Abstract
With the advancement of global carbon reduction efforts and the rapid development of battery industries, the scale of spent lithium-ion batteries (LIBs) has increased dramatically. Extracting lithium from spent LIBs to synthesize Li4SiO4 sorbents not only addresses the challenge of battery recycling but also reduces the production cost of CO2 sorbents, making it a research hotspot. However, the CO2 adsorption behavior of these sorbents under the effect of impurities may differ from the traditional Li4SiO4, and there is a lack of systematic research on the adsorption kinetics. To address this issue, two Li4SiO4 sorbents are prepared from spent ternary LIBs, and their adsorption kinetics are comprehensively investigated using classical kinetic models. Results show that the reaction order of LSO and Na-LSO is 0.41 and 1.63, respectively, with activation energies of 72.93 kJ/mol and 99.23 kJ/mol in the initial kinetic-controlled stage, and 323.15 kJ/mol and 176.79 kJ/mol in the following diffusion-controlled stage. In the cyclic processes, loss-in-capacity is observed on LSO due to the simultaneous decrease in rate constants in both the kinetic and diffusion-controlled stages, while Na-LSO could almost maintain its capacity by having a much bigger rate constant during the kinetic-controlled stage. This study reveals the adsorption kinetics of Li4SiO4 prepared from spent LIBs and could provide theoretical support for the targeted design of efficient and low-cost CO2 sorbents.
1. Introduction
The extensive use of various fossil fuels has released a large amount of CO2, intensifying the greenhouse effect [1,2,3]. To alleviate the pressure of carbon reduction, various renewable energy sources are accelerating their large-scale applications [4]. Batteries, as key energy storage carriers, have witnessed an explosive growth in production and consumption [5], leading to a simultaneous surge in the scale of spent LIBs. It is expected that, by 2035, the potential output of spent LIBs in China will reach 11.77 million tons [6]. If spent LIBs are not properly processed, it will not only cause the waste of various metallic resources within batteries, but also result in severe environmental pollution due to the leakage of heavy metals and evaporation of electrolytes [7].
Currently, the recycling of spent LIBs mainly involves pyrometallurgical [8] or hydrometallurgical [9] processes to recover precious metals such as Li and Ni for the preparation of battery raw materials or direct repair and regeneration of battery materials [10]. However, traditional methods have certain limitations. The former requires an extremely high purity of these metals [11], while the latter demands highly precise and complex recycling processes [12]. Therefore, it is necessary to explore a more suitable recycling path for spent LIBs. On the other hand, Li4SiO4, as an efficient high-temperature CO2 sorbent, Equation (1), has drawn significant attention owing to its high adsorption capacity (theoretical CO2 capacity of 0.367 g/g), relatively mild regeneration conditions, and good cycling performance [13]. However, its large-scale industrial application is limited by the expensive lithium source [14]. Under this circumstance, the lithium element rich in spent LIBs may provide a cheap lithium source for the synthesis of Li4SiO4. Our group pioneeringly proposed to extract lithium from spent LIBs to prepare Li4SiO4 sorbents through a pyrometallurgical recycling process. The obtained sorbent reached a stable CO2 adsorption capacity of 0.19 g/g at a cost of only 1/20 to 1/3 of the conventional method [14]. Since then, the preparation of Li4SiO4 sorbents using spent LIBs has attracted a growing attention [14,15,16,17,18,19,20,21]. For example, Liao et al. [18] synthesized Li4SiO4 from spent LIBs and iron tailings, and it showed an excellent CO2 capacity and cycling stability (0.25 g/g, 100 cycles), with a preparation cost of only 1/7 of the traditional method. The transformation path from batteries to sorbents not only overcomes the limitations of traditional LIBs recycling methods, endowing spent LIBs with a higher value, but also further reduces the cost of Li4SiO4 sorbents, achieving a win-win situation for the environment and resources.
However, research on Li4SiO4 sorbents prepared from spent LIBs is still in its infancy, especially regarding the lack of systematic and in-depth analysis of their adsorption kinetics. It is known that adsorption kinetics are crucial for revealing the essence of adsorption and optimizing the performance of sorbents. Our previous work [22] revealed that during the preparation of CO2 separation materials from spent LIBs, key impurity elements underwent migration, and the contents of these impurities were clarified. Due to the interference of such impurity elements, reaction kinetics of Li4SiO4 derived from spent LIBs often differ from those of traditional Li4SiO4. Therefore, it is urgent to further investigate the adsorption kinetic behaviors of Li4SiO4 sorbents derived from spent LIBs. For the CO2 adsorption process of Li4SiO4 sorbents, it usually involves complex physical and chemical steps. To describe the kinetics of such gas–solid reactions, various classical theoretical models have been developed. The grain model [23] is used to describe the kinetics of gas–solid reactions in porous materials composed of spherical grains, making it suitable for high-porosity sorbents. The shrinking core model [24,25,26] is often adopted to describe adsorption processes with a distinct reaction interface advancement, distinguishing between surface chemical reaction control and product layer diffusion control. The Jander model [27,28,29] is more suitable for describing reaction kinetics processes controlled by product layer diffusion, particularly focusing on diffusion activation energy. The Avrami–Erofeev [30,31,32] model is commonly used to describe the nucleation and growth mechanism of product crystals in Li4SiO4 adsorption. These models are crucial for revealing the rate-controlling steps of adsorption, calculating key kinetic parameters such as reaction orders and activation energies.
Overall, the Li4SiO4 sorbent prepared from low-cost spent LIBs is a highly promising material for CO2 adsorption, and a systematic analysis of its adsorption kinetics is a prerequisite for performance optimization. In this work, two Li4SiO4 sorbents with/without additional Na doping are prepared using spent ternary LIBs as the lithium source and fumed silica as the silicon source. Through kinetic analysis, adsorption behavior between the above two sorbents is compared, revealing the reaction order, activation energy, and the variation in kinetic parameters with different particle sizes and cyclic reactions. This work is expected to lay a theoretical foundation at the kinetic level for further regulation of the performance and doping modification design of Li4SiO4 sorbents.
2. Materials and Methods
2.1. Raw Materials and Sorbents Preparation
Spent 18650 cylindrical batteries were adopted as lithium raw materials in the work. The experiment also employed sodium hydroxide (AR, Chengdu Kelong Chemical Co., Ltd., Chengdu, China), sodium chloride (AR, Aladdin Reagent (Shanghai) Co., Ltd., Shanghai, China), and 20 vol.% CO/Ar for extracting lithium carbonate from spent LIBs. Fumed silica (AR, Aladdin Reagent (Shanghai) Co., Ltd., Shanghai, China) was selected as the silicon source to synthesize Li4SiO4. Sodium carbonate (AR, Shandong Keyuan Biochemical Co., Ltd., Shandong, China) was used for doping and modifying Li4SiO4.
To obtain lithium carbonate from the spent LIBs, they were placed in a 10 wt.% NaCl solution to be fully discharged. Then, cathode materials adhering to the aluminum foil were manually stripped. Subsequently, they were immersed in a 2 mol/L NaOH solution for 2 h and then filtered to obtain the cathode material powder with Al removed. Next, the cathode material was pyrolyzed at 550 °C for 1 h in 20 vol.% CO/Ar. The pyrolysis products were dispersed in deionized water, stirred at room temperature for 2 h, and then filtered. The filtrate was dried at 120 °C for 12 h to obtain Li2CO3. To synthesize Li4SiO4 sorbents, the obtained Li2CO3 and fumed silica were mixed in a molar ratio of 2.05:1 and calcined at 750 °C for 6 h in a muffle furnace, and the sorbent was named LSO. Moreover, the obtained Li2CO3 from spent LIBs, SiO2, and Na2CO3 were mixed in a molar ratio of 2.05:1:0.1 and calcined under the same conditions to obtain the Na-doped sorbent, which was named Na-LSO. Finally, sorbents of different sizes were obtained through sieving.
2.2. CO2 Adsorption Test Using TGA
CO2 adsorption kinetics of Li4SiO4 sorbents were tested using a thermogravimetric analyzer (NETZSCH TG209F3). To eliminate the influence of internal and external diffusion effects, the sorbents with different particle sizes and loading masses were first heated to 625 °C at a heating rate of 10 °C/min and then adsorbed for 30 min under 15 vol.% CO2/N2. To evaluate the kinetic behavior under different conditions, the following experimental designs were included: (1) effect of CO2 concentration: 5 mg sorbent (0.054–0.075 mm) at 600 °C with 15–50 vol.% CO2 balanced by N2; (2) effect of adsorption temperature: 5 mg sorbent (0.054–0.075 mm) under 15 vol.% CO2/N2 at 550 °C, 575 °C, and 600 °C; (3) effect of particle size: 5 mg sorbent with four discrete size fractions (0.054–0.075 mm, 0.1–0.2 mm, 0.5–0.6 mm, and 0.9–1.0 mm) at 625 °C under 15 vol.% CO2/N2; and (4) effect of cycle number: 5 mg sorbent (0.054–0.075 mm) for 20 cycles of adsorption at 625 °C in 15 vol.% CO2/N2 for 30 min and desorption at 700 °C in pure N2 for 10 min.
In this work, the adsorption conversion x is defined as follows:
where , and represent the initial mass, real-time mass, and theoretical maximum mass in adsorption, respectively.
2.3. Characterization Methods
The phase composition of the prepared Li4SiO4 sorbent was characterized by X-ray diffraction (Bruker D8 Advance, Bruker AXS GmbH, Karlsruhe, Germany). The microstructure and surface morphology were observed by environmental scanning electron microscopy (Hitachi Regulus 8100, Hitachi High-Technologies Corporation, Tokyo, Japan), and the distribution of various elements on the surface was obtained by energy dispersive spectroscopy mapping. The pore structure was characterized by an automatic physical adsorption instrument (ASAP 2460, Micromeritics Instrument Corporation, Norcross, GA, USA). Following degassing (200 °C, 8 h), the specific surface area, pore volume, and pore size distribution were determined based on the N2 adsorption/desorption isotherms.
3. Results and Discussion
3.1. Physicochemical Characteristics of Sorbents
The phase composition of the two sorbents prepared was analyzed by XRD. As depicted in Figure 1a, the major diffraction peaks are all associated with Li4SiO4. Additionally, a certain amount of NaLi3SiO4 is observed in both sorbents, indicating that the Na element tends to form new crystals with Li4SiO4. It should be noted that the presence of NaLi3SiO4 in the sorbent of LSO is due to the inflow of Na from spent LIBs during recycling. Figure 1b,c shows that the specific surface area and pore volume of LSO are both small at 0.1764 m2/g and 0.00099 cm3/g, respectively, implying a relatively dense surface. In contrast, the specific surface area and pore volume of Na-LSO increase to 1.5187 m2/g and 0.00688 cm3/g, respectively. This might be due to the lattice distortion induced by Na doping, which inhibits particle agglomeration and increases the specific surface area and porosity [33,34]. The pore size distribution in Figure 1c shows there are almost no pores in LSO, indicating an underdeveloped pore structure. Comparatively, there are obvious peaks at 2–3 nm and 10–50 nm for Na-LSO, representing the presence of meso-pores, which is consistent with the specific surface area of the sample. Furthermore, the surface morphologies of two sorbents seem to be very smooth in Figure 1d,e, though there are more gaps among the particles of Na-LSO. Turning to the energy spectrum, both O and Si are observed to be well dispersed, indicating a good quality of Li4SiO4 sorbents prepared from spent LIBs. The uniform distribution of Na in Na-LSO also proves the good doping of Na2CO3 in the sorbent.
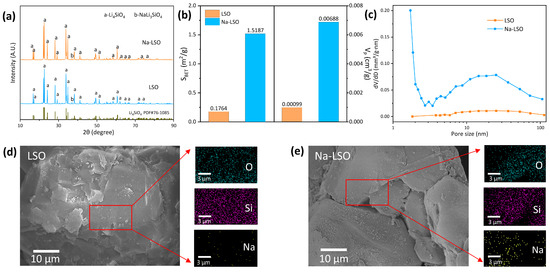
Figure 1.
Characterization results of the sorbents: (a) phase composition by XRD; (b) specific surface area and pore volume; (c) pore size distribution; and surface morphology and energy spectrum of (d) LSO and (e) Na-LSO.
3.2. Effect of CO2 Concentration and Temperature
3.2.1. Intrinsic Kinetics in the Kinetic-Controlled Stage
To eliminate the influence of internal and external diffusion of CO2 during adsorption, sorbents with different particle sizes and loading masses were tested firstly, as shown in Figure 2a–d. It is seen that the adsorption curves of sorbents with a particle size below 0.1 mm and mass less than 10 mg are almost the same, implying the absence of internal and external diffusion. It is also seen that the adsorption process consists of two stages: a kinetic-controlled fast reaction stage within the first few minutes and a diffusion-controlled slow reaction stage, where the former is close to the intrinsic chemical reaction situation.
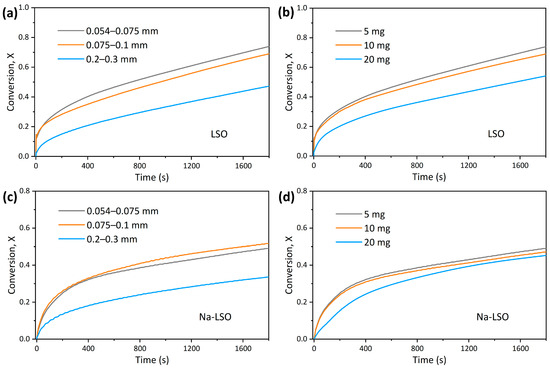
Figure 2.
CO2 adsorption conversion of (a,b) LSO and (c,d) Na-LSO with varying particle size and loading mass in TGA.
When the effects of external and internal diffusion resistance are eliminated, the adsorption behavior of CO2 sorbents can reflect its intrinsic reaction kinetics. Then, the relationship between the adsorption conversion and intrinsic reaction rate can be derived as follows based on the mechanism of surface reaction for gas–solid reactions and the assumption of uniform advancement of the reaction interface:
where k denotes the apparent reaction rate constant (s−1), while ki represents the intrinsic reaction rate (mol·m−2·s−1). M, ρ, and R0 are the molar mass, density, and initial radius of Li4SiO4, respectively.
By further combining the reaction rate equation and the Arrhenius equation, the intrinsic kinetic equations are established as follows [25,30]:
where k0 represents the pre-exponential factor (m3n−2·mol1−n·s−1), denotes the CO2 concentration (mol/m3), and R is the gas constant, which is 8.314 J·mol−1·K−1. T is the reaction temperature (K), Ea is the activation energy (J·mol−1), and n is the reaction order.
To obtain the reaction order in the kinetic-controlled stage, adsorption curves at different CO2 concentrations were tested, as shown in Figure 3a,d. The reaction order in the kinetic-controlled stage can be calculated according to the following formula:
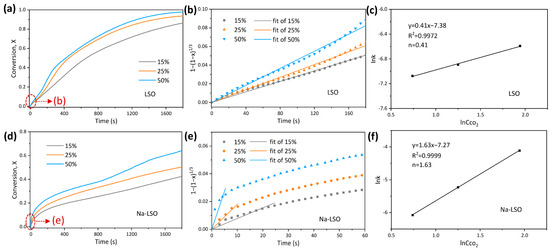
Figure 3.
(a,d) Adsorption conversion as a function of time at different CO2 concentrations, (b,e) initial fitting in the kinetic-controlled stage, and (c,f) Arrhenius fitting of the LSO and Na-LSO sorbent.
The apparent reaction rate constants (k) of LSO and Na-LSO at different CO2 concentrations are obtained from Figure 3b,e, with the relationships between lnk and plotted in Figure 3c,f. Slopes of the fitted lines are determined as the reaction orders, which are 0.41 and 1.63 for LSO and Na-LSO, respectively. It indicates that the two sorbents have different degrees of dependence on the CO2 concentration, and the adsorption by the Na-LSO sorbent is more susceptible to CO2 concentration.
To obtain the intrinsic activation energies of two sorbents during CO2 adsorption, tests were conducted at different temperatures of 550 °C, 575 °C, and 600 °C. Activation energy in the kinetic-controlled stage is described by the following formula:
Figure 4a,d demonstrates that the reaction rate and the final conversion increase significantly at higher temperatures under the conditions studied. The apparent reaction rate constants (k) of LSO and Na-LSO at different temperatures are obtained from Figure 4b,e, and the relationships between lnk and 1/T are shown in Figure 4c,f. Then, slopes are obtained through linear fitting, and activation energies can be calculated. Activation energies of LSO and Na-LSO during the kinetic-controlled stage are 72.93 kJ/mol and 99.23 kJ/mol, respectively, indicating that Na-LSO exhibits a stronger temperature dependence. It is reported in some of the literature that metal-doped Li4SiO4 has a higher reactivity and smaller activation energy than undoped Li4SiO4 [35,36]. Here, the larger activation energy of the Na-doped sorbent is mainly due to the fact that Na-doped sorbent has a “self-activation” property [35,37,38], and its reaction rate is inhibited in the first cycle. The activation energy for LSO during the kinetic-controlled stage is similar to the values of 94.14 kJ/mol and 102.93 kJ/mol reported by Lopez-Ortiz et al. [25] and Peltzer et al. [30]. The small deviation is possibly caused by the different methods in synthesizing Li4SiO4 and the impurity contents [26].
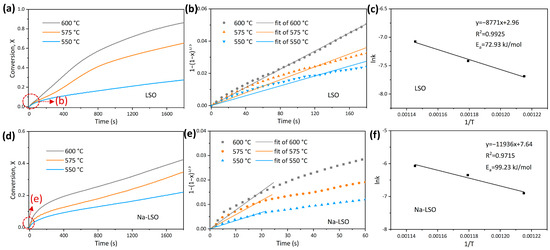
Figure 4.
(a,d) Adsorption conversion as a function of time at different temperatures, (b,e) initial fitting in the kinetic-controlled stage, and (c,f) Arrhenius fitting of the LSO and Na-LSO sorbent.
3.2.2. Reaction Kinetics in the Diffusion-Controlled Stage
The Jander model is specifically tailored to reaction processes controlled by product layer diffusion and is suitable for describing the stage in the middle and late phases of a reaction where diffusion resistance becomes dominant due to the increasing thickness of the product layer. In this work, after the adsorption reaction proceeds to a certain extent, the product layer formed on the surface of Li4SiO4 gradually thickens, and diffusion becomes the rate-limiting step. Therefore, the Jander model is adopted to conduct a kinetic analysis on the diffusion-controlled stage of adsorption at different temperatures, as detailed below:
where x represents the conversion of the sorbent, t stands for time, and kb refers to the diffusion-controlled reaction rate constant (s−1).
The model fitting results are shown in Figure 5a,c, and the kb at each temperature is obtained. The specific parameters obtained through fitting are listed in Table 1. By substituting kb into the Arrhenius equation, Equation (10), the activation energy in the diffusion-controlled stage can be obtained.
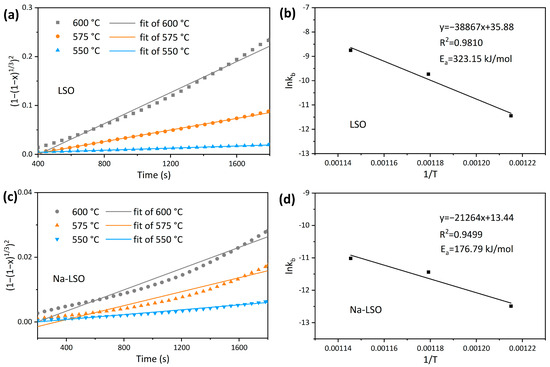
Figure 5.
(a,c) Jander model fitting in the diffusion-controlled stage, and (b,d) Arrhenius fitting of the LSO and Na-LSO sorbent.

Table 1.
Reaction fitting of the sorbents at different temperatures in the diffusion-controlled stage.
The activation energy of CO2 adsorption by LSO in the diffusion-controlled stage is 323.15 kJ/mol, while that of Na-LSO is 176.79 kJ/mol. It is seen that the activation energy of Na-LSO in this stage is significantly lower than that of LSO, which may be due to the structural changes introduced by Na doping that inhibit particle agglomeration and reduce the diffusion resistance in the product layer [33,39,40]. It is worth noting that the activation energy obtained here for the diffusion-controlled stage is higher than the reported values in the literature. For instance, Kaniwa et al. [28] obtained an activation energy of 201 kJ/mol using the Jander model, and Zhang et al. [41] reported a value of 272 kJ/mol using the Avrami–Erofeev model. The differences in activation energy among different studies, apart from the different models used, are attributed to the varying specific surface area and impurity content of the synthesized Li4SiO4. In this work, the LSO sorbent is prepared from the recycled lithium carbonate from spent LIBs, which has a relatively low specific surface area and contains more impurities [14,15]. These factors would affect the apparent reaction rate of the overall adsorption, leading to a higher activation energy in the diffusion stage.
3.3. Reaction Kinetics with Different Particle Sizes
CO2 adsorption curves with different particle sizes (0.054–0.075 mm, 0.1–0.2 mm, 0.5–0.6 mm, and 0.9–1 mm) are shown in Figure 6a,d. It can be observed that the conversion of LSO gradually increases with the decreasing particle size, from the final 43% at 0.9–1 mm to 74% at 0.054–0.075 mm after a reaction time of 30 min. For Na-LSO, the increase in conversion with the decreasing particle size is more significant, from 12% at 0.9–1 mm to 49% at 0.054–0.075 mm at the end of adsorption. Based on the methods described in prior studies [42] and considering the kinetic characteristics of CO2 adsorption in this work, the grain model Equation (11) is employed to describe gas–solid reaction kinetics during the kinetic-controlled fast reaction stage. This stage is characterized by a rapid CO2 reaction on Li4SiO4 surfaces and product layer formation. For the diffusion-controlled slow reaction stage, the Jander model is used to analyze the diffusion resistance-dominated process caused by product layer thickening:
where x represents the conversion of the sorbent, t stands for time, and ka is kinetic-controlled reaction rate constant (s−1).
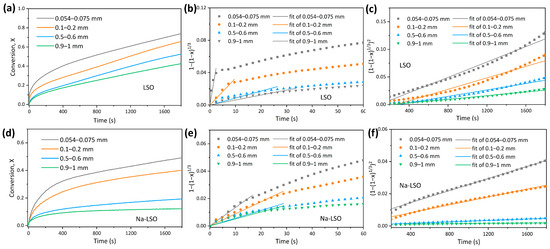
Figure 6.
(a,d) Adsorption conversion as a function of time at different particle sizes, (b,e) grain model fitting in the kinetic-controlled stage, and (c,f) Jander model fitting in the diffusion-controlled stage of the LSO and Na-LSO sorbent.
The results of grain model fitting for the kinetic-controlled stage of LSO and Na-LSO are shown in Figure 6b,e, and the fitting results using the Jander model are presented in Figure 6c,f. The high fitting degrees indicate the rationality of the model selection. The specific parameters obtained from the fitting are summarized in Table 2. It is seen that the kinetic change patterns of LSO and Na-LSO are similar. ka is about two orders of magnitude larger than kb. As the particle size decreases, both reaction rates in the kinetic and diffusion-controlled stages increase gradually. This is because a reduction in particle size increases the contact area between the sorbent and CO2, which means that more active sites on the surface of Li4SiO4 come into contact with CO2 molecules [43], thereby enhancing the reaction probability. On the other hand, a smaller particle size shortens the diffusion distance of CO2 in the product layer, which can also increase the reaction rate [28].

Table 2.
Reaction fitting of the sorbents at different particle sizes.
3.4. Reaction Kinetics at Different Reaction Cycles
To understand the variation in reaction kinetics during the cyclic reaction process, 20 cycles of adsorption and desorption were tested. Figure 7a shows that the sorbent of LSO achieves a conversion of 74% in the first adsorption cycle, which is at a relatively high level. However, the conversion decreases to 52% at the 10th cycle, and only 41% conversion remains after 20 adsorption cycles. To explore kinetic change during the cycling process, conversion curves at the 1st, 10th, and 20th adsorption cycle are plotted in Figure 7b, and a kinetic analysis is conducted on the kinetic and diffusion-controlled stages. The fitting results are shown in Figure 7c,d, and the detailed fitting parameters are listed in Table 3. Overall, as the cycle progresses, reaction rate constants in both the kinetic and diffusion-controlled stages decrease, indicating that the decline in CO2 capacity is due to the loss of surface active sites and the increase in diffusion resistance within the product layer [44].
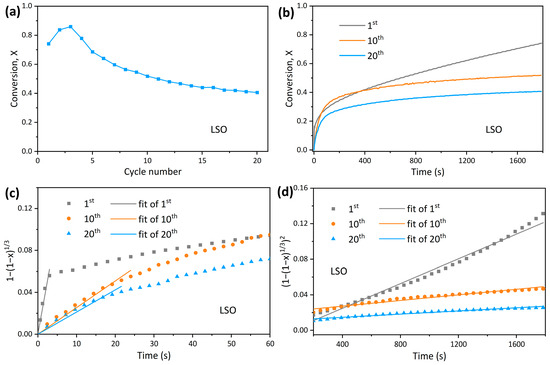
Figure 7.
LSO sorbent: (a) Adsorption conversion as a function of cycle number; (b) adsorption conversion curves at the 1st, 10th, and 20th cycle; (c) grain model fitting in the kinetic-controlled stage; and (d) Jander model fitting in the diffusion-controlled stage.

Table 3.
Reaction fitting of the sorbents in different cyclic processes.
The varying conversion of Na-LSO in 20 cycles of CO2 adsorption and desorption is depicted in Figure 8a. An initial increase in conversion is followed by a very slight decrease. The conversion is 49% in the 1st adsorption cycle, which becomes 65% in the 10th cycle, and a value of 62% is still maintained at the end of 20th cycle, indicating that Na doping significantly improves the cyclic stability of the sorbent [45]. The adsorption curves in the 1st, 10th, and 20th cycle are plotted in Figure 8b, and the model fitting results for the kinetic and diffusion-controlled stages are demonstrated in Figure 8c,d. Moreover, Table 3 summarizes the detailed model fitting parameters. It is seen that the ka of Na-LSO in the kinetic-controlled stage increases continuously during the cyclic reaction, while kb in the diffusion-controlled stage remains basically unchanged. Thus, the stable CO2 capacity maintained during the cycle is mainly attributed to the kinetic-controlled stage. The increase in surface activity during the cycle may be due to the structural reconstruction caused by Na doping, which reduces the degree of dense surface formation caused by sintering [39].
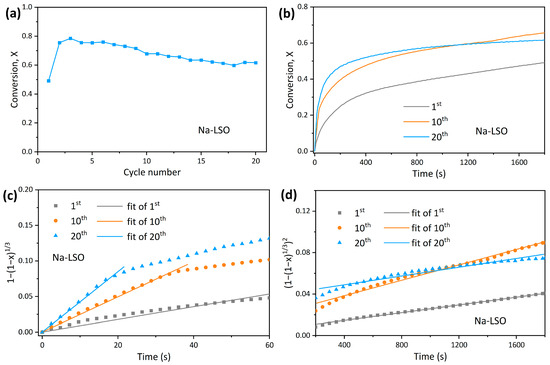
Figure 8.
Na-LSO sorbent: (a) Adsorption conversion as a function of cycle number; (b) adsorption conversion curves at the 1st, 10th, and 20th cycle; (c) grain model fitting in the kinetic-controlled stage; and (d) Jander model fitting in the diffusion-controlled stage.
4. Conclusions
This work conducts an adsorption kinetic analysis on two Li4SiO4 sorbents prepared from spent LIBs. After eliminating the influence of internal and external diffusion, adsorption tests are carried out at different CO2 concentrations and reaction temperatures. The reaction order of LSO is determined to be 0.41, with an initial activation energy of 72.93 kJ/mol, while that of Na-LSO is 1.63, with an initial activation energy of 99.23 kJ/mol. Then, the Jander model is used to fit the adsorption processes at different temperatures, and the activation energies for LSO and Na-LSO in the diffusion-controlled stage are calculated to be 323.15 kJ/mol and 176.79 kJ/mol, respectively. Tests on sorbents of different particle sizes reveal that particle size significantly affects the reaction rate and final conversion. Reducing particle size can effectively increase the reaction rate, thereby achieving a higher conversion. In 20 cycles of adsorption and desorption tests, it is found that the CO2 capacity of LSO decreases significantly with the increasing number of cycles, which is attributed to the reduction in rate constants of ka and kb in the kinetic and diffusion-controlled stages, respectively. In contrast, Na-LSO maintains better cycle stability as a result of the higher reaction rate constant ka in the kinetic-controlled stage.
Author Contributions
Conceptualization, X.W. and C.Q.; methodology, J.H. and J.N.; formal analysis, X.W.; investigation, J.H. and J.N.; writing, X.W.; writing—review and editing, X.W. and C.Q.; visualization, X.W., J.H., and J.N.; supervision, C.Q.; project administration, C.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 52376094) and the Venture and Innovation Support Program for Chongqing Overseas Returnees (No. cx2021108).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
There are no conflicts to declare.
References
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging Trends in Porous Materials for CO2 Capture and Conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.K.; Kim, J. Revolutionary Advancements in Carbon Dioxide Valorization via Metal-Organic Framework-Based Strategies. Carbon. Capture Sci. Technol. 2025, 15, 100405. [Google Scholar] [CrossRef]
- Li, J.; Gao, C.; Cheng, Y.; Ran, J.; Qin, C. Calcium Looping-Based Methane Reforming for Hydrogen and Syngas Production. Appl. Catal. Environ. Energy 2025, 379, 125674. [Google Scholar] [CrossRef]
- Jafarizadeh, H.; Yamini, E.; Zolfaghari, S.M.; Esmaeilion, F.; Assad, M.E.H.; Soltani, M. Navigating Challenges in Large-Scale Renewable Energy Storage: Barriers, Solutions, and Innovations. Energy Rep. 2024, 12, 2179–2192. [Google Scholar] [CrossRef]
- Global EV Outlook 2023–Analysis. Available online: https://www.iea.org/reports/global-ev-outlook-2023 (accessed on 28 June 2025).
- Yang, D.; Wang, M.; Luo, F.; Liu, W.; Chen, L.; Li, X. Evaluating the Recycling Potential and Economic Benefits of End-of-Life Power Batteries in China Based on Different Scenarios. Sustain. Prod. Consump. 2024, 47, 145–155. [Google Scholar] [CrossRef]
- Mrozik, W.; Ali Rajaeifar, M.; Heidrich, O.; Christensen, P. Environmental Impacts, Pollution Sources and Pathways of Spent Lithium-Ion Batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical Recycling of Li-Ion, Ni–Cd and Ni–MH Batteries: A Minireview. Curr. Opin. Green. Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Peng, C.; Liu, F.; Aji, A.T.; Wilson, B.P.; Lundström, M. Extraction of Li and Co from Industrially Produced Li-Ion Battery Waste–Using the Reductive Power of Waste Itself. Waste Manag. 2019, 95, 604–611. [Google Scholar] [CrossRef]
- Ji, H.; Wang, J.; Ma, J.; Cheng, H.-M.; Zhou, G. Fundamentals, Status and Challenges of Direct Recycling Technologies for Lithium Ion Batteries. Chem. Soc. Rev. 2023, 52, 8194–8244. [Google Scholar] [CrossRef]
- Linneen, N.; Bhave, R.; Woerner, D. Purification of Industrial Grade Lithium Chloride for the Recovery of High Purity Battery Grade Lithium Carbonate. Sep. Purif. Technol. 2019, 214, 168–173. [Google Scholar] [CrossRef]
- Lai, Z.; Long, J.; Lu, Y.; Luo, F.; Zeng, L.; Lai, W.; Li, Y.; Qian, Q.; Chen, Q.; Zhang, K.; et al. Direct Recycling of Retired Lithium-Ion Batteries: Emerging Methods for Sustainable Reuse. Adv. Energy Mater. 2025, 15, 2501009. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Yang, Y.; Qu, M.; Li, H. CO2 Capture by Li4SiO4 Sorbents and Their Applications: Current Developments and New Trends. Chem. Eng. J. 2019, 359, 604–625. [Google Scholar] [CrossRef]
- Tong, Y.; Qin, C.; Zhu, L.; Chen, S.; Lv, Z.; Ran, J. From Spent Lithium-Ion Batteries to Low-Cost Li4SiO4 Sorbent for CO2 Capture. Environ. Sci. Technol. 2022, 56, 5734–5742. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Qin, C.; Zhu, X.; Lv, Z.; Huang, X.; Chen, J. Recycling Spent LiFePO4 Battery to Prepare Low-Cost Li4SiO4 Sorbents for High-Temperature CO2 Capture. ACS Sustain. Chem. Eng. 2023, 11, 6722–6730. [Google Scholar] [CrossRef]
- Ruan, J.; Tong, Y.; Ran, J.; Qin, C. Simplifying and Optimizing Li4SiO4 Preparation from Spent LiFePO4 Batteries with Enhanced CO2 Adsorption. ACS Sustain. Chem. Eng. 2023, 11, 14158–14166. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Li, W.; Guo, X. High-Performance Li4SiO4 Sorbents Prepared from Solid Waste for CO2 Capture. Sep. Purif. Technol. 2023, 326, 124730. [Google Scholar] [CrossRef]
- Liao, T.; Qian, Y.; Yu, M.; Tang, A.; Yang, H. CO2 Capture Utilizing Li4SiO4 from Spent Lithium-Ion Batteries and Iron Tailings Offers Eco-Friendly Benefits. Chem. Eng. J. 2024, 493, 152756. [Google Scholar] [CrossRef]
- Ji, H.; Hu, G.; Wu, J.; Ma, W. Preparation of Li4SiO4 from Lithium-Ion Battery Cathode Waste and Diamond Wire Saw Silicon Powder Using a Two-Step Process. J. Environ. Chem. Eng. 2024, 12, 111865. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, X.; Wei, W.; Hu, W.; Xing, H.; Wang, S.; Liu, W. Synthesis of High-Performance Li4SiO4 Sorbent for CO2 Capture Using Li2CO3 Extracted from Spent Lithium Batteries. Sep. Purif. Technol. 2025, 353, 128605. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, W.; Sun, J.; Hu, W.; Xing, H.; Zhang, Z. High-Performance and Low-Cost Li4SiO4 Sorbent for CO2 Capture Synthesized from Spent LiCoO2 Battery and Pyrophyllite. Chem. Eng. J. 2025, 505, 159414. [Google Scholar] [CrossRef]
- Wang, X.; Han, J.; Ni, J.; Li, J.; Gao, C.; Qin, C. Recycling Spent Ternary Lithium-Ion Batteries into Li4SiO4 Sorbents and Oxygen Carriers for CO2 Separation. ACS Sustain. Chem. Eng. 2025, 13, 11835–11844. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, X.; Chen, X.; Yin, L.; Zhang, J.; Liu, W. Performance of Synthetic CaO-Based Sorbent Pellets for CO2 Capture and Kinetic Analysis. Fuel 2018, 232, 205–214. [Google Scholar] [CrossRef]
- Stefanelli, E.; Francalanci, F.; Vitolo, S.; Puccini, M. Insights into Adsorption Mechanism and Kinetic Modeling of K2CO3-Doped Li4SiO4 Pellets for CO2 Capture at High Temperature and Low Concentration. Fuel 2025, 380, 133161. [Google Scholar] [CrossRef]
- López Ortiz, A.; Escobedo Bretado, M.A.; Guzmán Velderrain, V.; Meléndez Zaragoza, M.; Salinas Gutiérrez, J.; Lardizábal Gutiérrez, D.; Collins-Martínez, V. Experimental and Modeling Kinetic Study of the CO2 Absorption by Li4SiO4. Int. J. Hydrogen Energy 2014, 39, 16656–16666. [Google Scholar] [CrossRef]
- Amorim, S.M.; Domenico, M.D.; Dantas, T.L.P.; José, H.J.; Moreira, R.F.P.M. Lithium Orthosilicate for CO2 Capture with High Regeneration Capacity: Kinetic Study and Modeling of Carbonation and Decarbonation Reactions. Chem. Eng. J. 2016, 283, 388–396. [Google Scholar] [CrossRef]
- Yang, Y.; Dai, P.; Chen, Z.; Sun, X.; Ren, X. Kinetic and Thermodynamic Investigations on the Cyclic CO2 Adsorption-Desorption Processes of Lithium Orthosilicate. Chem. Eng. J. 2023, 468, 143679. [Google Scholar] [CrossRef]
- Kaniwa, S.; Yoshino, M.; Niwa, E.; Hashimoto, T. Evaluation of Reaction Kinetics of CO2 and Li4SiO4 by Thermogravimetry under Various CO2 Partial Pressures. Mater. Res. Bull. 2018, 97, 56–60. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, D.; Zhang, S.; Ye, Q.; Wu, Y.; Ni, Y. Behaviors and Kinetic Models Analysis of Li4SiO4 under Various CO2 Partial Pressures. AIChE J. 2017, 63, 2153–2164. [Google Scholar] [CrossRef]
- Peltzer, D.; Salazar Hoyos, L.A.; Faroldi, B.; Múnera, J.; Cornaglia, L. Comparative Study of Lithium-Based CO2 Sorbents at High Temperature: Experimental and Modeling Kinetic Analysis of the Carbonation Reaction. J. Environ. Chem. Eng. 2020, 8, 104173. [Google Scholar] [CrossRef]
- Nair, S.; Raghavan, R. A Kinetic Study of CO2 Sorption/Desorption of Lithium Silicate Synthesized through a Ball Milling Method. Thermochim. Acta 2021, 699, 178918. [Google Scholar] [CrossRef]
- Stefanelli, E.; Puccini, M.; Vitolo, S.; Seggiani, M. CO2 Sorption Kinetic Study and Modeling on Doped-Li4SiO4 under Different Temperatures and CO2 Partial Pressures. Chem. Eng. J. 2020, 379, 122307. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, M.; Guo, M. Na-Doped Li4SiO4 as an Efficient Sorbent for Low-Concentration CO2 Capture at High Temperature: Superior Adsorption and Rapid Kinetics Mechanism. Sep. Purif. Technol. 2025, 352, 128268. [Google Scholar] [CrossRef]
- Wang, X.; Wei, J.; Jia, Y.; Geng, L.; Wang, D. Evaluation of Na2CO3-Doped MCM-48-Li4SiO4 Adsorbent for CO2 Capture: Performance and DFT Mechanism. Sep. Purif. Technol. 2025, 354, 129417. [Google Scholar] [CrossRef]
- Jia, Y.; Wei, J.; Yuan, Y.; Zhou, X.; Geng, L.; Liao, L. High Temperature Capture of Low Concentration CO2 by Na/Ca-Doped Lithium Orthosilicate with KIT-6 as Precursor. Mater. Today Commun. 2022, 33, 104685. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Wang, H.; Ni, Y.; Zhu, Z. Absorption Behaviors Study on Doped Li4SiO4 under a Humidified Atmosphere with Low CO2 Concentration. Int. J. Hydrogen Energy 2014, 39, 17913–17920. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Shen, C.; Ni, Y.; Wu, Y.; Wu, Q.; Zhu, Z. Self-Activation Mechanism Investigations on Large K2CO3-Doped Li4SiO4 Sorbent Particles. Ind. Eng. Chem. Res. 2015, 54, 7292–7300. [Google Scholar] [CrossRef]
- Zhao, D.; Geng, L.; Jia, Y.; Wei, J.; Zhou, X.; Liao, L. Adsorption of High-Temperature CO2 by Ca2+/Na+-Doped Lithium Orthosilicate: Characterization, Kinetics, and Recycle. Environ. Sci. Pollut. Res. 2024, 31, 21267–21278. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, X.; Chen, H.; Gu, X.; Cheng, Z.; Zhou, Z. Sol-Gel Derived, Na/K-Doped Li4SiO4-Based CO2 Sorbents with Fast Kinetics at High Temperature. Chem. Eng. J. 2020, 382, 122807. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Yang, Y.; Tong, X.; Chen, Q.; Zhou, Z. Synthesis of Highly Efficient, Structurally Improved Li4SiO4 Sorbents for High-Temperature CO2 Capture. Ceram. Int. 2018, 44, 16668–16677. [Google Scholar] [CrossRef]
- Qi, Z.; Daying, H.; Yang, L.; Qian, Y.; Zibin, Z. Analysis of CO2 Sorption/Desorption Kinetic Behaviors and Reaction Mechanisms on Li4SiO4. AIChE J. 2013, 59, 901–911. [Google Scholar] [CrossRef]
- Fu, J.; Huang, P.; Guo, Y.; Fan, K.; Wang, Z.; Xie, X.; Yu, J.; Zhao, C. Integrating Calcium-Looping and Reverse-Water–Gas-Shift Reaction for CO2 Capture and Conversion: Screening of Optimum Catalyst. Sep. Purif. Technol. 2023, 327, 124991. [Google Scholar] [CrossRef]
- Venegas, M.J.; Fregoso-Israel, E.; Escamilla, R.; Pfeiffer, H. Kinetic and Reaction Mechanism of CO2 Sorption on Li4SiO4: Study of the Particle Size Effect. Ind. Eng. Chem. Res. 2007, 46, 2407–2412. [Google Scholar] [CrossRef]
- Zhao, M.; Fan, H.; Yan, F.; Song, Y.; He, X.; Memon, M.Z.; Bhatia, S.K.; Ji, G. Kinetic Analysis for Cyclic CO2 Capture Using Lithium Orthosilicate Sorbents Derived from Different Silicon Precursors. Dalton Trans. 2018, 47, 9038–9050. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Peng, K.; Wang, Z.; Zou, X.; Cheng, H.; Lu, X. Elucidating the Promotion of Na2CO3 in CO2 Capture by Li4SiO4. Phys. Chem. Chem. Phys. 2021, 23, 26696–26708. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).