. Econ. Anal. Policy 2024, 81, 1484–1495. [Google Scholar] [CrossRef]
- Marouani, I.; Guesmi, T.; Alshammari, B.M.; Alqunun, K.; Alzamil, A.; Alturki, M.; Hadj Abdallah, H. Integration of Renewable-Energy-Based Green Hydrogen into the Energy Future. Processes 2023, 11, 2685. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-Hydrogen Production from Waste Materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Okajima, I.; Shimoyama, D.; Sako, T. Gasification and Hydrogen Production from Food Wastes Using High Pressure Superheated Steam in the Presence of Alkali Catalyst. J. Chem. Eng. Jpn. 2007, 40, 356–364. [Google Scholar] [CrossRef]
- Salimi, M.; Safari, F.; Tavasoli, A.; Shakeri, A. Hydrothermal Gasification of Different Agricultural Wastes in Supercritical Water Media for Hydrogen Production: A Comparative Study. Int. J. Ind. Chem. 2016, 7, 277–285. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Wilk, M. Thermophilic Co-Digestion of the Organic Fraction of Municipal Solid Wastes—The Influence of Food Industry Wastes Addition on Biogas Production in Full-Scale Operation. Molecules 2018, 23, 3146. [Google Scholar] [CrossRef]
- Falfushynska, H. Advancements and Prospects in Algal Biofuel Production: A Comprehensive Review. Phycology 2024, 4, 548–575. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Rajamehala, M.; Karthigadevi, G.; Praveenkumar, R.; Bharathiraja, B. A Review on Feedstock, Pretreatment Methods, Influencing Factors, Production and Purification Processes of Bio-Hydrogen Production. Case Stud. Chem. Environ. Eng. 2020, 2, 100038. [Google Scholar] [CrossRef]
- Kumar, G.; Eswari, A.P.; Kavitha, S.; Kumar, M.D.; Kannah, R.Y.; How, L.C.; Muthukaruppan, G.; Banu, J.R. Thermochemical Conversion Routes of Hydrogen Production from Organic Biomass: Processes, Challenges and Limitations. Biomass Conv. Bioref. 2023, 13, 8509–8534. [Google Scholar] [CrossRef]
- Gbiete, D.; Narra, S.; Mani Kongnine, D.; Narra, M.-M.; Nelles, M. Insights into Biohydrogen Production Through Dark Fermentation of Food Waste: Substrate Properties, Inocula, and Pretreatment Strategies. Energies 2024, 17, 6350. [Google Scholar] [CrossRef]
- Sher, F.; Hameed, S.; Smječanin Omerbegović, N.; Chupin, A.; Ul Hai, I.; Wang, B.; Heng Teoh, Y.; Joka Yildiz, M. Cutting-Edge Biomass Gasification Technologies for Renewable Energy Generation and Achieving Net Zero Emissions. Energy Convers. Manag. 2025, 323, 119213. [Google Scholar] [CrossRef]
- Sadeq, A.M.; Homod, R.Z.; Hussein, A.K.; Togun, H.; Mahmoodi, A.; Isleem, H.F.; Patil, A.R.; Moghaddam, A.H. Hydrogen Energy Systems: Technologies, Trends, and Future Prospects. Sci. Total Environ. 2024, 939, 173622. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.D.; Silva, V.B.; Rouboa, A. Assessment on Steam Gasification of Municipal Solid Waste against Biomass Substrates. Energy Convers. Manag. 2016, 124, 92–103. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable Hydrogen Production through Biomass Gasification: A Review and Future Prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Arku, P.; Dutta, A. Gasification of Plastic Solid Waste and Competitive Technologies. In Plastics to Energy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–293. ISBN 978-0-12-813140-4. [Google Scholar]
- Chen, W.-H.; Chen, C.-Y. Water Gas Shift Reaction for Hydrogen Production and Carbon Dioxide Capture: A Review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Wang, G.; Xu, S.; Wang, C.; Zhang, J.; Fang, Z. Desulfurization and Tar Reforming of Biogenous Syngas over Ni/Olivine in a Decoupled Dual Loop Gasifier. Int. J. Hydrogen Energy 2017, 42, 15471–15478. [Google Scholar] [CrossRef]
- Salam, M.A.; Ahmed, K.; Akter, N.; Hossain, T.; Abdullah, B. A Review of Hydrogen Production via Biomass Gasification and Its Prospect in Bangladesh. Int. J. Hydrogen Energy 2018, 43, 14944–14973. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Thermochemical Route for Biohydrogen Production. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2019; pp. 187–218. ISBN 978-0-444-64203-5. [Google Scholar]
- Su, H.; Hantoko, D.; Yan, M.; Cai, Y.; Kanchanatip, E.; Liu, J.; Zhou, X.; Zhang, S. Evaluation of Catalytic Subcritical Water Gasification of Food Waste for Hydrogen Production: Effect of Process Conditions and Different Types of Catalyst Loading. Int. J. Hydrogen Energy 2019, 44, 21451–21463. [Google Scholar] [CrossRef]
- Thunman, H.; Gustavsson, C.; Larsson, A.; Gunnarsson, I.; Tengberg, F. Economic Assessment of Advanced Biofuel Production via Gasification Using Cost Data from the GoBiGas Plant. Energy Sci. Eng. 2019, 7, 217–229. [Google Scholar] [CrossRef]
- NREL. Biomass Gasification Research Facilities. 2021. Available online: www.nrel.gov (accessed on 20 July 2025).
- Landrat, M.; Abawalo, M.; Pikoń, K.; Fufa, P.A.; Seyid, S. Assessing the Potential of Teff Husk for Biochar Production Through Slow Pyrolysis: Effect of Pyrolysis Temperature on Biochar Yield. Energies 2024, 17, 1988. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Dechlorination of Fuels in Pyrolysis of PVC Containing Plastic Wastes. Fuel Process. Technol. 2011, 92, 253–260. [Google Scholar] [CrossRef]
- Iribarren, D.; Susmozas, A.; Petrakopoulou, F.; Dufour, J. Environmental and Exergetic Evaluation of Hydrogen Production via Lignocellulosic Biomass Gasification. J. Clean. Prod. 2014, 69, 165–175. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Cheah, W.Y.; Kamaludin, N.H.; Tengku Ibrahim, T.N.B.; Sonne, C.; Peng, W.; Show, P.-L.; Lam, S.S. Progress in Waste Valorization Using Advanced Pyrolysis Techniques for Hydrogen and Gaseous Fuel Production. Bioresour. Technol. 2021, 320, 124299. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, M.; Zhu, Y.; Huhe, T.; Wang, Q.; Lei, T.; Zhou, Z.; Meng, X. Anaerobic Fermentation Integrated with Pyrolysis for Carbon Resource Recovery from Food Waste and Biogas Sludge: Effects of Inoculation Ratio and Pyrolysis Temperature. J. Environ. Manag. 2025, 379, 124879. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Song, Z.; Liu, H.; Li, L.; Ma, C. Microwave Pyrolysis of Straw Bale and Energy Balance Analysis. J. Anal. Appl. Pyrolysis 2011, 92, 43–49. [Google Scholar] [CrossRef]
- Demirbas, A. Waste Energy for Life Cycle Assessment; Green Energy and Technology; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-40550-6. [Google Scholar]
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Hydrogen-Rich Fuel Gas from Rice Straw via Microwave-Induced Pyrolysis. Bioresour. Technol. 2010, 101, 1968–1973. [Google Scholar] [CrossRef]
- Landrat, M.; Abawalo, M.T.; Pikoń, K.; Turczyn, R. Bio-Oil Derived from Teff Husk via Slow Pyrolysis Process in Fixed Bed Reactor and Its Characterization. Energies 2022, 15, 9605. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Barbarias, I.; Bilbao, J.; Olazar, M. Hydrogen Production from Biomass by Continuous Fast Pyrolysis and In-Line Steam Reforming. RSC Adv. 2016, 6, 25975–25985. [Google Scholar] [CrossRef]
- Anniwaer, A.; Chaihad, N.; Zhang, M.; Wang, C.; Yu, T.; Kasai, Y.; Abudula, A.; Guan, G. Hydrogen-Rich Gas Production from Steam Co-Gasification of Banana Peel with Agricultural Residues and Woody Biomass. Waste Manag. 2021, 125, 204–214. [Google Scholar] [CrossRef]
- Jerzak, W.; Acha, E.; Li, B. Comprehensive Review of Biomass Pyrolysis: Conventional and Advanced Technologies, Reactor Designs, Product Compositions and Yields, and Techno-Economic Analysis. Energies 2024, 17, 5082. [Google Scholar] [CrossRef]
- Holmen, A. Direct Conversion of Methane to Fuels and Chemicals. Catal. Today 2009, 142, 2–8. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Sci. 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Rahim Malik, F.; Yuan, H.-B.; Moran, J.C.; Tippayawong, N. Overview of Hydrogen Production Technologies for Fuel Cell Utilization. Eng. Sci. Technol. Int. J. 2023, 43, 101452. [Google Scholar] [CrossRef]
- ScienceDirect Topics. Partial Oxidation Gasification—An Overview. Available online: https://www.sciencedirect.com/topics/engineering/partial-oxidation-gasification (accessed on 2 June 2025).
- Aguilera, L.M.; Puig-Arnavat, M.; Ovtar, S.; Gurauskis, J.; Ahrenfeldt, J.; Henriksen, U.B.; Hendriksen, P.V.; Kiebach, R.; Haugen, A.B. Partial Oxidation of Biomass Gasification Tar with Oxygen Transport Membranes. J. Membr. Sci. 2023, 681, 121769. [Google Scholar] [CrossRef]
- Kong, L.; Li, G.; Zhang, B.; He, W.; Wang, H. Hydrogen Production from Biomass Wastes by Hydrothermal Gasification. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 1166–1178. [Google Scholar] [CrossRef]
- Jaafar, K.A. Biogas Production by Anaerobic Digestion of Date Palm Pulp Waste. Al-Khwarizmi Eng. J. 2010, 6, 14–20. [Google Scholar]
- Shanableh, A.; Radeef, W. Biogas Production from Raw and Oil-Spent Date Palm Seeds Mixed with Wastewater Treatment Sludge. Biofuels 2020, 11, 707–714. [Google Scholar] [CrossRef]
- Yong, Z.; Dong, Y.; Zhang, X.; Tan, T. Anaerobic Co-Digestion of Food Waste and Straw for Biogas Production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen Production from Renewable and Sustainable Energy Resources: Promising Green Energy Carrier for Clean Development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Qureshi, F.; Kamyab, H.; Rajendran, S.; Vo, D.-V.N.; Rajamohan, N.; Yusuf, M. Unveiling the Potentials of Biohydrogen as an Alternative Energy Source: Strategies, Challenges and Future Perspectives. Mater. Today Sustain. 2025, 31, 101133. [Google Scholar] [CrossRef]
- Mokhtarani, B.; Zanganeh, J.; Moghtaderi, B. A Review on Biohydrogen Production Through Dark Fermentation, Process Parameters and Simulation. Energies 2025, 18, 1092. [Google Scholar] [CrossRef]
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen Production Through Dark Fermentation: Recent Trends and Advances in Transition to a Circular Bioeconomy. Int. J. Hydrogen Energy 2024, 52, 335–357. [Google Scholar] [CrossRef]
- Koutra, E.; Kopsahelis, A.; Maltezou, M.; Grammatikopoulos, G.; Kornaros, M. Effect of Organic Carbon and Nutrient Supplementation on the Digestate-Grown Microalga, Parachlorella Kessleri. Bioresour. Technol. 2019, 294, 122232. [Google Scholar] [CrossRef]
- Castelló, E.; Nunes Ferraz-Junior, A.D.; Andreani, C.; Anzola-Rojas, M.D.P.; Borzacconi, L.; Buitrón, G.; Carrillo-Reyes, J.; Gomes, S.D.; Maintinguer, S.I.; Moreno-Andrade, I.; et al. Stability Problems in the Hydrogen Production by Dark Fermentation: Possible Causes and Solutions. Renew. Sustain. Energy Rev. 2020, 119, 109602. [Google Scholar] [CrossRef]
- Liu, Y.; Min, J.; Feng, X.; He, Y.; Liu, J.; Wang, Y.; He, J.; Do, H.; Sage, V.; Yang, G.; et al. A Review of Biohydrogen Productions from Lignocellulosic Precursor via Dark Fermentation: Perspective on Hydrolysate Composition and Electron-Equivalent Balance. Energies 2020, 13, 2451. [Google Scholar] [CrossRef]
- Dzulkarnain, E.L.N.; Audu, J.O.; Wan Dagang, W.R.Z.; Abdul-Wahab, M.F. Microbiomes of Biohydrogen Production from Dark Fermentation of Industrial Wastes: Current Trends, Advanced Tools and Future Outlook. Bioresour. Bioprocess. 2022, 9, 16. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the Biological Hydrogen Production Pathway of Dark Fermentation: Insight into the Impact of Strain Improvement. Microb. Cell Factories 2022, 21, 166. [Google Scholar] [CrossRef]
- Karaosmanoglu Gorgec, F.; Karapinar, I. Biohydrogen Production from Hydrolyzed Waste Wheat by Dark Fermentation in a Continuously Operated Packed Bed Reactor: The Effect of Hydraulic Retention Time. Int. J. Hydrogen Energy 2019, 44, 136–143. [Google Scholar] [CrossRef]
- Dessì, P.; Porca, E.; Lakaniemi, A.-M.; Collins, G.; Lens, P.N.L. Temperature Control as Key Factor for Optimal Biohydrogen Production from Thermomechanical Pulping Wastewater. Biochem. Eng. J. 2018, 137, 214–221. [Google Scholar] [CrossRef]
- Paillet, F.; Barrau, C.; Escudié, R.; Bernet, N.; Trably, E. Robust Operation Through Effluent Recycling for Hydrogen Production from the Organic Fraction of Municipal Solid Waste. Bioresour. Technol. 2021, 319, 124196. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Konopacka-Łyskawa, D.; Słupek, E.; Makoś, P.; Cieśliński, H.; Kamiński, M. Influence of Alkaline and Oxidative Pre-Treatment of Waste Corn Cobs on Biohydrogen Generation Efficiency via Dark Fermentation. Biomass Bioenergy 2020, 141, 105691. [Google Scholar] [CrossRef]
- Balachandar, G.; Varanasi, J.L.; Singh, V.; Singh, H.; Das, D. Biological Hydrogen Production via Dark Fermentation: A Holistic Approach from Lab-Scale to Pilot-Scale. Int. J. Hydrogen Energy 2020, 45, 5202–5215. [Google Scholar] [CrossRef]
- Teke, G.M.; Anye Cho, B.; Bosman, C.E.; Mapholi, Z.; Zhang, D.; Pott, R.W.M. Towards Industrial Biological Hydrogen Production: A Review. World J. Microbiol. Biotechnol. 2023, 40, 37. [Google Scholar] [CrossRef] [PubMed]
- Hitam, C.N.C.; Jalil, A.A. A Review on Biohydrogen Production Through Photo-Fermentation of Lignocellulosic Biomass. Biomass Conv. Bioref. 2023, 13, 8465–8483. [Google Scholar] [CrossRef]
- Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Photo-Fermentative Bacteria Used for Hydrogen Production. Appl. Sci. 2024, 14, 1191. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Zularisam, A.W. Outlook of Fermentative Hydrogen Production Techniques: An Overview of Dark, Photo and Integrated Dark-Photo Fermentative Approach to Biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Boran, E.; Ozgur, E.; Yucel, M.; Gündüz, U.; Eroğlu, İ. Biohydrogen production by Rhodobacter capsulatus Hup(-) mutant in pilot solar tubular photobioreactor. Int. J. Hydrogen Energy 2012, 37, 16437–16445. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L.; Sun, L.; Baeyens, J. Sequential Fermentation of Food Waste in an Integrated System to Improve N-Caproate Production. J. Clean. Prod. 2021, 313, 127771. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Ge, X.; Fu, X.; Dang, C.; Wang, J.; Liu, Y. Sequential fermentation with indigenous non-Saccharomyces yeasts and Saccharomyces cerevisiae for flavor and quality enhancement of Longyan dry white wine. Food Biosci. 2023, 55, 102952. [Google Scholar] [CrossRef]
- Niño-Navarro, C.; Chairez, I.; Christen, P.; Canul-Chan, M.; García-Peña, E.I. Enhanced Hydrogen Production by a Sequential Dark and Photo Fermentation Process: Effects of Initial Feedstock Composition, Dilution and Microbial Population. Renew. Energy 2020, 147, 924–936. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yang, M.-H.; Yeh, K.-L.; Liu, C.-H.; Chang, J.-S. Biohydrogen Production Using Sequential Two-Stage Dark and Photo Fermentation Processes. Int. J. Hydrogen Energy 2008, 33, 4755–4762. [Google Scholar] [CrossRef]
- Rashid, N.; Choi, W.; Lee, K. Optimization of Two-Staged Bio-Hydrogen Production by Immobilized Microcystis Aeruginosa. Biomass Bioenergy 2012, 36, 241–249. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Chen, S.-D.; Chen, C.-Y.; Huang, T.-I.; Lin, C.-Y.; Chang, J.-S. Combining enzymatic hydrolysis and dark–photo fermentation processes for hydrogen production from starch feedstock: A feasibility study. Int. J. Hydrogen Energy 2008, 33, 5224–5233. [Google Scholar] [CrossRef]
- Sikarwar, D.; Das, I.; Ganta, A.; Nambi, I.M.; Erable, B.; Das, S. Microbial Electrolysis Cells: Fuelling the Future with Biohydrogen—A Review. Sustain. Chem. Environ. 2025, 9, 100224. [Google Scholar] [CrossRef]
- Kundu, A.; Sahu, J.N.; Redzwan, G.; Hashim, M.A. An Overview of Cathode Material and Catalysts Suitable for Generating Hydrogen in Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2013, 38, 1745–1757. [Google Scholar] [CrossRef]
- Cho, S.-K.; Lee, M.-E.; Lee, W.; Ahn, Y. Improved Hydrogen Recovery in Microbial Electrolysis Cells Using Intermittent Energy Input. Int. J. Hydrogen Energy 2019, 44, 2253–2257. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A Comprehensive Review of Microbial Electrolysis Cells (MEC) Reactor Designs and Configurations for Sustainable Hydrogen Gas Production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef]
- Dange, P.; Pandit, S.; Jadhav, D.; Shanmugam, P.; Gupta, P.K.; Kumar, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K. Recent Developments in Microbial Electrolysis Cell-Based Biohydrogen Production Utilizing Wastewater as a Feedstock. Sustainability 2021, 13, 8796. [Google Scholar] [CrossRef]
- Shen, R.; Jiang, Y.; Ge, Z.; Lu, J.; Zhang, Y.; Liu, Z.; Ren, Z.J. Microbial Electrolysis Treatment of Post-Hydrothermal Liquefaction Wastewater with Hydrogen Generation. Appl. Energy 2018, 212, 509–515. [Google Scholar] [CrossRef]
- Joshi, N.C.; Gururani, P.; Talniya, N.C. An updated review on biomass-based hydrogen production. Biointerface Res. Appl. Chem. 2024, 14, 98. [Google Scholar] [CrossRef]
- Sharma, R.K.; Prabhakar, R.; Kumar, A.; Saha, M.; Bhaduri, S. Advanced processes of biohydrogen generation from lignocellulosic biomass: Review of thermochemical conversion. Energies 2023, 16, 6349. [Google Scholar] [CrossRef]
- Alvarado-Flores, J.J.; Alcaraz-Vera, J.V.; Ávalos-Rodríguez, M.L.; Guzmán-Mejía, E.; Rutiaga-Quiñones, J.G.; Pintor-Ibarra, L.F.; Guevara-Martínez, S.J. Thermochemical production of hydrogen from biomass: Pyrolysis and gasification pathways. Energies 2024, 17, 537. [Google Scholar] [CrossRef]
- Alptekin, F.M.; Celiktas, M.S. Review on catalytic biomass gasification for hydrogen production. ACS Omega 2022, 7, 24918–24941. [Google Scholar] [CrossRef]
- Zayer Kabeh, K.; Prussi, M.; Chiaramonti, D. Advances in Bio-Hydrogen Production: A Critical Review of Pyrolysis Gas Reforming. Appl. Sci. 2025, 15, 3995. [Google Scholar] [CrossRef]
- Osman, A.I.; Deka, T.J.; Baruah, D.C.; Rooney, D.W. Critical challenges in biohydrogen production: Dark fermentation, photofermentation and gasification from organic wastes. Biomass Conv. Bioref. 2023, 13, 123–145. [Google Scholar] [CrossRef]
- Albuquerque, M.M.; Sartor, G.d.B.; Martinez-Burgos, W.J.; Scapini, T.; Edwiges, T.; Soccol, C.R.; Medeiros, A.B.P. Biohydrogen Produced via Dark Fermentation: A Review. Methane 2024, 3, 500–532. [Google Scholar] [CrossRef]
- Galih Pangestu, M.R.; Razzak, S.A.; Uddin, S. Microbial Biomass Conversion for Hydrogen Production: A Review. Green Energy Resour. 2025, 3, 100131. [Google Scholar] [CrossRef]
- World Energy Outlook 2023–Analysis. Available online: https://www.iea.org/reports/world-energy-outlook-2023 (accessed on 22 June 2025).
- U.S. Department of Energy Hydrogen Program: 2022 Annual Merit Review and Peer Evaluation Report; 6–8 June 2022; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2023. Available online: https://www.osti.gov/biblio/1990033 (accessed on 17 May 2025).
- Bridgwater, A.V. Upgrading Biomass Fast Pyrolysis Liquids. Env Prog and Sustain Energy 2012, 31, 261–268. [Google Scholar] [CrossRef]
- Hamid, A.; Deris, R.R.R.; Shaffee, S.N.A.; Hin, T.Y.Y.; Ametefe, D.S.; Ibrahim, M.L. A Systematic Review on Environmentally Friendly Hydrogen Production Methods: Comparative Analysis of Reactor Technologies for Optimal Efficiency and Sustainability. Sustain. Chem. Clim. Action 2025, 6, 100088. [Google Scholar] [CrossRef]
- Global Hydrogen Review 2021—Analysis. Available online: https://www.iea.org/reports/global-hydrogen-review-2021 (accessed on 19 June 2025).
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life Cycle Assessment of Hydrogen Production via Electrolysis—A Review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Hajjaji, N.; Pons, M.-N.; Renaudin, V.; Houas, A. Comparative Life Cycle Assessment of Eight Alternatives for Hydrogen Production from Renewable and Fossil Feedstock. J. Clean. Prod. 2013, 44, 177–189. [Google Scholar] [CrossRef]
- Cetinkaya, E.; Dincer, I.; Naterer, G.F. Life Cycle Assessment of Various Hydrogen Production Methods. Int. J. Hydrogen Energy 2012, 37, 2071–2080. [Google Scholar] [CrossRef]
- Lee, G.N.; Kim, J.M.; Jung, K.H.; Park, H. Comparative Life Cycle Assessment of Various Hydrogen Supply Methods from Australia to the Republic of Korea in Environmental and Economic Aspects. Sci. Total Environ. 2024, 947, 174669. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative Assessment of Hydrogen Production Methods from Renewable and Non-Renewable Sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An Overview of Water Electrolysis Technologies for Green Hydrogen Production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Yagmur Goren, A.; Dincer, I.; Khalvati, A. A Comprehensive Review on Environmental and Economic Impacts of Hydrogen Production from Traditional and Cleaner Resources. J. Environ. Chem. Eng. 2023, 11, 111187. [Google Scholar] [CrossRef]
- Bensaid, S.; Ruggeri, B.; Saracco, G. Development of a Photosynthetic Microbial Electrochemical Cell (PMEC) Reactor Coupled with Dark Fermentation of Organic Wastes: Medium Term Perspectives. Energies 2015, 8, 399–429. [Google Scholar] [CrossRef]
- Munir, M.T.; Mardon, I.; Al-Zuhair, S.; Shawabkeh, A.; Saqib, N.U. Plasma Gasification of Municipal Solid Waste for Waste-to-Value Processing. Renew. Sustain. Energy Rev. 2019, 116, 109461. [Google Scholar] [CrossRef]
- Collet, P.; Flottes, E.; Favre, A.; Raynal, L.; Pierre, H.; Capela, S.; Peregrina, C. Techno-Economic and Life Cycle Assessment of Methane Production via Biogas Upgrading and Power to Gas Technology. Appl. Energy 2017, 192, 282–295. [Google Scholar] [CrossRef]
- Karaca, A.E.; Dincer, I. Development of a New Photoelectrochemical System for Clean Hydrogen Production and a Comparative Environmental Impact Assessment with Other Production Methods. Chemosphere 2023, 337, 139367. [Google Scholar] [CrossRef] [PubMed]
- Anand, C.; Chandraja, B.; Nithiya, P.; Akshaya, M.; Tamizhdurai, P.; Shoba, G.; Subramani, A.; Kumaran, R.; Yadav, K.K.; Gacem, A.; et al. Green Hydrogen for a Sustainable Future: A Review of Production Methods, Innovations, and Applications. Int. J. Hydrogen Energy 2025, 111, 319–341. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Shokrollahi, M.; Teymouri, N.; Ashrafi, O.; Navarri, P.; Khojasteh-Salkuyeh, Y. Methane Pyrolysis as a Potential Game Changer for Hydrogen Economy: Techno-Economic Assessment and GHG Emissions. Int. J. Hydrogen Energy 2024, 66, 337–353. [Google Scholar] [CrossRef]
- Jin, C.; Han, M.; Wu, Y.; Wang, S. Solar-Driven Photoelectrochemical Conversion of Biomass: Recent Progress, Mechanistic Insights and Potential Scalability. Energy Environ. Sci. 2024, 17, 7459–7511. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lin, C.Y.; Chang, J.S. Biohydrogen Production: Current Perspectives and the Way Forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Zhou, Y.; Remón, J.; Pang, X.; Jiang, Z.; Liu, H.; Ding, W. Hydrothermal Conversion of Biomass to Fuels, Chemicals and Materials: A Review Holistically Connecting Product Properties and Marketable Applications. Sci. Total Environ. 2023, 886, 163920. [Google Scholar] [CrossRef]
- Arena, U. Process and Technological Aspects of Municipal Solid Waste Gasification. A Review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial Electrolysis Cells for High Yield Hydrogen Gas Production from Organic Matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef]
- Techno-Economic Evaluation of SMR-Based Standalone (Merchant) Hydrogen Plant with CCS. IEAGHG. Available online: https://publications.ieaghg.org/technicalreports/2017-02%20Techno%20-%20Economic%20Evaluation%20of%20SMR%20Based%20Standalone%20(Merchant)%20Hydrogen%20Plant%20with%20CCS.pdf (accessed on 7 June 2025).
- Naeem, K.; Zghibi, A.; Elomri, A.; Mazzoni, A.; Triki, C. A Literature Review on System Dynamics Modeling for Sustainable Management of Water Supply and Demand. Sustainability 2023, 15, 6826. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Liang, M.; Karthick, R.; Wei, Q.; Dai, J.; Jiang, Z.; Chen, X.; Oo, T.Z.; Aung, S.H.; Chen, F. The Progress and Prospect of the Solar-Driven Photoelectrochemical Desalination. Renew. Sustain. Energy Rev. 2022, 155, 111864. [Google Scholar] [CrossRef]
- Giwa, S.; Xu, Y.; Wang, M. Characterization of VOCs during biomass pyrolysis: A review. Renew. Sustain. Energy Rev. 2022, 161, 112404. [Google Scholar]
- Shen, Y.; Yoshikawa, K.; Zhang, Y. Characteristics of syngas and tar formation during pyrolysis and gasification of biomass with different feedstocks. Energy 2015, 89, 667–675. [Google Scholar]
- Piskorz, J.; Scott, D.S.; Radlein, D.S. Production of chlorinated organics in biomass pyrolysis. Ind. Eng. Chem. Process Des. Dev. 1986, 25, 265–270. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.; Williams, P.T. Formation of chlorinated hydrocarbons during pyrolysis of plastic waste containing PVC. Waste Manag. 2019, 98, 119–126. [Google Scholar]
- Rahman, I.U.; Mohammed, H.J.; Bamasag, A. An Exploration of Recent Waste-to-Energy Advancements for Optimal Solid Waste Management. Discov. Chem. Eng. 2025, 5, 7. [Google Scholar] [CrossRef]
- The Future of Hydrogen: Seizing Today’s Opportunities—Event. Available online: https://www.iea.org/events/the-future-of-hydrogen-seizing-todays-opportunities (accessed on 22 June 2025).
- Global Hydrogen Review 2022. Available online: https://iea.blob.core.windows.net/assets/c5bc75b1-9e4d-460d-9056-6e8e626a11c4/GlobalHydrogenReview2022.pdf (accessed on 25 July 2025).
- IEA. The Future of Hydrogen. International Energy Agency. 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 17 July 2025).
- Hydrogen Council. Hydrogen Insights Report 2023. Available online: https://hydrogencouncil.com/en/hydrogen-insights-2023/ (accessed on 22 July 2025).
- Hallenbeck, P.C.; Ghosh, D. Advances in fermentative biohydrogen production. Biotechnol. J. 2009, 4, 431–447. [Google Scholar]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Techno-economic assessment of hydrogen production technologies. Renew. Sustain. Energy Rev. 2014, 29, 804–824. [Google Scholar]
- U.S. Department of Energy (DOE). Hydrogen Production Cost from Coal Gasification. Office of Fossil Energy. Available online: https://www.energy.gov (accessed on 27 April 2025).
- BloombergNEF (BNEF). Electrolyzer Market Outlook 2023. Available online: https://about.bnef.com (accessed on 25 July 2025).
- International Renewable Energy Agency (IRENA). Green Hydrogen Cost Reduction: Scaling Up Electrolysers to Meet the 1.5 °C Climate Goal; IRENA: Abu Dhabi, United Arab Emirates, 2020; Available online: https://www.irena.org/publications/2020/Dec/Green-hydrogen-cost-reduction (accessed on 12 July 2025).
- Al-Mufachi, N.A.; Shah, N. The Role of Hydrogen and Fuel Cell Technology in Providing Security for the UK Energy System. Energy Policy 2022, 171, 113286. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- E4tech. Fuel Cell Industry Review 2022. Available online: https://www.e4tech.com (accessed on 25 July 2025).
- Junne, S.; Kabbe, C.; Eder, T.; Wierckx, N.; Blank, L.M. Biohydrogen production from biomass gasification using microbial electrochemical technologies. Bioresour. Technol. 2019, 279, 393–403. [Google Scholar]
- Balat, M. Potential of biomass gasification for hydrogen production in Turkey. Energy Sources Part A 2008, 30, 1436–1446. [Google Scholar]
- Zhou, W.; Li, X.; Gong, X.; Wang, Z.; Yu, G. Hydrogen production from biomass pyrolysis: A review. Int. J. Hydrogren Energy 2021, 46, 18544–18561. [Google Scholar]
- Li, J.; Zhang, J.; Zhang, J.; Yu, J. Photoelectrochemical hydrogen production: Materials and system design. Energy Environ. Sci. 2019, 12, 1369–1392. [Google Scholar]
- Das, D.; Veziroglu, T.N. Fermentative hydrogen production. Int. J. Hydrogren Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Fabry, F.; Rehmet, C.; Rohani, V.; Fulcheri, L. Waste Gasification by Thermal Plasma: A Review. Waste Biomass Valor 2013, 4, 421–439. [Google Scholar] [CrossRef]
- Kruse, A.; Spieler, F.; Petschacher, U.; Steinhardt, J.; Seifert, H.; Wüst, D.; Heidelmann, M. Hydrothermal gasification of biomass and organic waste: A review of challenges and opportunities. J. Supercrit. Fluids 2020, 158, 104699. [Google Scholar]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Liu, G.; Logan, B.E. Hydrogen production with a microbial electrolysis cell using fermentation effluent. Environ. Sci. Technol. 2011, 45, 4380–4386. [Google Scholar]
- International Energy Agency (IEA). The Future of Hydrogen; IEA: Paris, France, 2021. [Google Scholar]
- International Renewable Energy Agency (IRENA). Green Hydrogen Supply: A Guide to Policy Making. Available online: https://h2chile.cl/wp-content/uploads/2024/02/IRENA_Green_Hydrogen_Supply_2021.pdf (accessed on 3 June 2025).
- International Renewable Energy Agency (IRENA). Global trade in green hydrogen derivatives: Trends in regulation, standardisation and certification. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2024/Oct/IRENA_Green_hydrogen_derivatives_trade_2024.pdf (accessed on 17 April 2025).
- Buchner, J.; Menrad, K.; Decker, T. Public Acceptance of Green Hydrogen Production in Germany. Renew. Sustain. Energy Rev. 2025, 208, 115057. [Google Scholar] [CrossRef]
- Bundesministerium für Wirtschaft und Klimaschutz (BMWK). Update of the National Hydrogen Strategy; BMWK: Berlin, Germany, 2023; Available online: https://usa-germany-cep.org/fileadmin/usa/Documents/adelphi_2023_Factsheet_Germany_s_updated_National_Hydrogen_Strategy__July_2023_.pdf (accessed on 22 March 2025).
- Government of Canada. Hydrogen Strategy for Canada; Natural Resources Canada: Ottawa, ON, Canada, 2020; Available online: https://natural-resources.canada.ca/sites/nrcan/files/environment/hydrogen/NRCan_Hydrogen-Strategy-Canada-na-en-v3.pdf (accessed on 25 May 2025).
- Government of Chile. National Green Hydrogen Strategy; Ministry of Energy: Santiago, Chile, 2022. Available online: https://energia.gob.cl/sites/default/files/national_green_hydrogen_strategy_-_chile.pdf (accessed on 22 July 2025).
- U.S. Department of Energy. National Clean Hydrogen Strategy and Roadmap; DOE: Washington, DC, USA, 2023. Available online: https://www.hydrogen.energy.gov/pdfs/clean-hydrogen-strategy-roadmap.pdf (accessed on 23 July 2025).
- European Commission. Directive (EU) 2023/2413 of the European Parliament and of the Council on the Promotion of the Use of Energy from Renewable Sources (RED III); European Commission: Brussels, Belgium, 2023; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32023L2413 (accessed on 24 July 2025).
- “Sectoral Agreement for the Development of the Hydrogen Economy in Poland” Signed—Ministry of Climate and Environment—Gov.Pl Website. Available online: https://www.gov.pl/web/climate/sectoral-agreement-for-the-development-of-the-hydrogen-economy-in-poland-signed (accessed on 13 February 2025).
- Guerra, O.J.; Eichman, J.; Kurtz, J.; Hodge, B.-M. Cost Competitiveness of Electrolytic Hydrogen. Joule 2019, 3, 2425–2443. [Google Scholar] [CrossRef]









| Scenario/Study | Reference Year | Food Waste Volume (Million Tonnes/Year) | H2 Yield (m3 H2/Tonne) | Total H2 Potential (Million Tonnes H2/Year) | Source/Notes |
|---|---|---|---|---|---|
| Baseline | 2019 | ~931 | 80 (~7.2 kg) | ~6.7 | [15,18,19] |
| Projection—moderate growth | 2030 | ~1200 | 80 | ~8.6 | [16,19,20] |
| Business as usual (BAU) | 2050 | ~2100 | 80 | ~15.1 | [16,19] |
| 2050 (25% Waste-to-H2 recovery) | 2050 | ~525 | 80 | ~3.7 | Assumes partial diversion to hydrogen recovery [16,18,19] |
| 2050 (50% Waste-to-H2 recovery | 2050 | ~1050 | 80 | ~7.5 | Feasible with aggressive circular bioeconomy interventions [19] |
| 2050—Optimized yield scenario | 2050 | ~2100 | 100 (optimized) | ~18.9 | Assumes optimized process conditions for higher yield [15,19] |
| Criteria | Gasification | Pyrolysis | POX | Hydrothermal Gasification | Dark Fermentation | Photofermentation | MECs |
|---|---|---|---|---|---|---|---|
| Feedstock Moisture Tolerance | Low–Moderate | Low | Moderate | High | High | Moderate | High |
| Operating Temperature (°C) | 800–1400 | 40–900 | 1000–1500 | 250–700 | 30–70 | 25–40 | Ambient |
| Hydrogen Yield (mol H2/mol) | 5–10 | 2–6 | 2–6 | 8–12 | 2–4 | 4–12 | 2–4 |
| Scalability/ Maturity | High | Medium | Medium | Low–Medium | Low–Medium | Low | Low |
| Energy Efficiency | Moderate–High | Moderate | Moderate | Moderate | Low–Moderate | Low | Moderate |
| Byproducts | CO, Tar, and CO2 | Char and Bio-oil | CO, H2O, and CO2 | CO2 and Salts | VFAs and CO2 | CO2 | Minimal Organics |
| Environmental Benefits | High (with CCS) | Moderate | Moderate | High | High | High (Solar-based) | High |
| Capital Cost | High | Medium | High | High | Low | Medium | Medium–High |
| Technology Complexity | High | Medium | High | Very High | Low | Medium | High |
| Suitability for Food Waste | Limited (Drying Needed) | Moderate | Moderate | Excellent | Excellent | Moderate | Good |
| Technology | CapEx (USD/kW) | OpEx (USD/kg H2) | LCOH (USD/kg H2) | Technology Status/Notes |
|---|---|---|---|---|
| SMR | 900–1200 | 0.8–1.2 | 1.0–2.0 | Mature, cost-effective, and high GHG emissions unless combined with CCS |
| Coal Gasification | 1300–1800 | 1.2–1.8 | 1.5–2.5 | High emissions and water use; feasible in coal-rich regions with CCS |
| Electrolysis (Grid) | 1500–2000 | 2.5–3.5 | 4.0–6.0 | Dependent on the grid mix; high operating costs |
| Electrolysis (Renewable) | 1800–2200 | 2.0–3.0 | 3.5–5.0 | Cleanest option; increasingly competitive as renewable costs fall |
| Biomass Gasification | 1800–2500 | 1.5–2.2 | 2.5–4.0 | Carbon-neutral potential; scalable |
| Pyrolysis | 2000–2500 | 1.8–2.5 | 3.0–4.5 | Produces solid carbon; still in early deployment |
| PEC | 3500–4500 | 2.5–3.5 | 6.0–8.0 | Experimental solar-driven technology; scalability issues |
| Dark Fermentation | 2500–3500 | 2.2–3.0 | 4.5–6.5 | Biowaste conversion: low yields and limited scalability |
| Photofermentation | 3000–4000 | 2.5–3.5 | 6.0–8.0 | Light-driven microbes; low efficiency |
| Biophotolysis | 3200–4500 | 3.0–4.0 | 7.0–9.0 | Early-stage alga-based process; low productivity |
| Plasma Gasification | 3000–3800 | 2.5–3.5 | 5.5–7.0 | High energy demand; useful for plastic and waste conversion |
| Hydrothermal Gasification | 2000–3000 | 2.0–2.8 | 4.0–6.0 | Ideal for wet biomass; catalyst and efficiency R&D are ongoing |
| Pathway | Prospect | Challenges | Future Research Directions |
|---|---|---|---|
| Biomass Gasification | Technological | Tar formation, catalyst deactivation, and feedstock variability | Low-temperature gasification, tar-resistant catalysts, and carbon capture integration |
| Environmental | GHG emissions, toxic byproducts, and ash handling | Lifecycle assessment (LCA), clean gas handling, and ash utilization | |
| Methane Pyrolysis | Technological | High energy demand, material degradation, and reactor design complexity | Renewable heat sources, robust reactors, and biogenic methane use |
| Environmental | Fossil methane dependency and thermal emissions | Biogas feedstock and green electricity input | |
| Hydrothermal Gasification | Technological | Corrosion, salt deposition, and energy efficiency | Anti-corrosion materials and energy recovery systems |
| Environmental | Effluent treatment and CO2 emissions | Closed-loop water reuse and process intensification | |
| Plasma Gasification | Technological | Expensive operation and plasma torch durability | Scalable systems, cost reduction, and decentralized design |
| Environmental | Emission control and slag disposal | Advanced gas cleaning and vitrified residue applications | |
| Dark Fermentation | Technological | Low H2 yield, microbial instability, and byproduct inhibition | Microbial engineering, hybrid fermentation, and pretreatment of food waste |
| Environmental | Acidic effluents and methane co-production | pH control systems and optimized reactor management | |
| Photofermentation | Technological | Low light efficiency and large surface area needed | High-efficiency photobioreactors and improved photosynthetic organisms |
| Environmental | Light source requirement and oxygen sensitivity | Use of solar-driven systems and oxygen-tolerant strains | |
| Sequential Fermentation | Technological | Synchronizing processes and intermediate management | Integrated bioreactors and kinetic modeling |
| Environmental | Complex resource use | Combined LCA and water–energy balance optimization | |
| Extraction | Technological | Low hydrogen recovery efficiency and solvent issues | Green solvent systems and integrated recovery |
| Environmental | Chemical waste and energy use | Sustainable extraction protocols | |
| MEC (Electrolysis) | Technological | High capital cost, membrane fouling, and low current density | Durable electrodes, anti-fouling strategies, and renewable voltage integration |
| Environmental | Energy demand and methane leakage | Solar/wind integration and methanogen suppression | |
| Biophotolysis | Technological | Low H2 rates, O2 inhibition, and enzyme degradation | Engineered algae/cyanobacteria and protective photoreactor designs |
| Environmental | Land and water use and fragile ecosystems | Compact bioreactor design and eco-friendly media | |
| SMR (Steam Reforming) | Technological | CO2-intensive and fossil dependency | CCS integration, biogas-based SMR, and reforming catalyst innovation |
| Environmental | High GHG emissions and natural gas reliance | Low-carbon feedstock reforming | |
| Transesterification | Technological | Limited direct H2 production and waste glycerol | Integrated H2–glycerol valorization and enzyme catalysts |
| Environmental | Chemical waste from catalysts | Eco-friendly biocatalysis and biorefinery integration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abawalo, M.; Pikoń, K.; Landrat, M.; Ścierski, W. Hydrogen Production from Biowaste: A Systematic Review of Conversion Technologies, Environmental Impacts, and Future Perspectives. Energies 2025, 18, 4520. https://doi.org/10.3390/en18174520
Abawalo M, Pikoń K, Landrat M, Ścierski W. Hydrogen Production from Biowaste: A Systematic Review of Conversion Technologies, Environmental Impacts, and Future Perspectives. Energies. 2025; 18(17):4520. https://doi.org/10.3390/en18174520
Chicago/Turabian StyleAbawalo, Mamo, Krzysztof Pikoń, Marcin Landrat, and Waldemar Ścierski. 2025. "Hydrogen Production from Biowaste: A Systematic Review of Conversion Technologies, Environmental Impacts, and Future Perspectives" Energies 18, no. 17: 4520. https://doi.org/10.3390/en18174520
APA StyleAbawalo, M., Pikoń, K., Landrat, M., & Ścierski, W. (2025). Hydrogen Production from Biowaste: A Systematic Review of Conversion Technologies, Environmental Impacts, and Future Perspectives. Energies, 18(17), 4520. https://doi.org/10.3390/en18174520






