Progress in Caking Mechanism and Regulation Technologies of Weakly Caking Coal
Abstract
1. Introduction
2. Caking Mechanism and Evaluation Indexes
2.1. Caking Mechanism
2.1.1. Plastic Mechanism of Coke Formation
2.1.2. Mesophase Mechanism of Coke Formation
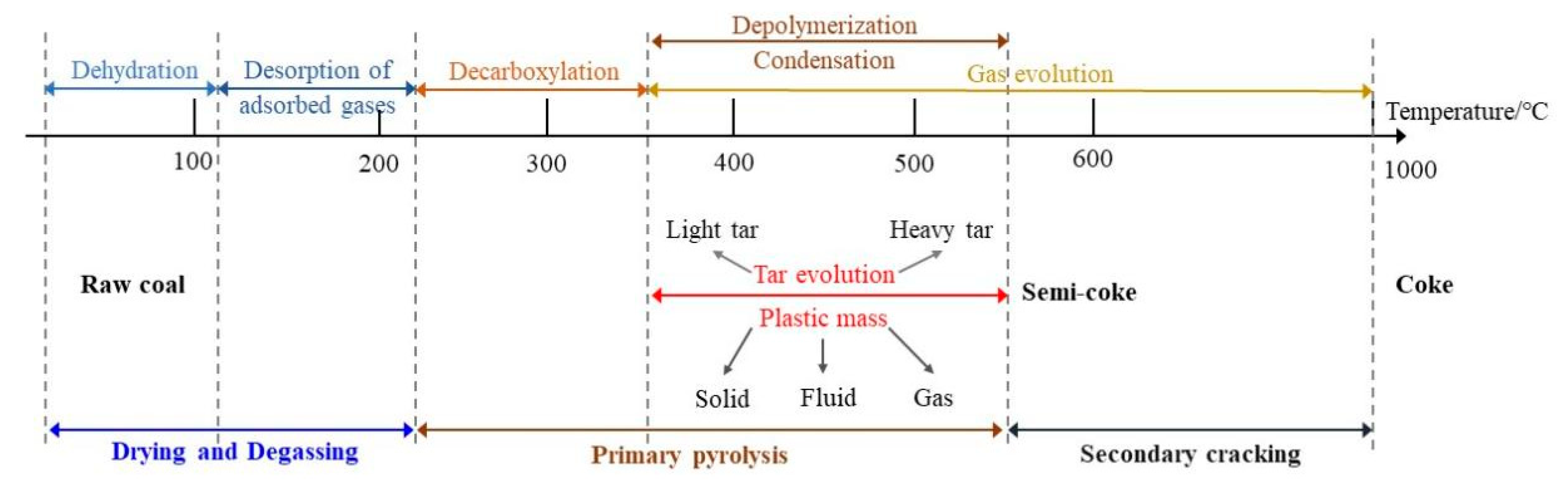
2.2. Plastic Mass
2.2.1. Formation of Plastic Mass
| Authors | Contents of Research |
|---|---|
| Qiu [32] | The liquid phase is predominantly composed of alkyl-substituted monocyclic compounds and long-chain unbranched alkanes. |
| Li [33] | The caking property is predominantly influenced by the structure of aliphatic compounds. A shorter and more branched aliphatic chain typically results in a stronger caking property. |
| Qin [34] | The primary constituents influencing the caking property are the series of compounds, including benzene, naphthalene, anthracene, and phenanthrene, along with long-chain alkanes. |
| Wang [35] | The release of a substantial quantity of volatile gases and tar results in an increase in the quantity of aromatic hydrocarbons. |
| Lee [36,37] | Aliphatic compounds are pivotal in the formation process of plastic mass. |
| Chen [38] | The cleavage of aliphatic bridge bonds and hydrogen transfer mechanisms are identified as predominant factors governing the formation of plastic mass. |
| Ibara [39] | Aliphatic structures and oxygen-containing functional groups are gradually eliminated during pyrolysis, which is accompanied by a substantial increase in the concentration of aromatic hydrogen. |
| Lee [40] | Within the thermoplastic temperature range, the extensive cleavage of bridge bonds generates free radicals that are effectively stabilized through hydrogen transfer mechanisms, leading to enhanced formation of plastic mass. |
| Zhang [41] | Alkyl chains with reactive hydrogen sites enhance the supply of aliphatic hydrogen (AlH), which actively participates in the formation of plastic mass. |
| Cui [42] | Aliphatic and aromatic substituents are identified as the predominant factors governing the maximum fluidity temperature and re-solidification temperature during pyrolysis, which are critical process parameters for the caking property. |
| Hammad [43] | The volatile compounds that are released from the vitrinite component of coal can be readily adsorbed by the porous structure of inertinite. This process leads to a reduction in the quantity of plastic mass, subsequently resulting in an increase in the caking property. |
| Chen [44] | Within the thermoplastic range, coal undergoes a series of chemical reactions—crosslinking, condensation, and re-polymerization—culminating in the depletion of oxygen. Consequently, this process facilitates the formation of condensed carbon-bearing crosslinking structures. |
| Soonho [45] | During early resolidification, structures of coking coal show a low degree of aromatic ring condensation and aromaticity but high CH2/CH3. |
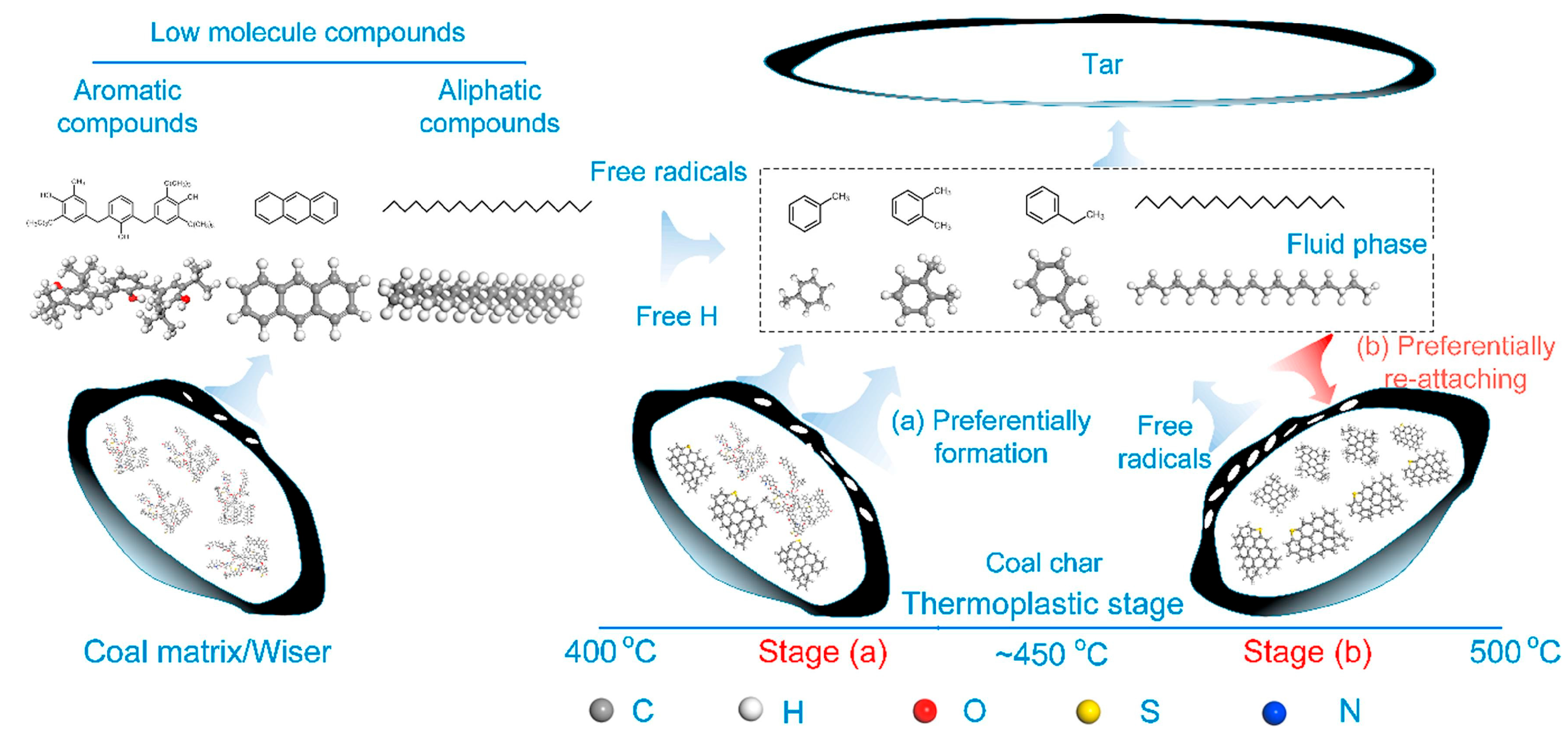
2.2.2. Property of Plastic Mass
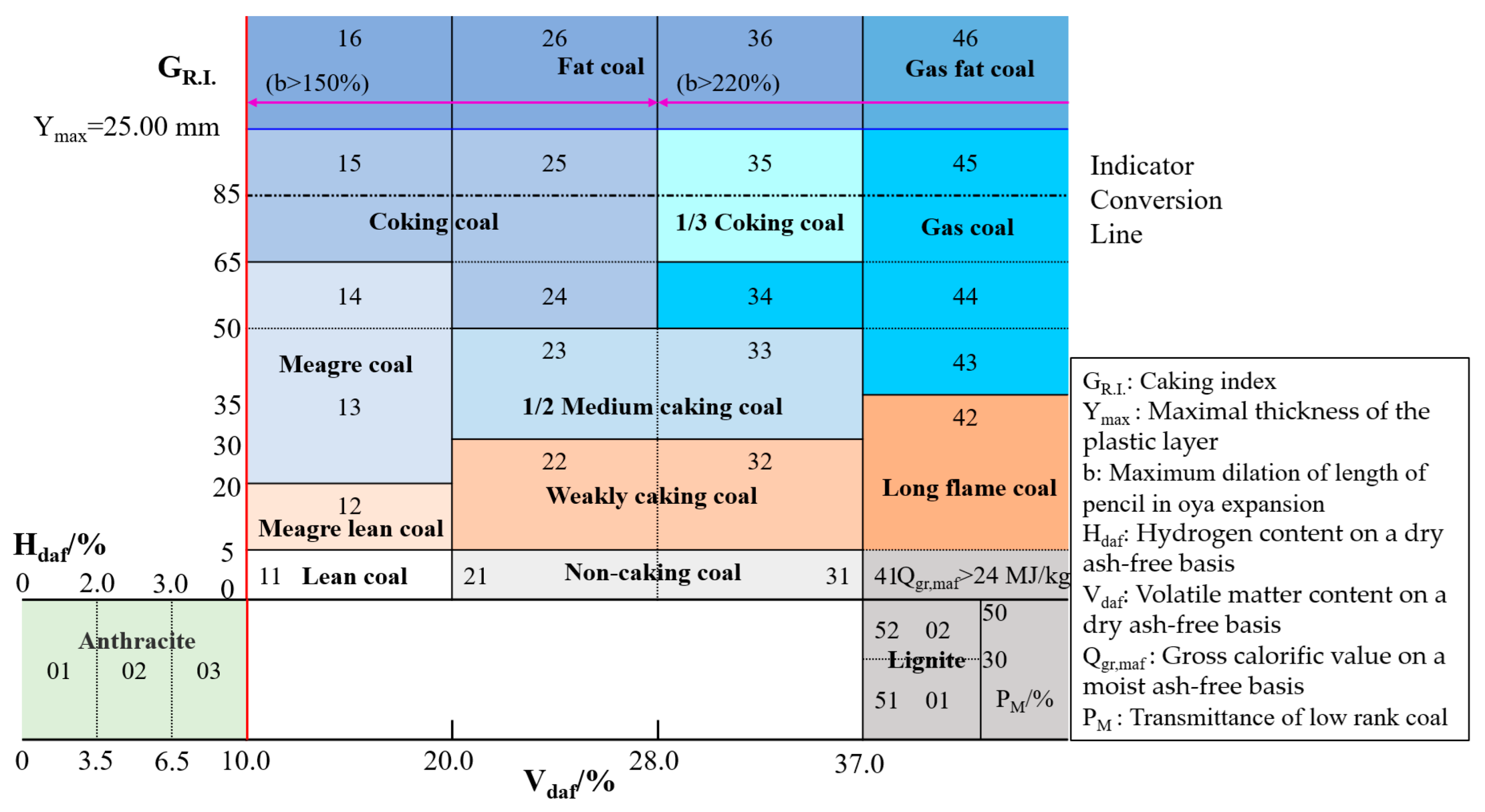
2.3. Evaluation Indexes of Caking Property
- Thermoplasticity: Thermoplasticity denotes the capacity to undergo flow deformation upon heating while retaining the shape post-cooling. Variations in this property directly affect characteristics of the plastic mass during pyrolysis, consequently differentiating pore–wall structures of coke formed through flow deformation and solidification. Based on this definition, evaluation indexes of thermoplasticity are divided into two categories. The first involves indirect determination through the shape and strength of coke, such as the crucible swelling number, Gray–King assay, and GR.I., and the second category directly quantifies the properties of plastic mass, such as Oya expansion, Gieseler fluidity, and Ymax [27,69,70].
- Coking property: The coking property refers to the capability to form coke of a specific lump size and strength under coking or simulated coking conditions. There are two distinct perspectives on the measurement of the coking property. The first perspective suggests that the plastic mass, as measured under simulated industrial coking heating rates, can serve as an effective measurement. Conversely, the second perspective posits that parameters such as the compressive strength of coke, obtained by simulating the coking process, can be utilized as a metric for measurement.
| Evaluation Indexes | Measuring Methods | Contents of Evaluation | Advantages | Disadvantages |
|---|---|---|---|---|
| Characteristic of char residue (CRC) | After the determination of volatile matter, coal samples are transformed into coke and remain within the crucible. Subsequently, they are categorized based on the shape of the coke residue. | Ability of coal to bind itself | Operational simplicity and rapid experimentation | Lack of strict quantitative concept |
| Crucible swelling number | Place a specified mass of coal samples in a specialized crucible and subject them to rapid heating (400 °C/min) to 800 °C. The resulting coke is then compared with standard coke to determine its crucible swelling number. | Expansibility and caking property of coal | Operational simplicity and rapid experimentation | Highly subjective and poorly able to discriminate between strongly caking coal |
| Gray–King assay | Coal samples are subjected to a heating process up to 600 °C in a high-temperature-resistant tube, with a rate of temperature increase maintained at 5 °C/min, in isolation from air, and held for a duration of 15 min. Subsequently, the coke residue preserved within the tube is evaluated against a standard coke type to ascertain its classification. | Expansibility and coking property of coal | Rapid experimentation | Complexity of measuring method |
| Roga index | A mixture of 1 g of bituminous coal and 5 g of anthracite with a particle size of 0.3~0.4 mm is rapidly heated to produce coke. The strength of the resulting coke is evaluated using a drum of defined specifications. Subsequently, the index is determined using a pre-established formula. | Ability of bituminous coal to bind inert additives (anthracite) when subjected to heat | Operational simplicity and rapid experimentation | Inaccurate results for strongly caking coal and weakly caking coal |
| GR.I. | A mixture of 3 g of bituminous coal and 3 g of anthracite with a particle size of 0.1~0.2 mm is rapidly heated to produce coke. The strength of the resulting coke is evaluated using a drum of defined specifications. Subsequently, the index is determined using a pre-established formula. | Ability of bituminous coal to bind inert additives (anthracite) when subjected to heat | Expanded the application range of the Roga index and reduced errors | Insufficient ability to differentiate between strongly caking coal |
| Oya expansion (Audibert–Arnu method) | Coal samples are shaped into coal pencils and positioned within expansion tubes, to which expansion rods are attached, then heated to 500~550 °C with a steady rate of 3 °C/min in a furnace preheated to 330 °C. Subsequent calculations of expansion and shrinkage are based on the maximum rise distance of the expansion rod. | Permeability and expansibility of coal | Distinguishes between coals of medium caking and above | Inaccurate results for strongly caking coal |
| Ymax | The coal sample is positioned within a coal cup and subsequently placed in a heating furnace, then heated at a steady rate of 3 °C/min. The thickness of the gelatinous layer, observed between the softening and curing points, is measured using a probe to determine the maximal plastic-layer thickness. | Quantity of plastic mass generated by coal | Visualizing the quantity of plastic mass | Highly subjective and unable to measure the property of plastic mass |
| Gieseler fluidity | Coal sample with a particle size of 0~0.43 mm is placed into a crucible. The stirring paddle is then rotated using a consistent torque (100 g·cm), while the crucible is heated at a steady rate of 3 °C/min. As the temperature escalated, the fluidity of the plastic mass altered, necessitating adjustments in the rotational speed of the stirring paddle, which is utilized to calculate the Gieseler fluidity. | Thermal stability, permeability, fluidity, and expansibility of plastic mass | Better ability to differentiate between different coals | Poor reproducibility and high costs |
| Swelling pressure | Depending on coal type, preparation method, and heating conditions, each coal develops a certain swelling pressure. Listed here is a measurement method for reference: Rapidly heat 2 g coal sample compressed at 218 MPa and constant 500 °C. Then, determine the swelling pressure dynamics in the context of 2 MPa initial external load. | Ability of coal to exert pressure within the limiting surface when heated in the fixed volume | Highly valuable for industrial applications | Complexity of measuring method |
3. Regulation Technologies for Weakly Caking Coal
3.1. Enhanced Caking Property
3.1.1. Rapid Heating Treatment
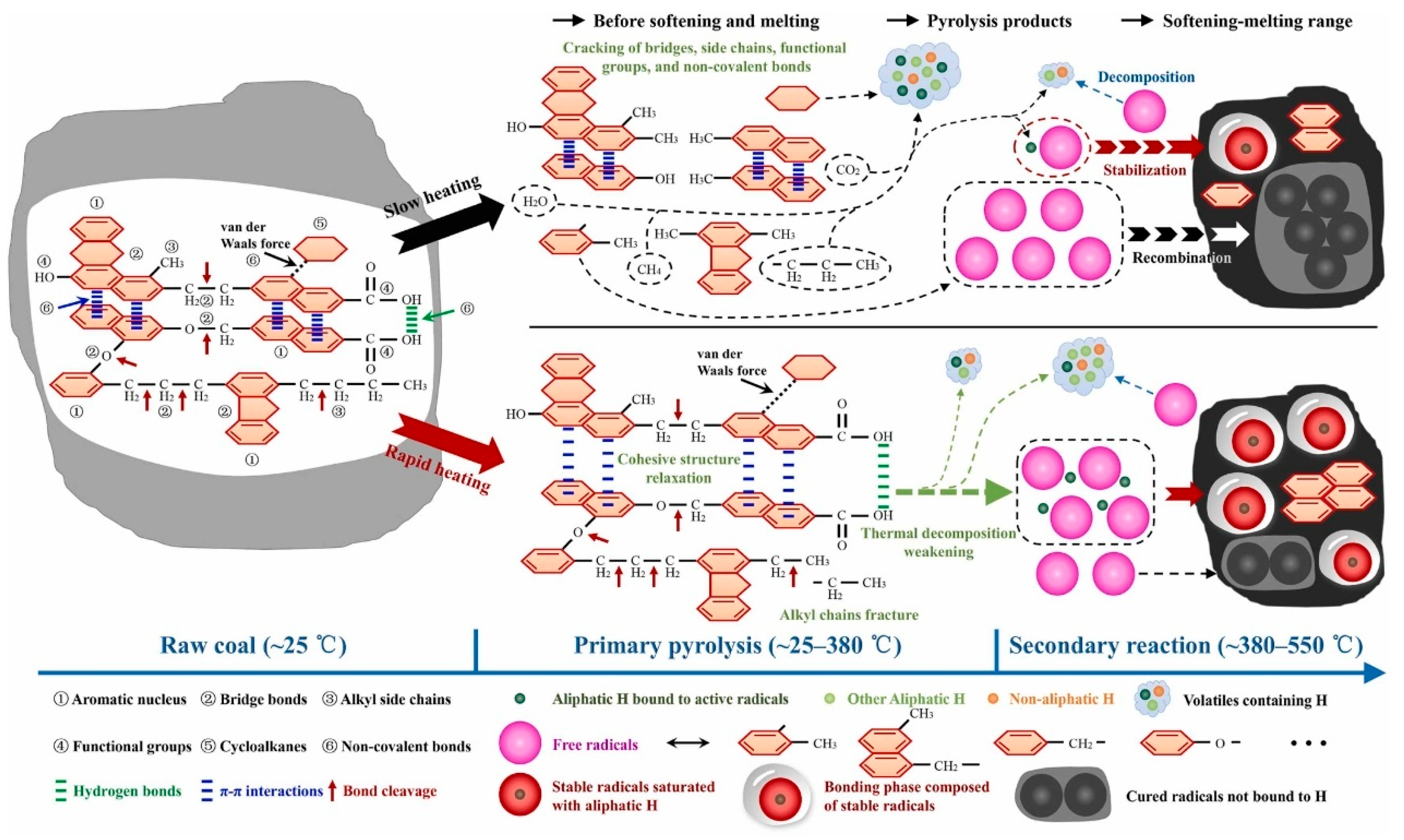
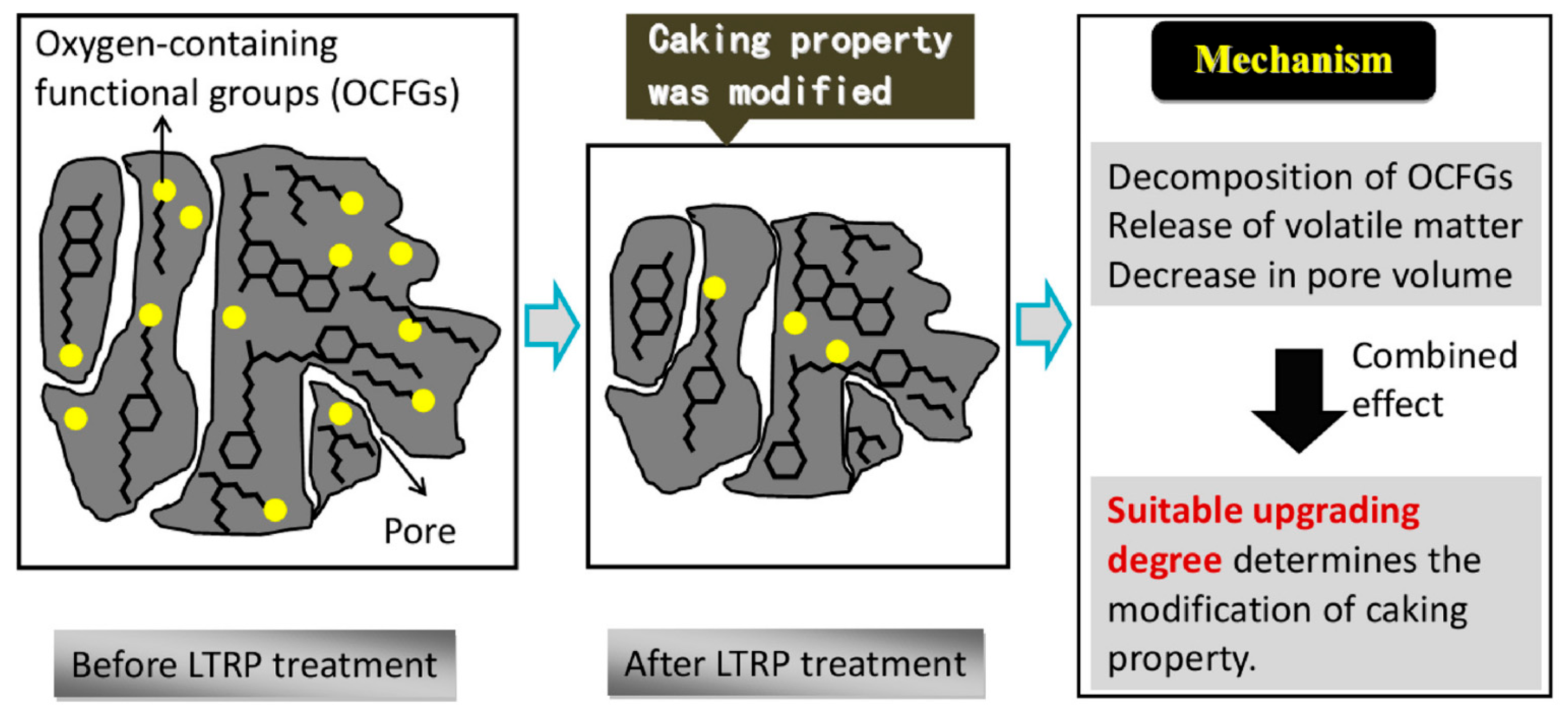
3.1.2. Hydrogenation Modification
3.2. Reduced Caking Property
3.2.1. Mechanical Breaking
3.2.2. Pre-Oxidation
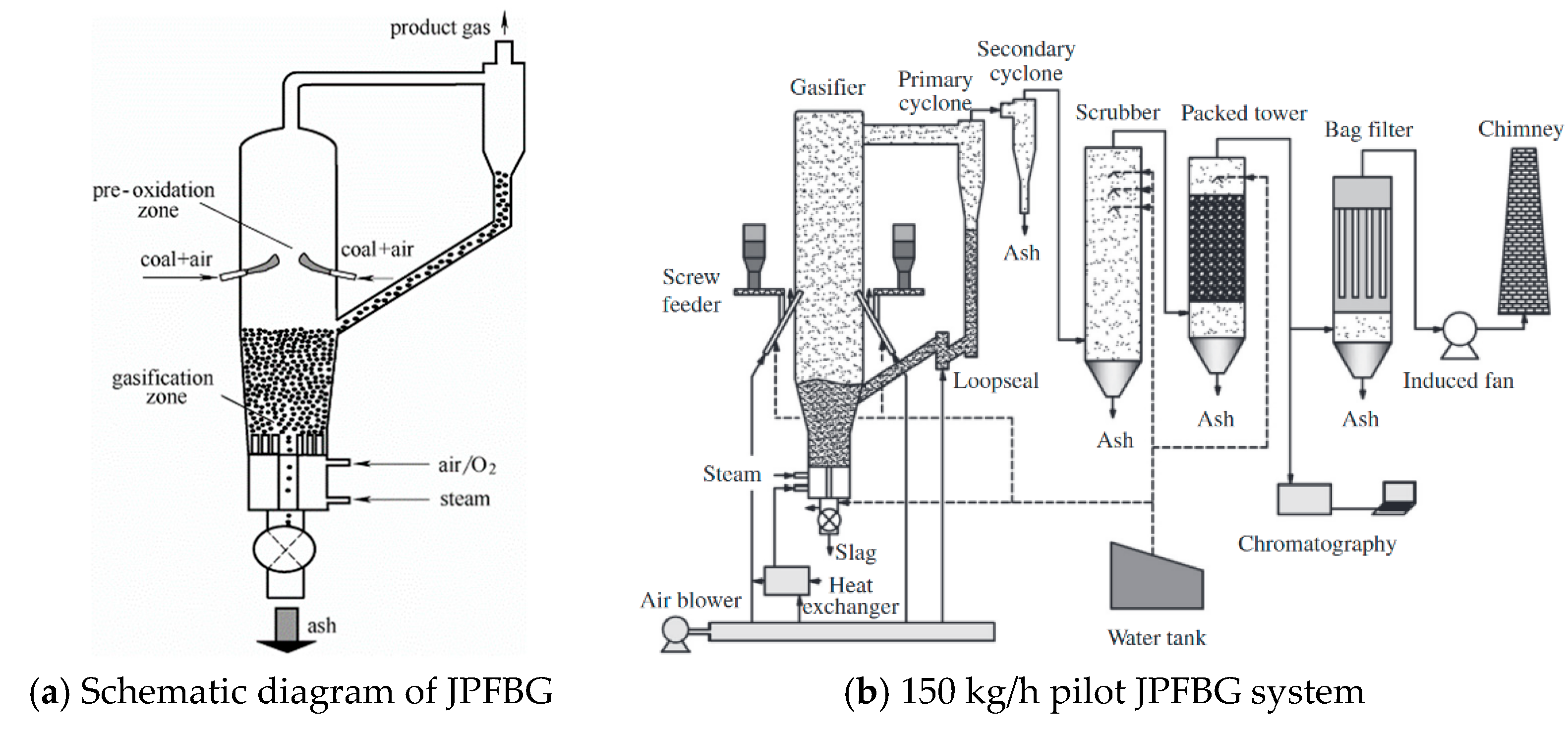
3.2.3. Other Technologies of Decaking
3.3. Shortcomings in Regulation Technologies
- On the one hand, existing technologies require energy consumption to regulate the caking property, such as heating to a specific temperature. On the other hand, they will also decrease the volumetric weight and volatile matter content of coal.
- Prior research has mostly focused on the variations in coal components, such bridge bonds and functional groups associated with the plastic mass. The impact of pretreatment on coal kinetics and activity, however, has received very little attention.
- Research in this field has primarily focused on the effects of parameters such as the temperature, heating rate, and atmosphere on the caking property. However, the influence of a scale effect, specifically the particle size of coal, has seldom been examined.
- Partial regulation technologies exhibit a significant impact on both strongly caking coal and non-caking coal. However, this effect becomes less pronounced when applied to weakly caking coal.
4. Conclusions and Prospects
4.1. Conclusions
- The caking mechanism primarily outlines the specific process involved in the transformation of coal into coke. During pyrolysis, the active component generates the plastic mass, in which gas, liquid, and solid phases coexist. With an increase in temperature, the liquid phase is diminished gradually, causing the inert components to bond. Therefore, the strength of the caking property is mainly determined by the plastic mass.
- Evaluation indexes such as CRC, GR.I., and Ymax can be utilized to distinguish the strength of the caking property and clarify the type of coal. However, due to the complexity of the caking mechanism and measuring conditions, the existing evaluation indexes can only be utilized to assess the caking property under specific circumstances, and they cannot perform an accurate assessment in actual applications.
- Technologies such as rapid heating treatment and hydrogenation modification can increase the amount of plastic mass generated, thereby improving the caking property. Technologies such as mechanical breaking and pre-oxidation reduce the caking property by destroying agglomerates or consuming plastic mass.
4.2. Future Work
- Regarding research on the mechanism of coal caking, future efforts should focus on breakthroughs in the visualization of the caking process and the accurate identification of key substances. Through dynamic characterization methods such as in situ thermal stage microscopy and real-time infrared imaging, the entire process from the onset of caking to the agglomeration of particles can be tracked. Combined with analytical methods such as thermogravimetric-mass spectrometry (TG-MS), caking-related substances can be accurately extracted and their chemical composition, microstructure, and formation path can be clarified.
- Given that existing evaluation indexes mostly describe the caking property at the macro level, lacking micro-level mechanisms and quantitative characterization, follow-up research is needed to construct a multi-dimensional evaluation system covering basic coal quality parameters, reaction characteristics, and caking processes to achieve an accurate classification and prediction of the caking property.
- In terms of the development of regulation technologies, on the one hand, energy loss caused by the pretreatment process can be reduced by optimizing the parameters of existing technologies. On the other hand, based on an in-depth analysis of the caking mechanism, low-energy and high-efficiency technologies should be developed for specific links in the production of key materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Vdaf | Dry ash-free basis |
| GR.I. | Caking index |
| Ymax | Maximal thickness of plastic layer |
| CRC | Characteristic of char residue |
| OCFGs | Oxygen-containing functional groups |
| LTRP | Low-temperature rapid pyrolysis |
| JPFBG | Jetting pre-oxidation fluidized bed gasification |
| LTPT | Low-temperature pyrolysis treatment |
| AlH | Aliphatic hydrogen |
References
- IEA. Global Energy Review 2025. 2025. Available online: https://www.iea.org/reports/global-energy-review-2025 (accessed on 26 July 2025).
- Pranckevičius, D.; Marčiukaitis, M.; Kairaitis, G.; Radziukynas, V. Investigation of hybrid renewable energy system application in the industrial sector. Energy 2025, 328, 136553. [Google Scholar] [CrossRef]
- Liang, Y.; Song, Y.; Chen, Z. Correlation effects, driving forces and evolutionary paths of cross-Industry transfer of energy consumption in china: A new analytical framework. Energies 2025, 18, 3128. [Google Scholar] [CrossRef]
- Hui, J.; Zhu, S.; Zhang, X.; Liu, Y.; Lin, J.; Ding, H.; Su, K.; Cao, X.; Lyu, Q. Experimental study of deep and flexible load adjustment on pulverized coal combustion preheated by a circulating fluidized bed. J. Clean. Prod. 2023, 418, 138040. [Google Scholar] [CrossRef]
- Li, B.; Cong, R.; Matsumoto, T.; Li, Y. Research on different energy transition pathway analysis and low-carbon electricity development: A case study of an energy system in Inner Mongolia. Energies 2025, 18, 3129. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, H.; Li, Z.; Zeng, X.; Ouyang, Z.; Hui, J.; Lin, J.; Su, K.; Wang, H.; Ding, H.; et al. Wide-load combustion characteristics of lean coal tangential preheating combustion. Energy 2025, 323, 135845. [Google Scholar] [CrossRef]
- GB/T 5751-2009; Chinese Classification of Coals. Chinese Standard; China National Coal Association: Beijing, China, 2009.
- Williams, A.; Pourkashanian, M.; Jones, J. Combustion of pulverised coal and biomass. Prog. Energy Combust. Sci. 2001, 27, 587–610. [Google Scholar] [CrossRef]
- Appiah, J.; Tian, L.; Dou, J.X.; Chen, Y.; Chen, X.; Xu, X.; Yu, J. Investigation into the impact of coal blending on the carbon structure of chars obtained from Chinese coals during coking. J. Anal. Appl. Pyrolysis 2024, 179, 106504. [Google Scholar] [CrossRef]
- Zeng, S.; Su, B.; Zhang, M.; Zhang, M.; Gao, Y.; Liu, J.; Luo, S.; Tao, Q. Analysis and forecast of China’s energy consumption structure. Energy Policy 2021, 159, 112630. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Wu, W. Assessing the impact of population, income and technology on energy consumption and industrial pollutant emissions in China. Appl. Energy 2015, 155, 904–917. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, X.; Li, B.; Gonzalez, P.; Kammen, D.; Zou, J.; Wang, K. Immediate actions on coal phaseout enable a just low-carbon transition in China’s power sector. Appl. Energy 2022, 308, 118401. [Google Scholar] [CrossRef]
- Meng, Y.; Cao, Y.; Li, J.; Liu, C.; Li, J.; Wang, Q.; Cai, G.; Zhao, Q.; Liu, Y.; Meng, X.; et al. The real cost of deep peak shaving for renewable energy accommodation in coal-fired power plants: Calculation framework and case study in China. J. Clean. Prod. 2022, 367, 132913. [Google Scholar] [CrossRef]
- Jia, J.; Xing, Y.; Li, B.; Zhao, D.; Wu, Y.; Chen, Y.; Wang, D. Study on the occurrence difference of functional groups in coals with different metamorphic degrees. Molecules 2023, 28, 2264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Z.; Zhang, G.; Xi, Z.; Zhao, F.; Dong, L.; Xu, G. Pilot study on jetting pre-oxidation fluidized bed gasification adapting to caking coal. Appl. Energy 2013, 110, 276–284. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, J.; Lyu, Q.; Man, C.; Ouyang, Z.; Liu, J.; Ding, H.; Liu, Y.; Zhang, X. Experimental study on weakly caking coal combustion preheated by circulating fluidized bed. Fuel 2021, 295, 120592. [Google Scholar] [CrossRef]
- Xiao, Y.; Lü, H.; Huang, A.; Deng, J.; Shu, C. A new numerical method to predict the growth temperature of spontaneous combustion of 1/3 coking coal. Appl. Therm. Eng. 2018, 131, 221–229. [Google Scholar] [CrossRef]
- Wang, R.; Ma, Q.; Ye, X.; Li, C.; Zhao, Z. Preparing coal slurry from coking wastewater to achieve resource utilization: Slurrying mechanism of coking wastewater–coal slurry. Sci. Total Environ. 2019, 650, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, J.; Zhao, F.; Zeng, X.; Liu, X.; Xu, G. Destruction of caking properties of bituminous coal by jetting pre-oxidation in a fluidized bed. Fuel 2014, 133, 45–51. [Google Scholar] [CrossRef]
- Liu, X.; Ling, Q.; Zhao, Z.; Xie, R.; Yu, D.; Ke, Q.; Lei, Z.; Cui, P. Effects of low-temperature rapid pyrolysis treatment on the improvement in caking property of a Chinese sub-bituminous coal. J. Anal. Appl. Pyrolysis 2018, 135, 319–326. [Google Scholar] [CrossRef]
- Shui, H.; Li, H.; Chang, H.; Wang, Z.; Gao, Z.; Lei, Z.; Ren, S. Modification of sub-bituminous coal by steam treatment: Caking and coking properties. Fuel Process. Technol. 2011, 92, 2299–2304. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Cui, X.; Dong, D.; Wang, Q.; Zhang, Y. Converting lignite to caking coal via hydro-modification in a subcritical water–CO system. Fuel 2016, 167, 1–8. [Google Scholar] [CrossRef]
- Williams, O.; Eastwick, C.; Kingman, S.; Giddings, D.; Lormor, S.; Lester, E. Overcoming the caking phenomenon in olive mill wastes. Ind. Crops Prod. 2017, 101, 92–102. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, J.; Song, S.; Wang, Z.; Fang, Y. Insight into the effects of additive water on caking and coking behaviors of coal blends with low-rank coal. Fuel 2019, 238, 10–17. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, T.; Sun, C.; Song, Q.; Li, Y.; Wang, X.; Shu, X. Strengthening the results of destroying the caking property of CBC in weak oxygen and upgrading pyrolysis products. Fuel 2017, 205, 90–99. [Google Scholar] [CrossRef]
- Jüntgen, H. Review of the kinetics of pyrolysis and hydropyrolysis in relation to the chemical constitution of coal. Fuel 1984, 63, 731–737. [Google Scholar] [CrossRef]
- Guelton, N. The prediction of the Gieseler characteristics of coal blends. Fuel 2017, 209, 661–673. [Google Scholar] [CrossRef]
- Nomura, S.; Mahoney, M.; Fukuda, K.; Kato, K.; Bas, A.; McGuire, S. The mechanism of coking pressure generation I: Effect of high volatile matter coking coal, semi-anthracite and coke breeze on coking pressure and plastic coal layer permeability. Fuel 2010, 89, 1549–1556. [Google Scholar] [CrossRef]
- Mahoney, M.; Nomura, S.; Fukuda, K.; Kato, K.; Bas, A.; Jenkins, D.; McGuire, S. The mechanism of coking pressure generation II: Effect of high volatile matter coking coal, semi-anthracite and coke breeze on coking pressure and contraction. Fuel 2010, 89, 1557–1565. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Zhao, H.; Ye, Y.; Xie, R.; Zhao, Z.; Lei, Z.; Cui, P. Changes in caking properties of caking bituminous coals during low-temperature pyrolysis process. Fuel 2022, 321, 124023. [Google Scholar] [CrossRef]
- He, Y.; Zhao, R.; Yan, L.; Bai, Y.; Li, F. The effect of low molecular weight compounds in coal on the formation of light aromatics during coal pyrolysis. J. Anal. Appl. Pyrolysis 2017, 123, 49–55. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, S.; Wu, Y.; Qiu, G.; Sun, C.; Zhang, Q.; Dang, J.; Wen, L.; Hu, M.; Xu, J.; et al. Structural transformation of fluid phase extracted from coal matrix during thermoplastic stage of coal pyrolysis. Fuel 2018, 232, 374–383. [Google Scholar] [CrossRef]
- Li, X.; Qin, Z.; Bu, L.; Yang, Z.; Shen, C. Structural analysis of functional group and mechanism investigation of caking property of coking coal. J. Fuel Chem. Technol. 2016, 44, 385–393. [Google Scholar] [CrossRef]
- Qin, Z. New advances in coal structure model. Int. J. Min. Sci. Technol. 2018, 28, 541–559. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, H.; Zhao, X.; Zhang, S.; Hu, W. Relevance between various phenomena during coking coal carbonization. Part 1: A new testing method developed on a sapozhnikov plastometer. Energy Fuels 2018, 32, 7438–7443. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.; Mahoney, M.; Tremain, P.; Tremain, P.; Moghtaderi, B.; Tahmasebi, A.; Stanger, R.; Wall, T.; Lucas, J. Study of chemical structure transition in the plastic layers sampled from a pilot-scale coke oven using a thermogravimetric analyzer coupled with Fourier transform infrared spectrometer. Fuel 2019, 242, 277–286. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.; Mahoney, M.; Tahmasebi, A.; Stanger, R.; Wall, T.; Lucas, J. In-situ study of plastic layers during coking of six Australian coking coals using a lab-scale coke oven. Fuel Process. Technol. 2019, 188, 51–59. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, S.; Tahmasebi, A.; Bai, J.; Vongsvivut, J.; Yu, J. Chemical structure transformation during the later stage of plastic layers during coking using Synchrotron infrared microspectroscopy technique. Fuel 2020, 273, 117764. [Google Scholar] [CrossRef]
- Ibarra, J.; Moliner, R.; Bonet, A. FT-IR investigation on char formation during the early stages of coal pyrolysis. Fuel 1994, 73, 918–924. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.; Mahoney, M.; Tremain, P.; Moghtaderi, B.; Tahmasebi, A. A study on the structural transition in the plastic layer during coking of Australian coking coals using Synchrotron micro-CT and ATR-FTIR. Fuel 2018, 233, 877–884. [Google Scholar] [CrossRef]
- Zhang, L.; Qiao, Z.; Wang, J.; Wang, G.; Zuo, J.; She, X.; Xue, Q. Mechanism of aliphatic hydrogen on the caking property of 1/3 coking coal during rapid preheating. J. Anal. Appl. Pyrolysis 2022, 168, 105705. [Google Scholar] [CrossRef]
- Cui, B.; Shen, Y.; Guo, J.; Jin, X.; Wang, M.; Xie, W.; Chang, L. A study of coking mechanism based on the transformation of coal structure. Fuel 2022, 328, 125360. [Google Scholar] [CrossRef]
- Hammad, A.; Tara, C.; Karen, M. Understanding the multiple interactions of inertinite during pyrolysis/carbonisation with vitrinite: A study of two Australian coals of different rank. Fuel Process. Technol. 2021, 217, 106823. [Google Scholar] [CrossRef]
- Chen, Y.; Soonho, L.; Arash, T.; Liu, M.; Zhang, T.; Bai, J.; Tian, L.; Yu, J. Mechanism of carbon structure transformation in plastic layer and semi-coke during coking of Australian metallurgical coals. Fuel 2022, 315, 123205. [Google Scholar] [CrossRef]
- Soonho, L.; Arash, T.; Richard, P.; Vongsvivut, J.; Jo, J. Chemical characterization of microtexture transformation during plastic layer formation via synchrotron macro ATR-FTIR microspectroscopy. Fuel 2025, 402, 135948. [Google Scholar] [CrossRef]
- Lee, S.; Mahoney, M.; Yu, J. Advances in the understanding of the formation and chemistry of the plastic layer during coke-making, A comprehensive review. Fuel 2020, 263, 116655. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, S.; Tahmasebi, A.; Bai, J.; Mahoney, M.; Yu, J. A review of the state-of-the-art research on carbon structure evolution during the coking process: From plastic layer chemistry to 3D carbon structure establishment. Fuel 2020, 271, 117657. [Google Scholar] [CrossRef]
- Walker, R.; Mastalerz, M. Functional group and individual maceral chemistry of high volatile bituminous coals from southern Indiana: Controls on coking. Int. J. Coal Geol. 2004, 58, 181–191. [Google Scholar] [CrossRef]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Kawashima, H.; Yamashita, Y.; Saito, I. Studies on structural changes of coal density-separated components during pyrolysis by means of solid-state 13C NMR spectra. J. Anal. Appl. Pyrolysis 2000, 53, 35–50. [Google Scholar] [CrossRef]
- Mastalerz, M.; Bustin, R. Variation in elemental composition of macerals; an example of the application of electron microprobe to coal studies. Int. J. Coal Geol. 1993, 22, 83–99. [Google Scholar] [CrossRef]
- Mastalerz, M.; Marc Bustin, R. Electron microprobe and micro-FTIR analyses applied to maceral chemistry. Int. J. Coal Geol. 1993, 24, 333–345. [Google Scholar] [CrossRef]
- Mastalerz, M.; Bustin, R. Application of reflectance micro-Fourier transform infrared spectrometry in studying coal macerals: Comparison with other Fourier transform infrared techniques. Fuel 1995, 74, 536–542. [Google Scholar] [CrossRef]
- Michaelian, K.; Friesen, W. Photoacoustic FT-i.r. spectra of separated western Canadian coal macerals: Analysis of the CH stretching region by curve-fitting and deconvolution. Fuel 1990, 69, 1271–1275. [Google Scholar] [CrossRef]
- Zilm, K.; Pugmire, R.; Larter, S.; Allan, J.; Grant, D.M. Carbon-13 CP/MAS spectroscopy of coal macerals. Fuel 1981, 60, 717–722. [Google Scholar] [CrossRef]
- Kidena, K.; Katsuyama, M.; Murata, S.; Nomura, M. Study on plasticity of maceral concentrates in terms of their structural features. Energy Fuels 2002, 16, 1231–1238. [Google Scholar] [CrossRef]
- Xie, K.; Zhang, Y.; Li, C.; Ling, D. Pyrolysis characteristics of macerals separated from a single coal and their artificial mixture. Fuel 1991, 70, 474–479. [Google Scholar] [CrossRef]
- Malumbazo, N.; Wagner, N.; Bunt, J.; Niekerk, D.; Assumption, H. Structural analysis of chars generated from South African inertinite coals in a pipe-reactor combustion unit. Fuel Process. Technol. 2011, 92, 743–749. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, H.; Jin, L.; He, X.; Wu, B. Pyrolysis behavior of vitrinite and inertinite from Chinese Pingshuo coal by TG–MS and in a fixed bed reactor. Fuel Process. Technol. 2011, 92, 780–786. [Google Scholar] [CrossRef]
- Xie, K.; Lin, J.; Li, W.; Chang, L.; Feng, J.; Zhao, W. Formation of HCN and NH3 during coal macerals pyrolysis and gasification with CO2. Fuel 2005, 84, 271–277. [Google Scholar] [CrossRef]
- Sun, Q.; Li, W.; Chen, H.; Li, B. The variation of structural characteristics of macerals during pyrolysis. Fuel 2003, 82, 669–676. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, B.; Mathews, J.; Yan, G.; Li, F. Structural transformations and hydrocarbon generation of low-rank coal (vitrinite) during slow heating pyrolysis. Fuel Process. Technol. 2017, 167, 535–544. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, Y.; Qiu, P.; Lin, D.; Qian, J.; Hou, H.; Pei, J. Investigation of the relationship between infrared structure and pyrolysis reactivity of coals with different ranks. Fuel 2018, 216, 521–530. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Y.; Li, Z.; Shi, Q.; Wei, C.; Wu, C.; Cao, C.; Qu, Z. Differences in desorption rate and composition of desorbed gases between undeformed and mylonitic coals in the Zhina Coalfield, Southwest China. Fuel 2019, 239, 905–916. [Google Scholar] [CrossRef]
- Mählmann, R.F.; Le Bayon, R. Vitrinite and vitrinite like solid bitumen reflectance in thermal maturity studies: Correlations from diagenesis to incipient metamorphism in different geodynamic settings. Int. J. Coal Geol. 2016, 157, 52–73. [Google Scholar] [CrossRef]
- Unsworth, J.; Gough, H. Characterization of coals by automated optical image analysis 2. Inertinite reflectance. J. Microsc. 1989, 156, 327–342. [Google Scholar] [CrossRef]
- Sun, Z.; Ni, Z.; Wei, Q.; Liang, Y. Pyrolysis characteristics of vitrinites in coking coals with different coal ranks. Int. J. Coal Prep. Util. 2018, 38, 271–279. [Google Scholar] [CrossRef]
- James, C.; Hower; Alan, D. Application of vitrinite reflectance anisotropy in the evaluation of coal metamorphism. GSA Bull. 1981, 92, 350–366. [Google Scholar] [CrossRef]
- Gayo, F.; García, R.; Diez, M. Modelling the Gieseler fluidity of coking coals modified by multicomponent plastic wastes. Fuel 2016, 165, 134–144. [Google Scholar] [CrossRef]
- Rejdak, M.; Wojtaszek-Kalaitzidi, M.; Gałko, G.; Mertas, B.; Radko, T.; Baron, R.; Książek, M.; Yngve Larsen, S.; Sajdak, M.; Kalaitzidis, S. A Study on Bio-Coke Production—The Influence of Bio-Components Addition on Coke-Making Blend Properties. Energies 2022, 15, 6847. [Google Scholar] [CrossRef]
- Qin, Z.; Li, X.; Sun, H.; Zhao, C.; Rong, L. Caking property and active components of coal based on group component separation. Int. J. Min. Sci. Technol. 2016, 26, 571–575. [Google Scholar] [CrossRef]
- Mohammad, Z.; Maria, M.; Trevor, A. Biomass for iron ore sintering. Miner. Eng. 2010, 23, 1139–1145. [Google Scholar] [CrossRef]
- Andrii, K.; Lina, K.; Andrii, U.; Artem, S. Study of cellulose additive effect on the caking properties of coal. Min. Miner. Depos. 2023, 17, 1–8. [Google Scholar] [CrossRef]
- Su, X.; Wang, Z.; Li, N.; Li, L.; Zhang, P.; Sun, M.; Ma, X. Study on coal pyrolysis characteristics by combining different pyrolysis reactors. Chin. J. Chem. Eng. 2024, 76, 1–9. [Google Scholar] [CrossRef]
- Lv, Y.; Sun, Z.; Hu, W.; Han, J.; Shang, N. Study on the relationship between chemical structure and thermoplasticity of moderately metamorphic coking coal. Coke Chem. 2023, 66, 526–535. [Google Scholar] [CrossRef]
- Bambalaza, S.; Xakalashe, S.; Coetsee, Y.; van Zyl, P.G.; Dyosiba, X.L.; Musyoka, N.M.; Steenkamp, J.D. Co-Carbonization of discard coal with waste polyethylene terephthalate towards the preparation of metallurgical coke. Materials 2023, 16, 2782. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Q.; Zhao, X.; Yang, S.; Wu, H.; Zhang, S.; Sun, J. Relevance between various phenomena during coking coal carbonization. Part 3: Understanding the properties of the plastic layer during coal carbonization. Fuel 2021, 292, 120371. [Google Scholar] [CrossRef]
- Yoshida, T.; Takanohashi, T.; Iino, M.; Katoh, K. Temperature-variable dynamic viscoelastic measurements for coal blends of coking coal with slightly coking coal. Fuel Process. Technol. 2002, 77–78, 275–283. [Google Scholar] [CrossRef]
- Saito, K.; Hatakeyama, M.; Komaki, I.; Katoh, K. Solid state NMR studies for a new carbonization process with high temperature preheating. J. Mol. Struct. 2002, 602, 89–103. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Xue, Q.; Zuo, H.; She, X.; Wang, J. Effect of preheating on coking coal and metallurgical coke properties: A review. Fuel Process. Technol. 2021, 221, 106942. [Google Scholar] [CrossRef]
- Takanohashi, T.; Yoshida, T.; Iino, M.; Toyoda, M.; Kojima, T.; Kato, K. Effect of heating rate on structural changes of heat-treated coals. Tetsu Hagane-J. Iron Steel Inst. Jpn. 2001, 87, 454–458. [Google Scholar] [CrossRef]
- Matsuura, M.; Sasaki, M.; Saito, K.; Kato, K.; Komaki, I. Effects of rapid preheating on coal structure and coke strength. Tetsu Hagane-J. Iron Steel Inst. Jpn. 2003, 89, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Song, H.; Wang, S.; Ling, Q.; Zhao, Z.; Xie, R.; Liu, X.; Cui, P. Modification of Caking and Coking Properties of Shenmu Subbituminous Coal by Low-temperature Rapid Pyrolysis Treatment under CO2 Atmosphere. Coke Chem. 2022, 65, 371–380. [Google Scholar] [CrossRef]
- Collin, G.; Bujnowska, B.; Polaczek, J. Co-coking of coal with pitches and waste plastics. Fuel Process. Technol. 1997, 50, 179–184. [Google Scholar] [CrossRef]
- Grint, A.; Marsh, H. Carbonization and liquid-crystal (mesophase) development. 20. Co-carbonization of a high-volatile caking coal with several petroleum pitches. Fuel 1981, 60, 513–518. [Google Scholar] [CrossRef]
- Clemens, A.; Matheson, T. The effect of selected additives and treatments on Gieseler fluidity in coals. Fuel 1995, 74, 57–62. [Google Scholar] [CrossRef]
- Forney, A.; Kenny, R.; Field, J. Effect of air oxidation on the caking properties of Pittsburgh-seam coal. Am. Chem. Soc. Div. Fuel Chem. 1976, 9, 66–67. [Google Scholar]
- Gasior, S.; Forney, A.; Field, J.H. Destruction of caking quality of bituminous coal in a fixed bed. Ind. Eng. Chem. Prod. Res. Dev. 1964, 3, 43–47. [Google Scholar] [CrossRef]
- Forney, A.; Kenny, R.; Gasior, S.; Field, J.H. Destruction of caking properties of coal by pretreatment in a fluidized bed. Ind. Eng. Chem. Prod. Res. Dev. 1964, 3, 48–53. [Google Scholar] [CrossRef]
- Ren, L.; Tao, F.; Weng, T.; Li, Q.; Yu, X.; Zhai, X.; Ma, T. Investigating how oxygen levels and particle size impact the thermodynamics of low temperature coal oxidation: A case study using weakly caking coal. Fuel 2024, 378, 132914. [Google Scholar] [CrossRef]
- Orchin, M.; Golumbic, C.; Anderson, J.; Storch, H. Studies of the Extraction and Coking of Coal and Their Significance in Relation to Its Structure; United States Government Printing Office: Washington, DC, USA, 1951.
- Sánchez, J.; Rincón, J. Oxidation paths of a coking coal and comparison of its oxidized product with a non-caking coal. Fuel 1997, 76, 1137–1142. [Google Scholar] [CrossRef]
- Ignasiak, B.; Szladow, A.; Montgomery, D. Oxidation studies on coking coal related to weathering. 3. The influence of acidic hydroxyl groups, created during oxidation, on the plasticity and dilatation of the weathered coking coal. Fuel 1974, 53, 12–15. [Google Scholar] [CrossRef]
- Rhoads, C.; Senftle, J.; Coleman, M.; Davis, A.; Painter, P.C. Further studies of coal oxidation. Fuel 1983, 62, 1387–1392. [Google Scholar] [CrossRef]
- Painter, P.; Snyder, R.; Pearson, D.; Kwong, J. Fourier transform infrared study of the variation in the oxidation of a coking coal. Fuel 1980, 59, 282–286. [Google Scholar] [CrossRef]
- Liotta, R.; Brons, G.; Isaacs, J. Oxidative weathering of Illinois No.6 coal. Fuel 1983, 62, 781–791. [Google Scholar] [CrossRef]
- Larsen, J.; Lee, D.; Schmidt, T.; Grint, A. Multiple mechanisms for the loss of coking properties caused by mild air oxidation. Fuel 1986, 65, 595–596. [Google Scholar] [CrossRef]
- Wang, D.; Xin, H.; Qi, X.; Dou, G.; Qi, G.; Ma, L. Reaction pathway of coal oxidation at low temperatures: A model of cyclic chain reactions and kinetic characteristics. Combust. Flame 2016, 163, 447–460. [Google Scholar] [CrossRef]
- Worasuwannarak, N.; Nakagawa, H.; Miura, K. Effect of pre-oxidation at low temperature on the carbonization behavior of coal. Fuel 2002, 81, 1477–1484. [Google Scholar] [CrossRef]
- Ruiz, B.; Parra, J.; Pajares, J.; Pis, J. Effect of coal pre-oxidation on the optical texture and porosity of pyrolysis chars. J. Anal. Appl. Pyrolysis 2006, 75, 27–32. [Google Scholar] [CrossRef]
- Gasior, S.; Forney, A.; Field, J. Decaking of coal in free fall. Fuel Gasif. 1967, 69, 1–7. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, X.; Sun, Y.; Zhang, J.; Zhao, Z.; Wang, Y.; Xu, G. Jetting pre-oxidation fluidized bed gasification process for caking coal: Fundamentals and pilot test. Appl. Energy 2015, 160, 80–87. [Google Scholar] [CrossRef]
- Seki, H.; Kumagai, J.; Matsuda, M.; Ito, O.; Iino, M. Fluidity of coal residues after extraction with mixed solvents. Fuel 1989, 68, 978–982. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Song, H.; Cui, P. Modification mechanism of caking property of polystyrene waste using low-temperature pyrolysis and its use in coal-blending coking. Chin. J. Chem. Eng. 2025, 77, 93–101. [Google Scholar] [CrossRef]
| Samples | Temperature (°C) | GR.I. 1 | Extraction Yielddaf (wt.%) | Vdaf (wt.%) |
|---|---|---|---|---|
| Raw coal | / | 34.6 | 4.8 | 38.3 |
| 1# | 100 | 38.4 | 10.3 | 36.5 |
| 2# | 150 | 42.4 | 15.2 | 31.4 |
| 3# | 200 | 41.2 | 16.0 | 30.2 |
| 4# | 250 | 39.2 | 15.6 | 28.7 |
| Authors | Contents of Research |
|---|---|
| Forney [87] | When strongly caking coals were heated to 120~250 °C in air and kept for a certain time, the caking property decreased significantly with increasing temperature and time. |
| Gasior [88] | The caking property could be drastically reduced by using an inert gas with 1% oxygen by volume, maintaining the coal at the softening temperature for 1~3 h, and then slowly heating it to the plastic temperature range. |
| Forney [89] | The pre-oxidation of coal using an inert gas containing 0.2% oxygen by volume, conducted at temperatures between ~400 and 425 °C, demonstrated a significant reduction in the caking property. |
| Ren [90] | A lower oxygen concentration delays the heat flow curve, raises the characteristic temperature, and slows the oxidation reaction. In contrast, smaller coal particle sizes increase heat release intensity. |
| Zhao [25] | The temperature, gas flow rate, and oxygen volume fraction of pre-oxidation had a more pronounced effect on the caking property. |
| Zhao [19] | The jetting pre-oxidation fluidized bed gasification technology could be applied to achieve stable gasification of coal with a GR.I. of 20. |
| Technologies | Introduction | Evaluate |
|---|---|---|
| Blending with non-caking fuels [24] | Blending the weakly caking coal with non-caking fuels in specific proportions to change the caking property. | The caking property changes nonlinearly after blending and this method is less economical. |
| Additive method [103] |
| Due to the poor effect of decaking when the additive is used alone, it needs to be used in conjunction with other technologies. |
| Extraction [71] | Components with caking property can be extracted from coal by organic solvents (CS2-NMP). | The extraction process significantly impacts the structure of residual organic components. Moreover, extractants present issues including high costs and environmental pollution. |
| Weathering [93] | The oxidation of weakly caking coal at ambient temperature with natural air can effectively reduce the caking property. | While this method is cost-effective and straightforward to implement, it necessitates a more extended processing time. |
| Low-temperature pyrolysis treatment (LTPT) [104] | When coal is heated to a specified temperature at a slow heating rate within an inert atmosphere, the aliphatic ester compounds and long-chain aliphatic are effectively reduced. This process subsequently minimizes the formation of plastic mass. | LTPT consumes organic components and has a certain amount of energy consumption, which makes it less economical. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhu, S.; Ouyang, Z.; Zhu, Z.; Lyu, Q. Progress in Caking Mechanism and Regulation Technologies of Weakly Caking Coal. Energies 2025, 18, 4178. https://doi.org/10.3390/en18154178
Li Z, Zhu S, Ouyang Z, Zhu Z, Lyu Q. Progress in Caking Mechanism and Regulation Technologies of Weakly Caking Coal. Energies. 2025; 18(15):4178. https://doi.org/10.3390/en18154178
Chicago/Turabian StyleLi, Zhaoyang, Shujun Zhu, Ziqu Ouyang, Zhiping Zhu, and Qinggang Lyu. 2025. "Progress in Caking Mechanism and Regulation Technologies of Weakly Caking Coal" Energies 18, no. 15: 4178. https://doi.org/10.3390/en18154178
APA StyleLi, Z., Zhu, S., Ouyang, Z., Zhu, Z., & Lyu, Q. (2025). Progress in Caking Mechanism and Regulation Technologies of Weakly Caking Coal. Energies, 18(15), 4178. https://doi.org/10.3390/en18154178






