Abstract
Various pretreatment methods for the valorization of sunflower husks (SHs) for H2 gas generation through fermentation by Escherichia coli were investigated. We analyzed thermal treatment (TT), acid hydrolysis (AH), and alkaline hydrolysis (AlkH) at different substrate concentrations (50 g L−1, 75 g L−1, 100 g L−1, and 150 g L−1) and dilution levels (undiluted, 2× diluted, and 5× diluted). A concentration of 75 g L−1 SH that was acid-hydrolyzed and dissolved twice in the medium yielded optimal microbial growth, reaching 0.3 ± 0.1 g cell dry weight (CDW) L−1 biomass. The highest substrate level enabling effective fermentation was 100 g L−1, producing 0.37 ± 0.13 (g CDW) × L−1 biomass after complete fermentation, while 150 g L−1 exhibited pronounced inhibitory effects. It is worth mentioning that the sole alkaline treatment was not optimal for growth and H2 production. Co-fermentation with glycerol significantly enhanced both biomass formation (up to 0.42 ± 0.15 (g CDW) × L−1)) and H2 production. The highest H2 yield was observed during batch growth at 50 g L−1 SH hydrolysate with 5× dilution, reaching up to 5.7 mmol H2 (g sugar)−1 with glycerol supplementation. This study introduces a dual-waste valorization strategy that combines agricultural and biodiesel industry residues to enhance clean energy generation. The novelty lies in optimizing pretreatment and co-substrate fermentation conditions to maximize both biohydrogen yield and microbial biomass using E. coli, a widely studied and scalable host.
1. Introduction
The sunflower is a globally significant oil-producing plant, valued for its high oil content (40–50%), nutritional benefits, and economic importance, with more than 70 species known worldwide [1]. It is considered the fourth leading source of vegetable oil globally, after soybean, oil palm, and canola, with sunflower oil being a premium edible oil because of its high unsaturated fat content and health benefits [1,2]. The crop is drought-tolerant and widely cultivated for both food and industrial applications.
Sunflower oil production primarily uses solvent extraction, generating large quantities of husk as a byproduct. Since sunflower oil has a significantly higher economic value than sunflower meal, maximizing oil yield is crucial for process efficiency [3]. A major byproduct of sunflower seed processing is the sunflower husk (SH), which constitutes 20–30% of the seed [4], in specific cases up to 60% [5,6], and mainly comprises cellulose, hemicellulose, and lignin. While some hulls are used in animal feed or biomass energy, a large proportion remains underutilized and is often discarded or landfilled, representing a lost opportunity for valorization.
Due to its high energy density, this biomass residue holds strong potential as a renewable heating fuel. Nevertheless, a significant portion is still discarded and landfilled without being repurposed sustainably. Given its carbon-neutral nature, it offers promising opportunities for contributing to climate change mitigation [7]. The gross calorific value (GCV) of sunflower husk is 17.844 MJ/kg [7], making it a viable but relatively low-efficiency biomass fuel. Sunflower husks are often burned for energy [6,8], but this process can still produce toxic emissions, leading to air pollution and having negative health impacts [9]. Despite its energy potential, a large portion of sunflower husk is landfilled without sustainable use.
In contrast, hydrogen (H2) has a much higher energy density, with an HHV of 141.6 MJ/kg—nearly eight times greater than that of sunflower husk [10]. Unlike biomass combustion, H2 burns cleanly, producing only water as a byproduct, making it a far more sustainable and environmentally friendly energy carrier. By converting sunflower husks into bio-H2 through microbial fermentation using Escherichia coli instead of burning them, the energy yield per kilogram of feedstock can be significantly enhanced, offering a superior alternative for renewable energy production.
Beyond efficiency, H2 production presents multiple advantages over direct combustion. It offers a higher energy output per unit mass, making it a more efficient fuel source. Additionally, hydrogen is a zero-carbon fuel, whereas burning husks still releases CO2 into the atmosphere [11,12]. Instead of using husks as a low-value biomass fuel, microbial fermentation allows them to be transformed into high-value hydrogen, unlocking greater economic and environmental benefits. This process also supports waste valorization, ensuring that SH—typically seen as industrial waste—is repurposed into clean, renewable energy, aligning with circular bioeconomy principles.
The use of microbial fermentation to convert agricultural waste into valuable products, in particular to biohydrogen, is of considerable interest to a variety of research groups [13].
E. coli, a widely studied facultative anaerobe, has demonstrated strong potential in bio-H2 production through dark fermentation. It performs mixed acid fermentation, whereas H2 evolution occurs as one of the end products of its anaerobic metabolism. E. coli establishes rapid growth phases and can thrive across a wide pH range [14]. Its flexible metabolism, capable of fermentation, aerobic, and anaerobic respiration, makes it an ideal candidate for biotechnological applications.
Furthermore, E. coli is genetically and metabolically well-characterized, making it easy to manipulate for strain optimization. By metabolizing sugars derived from hydrolyzed biomass, E. coli can efficiently generate H2 gas, and optimizing substrate pretreatment, microbial strains, and fermentation conditions is crucial for maximizing hydrogen yield and ensuring industrial feasibility.
In this study, we studied the potential of SH hydrolysates as a substrate for biohydrogen evolution using a pure culture of Escherichia coli. We assess the effects of thermal, acid, and alkaline pretreatment methods on hydrolysate quality and microbial fermentability. In addition, we examine biomass growth and hydrogen production under varying SH concentrations, dilution levels, and co-fermentation conditions with glycerol.
This study’s originality lies in its integrated approach, optimizing both pretreatment strategies and co-substrate supplementation to enhance hydrogen and biomass yields from an underutilized agrowaste. Unlike previous studies, this research systematically evaluates the effects of process variables using a single microbial platform.
The following objectives were set: (1) determine how different pretreatment methods affect SH fermentability and microbial growth; (2) identify optimal substrate concentrations and dilution conditions for effective fermentation; (3) evaluate the impact of glycerol co-fermentation on enhancing biohydrogen and biomass production. These insights aim to support sustainable waste-to-energy conversion and demonstrate a scalable dual-waste valorization strategy aligned with circular bioeconomy goals.
2. Materials and Methods
2.1. Waste, Pretreatment, and Bacterial Strain Cultivation
Sunflower husks (SHs) were collected from small-scale oil-processing facilities in Armenia. These residues were oven-dried at 105 °C to remove moisture and stored at 4 °C until use.
Pretreatment of the biomass was carried out on suspensions containing 50, 75, 100, and 150 g L−1 of SH. Two main strategies were applied: thermal (hydrothermal) and physicochemical methods. Physicochemical pretreatment included either acidic or alkaline hydrolysis. In these cases, sulfuric acid or sodium hydroxide was added at final concentrations of 0.5% and 1.5% [15,16], respectively, followed by autoclaving at 121 °C for 45 min using a Daihan Scientific system (Seoul, Republic of Korea). Hydrothermal treatment was performed under the same temperature and time conditions, but without the addition of any chemical agents. Details of the treatment and reagent combinations are provided in Table 1.

Table 1.
Pretreatment strategies, hydrolyzing agents, and process conditions used in sunflower husk valorization. This table summarizes the types of pretreatments applied, the chemicals used for hydrolysis, and the corresponding process conditions, including temperature, duration, and pH adjustment methods.
After hydrolysis, all samples were filtered, and their pH was adjusted to 7.5. The liquid fractions were then brought to a boil for 15–20 min to further eliminate sediment, allowed to cool, and centrifuged at 7500× g for 15 min. The clarified hydrolysates were diluted with distilled water either in a 1:1 ratio (denoted as 2×) or a 1:4 ratio (labeled as 5×). These final media preparations were autoclaved for 15 min at 121 °C before use in fermentation assays [17,18].
Cell dry weight (CDW) was estimated from optical density readings at 600 nm using a conversion coefficient of 0.3 ± 0.1 g CDW per unit of OD600, as described previously [19,20].
2.2. Physicochemical Analysis
To evaluate the composition of SH material, total solids (TS) and volatile solids (VS) were determined on a fresh mass (FM) basis [21]. Samples were oven-dried at 105 °C for 18–24 h (WiseVen, Daihan Scientific, Seoul, Republic of Korea) until reaching constant weight. The residual mass was then combusted at 550 °C for 4–5 h using a Daihan FHX-05 (Daihan Scientific, Seoul, Republic of Korea) muffle furnace [21]. Chemical oxygen demand (COD) of the SH hydrolysate—with or without added glycerol—was assessed using a modified dichromate-based protocol for solid-rich samples, following Raposo et al. (2008) [22]. The method involved oxidation using potassium dichromate and silver sulfate in sulfuric acid, with heating in a digestion block system (KJELDATHERM, C. Gerhardt Analytical Systems, Königswinter, Germany).
COD was calculated from the volume of ferrous ammonium sulfate (FAS) consumed during titration, using the following equation:
where FASBl is the volume (mL) of ferrous ammonium sulfate (FAS) solution required to titrate the blank, FASLiquid sample is the volume (mL) of FAS used to titrate the actual liquid sample, NFAS represents the normality of the FAS solution (eq/L), and Vliquid sample is the volume (mL) of the tested liquid sample.
The total nitrogen content was determined via the Kjeldahl method. Digestion was conducted using concentrated sulfuric acid and a catalyst mixture (CuSO4 + K2SO4) in a TURBOTHERM infrared unit (C. Gerhardt, Königswinter, Germany), and distillation was performed with a VAPODEST® 300 system (C. Gerhardt, Königswinter, Germany). Boric acid (4%) containing Tashiro indicator was used to collect distillate, which was titrated with 0.1 N HCl [23].
The total carbohydrate concentration (g/L) in the hydrolysates was assessed following the phenol–sulfuric acid method described by Dubois et al. [24]. The carbon conversion efficiency (CCE) into biomass was calculated using the following formula:
where C1 is the total amount of carbon before fermentation (g L−1), and C2 is the amount of carbon in biomass (g CDW) × L−1) [25]. It was experimentally shown that carbon constitutes about 48–50% of cell dry weight (CDW) [26]. Moreover, carbon from sugar was calculated based on the structure of glucose, in which carbon is 40%. In the presence of glycerol, supplemented carbon was taken into consideration.
CCE = C2 × 100%/C1
2.3. Hydrogen Production Assays
The concentration of molecular hydrogen accumulated during fermentation was determined using gas chromatography. The analyses were conducted with an Agilent 7820A GC system (Santa Clara, CA, USA) fitted with a thermal conductivity detector (TCD). Bacterial cultures were incubated in Hungate tubes that maintained a 1:2 liquid-to-gas-phase ratio to ensure sufficient headspace for gas sampling.
At designated time points, 200 μL of headspace gas was extracted using a manual gas-tight syringe (Agilent 5190-1531) and introduced into a CP-Molsieve 5A column (10 μm × 0.32 μm × 30 μm, Agilent Technologies, Mulgrave, Australia). Chromatographic separation occurred at an oven temperature of 50 °C, with the injector held at 150 °C. Nitrogen was used as the carrier gas at a flow rate of 1 mL min−1. Each run was completed in 4 min, and a brief post-run period at 100 °C ensured column cleaning.
Calibration of the system was performed using injections of known volumes of pure hydrogen gas (40, 60, 100, 200, and 300 μL), which corresponded to hydrogen concentrations ranging from 1.6 to 12.3 mmol L−1. These standards were used to construct a calibration curve that exhibited strong linearity (R2 > 0.99), allowing accurate conversion of peak areas into hydrogen concentrations.
For each sample, the standard point whose peak area was most similar to that of the sample was used for quantification. This approach minimized errors in cases of non-linear detector response at low or high concentrations. Peak integration and hydrogen quantification were carried out using the OpenLAB software 3.6 package [18]. Gas-phase concentrations were then converted to molar units using the ideal gas law.
Measurements were performed in triplicate for all experimental conditions to ensure reproducibility. The amount of hydrogen produced was reported as millimoles per liter of culture (mmol L−1), while the hydrogen yield was calculated as millimoles of H2 generated per gram of sugar consumed (mmol (g sugar)−1).
2.4. Statistical Analysis
All fermentation experiments were conducted in triplicate biological replicates (n = 3), with each replicate derived from an independent inoculation and culture run. For measurements such as OD600, pH, and ORP, technical duplicates were also used to evaluate intra-assay consistency. The results are presented as mean ± standard deviation (SD).
The statistical tests were conducted using GraphPad Prism 8.0.2 software (San Diego, CA, USA) for time-course data visualization (e.g., biomass growth and redox potential curves), and Python 3.9.19 for bar plots and hydrogen yield summaries.
To evaluate the effects of pretreatment, dilution, and co-fermentation, a two-way ANOVA was performed, followed by Tukey’s post hoc test for pairwise comparisons. Statistical significance was set at p < 0.05 [18]
To avoid visual overcrowding, statistical significance indicators (e.g., asterisks or superscripts) were not included directly on graphs or tables. Instead, all statistical comparisons are described in the Results and Discussion section where relevant. For calculated metrics such as hydrogen yield (mmol·g−1 sugar) and carbon conversion efficiency, we estimated the associated uncertainty using standard error propagation formulas, assuming that the measured input values (e.g., sugar consumed and hydrogen produced) were statistically independent.
3. Results
3.1. Impact of Different Pretreatment Methods on Sunflower Husk Fermentation by E. coli
Sunflower husks (SHs) are an abundant agricultural byproduct with significant potential for valorization in bio-based processes, particularly fermentation. Their composition primarily includes cellulose, hemicellulose, and lignin, with moderate protein and oil contents [27,28]. Their composition makes them a promising feedstock for microbial fermentation, particularly for bio-H2 production. Table 2 summarizes the chemical composition, proximate and ultimate analyses, and fermentation-relevant properties of SH based on this study and the literature survey.

Table 2.
Comparative composition and characteristics of sunflower husk (SH) used in biohydrogen production. Data on the basic and elemental composition, along with the chemical profile, are presented for untreated sunflower husk waste, with a comparison to the literature values. The results are shown as mean ± SD (n = 3).
The results of raw SH show a comparable protein content of 8.7 ± 0.27%, which aligns well with the values reported in the literature, supporting the consistency of SH as a biomass source. Additionally, the total solids (TS) were 59 ± 1.8%, with volatile solids (VS) comprising 98 ± 2.5%, indicating a high proportion of organic matter suitable for microbial metabolism. The chemical oxygen demand (COD) was recorded at 1.256 ± 0.04 g (g VS)−1, further emphasizing its potential as a fermentation substrate. These findings highlight SH as a viable feedstock for H2 production and underscore the importance of optimizing pretreatment strategies to maximize yield.
However, the extraction of simple carbohydrates from lignocellulosic structures still stands as an unsolved problem for optimal organic waste usage. Here, we analyze the effect of differently carried thermal (hydrothermal) and thermochemical hydrolysis on E. coli growth and H2 production. For the first stage of the research, two initial waste concentrations were selected: 50 g L−1 and 75 g L−1 of SH. The latter was hydrolyzed using thermal treatment as well as dilute acid and alkaline solutions. The alkaline treatment of SH has been previously reported by Soto et al. [16], showing that maximum delignification occurred with 1.5% NaOH, maintaining a substrate-to-medium ratio of 0.1 g per mL−1. Furthermore, Fox et al. [29] demonstrated that treatment yield remained stable across a solid-to-liquid ratio range of 0.05–0.25 g mL−1. Based on these findings, our study used the lowest tested concentration of 50 g L−1, along with a slightly higher concentration of 75 g L−1 as a reference point, ensuring a balanced approach to evaluating the effects of pretreatment. A similar amount of SH was subjected to thermal and acid hydrolysis, using 0.5% sulfuric acid, the slight variations in which have been reported as optimal for feedstocks such as spent coffee grounds, coffee silverskin, brewery waste, etc. [15,17,18,30].
In lignocellulosic biomass, polysaccharides (cellulose and hemicellulose) are embedded within the cell wall and protected by lignin, which serves as a physical barrier limiting enzymatic and chemical accessibility. Lignin makes biomass water-resistant and difficult for microbes to break down, presenting a significant challenge during processing. Cellulose provides rigidity through microfibrils, while hemicellulose forms a flexible matrix surrounding the cellulose [31].
Previous investigations have mainly focused on removing lignin, followed by enzymatic or microbial treatment of the remaining cellulose and hemicellulose [32,33]. However, one-step treatments remain largely unexplored, especially when taking into consideration that the efficiency of different pretreatment strategies varies depending on the biomass feedstock used. Additionally, studies have primarily concentrated on lignin removal, while the direct treatment of cellulose or hemicellulose alone with SH has not been extensively evaluated. Pretreatment alters biomass structure in several ways: alkaline hydrolysis effectively removes lignin and partially solubilizes hemicellulose; acid hydrolysis breaks down hemicellulose into simple sugars; thermal treatment increases biomass porosity, improving accessibility for further conversion.
The results in Figure 1 illustrate how different pretreatment methods affect microbial growth and redox potential (ORP). Thermal treatment (TT) generally provided stable microbial growth, as indicated by increasing optical density (OD600) over time (Figure 1a,b). Accurately determining the cell-specific growth rate is difficult with concentrated wastes; therefore, we monitored changes in the optical density (OD) and total biomass production, as outlined in the Section 2. This challenge is further amplified by the presence of multiple sugars in the medium, which are likely utilized both sequentially and simultaneously [34]. Traditional growth models (e.g., Monod kinetics) typically assume one limiting substrate, whereas multiple carbon sources introduce complex utilization patterns, including diauxic shifts or partial overlap of consumption. As a result, we opted to report overall growth trends via OD measurements rather than attempt a single μ value that may not accurately capture these multi-substrate dynamics. Thus, when 50 g L−1 SH was initially applied for thermal treatment, the final hydrolysate with 2× and 5× dilutions showed no concentration-dependent improvement in growth. Specifically, at the 24th hour of growth in all cases OD600 reached 0.4 ± 0.012 values (Figure 1a), which is equal to the biomass of 0.12 ± 0.05 (g CDW) × L−1, and at the end of fermentation (96–120 h), it reached up to 0.17 ± 0.05 (g CDW) × L−1. These observations suggest that TT alone does not substantially enhance substrate availability, leaving the accessibility of fermentable sugars relatively limited.
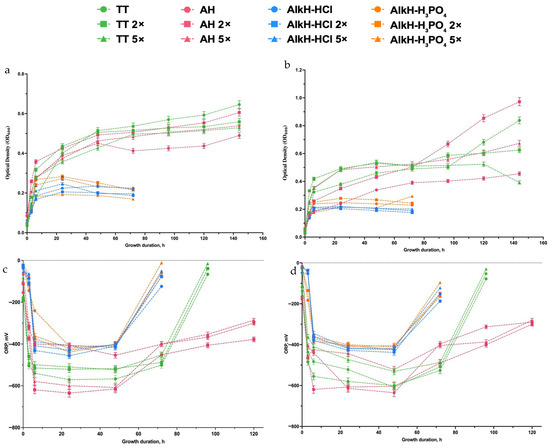
Figure 1.
Impact of different pretreatment methods on growth and ORP changes during batch growth of E. coli using sunflower husk hydrolysates. Growth was measured by optical density (OD600) for 50 g L−1 (a) and 75 g L−1 (b) SH, ORP changes expressed in mV over time for 50 g L−1 (c) and 75 g L−1 (d) SH concentrations, respectively. Treatments include thermal (TT), acid (AH), and alkaline hydrolysis with HCl (AlkH-HCl) or H3PO4 (AlkH-H3PO4), each at different dilutions (0×, 2×, and 5×). Cells were grown at 37 °C under anaerobic conditions. Data points represent the mean ± SD from triplicate experiments (n = 3). Statistical details are provided in the Section 2.
This limited effect may be attributed to the temperature-dependent solubilization behavior of lignocellulosic components. Hemicelluloses begin to solubilize above 150–180 °C [35], but excessive heating can cause the release of phenolic and heterocyclic inhibitory compounds, such as furfural and HMF, especially in acidic environments [36]. Although TT in our study did not result in complete inhibition, the lack of improved growth across concentrations suggests that the formation or redeposition of soluble lignin compounds may have limited microbial access to sugars. Additionally, if TT conditions were too mild, insufficient hemicellulose solubilization may have occurred, resulting in low sugar availability.
Acid hydrolysis (AH) exhibited more complex, concentration-dependent behavior. Undiluted AH led to lower microbial growth, likely because of inhibitory byproducts (e.g., furfural and hydroxymethylfurfural) [37], whereas moderate dilution (2×) improved microbial growth, but the differences were significant only during later hours of growth (Figure 1a). However, the difference between TT and AH while using a low concentration of SH was not significant.
Similarly, at 75 g L−1, we observed a comparable trend: TT-treated samples showed no marked improvement in growth when diluted 2× or 5×, whereas AH at moderate dilution again provided better performance than the undiluted condition reaching up to 1 ± 0.03 OD600 in 2× diluted hydrolysate (Figure 1b) which is equal to 0.3 ± 0.1 (g CDW) × L−1 biomass. These results reinforce that AH benefits from an optimal dilution range, balancing effective hemicellulose/cellulose breakdown with minimal inhibitor production. Overall, Figure 1 demonstrates that while TT is consistent and relatively free of toxic byproducts, it does not significantly improve microbial growth regardless of dilution. By contrast, AH shows a beneficial effect at moderate dilutions, pointing to an optimal acid concentration that promotes fermentable sugar generation while limiting inhibitor accumulation. Alkaline hydrolysis (AlkH), whether using HCl or H3PO4 as a pH-adjusting agent, resulted in intermediate microbial growth. It was shown that alkaline media are favorable for lignin solubilization.
Initially, growth was lower compared with TT and AH 2×, but it gradually improved over time, suggesting that inhibitory compounds were either metabolized or diluted during the culture period. Between the two alkaline hydrolysis treatments, AlkH-H3PO4 performed slightly better than AlkH-HCl, possibly because the phosphoric acid treatment preserves more fermentable sugars or provides additional nutrients. However, the lignin treatment alone does not affect the growth.
The moderate performance observed may also be linked to the presence of lignin-derived phenolic compounds generated during alkaline treatment. Alkaline fractionation is known to fragment lignin polymers, releasing lignin oligomers and phenolic acids such as p-coumaric, ferulic, and vanillic acids [38,39], which are known microbial inhibitors at elevated concentrations.
In contrast, cultures grown with isolated lignin liquor—without sugars or detoxification—showed negligible growth, with OD600 values remaining near baseline (Figure 1a). This confirms that lignin-derived compounds alone are not fermentable by E. coli under these conditions and may exert inhibitory effects.
The ORP trends (Figure 1c,d) support microbial growth observations. No major differences were observed between the 50 g L−1 and 75 g L−1 SH hydrolysates. The first measurement at 3 h of growth revealed a sharp ORP drop (≤−400 mV) in all diluted TT and AH samples, aligning with conditions favorable for H2 production [40]. H2 production was sustained for up to 72 h in TT samples and up to 120 h in acid-hydrolyzed (AH) samples, regardless of SH concentration. Meanwhile, AlkH samples only maintained such ORP values between 6 and 48 h, with no significant influence from pH-calibrating agents or dilution. Notably, ORP changes remained similar at both 50 g L−1 and 75 g L−1, suggesting that substrate concentration alone did not significantly impact redox dynamics. In contrast, undiluted AH and all AlkH treatments exhibited a slower ORP recovery, pointing to greater metabolic stress and delayed substrate utilization. These results suggest that while pretreatment type plays a major role in microbial activity and H2 production, increasing the SH concentration from 50 g L−1 to 75 g L−1 does not further influence redox behavior. Thus, for further experiments, higher substrate concentrations such as 100 g L−1 and 150 g L−1 were selected for further experimental set-ups only by using AH.
At higher substrate concentrations, microbial growth followed similar trends as observed for 50 g L −1 and 75 g L−1 SH, but with more pronounced inhibitory effects. At 100 g L−1 SH, microbial growth showed a distinct trend where the undiluted 0× and 2× diluted samples exhibited similar OD600 values over time making up to 0.195 ± 0.07 (g CDW) × L−1 of biomass at the 24th hour of growth and 0.37 ± 0.13 (g CDW) × L−1 of biomass, while the 5× diluted condition resulted in lower growth. This suggests that at this concentration, a moderate dilution (2×) does not significantly impact biomass formation, whereas excessive dilution (5×) likely reduces the availability of essential nutrients, limiting (Figure 2a).
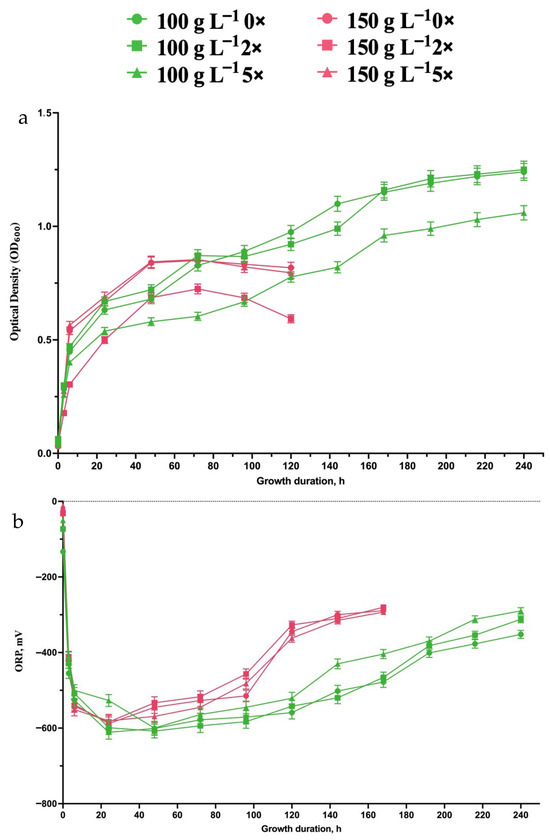
Figure 2.
Growth (a) and ORP changes (b) during batch growth of E. coli using 100 g L−1 and 150 g L−1 SH hydrolysates at different dilutions (0×, 2×, and 5×). Data represent the mean ± SD from triplicate experiments (n = 3). For further details, refer to the Section 2.
At an initial 150 g L−1 SH containing hydrolysate, the inhibitory effects became more apparent, with microbial growth being lower across all conditions compared with 100 g L−1 SH. In undiluted and 2× diluted samples, OD600 increased initially but plateaued earlier than at lower substrate concentrations (Figure 2a). The 5× dilution condition showed even weaker growth, further reinforcing that at such high concentrations, dilution alone is insufficient to fully counteract the inhibitory effects. These results indicate that while some level of dilution helps mitigate substrate inhibition, beyond a certain threshold (150 g L−1 SH), microbial adaptation is severely limited.
The ORP profiles follow a similar trend to the observed microbial growth. At 100 g L−1 SH hydrolysate, ORP dropped rapidly to ≤−600 mV, indicating active microbial metabolism and H2 production. In the undiluted and 2× diluted samples, ORP remained low for an extended period, sustaining metabolic activity throughout most of the 240-h incubation (Figure 2b). However, at 150 g L−1 SH, ORP values started increasing earlier, especially after 120 h, indicating that microbial activity was suppressed sooner, leading to a shorter H2 production phase. Thus, no further substrate levels were tested. However, these data clearly show that for bacterial growth and biomass generation, a 100 g L−1 SH concentration was the most optimal one.
3.2. Enhanced Hydrogen Production Through Co-Fermentation of SH and Glycerol
The goal of this research is to improve the use of sunflower husk (SH) in producing biohydrogen and biomass. Glycerol, a biodiesel byproduct [41,42], was added to increase yields. This dual-waste method enhances sustainable bioenergy production while helping to minimize waste by utilizing byproducts from the sunflower oil and biodiesel industries. The co-fermentation of SH hydrolysates with glycerol revealed a clear improvement in both H2 yield and overall fermentation efficiency compared with SH-only substrates. When glycerol was introduced as a co-substrate, E. coli cultures showed ORP values sustained for longer durations and increased biomass accumulation. Microbial growth showed a continuous increase in OD600 from 6 to 150 h, suggesting that cell division was sustained over a long period rather than following a typical exponential phase (Figure 3a). This indicates that co-fermentation with glycerol contributed to prolonged biomass formation, likely by improving substrate utilization and reducing growth limitations.
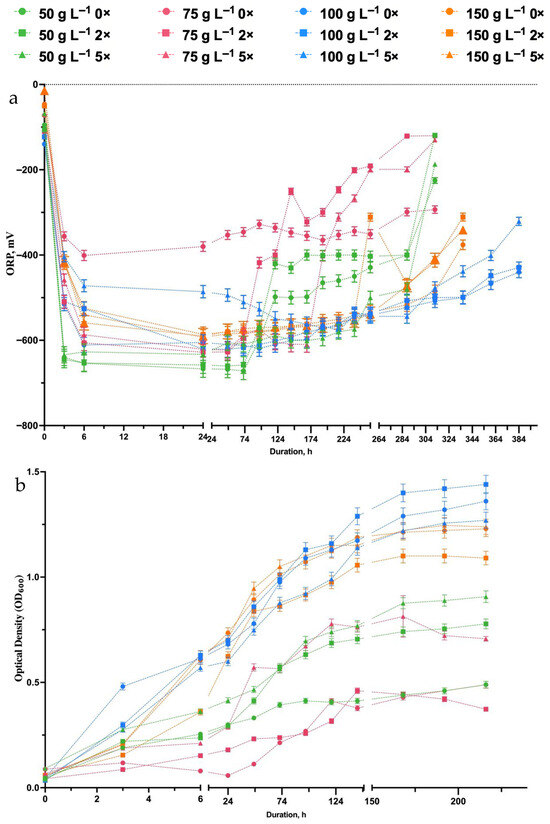
Figure 3.
Growth (a) and ORP changes (b) during batch growth of E. coli using different SH concentrations (50–150 g L−1) at various dilutions (0×, 2×, and 5×) with co-fermentation on 10 mL L−1 glycerol. Data represent the mean ± SD from triplicate experiments (n = 3). For statistical analysis, refer to the Section 2.
Growth kinetics were significantly enhanced in co-fermentation conditions, particularly at 100 g L−1 SH containing hydrolysate. The biomass yield reached 0.42 ± 0.15 (g CDW) × L−1 at the end of fermentation under co-fermentation conditions, representing a ~15% increase compared with SH fermentation alone (Figure 4). This synergy likely stems from glycerol’s role as a more readily metabolizable substrate compared with the complex sugars present in SH hydrolysates. Biochemically, glycerol is readily metabolized via glycolysis and the glycerol dehydrogenase pathway, providing additional NADH and pyruvate that feed into mixed-acid fermentation [14].
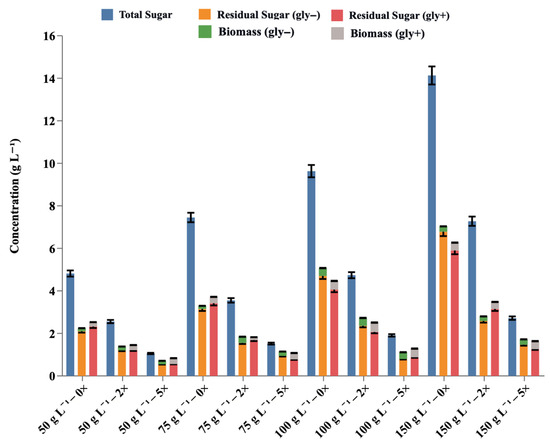
Figure 4.
Sugar consumption and biomass generation of E. coli during batch fermentation of SH hydrolysate at different dilutions (0×, 2×, and 5×) either in the absence (gly−) or presence (gly+) of glycerol. The x-axis shows experimental conditions with varying substrate concentrations (50, 75, 100, and 150 g L−1) and dilution factors. The blue bars indicate the initial total sugar concentration prior to fermentation, while the orange and red bars represent the remaining sugar content under Gly− and Gly+ conditions, respectively. Biomass production after fermentation is shown by the green bars for Gly− and gray bars for Gly+ conditions. Data are presented as mean ± SD from triplicate experiments (n = 3).
Furthermore, co-fermentation appeared to mitigate some of the inhibitory effects observed at higher SH concentrations. At 150 g L−1 SH, where single-substrate fermentation showed significant growth inhibition, the addition of glycerol helped maintain more robust growth and metabolic activity (compare Figure 2a and Figure 3a). The benefits of glycerol supplementation on H2 production were evident across all tested conditions, with cumulative H2 production consistently higher in co-fermentation conditions. However, when considering CCE to biomass (Table 3) in SH-only assays, CCE was significantly higher compared with co-fermenting conditions. Higher CCE conditions can be used for obtaining biomass as a source of single-cell protein, աs recent research has increasingly focused on improving technologies for extracting single-cell proteins from bacteria [43]. However, lower CCE in glycerol co-fermenting conditions leads to the maximization of product formation.

Table 3.
Carbon conversion efficiency to biomass during batch fermentation of acid-hydrolyzed sunflower husk in the presence or absence of glycerol. For calculation details, see Section 2. Carbon conversion efficiency (CCE) is expressed as a percentage, calculated from the biomass yield and initial carbon content. Glycerol supplementation impacts carbon conversion during fermentation at different SH concentrations and dilutions. Values are presented as mean ± SD (n = 3).
Particularly, at 50 g L−1 SH with 5× dilution, the H2 yield increased dramatically from 1.1 to 5.7 mmol (g sugar)−1 when supplemented with glycerol (Table 4), representing a more than 5-fold improvement.

Table 4.
Gas chromatography (GC) analysis of cumulative hydrogen production and yield during batch fermentation of E. coli using sunflower husk hydrolysate with and without glycerol supplementation. Hydrogen yield (mmol H2 per g sugar) and cumulative H2 production (mM) were measured after fermentation, with and without glycerol supplementation. The table shows results for various SH concentrations and dilution levels. Data represent the mean ± SD (n = 3).
This remarkable enhancement suggests that at lower substrate concentrations with appropriate dilution, the co-fermentation strategy can significantly optimize the conversion efficiency of available sugars to H2. At higher concentrations (150 g L−1 SH, undiluted), cumulative H2 production increased from 2.14 mM to 4.6 mM with glycerol addition, while sugar consumption increased from 7.35 to 8.25 mg mL−1, indicating that glycerol not only enhances hydrogen production directly but may also improve the utilization of SH-derived sugars. A particularly interesting trend emerged in the relationship between substrate concentration, dilution, and H2 yield. While undiluted samples generally showed higher cumulative H2 production, the specific H2 yields (mmol H2 per g sugar consumed) were typically superior in more diluted conditions. This pattern was consistently amplified in the presence of glycerol, suggesting that dilution helps optimize the metabolic balance between the two substrates. For example, at 75 g L−1 SH, the specific H2 yield in 5× diluted samples with glycerol reached 4.3 mmol (g sugar)−1, compared with 0.9 mmol (g sugar)−1 in undiluted conditions. The normal maximum H2 yield in mesophilic biohydrogen fermentation is ~2 mol of H2 (mol of glucose)−1 [44]. Different research groups showed multiple different yields depending on the microbial culture and biomass source, particularly for E. coli; it varies from 0.75 to 3.12 mol H2 (mol hexose from glucose)−1 [45]. In our recently published paper, 12 mmol H2 (g sugar)−1 was recorded using sugar beet pulp as waste [46]. Our obtained data demonstrates strong potential for further improvement, as the highest H2 yield was achieved using 50 g L−1 SH 5× diluted hydrolysate, with an estimated yield of approximately 1 mol of H2 per mol of sugar (calculated based on Table 3 using the molar mass of glucose). Further research will be focused on improving the technology and on large-scale analysis.
The improved performance under co-fermentation conditions can be attributed to several factors. The improved sugar consumption rates in the presence of glycerol (Figure 4) also suggest that co-substrate addition helps maintain more robust cellular metabolism, possibly by balancing the redox state of the cells and providing an additional energy source that supports the breakdown of more complex SH-derived sugars.
Furthermore, these data revealed that optimal H2 production conditions might differ depending on whether the goal is maximizing total hydrogen output or achieving the highest conversion efficiency of sugars to hydrogen. While higher substrate concentrations (150 g L−1) produced the greatest cumulative hydrogen amounts, the most efficient sugar-to-hydrogen conversion was achieved at lower concentrations with higher dilution factors, particularly in co-fermentation conditions.
4. Conclusions
This investigation assessed sunflower husk’s potential as a substrate for E. coli-mediated biomass and biohydrogen generation, concentrating on optimizing pretreatment and glycerol co-fermentation. These data support the following conclusions:
- In the sole-SH fermentation process, optimal pretreatment and substrate concentration play key roles in biomass yield. The highest biomass yield of 0.37 ± 0.13 (g CDW) × L−1 was achieved with a 100 g L−1 SH concentration, following a 2× dilution after acid hydrolysis. A 75 g L−1 SH concentration resulted in a similar yield of 0.3 ± 0.1 (g CDW) × L−1, indicating that the latter is also a highly efficient and potentially more resource-effective concentration. In contrast, a 50 g L−1 SH concentration, with the same pretreatment and dilution, produced a lower yield of 0.17 ± 0.05 (g CDW) × L−1, highlighting the significant impact of substrate concentration on biomass production.
- The most significant improvement in the biomass yield was observed at 100 g L−1 SH with 2× dilution, constituting 0.42 ± 0.012 (g CDW) × L−1 when supplemented with glycerol. This represents a ~15% enhancement, demonstrating that co-fermentation can substantially optimize the production of biomass.
- The most significant improvement in H2 yield was observed at 50 g L−1 SH with 5× dilution, where it increased from 1.050 to 5.685 mmol H2 (g sugar)−1 when supplemented with glycerol.
However, scaling up this method for industrial applications will require addressing several key challenges:
- The presence and variability of inhibitory compounds such as furfural and hydroxymethylfurfural (HMF) in glycerol utilization and hydrolysates may affect microbial performance at larger volumes.
- Complexities in maintaining uniform conditions, such as pH, oxygen transfer, and substrate distribution, can influence fermentation efficiency and consistency.
- Efficient product recovery becomes increasingly critical at scale. For hydrogen, this includes gas capture, purification (e.g., CO2 removal), and safe storage or compression. For biomass, downstream handling may involve separation, concentration, and drying—steps that can be energy- and cost-intensive if not optimized.
Future studies will focus on:
- Combining sunflower husk with other agricultural or industrial wastes to broaden the feedstock base;
- Exploring microbial co-cultures or genetically engineered strains to improve substrate utilization and inhibitor tolerance;
- Conducting continuous or pilot-scale fermentations to validate scalability, process robustness, and economic feasibility;
- Implementing detoxification strategies or pretreatment refinements to further reduce inhibitory compound formation.
Overall, the study provides a foundation for sustainable bioconversion of lignocellulosic and glycerol-rich wastes, with promising implications for biohydrogen and biomass production at a larger scale.
Author Contributions
G.M., A.T., L.Z., K.Y. and A.D.—Experimental analysis, data collection, methodology, and investigation; L.V. and A.V.—Formal analysis and writing—original draft; K.B.—Funding acquisition, project administration, writing—review and editing; A.P. and K.T.—Funding acquisition, writing—review and editing, validation, supervision, and conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. BR24992914) and by Basic support and a research grant from the Higher Education and Science Committee of the Ministry of Education, Science, Culture and Sports of Armenia (24FP-3D020).
Data Availability Statement
Data will be made available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- Adeleke, B.S.; Babalola, O.O. Oilseed Crop Sunflower (Helianthus annuus) as a Source of Food: Nutritional and Health Benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K. Sunflower Oil and Its Applications. Lipid Technol. 2014, 26, 260–263. [Google Scholar] [CrossRef]
- Le Clef, E.; Kemper, T. Sunflower Seed Preparation and Oil Extraction. In Sunflower; Elsevier: Amsterdam, The Netherlands, 2015; pp. 187–226. [Google Scholar]
- Puttha, R.; Venkatachalam, K.; Hanpakdeesakul, S.; Wongsa, J.; Parametthanuwat, T.; Srean, P.; Pakeechai, K.; Charoenphun, N. Exploring the Potential of Sunflowers: Agronomy, Applications, and Opportunities within Bio-Circular-Green Economy. Horticulturae 2023, 9, 1079. [Google Scholar] [CrossRef]
- Milovanov, O.; Klimov, D.; Kuzmin, S.; Grigoriev, S.; Mikhalev, A.; Isemin, R.; Brulé, M. Application of Torrefaction for Improved Fuel Properties of Sunflower Husks. Energies 2024, 17, 4643. [Google Scholar] [CrossRef]
- Turzyński, T.; Kluska, J.; Ochnio, M.; Kardaś, D. Comparative Analysis of Pelletized and Unpelletized Sunflower Husks Combustion Process in a Batch-Type Reactor. Materials 2021, 14, 2484. [Google Scholar] [CrossRef] [PubMed]
- Perea-Moreno, M.-A.; Manzano-Agugliaro, F.; Perea-Moreno, A.-J. Sustainable Energy Based on Sunflower Seed Husk Boiler for Residential Buildings. Sustainability 2018, 10, 3407. [Google Scholar] [CrossRef]
- Havrysh, V.; Kalinichenko, A.; Mentel, G.; Mentel, U.; Vasbieva, D.G. Husk Energy Supply Systems for Sunflower Oil Mills. Energies 2020, 13, 361. [Google Scholar] [CrossRef]
- Osman, A.I.; Lai, Z.Y.; Farghali, M.; Yiin, C.L.; Elgarahy, A.M.; Hammad, A.; Ihara, I.; Al-Fatesh, A.S.; Rooney, D.W.; Yap, P.-S. Optimizing Biomass Pathways to Bioenergy and Biochar Application in Electricity Generation, Biodiesel Production, and Biohydrogen Production. Environ. Chem. Lett. 2023, 21, 2639–2705. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. ‘Renewable’ Hydrogen: Prospects and Challenges. Renew. Sustain. Energy Rev. 2011, 15, 3034–3040. [Google Scholar] [CrossRef]
- Kamran, M.; Turzyński, M. Exploring Hydrogen Energy Systems: A Comprehensive Review of Technologies, Applications, Prevailing Trends, and Associated Challenges. J. Energy Storage 2024, 96, 112601. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Biofuels, Biodiesel and Biohydrogen Production Using Bioprocesses. A Review. Environ. Chem. Lett. 2020, 18, 1049–1072. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Mat Aron, N.S.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R.; Chew, K.W.; Show, P.L. A Review on Bioconversion Processes for Hydrogen Production from Agro-Industrial Residues. Int. J. Hydrogen Energy 2022, 47, 37302–37320. [Google Scholar] [CrossRef]
- Trchounian, K.; Poladyan, A.; Vassilian, A.; Trchounian, A. Multiple and Reversible Hydrogenases for Hydrogen Production by Escherichia coli: Dependence on Fermentation Substrate, PH and the F0F1-ATPase. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Poladyan, A.; Trchounian, K.; Vassilian, A.; Trchounian, A. Hydrogen Production by Escherichia coli Using Brewery Waste: Optimal Pretreatment of Waste and Role of Different Hydrogenases. Renew. Energy 2018, 115, 931–936. [Google Scholar] [CrossRef]
- Soto, M.L.; Domínguez, H.; Núñez, M.J.; Lema, J.M. Enzymatic Saccharification of Alkali-Treated Sunflower Hulls. Bioresour. Technol. 1994, 49, 53–59. [Google Scholar] [CrossRef]
- Mirzoyan, S.; Aghekyan, H.; Vanyan, L.; Vassilian, A.; Trchounian, K. Coffee Silverskin as a Substrate for Biobased Production of Biomass and Hydrogen by Escherichia coli. Int. J. Energy Res. 2022, 46, 23110–23121. [Google Scholar] [CrossRef]
- Vanyan, L.; Aghekyan, H.; Vassilian, A.; Poladyan, A.; Trchounian, K. Biotechnological Potential of Spent Coffee Grounds for Biohydrogen Production by Escherichia coli. Int. J. Hydrogen Energy 2024, 142, 1121–1131. [Google Scholar] [CrossRef]
- Gleizer, S.; Ben-Nissan, R.; Bar-On, Y.M.; Antonovsky, N.; Noor, E.; Zohar, Y.; Jona, G.; Krieger, E.; Shamshoum, M.; Bar-Even, A.; et al. Conversion of Escherichia coli to Generate All Biomass Carbon from CO2. Cell 2019, 179, 1255–1263.e12. [Google Scholar] [CrossRef] [PubMed]
- Aristidou, A.A.; San, K.-Y.; Bennett, G.N. Improvement of Biomass Yield and Recombinant Gene Expression in Escherichia coli by Using Fructose as the Primary Carbon Source. Biotechnol. Prog. 1999, 15, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Sołowski, G.; Konkol, I.; Cenian, A. Methane and Hydrogen Production from Cotton Waste by Dark Fermentation under Anaerobic and Micro-Aerobic Conditions. Biomass Bioenergy 2020, 138, 105576. [Google Scholar] [CrossRef]
- Raposo, F.; Delarubia, M.; Borja, R.; Alaiz, M. Assessment of a Modified and Optimised Method for Determining Chemical Oxygen Demand of Solid Substrates and Solutions with High Suspended Solid Content. Talanta 2008, 76, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Bradstreet, R.B. Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Gevorgyan, H.K. THE UTILIZATION OF CARBON SOURCES MIXTURE (GLUCOSE, GLYCEROL AND FORMATE) AND GENERATION OF FERMENTATION END-PRODUCTS BY ESCHERICHIA COLI. Proc. YSU B Chem. Biol. Sci. 2020, 54, 55–62. [Google Scholar] [CrossRef]
- Simensen, V.; Schulz, C.; Karlsen, E.; Bråtelund, S.; Burgos, I.; Thorfinnsdottir, L.B.; García-Calvo, L.; Bruheim, P.; Almaas, E. Experimental Determination of Escherichia coli Biomass Composition for Constraint-Based Metabolic Modeling. PLoS ONE 2022, 17, e0262450. [Google Scholar] [CrossRef] [PubMed]
- Taha, F.S.; Wagdy, S.M.; Hassanein, M.M.M.; Hamed, S.F. Evaluation of the Biological Activity of Sunflower Hull Extracts. Grasas Aceites 2012, 63, 184–192. [Google Scholar] [CrossRef]
- Casoni, A.I.; Gutierrez, V.S.; Volpe, M.A. Conversion of Sunflower Seed Hulls, Waste from Edible Oil Production, into Valuable Products. J. Environ. Chem. Eng. 2019, 7, 102893. [Google Scholar] [CrossRef]
- Fox, D.J.; Gray, P.P.; Dunn, N.W.; Marsden, W.L. Comparison of Alkali and Steam (Acid) Pretreatments of Lignocellulosic Materials to Increase Enzymic Susceptibility: Evaluation under Optimised Pretreatment Conditions. J. Chem. Technol. Biotechnol. 1989, 44, 135–146. [Google Scholar] [CrossRef]
- Bekbayev, K.; Mirzoyan, S.; Toleugazykyzy, A.; Tlevlessova, D.; Vassilian, A.; Poladyan, A.; Trchounian, K. Growth and Hydrogen Production by Escherichia coli during Utilization of Sole and Mixture of Sugar Beet, Alcohol, and Beer Production Waste. Biomass Convers. Biorefin. 2022, 14, 909–919. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, S.; Yang, S.; Ding, S.-Y. Lignin Plays a Negative Role in the Biochemical Process for Producing Lignocellulosic Biofuels. Curr. Opin. Biotechnol. 2014, 27, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, C.; Tang, W.; Huang, M.; Ma, C.; He, Y.-C. Enhancing Enzymatic Saccharification of Sunflower Straw through Optimal Tartaric Acid Hydrothermal Pretreatment. Bioresour. Technol. 2023, 385, 129279. [Google Scholar] [CrossRef] [PubMed]
- Ohgren, K.; Bura, R.; Saddler, J.; Zacchi, G. Effect of Hemicellulose and Lignin Removal on Enzymatic Hydrolysis of Steam Pretreated Corn Stover. Bioresour. Technol. 2007, 98, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Kovárová-Kovar, K.; Egli, T. Growth Kinetics of Suspended Microbial Cells: From Single-Substrate-Controlled Growth to Mixed-Substrate Kinetics. Microbiol. Mol. Biol. Rev. 1998, 62, 646–666. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.P. The Chemistry Involved in the Steam Treatment of Lignocellulosic Materials. Quim. Nova 2003, 26, 863–871. [Google Scholar] [CrossRef]
- Kabir, F.; Katayama, S.; Tanji, N.; Nakamura, S. Antimicrobial Effects of Chlorogenic Acid and Related Compounds. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 359–365. [Google Scholar] [CrossRef]
- Nenkova, S.; Vasileva, T.; Stanulov, K. Production of Phenol Compounds by Alkaline Treatment of Technical Hydrolysis Lignin and Wood Biomass. Chem. Nat. Compd. 2008, 44, 182–185. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Piskarev, I.M.; Ushkanov, V.A.; Aristova, N.A.; Likhachev, P.P.; Myslivets, T.S. Establishment of the Redox Potential of Water Saturated with Hydrogen. Biophysics 2010, 55, 13–17. [Google Scholar] [CrossRef]
- Murarka, A.; Dharmadi, Y.; Yazdani, S.S.; Gonzalez, R. Fermentative Utilization of Glycerol by Escherichia coli and Its Implications for the Production of Fuels and Chemicals. Appl. Environ. Microbiol. 2008, 74, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, K.; Flores, N.; Castañeda, H.M.; Martínez-Batallar, G.; Hernández-Chávez, G.; Ramírez, O.T.; Gosset, G.; Encarnación, S.; Bolivar, F. New Insights into Escherichia coli Metabolism: Carbon Scavenging, Acetate Metabolism and Carbon Recycling Responses during Growth on Glycerol. Microb. Cell Fact. 2012, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Ahmadi, F.; Kariman, K.; Lackner, M. Recent Advances and Challenges in Single Cell Protein (SCP) Technologies for Food and Feed Production. npj Sci. Food 2024, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Salerno, M.B.; Rittmann, B.E. Thermodynamic Evaluation on H2 Production in Glucose Fermentation. Environ. Sci. Technol. 2008, 42, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Kumar, P.; Kalia, V.C. Enhancing Biological Hydrogen Production through Complementary Microbial Metabolisms. Int. J. Hydrogen Energy 2012, 37, 10590–10603. [Google Scholar] [CrossRef]
- Mikoyan, G.; Vanyan, L.; Toleugazykyzy, A.; Bekbayeva, R.; Baichiyeva, K.; Bekbayev, K.; Trchounian, K. Fermentation of Sugar Beet Pulp by E. coli for Enhanced Biohydrogen and Biomass Production. Energies 2025, 18, 2648. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).