Cascade Processing of Agricultural, Forest, and Marine Waste Biomass for Sustainable Production of Food, Feed, Biopolymers, and Bioenergy
Abstract
1. Introduction
Alignment with the SDGs
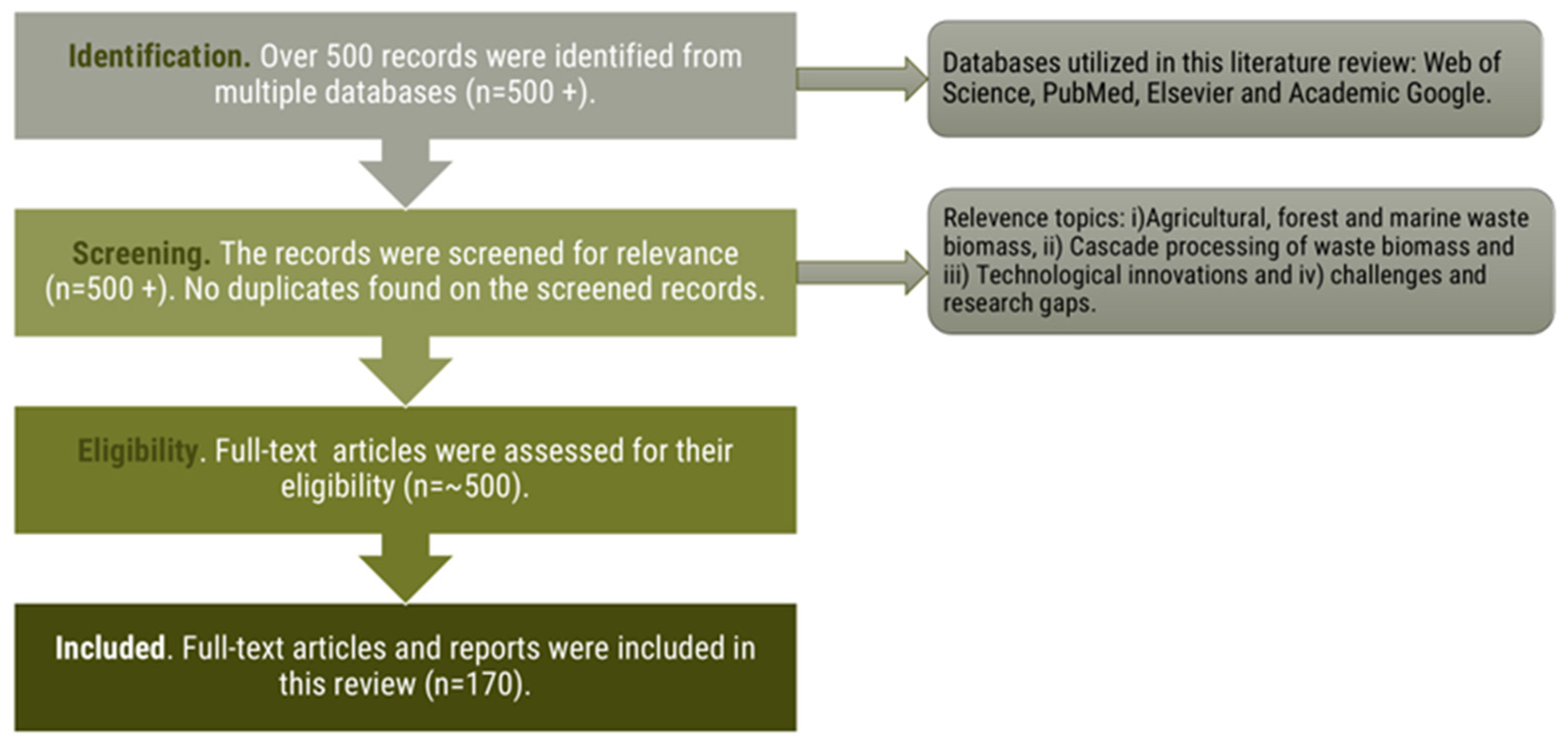
2. Methodology for Data Collection, Selection, and Review
3. Waste Biomass
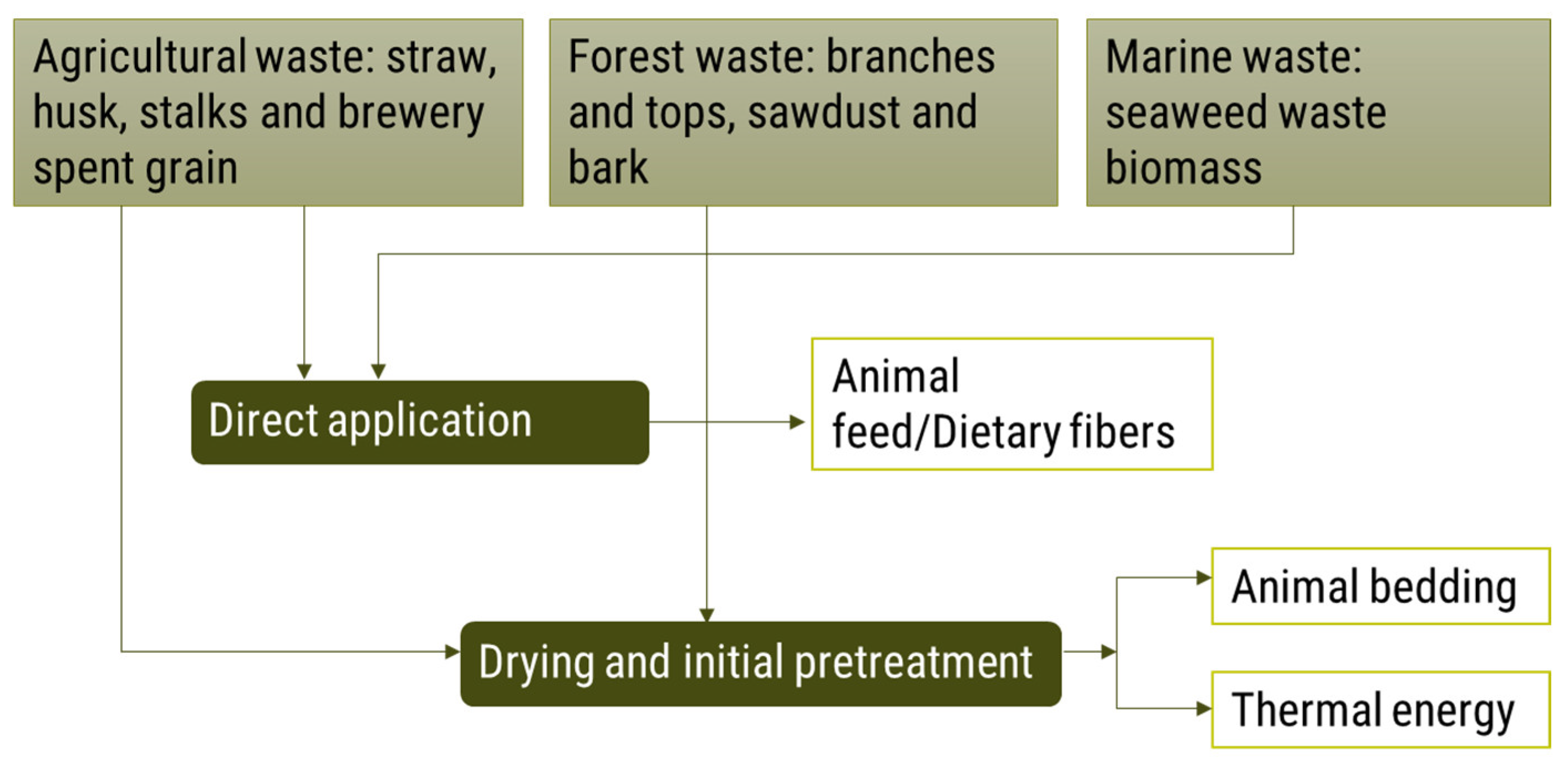
3.1. Agricultural Waste
3.2. Forest Waste
3.3. Marine Waste Biomass
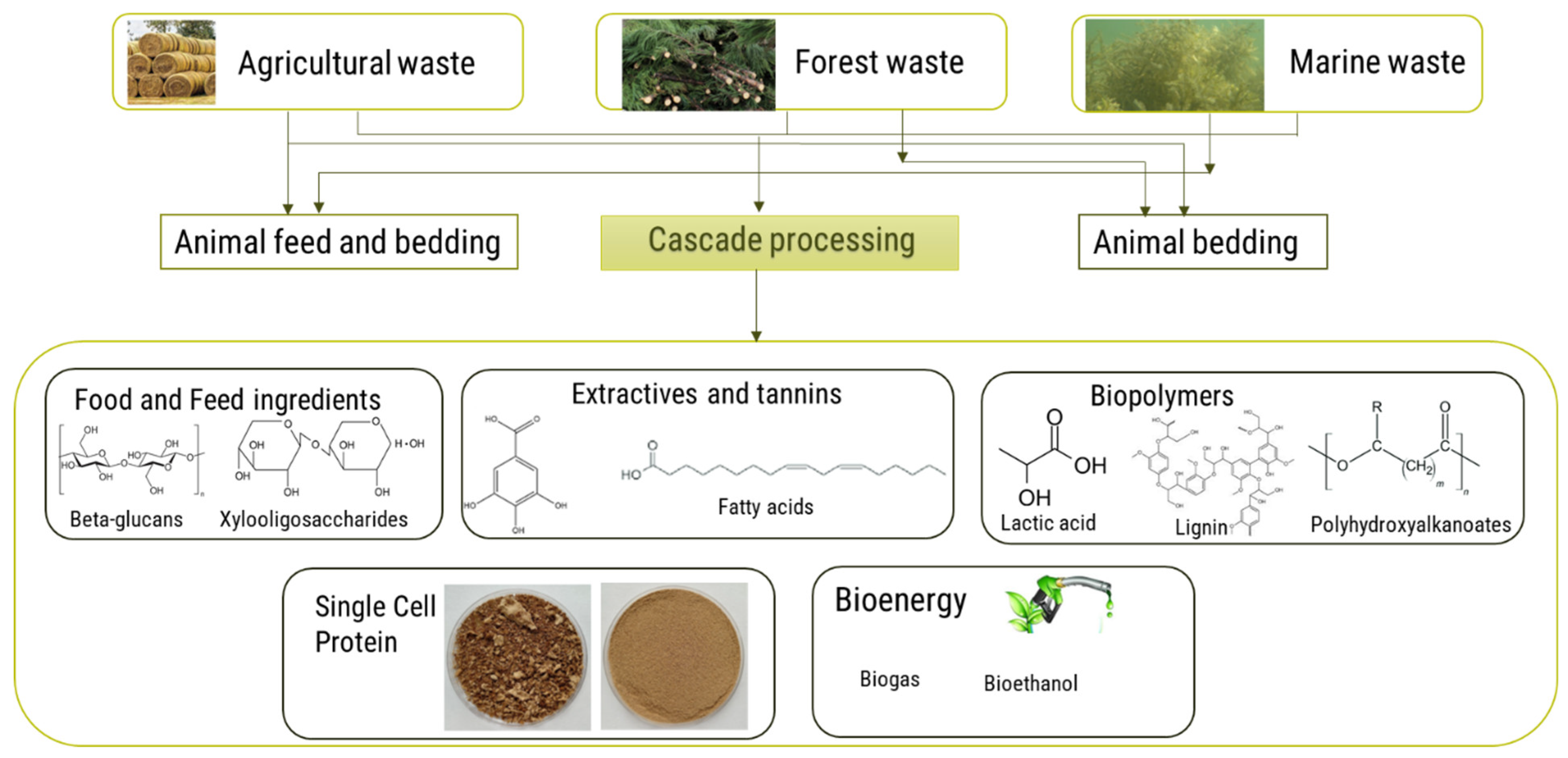
4. Cascade Processing of Waste Biomass
4.1. Principles and Importance of Cascade Processing
4.1.1. Initial Pretreatment
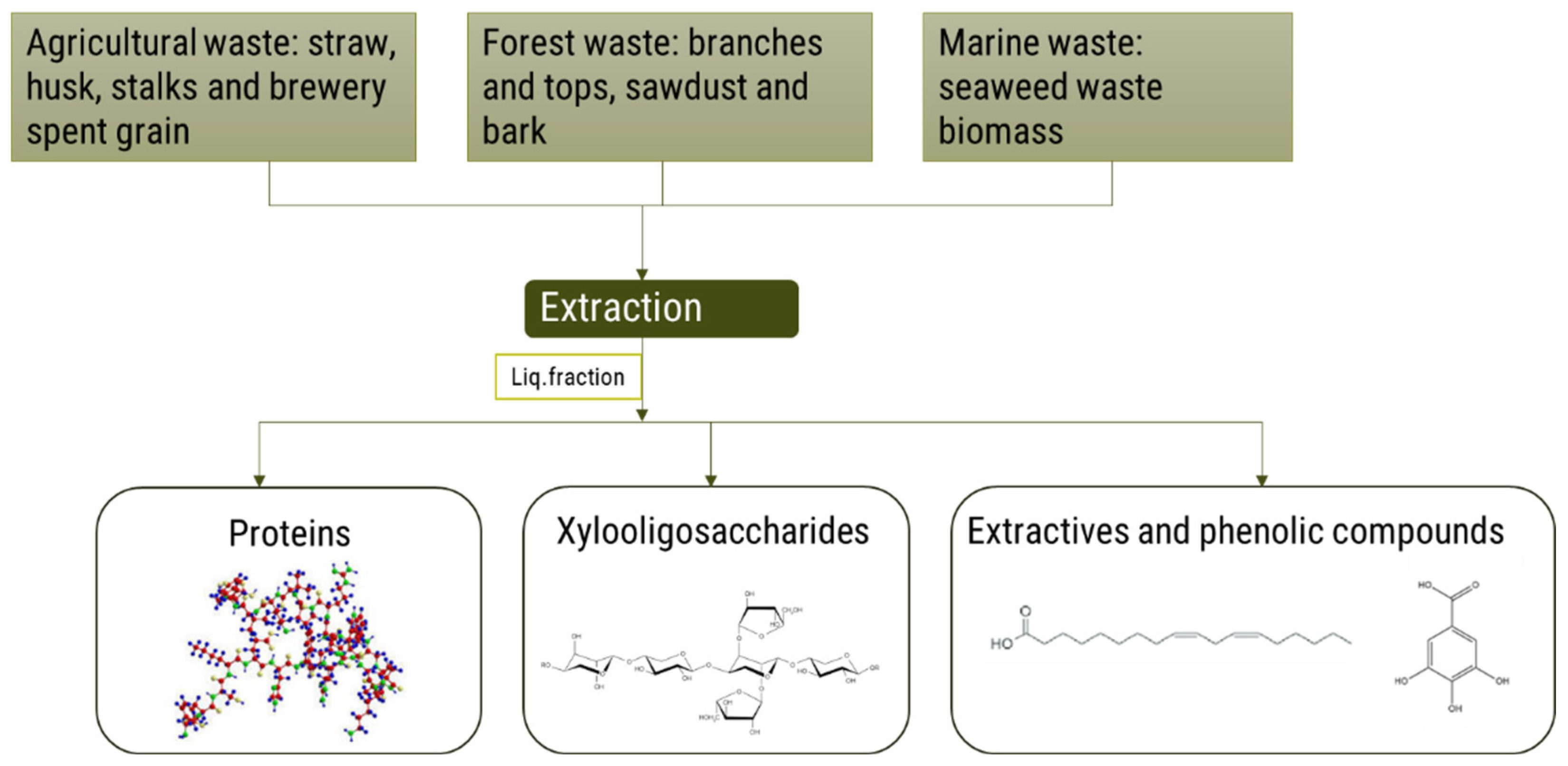
4.1.2. Extraction of High-Value Components
Proteins
Xylooligosaccharides
Extractives and Phenolic Compounds
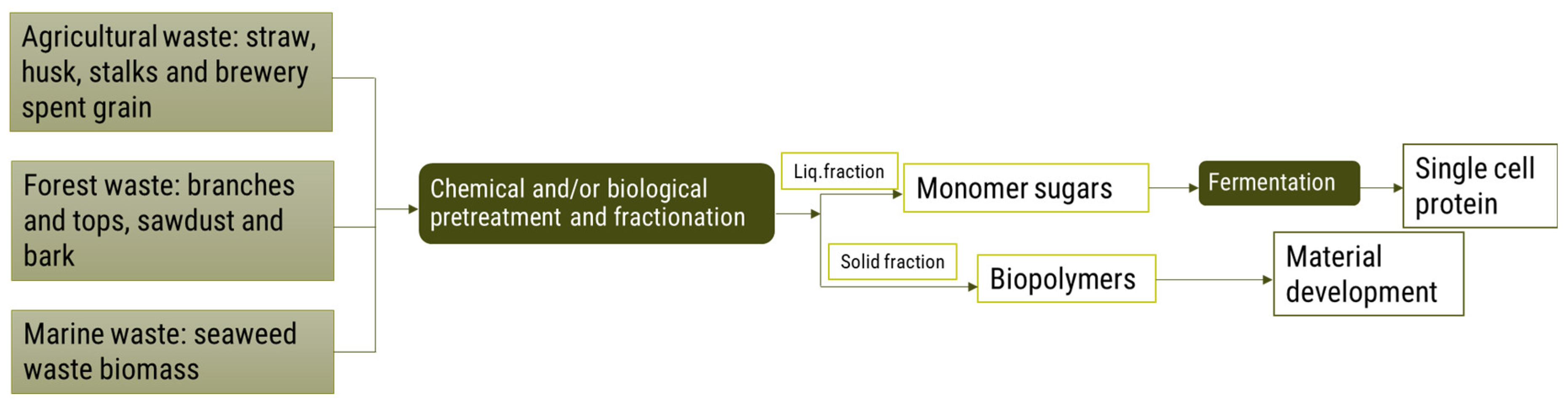
4.1.3. Chemical and/or Biological Pretreatment and Fractionation
Pretreatment and Fractionation
Biopolymers
Bioplastics
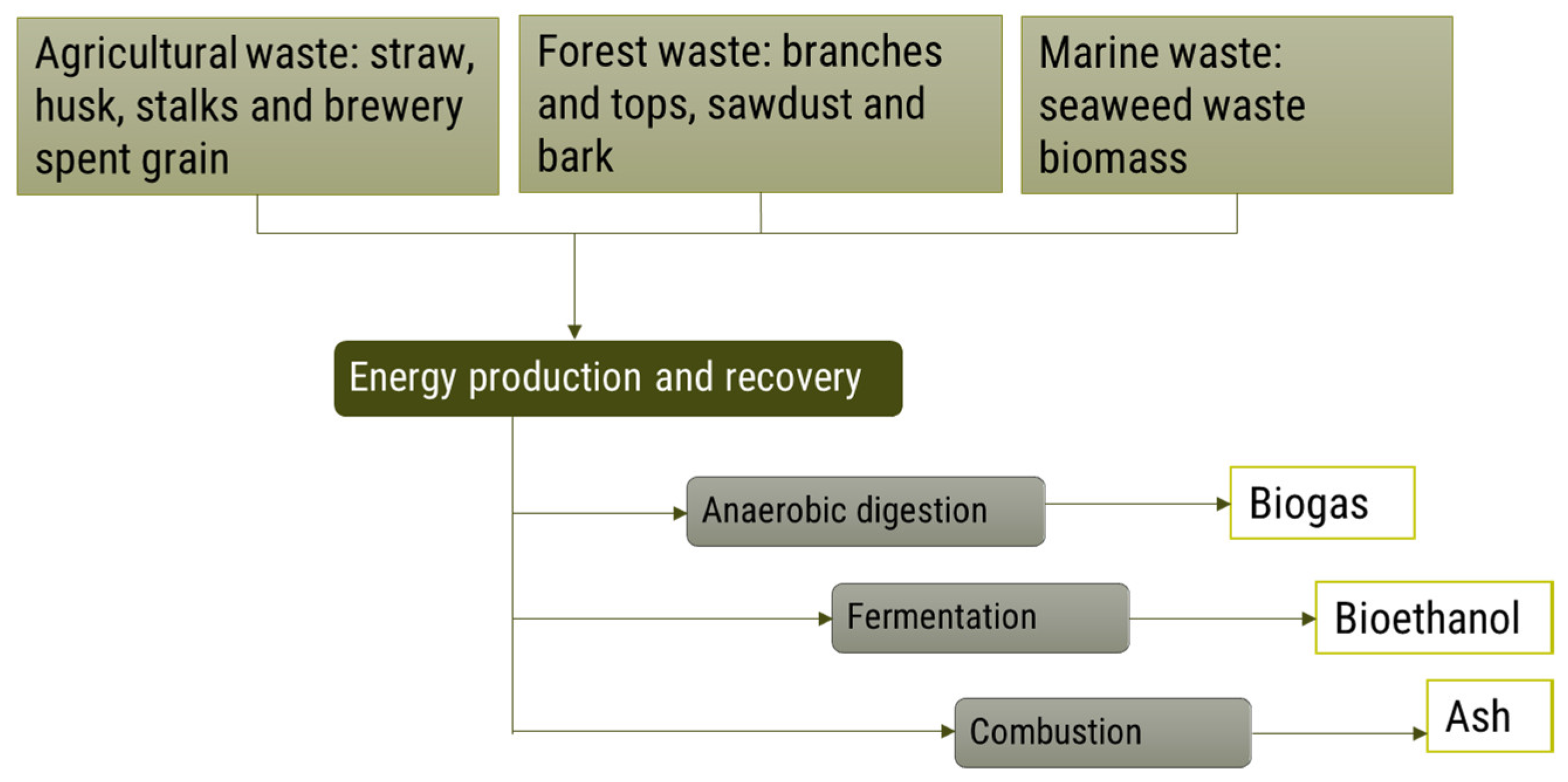
4.1.4. Energy Production and Recovery
Biogas
Bioethanol
Ash
4.2. Advantages of Cascade Processing
4.3. Integration of Energy Recovery in Cascade Systems
5. Technological Innovations
5.1. Innovative Bioreactor Designs for Multi-Product Recovery
5.2. Integrated Upstream and Downstream Processing for Enhanced Bioproduct Recovery
6. Challenges and Research Gaps
Health and Safety Considerations
7. Future Perspectives
7.1. Innovations in Process Optimization
7.2. Role of Public–Private Partnerships and Funding Opportunities
7.3. Potential to Align with Circular Bioeconomy Principles
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. How to Feed the World in 2050; FAO: Rome, Italy, 2009. [Google Scholar]
- UNEP. From Waste to Wealth: Circular Economy as a Catalyst for Jobs and Sustainability; UNEP: Nairobi, Kenya, 2025. [Google Scholar]
- Gottinger, A.; Ladu, L.; Quitzow, R. Studying the Transition towards a Circular Bioeconomy—A Systematic Literature Review on Transition Studies and Existing Barriers. Sustainability 2020, 12, 8990. [Google Scholar] [CrossRef]
- Partnership for Policy Integrity (PFPI). Global Demand for Biomass Energy is Destroying Forests Around the World; PFPI: Pelham, MA, USA, 2023. [Google Scholar]
- Dace, E.; Cascavilla, A.; Bianchi, M.; Chioatto, E.; Zecca, E.; Ladu, L.; Yilan, G. Barriers to transitioning to a circular bio-based economy: Findings from an industrial perspective. Sustain. Prod. Consum. 2024, 48, 407–418. [Google Scholar] [CrossRef]
- Dulce María, D.-M. Valorization of Biomass as a Raw Material to Obtain Products of Industrial Interest. In Biomass, Biorefineries and Bioeconomy; Mohamed, S., Ed.; IntechOpen: Rijeka, Croatia, 2022; Chapter 11. [Google Scholar]
- Kataya, G.; Cornu, D.; Bechelany, M.; Hijazi, A.; Issa, M. Biomass Waste Conversion Technologies and Its Application for Sustainable Environmental Development—A Review. Agronomy 2023, 13, 2833. [Google Scholar] [CrossRef]
- Varalakksmi, V.; Sudalai, S.; Arumugam, A. Sustainable Utilization of Biomass Resources. In Clean Energy Transition-via-Biomass Resource Utilization: A Way to Mitigate Climate Change; Kumar, S., Sundaramurthy, S., Kumar, D., Chandel, A.K., Eds.; Springer Nature: Singapore, 2024; pp. 1–27. [Google Scholar]
- Zhu, J.; Guo, Y.; Chen, N.; Chen, B. A Review of the Efficient and Thermal Utilization of Biomass Waste. Sustainability 2024, 16, 9506. [Google Scholar] [CrossRef]
- Grand View Research. Biopolymers Market Size, Share & Trends Analysis Report by Product (Bio-PE, Bio-PET), by End-Use (Packaging, Consumer Goods), by Application, by Region, and Segment Forecasts, 2024–2030; Grand View Research: San Francisco, CA, USA, 2024. [Google Scholar]
- Kumar, S.; Lohan, S.K.; Parihar, D. Biomass Energy from Agriculture: Conversion Techniques and Use. In Handbook of Energy Management in Agriculture; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–19. [Google Scholar]
- Wijerathna, K.; Kumarasinghe, U.; Idroos, F. Waste Biomass Valorization and Its Application in the Environment. In Sustainable Valorization of Agriculture & Food Waste Biomass; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–28. [Google Scholar]
- Santoro, M.; Cartus, O.; Carvalhais, N.; Rozendaal, D.M.A.; Avitabile, V.; Araza, A.; de Bruin, S.; Herold, M.; Quegan, S.; Rodríguez-Veiga, P.; et al. The global forest above-ground biomass pool for 2010 estimated from high-resolution satellite observations. Earth Syst. Sci. Data 2021, 13, 3927–3950. [Google Scholar] [CrossRef]
- Joint Research Centre, European Commission. Brief on Biomass Production of Fisheries and Aquaculture; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Ginni, G.; Kavitha, S.; Kannah, R.Y.; Bhatia, S.K.; Kumar, S.A.; Rajkumar, M.; Kumar, G.; Pugazhendhi, A.; Chi, N.T.L.; Banu, J.R. Valorization of agricultural residues: Different biorefinery routes. J. Environ. Chem. Eng. 2021, 9, 105435. [Google Scholar] [CrossRef]
- Song, B.; Lin, R.C.; Lam, C.H.; Wu, H.; Tsui, T.H.; Yu, Y. Recent advances and challenges of inter-disciplinary biomass valorization by integrating hydrothermal and biological techniques. Renew. Sustain. Energy Rev. 2021, 135, 110370. [Google Scholar] [CrossRef]
- Campbell-Johnston, K.; Vermeulen, W.J.V.; Reike, D.; Brullot, S. The Circular Economy and Cascading: Towards a Framework. Resour. Conserv. Recycl. X 2020, 7, 100038. [Google Scholar] [CrossRef]
- Smit, A.T.; van Zomeren, A.; Dussan, K.; Riddell, L.A.; Huijgen, W.J.J.; Dijkstra, J.W.; Bruijnincx, P.C.A. Biomass Pre-Extraction as a Versatile Strategy to Improve Biorefinery Feedstock Flexibility, Sugar Yields, and Lignin Purity. ACS Sustain. Chem. Eng. 2022, 10, 6012–6022. [Google Scholar] [CrossRef]
- Blair, J.; Gagnon, B.; Klain, A. Biomass Supply and the Sustainable Development Goals: International Case Studies; IEA Bioenergy: Dublin, Ireland, 2021. [Google Scholar]
- Keegan, D.; Kretschmer, B.; Elbersen, B.; Panoutsou, C. Cascading use: A systematic approach to biomass beyond the energy sector. Biofuels Bioprod. Biorefining 2013, 7, 193–206. [Google Scholar] [CrossRef]
- Mair, C.; Stern, T. Cascading Utilization of Wood: A Matter of Circular Economy? Curr. For. Rep. 2017, 3, 281–295. [Google Scholar] [CrossRef]
- Besserer, A.; Troilo, S.; Girods, P.; Rogaume, Y.; Brosse, N. Cascading Recycling of Wood Waste: A Review. Polymers 2021, 13, 1752. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.; Malins, C. Availability of Cellulosic Residues and Wastes in the EU; ICCT: Washington, DC, USA, 2013. [Google Scholar]
- Van Hung, N.; Maguyon-Detras, M.C.; Migo, M.V.; Quilloy, R.; Balingbing, C.; Chivenge, P.; Gummert, M. Rice Straw Overview: Availability, Properties, and Management Practices. In Sustainable Rice Straw Management; Gummert, M., Hung, N.V., Chivenge, P., Douthwaite, B., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–13. [Google Scholar]
- Kapoor, M.; Panwar, D.; Kaira, G.S. Chapter 3—Bioprocesses for Enzyme Production Using Agro-Industrial Wastes: Technical Challenges and Commercialization Potential. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Dhillon, G.S., Kaur, S., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 61–93. [Google Scholar]
- Dancker, P.; Glas, K.; Gastl, M. Potential utilisation methods for brewer’s spent grain: A review. Int. J. Food Sci. Technol. 2025, 60, vvae022. [Google Scholar] [CrossRef]
- Lima Moraes dos Santos, A.; de Sousa e Silva, A.; Sales Morais, N.W.; Bezerra dos Santos, A. Brewery Spent Grain as sustainable source for value-added bioproducts: Opportunities and new insights in the integrated lignocellulosic biorefinery concept. Ind. Crops Prod. 2023, 206, 117685. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Belbo, H.; Gjølsjo, S.; Hohle, E.E. Market survey—Biomass from forestry for energy and industrial purposes. In Markedsundersøkelse—Skogsbasert Biomasse til Energi og Industriformål; 2464-1162: NIBIO-Report; NIBIO: Osaka, Japan, 2023. [Google Scholar]
- Leso, L.; Barbari, M.; Lopes, M.A.; Damasceno, F.A.; Galama, P.; Taraba, J.L.; Kuipers, A. Invited review: Compost-bedded pack barns for dairy cows. J. Dairy Sci. 2020, 103, 1072–1099. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, S.; Tuan, Z.; Leitch, M.; Xu, C. Valorization of bark for chemicals and materials: A review. Renew. Sustain. Energy Rev. 2013, 26, 560–578. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef]
- El-Said, G.F.; El-Sikaily, A. Chemical composition of some seaweed from Mediterranean Sea coast, Egypt. Environ. Monit. Assess. 2013, 185, 6089–6099. [Google Scholar] [CrossRef]
- van Oirschot, R.; Thomas, J.-B.E.; Gröndahl, F.; Fortuin, K.P.J.; Brandenburg, W.; Potting, J. Explorative environmental life cycle assessment for system design of seaweed cultivation and drying. Algal Res. 2017, 27, 43–54. [Google Scholar] [CrossRef]
- Stedt, K.; Trigo, J.P.; Steinhagen, S.; Nylund, G.M.; Forghani, B.; Pavia, H.; Undeland, I. Cultivation of seaweeds in food production process waters: Evaluation of growth and crude protein content. Algal Res. 2022, 63, 102647. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Lim, S.-R.; Kim, Y.; Park, J.M. Opportunity and challenge of seaweed bioethanol based on life cycle CO2 assessment. Environ. Prog. Sustain. Energy 2017, 36, 200–207. [Google Scholar] [CrossRef]
- Susanto, E.; Fahmi, A.S.; Abe, M.; Hosokawa, M.; Miyashita, K. Lipids, Fatty Acids, and Fucoxanthin Content from Temperate and Tropical Brown Seaweeds. Aquat. Procedia 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed biorefinery. Rev. Environ. Sci. Bio/Technol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Johnston, K.G.; Abomohra, A.; French, C.E.; Zaky, A.S. Recent Advances in Seaweed Biorefineries and Assessment of Their Potential for Carbon Capture and Storage. Sustainability 2023, 15, 3193. [Google Scholar] [CrossRef]
- Castilla-Archilla, J.; Cermeño, M.; Tuohy, M.; FitzGerald, R.J.; Lens, P.N. Fractionation of Brewer’s Spent Grain Using a Cascade Process for Carbohydrate Release and the Simultaneous Production of Protein and Fiber Fractions Targeting the Food Industry. ACS Sustain. Resour. Manag. 2024, 1, 2350–2360. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Pacheco, D.; Gonçalves, A.M.; Bahcevandziev, K.; Pereira, L.; Figueirinha, A.; Dias, J.M. Valorisation of marine macroalgae waste using a cascade biorefinery approach: Exploratory study. J. Clean. Prod. 2023, 385, 135672. [Google Scholar] [CrossRef]
- SRavi, B. Developments in seaweed biorefinery research: A comprehensive review. Chem. Eng. J. 2023, 454, 140177. [Google Scholar]
- Olle Olsson, L.B.; Hektor, B.; Roos, A.; Guisson, R.; Lamers, P.; Hartley, D.; Ponitka, J.; Hildebrandt, J.; Thrän, D. Cascading of Woody Biomass: Definitions Policies Effects on International Trade; I.B.T., 40, Ed.; IEA Bioenergy: Dublin, Ireland, 2016. [Google Scholar]
- Sudar, M.; Blažević, Z.F. Enzyme Cascade Kinetic Modelling. In Enzyme Cascade Design and Modelling; Kara, S., Rudroff, F., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 91–108. [Google Scholar]
- Tuula Jyske, K.R.; Korkalo, P.; Kohl, J. Cascade vision: Regionally adaptive circular bioeconomy—Added value, wellbeing and resource wisdom with cascade processing. In Natural Resources and Bioeconomy Studies 52/2023; Natural Resource Institute Finland (Luke): Helsinki, Finland, 2023; p. 29. [Google Scholar]
- Fehrenbach, H.; Köppen, S.; Kauertz, B.; Detzel, A.; Wellenreuther, F. Biomass Cascades: Increasing Resource Efficiency by Cascading Use of Biomass—From Theory to Practice; TEXTE 53/2017; Umweltbundesamt: Dessau-Roßlau, Germany, 2017. [Google Scholar]
- Veluchamy, C.; Kalamdhad, A.S.; Gilroyed, B.H. Advanced Pretreatment Strategies for Bioenergy Production from Biomass and Biowaste. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–19. [Google Scholar]
- Azizan, A.; Jusri, N.A.A. Mechanical Pretreatment Options on Biofuel Biomass Feedstock Discussing on Biomass Grindability Index Relating to Particle Size reduction—A Review. In Recent Trends in Manufacturing and Materials Towards Industry 4.0; Springer: Singapore, 2021. [Google Scholar]
- Haynes, R.D.; Greschick, T. Using oxidative chemistry for Mechanical Pretreatments. In Proceedings of the International Bioenergy and Bioproducts Conference 2014 (IBBC 2014), Unlocking the Forest Biorefinery, Tacoma, WA, USA, 17–19 September 2014. [Google Scholar]
- Tedesco, S.; Montingelli, M.E.; Olabi, A.G. Hollander beater operational parameters ‘effect on macroalgal biogas production. In Proceedings of the 26th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, ECOS 2013, Guilin, China, 16–19 July 2013. [Google Scholar]
- Patil, R.S.; Upadhyay, N.; Rathore, A.S. Optimization of Process Parameters for Enhanced Production of Ranibizumab in Escherichia coli. Biotechnol. Bioprocess Eng. 2023, 28, 386–397. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Quint, J.; Rieder, A.; Ballance, S.; Dreiss, C.A.; Butterworth, P.J.; Ellis, P.R. Impact of hydrothermal and mechanical processing on dissolution kinetics and rheology of oat β-glucan. Carbohydr. Polym. 2017, 166, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Outlook, F. Biannual Report on Global Food Markets. In Food Outlook; FAO: Rome, Italy, 2020. [Google Scholar]
- Brack, D.; Wellesley, L.; Glover, A. Agricultural Commodity Supply Chains: Trade, Consumption and Deforestation; The Royal Institute of International Affairs: London, UK, 2016. [Google Scholar]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef]
- Spalvins, K.; Ivanovs, K.; Blumberga, D. Single cell protein production from waste biomass: Review of various agricultural by-products. Agron. Res. 2018, 16, 1493–1508. [Google Scholar]
- Bajic, B.; Vučurović, D.; Vasic, D.; Jevtic-Mucibabic, R.; Dodic, S. Biotechnological production of sustainable Microbial proteins from agro-industrial residues and by-products. Foods 2023, 12, 107. [Google Scholar] [CrossRef]
- Pratima, B. Single Cell Protein Production from Lignocellulosic Biomass; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Hooft, J.M.; Montero, R.; Morales-Lange, B.; Blihovde, V.F.; Purushothaman, K.; Press, C.M.; Mensah, D.D.; Agboola, J.O.; Javed, S.; Mydland, L.T.; et al. Paecilomyces variotii (PEKILO®) in novel feeds for Atlantic salmon: Effects on pellet quality, growth performance, gut health, and nutrient digestibility and utilization. Aquaculture 2024, 589, 740905. [Google Scholar] [CrossRef]
- Ee, K.Y.; Lam, M.Q.; Mah, J.K.; Merican, A. Black soldier fly (Hermetia illucens L.) larvae in degrading agricultural waste as a sustainable protein production: Feedstock modification and challenges. Int. J. Trop. Insect Sci. 2022, 42, 3847–3854. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Aroso, I.M.; Araújo, A.R.; Pires, R.A.; Reis, R.L. Cork: Current Technological Developments and Future Perspectives for this Natural, Renewable, and Sustainable Material. ACS Sustain. Chem. Eng. 2017, 5, 11130–11146. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Zembrzuska, J. Advances in Biological Activities and Application of Plant Extracts. Appl. Sci. 2023, 13, 9324. [Google Scholar] [CrossRef]
- Wang, L.-X.; Wang, H.-L.; Huang, J.; Chu, T.-Z.; Peng, C.; Zhang, H.; Chen, H.-L.; Xiong, Y.-A.; Tan, Y.-Z. Review of lignans from 2019 to 2021: Newly reported compounds, diverse activities, structure-activity relationships and clinical applications. Phytochemistry 2022, 202, 113326. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Tannin Market Size, Share & Trends Analysis Report by Source (Plants, Brown Algae), by Product (Hydrolysable, Non-hydrolysable, Phlorotannins), by Application (Leather Tanning, Wine Production), by Region, and Segment Forecasts, 2023–2030; Grand View Research: San Francisco, CA, USA, 2022; p. 122. [Google Scholar]
- IMARC. Tannin Market Report by Source (Plants, Brown Algae), Product (Hydrolysable Tannins, Condensed Tannins, Phlorotannins), Application (Food and Beverages, Leather Tanning, Wood Adhesives, and Other), and Region 2025–2033; IMARC: Sydney, Australia, 2024. [Google Scholar]
- Saad, M.B.W.; Gonçalves, A.R. Industrial pretreatment of lignocellulosic biomass: A review of the early and recent efforts to scale-up pretreatment systems and the current challenges. Biomass Bioenergy 2024, 190, 107426. [Google Scholar] [CrossRef]
- Chen, X.; Zhai, R.; Shi, K.; Yuan, Y.; Dale, B.E.; Gao, Z.; Jin, M. Mixing alkali pretreated and acid pretreated biomass for cellulosic ethanol production featuring reduced chemical use and decreased inhibitory effect. Ind. Crops Prod. 2018, 124, 719–725. [Google Scholar] [CrossRef]
- Loow, Y.L.; Wu, T.Y.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- Karp, E.M.; Donohoe, B.S.; O’Brien, M.H.; Ciesielski, P.N.; Mittal, A.; Biddy, M.J.; Beckham, G.T. Alkaline pretreatment of corn stover: Bench-scale fractionation and stream characterization. ACS Sustain. Chem. Eng. 2014, 2, 1481–1491. [Google Scholar] [CrossRef]
- Kuhn, E.M.; O’Brien, M.H.; Ciesielski, P.N.; Schell, D.J. Pilot-Scale Batch Alkaline Pretreatment of Corn Stover. ACS Sustain. Chem. Eng. 2016, 4, 944–956. [Google Scholar] [CrossRef]
- Lobato-Rodríguez, Á.; Gullón, B.; Romaní, A.; Ferreira-Santos, P.; Garrote, G.; Del-Río, P.G. Recent advances in biorefineries based on lignin extraction using deep eutectic solvents: A review. Bioresour. Technol. 2023, 388, 129744. [Google Scholar] [CrossRef]
- Ullah, A.; Zhang, Y.; Liu, C.; Qiao, Q.; Shao, Q.; Shi, J. Process intensification strategies for green solvent mediated biomass pretreatment. Bioresour. Technol. 2023, 369, 128394. [Google Scholar] [CrossRef]
- Pereira, E.; Pereira, D.T.V.; Rabelo, S.C.; Ceriani, R.; Costa, A.C.D. Green solvent pretreatments for lignocellulosic biorefineries: A review. J. Environ. Chem. Eng. 2025, 13, 115303. [Google Scholar] [CrossRef]
- Agnihotri, S.; Johnsen, I.; Bøe, M.; Øyaas, K.; Moe, S. Ethanol organosolv pretreatment of softwood (Picea abies) and sugarcane bagasse for biofuel and biorefinery applications. Wood Sci. Technol. 2015, 49, 881–896. [Google Scholar] [CrossRef]
- Parchami, M.; Agnihotri, S.; Taherzadeh, M.J. Aqueous ethanol organosolv process for the valorization of Brewer’s spent grain (BSG). Bioresour. Technol. 2022, 362, 127764. [Google Scholar] [CrossRef]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels Bioprod. Biorefining 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Sidiras, D.; Politi, D.; Giakoumakis, G.; Salapa, I. Simulation and optimization of organosolv based lignocellulosic biomass refinery: A review. Bioresour. Technol. 2022, 343, 126158. [Google Scholar] [CrossRef] [PubMed]
- Monção, M.; Anukam, A.I.; Hrůzová, K.; Rova, U.; Christakopoulos, P.; Matsakas, L. A Parametric Study of the Organosolv Fractionation of Norway Spruce Sawdust. Energies 2024, 17, 3276. [Google Scholar] [CrossRef]
- Xu, J.K.; Sun, Y.C.; Xu, F.; Sun, R.C. Characterization of hemicelluloses obtained from partially delignified eucalyptus using ionic liquid pretreatment. BioResources 2013, 8, 1946–1962. [Google Scholar] [CrossRef]
- Forsberg, Z.; Sorlie, M.; Petrovic, D.; Courtade, G.; Aachmann, F.L.; Vaaje-Kolstad, G.; Bissaro, B.; Rohr, A.K.; Eijsink, V.G. Polysaccharide degradation by lytic polysaccharide monooxygenases. Curr. Opin. Struct. Biol. 2019, 59, 54–64. [Google Scholar] [CrossRef]
- Yang, J.; Gao, C.; Yang, X.; Su, Y.; Shi, S.; Han, L. Effect of combined wet alkaline mechanical pretreatment on enzymatic hydrolysis of corn stover and its mechanism. Biotechnol. Biofuels Bioprod. 2022, 15, 31. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef]
- Chen, Z.; Aziz, T.; Sun, H.; Ullah, A.; Ali, A.; Cheng, L.; Ullah, R.; Khan, F.U. Advances and Applications of Cellulose Bio-Composites in Biodegradable Materials. J. Polym. Environ. 2023, 31, 2273–2284. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Chitin and Chitosan Based Composites for Energy and Environmental Applications: A Review. Waste Biomass Valorization 2021, 12, 4777–4804. [Google Scholar] [CrossRef]
- Yu, Z.; Ji, Y.; Bourg, V.; Bilgen, M.; Meredith, J.C. Chitin- and cellulose-based sustainable barrier materials: A review. Emergent Mater. 2020, 3, 919–936. [Google Scholar] [CrossRef]
- Rath, S.; Pradhan, D.; Du, H.; Mohapatra, S.; Thatoi, H. Transforming lignin into value-added products: Perspectives on lignin chemistry, lignin-based biocomposites, and pathways for augmenting ligninolytic enzyme production. Adv. Compos. Hybrid Mater. 2024, 7, 27. [Google Scholar] [CrossRef]
- Agnihotri, S.; Horváth, I.S. Integrated products biorefinery options within the Swedish pulp and paper industry: Current status. Sustain. Chem. Environ. 2024, 7, 100128. [Google Scholar] [CrossRef]
- Mainka, H.; Täger, O.; Körner, E.; Hilfert, L.; Busse, S.; Edelmann, F.T.; Herrmann, A.S. Lignin—An alternative precursor for sustainable and cost-effective automotive carbon fiber. J. Mater. Res. Technol. 2015, 4, 283–296. [Google Scholar] [CrossRef]
- Miao, B.H.; Headrick, R.J.; Li, Z.; Spanu, L.; Loftus, D.J.; Lepech, M.D. Development of biopolymer composites using lignin: A sustainable technology for fostering a green transition in the construction sector. Clean. Mater. 2024, 14, 100279. [Google Scholar] [CrossRef]
- Cline, S.P.; Smith, P.M. Opportunities for lignin valorization: An exploratory process. Energy Sustain. Soc. 2017, 7, 26. [Google Scholar] [CrossRef]
- Margarida Martins, M.; Carvalheiro, F.; Gírio, F. An overview of lignin pathways of valorization: From isolation to refining and conversion into value-added products. Biomass Convers. Biorefinery 2024, 14, 3183–3207. [Google Scholar] [CrossRef]
- Grand View Research. Lignin Market Size, Share & Trend Analysis Report by Product (Lignosulfonates, Kraft Lignin, Organosolv Lignin, Others), by Application, by Region, and Segment Forecasts, 2024–2030; Grand View Research: San Francisco, CA, USA, 2023; p. 140. [Google Scholar]
- Global Market Insights. Lignin Market—By Raw Material (Hardwood, Softwood, Straw, Sugarcane Bagasse, Corn Stover), by Product (Kraft Lignin, Lignosulphonates, Organosolv Lignin), by Application (Aromatics, Macromolecules), by Downstream Potential & Forecast, 2024–2032; Global Market Insights: Selbyville, DE, USA, 2023. [Google Scholar]
- Jayasekara, T.; Wickrama Surendra, Y.; Rathnayake, M. Polylactic Acid Pellets Production from Corn and Sugarcane Molasses: Process Simulation for Scaled-Up Processing and Comparative Life Cycle Analysis. J. Polym. Environ. 2022, 30, 4590–4604. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic acid (PLA): Properties, synthesis, and biomedical applications—A review of the literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Saravanan, K.; Umesh, M.; Kathirvel, P. Microbial Polyhydroxyalkanoates (PHAs): A Review on Biosynthesis, Properties, Fermentation Strategies and Its Prospective Applications for Sustainable Future. J. Polym. Environ. 2022, 30, 4903–4935. [Google Scholar] [CrossRef]
- Surendran, A.; Lakshmanan, M.; Chee, J.Y.; Sulaiman, A.M.; Thuoc, D.V.; Sudesh, K. Can Polyhydroxyalkanoates Be Produced Efficiently From Waste Plant and Animal Oils? Front. Bioeng. Biotechnol. 2020, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Perez-Zabaleta, M.; Atasoy, M.; Khatami, K.; Eriksson, E.; Cetecioglu, Z. Bio-based conversion of volatile fatty acids from waste streams to polyhydroxyalkanoates using mixed microbial cultures. Bioresour. Technol. 2021, 323, 124604. [Google Scholar] [CrossRef]
- Vu, D.; Wainaina, S.; Taherzadeh, M.; Åkesson, D.; A Ferreira, J. Production of polyhydroxyalkanoates (PHAs) by Bacillus megaterium using food waste acidogenic fermentation-derived volatile fatty acids. Bioengineered 2021, 12, 2480–2498. [Google Scholar] [CrossRef]
- Stout, B.A. Handbook of Energy for World Agriculture; Elsevier Applied Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Alvarado-Ramírez, L.; Santiesteban-Romero, B.; Poss, G.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Parra-Saldívar, R.; Bonaccorso, A.D.; Melchor-Martínez, E.M. Sustainable production of biofuels and bioderivatives from aquaculture and marine waste. Front. Chem. Eng. 2023, 4, 1072761. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Camia, A.; Giuntoli, J.; Jonsson, R.; Robert, N.; Cazzaniga, N.E.; Jasinevičius, G.; Avitabile, V.; Grassi, G.; Barredo, J.I.; Mubareka, S. The Use of Woody Biomass for Energy Production in the EU; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- IEA Bioenergy. Implementation of Bioenergy in the European Union–2024 Update; Pelkmans, L., Ed.; IEA Bioenergy: Dublin, Ireland, 2024. [Google Scholar]
- Mignogna, D.; Ceci, P.; Cafaro, C.; Corazzi, G.; Avino, P. Production of Biogas and Biomethane as Renewable Energy Sources: A Review. Appl. Sci. 2023, 13, 10219. [Google Scholar] [CrossRef]
- IRENA. Renewable Capasity Statistics 2024; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2024. [Google Scholar]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Estevez, M.M.; Tomczak-Wandzel, R.; Kvamme, K. Fish sludge as a co-substrate in the anaerobic digestion of municipal sewage sludge- maximizing the utilization of available organic resources. EFB Bioeconomy J. 2022, 2, 100027. [Google Scholar] [CrossRef]
- Haugland, B.T.; Armitage, C.S.; Kutti, T.; Husa, V.; Skogen, M.D.; Bekkby, T.; Carvajalino-Fernández, M.A.; Bannister, R.J.; White, C.A.; Norderhaug, K.M.; et al. Large-scale salmon farming in Norway impacts the epiphytic community of Laminaria hyperborea. Aquac. Environ. Interact. 2021, 13, 81–100. [Google Scholar]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.A.; Esmaeilion, F.; Assad, M.E.; Hajilounezhad, T.; Jamali, D.H.; Hmida, A.; et al. A critical review of biogas production and usage with legislations framework across the globe. Int. J. Environ. Sci. Technol. 2022, 19, 3377–3400. [Google Scholar] [CrossRef] [PubMed]
- Awe, O.W.; Zhao, Y.Q.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valorization 2017, 8, 267–283. [Google Scholar] [CrossRef]
- Czekala, W.; Jasinski, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas Plant Operation: Digestate as the Valuable Product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- Golovko, O.; Ahrens, L.; Schelin, J.; Sörengård, M.; Bergstrand, K.J.; Asp, H.; Hultberg, M.; Wiberg, K. Organic micropollutants, heavy metals and pathogens in anaerobic digestate based on food waste. J. Environ. Manag. 2022, 313, 114997. [Google Scholar] [CrossRef] [PubMed]
- Jameel, M.K.; Mustafa, M.A.; Ahmed, H.S.; Mohammed, A.J.; Ghazy, H.; Shakir, M.N.; Lawas, A.M.; Mohammed, S.K.; Idan, A.H.; Mahmoud, Z.H.; et al. Biogas: Production, properties, applications, economic and challenges: A review. Results Chem. 2024, 7, 101549. [Google Scholar] [CrossRef]
- Gupta, P.; Kurien, C.; Mittal, M. Biogas (a promising bioenergy source): A critical review on the potential of biogas as a sustainable energy source for gaseous fuelled spark ignition engines. Int. J. Hydrogen Energy 2023, 48, 7747–7769. [Google Scholar] [CrossRef]
- Novia, N.; Melwita, E.; Jannah, M.A.; Selpiana, S.; Yandriani, Y.; Afrah, D.B.; Rendana, M. Current advances in bioethanol synthesis from lignocellulosic biomass: Sustainable methods, technological developments, and challenges. J. Umm Al-Qura Univ. Appl. Sci. 2025, 1–8. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, S. A comprehensive review of bioethanol production from diverse feedstocks: Current advancements and economic perspectives. Energy 2024, 296, 131130. [Google Scholar] [CrossRef]
- Ghazali, M.F.S.M.; Mustafa, M. Bioethanol as an alternative fuels: A review on production strategies and technique for analysi. Energy Convers. Manag. 2025, 26, 100933. [Google Scholar] [CrossRef]
- Tirath Raj, K.C.; ANaresh Kumar, J.; Banu, R.; Yoon, J.-J.; Bhatia, S.K.; Yang, Y.-H.; Varjani, S.; Kim, S.-H. Recent advances in commercial biorefineries for lignocellulosic ethanol production: Current status, challenges and future perspectives. Bioresour. Technol. 2022, 344, 126292. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.H.; Burke, I.T.; Stewart, D.I. Beneficial management of biomass combustion ashes. Renew. Sustain. Energy Rev. 2021, 151, 111555. [Google Scholar] [CrossRef]
- Tosti, L.; van Zomeren, A.; Pels, J.R.; Dijkstra, J.J.; Comans, R.N.J. Assessment of biomass ash applications in soil and cement mortars. Chemosphere 2019, 223, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.; Eichermüller, J.; Endriss, F.; Baumgarten, B.; Kirchhof, R.; Tejada, J.; Kappler, A.; Thorwarth, H. Utilization and recycling of wood ashes from industrial heat and power plants regarding fertilizer use. Waste Manag. 2022, 141, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Eichermüller, J.; Scheuber, M.; Kappler, A.; Thorwarth, H. Mobility of Elements in Ashes from a Wood-Fired Heat and Power Plant with a Grate-Fired Furnace. Energy Fuels 2024, 38, 22245–22265. [Google Scholar] [CrossRef]
- Toraldo, E.; Saponaro, S.; Careghini, A.; Mariani, E. Use of stabilized bottom ash for bound layers of road pavements. J. Environ. Manag. 2013, 121, 117–123. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Losada-Garcia, N.; Cabrera, Z.; Urrutia, P.; Garcia-Sanz, C.; Andreu, A.; Palomo, J.M. Recent Advances in Enzymatic and Chemoenzymatic Cascade Processes. Catalysts 2020, 10, 1258. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Madzimbamuto, T.F.; Ojumu, T.V. Development of an Integrated Process for the Production and Recovery of Some Selected Bioproducts From Lignocellulosic Materials. In Valorization of Biomass to Value-Added Commodities: Current Trends, Challenges, and Future Prospects; Daramola, M.O., Ayeni, A.O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 439–467. [Google Scholar]
- Rødsrud, G.; Lersch, M.; Sjöde, A. History and future of world’s most advanced biorefinery in operation. Biomass Bioenergy 2012, 46, 46–59. [Google Scholar] [CrossRef]
- De Brabander, P.; Uitterhaegen, E.; Verhoeven, E.; Vander Cruyssen, C.; De Winter, K.; Soetaert, W. In Situ Product Recovery of Bio-Based Industrial Platform Chemicals: A Guideline to Solvent Selection. Fermentation 2021, 7, 26. [Google Scholar] [CrossRef]
- Heerema, L. In-situ product removal by membrane extraction. In IEEE Transactions on Plasma Science—IEEE TRANS PLASMA SCI; IEEE: New York, NY, USA, 2012. [Google Scholar]
- Buque-Taboada, E.M.; Straathof, A.J.J.; Heijnen, J.J.; van der Wielen, L.A.M. In situ product recovery (ISPR) by crystallization: Basic principles, design, and potential applications in whole-cell biocatalysis. Appl. Microbiol. Biotechnol. 2006, 71, 1–12. [Google Scholar] [CrossRef]
- Palladino, F.; Marcelino, P.R.; Schlogl, A.E.; José, Á.H.; Rodrigues, R.D.; Fabrino, D.L.; Santos, I.J.; Rosa, C.A. Bioreactors: Applications and Innovations for a Sustainable and Healthy Future—A Critical Review. Appl. Sci. 2024, 14, 9346. [Google Scholar] [CrossRef]
- Guimaraes, B.; de Boer, K.; Gremmen, P.; Drinkwaard, A.; Wieggers, R.; Wijffels, R.; Barbosa, M.J.; Dadamo, S. Selenium enrichment in the marine microalga Nannochloropsis oceanica. Algal Res. 2021, 59, 102427. [Google Scholar] [CrossRef]
- Pruvost, J.; Cornet, J.-F.; Pilon, L. Large-Scale Production of Algal Biomass: Photobioreactors; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Chisti, Y. Large-Scale Production of Algal Biomass: Raceway Ponds; Springer: Berlin/Heidelberg, Germany, 2016; pp. 21–40. [Google Scholar]
- Nadal-Rey, G.; Kavanagh, J.; Cassells, B.; Cornelissen, S.; Fletcher, D.; Gernaey, K.; McClure, D. Modelling of industrial-scale bioreactors using the particle lifeline approach. Biochem. Eng. J. 2023, 198, 108989. [Google Scholar] [CrossRef]
- Banerjee, S.; Dasgupta, S.; Das, D.; Atta, A. Influence of photobioreactor configuration on microalgal biomass production. Bioprocess Biosyst. Eng. 2020, 43, 1487–1497. [Google Scholar] [CrossRef]
- Zhu, C.; Chi, Z.; Bi, C.; Zhao, Y.; Cai, H. Hydrodynamic performance of floating photobioreactors driven by wave energy. Biotechnol. Biofuels 2019, 12, 54. [Google Scholar] [CrossRef]
- Iragavarapu, G.; Imam, S.; Sarkar, O.; Mohan, S.; Chang, Y.-C.; Reddy, M.; Kim, S.-H.; Amradi, N. Bioprocessing of Waste for Renewable Chemicals and Fuels to Promote Bioeconomy. Energies 2023, 16, 3873. [Google Scholar] [CrossRef]
- Shanu, K.; Choudhary, S.; Kumari, S.; Anu, K.; Devi, S. Downstream Processing for Bio-product Recovery and Purification. In Recent Advances in Bioprocess Engineering and Bioreactor Design; Dhagat, S., Jujjavarapu, S.E., Sampath Kumar, N.S., Mahapatra, C., Eds.; Springer Nature: Singapore, 2024; pp. 139–169. [Google Scholar]
- Boodhoo, K.V.K.; Flickinger, M.C.; Woodley, J.M.; Emanuelsson, E.A.C. Bioprocess intensification: A route to efficient and sustainable biocatalytic transformations for the future. Chem. Eng. Process.—Process Intensif. 2022, 172, 108793. [Google Scholar] [CrossRef]
- Kiss, A.A.; Lange, J.-P.; Schuur, B.; Brilman, D.W.F.; van der Ham, A.G.J.; Kersten, S.R.A. Separation technology—Making a difference in biorefineries. Biomass Bioenergy 2016, 95, 296–309. [Google Scholar] [CrossRef]
- Demirbas, A. Competitive liquid biofuels from biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Makepa, D.C.; Chihobo, C.H. Barriers to commercial deployment of biorefineries: A multi-faceted review of obstacles across the innovation chain. Heliyon 2024, 10, e32649. [Google Scholar] [CrossRef]
- Page-Dumroese, D.S.; Franco, C.R.; Archuleta, J.G.; Taylor, M.E.; Kidwell, K.; High, J.C.; Adam, K. Forest Biomass Policies and Regulations in the United States of America. Forests 2022, 13, 1415. [Google Scholar] [CrossRef]
- Directorate-General Climate Action, European Commission. Biomass Issues in the EU ETS: MRR Guidance Document No. 3; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Milledge, J.; Harvey, P. Potential process ‘hurdles’ in the use of macroalgae as feedstock for biofuel production in the British Isles. J. Chem. Technol. Biotechnol. 2016, 91, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Abels, C.; Carstensen, F.; Wessling, M. Membrane processes in biorefinery applications. J. Membr. Sci. 2013, 444, 285–317. [Google Scholar] [CrossRef]
- EBIA. Challenges Related to Biomass; European Biomass Industry Association: Brussels, Belgium, 2025. [Google Scholar]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Moskalenko, V.; Fonta, N. The Cascading Subsystem of Key Performance Indicators in the Enterprise Performance Management System. In Integrated Computer Technologies in Mechanical Engineering—2020; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Schlosser, O. Bioaerosols and Health: Current Knowledge and Gaps in the Field of Waste Management. Detritus 2019, 5, 111–125. [Google Scholar] [CrossRef]
- Agarwal, P.; Goyal, A.; Vaishnav, R. Chemical hazards in pharmaceutical industry: An overview. Asian J. Pharm. Clin. Res. 2018, 11, 27. [Google Scholar] [CrossRef][Green Version]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, M.; Olsen, A.; Bar, E.M.S. A Review of Food Contaminants and Their Pathways Within Food Processing Facilities Using Open Food Processing Equipment. J. Food Prot. 2023, 86, 100184. [Google Scholar] [CrossRef]
- Chauhan, S.; Solanki, P.; Putatunda, C.; Walia, A.; Keprate, A.; Kumar Bhatt, A.; Kumar Thakur, V.; Kant Bhatia, R. Recent advancements in biomass to bioenergy management and carbon capture through artificial intelligence integrated technologies to achieve carbon neutrality. Sustain. Energy Technol. Assess. 2025, 73, 104123. [Google Scholar] [CrossRef]
- Liao, M.; Yao, Y. Applications of Artificial Intelligence-Based Modeling for Bioenergy Systems: A Review. GCB Bioenergy 2021, 13, 774–802. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Anderson, N. Machine learning applications in forest and biomass supply chain management: A review. Int. J. For. Eng. 2024, 35, 371–380. [Google Scholar] [CrossRef]
- Abeywardhana, A. Application of AI and Machine Learning in Enhancing the Efficiency of Bio Mass Gasification Process. Int. J. Eng. Dev. Res. 2024, 12, 68–85. [Google Scholar]
- European Commission. Funding Programmes and Open Calls; Directorate-General for Research and Innovation; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Foundation, E.M. The Circular Economy in Detail; The Ellen MacArthur Foundation: Isle of Wight, UK, 2019. [Google Scholar]

| Sector | Waste Generated (Million Metric Tons/Year) | Waste Valorized (Estimated %) |
|---|---|---|
| Agriculture | 140,000 | <10% |
| Forestry | 522,000 | ~5% |
| Aquaculture | 80 | <15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnihotri, S.; Heggset, E.B.; de Lima, J.A.; Horváth, I.S.; Tanase-Opedal, M. Cascade Processing of Agricultural, Forest, and Marine Waste Biomass for Sustainable Production of Food, Feed, Biopolymers, and Bioenergy. Energies 2025, 18, 4093. https://doi.org/10.3390/en18154093
Agnihotri S, Heggset EB, de Lima JA, Horváth IS, Tanase-Opedal M. Cascade Processing of Agricultural, Forest, and Marine Waste Biomass for Sustainable Production of Food, Feed, Biopolymers, and Bioenergy. Energies. 2025; 18(15):4093. https://doi.org/10.3390/en18154093
Chicago/Turabian StyleAgnihotri, Swarnima, Ellinor B. Heggset, Juliana Aristéia de Lima, Ilona Sárvári Horváth, and Mihaela Tanase-Opedal. 2025. "Cascade Processing of Agricultural, Forest, and Marine Waste Biomass for Sustainable Production of Food, Feed, Biopolymers, and Bioenergy" Energies 18, no. 15: 4093. https://doi.org/10.3390/en18154093
APA StyleAgnihotri, S., Heggset, E. B., de Lima, J. A., Horváth, I. S., & Tanase-Opedal, M. (2025). Cascade Processing of Agricultural, Forest, and Marine Waste Biomass for Sustainable Production of Food, Feed, Biopolymers, and Bioenergy. Energies, 18(15), 4093. https://doi.org/10.3390/en18154093










