Environmental Performance of the Sewage Sludge Gasification Process Considering the Recovered CO2
Abstract
1. Introduction
2. Materials and Methods
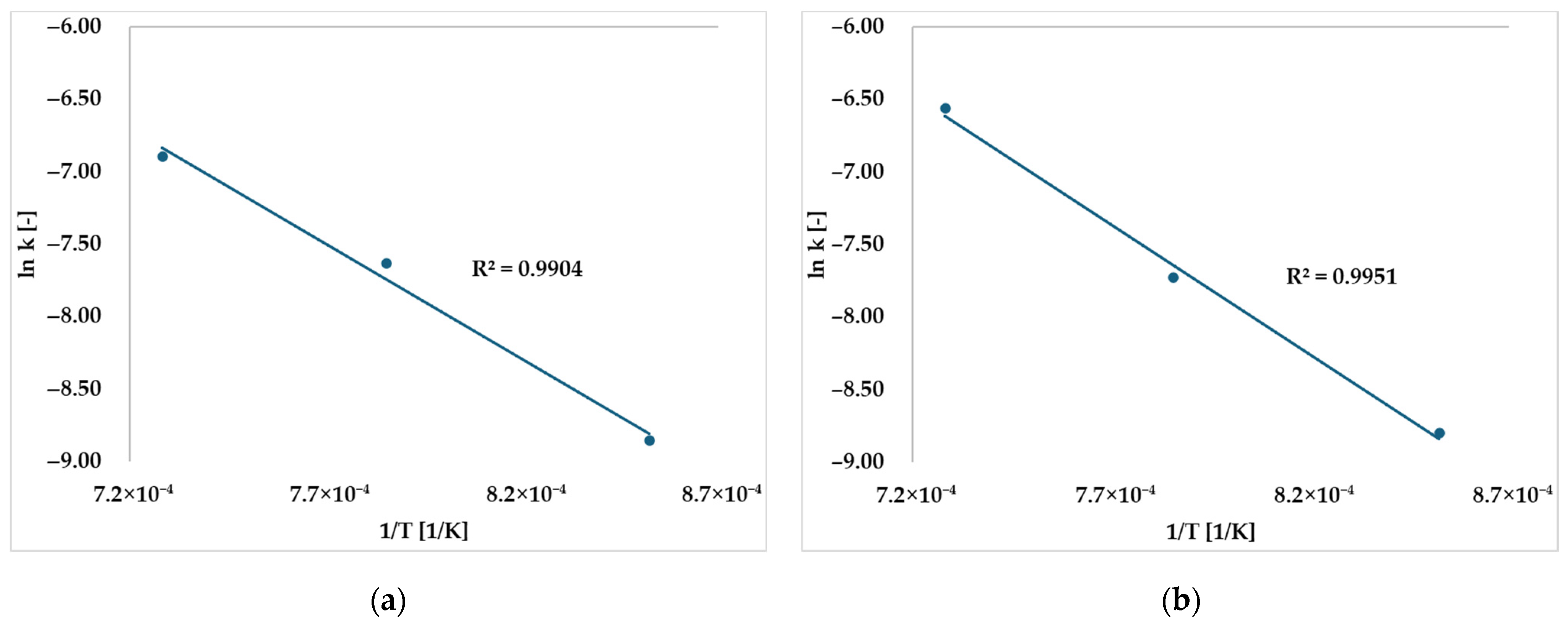
2.1. Preliminary Experiments: Estimation of Gasification Rate Equation for Sewage Sludge Tar
2.2. Design of Process Simulation
2.3. LCA
2.4. Evaluation Method
3. Results
3.1. Sensitivity Analysis Results
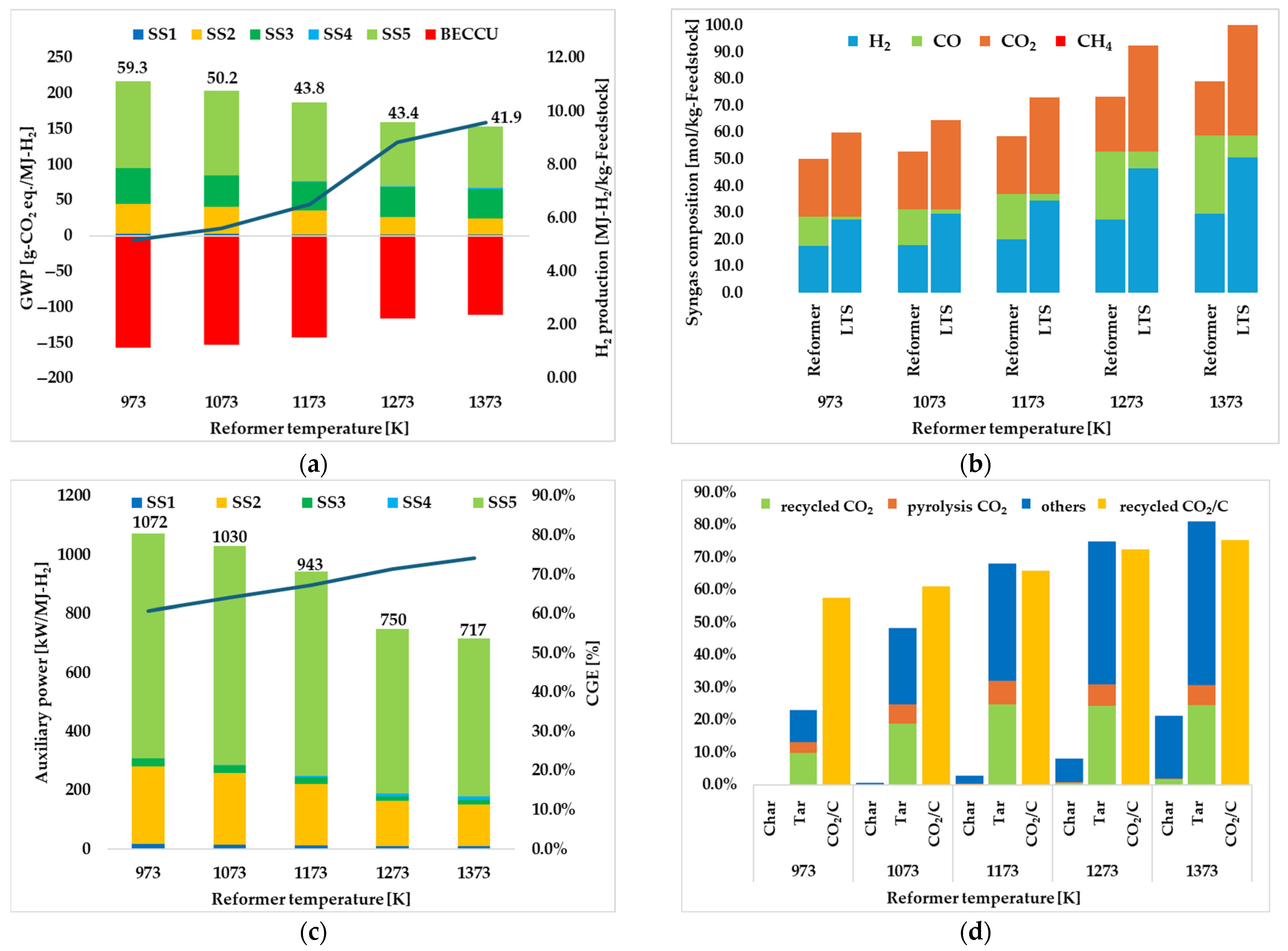
3.1.1. Effect of Reformer Temperature
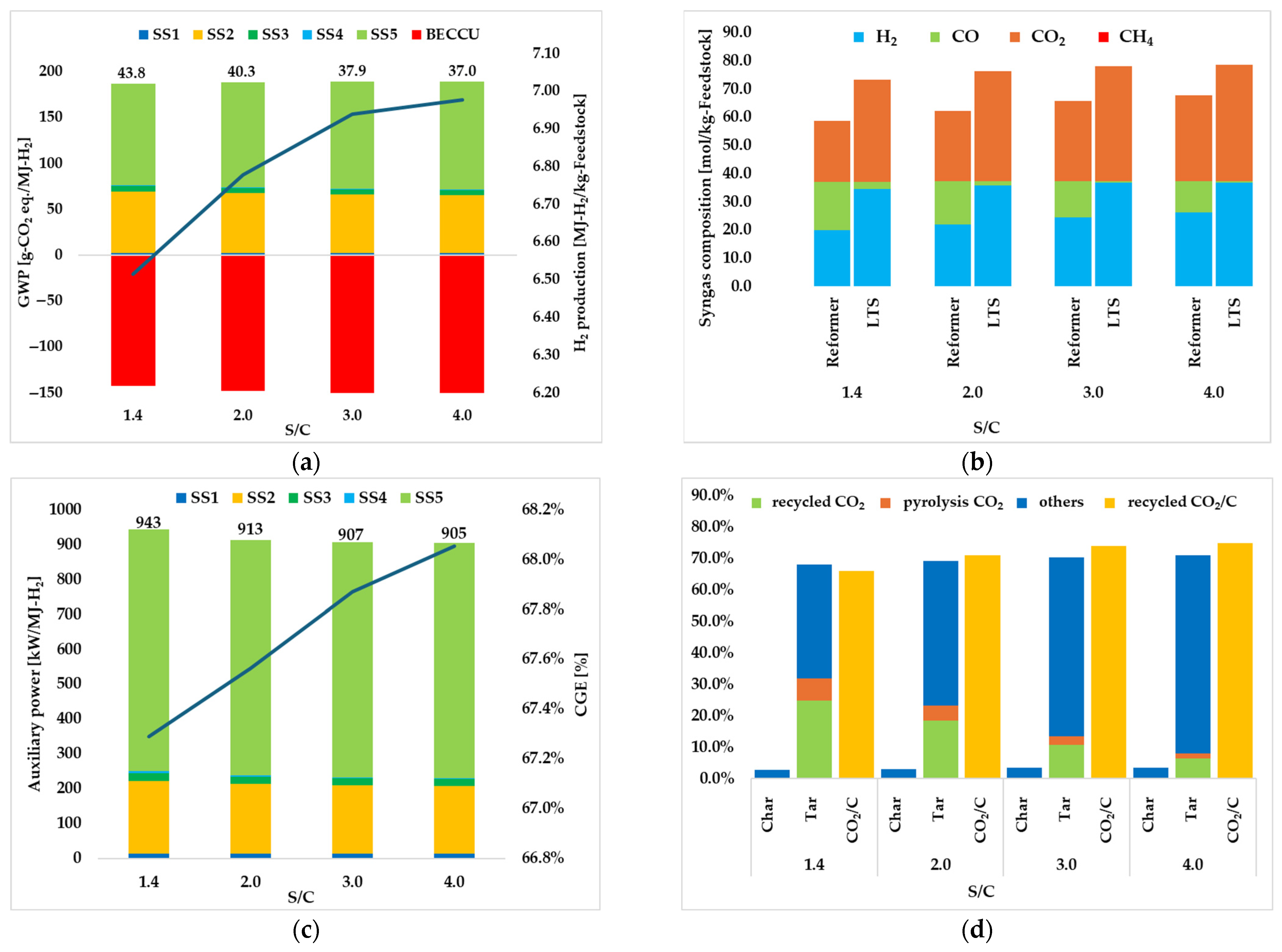
3.1.2. Effect of S/C
3.2. Effectiveness of 2-Step PSA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Schematic of the Process and Stream Data Table
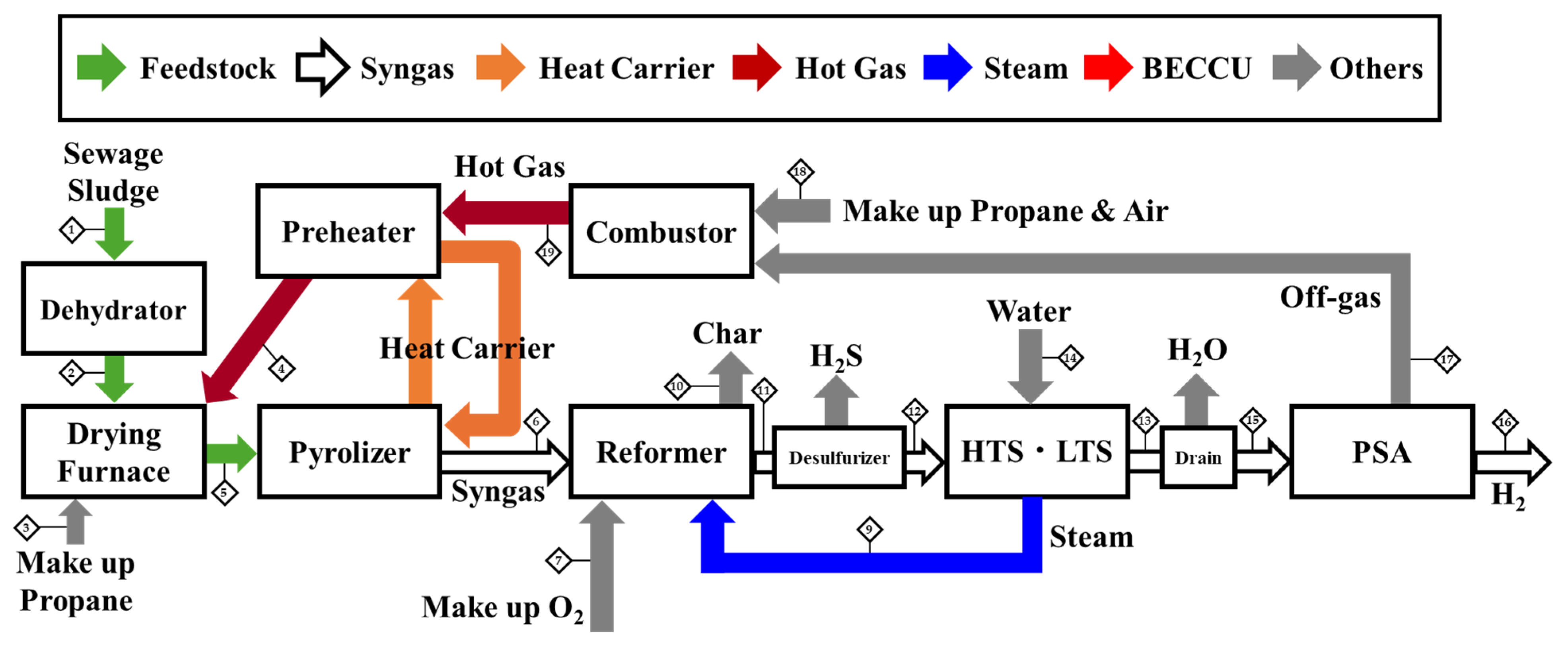
| Case 1 | Units | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure | kPa | 101.3 | 101.3 | 101.3 | 104.0 | 106.0 | 106.0 | 106.0 | - | 106.0 | 106.0 | 106.0 | 105.0 | 102.0 | 109.0 | 101.0 | 101.0 | 106.0 | 106.0 | 106.0 |
| Temperature | K | 298.2 | 298.2 | 298.2 | 423.2 | 298.2 | 957.7 | 303.6 | - | 423.2 | 1173.2 | 1173.2 | 623.2 | 630.1 | 303.2 | 303.2 | 313.2 | 315.1 | 298.2 | 1273.2 |
| Mass Flows | kg/s | 0.1995 | 0.0349 | 0.0000 | 0.3318 | 0.0083 | 0.0074 | 0.0002 | - | 0.0054 | 0.0009 | 0.0122 | 0.0187 | 0.0187 | 0.0054 | 0.0145 | 0.0004 | 0.0073 | 0.1716 | 0.1789 |
| Mole Flows | mol/s | 11.5739 | 2.4367 | 0.0000 | 11.4559 | 0.9853 | 0.3411 | 0.0092 | - | 0.2995 | 0.0742 | 0.7053 | 0.7014 | 0.7014 | 0.2995 | 0.4780 | 0.2203 | 0.2577 | 5.9352 | 6.1748 |
| C | mol/s | 0.2674 | 0.2674 | 0.0000 | 0.0000 | 0.2674 | 0.0000 | 0.0000 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H | mol/s | 0.4711 | 0.4711 | 0.0000 | 0.0000 | 0.4711 | 0.0000 | 0.0000 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| N | mol/s | 0.0241 | 0.0241 | 0.0000 | 0.0000 | 0.0241 | 0.0000 | 0.0000 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| O | mol/s | 0.1213 | 0.1213 | 0.0000 | 0.0000 | 0.1213 | 0.0000 | 0.0000 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| S | mol/s | 0.0019 | 0.0019 | 0.0000 | 0.0000 | 0.0019 | 0.0000 | 0.0000 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Cl | mol/s | 0.0002 | 0.0002 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0330 | 0.0000 | - | 0.0000 | 0.0000 | 0.2231 | 0.2231 | 0.2977 | 0.0000 | 0.2977 | 0.2203 | 0.0774 | 0.0000 | 0.0000 |
| CO | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0176 | 0.0000 | - | 0.0000 | 0.0000 | 0.0818 | 0.0818 | 0.0072 | 0.0000 | 0.0072 | 0.0000 | 0.0072 | 0.0000 | 0.0000 |
| CO2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.3258 | 0.0000 | 0.0506 | 0.0000 | - | 0.0000 | 0.0000 | 0.0658 | 0.0658 | 0.1404 | 0.0000 | 0.1404 | 0.0000 | 0.1404 | 0.0389 | 0.2730 |
| CH4 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0414 | 0.0000 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| C2H4 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0017 | 0.0000 | - | 0.0000 | 0.0000 | 0.0013 | 0.0013 | 0.0013 | 0.0000 | 0.0013 | 0.0000 | 0.0013 | 0.0000 | 0.0000 |
| C2H6 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0286 | 0.0000 | - | 0.0000 | 0.0000 | 0.0225 | 0.0225 | 0.0225 | 0.0000 | 0.0225 | 0.0000 | 0.0225 | 0.0000 | 0.0000 |
| N2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 8.8893 | 0.0000 | 0.0088 | 0.0000 | - | 0.0000 | 0.0000 | 0.0088 | 0.0088 | 0.0088 | 0.0000 | 0.0088 | 0.0000 | 0.0088 | 4.7612 | 4.7700 |
| C6H6 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0051 | 0.0000 | - | 0.0000 | 0.0000 | 0.0019 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| C10H8 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0006 | 0.0000 | - | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H2O | mol/s | 10.6878 | 1.5506 | 0.0000 | 0.1994 | 0.0749 | 0.0749 | 0.0000 | - | 0.2995 | 0.0000 | 0.2980 | 0.2980 | 0.2234 | 0.2995 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.1994 |
| H2S | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0017 | 0.0000 | - | 0.0000 | 0.0000 | 0.0017 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| O2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 2.0414 | 0.0000 | 0.0000 | 0.0092 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.1222 | 0.9324 |
| HCl | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | - | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| C-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0610 | 0.0000 | - | 0.0000 | 0.0589 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0113 | 0.0000 | - | 0.0000 | 0.0106 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| O-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0013 | 0.0000 | - | 0.0000 | 0.0013 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| N-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0032 | 0.0000 | - | 0.0000 | 0.0032 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| S-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | - | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Cl-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Ash | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0243 | 0.0000 | 0.0000 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| PROPANE | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | - | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0130 | 0.0000 |
| Case 2 | Units | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure | kPa | 101.3 | 101.3 | 101.3 | 104.0 | 106.0 | 106.0 | 106.0 | 106.0 | 106.0 | 106.0 | 106.0 | 105.0 | 102.0 | 109.0 | 101.0 | 101.0 | 106.0 | 106.0 | 106.0 |
| Temperature | K | 298.2 | 298.2 | 298.2 | 423.2 | 298.2 | 957.7 | 303.6 | 307.6 | 423.2 | 1173.2 | 1173.2 | 623.2 | 630.1 | 303.2 | 303.2 | 313.2 | 315.1 | 298.2 | 1273.2 |
| Mass Flows | kg/s | 0.1995 | 0.0349 | 0.0000 | 0.3318 | 0.0083 | 0.0074 | 0.0002 | 0.0036 | 0.0054 | 0.0009 | 0.0122 | 0.0187 | 0.0187 | 0.0054 | 0.0145 | 0.0004 | 0.0073 | 0.1716 | 0.1789 |
| Mole Flows | mol/s | 11.5739 | 2.4367 | 0.0000 | 11.2282 | 0.9853 | 0.3411 | 0.0092 | 0.0001 | 0.2995 | 0.0742 | 0.7053 | 0.7014 | 0.7014 | 0.2995 | 0.4780 | 0.2326 | 0.1634 | 6.0612 | 6.2171 |
| C | mol/s | 0.2674 | 0.2674 | 0.0000 | 0.0000 | 0.2674 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H | mol/s | 0.4711 | 0.4711 | 0.0000 | 0.0000 | 0.4711 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| N | mol/s | 0.0241 | 0.0241 | 0.0000 | 0.0000 | 0.0241 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| O | mol/s | 0.1213 | 0.1213 | 0.0000 | 0.0000 | 0.1213 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| S | mol/s | 0.0019 | 0.0019 | 0.0000 | 0.0000 | 0.0019 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Cl | mol/s | 0.0002 | 0.0002 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0330 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.2231 | 0.2231 | 0.2977 | 0.0000 | 0.2977 | 0.2326 | 0.0651 | 0.0000 | 0.0000 |
| CO | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0176 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0818 | 0.0818 | 0.0072 | 0.0000 | 0.0072 | 0.0000 | 0.0072 | 0.0000 | 0.0000 |
| CO2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.2556 | 0.0000 | 0.0506 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0658 | 0.0658 | 0.1404 | 0.0000 | 0.1404 | 0.0000 | 0.0584 | 0.0397 | 0.2055 |
| CH4 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0414 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| C2H4 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0017 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0013 | 0.0013 | 0.0013 | 0.0000 | 0.0013 | 0.0000 | 0.0013 | 0.0000 | 0.0000 |
| C2H6 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0286 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0225 | 0.0225 | 0.0225 | 0.0000 | 0.0225 | 0.0000 | 0.0225 | 0.0000 | 0.0000 |
| N2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 8.7762 | 0.0000 | 0.0088 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0088 | 0.0088 | 0.0088 | 0.0000 | 0.0088 | 0.0000 | 0.0088 | 4.8588 | 4.8676 |
| C6H6 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0051 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0019 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| C10H8 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0006 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H2O | mol/s | 10.6878 | 1.5506 | 0.0000 | 0.2053 | 0.0749 | 0.0749 | 0.0000 | 0.0000 | 0.2995 | 0.0000 | 0.2980 | 0.2980 | 0.2234 | 0.2995 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.2053 |
| H2S | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0017 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0017 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| O2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 1.9911 | 0.0000 | 0.0000 | 0.0092 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.1452 | 0.9388 |
| HCl | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| C-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0610 | 0.0000 | 0.0000 | 0.0000 | 0.0589 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0113 | 0.0000 | 0.0000 | 0.0000 | 0.0106 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| O-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0013 | 0.0000 | 0.0000 | 0.0000 | 0.0013 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| N-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0032 | 0.0000 | 0.0000 | 0.0000 | 0.0032 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| S-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Cl-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Ash | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0243 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| PROPANE | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0175 | 0.0000 |
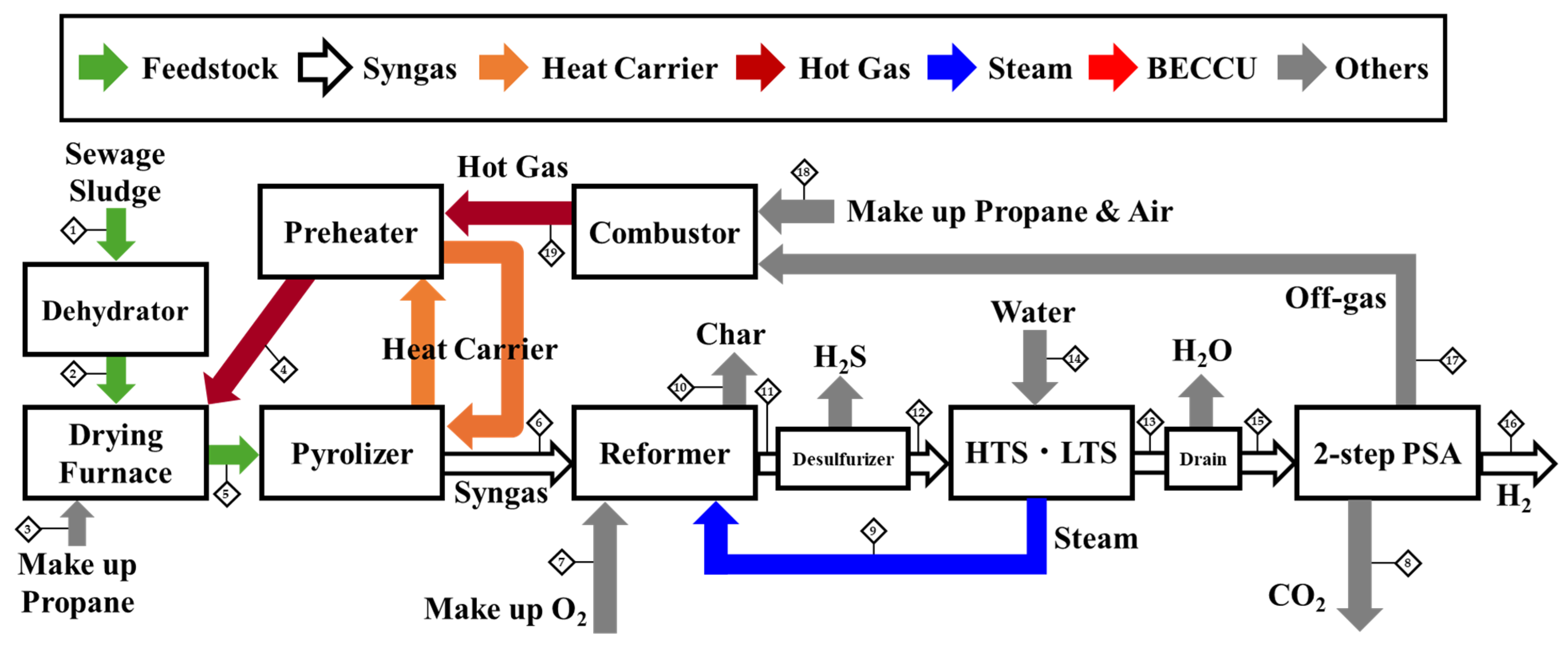
References
- Garzón Baquero, J.E.; Bellon Monsalve, D. From fossil fuel energy to hydrogen energy: Transformation of fossil fuel energy economies into hydrogen economies through social entrepreneurship. Int. J. Hydrogen Energy 2024, 54, 574–585. [Google Scholar] [CrossRef]
- Gaudernack, B.; Steinar, L. Hydrogen from natural gas without release of CO2 to the atmosphere. Int. J. Hydrogen Energy 1998, 23, 1087–1093. [Google Scholar] [CrossRef]
- Agency for Natural Resources and Energy. Available online: https://www.enecho.meti.go.jp/about/special/johoteikyo/suisohou_01.html (accessed on 22 June 2025).
- Tarkowski, R.; Uliasz-Misiak, B. Towards underground hydrogen storage: A review of barriers. Renew. Sustain. Energy Rev. 2022, 162, 112451. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Li, P.; Shi, X.; Wang, X.; Song, J.; Fang, S.; Bai, J.; Zhang, G.; Chang, C.; Pang, S. Bio-oil from biomass fast pyrolysis: Yields, related properties and energy consumption analysis of the pyrolysis system. J. Clean. Prod. 2021, 328, 129613. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kaneko, S. Study on Small-Sized Gas Engine with Biomass Gas. J. Combust. Soc. Jpn. 2007, 49, 236–245. [Google Scholar] [CrossRef]
- Kuroda, K.; Kim, H.B.; Yoshioka, T. Forest resource distribution and transport cost optimization-based economic evaluation of gasification and steam-turbine biomass power generation systems. Renew. Energy Focus 2024, 50, 100595. [Google Scholar] [CrossRef]
- Renganathan, T.; Yadav, M.V.; Pushpavanam, S.; Voolapalli, R.K.; Cho, Y.S. CO2 utilization for gasification of carbonaceous feedstocks: A thermodynamic analysis. Chem. Eng. Sci. 2012, 83, 159–170. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Owusu, A.B. A modified Ester-branched thickener for rheology and wettability during CO2 fracturing for improved fracturing property. Environ. Sci. Pollut. Res. Int. 2019, 26, 20787–20797. [Google Scholar] [CrossRef]
- Natsumi, Y.; Nochi, K.; Otsuka, M. Immobilization Experiment of the Carbon Dioxide by the Minute Algal Biomass Apparatus. Soc. Heating. Air Cond. Sanit. Eng. Jpn. 2011, 45–48. [Google Scholar] [CrossRef]
- Hayashi, A.; Akimoto, K.; Sano, F. An Evaluation on Bioenergy Potentials for Negative CO2 Emissions. Dai 25 Kai Nihon Enerugī Gakkai Taikai 2016, 25, 210–211. [Google Scholar] [CrossRef]
- Guntermann, N.; Mengers, H.G.; Franciò, G.; Blank, L.M.; Leitner, W. Bio-energy conversion with carbon capture and utilization (BECCU): Integrated biomass fermentation and chemo-catalytic CO2 hydrogenation for bioethanol and formic acid co-production. Green Chem. 2021, 23, 9860–9864. [Google Scholar] [CrossRef]
- Artz, J.; Múller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- New Energy and Industrial Technology Development Organization. Available online: https://www.nedo.go.jp/content/100949869.pdf (accessed on 22 June 2025).
- Kuroda, S.; Dowaki, K.; Mori, S.; Harada, T. Design of an Impurity Removal System for H2S in Biohydrogen Using HAS-Clay/Zeolite A5 (HAS-Clay/Zeolite A5を用いたバイオ水素中のH2Sを含む不純物除去システムの設計). Master’s Thesis, Tokyo University of Science, Tokyo, Japan, 2018. [Google Scholar]
- Maran, K.; Sinha, R.; Brandão, M.M.R. LCA of Advanced Biofuel Production. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2024. [Google Scholar]
- Nakayama, H.; Kameyama, M.; Long, Y.; Dowaki, K. An Environmental Analysis of Bio-H2 Production Introducing the 2-step PSA and Waste Heat Recovery. J. Jpn. Inst. Energy 2020, 99, 129–135. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Huang, S.; Wu, S.; Li, Y.; Wu, Y.; Lei, T. Study on the mechanism of syngas production from catalytic pyrolysis of biomass tar by Ni–Fe catalyst in CO2 atmosphere. Fuel 2023, 335, 126705. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Guo, X.; Hu, Z.; Liu, S.; He, M. Hydrogen-rich gas from catalytic steam gasification of biomass in a fixed bed reactor: Influence of particle size on gasification performance. Int. J. Hydrogen Energy 2009, 34, 1260–1264. [Google Scholar] [CrossRef]
- Murakami, T. Detailed Analysis of Tar Components in Syngas Generated by Lignite Gasification in a Fluidized Bed Gasifier. J. Jpn. Pet. Inst. 2025, 68, 69–80. [Google Scholar] [CrossRef]
- Dowaki, K. Life Cycle Analysis of Biomass Energy Systems Using Gasification Processes (ガス化プロセスによるバイオマスエネルギーシステムのライフサイクル分析). Ph.D. Thesis, The University of Tokyo, Tokyo, Japan, 2001. [Google Scholar]
- The Society of Chemical Engineers, Japan. Kagaku Kogaku Binran (Chemical Engineering Handbook), 6th ed.; Maruzen Publishing: Tokyo, Japan, 1999; pp. 952–955. [Google Scholar]
- Asaki, Z.; Kondo, Y. Gas-solid phase reaction models in metal refining. Denki Kagaku 1975, 43, 352–359. [Google Scholar] [CrossRef]
- Fujioka, Y.; Jung, W.S. Simplified Coal Gasification Rate for Fluidzed Bed Coal Gasifier. J. Chem. Eng. Jpn. 1996, 22, 433–445. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Ikoda, K. Study on heterogeneous reactions between tar and ash during coal gasification. Sci. Eng. Rep. Saitama Univ. 2008, 41, 10–14. [Google Scholar][Green Version]
- Kobayashi, N.; Itaya, Y.; Suami, A. Case Study of Energy Recovery and CO2 Reduction in Sewage Sludge Drying Process. Mater. Cycles Waste Manag. Res. 2022, 33, 242–253. [Google Scholar] [CrossRef]
- Nakakubo, T. Analysis of energy balances for a recycling system to input dewatered sludge directly in cement kiln. J. Jpn. Soc. Civ. Eng. Ser. G Environ. Res. 2017, 73, II_233–II_244. [Google Scholar] [CrossRef]
- Hamazaki, M.; Torii, K.; Shan, M.; Kameyama, M.; Mercado, J.V.L.; Dowaki, K. Discussions on the heat transfer performance of the indirect pyrolysis plant using CFD modeling. In IOP Conference Series: Earth and Environmental Science; IOP: Bristol, UK, 2023; Volume 1187, p. 012026. [Google Scholar] [CrossRef]
- Fujitsuka, H.; Kobayashi, T.; Tago, T. Preparation of Birdcage-type zeolite catalyst and its application in dry reforming of methane. Zeoraito 2019, 36, 106–114. [Google Scholar] [CrossRef]
- Urdampilleta, I.; Uribe, F.; Rockward, T.; Brosha, E.L.; Pivovar, B.; Garzon, F.H. PEMFC poisoning with H2S: Dependence on operating conditions. ECS Trans. 2007, 11, 831. [Google Scholar] [CrossRef]
- Kitayama, S.; Hamazaki, M.; Kumon, S.; Sato, K.; Dowaki, K. Enhancement of Bio-H2 Purification Performance in a Multi-Stage Desulfurization Process Using Mining Waste and LaNi5. Energies 2025, 18, 1000. [Google Scholar] [CrossRef]
- Inaba, A. Kiso Kara Manabu LCA -LCA no Jisshi to Katsuyo (Learning LCA from the Basics—Implementing and Utilizing LCA Life Cycle Assessment; Japan Life Cycle Assessment Facilitation Centre LCAF: Tokyo, Japan, 2023; pp. 96–97. [Google Scholar]
- Tamura, M. History of Air Separation Unit Technologies Developed by TNSC. TAIYO NIPPON SANSO Technical Report No. 41. 2022. Available online: https://www.tn-sanso.co.jp/Portals/0/resources/en/rd/giho/pdf/41/tnscgiho41_E00.pdf (accessed on 23 June 2025).
- Nishihiro, K. Study on the Mechanism of Carbon Precipitation from CO-H2 Mixed Gas Using Iron as a Catalyst and the Gasification Behavior of Precipitated Carbon (鉄を触媒としたCO-H2混合ガスからの炭素析出反応メカニズムおよび析出炭素のガス化挙動に関する研究). Ph.D. Thesis, Kyushu University, Fukuoka, Japan, 2020. [Google Scholar] [CrossRef]
- Takahashi, Y. Recent Movements in Structural Integrity Evaluation for High-Temperature Plant Components. Jpn. Soc. Mech. Eng. 2011, 77, 1156–1168. [Google Scholar] [CrossRef][Green Version]
- Khoshnoudı, A.; Akay, R. Simulation and optimization of hydrogen production by steam reforming of natural gas. J. Turk. Chem. Soc. Sect. B Chem. Eng. 2023, 6, 123–136. [Google Scholar] [CrossRef]
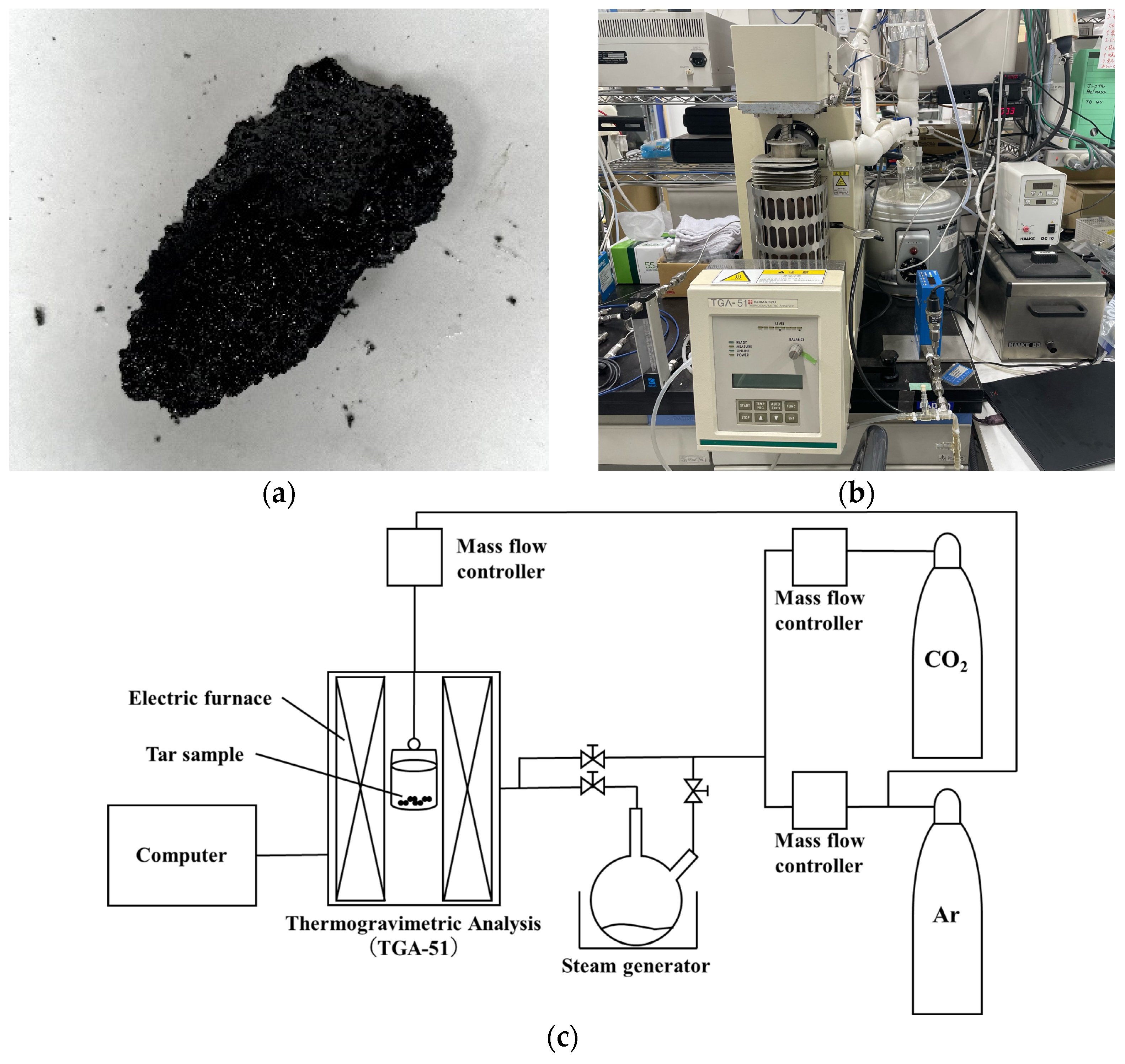
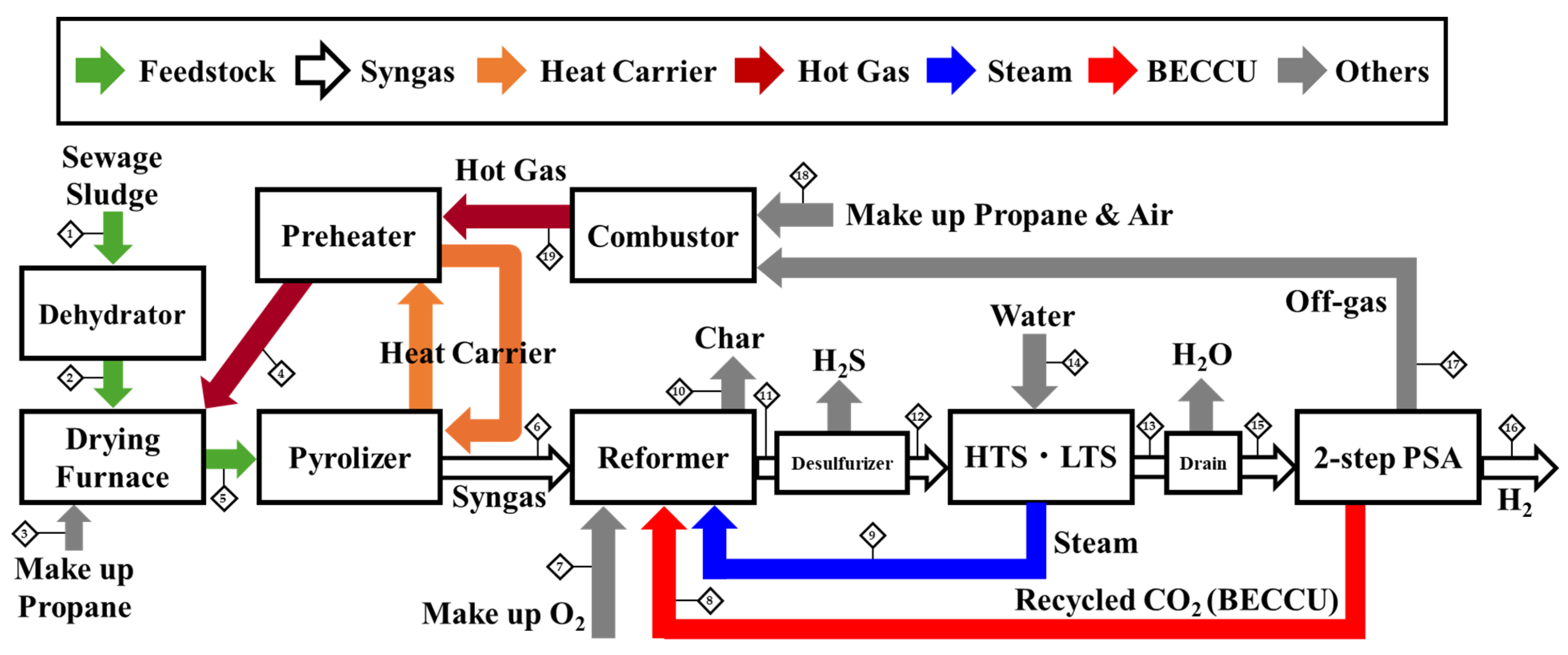
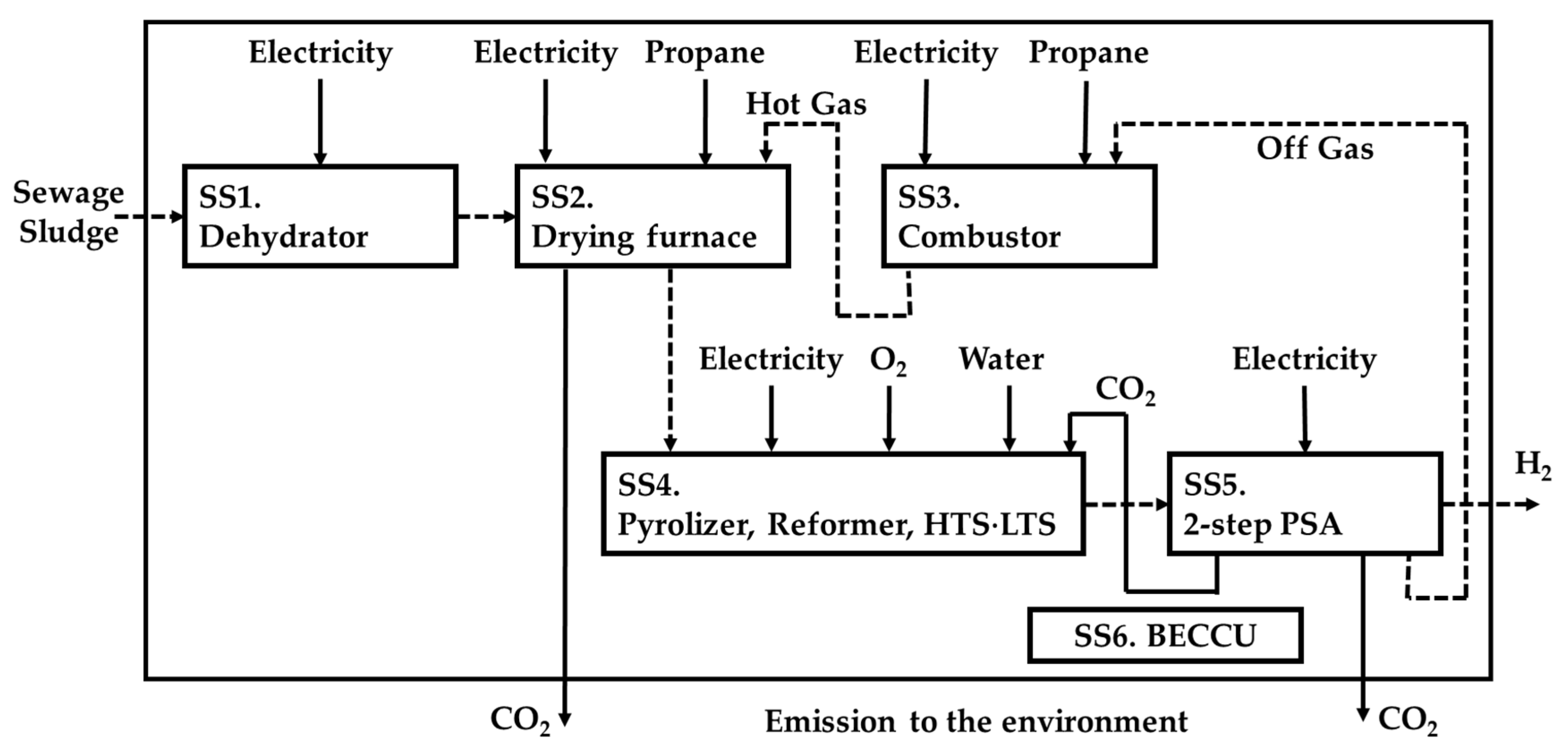
| C * [wt.%] | 72.37 |
| H * [wt.%] | 7.07 |
| N * [wt.%] | 9.08 |
| O *,** [wt.%] | 11.49 |
| S * [wt.%] | 0.00 |
| Cl * [wt.%] | 0.00 |
| Higher heating value (HHV) [kJ/kg] | 30,711 |
| Gasifying Agent | CO2, Steam |
|---|---|
| Temperature [K] | 1173, 1273, 1373 |
| Heating rate [K/min] | 30 |
| Sample particle size [mm] | ≤1 |
| Sample weight [mg] | 10 |
| Gasifying Agent | [1/s] | [kJ/mol] |
|---|---|---|
| CO2 | ||
| Steam |
| Units | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure | kPa | 101.3 | 101.3 | 101.3 | 104.0 | 106.0 | 106.0 | 106.0 | 106.0 | 106.0 | 106.0 | 106.0 | 105.0 | 102.0 | 109.0 | 101.0 | 101.0 | 106.0 | 106.0 | 106.0 |
| Temperature | K | 298.2 | 298.2 | 298.2 | 423.2 | 298.2 | 957.7 | 303.6 | 307.6 | 423.2 | 1173.2 | 1173.2 | 623.2 | 630.1 | 303.2 | 303.2 | 313.2 | 315.1 | 298.2 | 1273.2 |
| Mass Flows | kg/s | 0.1995 | 0.0349 | 0.0000 | 0.3318 | 0.0083 | 0.0074 | 0.0002 | 0.0068 | 0.0054 | 0.0009 | 0.0188 | 0.0187 | 0.0187 | 0.0054 | 0.0145 | 0.0004 | 0.0073 | 0.1716 | 0.1789 |
| Mole Flows | mol/s | 11.5739 | 2.4367 | 0.0000 | 11.4453 | 0.9853 | 0.3411 | 0.0047 | 0.1764 | 0.2995 | 0.0749 | 0.8781 | 0.8744 | 0.8744 | 0.2995 | 0.6438 | 0.2244 | 0.2430 | 5.9474 | 6.1745 |
| C | mol/s | 0.2674 | 0.2674 | 0.0000 | 0.0000 | 0.2674 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H | mol/s | 0.4711 | 0.4711 | 0.0000 | 0.0000 | 0.4711 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| N | mol/s | 0.0241 | 0.0241 | 0.0000 | 0.0000 | 0.0241 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| O | mol/s | 0.1213 | 0.1213 | 0.0000 | 0.0000 | 0.1213 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| S | mol/s | 0.0019 | 0.0019 | 0.0000 | 0.0000 | 0.0019 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Cl | mol/s | 0.0002 | 0.0002 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0330 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.1660 | 0.1660 | 0.2872 | 0.0000 | 0.2872 | 0.2244 | 0.0628 | 0.0000 | 0.0000 |
| CO | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0176 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.1417 | 0.1417 | 0.0206 | 0.0000 | 0.0206 | 0.0000 | 0.0206 | 0.0000 | 0.0000 |
| CO2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.3221 | 0.0000 | 0.0506 | 0.0000 | 0.1764 | 0.0000 | 0.0000 | 0.1810 | 0.1810 | 0.3021 | 0.0000 | 0.3021 | 0.0000 | 0.1257 | 0.0389 | 0.2770 |
| CH4 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0414 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| C2H4 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0017 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0014 | 0.0014 | 0.0014 | 0.0000 | 0.0014 | 0.0000 | 0.0014 | 0.0000 | 0.0000 |
| C2H6 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0286 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0237 | 0.0237 | 0.0237 | 0.0000 | 0.0237 | 0.0000 | 0.0237 | 0.0000 | 0.0000 |
| N2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 8.8841 | 0.0000 | 0.0088 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0088 | 0.0088 | 0.0088 | 0.0000 | 0.0088 | 0.0000 | 0.0088 | 4.7703 | 4.7791 |
| C6H6 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0051 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0016 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| C10H8 | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0006 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H2O | mol/s | 10.6878 | 1.5506 | 0.0000 | 0.2062 | 0.0749 | 0.0749 | 0.0000 | 0.0000 | 0.2995 | 0.0000 | 0.3517 | 0.3517 | 0.2306 | 0.2995 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.1922 |
| H2S | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0017 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0017 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| O2 | mol/s | 0.0000 | 0.0000 | 0.0000 | 2.0330 | 0.0000 | 0.0000 | 0.0047 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.1244 | 0.9262 |
| HCl | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| C-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0610 | 0.0000 | 0.0000 | 0.0000 | 0.0593 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| H-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0113 | 0.0000 | 0.0000 | 0.0000 | 0.0108 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| O-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0013 | 0.0000 | 0.0000 | 0.0000 | 0.0013 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| N-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0032 | 0.0000 | 0.0000 | 0.0000 | 0.0032 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| S-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Cl-CHAR | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Ash | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0243 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| PROPANE | mol/s | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0139 | 0.0000 |
| Sewage Sludge | Sewage Sludge Char | |
|---|---|---|
| C * [wt.%] | 46.0 | 41.7 |
| H * [wt.%] | 6.80 | 1.30 |
| N * [wt.%] | 4.84 | 5.15 |
| O *,** [wt.%] | 27.8 | 2.36 |
| S * [wt.%] | 0.860 | 0.340 |
| Cl * [wt.%] | 0.100 | 0.0500 |
| Ash * [wt.%] | 13.6 | 49.1 |
| HHV [kJ/kg] | 17,220 | 14,644 |
| H2 [wt.%] | 0.797 |
| CO [wt.%] | 5.92 |
| CO2 [wt.%] | 26.7 |
| CH4 [wt.%] | 7.96 |
| C2H4 [wt.%] | 0.566 |
| C2H6 [wt.%] | 10.3 |
| N2 [wt.%] | 2.97 |
| C6H6 [wt.%] | 4.77 |
| C10H8 [wt.%] | 0.904 |
| H2O [wt.%] | 16.2 |
| H2S [wt.%] | 0.690 |
| O2 [wt.%] | 0.00 |
| HCl [wt.%] | 0.0753 |
| C-Char [wt.%] | 8.79 |
| H-Char [wt.%] | 0.274 |
| N-Char [wt.%] | 1.09 |
| O-Char [wt.%] | 0.497 |
| S-Char [wt.%] | 0.0716 |
| Cl-Char [wt.%] | 0.0105 |
| Ash [wt.%] | 11.4 |
| Total [wt.%] | 100 |
| Partial oxidation | C-Char + 1/2O2 → CO |
| CO + 1/2O2 → CO2 | |
| H2 + 1/2O2 → H2O | |
| H-Char + 1/2O2 → H2O | |
| CH4 + 1/2O2 → CO + 2H2 | |
| C2H4 + O2 → 2CO + 2H2 | |
| C2H6 + O2 → 2CO + 3H2 | |
| Primary water gas | C-Char + H2O → CO + H2 |
| Tar steam reforming | C6H6 + 6H2O → 6CO + 9H2 |
| C10H8 + 10H2O → 10CO + 14H2 | |
| Boudouard | C-Char + CO2 → 2CO |
| Tar dry reforming | C6H6 + 6CO2 → 12CO + 3H2 |
| C10H8 + 10CO2 → 20CO + 4H2 | |
| Dry reforming of methane | CH4 + CO2 → 2CO + 2H2 |
| Methane reforming | CH4 + H2O ↔ CO + 3H2 |
| Water–gas shift | CO + H2O ↔ H2 + CO2 |
| Item | Intensity | Unit | Reference |
|---|---|---|---|
| Electricity | 0.574 | kg-CO2/kWh | [33] |
| Propane (production) | 0.508 | kg-CO2/Nm3 | [33] |
| Propane (combustion) | 0.0499 | kg-CO2/MJ | [33] |
| Oxygen production | 0.350 | kWh/Nm3 | [34] |
| Water | 0.000366 | kg-CO2/L | [33] |
| 2-step PSA (Auxiliary power) | 2.83 | MJ/kg-CO2 | [16] |
| Reformer temperature [K] | 973 | 1073 | 1173 | 1273 | 1373 |
| GWP [g-CO2 eq./MJ-H2] | |||||
| SS1 | 2.70 | 2.48 | 2.14 | 1.58 | 1.46 |
| SS2 | 84.1 | 74.9 | 67.0 | 61.3 | 58.3 |
| SS3 | 7.94 | 7.09 | 6.30 | 5.63 | 5.34 |
| SS4 | 0.313 | 0.551 | 0.92 | 1.28 | 2.03 |
| SS5 | 122 | 119 | 111 | 89.5 | 85.8 |
| BECCU | −157 | −153 | −143 | −116 | −111 |
| Total | 59.3 | 50.2 | 43.8 | 43.4 | 41.9 |
| H2 production [MJ-H2/kg-SS] | 5.17 | 5.62 | 6.51 | 8.83 | 9.59 |
| CGE [%] | 60.9 | 64.1 | 67.3 | 71.4 | 74.2 |
| Auxiliary power [kW/MJ-H2] | |||||
| SS1 | 16.9 | 15.6 | 13.4 | 9.9 | 9.1 |
| SS2 | 263 | 243 | 209 | 155 | 142 |
| SS3 | 27.4 | 25.2 | 21.7 | 15.9 | 14.6 |
| SS4 | 1.68 | 3.19 | 5.51 | 7.9 | 12.6 |
| SS5 | 762 | 744 | 693 | 562 | 538 |
| Total | 1072 | 1030 | 943 | 750 | 717 |
| recycled CO2 [%] | 57.6 | 61.0 | 66.0 | 72.4 | 75.4 |
| Char reaction rate [%] | |||||
| recycled CO2 | 0.00546 | 0.0444 | 0.225 | 0.627 | 1.60 |
| pyrolysis CO2 | 0.00179 | 0.0137 | 0.0646 | 0.164 | 0.402 |
| others | 0.0773 | 0.505 | 2.47 | 7.23 | 19.2 |
| Total | 0.0846 | 0.563 | 2.76 | 8.02 | 21.2 |
| Tar reaction rate [%] | |||||
| recycled CO2 | 9.81 | 18.9 | 24.9 | 24.4 | 24.6 |
| pyrolysis CO2 | 3.22 | 5.85 | 7.13 | 6.38 | 6.17 |
| others | 10.0 | 23.5 | 36.0 | 44.1 | 52.6 |
| Total | 23.0 | 48.3 | 68.0 | 74.9 | 83.4 |
| S/C ratio | 1.4 | 2.0 | 3.0 | 4.0 |
| GWP [g-CO2 eq./MJ-H2] | ||||
| SS1 | 2.14 | 2.06 | 2.01 | 2.00 |
| SS2 | 67.0 | 65.0 | 63.6 | 63.0 |
| SS3 | 6.30 | 6.10 | 5.96 | 5.91 |
| SS4 | 0.915 | 0.721 | 0.566 | 0.492 |
| SS5 | 111 | 114 | 116 | 117 |
| BECCU | −143 | −148 | −151 | −152 |
| Total | 43.8 | 40.3 | 37.9 | 37.0 |
| H2 production [MJ-H2/kg-SS] | 6.51 | 6.78 | 6.94 | 6.98 |
| CGE [%] | 67.3 | 67.6 | 67.9 | 68.1 |
| Auxiliary power [kW/MJ-H2] | ||||
| SS1 | 13.4 | 12.9 | 12.6 | 12.5 |
| SS2 | 209 | 201 | 197 | 195 |
| SS3 | 21.7 | 20.8 | 20.3 | 20.2 |
| SS4 | 5.51 | 4.19 | 3.03 | 2.38 |
| SS5 | 693 | 674 | 675 | 675 |
| Total | 943 | 913 | 907 | 905 |
| recycled CO2 [%] | 66.0 | 71.0 | 74.0 | 74.9 |
| Char reaction rate [%] | ||||
| recycled CO2 | 0.225 | 0.178 | 0.116 | 0.0772 |
| pyrolysis CO2 | 0.0646 | 0.0474 | 0.0296 | 0.0195 |
| others | 2.47 | 2.86 | 3.21 | 3.32 |
| Total | 2.76 | 3.09 | 3.35 | 3.42 |
| Tar reaction rate [%] | ||||
| recycled CO2 | 24.9 | 18.4 | 10.64 | 6.33 |
| pyrolysis CO2 | 7.13 | 4.91 | 2.72 | 1.60 |
| others | 36.0 | 45.9 | 57.1 | 63.2 |
| Total | 68.0 | 69.2 | 70.4 | 71.1 |
| Case | Case 1 | Case 2 | Case 3 (This Study) |
|---|---|---|---|
| AGM + PSA | AGM + 2-Step PSA | AGM + 2-Step PSA + Reuse As a Gasifying Agent | |
| GWP [g-CO2 eq./MJ-H2] | 156 | 140 | 43.8 |
| H2 production [MJ-H2/kg-Feedstock] | 6.39 | 6.75 | 6.51 |
| CGE [%] | 65.4 | 65.4 | 67.3 |
| Auxiliary power [kW/MJ-H2] | 759 | 556 | 943 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terasawa, D.; Hamazaki, M.; Kumagai, K.; Dowaki, K. Environmental Performance of the Sewage Sludge Gasification Process Considering the Recovered CO2. Energies 2025, 18, 4460. https://doi.org/10.3390/en18174460
Terasawa D, Hamazaki M, Kumagai K, Dowaki K. Environmental Performance of the Sewage Sludge Gasification Process Considering the Recovered CO2. Energies. 2025; 18(17):4460. https://doi.org/10.3390/en18174460
Chicago/Turabian StyleTerasawa, Daichi, Mayu Hamazaki, Kanato Kumagai, and Kiyoshi Dowaki. 2025. "Environmental Performance of the Sewage Sludge Gasification Process Considering the Recovered CO2" Energies 18, no. 17: 4460. https://doi.org/10.3390/en18174460
APA StyleTerasawa, D., Hamazaki, M., Kumagai, K., & Dowaki, K. (2025). Environmental Performance of the Sewage Sludge Gasification Process Considering the Recovered CO2. Energies, 18(17), 4460. https://doi.org/10.3390/en18174460






