Low-Emission Hydrogen for Transport—A Technology Overview from Hydrogen Production to Its Use to Power Vehicles
Abstract
1. Introduction
2. Research Methods and Tools
3. Low-Emission Hydrogen Production
3.1. Generating Power from Renewable Energy Sources for Green Hydrogen Production
3.2. Production of Green Hydrogen by Water Electrolysis Methods Powered by Renewable Energy Sources
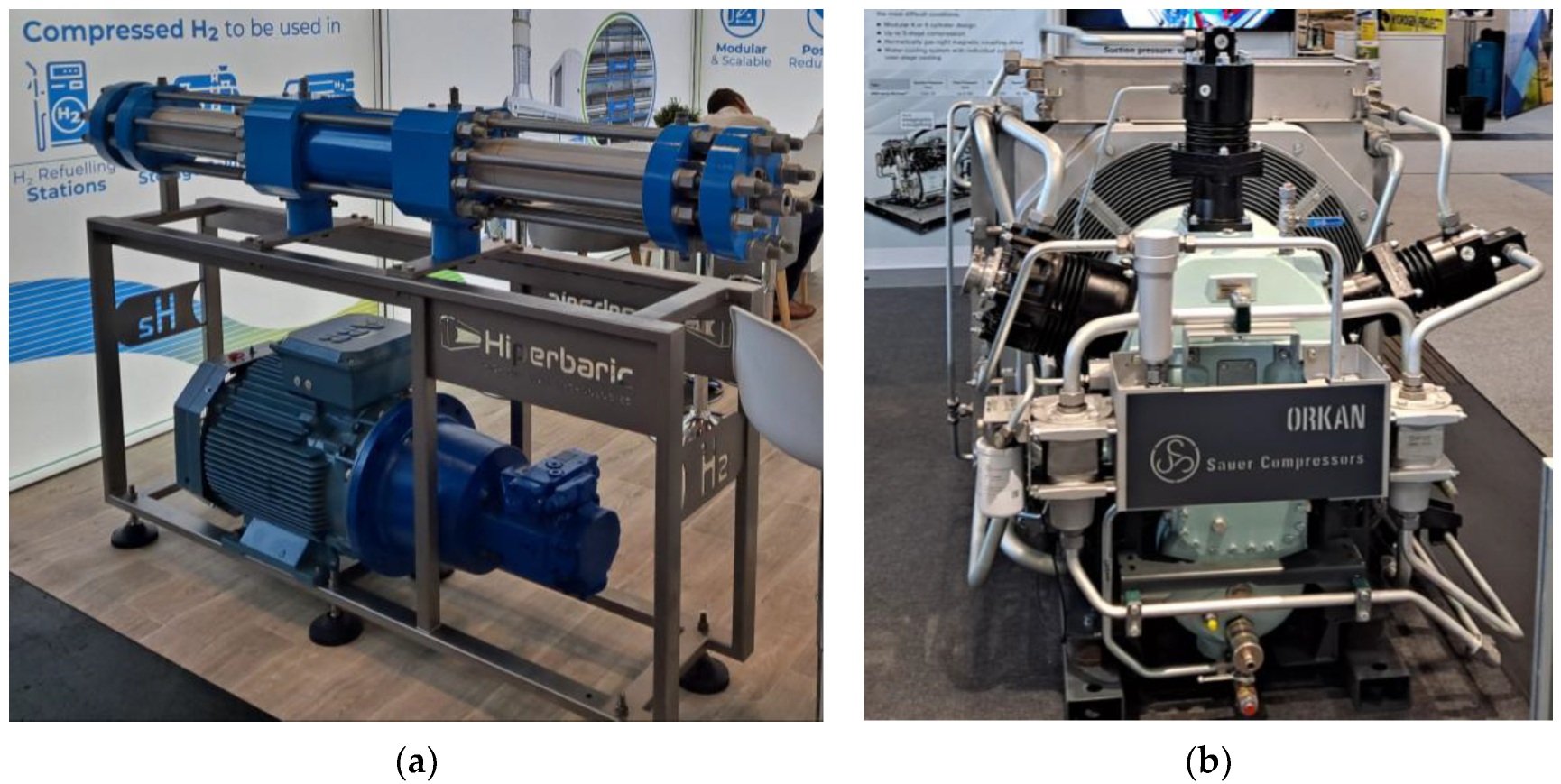
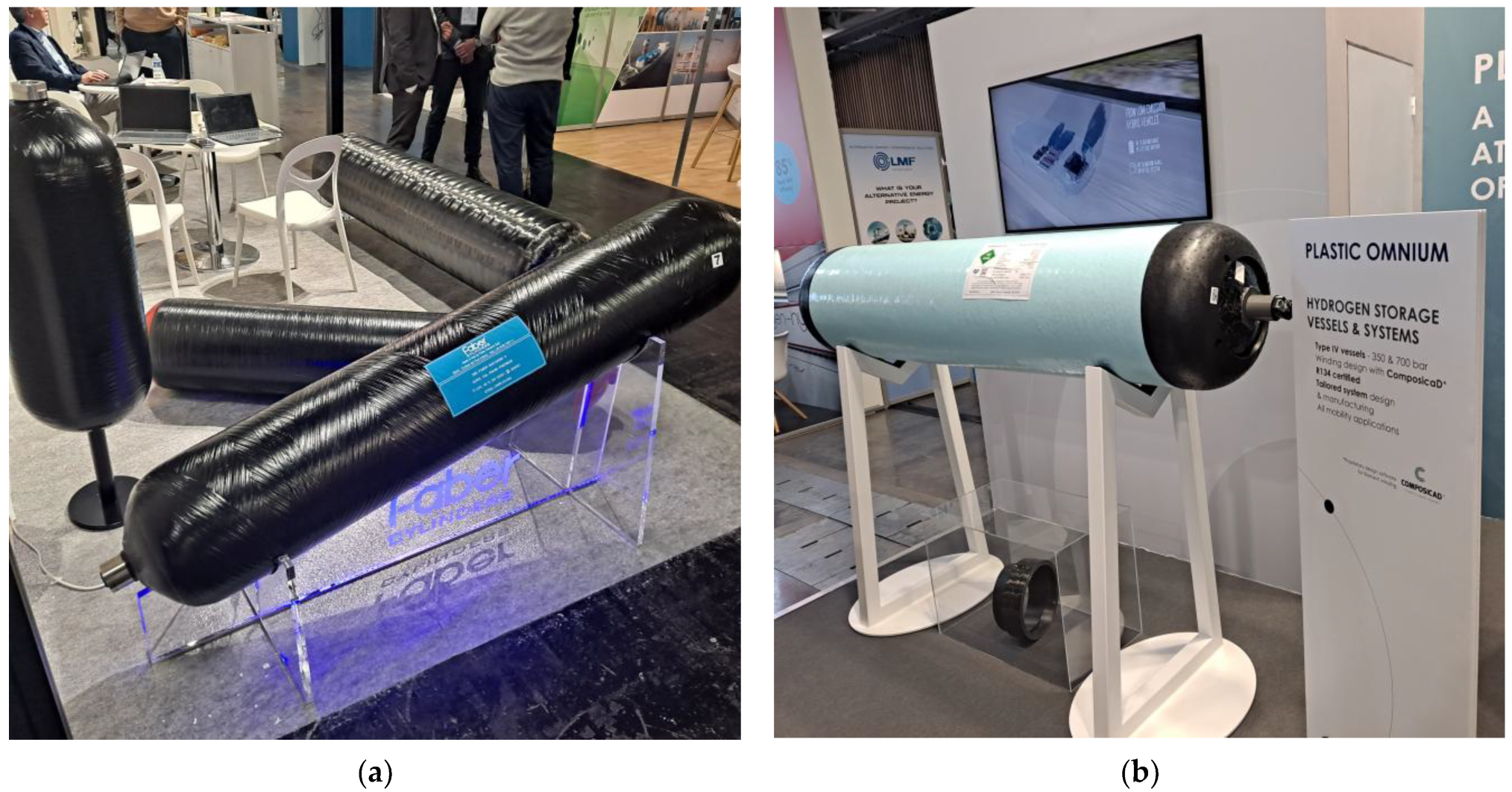
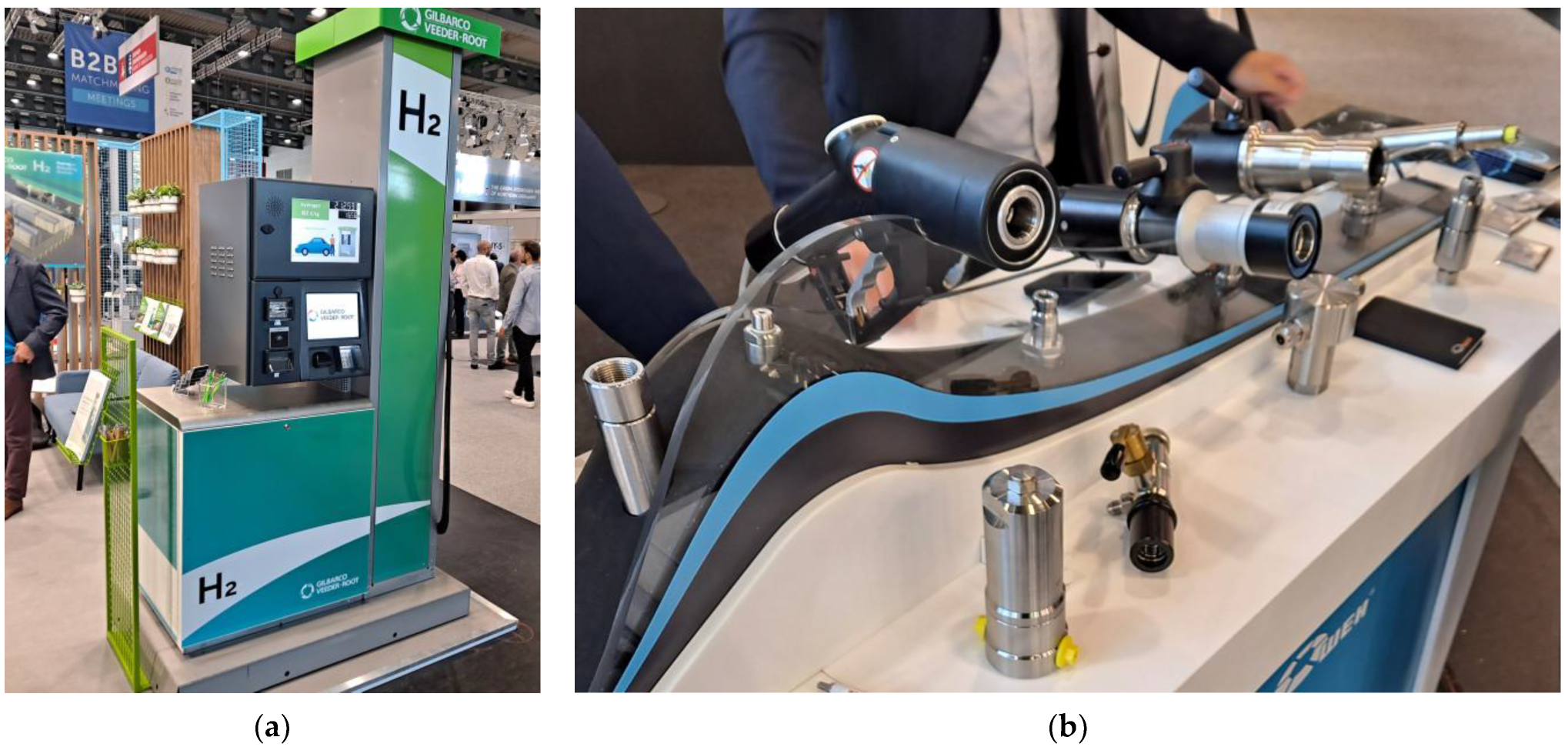
4. Compressing, Transporting, and Refueling Hydrogen
5. Hydrogen as a Fuel for Hydrogen Fuel Cells and Internal Combustion Engines
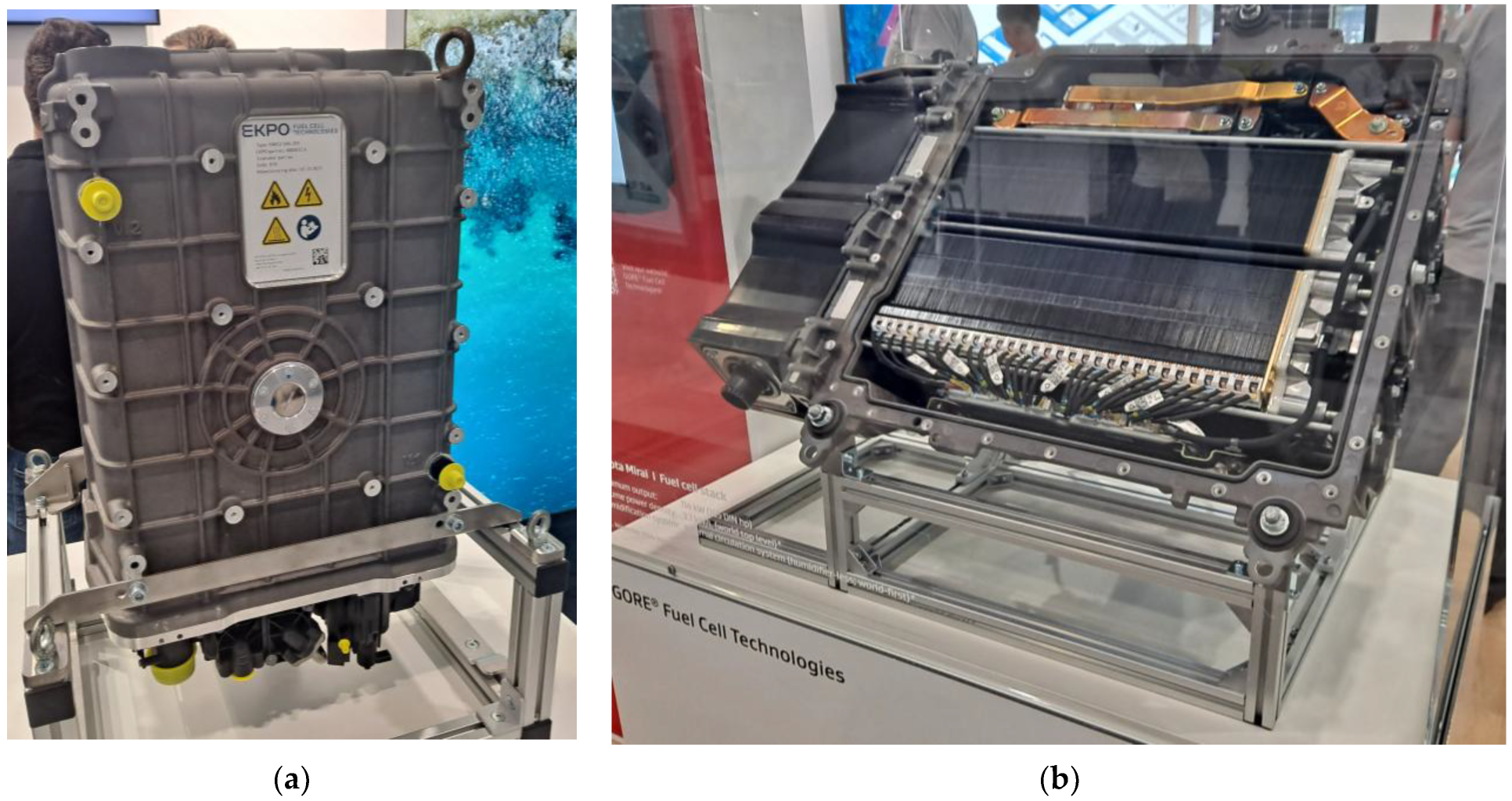
5.1. Hydrogen as a Fuel for Hydrogen Fuel Cells
5.2. Selected Problems in the Implementation of Hydrogen Fuel Cell Vehicles
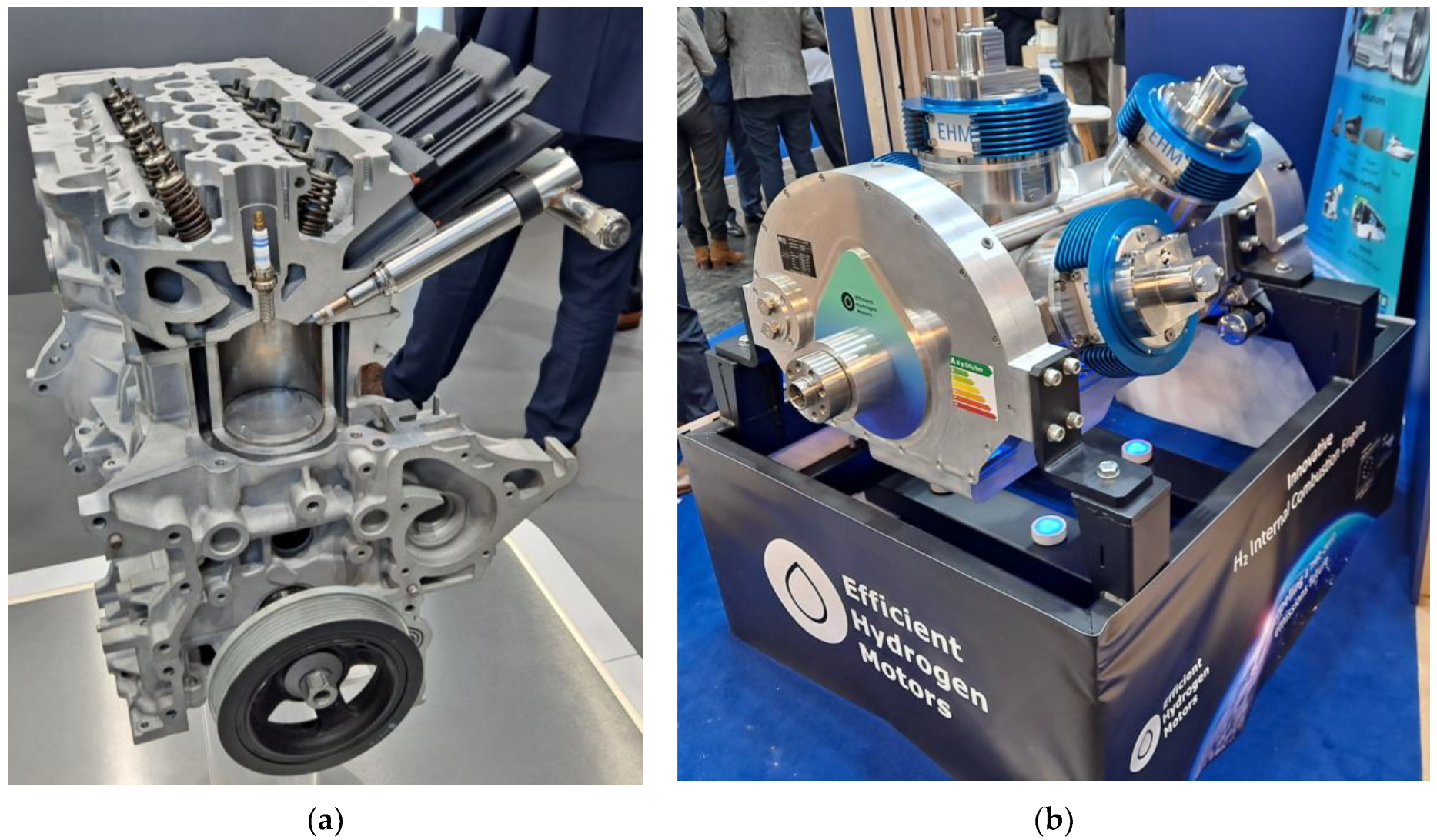
5.3. Hydrogen as a Fuel for Internal Combustion Engines
5.4. Hydrogen Fuel Cell Vehicles and Hydrogen Combustion Engine Vehicles—Bottlenecks in Commercialization
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEM | Anion Exchange Membrane |
| ATA | Air Transport Association |
| CCS | Carbon Capture and Storage |
| CFD | Computational Fluid Dynamics |
| CO2 | Carbon Dioxide |
| EMC | Electromagnetic Compatibility |
| EMS | Energy Management System |
| ENTSO | European Network of Transmission System Operators |
| ESS | Energy Storage System |
| EU | European Union |
| EXPO | International Exposition |
| HD | Heavy Duty |
| HRS | Hydrogen Refueling Station |
| HTGR | High-Temperature Gas-cooled Reactor |
| HTR | High-Temperature Reactor |
| IEC | International Electrotechnical Commission |
| IET | Institution of Engineering and Technology |
| IPCC | Intergovernmental Panel on Climate Change |
| IRENA | International Renewable Energy Agency |
| ISO | International Organization for Standardization |
| LHV | Lower Heating Value |
| LTO | Lithium Titanate (Battery) |
| NASA | National Aeronautics and Space Administration |
| NEDC | New European Driving Cycle |
| NREL | National Renewable Energy Laboratory |
| PEM | Proton Exchange Membrane |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| PEMWE | Proton Exchange Membrane Water Electrolyzer |
| PM | Particulate Matter |
| PV | Photovoltaic |
| SOEC | Solid-Oxide Electrolysis Cell |
| SOFC | Solid-Oxide Fuel Cell |
| TCO | Total Cost of Ownership |
| TEN | Trans-European Networks |
| WLTP | Worldwide Harmonized Light Vehicles Test Procedure |
References
- Sharma, V.K.; Monteleone, G.; Braccio, G.; Anyanwu, C.N.; Aneke, N.N. A Comprehensive Review of Green Energy Technologies: Towards Sustainable Clean Energy Transition and Global Net-Zero Carbon Emissions. Processes 2025, 13, 69. [Google Scholar] [CrossRef]
- Kotowicz, J.; Baszczeńska, O.; Niesporek, K. Cost of Green Hydrogen. Energies 2024, 17, 4651. [Google Scholar] [CrossRef]
- Pivetta, D.; Dall’Armi, C.; Taccani, R. Multi-Objective Optimization of a Hydrogen Hub for the Decarbonization of a Port Industrial Area. J. Mar. Sci. Eng. 2022, 10, 231. [Google Scholar] [CrossRef]
- Ustolin, F.; Campari, A.; Taccani, R. An Extensive Review of Liquid Hydrogen in Transportation with Focus on the Maritime Sector. J. Mar. Sci. Eng. 2022, 10, 1222. [Google Scholar] [CrossRef]
- Algburi, S.; Munther, H.; Fakhruldeen, H.F.; Sapaev, I.B.; Al-Dulaimi, O.; Al Seedi, K.F.K.; Khalaf, D.H.; Jabbar, F.I.; Hassan, Q.; Khudhair, A.; et al. Green Hydrogen Role in Sustainable Energy Transformations: A Review. Results Eng. 2025, 19, 105109. [Google Scholar] [CrossRef]
- Gómez, J.; Castro, R. Green Hydrogen Energy Systems: A Review on Their Contribution to a Renewable Energy System. Energies 2024, 17, 3110. [Google Scholar] [CrossRef]
- Dai, S.; Shen, P.; Deng, W.; Yu, Q. Hydrogen Energy in Electrical Power Systems: A Review and Future Outlook. Electronics 2024, 13, 3370. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Zhuo, G.; Xu, C. Configuration Optimization of Hydrogen-Based Multi-Microgrid Systems under Electricity Market Trading and Different Hydrogen Production Strategies. Sustainability 2023, 15, 6753. [Google Scholar] [CrossRef]
- Olabi, A.G.; Sayed, E.T. Developments in Hydrogen Fuel Cells. Energies 2023, 16, 2431. [Google Scholar] [CrossRef]
- Cărăușan, H.; Varga, B.O.; Moldovanu, D.; Prunean, G.; Oargă, I.-T. Energy Efficiency Analysis of a Fuel Cell Bus Model Using Real Scenarios Generated by Data Collection. Sustainability 2024, 16, 1863. [Google Scholar] [CrossRef]
- Kim, M.-S.; Jeon, H.-K.; Lee, K.-W.; Ryu, J.-H.; Choi, S.-W. Analysis of Hydrogen Filling of 175 Liter Tank for Large-Sized Hydrogen Vehicle. Appl. Sci. 2022, 12, 4856. [Google Scholar] [CrossRef]
- Gul, W.; Xia, Y.E.; Gérard, P.; Ha, S.K. Characterization of Polymeric Composites for Hydrogen Tank. Polymers 2023, 15, 3716. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H. Total Cost of Ownership Analysis of Fuel Cell Electric Bus with Different Hydrogen Supply Alternatives. Sustainability 2024, 16, 259. [Google Scholar] [CrossRef]
- SAE J2572; Recommended Practice for Measuring Fuel Consumption and Range of Fuel Cell and Hybrid Fuel Cell Vehicles Fueled by Compressed Gaseous Hydrogen. SAE International: Warrendale, Pennsylvania, PA, USA, 2024.
- Borghetti, F.; Longo, M.; Bonera, M.; Libretti, M.; Somaschini, C.; Martinelli, V.; Medeghini, M.; Mazzoncini, R. Battery Electric Buses or Fuel Cell Electric Buses? A Decarbonization Case Study in the City of Brescia, Italy. Infrastructures 2023, 8, 178. [Google Scholar] [CrossRef]
- Bae, S.; Lee, E.; Han, J. Multi-Period Planning of Hydrogen Supply Network for Refuelling Hydrogen Fuel Cell Vehicles in Urban Areas. Sustainability 2020, 12, 4114. [Google Scholar] [CrossRef]
- Wu, Q.; Dong, Z.; Zhang, X.; Zhang, C.; Iqbal, A.; Chen, J. Towards More Efficient PEM Fuel Cells Through Advanced Thermal Management: From Mechanisms to Applications. Sustainability 2025, 17, 943. [Google Scholar] [CrossRef]
- Padovan, C.; Fagundes, J.A.G.; D’Agosto, M.d.A.; Angelo, A.C.M.; Carneiro, P.J.P. Impact of Fuel Production Technologies on Energy Consumption and GHG Emissions from Diesel and Electric–Hydrogen Hybrid Buses in Rio de Janeiro, Brazil. Sustainability 2023, 15, 7400. [Google Scholar] [CrossRef]
- Muñoz Díaz, M.T.; Chávez Oróstica, H.; Guajardo, J. Economic Analysis: Green Hydrogen Production Systems. Processes 2023, 11, 1390. [Google Scholar] [CrossRef]
- Hidouri, D.; Ben Omrane, I.; Khalil, K.; Cherif, A. Energy Management of a 1 MW Photovoltaic Power-to-Electricity and Power-to-Gas for Green Hydrogen Storage Station. World Electr. Veh. J. 2025, 16, 227. [Google Scholar] [CrossRef]
- Ciuła, J.; Generowicz, A.; Gronba-Chyła, A.; Wiewiórska, I.; Kwaśnicki, P.; Cygnar, M. Analysis of the Efficiency of Landfill Gas Treatment for Power Generation in a Cogeneration System in Terms of the European Green Deal. Sustainability 2024, 16, 1479. [Google Scholar] [CrossRef]
- Jeje, S.O.; Marazani, T.; Obiko, J.; Shongwe, M.B. Advancing the hydrogen production economy: A comprehensive review of technologies, sustainability, and future prospects. Int. J. Hydrogen Energy 2024, 49, 642–661. [Google Scholar] [CrossRef]
- Brandt, J.; Iversen, T.; Eckert, C.; Peterssen, F.; Bensmann, B.; Bensmann, A.; Beer, M.; Weyer, H.; Hanke-Rauschenbach, R. Cost and competitiveness of green hydrogen and the effects of the European Union regulatory framework. Nat. Energy 2024, 9, 703–713. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, H.; Baimbetov, D.; Syrlybekkyzy, S.; Akhmetov, B.B.; Abbas, Q. Development Status and Future Prospects of Hydrogen Energy Technology: Production, Storage, and Cost Analysis. Adv. Energy Sustain. Res. 2025, 2400451. [Google Scholar] [CrossRef]
- Mekuye, B.; Mebratie, G.; Abera, B.; Yibelital, A.; Lake, A.; Tolesa, A. Energy: An Overview of Type, Form, Storage, Advantages, Efficiency, and Their Impact. Highlights Sci. Eng. Technol. CEAM 2025, 116, 237–245. [Google Scholar] [CrossRef]
- Sebbagh, T.; Şahin, M.E.; Beldjaatit, C. Green hydrogen revolution for a sustainable energy future. Clean Technol. Environ. Policy 2024, 26, 4017–4040. [Google Scholar] [CrossRef]
- Akpasi, S.O.; Anekwe, I.M.S.; Tetteh, E.; Amune, U.; Mustapha, S.I.; Elshafie, A.E. Hydrogen as a Clean Energy Carrier: Advancements, Challenges, and its Role in a Sustainable Energy Future. Clean Energy 2025, 9, 52–88. [Google Scholar] [CrossRef]
- Münster, M.; Bramstoft, R.; Kountouris, I.; Langer, L.; Keles, D.; Schlautmann, R.; Mörs, F.; Saccani, C.; Guzzini, A.; Pellegrini, M.; et al. Perspectives on green hydrogen in Europe—During an energy crisis and towards future climate neutrality. Oxf. Open Energy 2024, 3, oiae001. [Google Scholar] [CrossRef]
- Lebepe, M.C.; Oviroh, P.O.; Jen, T.C. Techno-economic optimisation modelling of a solar-powered hydrogen production system for green hydrogen generation. Sustain. Energy Res. 2025, 12, 11. [Google Scholar] [CrossRef]
- Fan, Z. The Role of Hydrogen in Achieving Sustainable Energy Systems: Challenges and Opportunities. Highlights Sci. Eng. Technol. CEAM 2024, 116, 237–245. [Google Scholar] [CrossRef]
- Perissi, I.; Jones, A. Investigating European Union Decarbonization Strategies: Evaluating the Pathway to Carbon Neutrality by 2050. Sustainability 2022, 14, 4728. [Google Scholar] [CrossRef]
- Muhsen, H.; Hamida, F.; Tarawneh, R. The Potential of Green Hydrogen and Power-to-X to Decarbonize the Fertilizer Industry in Jordan. Agriculture 2025, 15, 608. [Google Scholar] [CrossRef]
- Blažek, J.; Toullis, D.; Straka, P.; Staš, M.; Šimáček, P. Influence of Pressure on Product Composition and Hydrogen Consumption in Hydrotreating of Gas Oil and Rapeseed Oil Blends over a NiMo Catalyst. Catalysts 2021, 11, 1093. [Google Scholar] [CrossRef]
- Tuluhong, A.; Chang, Q.; Xie, L.; Xu, Z.; Song, T. Current Status of Green Hydrogen Production Technology: A Review. Sustainability 2024, 16, 9070. [Google Scholar] [CrossRef]
- Pinchao, P.; Torres, A.; Yánez, M.; Reina, S.; Cando, E. Analysis for the Implementation of Surplus Hydropower for Green Hydrogen Production in Ecuador. Energies 2024, 17, 6051. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Saars, L.; Madsen, M.; Meyer, J. Optimizing the Operation of an Electrolyzer with Hydrogen Storage Using Two Different Methods: A Trade-Off Between Simplicity and Precision in Minimizing Hydrogen Production Costs Using Day-Ahead Market Prices. Energies 2024, 17, 5546. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Chan, E.Y.H.; Adi Putra, Z.; Tan, R.R.; Wan, Y.K.; Foo, D.C.Y. Carbon Footprint Analysis of Chemical Production: A Case Study of Blue Hydrogen Production. Processes 2025, 13, 1254. [Google Scholar] [CrossRef]
- Scheiblehner, D.; Antrekowitsch, H.; Neuschitzer, D.; Wibner, S.; Sprung, A. Hydrogen Production by Methane Pyrolysis in Molten Cu-Ni-Sn Alloys. Metals 2023, 13, 1310. [Google Scholar] [CrossRef]
- Al-Shikh, S.A.; Al-Ammar, E.A.; Alomari, A.S. Economic Feasibility of Hydrogen Generation Using HTR-PM Technology in Saudi Arabia. Sustainability 2025, 17, 1730. [Google Scholar] [CrossRef]
- Hildebrand, J.; Kortsch, T.; Rau, I. The Public Acceptance of Power-to-X Technologies—Results from Environmental–Psychological Research Using a Representative German Sample. Sustainability 2025, 17, 6574. [Google Scholar] [CrossRef]
- Franco, A.; Carcasci, C.; Ademollo, A.; Calabrese, M.; Giovannini, C. Integrated Plant Design for Green Hydrogen Production and Power Generation in Photovoltaic Systems: Balancing Electrolyzer Sizing and Storage. Hydrogen 2025, 6, 7. [Google Scholar] [CrossRef]
- Schleifer Anna, H.; Harrison-Atlas Dylan Cole Wesley, J.; Murphy Caitlin, A. Hybrid renewable energy systems: The value of storage as a function of PV-wind variability. Front. Energy Res. 2023, 11, 1036183. [Google Scholar] [CrossRef]
- Osman, M.G.; Lazaroiu, G.; Hamad, S.A.; Messaoud, H.; Mohammed, D.; Stoica, D. Analysis of Photovoltaic Systems with Battery Storage, Electric Vehicle Charging, and Smart Energy Management. Sustainability 2025, 17, 3887. [Google Scholar] [CrossRef]
- Wang, W.; Qi, Y.; Zhang, X.; Xie, P.; Guo, Y.; Sun, H. Carbon Emission Optimization of the Integrated Energy System in Industrial Parks with Hydrogen Production from Complementary Wind and Solar Systems. Hydrogen 2025, 6, 8. [Google Scholar] [CrossRef]
- Tumse, S.; Bilgili, M.; Yildirim, A.; Sahin, B. Comparative Analysis of Global Onshore and Offshore Wind Energy Characteristics and Potentials. Sustainability 2024, 16, 6614. [Google Scholar] [CrossRef]
- Olczak, P.; Surma, T. Energy Productivity Potential of Offshore Wind in Poland and Cooperation with Onshore Wind Farm. Appl. Sci. 2023, 13, 4258. [Google Scholar] [CrossRef]
- Azarbakhsh, G.; Mahmoudi, A.; Kahourzade, S.; Yazdani, A.; Mahmud, A. Optimal Sizing of Photovoltaic and Battery Energy Storage for Residential Houses in South Australia by Considering Vehicle-to-Home Operation. IET Renew. Power Gener. 2025, 19, e70053. [Google Scholar] [CrossRef]
- Golub, G.; Blažauskas, E.; Tsyvenkova, N.; Šarauskis, E.; Jasinskas, A.; Kukharets, S.; Nadykto, V.; Holubenko, A. Determination of the Installation Efficiency of Vertical Stationary Photovoltaic Modules with a Double-Sided “East–West”-Oriented Solar Panel. Appl. Sci. 2025, 15, 1635. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Aly, M.; Mostafa, M.; Rezk, H.; Alnuman, H.; Alhosaini, W. An Accurate Model for Bifacial Photovoltaic Panels. Sustainability 2023, 15, 509. [Google Scholar] [CrossRef]
- Ross, K.; Matuszewska, D.; Olczak, P. Analysis of Using Hybrid 1 MWp PV-Farm with Energy Storage in Poland. Energies 2023, 16, 7654. [Google Scholar] [CrossRef]
- de Luis-Ruiz, J.M.; Salas-Menocal, B.R.; Pereda-García, R.; Pérez-Álvarez, R.; Sedano-Cibrián, J.; Ruiz-Fernández, C. Optimal Location of Solar Photovoltaic Plants Using Geographic Information Systems and Multi-Criteria Analysis. Sustainability 2024, 16, 2895. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X. Numerical Investigation of Aerodynamic Performances for NREL 5-MW Offshore Wind Turbine. Wind 2023, 3, 191–212. [Google Scholar] [CrossRef]
- Saenz-Aguirre, A.; Fernandez-Resines, S.; Aramendia, I.; Fernandez-Gamiz, U.; Zulueta, E.; Lopez-Guede, J.M.; Sancho, J. 5 MW Wind Turbine Annual Energy Production Improvement by Flow Control Devices. Proceedings 2018, 2, 1452. [Google Scholar] [CrossRef]
- Reddy, Y.-s.; Hur, S.-h. Comparison of Optimal Control Designs for a 5 MW Wind Turbine. Appl. Sci. 2021, 11, 8774. [Google Scholar] [CrossRef]
- Małek, A.; Marciniak, A.; Bednarczyk, T. Probabilistic Analysis of Electricity Production from a Photovoltaic–Wind Energy Mix for Sustainable Transport Needs. Sustainability 2024, 16, 10164. [Google Scholar] [CrossRef]
- Małek, A.; Dudziak, A.; Caban, J.; Matijošius, J. Probabilistic Analysis of Low-Emission Hydrogen Production from a Photovoltaic Carport. Appl. Sci. 2024, 14, 9531. [Google Scholar] [CrossRef]
- Šarkan, B.; Caban, J.; Małek, A.; Marciniak, A. Determining Signatures for Energy Mix Produced by Photovoltaic Systems and Wind Turbines. Appl. Sci. 2025, 15, 1800. [Google Scholar] [CrossRef]
- Abdelkader, S.; Amissah, J.; Abdel-Rahim, O. Virtual power plants: An in-depth analysis of their advancements and importance as crucial players in modern power systems. Energ. Sustain. Soc. 2024, 14, 52. [Google Scholar] [CrossRef]
- Luis, R.; Tiago, S.; Igor, R.; João, F.; Vladimiro, M. Virtual power plant optimal dispatch considering power-to-hydrogen systems. Int. J. Hydrogen Energy 2024, 68, 1019–1032. [Google Scholar] [CrossRef]
- Yang, Z.; Li, K.; Chen, J. Robust scheduling of virtual power plant with power-to-hydrogen considering a flexible carbon emission mechanism. Electr. Power Syst. Res. 2024, 226, 109868. [Google Scholar] [CrossRef]
- Małek, A.; Dudziak, A.; Marciniak, A.; Słowik, T. Designing a Photovoltaic–Wind Energy Mix with Energy Storage for Low-Emission Hydrogen Production. Energies 2025, 18, 846. [Google Scholar] [CrossRef]
- Maheshwari, K.U.; Lakshmi, P.V.; Nagaraju, A.; Kothagundu, S. Optimal power dispatch capability and reliability analysis of hybrid renewable energy system. Multidiscip. Sci. J. 2023, 6, 2024046. [Google Scholar] [CrossRef]
- Gullu, S.; Djaho, A.-c.; Mensah, A.; Batarseh, I. Demonstration Project: 1.86 MWH Battery Energy Storage System and 540 KVA Inverter Integration. Electronics 2024, 13, 2596. [Google Scholar] [CrossRef]
- He, M.; Chartouni, D.; Landmann, D.; Colombi, S. Safety Aspects of Stationary Battery Energy Storage Systems. Batteries 2024, 10, 418. [Google Scholar] [CrossRef]
- Castillo-Calzadilla, T.; Oroya-Villalta, J.; Borges, C.E. Energy Management System for a Residential Positive Energy District Based on Fuzzy Logic Approach (RESTORATIVE). Smart Cities 2024, 7, 1802–1835. [Google Scholar] [CrossRef]
- Clean Hydrogen Production Pathways Report 2024. Available online: https://hydrogeneurope.eu/wp-content/uploads/2024/06/2024_H2E_CleanH2ProductionPathwaysReport.pdf (accessed on 16 July 2025).
- Global Trade in Green Hydrogen Derivatives. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2024/Oct/IRENA_Green_hydrogen_derivatives_trade_2024.pdf (accessed on 16 July 2025).
- Kim, Y.; Yang, H. Hydrogen Purity: Influence of Production Methods, Purification Techniques, and Analytical Approaches. Energies 2025, 18, 741. [Google Scholar] [CrossRef]
- Dumančić, A.; Vlahinić Lenz, N.; Wagmann, L. Profitability Model of Green Hydrogen Production on an Existing Wind Power Plant Location. Sustainability 2024, 16, 1424. [Google Scholar] [CrossRef]
- Marouani, I.; Guesmi, T.; Alshammari, B.M.; Alqunun, K.; Alzamil, A.; Alturki, M.; Hadj Abdallah, H. Integration of Renewable-Energy-Based Green Hydrogen into the Energy Future. Processes 2023, 11, 2685. [Google Scholar] [CrossRef]
- Araújo, H.F.; Gómez, J.A.; Santos, D.M.F. Proton-Exchange Membrane Electrolysis for Green Hydrogen Production: Fundamentals, Cost Breakdown, and Strategies to Minimize Platinum-Group Metal Content in Hydrogen Evolution Reaction Electrocatalysts. Catalysts 2024, 14, 845. [Google Scholar] [CrossRef]
- Pg Haji Omar Ali, D.N.H.A.; Suhaimi, H.; Abas, P.E. Membrane-Based Hydrogen Production: A Techno-Economic Evaluation of Cost and Feasibility. Hydrogen 2025, 6, 9. [Google Scholar] [CrossRef]
- Kim, Y.; Min, I.; Lee, J.; Yang, H. An Analysis of Greenhouse Gas Emissions in Electrolysis for Certifying Clean Hydrogen. Energies 2024, 17, 3698. [Google Scholar] [CrossRef]
- Snehasish, D.; Arjun, S.K.; Jose, S.; Vincent, H.W.D.; Elangovan, D.; Subbarama, K.S.; Sendhil, K.N. Advances in green hydrogen production through alkaline water electrolysis: A comprehensive review. Int. J. Hydrogen Energy 2024, 83, 614–629. [Google Scholar] [CrossRef]
- Şahin, M.E. An Overview of Different Water Electrolyzer Types for Hydrogen Production. Energies 2024, 17, 4944. [Google Scholar] [CrossRef]
- Guerrero-Rodríguez, N.F.; De La Rosa-Leonardo, D.A.; Tapia-Marte, R.; Ramírez-Rivera, F.A.; Faxas-Guzmán, J.; Rey-Boué, A.B.; Reyes-Archundia, E. An Overview of the Efficiency and Long-Term Viability of Powered Hydrogen Production. Sustainability 2024, 16, 5569. [Google Scholar] [CrossRef]
- Ansari, S.A.; Alam, M.W.; Dhanda, N.; Abbasi, M.S.; Ahmed, M.E.; Alrashidi, A.B.; Al-Farhan, A.M.; Abebe, B. Sustainable Hydrogen Production, a Review of Methods, Types, Applications, Challenges, and Future Perspectives. Glob. Chall. 2025, 9, 2500086. [Google Scholar] [CrossRef]
- Spatolisano, E.; Pellegrini, L.A. A Technical Analysis of the H2 Purification Trains Downstream of Alkaline Electrolyzers. Energies 2025, 18, 2886. [Google Scholar] [CrossRef]
- Bayat, A.; Das, P.K.; Saha, G.; Saha, S.C. Proton Exchange Membrane Electrolysis Revisited: Advancements, Challenges, and Two-Phase Transport Insights in Materials and Modelling. Eng 2025, 6, 72. [Google Scholar] [CrossRef]
- Zun, M.T.; McLellan, B.C. Cost Projection of Global Green Hydrogen Production Scenarios. Hydrogen 2023, 4, 932–960. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Z.; Song, C.; Xu, L.; Zhang, W. Seawater Membrane Distillation Coupled with Alkaline Water Electrolysis for Hydrogen Production: Parameter Influence and Techno-Economic Analysis. Membranes 2025, 15, 60. [Google Scholar] [CrossRef]
- Hou, D.; Ma, W.; Hu, L.; Huang, Y.; Yu, Y.; Wan, X.; Wu, X.; Li, X. Modeling of Nonlinear SOEC Parameter System Based on Data-Driven Method. Atmosphere 2023, 14, 1432. [Google Scholar] [CrossRef]
- Maggio, G.; Vasta, S.; Nicita, A.; Trocino, S.; Giorgianni, M. Green Hydrogen Generation by Water Photoelectrolysis: Economic and Environmental Analysis. Energies 2025, 18, 1439. [Google Scholar] [CrossRef]
- Noor Azam, A.M.I.; Ragunathan, T.; Zulkefli, N.N.; Masdar, M.S.; Majlan, E.H.; Mohamad Yunus, R.; Shamsul, N.S.; Husaini, T.; Shaffee, S.N.A. Investigation of Performance of Anion Exchange Membrane (AEM) Electrolysis with Different Operating Conditions. Polymers 2023, 15, 1301. [Google Scholar] [CrossRef]
- European Made Alkaline Electrolysers for Enabling the Industry of Tomorrow. Available online: https://stargatehydrogen.com/ (accessed on 16 July 2025).
- Produce Maximum Green Hydrogen from Renewables. Available online: https://www.enapter.com/en/ (accessed on 16 July 2025).
- Large-Scale Water Electrolysis for Green Hydrogen Production. Available online: https://www.thyssenkrupp-nucera.com/wp-content/uploads/2022/11/thyssenkrupp-nucera-green-hydrogen-solutions-brochure.pdf (accessed on 16 July 2025).
- Modular PEM Electrolyzers 2-50 MW. Available online: https://fest-group.de/en/solutions/pem-electrolyzers/ (accessed on 16 July 2025).
- Denizhan, B.; Özçelik, C.E. Selection of a green hydrogen production facility location with a novel heuristic approach. Int. J. Hydrogen Energy 2025, 115, 198–213. [Google Scholar] [CrossRef]
- Malek, A.; Dreger, M.; Shaigan, N.; Song, C.; Malek, K.; Jankovic, J.; Eikerling, M. Data-driven modeling of polymer electrolyte fuel cells: Towards predictive analytics with explainable artificial intelligence. Energy AI 2025, 21, 100577. [Google Scholar] [CrossRef]
- Mao, J.; Li, Z.; Xuan, J.; Du, X.; Ni, M.; Xing, L. A review of control strategies for proton exchange membrane (PEM) fuel cells and water electrolysers: From automation to autonomy. Energy AI 2024, 17, 100406. [Google Scholar] [CrossRef]
- LTE Electrolyzer Data Collection. Available online: https://docs.nrel.gov/docs/fy24osti/89518.pdf (accessed on 16 July 2025).
- Franco, A.; Giovannini, C. Hydrogen Gas Compression for Efficient Storage: Balancing Energy and Increasing Density. Hydrogen 2024, 5, 293–311. [Google Scholar] [CrossRef]
- Alssalehin, E.; Holborn, P.; Pilidis, P. Assessment of Hydrogen Storage and Pipelines for Hydrogen Farm. Energies 2025, 18, 1167. [Google Scholar] [CrossRef]
- Davies, E.; Ehrmann, A.; Schwenzfeier-Hellkamp, E. Safety of Hydrogen Storage Technologies. Processes 2024, 12, 2182. [Google Scholar] [CrossRef]
- Naquash, A.; Agarwal, N.; Lee, M. A Review on Liquid Hydrogen Storage: Current Status, Challenges and Future Directions. Sustainability 2024, 16, 8270. [Google Scholar] [CrossRef]
- Genovese, M.; Cigolotti, V.; Jannelli, E.; Fragiacomo, P. Hydrogen Refueling Process: Theory, Modeling, and In-Force Applications. Energies 2023, 16, 2890. [Google Scholar] [CrossRef]
- How China’s ‘Hydrogen into Ten Thousand Homes’ Initiative Is Taking Off. Available online: https://www.hydrogenfuelnews.com/hydrogen-into-ten-thousand-homes/8568556/ (accessed on 16 July 2025).
- Milestone Reached: Over 1000 Hydrogen Refuelling Stations in Operation Worldwide in 2024. Available online: https://www.h2stations.org/press-release-2025-milestone-reached-over-1000-hydrogen-refuelling-stations-in-op-eration-worldwide-in-2024/ (accessed on 16 July 2025).
- Pereira, J.; Souza, R.; Oliveira, J.; Moita, A. Hydrogen Production, Transporting and Storage Processes—A Brief Review. Clean Technol. 2024, 6, 1260–1313. [Google Scholar] [CrossRef]
- Magliano, A.; Perez Carrera, C.; Pappalardo, C.M.; Guida, D.; Berardi, V.P. A Comprehensive Literature Review on Hydrogen Tanks: Storage, Safety, and Structural Integrity. Appl. Sci. 2024, 14, 9348. [Google Scholar] [CrossRef]
- Pereira, R.; Monteiro, V.; Afonso, J.L.; Teixeira, J. Hydrogen Refueling Stations: A Review of the Technology Involved from Key Energy Consumption Processes to Related Energy Management Strategies. Energies 2024, 17, 4906. [Google Scholar] [CrossRef]
- Filina-Dawidowicz, L.; Miłek, D.; Baziukė, D. The Concept of an Infrastructure Location to Supply Buses with Hydrogen: A Case Study of the West Pomeranian Voivodeship in Poland. Energies 2025, 18, 3026. [Google Scholar] [CrossRef]
- Giza, K.; Owczarek, E.; Piotrowska-Woroniak, J.; Woroniak, G. Hydrogen Materials and Technologies in the Aspect of Utilization in the Polish Energy Sector. Appl. Sci. 2024, 14, 10024. [Google Scholar] [CrossRef]
- Wróblewski, P.; Drożdż, W.; Lewicki, W.; Dowejko, J. Total Cost of Ownership and Its Potential Consequences for the Development of the Hydrogen Fuel Cell Powered Vehicle Market in Poland. Energies 2021, 14, 2131. [Google Scholar] [CrossRef]
- Hassan, Q.; Azzawi, I.D.J.; Sameen, A.Z.; Salman, H.M. Hydrogen Fuel Cell Vehicles: Opportunities and Challenges. Sustainability 2023, 15, 11501. [Google Scholar] [CrossRef]
- Chiang, P.-H.; Ke, B.-R.; Yen, S.-J.; Chien, W.-C. Minimization of Construction and Operation Costs of the Fuel Cell Bus Transportation System. Systems 2024, 12, 573. [Google Scholar] [CrossRef]
- Kishore, S.C.; Perumal, S.; Atchudan, R.; Alagan, M.; Sundramoorthy, A.K.; Lee, Y.R. A Critical Review on Artificial Intelligence for Fuel Cell Diagnosis. Catalysts 2022, 12, 743. [Google Scholar] [CrossRef]
- Lu, D.; Yi, F.; Li, J. Optimization of the Adaptability of the Fuel Cell Vehicle Waste Heat Utilization Subsystem to Extreme Cold Environments. Sustainability 2022, 14, 11570. [Google Scholar] [CrossRef]
- Mo, T.; Li, Y.; Luo, Y. Advantages and Technological Progress of Hydrogen Fuel Cell Vehicles. World Electr. Veh. J. 2023, 14, 162. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef]
- Pamungkas, E.D.; Kurniyanto, F.W.; Putra, F.A.; Marpaung, H.T.R.; Silvia; Priyono, B. PEMFC Technology as a Mobile Generator and Back-Up Power Supply Environmentally Friendly. Int. J. Educ. Sci. Technol. Eng. 2024, 7, 75–86. [Google Scholar] [CrossRef]
- Burchart, D.; Przytuła, I. Review of Environmental Life Cycle Assessment for Fuel Cell Electric Vehicles in Road Transport. Energies 2025, 18, 1229. [Google Scholar] [CrossRef]
- Syré, A.M.; Shyposha, P.; Freisem, L.; Pollak, A.; Göhlich, D. Comparative Life Cycle Assessment of Battery and Fuel Cell Electric Cars, Trucks, and Buses. World Electr. Veh. J. 2024, 15, 114. [Google Scholar] [CrossRef]
- Xing, H.; Stuart, C.; Spence, S.; Chen, H. Fuel Cell Power Systems for Maritime Applications: Progress and Perspectives. Sustainability 2021, 13, 1213. [Google Scholar] [CrossRef]
- Karsan e-ATA 12 Hydrogen Completes ‘Winter Challenge’—Powered by Ballard. Available online: https://www.ballard.com/karsan-e-ata-12-hydrogen-completes-winter-challenge-powered-by-ballard/ (accessed on 16 July 2025).
- Nguyen, H.-L.; Lee, S.-M.; Yu, S. A Comprehensive Review of Degradation Prediction Methods for an Automotive Proton Exchange Membrane Fuel Cell. Energies 2023, 16, 4772. [Google Scholar] [CrossRef]
- Tang, X.; Shi, L.; Li, M.; Xu, S.; Sun, C. Health State Estimation and Long-Term Durability Prediction for Vehicular PEM Fuel Cell Stacks Under Dynamic Operational Conditions. IEEE Trans. Power Electron. 2025, 40, 4498–4509. [Google Scholar] [CrossRef]
- Yan, S.; Yang, M.; Sun, C.; Xu, S. Liquid Water Characteristics in the Compressed Gradient Porosity Gas Diffusion Layer of Proton Exchange Membrane Fuel Cells Using the Lattice Boltzmann Method. Energies 2023, 16, 6010. [Google Scholar] [CrossRef]
- Song, K.; Hou, T.; Jiang, J.; Grigoriev, S.A.; Fan, F.; Qin, J.; Wang, Z.; Sun, C. Thermal management of liquid-cooled proton exchange membrane fuel cell: A review. J. Power Sources 2025, 648, 237227. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, X.; Li, Y.; Ma, H.; Zhang, K.; Song, K. Degradation Prediction of Proton Exchange Membrane Fuel Cell Based on Multi-Head Attention Neural Network and Transformer Model. Energies 2025, 18, 3177. [Google Scholar] [CrossRef]
- Yao, Q.; Lu, H.; Chen, J.; Kwan, T.H. Numerical Investigation of the Combustion Characteristics of a Hydrogen-Fueled Engine with Water Injection. Fire 2024, 7, 289. [Google Scholar] [CrossRef]
- Yip, H.L.; Srna, A.; Yuen, A.C.Y.; Kook, S.; Taylor, R.A.; Yeoh, G.H.; Medwell, P.R.; Chan, Q.N. A Review of Hydrogen Direct Injection for Internal Combustion Engines: Towards Carbon-Free Combustion. Appl. Sci. 2019, 9, 4842. [Google Scholar] [CrossRef]
- Pukalskas, S.; Rimkus, A.; Vipartas, T.; Stravinskas, S.; Kriaučiūnas, D.; Mejeras, G.; Ušinskas, A. Hydrogen Supplementation in SI Engines: Enhancing Efficiency and Reducing Emissions with a Focus on Knock Phenomena. Machines 2025, 13, 571. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, K.; Luo, L.; Ma, Z.; Hu, Z.; Li, X.; Qu, P.; Pei, Y.; An, Y.; Gao, Z. A Numerical Simulation of Mixture Formation in a Hydrogen Direct-Injection Internal Combustion Engine. Appl. Sci. 2024, 14, 11317. [Google Scholar] [CrossRef]
- Ozkara, M.; Gul, M.Z. Optimization of a Heavy-Duty Hydrogen-Fueled Internal Combustion Engine Injector for Optimum Performance and Emission Level. Appl. Sci. 2025, 15, 8131. [Google Scholar] [CrossRef]
- Durkin, K.; Khanafer, A.; Liseau, P.; Stjernström-Eriksson, A.; Svahn, A.; Tobiasson, L.; Andrade, T.S.; Ehnberg, J. Hydrogen-Powered Vehicles: Comparing the Powertrain Efficiency and Sustainability of Fuel Cell versus Internal Combustion Engine Cars. Energies 2024, 17, 1085. [Google Scholar] [CrossRef]
- Sipos, G.; Bukovácz, K.; Istvánkó, K.; Sebestyén, L.Á. Hydrogen Engine Conversion Aspects. Eng. Proc. 2024, 79, 6. [Google Scholar] [CrossRef]
- Stępień, Z. A Comprehensive Overview of Hydrogen-Fueled Internal Combustion Engines: Achievements and Future Challenges. Energies 2021, 14, 6504. [Google Scholar] [CrossRef]
- Wróbel, K.; Wróbel, J.; Tokarz, W.; Lach, J.; Podsadni, K.; Czerwiński, A. Hydrogen Internal Combustion Engine Vehicles: A Review. Energies 2022, 15, 8937. [Google Scholar] [CrossRef]
- Suatean, B.; Cican, G.; Guilain, S.; De-Paz-Alcolado, G. Optimization of Hydrogen Combustion in Diesel Engines: A CFD-Based Approach for Efficient Hydrogen Mixing and Emission Reduction. Fuels 2025, 6, 27. [Google Scholar] [CrossRef]
- Okonkwo, P.C. Proton Exchange Membrane Fuel Cell Catalyst Layer Degradation Mechanisms: A Succinct Review. Catalysts 2025, 15, 97. [Google Scholar] [CrossRef]
- Helsel, N.; Choudhury, P. Non-Platinum Group Metal Oxygen Reduction Catalysts for a Hydrogen Fuel Cell Cathode: A Mini-Review. Catalysts 2025, 15, 588. [Google Scholar] [CrossRef]
- Martinaiou, I.; Daletou, M.K. Enhancing Electrode Efficiency in Proton Exchange Membrane Fuel Cells with PGM-Free Catalysts: A Mini Review. Energies 2024, 17, 3443. [Google Scholar] [CrossRef]
- Meng, G.; Li, X.; Liu, M.; Grigoriev, S.A.; Tolj, I.; Shen, J.; Yue, C.; Sun, C. Investigations of Dongyue Series Perfluorosulfonic Acid Membranes for Applications in Proton Exchange Membrane Fuel Cells (PEMFCs). Batteries 2025, 11, 277. [Google Scholar] [CrossRef]
- Zhang, Z.; Dou, Y.; Zhang, W.; Xu, L.; Wang, Y. A Fiberglass-Cloth-Reinforced Perfluorosulfonic Acid Membrane. Membranes 2025, 15, 166. [Google Scholar] [CrossRef]
- Li, X.; Ye, T.; Meng, X.; He, D.; Li, L.; Song, K.; Jiang, J.; Sun, C. Advances in the Application of Sulfonated Poly(Ether Ether Ketone) (SPEEK) and Its Organic Composite Membranes for Proton Exchange Membrane Fuel Cells (PEMFCs). Polymers 2024, 16, 2840. [Google Scholar] [CrossRef]
- Teixeira, F.C.; Teixeira, A.P.S.; Rangel, C.M. New Modified SPEEK-Based Proton Exchange Membranes. Polymers 2025, 17, 1646. [Google Scholar] [CrossRef]
- Wang, X.; Shi, B. Simultaneously Promoting Proton Conductivity and Mechanical Stability of SPEEK Membrane by Incorporating Porous g–C3N4. Membranes 2025, 15, 194. [Google Scholar] [CrossRef]
- Tanno, S.; Ito, Y.; Michikawauchi, R.; Nakamura, M.; Tomita, H. High-Efficiency and Low-NOx Hydrogen Combustion by High Pressure Direct Injection. SAE Int. J. Engines 2010, 3, 259–268. [Google Scholar] [CrossRef]
- Zöldy, M.; Virt, M.; Lukács, K.; Szabados, G. A Comprehensive Analysis of Characteristics of Hydrogen Operation as a Preparation for Retrofitting a Compression Ignition Engine to a Hydrogen Engine. Processes 2025, 13, 718. [Google Scholar] [CrossRef]
- Markowska, K.; Wittek, K.; Kabiesz, P.; Stecuła, K.; Aydın, B.; Pawlak, S.; Markowska, A. Hydrogen-Powered Engines: A Study on Selected Technological and Emissions Issues. Energies 2025, 18, 1675. [Google Scholar] [CrossRef]
- Danielis, R.; Scorrano, M.; Masutti, M.; Awan, A.M.; Niazi, A.M.K. The Economic Competitiveness of Hydrogen Fuel Cell-Powered Trucks: A Review of Total Cost of Ownership Estimates. Energies 2024, 17, 2509. [Google Scholar] [CrossRef]







| Technology | Efficiency (LHV) | Operating Temperature | Typical H2 Output Pressure | Key Advantages | Key Limitations |
|---|---|---|---|---|---|
| PEM Electrolyzer | 60–70% | 50–80 °C | 30–80 bar | High purity H2, dynamic operation, compact | Higher cost, uses precious metal catalysts |
| Alkaline Electrolyzer | 55–65% | 60–90 °C | ~1–30 bar | Mature tech, low cost, durable | Lower current density, slower response time |
| SOEC | 75–85% | 600–850 °C | ~1–30 bar | Very high efficiency with heat integration | High-temp materials challenges, early stage |
| Anion Exchange Membrane | 55–65% | 50–70 °C | ~1–30 bar | Non-precious catalysts, compact | Lower maturity, durability under development |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małek, A. Low-Emission Hydrogen for Transport—A Technology Overview from Hydrogen Production to Its Use to Power Vehicles. Energies 2025, 18, 4425. https://doi.org/10.3390/en18164425
Małek A. Low-Emission Hydrogen for Transport—A Technology Overview from Hydrogen Production to Its Use to Power Vehicles. Energies. 2025; 18(16):4425. https://doi.org/10.3390/en18164425
Chicago/Turabian StyleMałek, Arkadiusz. 2025. "Low-Emission Hydrogen for Transport—A Technology Overview from Hydrogen Production to Its Use to Power Vehicles" Energies 18, no. 16: 4425. https://doi.org/10.3390/en18164425
APA StyleMałek, A. (2025). Low-Emission Hydrogen for Transport—A Technology Overview from Hydrogen Production to Its Use to Power Vehicles. Energies, 18(16), 4425. https://doi.org/10.3390/en18164425





