High-Efficiency Polysulfide Trapping with g-C3N4/CNT Hybrids for Superior Lithium-Sulfur Batteries
Abstract
1. Introduction
2. Materials and Methods
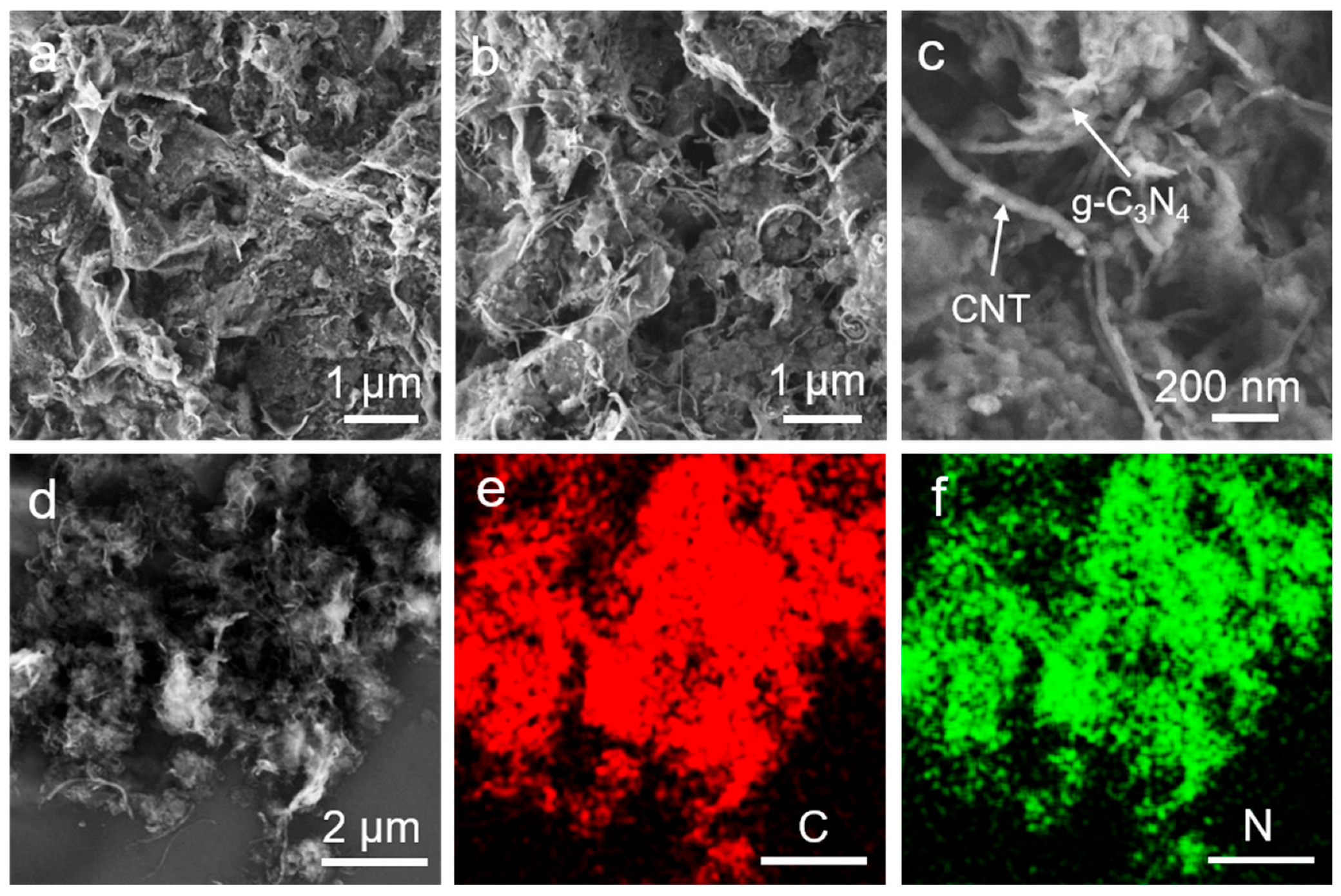
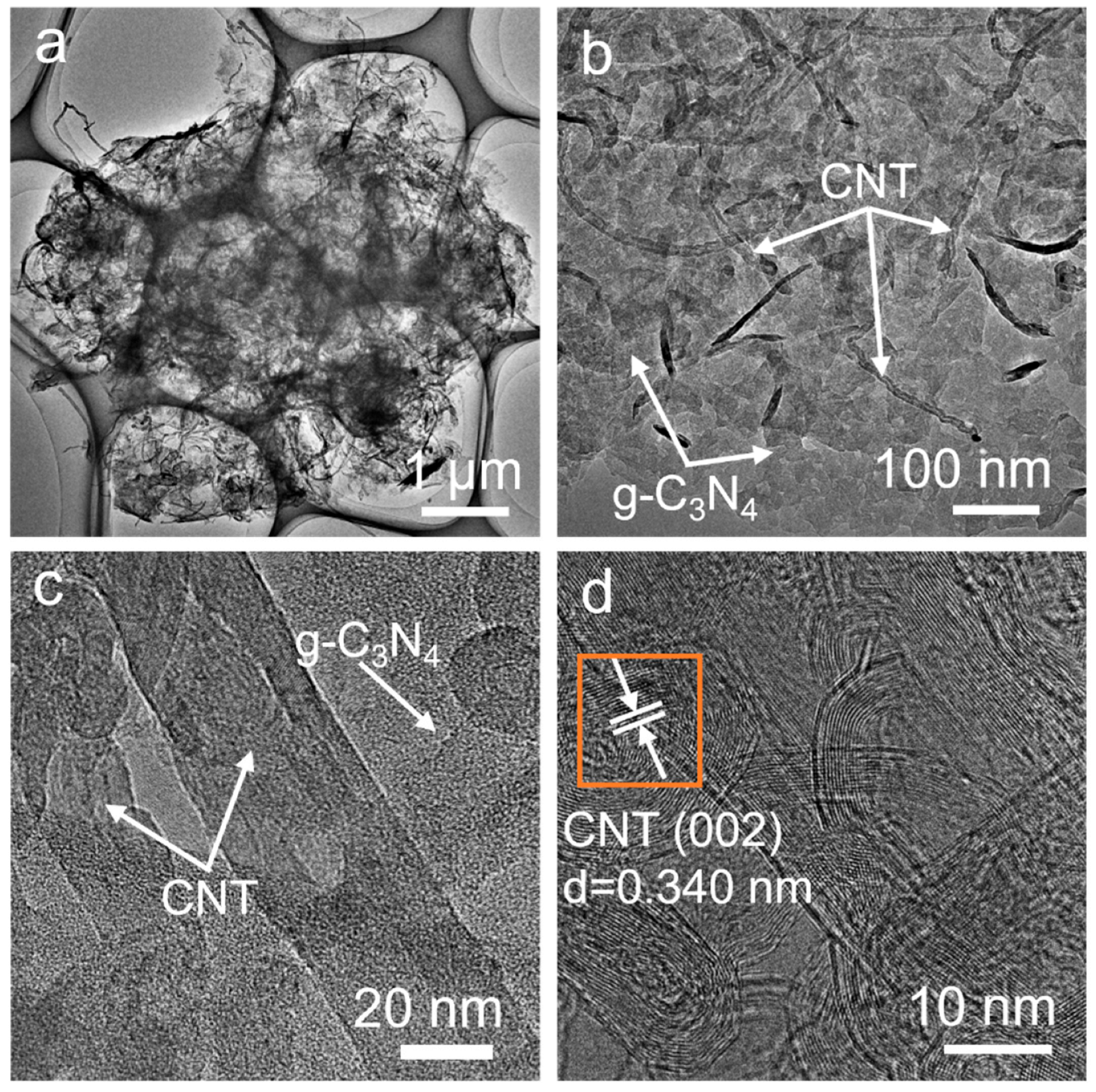
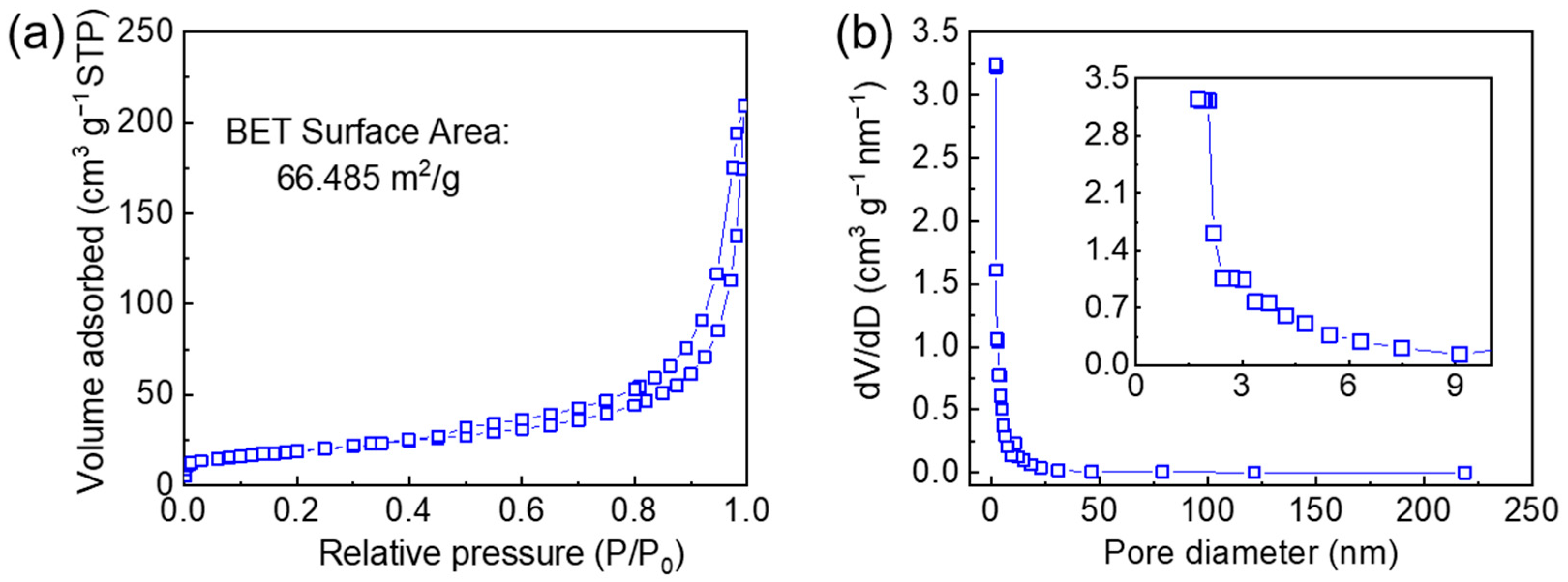
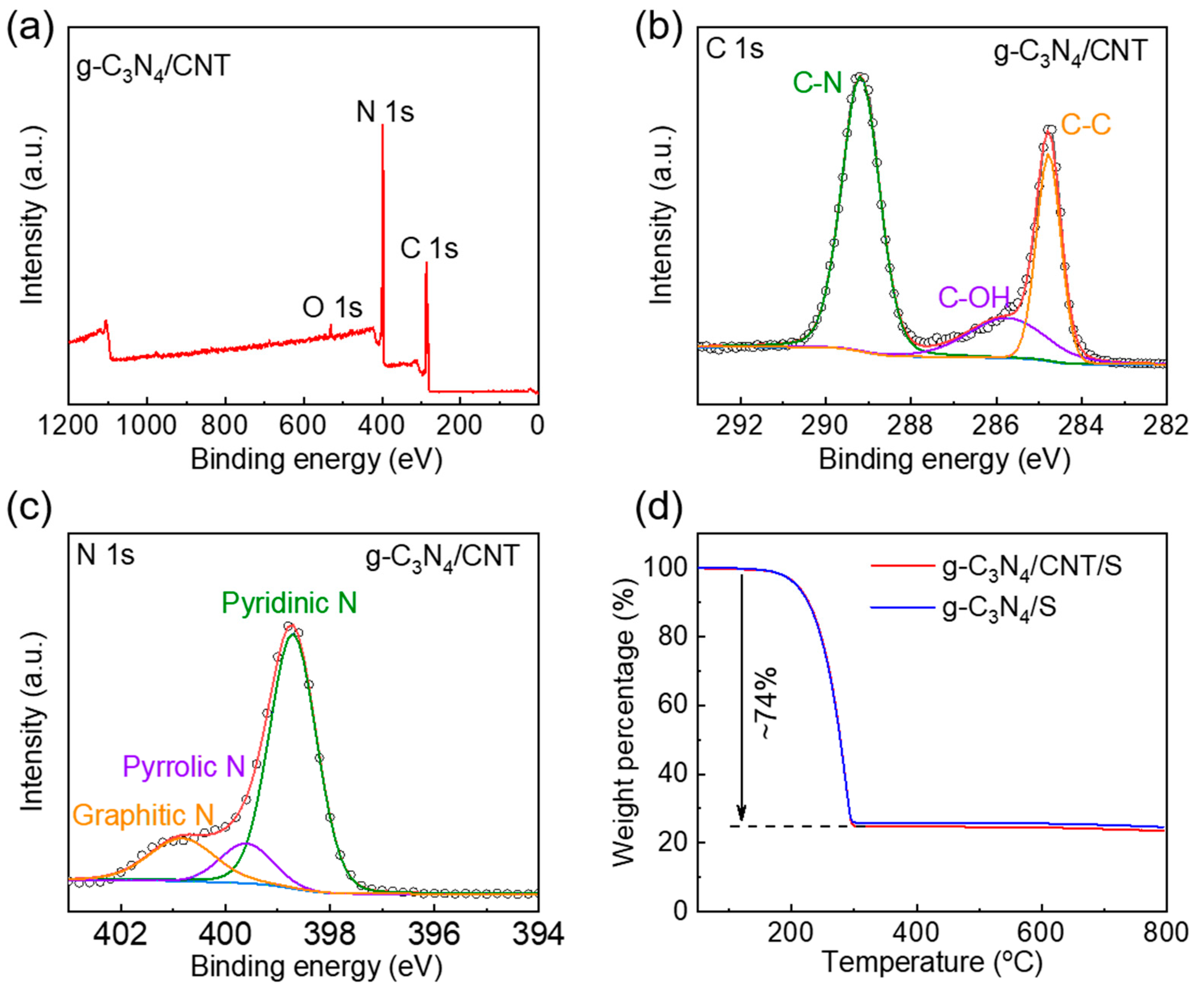
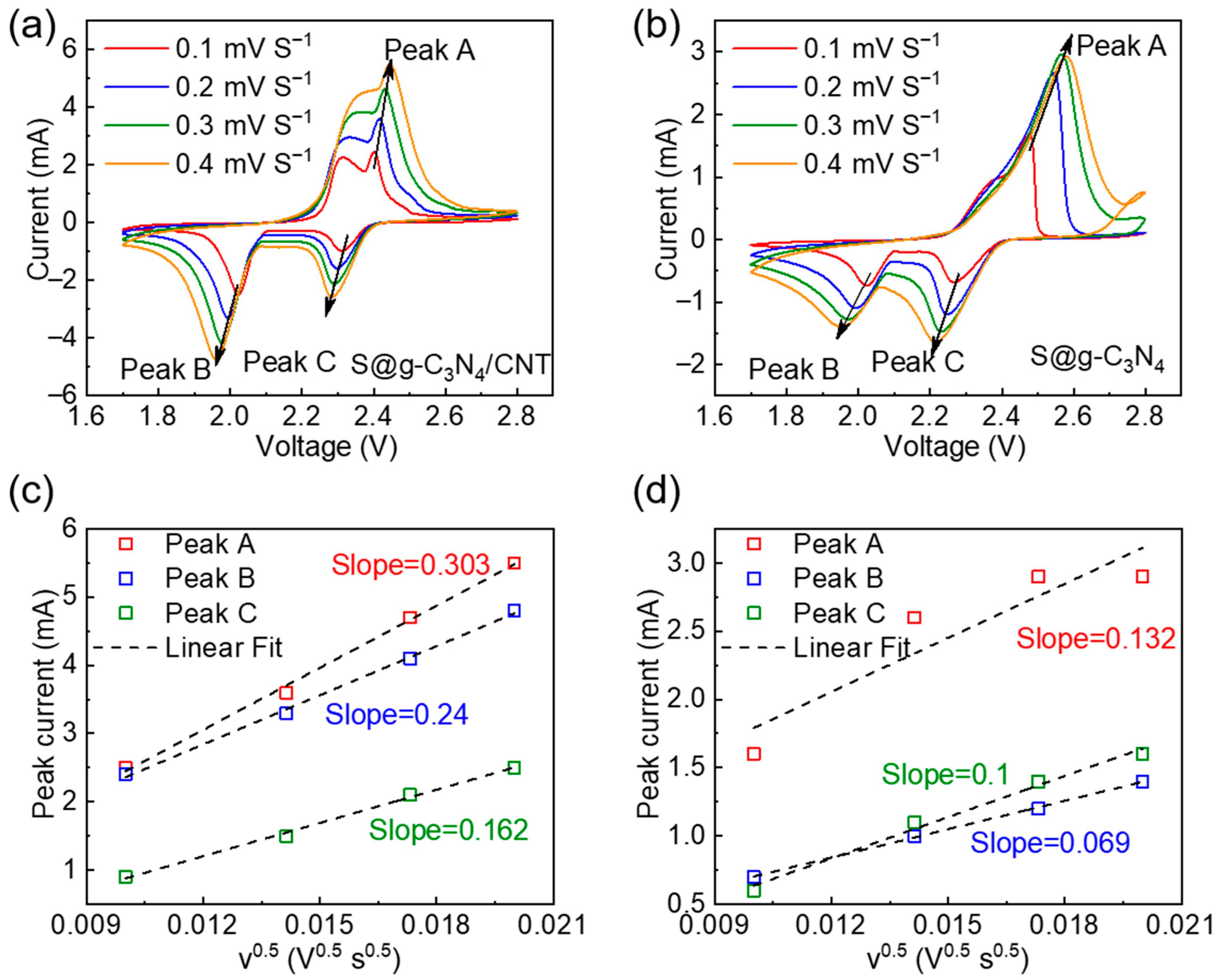
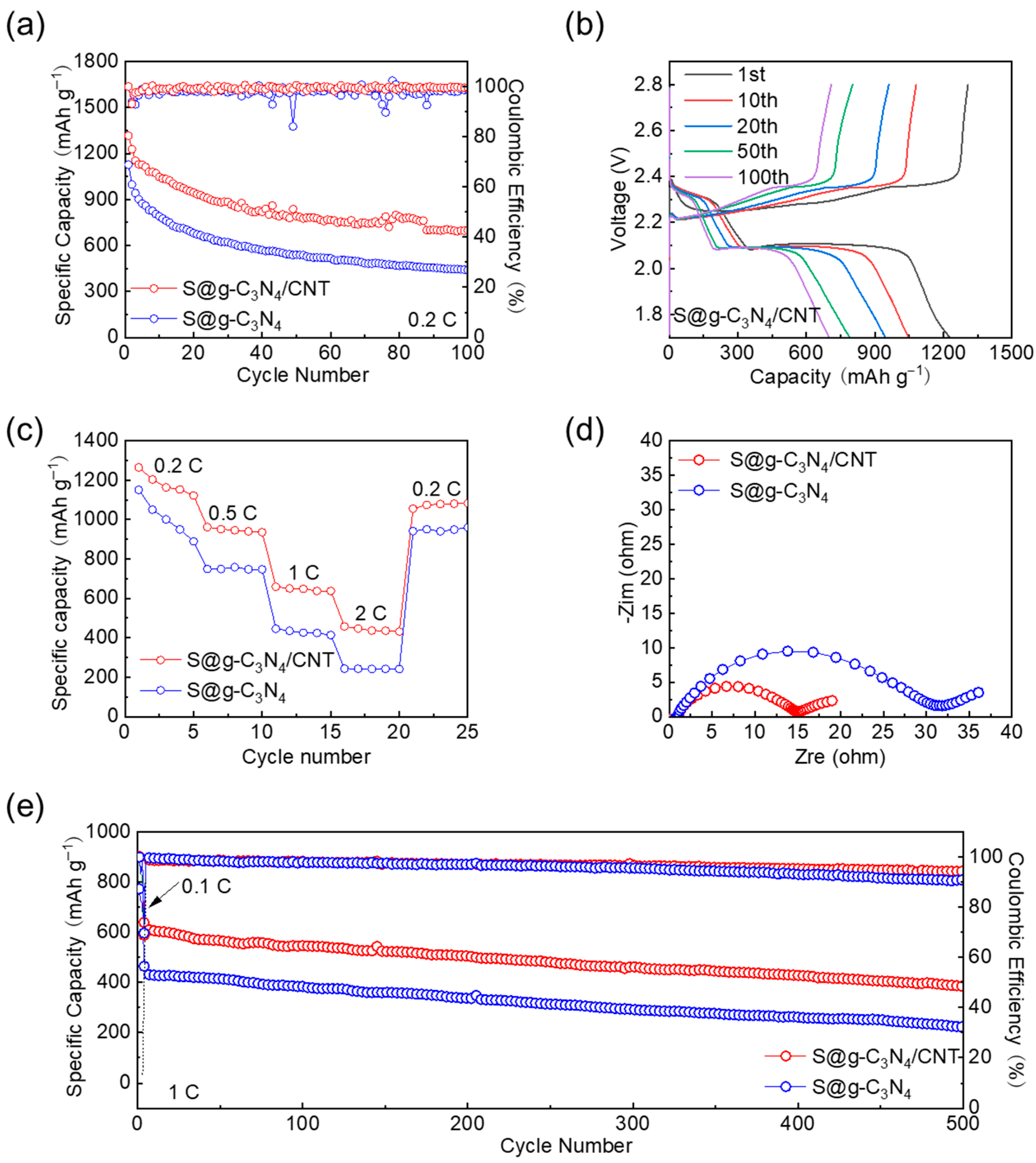
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.; Wang, Y.; Kang, Y.; Zhao, Y.; Tian, Y.; Wang, X.; Tan, Y.; Liang, Z.; Wozny, J.; Li, T.; et al. Side Reactions/Changes in Lithium-Ion Batteries: Mechanisms and Strategies for Creating Safer and Better Batteries. Adv. Mater. 2024, 36, 2401482. [Google Scholar] [CrossRef]
- Zheng, M.; You, Y.; Lu, J. Understanding materials failure mechanisms for the optimization of lithium-ion battery recycling. Nat. Rev. Mater. 2025, 10, 355. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, S.; Mishra, P.K. A critical review of recent progress on lithium-ion batteries: Challenges, applications, and future prospects. Microchem. J. 2025, 212, 113494. [Google Scholar] [CrossRef]
- Yamada, A. Hidden Negative Issues and Possible Solutions for Advancing the Development of High-Energy-Density in Lithium Batteries: A Review. Adv. Sci. 2024, 11, 2401739. [Google Scholar] [CrossRef]
- Fei, Y.; Li, G. Unveiling the Pivotal Parameters for Advancing High Energy Density in Lithium-Sulfur Batteries: A Comprehensive Review. Adv. Funct. Mater. 2024, 34, 2312550. [Google Scholar] [CrossRef]
- Li, S.; Chen, Z.; Chen, J.; Luo, X.; Qiu, X.; Qian, Y. Engineering of Lignocellulose Pulp Binder for Ah-Scale Lithium–Sulfur Batteries. Adv. Energy Mater. 2025, 15, 2405461. [Google Scholar] [CrossRef]
- Min, Y.; Zou, X.; Lu, Q.; Cai, W.; Bu, Y. Advances in the Catalytic Mechanism of Metal Oxides for Lithium–Sulfur Batteries. Small 2025, 21, 2411794. [Google Scholar] [CrossRef]
- Chen, J.; Fu, Y.; Guo, J. Development of Electrolytes under Lean Condition in Lithium–Sulfur Batteries. Adv. Mater. 2024, 36, 2401263. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.D.; Cha, E.; Choi, W. Towards the commercialization of Li-S battery: From lab to industry. Energy Storage Mater. 2024, 72, 103711. [Google Scholar] [CrossRef]
- Li, B.Q.; Zhang, S.Y.; Kong, L.; Peng, H.J.; Zhang, Q. Porphyrin organic framework hollow spheres and their applications in lithium-sulfur batteries. Adv. Mater. 2018, 30, 1707483. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, C.; Liu, W.; Yu, S.; Wang, L.; Wang, T.; Shi, C.; Zhao, X.; Chen, S.; Chou, S.; et al. Enabling Efficient Anchoring-Conversion Interface by Fabricating Double-Layer Functionalized Separator for Suppressing Shuttle Effect. Angew. Chem. Int. Ed. 2024, 136, e202407042. [Google Scholar] [CrossRef]
- Fu, Y.Z.; Su, Y.S.; Manthiram, A. Highly reversible lithium/dissolved polysulfide batteries with carbon nanotube electrodes. Angew. Chem. Int. Ed. 2013, 52, 6930–6935. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.R.; Ma, Z.; Wang, X.; Han, F.; Gao, T.; Fan, X.; Wang, J.Z.; Liu, H.K.; Dou, S.; Wang, C. Reverse microemulsion synthesis of sulfur/graphene composite for lithium/sulfur batteries. ACS Nano 2017, 11, 9048–9056. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Yu, F.; Wei, S.; Liu, J.; Que, L.; Wu, Y.; Pang, Q.; Lu, C.; Xie, Y. Two-in-one linkage reaction of aloe vera peel-derived porous carbon in lithium–sulfur batteries for synergistic enhancement of performance and stability. Chem. Eng. J. 2025, 513, 162707. [Google Scholar] [CrossRef]
- Nong, S.; Huang, D.; Li, Y.; Yang, R.; Xie, J.; Li, J.; Huang, H.; Liang, X.; Li, G.; Lan, Z.; et al. Multifunction-balanced porous carbon and its application in sulfur-loading host and separator modification for lithium–sulfur batteries. J. Energy Storage 2024, 81, 110296. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, D.; Muhammad, Z.; Wan, F.; Xie, W.; Wang, Y.; Song, L.; Niu, Z.; Chen, J. Single Nickel Atoms on Nitrogen-Doped Graphene Enabling Enhanced Kinetics of Lithium–Sulfur Batteries. Adv. Mater. 2019, 31, 1903955. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Y.; Zhang, S.; Wang, L.; Zhang, C.; Chen, Y.; Wu, Q.; Chen, L.; Zhou, L.; Wei, W. Integrating Energy Band Alignment and Oxygen Vacancies Engineering of TiO2 Anatase/Rutile Homojunction for Kinetics-Enhanced Li–S Batteries. Adv. Funct. Mater. 2023, 33, 2305788. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, H.; Hui, K.S.; Ye, Z.; Zha, C.; Lin, Z.; Zheng, M.; Lu, J.; Hui, K.N. Breaking the Passivation Effect for MnO2 Catalysts in Li−S Batteries by Anion-Cation Doping. Angew. Chem. Int. Ed. 2024, 63, e202408474. [Google Scholar] [CrossRef]
- Liang, J.; Sun, J.; Cao, X.; Li, X.; Chen, X.; Xing, R.; Kong, J. Enhanced Reaction Kinetics in Sodium-Ion Batteries Achieved by 3D Heterostructure CoS2/CoS with Self-Induced Internal Electric Field. Adv. Sci. 2025, 12, 2502241. [Google Scholar] [CrossRef]
- Ye, Z.; He, L.; Liao, J.; Huang, Y.; Jiang, Q. Preparation of multilayer core-shell WS2@CZIF-8@CZIF-67 and application in lithium-sulfur batteries. J. Alloys Compd. 2025, 1022, 179754. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Zhao, Y.; Yang, H.; Tong, H.; Fan, S.; Wang, Z.; Xu, W. Modulating the d-band center of single-atom catalysts for efficient Li2S2-Li2S conversion in durable lithium-sulfur batteries. Energy Storage Mater. 2024, 70, 103477. [Google Scholar] [CrossRef]
- Huang, C.; Sun, T.; Shu, H.; Chen, M.; Lian, Q.; Zhou, Y.; Gao, P.; Xu, S.; Yang, X.; Wu, M.; et al. Multifunctional reaction interfaces for capture and boost conversion of polysulfide in lithium-sulfur batteries. Electrochim. Acta 2020, 334, 135658. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Q.; Zhang, B.; Chen, C.; Lin, Z. Integrating conductivity, immobility, and catalytic ability into high-n carbon/graphene sheets as an effective sulfur host. Adv. Mater. 2020, 32, 1906357. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Li, L.; Liao, Y.; Luo, S.; Wu, Y.; Qing, Y. Polysulfides manipulation: Constructing g-C3N4 networks encapsulated into natural wood fibers for high-performance lithium–sulfur batteries. Chem. Eng. J. 2023, 461, 141988. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Yang, J.-L.; Tang, W.; Ge, B.; Liu, R. Bidirectional Catalyst with Robust Lithiophilicity and Sulfiphilicity for Advanced Lithium–Sulfur Battery. Adv. Funct. Mater. 2023, 33, 2302267. [Google Scholar] [CrossRef]
- Khasim, S.; Pasha, A.; Lakshmi, M.; Panneerselvam, C.; Ullah, M.F.; Darwish, A.A.A.; Hamdalla, T.A.; Alfadhli, S.; Al-Ghamdi, S.A. Synthesis of g-C3N4/CuO Nanocomposite as a Supercapacitor with Improved Electrochemical Performance for Energy Storage applications. Int. J. Electrochem. Sci. 2022, 17, 220838. [Google Scholar] [CrossRef]
- Huang, F.Y.; Zheng, T.; Zhang, K.; She, X.; Xu, H.; Fang, Z.; Xie, K. Facile fabrication of permselective g-C3N4 separator for improved lithium-sulfur batteries. Electrochim. Acta 2018, 272, 60–67. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, H.; Wang, Z.; Qin, N.; Huang, Y.; Cao, Y.; Li, Y.; Lu, W.; Zeng, C.; Liu, Q.; et al. Cu single atoms regulating nitrogen active-sites of g-C3N4 for sodium ion storage. Energy Storage Mater. 2024, 71, 103608. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.; Zhou, J.; Yao, X.; Li, J.; Zhuang, H.; Chen, Y.; Chen, Y.; Li, S.L.; Lan, Y.Q. Anisotropically Hybridized Porous Crystalline Li-S Battery Separators. Small 2023, 19, 2206616. [Google Scholar] [CrossRef]
- Jin, L.; Li, B.; Zhang, K.; Qian, X.; Zhao, S.; Xu, H. Sn Nanoparticles Encapsulated in Nanoporous Nitrogen-Rich Carbon Derived from Sn-MOF@ZIF-8 for Lithium–Sulfur Battery Separators. ACS Appl. Nano Mater. 2024, 7, 19538–19547. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, W.; Zhu, Y.; Ma, J.; Zhou, M.; Shi, Y.; Yan, P.; Liu, R. Catalytic VS2–VO2 Heterostructure that Enables a Self-Supporting Li2S Cathode for Superior Lithium–Sulfur Batteries. Small Methods 2023, 7, 2300186. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Dong, L.; Gao, X.; Wang, G.; Shao, G. Enabling immobilization and conversion of polysulfides through a nitrogen-doped carbon nanotubes/ultrathin MoS2 nanosheet core-shell architecture for lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 13103–13112. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Reyhani, R.; Zadhoush, A.; Tabrizi, N.S.; Nazockdast, H.; Naeimirad, M. Synthesis and characterization of powdered CNT-doped carbon aerogels. J. Non-Cryst. Solids 2021, 571, 121058. [Google Scholar] [CrossRef]
- Fang, S.; Xia, Y.; Lv, K.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Wei, Z.Z.; Zhang, N.X.; Feng, T.; Wu, F.; Zhao, T.; Chen, R.J. A copolymer microspheres-coated separator to enhance thermal stability of lithium-sulfur batteries. Chem. Eng. J. 2022, 430, 10. [Google Scholar] [CrossRef]
- Saini, R.; Farooq, U.; Imam, F. Investigation of the Synergistic Effect of Doping and Composite Formation on Electrochemical OER Performance of g-C3N4 for Sustainable Energy. Energy Fuels 2025, 39, 1327. [Google Scholar] [CrossRef]
- Kaya, S. Synthesis, characterization and 1-propanol electrooxidation application of carbon nanotube supported bimetallic catalysts. Int. J. Hydrogen Energy 2023, 48, 18398–18404. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, M.; Wei, C.; Yuan, Z.; Gu, X.; Wang, J.; Wu, L.; Hu, Y.; Yang, L.; Wang, X.; et al. Design of self-supporting porous soft-hard carbon hybrid materials for ultra-long cycling lithium-ion storage. J. Power Sources 2025, 649, 237249. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Ouyang, S.; Lu, D.; Wang, X.; Wang, D.; Ye, J. In situ surface alkalinized g-C3N4 toward enhancement of photocatalytic H2 evolution under visible-light irradiation. J. Mater. Chem. A 2016, 4, 2943–2950. [Google Scholar] [CrossRef]
- Jiang, D.; Ma, W.; Xiao, P.; Shao, L.; Li, D.; Chen, M. Enhanced photocatalytic activity of graphitic carbon nitride/carbon nanotube/Bi2WO6 ternary Z-scheme heterojunction with carbon nanotube as efficient electron mediator. J. Colloid Interface Sci. 2018, 512, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater. 2017, 29, 1605148–1605155. [Google Scholar] [CrossRef]
- Cai, D.Q.; Yang, J.L.; Liu, T.; Zhao, S.X.; Cao, G. Interfaces-dominated Li2S nucleation behavior enabled by heterostructure catalyst for fast kinetics Li-S batteries. Nano Energy 2021, 89, 106452. [Google Scholar] [CrossRef]
- Sun, R.; Qu, M.; Peng, L.; Yang, W.; Wang, Z.; Bai, Y.; Sun, K. Regulating electrochemical kinetics of CoP by incorporating oxygen on surface for high-performance Li–S batteries. Small 2023, 19, 2302092. [Google Scholar] [CrossRef] [PubMed]
- Talapaneni, S.N.; Hwang, T.H.; Je, S.H.; Buyukcakir, O.; Choi, J.W.; Coskun, A. Elemental-sulfur-mediated facile synthesis of a covalent triazine framework for high-performance lithium–sulfur batteries. Angew. Chem. Int. Ed. 2016, 55, 3106–3111. [Google Scholar] [CrossRef]
- Xu, F.; Yang, S.; Jiang, G.; Ye, Q.; Wei, B.; Wang, H. Fluorinated, sulfur-rich, covalent triazine frameworks for enhanced confinement of polysulfides in lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 37731–37738. [Google Scholar] [CrossRef]
- Je, S.H.; Kim, H.J.; Kim, J.; Choi, J.W.; Coskun, A. Perfluoroaryl-elemental sulfur SNAr chemistry in covalent triazine frameworks with high sulfur contents for lithium–sulfur batteries. Adv. Funct. Mater. 2017, 27, 1703947. [Google Scholar] [CrossRef]
- Meng, Y.; Lin, G.; Ding, H.; Liao, H.; Wang, C. Impregnation of sulfur into a 2D pyrene-based covalent organic framework for high-rate lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 17186–17191. [Google Scholar] [CrossRef]
- Wang, D.G.; Tan, L.; Wang, H.; Song, M.; Wang, J.; Kuang, G.C. Multiple Covalent Triazine Frameworks with Strong Polysulfide Chemisorption for Enhanced Lithium-Sulfur Batteries. ChemElectroChem 2019, 6, 2777–2781. [Google Scholar] [CrossRef]
- Xu, F.; Yang, S.; Chen, X.; Liu, Q.; Li, H.; Wang, H.; Wei, B.; Jiang, D. Energy-storage covalent organic frameworks: Improving performance via engineering polysulfide chains on walls. Chem. Sci. 2019, 10, 6001–6006. [Google Scholar] [CrossRef]
- Hu, X.; Jian, J.; Fang, Z.; Zhong, L.; Yuan, Z.; Yang, M.; Ren, S.; Zhang, Q.; Chen, X.; Yu, D. Hierarchical assemblies of conjugated ultrathin COF nanosheets for high-sulfur-loading and long-lifespan lithium–sulfur batteries: Fully-exposed porphyrin matters. Energy Storage Mater. 2019, 22, 40–47. [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, H.; Wang, R.; Zang, S.Q.; Mak, T.C. Cationic Covalent-Organic Framework as Efficient Redox Motor for High-Performance Lithium–Sulfur Batteries. Small 2020, 16, 2002932. [Google Scholar] [CrossRef]
- Gao, G.; Jia, Y.; Gao, H.; Shi, W.; Yu, J.; Yang, Z.; Dong, Z.; Zhao, Y. New covalent triazine framework rich in nitrogen and oxygen as a host material for lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2021, 13, 50258–50269. [Google Scholar] [CrossRef]
- Wang, D.G.; Li, N.; Hu, Y.; Wan, S.; Song, M.; Yu, G.; Jin, Y.; Wei, W.; Han, K.; Kuang, G.C. Highly fluoro-substituted covalent organic framework and its application in lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 42233–42240. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, C.; Meng, R.; Zu, L.; Feng, Y.; Chen, B.; Mi, Y.; Zhang, C.; Yang, J. Hybrid anatase/rutile nanodots-embedded covalent organic frameworks with complementary polysulfide adsorption for high-performance lithium–sulfur batteries. ACS Cent. Sci. 2019, 5, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Elabd, A.; Chung, S.Y.; Coskun, A.; Choi, J.W. Covalent triazine frameworks incorporating charged polypyrrole channels for high-performance lithium–sulfur batteries. Chem. Mater. 2020, 32, 4185–4193. [Google Scholar] [CrossRef]
- Haldar, S.; Wang, M.; Bhauriyal, P.; Hazra, A.; Khan, A.H.; Bon, V.; Isaacs, M.A.; De, A.; Shupletsov, L.; Boenke, T. Porous dithiine-linked covalent organic framework as a dynamic platform for covalent polysulfide anchoring in lithium–sulfur battery cathodes. J. Am. Chem. Soc. 2022, 144, 9101–9112. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, J.; Wan, T.; Luo, Y.; Liu, G.; Li, J. A COF-coated ordered porous framework as multifunctional polysulfide barrier towards high-performance lithium-sulfur batteries. J. Colloid Interface Sci. 2023, 638, 542–551. [Google Scholar] [CrossRef]
- Yan, R.; Mishra, B.; Traxler, M.; Roeser, J.; Chaoui, N.; Kumbhakar, B.; Schmidt, J.; Li, S.; Thomas, A.; Pachfule, P. A Thiazole-linked Covalent Organic Framework for Lithium-Sulphur Batteries. Angew. Chem. Int. Ed. 2023, 62, e202302276. [Google Scholar] [CrossRef]
- Liu, X.; Ding, X.; Zheng, T.; Jin, Y.; Wang, H.; Yang, X.; Yu, B.; Jiang, J. Single Cobalt Ion-Immobilized Covalent Organic Framework for Lithium–Sulfur Batteries with Enhanced Rate Capabilities. ACS Appl. Mater. Interfaces 2024, 16, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhu, M.; Jin, Z.; Zhang, Y.; Yan, X.; Peng, H.; Song, Y.; Dong, Y.; Zhang, J. Regulation of covalent organic framework pore structure for boosting electrochemical performances of lithium-sulfur battery. J. Colloid Interface Sci. 2025, 138154. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.D.; Neem, M.; Kumar, G.; Modak, B.; Neogi, S.; Mandal, B.P. Diamondoid covalent organic framework-MXene composite for cathode host in lithium sulfur battery. J. Energy Storage 2025, 117, 116176. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Meng, H.; Wang, J.; Yang, L.; Wang, X.; Chen, Z. High-Efficiency Polysulfide Trapping with g-C3N4/CNT Hybrids for Superior Lithium-Sulfur Batteries. Energies 2025, 18, 4462. https://doi.org/10.3390/en18174462
Chen Z, Meng H, Wang J, Yang L, Wang X, Chen Z. High-Efficiency Polysulfide Trapping with g-C3N4/CNT Hybrids for Superior Lithium-Sulfur Batteries. Energies. 2025; 18(17):4462. https://doi.org/10.3390/en18174462
Chicago/Turabian StyleChen, Zhen, Hao Meng, Jiayi Wang, Lin Yang, Xin Wang, and Zhongwei Chen. 2025. "High-Efficiency Polysulfide Trapping with g-C3N4/CNT Hybrids for Superior Lithium-Sulfur Batteries" Energies 18, no. 17: 4462. https://doi.org/10.3390/en18174462
APA StyleChen, Z., Meng, H., Wang, J., Yang, L., Wang, X., & Chen, Z. (2025). High-Efficiency Polysulfide Trapping with g-C3N4/CNT Hybrids for Superior Lithium-Sulfur Batteries. Energies, 18(17), 4462. https://doi.org/10.3390/en18174462




