Abstract
As an important component of current power and energy storage systems, lithium-ion batteries have essential scientific significance and application value in terms of accurately and reliably diagnosing their aging to determine system performance, identify potential faults in modules, and prolong their service life. For this purpose, this paper first briefly describes the working principle of lithium-ion batteries and illustrates the possible impacts of various aging mechanisms on the state of battery capacity. Secondly, starting from both implementable and laboratory perspectives, it sorts out and summarizes the diagnostic mechanisms and applicable scenarios of current typical battery aging state assessment and diagnosis methods. Then, targeting the specific aging mechanisms involved in batteries, it elaborates on the targeted diagnosis processes for each aging mechanism. Finally, combined with implementable and laboratory diagnosis methods, it systematically summarizes a highly standardized and universal routine diagnosis process for battery aging. In addition, in combination with the latest development of aging diagnosis and related technologies, this paper reflects on and discusses the possible future development directions of battery diagnosis technologies.
1. Preface
With the implementation of the “dual-carbon” policy (the national strategy to achieve carbon emission peaking by 2030 and carbon neutrality by 2060 [1,2]), the battery industry has entered an unprecedented period of development. As the direct energy supply device for electric vehicles and energy storage systems, its performance directly affects the overall efficiency of the systems it serves [3]. Lithium-ion batteries are widely used due to their advantages, such as high energy density, long lifespan, and lightweight characteristics. However, as the number of charge–discharge cycles increases, their performance in various aspects gradually declines, which is referred to as battery aging [4]. Aging not only affects the battery’s storage capability but may also lead to a reduction in battery safety. Therefore, achieving the effective diagnosis of battery aging holds significant importance for the entire battery industry.
To comprehensively and accurately diagnose battery aging status, it is essential to first understand the aging mechanisms of batteries. Over the past decade, researchers have employed experimental and modeling approaches [5,6]. A deep exploration of the internal mechanisms and corresponding external characteristics of battery aging has been conducted, leading to the development of diagnostic methods that correspond to each aging mechanism. In terms of approach, these methods can be categorized into implementable diagnostic techniques, which primarily observe external battery characteristic curves (such as current, voltage, impedance), and laboratory diagnostic techniques, which mainly involve observing internal materials using precision instruments (such as electron microscopes, energy-dispersive spectrometers).
Among these, implementable diagnosis can preliminarily determine the aging process and degradation direction of batteries, while microdiagnosis can further utilize instruments and image information to verify and refine the results of implementable diagnosis. For each specific aging direction, in-depth research can be conducted through the above combination methods. In practical applications, based on these diagnostic techniques, every aged battery sample can be evaluated for its aging status by following a fixed diagnostic procedure. Nowadays, with the gradual development of computer technology and the increasing production of batteries, modern diagnostic technologies are beginning to align with big data and machine learning. By analyzing historical operation data of batteries, machine learning can achieve online multi-state joint estimation of batteries, transmitting the estimated results to the cloud for analysis and storage to enable data sharing. This cost-effective and efficient diagnostic method is gradually gaining market favor and has significant application value.
To fully and timely summarize the field of battery aging diagnosis, we can also draw on knowledge from many other fields. For example, collagen peptides can be explored for their potential applications in improving material stability or slowing down material aging, which can be analogized to the strategy of slowing down lithium-ion battery aging [7]. For example, the stability and extraction efficiency of antioxidants coincide with the research on aging diagnosis methods for lithium-ion batteries. Just as antioxidants can prevent the oxidation of food ingredients, similar studies on chemical stability may provide new ideas for extending the life of lithium-ion batteries [8]. Precise temperature management is critical for lithium-ion battery operation, as thermal fluctuations accelerate aging mechanisms. Cold chain logistics monitoring technologies—including real-time thermal mapping and adaptive control algorithms—can be adapted to develop predictive battery aging models [9]. The importance of real-time monitoring and early warning systems for microbial growth environments is similar to that of state monitoring and fault prediction technologies in the aging process of lithium-ion batteries. By comparing these two monitoring methods, we may find new ways to improve the accuracy of the aging diagnosis of lithium-ion batteries. This paper first analyzes the aging mechanism in detail during the working process of batteries, then summarizes the existing battery aging diagnosis methods from implementable and micro perspectives, and introduces the diagnostic methods for specific aging mechanisms. Finally, from an overall perspective, a general diagnostic process is proposed, and the future diagnostic trend is predicted.
2. Analysis of the Aging Mechanism of Lithium-Ion Battery
2.1. The Basic Working Principle of Batteries
During the operation of batteries, multiple complex chemical reactions occur internally, which can be compared to the impact of new food packaging materials on the shelf life of food. The research and application of new materials share similar goals with the study of lithium-ion battery materials, namely, to enhance product stability and durability. By studying food packaging materials, we may gain new insights into the diagnosis of lithium-ion battery aging [10]. Taking lithium cobalt oxide/graphite batteries as an example, both the positive- and negative-electrode active materials have a layered structure, consisting of lithium cobalt oxide and graphite, respectively, as shown in Figure 1. During charging, lithium-ions diffuse from the inside of the lithium-containing positive-electrode material to the surface, then enter the electrolyte, pass through the separator, and move toward the negative electrode. Finally, they reach the graphite at the negative electrode, where electrons travel directly from the positive electrode to the negative electrode via the external circuit to combine with the lithium-ions. The discharge process is the reverse of the charging process; during discharge, electrons move from the negative electrode to the positive electrode through the external circuit, generating current for the load.

Figure 1.
Schematic diagram of working principles of lithium-ion battery (lithium cobalt oxide/graphite as example) [5,6].
2.2. Analysis of Battery Aging Mechanism
Each time a battery undergoes a charge–discharge cycle, corresponding chemical and physical changes occur inside. These changes can lead to varying degrees of loss in the active lithium or materials within the battery, a process known as battery aging. As the number of charge–discharge cycles increases, the rate of loss also continues to rise. The mechanism of aging can be summarized as shown in Figure 2. From right to left, this shows metal dissolution, electrolyte decomposition, binder degradation, structural disordering, lithium dendrite formation, the microcracking of electrode particles, the growth and decomposition of solid electrolyte interface (SEI)/cathode electrolyte interphase CEI films, graphite spalling, the alteration of electrode composition, and the corrosion of current collectors.
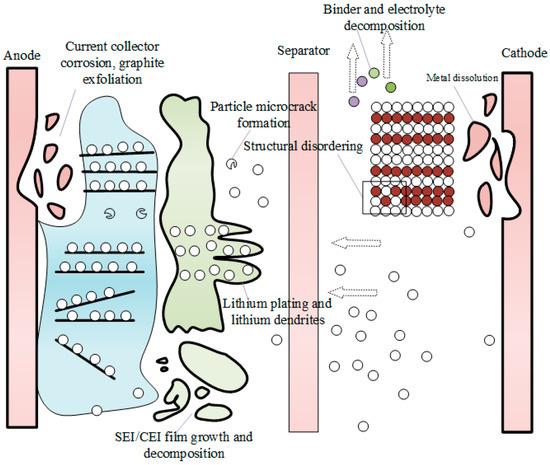
Figure 2.
Diagrammatic sketch of internal battery loss [11].
Different loss mechanisms can lead to active lithium loss (LLI) or active material loss (LAM) to some extent, as shown in Figure 3. The losses can be divided into three parts: LLI, negative-electrode LAM, and positive-electrode LAM. Among these, the causes of LLI include the growth and decomposition of SEI/CEI films, electrolyte breakdown, and lithium dendrites. The causes of negative-electrode LAM and positive-electrode LAM include graphite spalling, the microcracking of electrode particles, metal dissolution, structural disordering, changes in electrode composition, binder decomposition, and current collector corrosion. LLI and the LAMs of both electrodes subsequently lead to capacity loss and the power degradation of the battery.
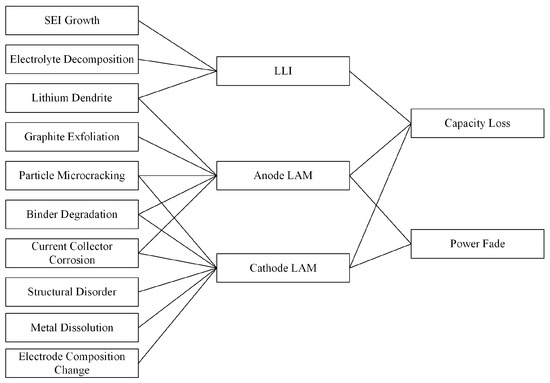
Figure 3.
Aging mechanism of batteries and corresponding capacity loss mechanism [12].
3. Battery Aging Diagnosis Means
Existing battery aging diagnostic methods can be divided into implementable and laboratory diagnostic approaches. Implementable diagnosis involves using the external characteristic curves of the battery to make an initial assessment of the entire aging process. Laboratory diagnosis, on the other hand, relies on equipment such as scanning electron laboratory and energy-dispersive spectrometers to observe specific aging conditions inside the battery, including element distribution and material structure changes. We have developed a ratio fluorescence probe based on this paper, providing unique insights for constructing cost-effective and environmentally friendly fluorescence sensors, which are used for high-performance self-absorption (SA) detection. Similar methods are also employed in battery aging diagnosis [13]. Additionally, such techniques are used in the creative reference screening of appropriate microbial fermenters (Livor and Lactobacillus intestinalis) to produce additional fermented fish, the optimization of the appropriate fermentation process, and the use of certain catalysts in battery aging diagnosis to assist in the observation of test results [14]. Pulsed light (PL) irradiation can also be used to degrade harmful substances by removing hydroxyl or carboxyl groups from benzene rings, and new treatment methods can be used for aging diagnosis [15]. At the same time, the traditional testing components are mainly destructive tests, which are time-consuming, inefficient, and cumbersome. Non-destructive testing (NDT), which is fast and simple, has become a new technology, and we can also refer to this testing method for aging diagnosis testing [16].
3.1. Implementable Methods
Implementable characteristics are more intuitive than micro characteristics and serve as specific manifestations of the latter. The classification of implementable diagnostic methods is shown in Figure 4 and can be divided into five categories: based on battery charge–discharge curves, based on open-circuit voltage (OCV) curves, electrochemical impedance spectra, and other characteristic curves. Other characteristics include initial Coulombic efficiency (ICE). A critical factor in battery aging is initial Coulombic efficiency, defined as the ratio of discharge capacity to charge capacity during the first cycle. For commercial batteries, ICE directly reflects irreversible capacity loss caused by solid electrolyte interphase (SEI) formation and lithium consumption. Lower ICE values typically correlate with accelerated aging, as they indicate greater initial active lithium loss compared to compound degradation during cycling. However, due to the extensive variety of the fifth category (other characteristic curves), this section primarily details the first four types.
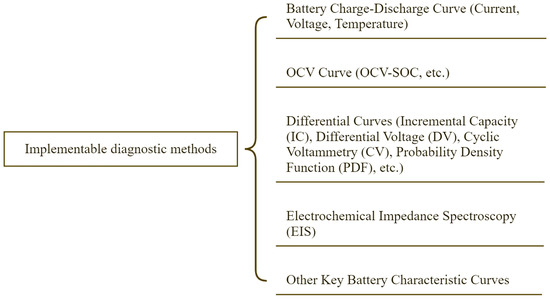
Figure 4.
Classification of implementable diagnostic methods.
3.1.1. Based on Battery Charge and Discharge Curve
The most basic implementable diagnostic method is to determine the aging state of a battery through its charge–discharge curve. The charge–discharge curve describes how current, voltage, and temperature change over time during charging or discharging, as shown in Figure 5a [17]. It is necessary to connect the battery to the charging and discharging equipment, set up the test conditions via a computer, and conduct charge–discharge tests on the battery to obtain the required curve. By comparing the charge–discharge curves at different times, an initial assessment of the battery’s aging state can be made.

Figure 5.
Aging mechanism of batteries and corresponding capacity loss mechanism. (a) Current, voltage and temperature curves of battery CC-CV charging condition from top to bottom [17]. (b) Charging rate test, from top to bottom: terminal voltage, first derivative of voltage, second derivative of voltage [18]. (c) IC curves (top) and DV curves (bottom) of nickel-cobalt-aluminum oxide (NCA) batteries under different cycles [11]. (d) 1/3 C cycle data of LiMn2O4/Li battery (top) PDF curve and (bottom) CV curve [19]. (e) From top to bottom: classical EIS, linear EIS, single-frequency EIS, nonlinear-frequency domain response (NFRA), and odd random response (ORP-EIS) [20].
3.1.2. Based on OCV Curve
OCV stands for open-circuit voltage. This is calculated as the potential difference between the two ends of an electrode when the battery has been left undisturbed for a sufficient period. The OCV curve typically refers to the curve showing how the open-circuit voltage changes with a variable (such as the state of charge or temperature). Commonly used curves include the OCV-SOC curve, where the state of charge (SOC) represents the state of charge of the battery. As the battery transitions from a fully charged state (SOC = 100%) to a completely discharged state (SOC = 0%), the corresponding OCV values will gradually decrease, and the rate of OCV reduction will vary across different SOC ranges, as shown in Figure 5b [18].
When the battery ages, the whole OCV-SOC curve will show a tendency towards inward compression, which is specifically manifested as the change of slope in different SOC intervals, so the property of slope change can help us to preliminarily judge the aging degree of the battery. Furthermore, the temporal evolution of OCV can be quantified by the parameter K, calculated as , where is the time interval between two measurements. The K value serves as an indicator of aging-related phenomena, such as capacity fading or internal self-discharge caused by particle degradation, thereby offering additional diagnostic granularity for the aging process.
3.1.3. Based on Differential Curves
A differential curve refers to a curve where at least one of the coordinates on either the x-axis or the y-axis is derived through differential calculations. Differential curves can clearly show the trend of how one characteristic quantity changes with another, as shown in Figure 5c [11]. Commonly used differential curves for analyzing battery aging include the capacity increment (IC) curve, differential voltage (DV) curve, cyclic voltammetry (CV) curve, and probability density (PDF) curve.
- IC Curve
The IC curve of a battery represents the relationship between the differential of charge (dQ/dV) and voltage (V), describing how capacity changes with voltage during charging or discharging. Typically, the IC curve displays voltage on the x-axis and the differential of capacity with respect to voltage on the y-axis. The IC curve will show “peaks” regardless of whether the battery is charging or discharging, each corresponding to different chemical reactions. However, as the number of cycles increases, these “peaks” tend to become smaller and shift downward to the right. This is because the active material or lithium becomes less active over time due to battery aging, leading to a decrease in internal chemical reaction intensity. By using the characteristic that the position of the “peaks” changes with battery aging and comparing the “peaks” of new and old batteries, one can roughly determine the aging process of the battery.
- 2.
- DV Curve
The “peaks” of the IC curve introduced in (1) are conducive to observing battery aging conditions and mechanisms. The DV curve described here can also clearly distinguish the state of battery aging. The DV curve typically refers to the relationship between dQ/dV and capacity (Ah). Its usage is like that of the IC curve, with corresponding “peaks.” After multiple charge–discharge cycles, the position of these “peaks” will shift to the right. This shifting pattern can also be used to observe the state of battery aging.
- 3.
- CV Curve
By applying a specific voltage signal to the battery electrode and measuring the corresponding current response, the resulting curve is known as a CV curve. The curve displays voltage on the x-axis and current on the y-axis, providing the current response of the battery under different potential conditions, reflecting the electrochemical reaction process within the battery. In cyclic voltammetry tests, the potential is typically scanned back and forth within a certain voltage range to obtain current responses for both forward and reverse scans, allowing the observation of the redox reactions at different potentials.
- 4.
- PDF Curve
Like the IC and DV curves, the PDF curve describes the probability distribution of random variables and can be used to represent the distribution of specific battery parameters. When evaluating the health status of lithium-ion batteries, common parameters include battery capacity, internal resistance, and open-circuit voltage. By collecting a large amount of battery test data, probability density functions for these parameters can be established and used to assess the health status of other battery parameters. The PDF curve also has corresponding “peaks,” as shown in Figure 5d. By combining the position information of these “peaks” for both new and old batteries, the degree of battery aging can be analyzed.
3.1.4. Electrochemical Impedance Spectrum
Impedance represents the ability of ions to be hindered as they shuttle through the battery, which increases with battery aging [21]. Electrochemical impedance spectroscopy (EIS) quantifies aging mechanisms by probing interfacial resistances, such as SEI film impedance and charge-transfer resistance. For example, EIS Nyquist plots can distinguish between LLI-dominated aging and LAM-dominated aging, providing a diagnostic framework for degradation modes. During the test, a scan is performed over a range of frequencies, from low to high, to obtain impedance values at different frequencies. As the battery ages, changes occur in both the low-frequency and high-frequency regions of the impedance spectrum, allowing for an observation of its general aging state. EIS can be further categorized into classical EIS, linear EIS, single-frequency EIS, nonlinear-frequency domain response (NFRA), and odd random response (ORP-EIS), as shown in Figure 5e.
3.2. Laboratory Techniques
According to the diagnostic content, the existing microdiagnostic means can be divided into seven categories: element composition and valence state analysis, morpho-logical characteristics, material crystal structure characterization, material functional group characterization, material ion transport process observation, material micro-mechanical properties, and other microdiagnostic techniques, as shown in Figure 6.
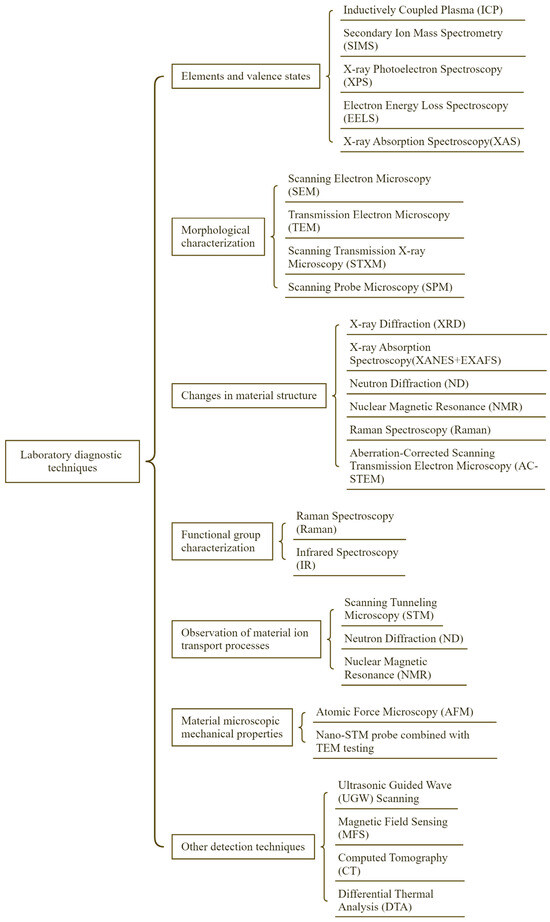
Figure 6.
Classification of laboratory diagnostic methods.
3.2.1. Analysis of Elemental Composition and Valence State
The determination of elemental composition and valence is mainly based on energy spectrum technology. Different beams are used as excitation sources to irradiate or bombard the surface of the sample. After the action, relevant particles (such as electrons, etc.) will be produced. The elemental composition and valence state can be determined by measuring the binding energy of particles in atoms and analyzing their chemical shift.
- Inductively Coupled Plasma
Inductively coupled plasma (ICP) uses high-frequency electric fields to discharge gases (such as argon) and generate plasma, which passes through the sample, ionizing atoms and molecules within it for elemental analysis. ICP quantitatively analyzes dissolved transition metals in electrolytes or electrodes, correlating their concentration with capacity fade. ICP-MS further detects trace isotopes, while ICP-OES optimizes testing parameters for aging diagnostics. Inductively coupled plasma spectrometers and mass spectrometers are two commonly used analytical instruments. Inductively coupled plasma optical emission spectrometry (ICP-OES) collects and analyzes specific wavelength light signals emitted by the sample using an optical system, with wavelength resolution achieved through gratings or interferometers [22]. It can measure multiple elements simultaneously, offering high sensitivity and a wide linear range; this method is applied to study the impact of drone spray parameters on pesticide droplet deposition and control effectiveness, optimizing parameters through experiments. In the aging diagnosis of lithium-ion batteries, similar experimental methods can be used to optimize various parameters during battery testing, such as optimizing charging and discharging test conditions in implementable diagnostic approaches to improve diagnostic accuracy [23]. Inductively coupled plasma mass spectrometer (ICP-MS) can determine the isotope ratio of multiple elements and detect the limit of detection by the mass analysis of these particles and diagnosis according to the mass-to-charge ratio of ions [24].
- 2.
- Secondary Ion Mass Spectrometry and Hyperspectral Imaging
Secondary ion mass spectrometry (SIMS) bombards the sample surface with high-energy particles to generate secondary particles [25]. These particles are then analyzed using a mass spectrometer to obtain chemical information about the sample surface. Subsequently, hyperspectral imaging technology combined with data reduction algorithms is employed to non-destructively detect the total volatile basic nitrogen (TVB-N) content in chicken meat. This process is like the microdiagnostic techniques used in lithium-ion batteries. For instance, after obtaining extensive internal information about a battery using laboratory inspection equipment, one can apply the data processing methods described in this literature to reduce the complexity of micro-data, extract key information, and assist in assessing the battery’s aging status [26]. The schematic diagram is shown in Figure 7a. In SIMS, high-energy particle beams first bombard the sample surface, causing secondary ions to be emitted from the sample surface with information on elemental composition, compound formation, distribution, and concentration. The emitted secondary ions are then introduced into a mass spectrometer for analysis, which involves a series of ion lenses and a mass analyzer to determine the elemental and compound information on the material’s surface. Specific diagnostic methods can be divided into static SIMS and dynamic SIMS, distinguishing between stable ion bombardment and transient ion bombardment on the sample surface. Static SIMS is primarily used to study elemental and compound information on the sample surface, while dynamic SIMS is suitable for studying the microstructure and interface reactions of materials.
- 3.
- X-ray Photoelectron Spectroscopy
Similar to SIMS is the X-ray photoelectron spectroscopy (XPS), XPS determines chemical states of elements and tracks interfacial reactions, revealing decomposition pathways in aged batteries. XPS uses an X-ray beam with sufficient energy to irradiate samples, interacting with surface atoms and exciting inner electrons to higher energy levels. The excited electrons are then emitted from the sample surface through the photoelectric effect. The emitted photoelectrons carry chemical information about the sample and enter an electron energy analyzer, where their energies are measured to determine their binding energy with atoms [27]. Some researchers have used XPS techniques to determine the valence states of elements in the cubic perovskite material Li7La3Zr2O12 (c-LLZO) after it reacts with H2O and CO2 in air, thereby suggesting the specific substances. The crystal structure diagram and XPS spectrum are shown in Figure 7b.
- 4.
- Electron Energy Loss Spectroscopy
The electron energy loss spectroscopy (EELS) technique uses high-energy electron beams to bombard samples, causing energy loss in the electrons within the sample. This energy loss can be categorized into elastic and inelastic losses (the distinction between elastic and inelastic losses lies in whether the lost energy can be fully recovered; if it can, it is considered an elastic loss; otherwise, it is an inelastic loss). The electrons are then introduced into a spectrometer for analysis, providing information on the electronic structure of the sample. Specifically, EELS probes electronic structures and local bonding environments, enabling the nanoscale analysis of material properties such as electrode degradation and phase transformations in batteries.
EELS diagnostic methods primarily include synchrotron radiation EELS (SR-EELS) and transmission electron microscopy EELS (TEM-EELS). The former uses high-brightness and wide-energy-range electromagnetic radiation as the electron source, while the latter employs a high-spatial-resolution transmission electron microscope to analyze electron energy loss in specific regions of the sample [28]. Additionally, the method can be combined with short-wave and long-wave near-infrared spectroscopy, using color compensation techniques to detect soluble solids content in apples. In battery diagnostics, for some battery information obtained through spectral techniques; the methods described in this literature can be referenced to process and analyze spectral data, enhancing the accuracy and reliability of the diagnosis [29].
3.2.2. Morphological Representation
The morphological characteristics of the internal materials of batteries are usually observed by instruments with precision down to nanometers, mainly using high-resolution electron microscopes such as scanning electron microscopes, transmission electron microscopes, scanning transmission X-ray microscopes, and scanning probe microscopes.
- Scanning Electron Microscopy
Scanning electron microscopy (SEM) is indispensable in characterizing battery degradation, as it visualizes morphological changes such as electrode cracking, SEI film growth, and lithium dendrite formation. For instance, the SEM imaging of aged graphite anodes reveals particle fractures and SEI layer thickening, which correlate with active lithium loss (LLI). SEM uses an electron beam emitted by an electron gun to scan the sample surface, measuring the reflection, scattering, and absorption characteristics of electrons to obtain relevant information about the sample surface. The schematic diagram of the lithium metal surface image observed by SEM is shown in Figure 7d.
- 2.
- Transmission Electron Microscopy
Transmission electron microscopy (TEM) enables the atomic-scale analysis of battery materials, revealing crystallographic defects and phase transformations. For instance, TEM has identified Mn dissolution from cathodes and its deposition on anodes, a key contributor to capacity loss. The main principle is that the electron beam penetrates the sample and forms an image after focusing. Due to this characteristic, TEM can observe the internal structure of samples with higher resolution than SEM.
- 3.
- Scanning Transmission X-ray Microscopy
The core components of the scanning transmission X-ray microscope (STXM) technology include the X-ray source and detector. The X-ray source generates rays that pass through the sample, and the detector measures the electron signals from X-ray transmission and characteristic X-ray absorption signals to visualize the fine morphology of the sample. This technique, known as scanning transmission X-ray microscopy (STXM), combines X-ray spectroscopy with high spatial resolution, making it particularly useful for mapping chemical composition changes in aged electrodes and studying heterogeneous degradation patterns or localized failure sites. The schematic diagram is shown in Figure 7e.
- 4.
- Scanning Probe Microscopy
The scanning probe microscopes (SPMs) used include the scanning tunneling microscope (STM) and the atomic force microscope (AFM). These instruments combine the advantages of optical and electron microscopes. The two most critical components of SPM are the probe and the scanning tube. The probe makes direct contact with the sample to sense its surface properties, while the scanning tube precisely controls the relative position between the probe and the surface. By quantifying surface topography changes and electronic state variations at electrode interfaces, SPM techniques (AFM/STM) provide critical insights into SEI growth dynamics and lithium deposition behavior during battery aging. By using suitable probes to scan the sample surface (AFM can detect the topography of the sample surface and STM can provide images of the electronic states on the sample surface), these instruments can characterize the morphology of the material being studied. Detailed discussions on AFM and STM are provided in Section 3.2.5, “Observation of Material Ion Transport Processes,” and Section 3.2.6, “Microscopic Mechanical Properties of Materials.”
3.2.3. Material Crystal Structure Change
If you want to further observe the crystal structure of materials, most electron microscopes cannot meet the requirements of accuracy, and more accurate spectroscopic techniques or optical microscopes are needed. Representative technologies include X-ray diffraction, extended X-ray fine spectrum, neutron diffraction, nuclear magnetic resonance, Raman spectrum, etc., and representative electron microscopes include the spherical aberration-corrected scanning transmission electron microscope.
These techniques collectively establish structure–degradation correlations across multiple scales: from bulk phase transitions (XRD/ND) to local atomic environments (XAS/NMR) and atomic-scale defects (AC-STEM). Their complementary applications enable comprehensive diagnosis of the crystallographic origins of battery aging.
- X-ray Diffraction
X-ray diffraction (XRD) is a critical tool for studying battery materials, as it can monitor real-time structural evolution during cycling or analyze crystalline changes after aging. By analyzing the diffraction pattern, information about the structure and composition of the material can be obtained [30]. This method can also be used to study the inactivation effects of temperature-controlled short-wave infrared radiation on lipase and lipoxygenase in wheat germ, as well as their impact on the storage stability and quality of wheat germ oil [31]. In battery research, this approach to studying the effects of temperature and other factors on battery material performance and aging processes can be analogously applied [32]. The energy spectrum of LixMn0.25Fe0.75PO4 energy spectrum is shown in Figure 7f.
- 2.
- X-ray Absorption Spectroscopy
X-ray Absorption Spectroscopy (XAS) encompasses two complementary techniques for analyzing materials: X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine-structure (EXAFS) analysis. These methods provide detailed information about the electronic structure and local atomic environments in battery materials.
The method for determining the elemental composition and valence state of elements also includes X-ray absorption near-edge structure (XANES). This technique uses X-rays to irradiate samples, causing their atoms to absorb energy. Inner electrons are excited to higher energy levels and then emitted from the sample through the photoelectric effect. Information about the electron structure information of atoms and molecules in the sample is obtained via measurement with an energy analyzer. A schematic diagram of XANES is shown in Figure 7c, where the left image shows the sulfur K-edge XANES spectra of samples containing different concentrations of sulfur added to carbon black (CB) and boron nitride (BN); the right image shows the same spectrum after SA correction.
Extended X-ray absorption fine-structure (EXAFS) techniques are used to analyze the local structure and chemical environment of atoms in materials. This technique utilizes synchrotron radiation X-rays to irradiate samples, causing inner-shell electrons to be ionized after absorbing photons and releasing them as spherical light electrons. If other particles are present around the absorbing atom, the light electrons will be scattered; if no other particles are present, the light electrons will disappear. By measuring the absorption spectrum of X-rays, information about the local structure and chemical environment of sample atoms can be obtained. For samples with long-range disorder but short-range order in their crystal structures (such as catalysts, solutions, or samples containing trace elements), EXAFS is a relatively effective analytical method. The schematic diagram is shown in Figure 7g, where (A) is a representative TEM image of Pd1/TiO2, showing the cross-section of an ultra-thin TiO2 after aberration correction in scanning transmission electron microscopy (STEM) mode; (B) is a scanning transmission electron microscopy-energy-dispersive X-ray spectroscopy (STEM-EDS) elemental map of a single-Pd1/TiO2 nanosheet; (C) is the Fourier-transform extended X-ray absorption fine-structure (FT-EXAFS) spectrum of Pd1/TiO2 displaying atoms adjacent to Pd atoms; (D) is a high-resolution high-angle annular dark-field (HAADF) STEM image of Pd1/TiO2.
- 3.
- Neutron Diffraction
Neutron diffraction (ND) technology leverages the principle of neutron interaction with matter. Neutrons, one of the fundamental particles that make up atoms, can be produced through nuclear fission or other methods. In experiments, neutron sources are commonly used to generate neutrons. When neutrons pass through matter, they interact with atomic nuclei and electrons within the material, producing diffraction phenomena. Unlike X-ray diffraction, neutron diffraction is related to the interaction between atomic nuclei in crystals.
While both neutron diffraction (ND) and X-ray diffraction (XRD) probe material structures, ND offers unique advantages for battery research through its exceptional sensitivity to light elements. In lithium-ion battery studies, ND can directly determine lithium atomic positions and detect hydrogen-containing species, capabilities that are severely limited in conventional XRD analysis. These characteristics make ND an indispensable tool for investigating lithium migration pathways and electrolyte decomposition mechanisms.
The wavelength of neutrons is typically in the order of the lattice spacing of materials, making neutron diffraction more sensitive to crystal lattices. This phenomenon can be used to observe the crystal structure of materials.
- 4.
- Nuclear Magnetic Resonance
The fundamental principle of nuclear magnetic resonance (NMR) technology is that when atomic nuclei are subjected to an external magnetic field, they undergo energy-level splitting. When the frequency of the applied radiofrequency field numerically equals the distance between the energy levels, the atomic nuclei absorb the radiofrequency energy, resulting in resonance absorption. By measuring the resonance absorption signal, information such as the number of atomic nuclei in the sample, chemical shift, coupling constants, and relaxation time can be obtained. In the study of battery aging, NMR technology can be used to determine the NMR spectrum of active substances, thereby obtaining their structural information. As shown in Figure 7h, this figure illustrates the NMR spectra of LiPF6 carbonyl region 13C with different molar ratios.
- 5.
- Raman Spectroscopy
The principle of Raman spectroscopy is based on Raman scattering. When high-intensity incident light interacts with a substance, a small portion of the scattered light has a different wavelength from the incident light. This change in wavelength is determined by the chemical structure of the sample being measured, and this scattered light is called Raman scattering. The frequency changes in this scattered light are related to the vibration modes of the molecules in the material. This makes Raman spectroscopy particularly valuable for identifying bond vibration changes in electrode materials and SEI composition through characteristic fingerprint spectra, enabling the non-destructive monitoring of chemical degradation processes. As shown in Figure 7i, which illustrates the Raman spectra of three substances on the cathode, this can be used to further observe the battery structure by referencing the analysis of sugar diffusion and optimization in Gaido Seak based on finite element analysis, as well as hyperspectral imaging techniques [33].
- 6.
- Scanning Transmission Electron Microscopy with Aberration Correction
Aberration correction scanning transmission electron microscopy (AC-STEM) refers to an optical defect in transmission electron microscopy that causes blurring and distortion in imaging. By introducing specific electric or magnetic fields in the electron transmission microscope, the effect of aberration can be corrected, thereby improving the resolution and quality of the image. AC-STEM uses an electron beam to scan through a sample; it then reconstructs the three-dimensional structure of the sample from the transmitted electron images collected from the sample. These images can be processed using computer algorithms and image processing techniques to obtain high-resolution three-dimensional information.
This aberration-corrected technique provides atomic-resolution imaging capabilities that are particularly valuable for observing defect formation and interface reconstruction, crucial for understanding nanoscale degradation initiation mechanisms in battery materials.
Compared to traditional X-ray crystallography and nuclear magnetic resonance techniques, aberration-corrected scanning transmission electron microscopy offers higher resolution and broader applicability. As shown in Figure 7j, the top row displays an AC-STEM image of a two-dimensional WS2 sheet containing nanoholes irradiated by ions (the dark area in the image), while the bottom row shows an artificially identified image of the nanoholes (bright green).
3.2.4. Characterization of Material Functional Groups
Compared with the elemental composition and crystal structure mentioned above, the chemical properties of material functional groups are more active. Although the specific components of functional groups can be intuitively observed by acid–base neutralization, oxidation–reduction, precipitation formation, etc., it will change the battery material and may even cause safety problems. Raman spectroscopy and infrared spectroscopy are commonly used in laboratories to diagnose the required functional groups.
- Raman Spectroscopy
This technology has been described in detail in the previous article, so it will not be repeated here.
- 2.
- Infrared Spectroscopy
Infrared (IR) spectroscopy is an absorption spectrum that uses infrared radiation at specific wavelengths (typically divided into three regions: near-infrared 0.75–2.5 μm, mid-infrared 2.5–25 μm, and far-infrared 25–1000 μm) to interact with samples. The infrared radiation within these wavelength ranges is associated with the vibration modes of chemical bonds and the rotational motions of molecules, causing substances to absorb specific infrared spectra. The form of motion corresponding to infrared light is molecular vibration, which alters the dipole moment and thus produces infrared absorption. The frequency of vibration is related to the strength of chemical bonds and atomic mass, so infrared spectroscopy determines functional groups by measuring the vibration frequencies of chemical bonds in molecules. This makes IR spectroscopy particularly powerful for battery diagnostics, where it can identify organic functional groups in degraded electrolytes and binder degradation products. The technique also probes inorganic components in SEI layers through lattice vibrations, while resolving dynamic interactions like Li+ solvation structure changes, as demonstrated in Figure 7k for LiTFSI systems. These capabilities are critical for understanding electrolyte aging mechanisms.
3.2.5. Observation of Ion Transport Process in Materials
- Scanning Tunneling Microscopy
Scanning tunneling microscopy (STM) uses the tunneling effect to observe the surface of materials. STM can closely examine and locate individual atoms; it is a scanning microscopy tool where the tip consists of just one atom. When the tip scans the sample at a height of less than one nanometer, the electron cloud overlaps and produces a tunneling effect under an applied voltage, causing electrons to escape. As the distance between the probe and the sample surface continuously changes, the current fluctuates, revealing the topography of the sample at the atomic scale and allowing for detailed contouring. These unique capabilities make STM particularly valuable for battery research, enabling the atomic-scale visualization of electrode surface evolution during cycling. The technique directly images SEI layer formation, lithium deposition patterns, and graphite anode morphological changes (as demonstrated in Figure 7l). With its ultra-high resolution, STM reveals localized degradation hotspots and quantifies surface roughness changes that critically impact ion transport processes.
Neutron diffraction and nuclear magnetic resonance are described in detail in Section 3.2.3 (3) and Section 3.2.3 (4), and so they will not be repeated here.
3.2.6. Microscopic Mechanical Properties of Materials
The characterization of microscopic mechanical properties provides crucial insights into structural degradation and interface evolution in aged lithium-ion batteries. These techniques enable the quantitative assessment of mechanical property changes that contribute to performance degradation.
- Atomic Force Microscopy
Atomic force microscopy (AFM) acquires the surface morphology and mechanical properties of samples by scanning their surfaces. It can measure surface height differences at the nanoscale and obtain atomic-level information about the surface through the interaction between the probe and the sample. Beyond topography, AFM precisely characterizes nanoscale mechanical property changes in electrode materials and interfaces, including SEI layer stiffness evolution, particle fracture toughness reduction, and binder degradation. When the sample’s morphology changes, the interaction forces between the tip and the sample also change (for example, from attraction to repulsion), causing the slight displacement of the AFM’s microcantilever. The photodetector on the AFM precisely amplifies the displacement changes of the microcantilever via a light spot. Finally, data processing yields images and related data of the sample’s surface structure. The schematic diagram observed by AFM is shown in Figure 7m, which includes (a) highly oriented pyrolytic graphite (HOPG) and (b) composite electrode AFM images.
- 2.
- Nanometer, Scanning Tunneling Microscopy Probe Combined with Transmission Electron Microscopy Test
In traditional transmission electron microscopy (TEM), nanoprobes or scanning tunneling microscopy (STM) probes are usually used in combination to achieve more detailed sample analysis. Two test method are listed as follows:
- Nanoprobe combined with TEM test method: Nanoprobe can be introduced into TEM to achieve mechanical operation of samples at the nanoscale, and to achieve accurate analysis of samples. Common nanoprobe includes ion beam etching probe, atomic force microscope probe, etc. Nanoprobe-TEM enables the in situ mechanical testing of electrode materials at nanoscale resolution, revealing crack propagation mechanisms and phase-dependent mechanical property changes during cycling.
- STM probe combined with TEM test method: The STM probe can realize the in situ electrical property analysis of samples in TEM. The electrical property information of samples can be obtained by scanning the surface of the samples and detecting the electronic structure of the samples. STM-TEM combines atomic-scale imaging with simultaneous electrical characterization, allowing the direct correlation of structural defects with local conductivity changes in aged electrodes.
3.2.7. Other Microscopic Diagnostic Techniques
- Ultrasonic Guided Wave Scanning
Ultrasound-guided wave (UGW) scanning is a non-destructive testing technique that uses the principle of ultrasonic waves propagating and reflecting within structures to diagnose and evaluate internal defects in materials. It can determine the location, size, and type of defects in structures through changes in the signal along the propagation path. By applying ultrasonic excitation to the surface of a structure, sound waves are introduced into the interior, where they propagate as guided waves, reflecting and scattering at interfaces between the surface and the interior. When ultrasonic waves encounter structural defects caused by battery aging, such as cracks, protrusions, or material changes, they undergo reflection, scattering, and energy loss. By analyzing the signal characteristics of ultrasonic waves propagating within the structure, such as amplitude, time delay, and spectrum, the position and size of defects can be determined. This is then compared with reference signals from defect-free structures for analysis and interpretation. The schematic diagram is shown in Figure 7n, which illustrates the UGW spectra of lithium-ion batteries at different aging cycles.
- 2.
- Magnetic Sensing
Magnetic field sensing (MFS) detects batteries based on the principles of battery electrochemical reactions and electromagnetic theory. When a battery is charging or discharging, an induced magnetic field is generated both inside and around the battery. By placing a magnetometer around the battery to measure the magnetic field strength, changes in the battery’s performance and state can affect the surrounding magnetic field [34]. Analyzing and comparing the changes in magnetic field strength recorded by the magnetometer with those of a reference battery allows for the evaluation of differences between the two.
- 3.
- Computerized Tomography
Computed tomography (CT) is a non-destructive diagnostic technique that reconstructs the three-dimensional image of an object by scanning it from multiple angles with X-rays and then reconstructing it by computer. It can provide detailed information about the internal structure and composition of an object, as shown in Figure 7o, which is a CT image of a lithium cobalt oxide battery during charging.
- 4.
- Differential Thermal Analysis
Differential thermal analysis (DTA) techniques study the thermal properties and phase transition behaviors of materials by measuring the temperature differences between samples and reference materials. During the experiment, the differential thermal analyzer measures the temperature difference between the sample and the reference sample to calculate the heat difference. By analyzing the measured data, one can observe the thermal effects of the sample at different temperature ranges, such as exothermic peaks and endothermic peaks. By comparing the position, shape, and intensity of these thermal effect peaks, it is possible to infer potential phase transitions, chemical reactions, or pyrolysis phenomena in the sample. For lithium-ion battery cathode materials, these thermal effect peaks may be related to lithium-ion insertion and extraction, lattice structure phase changes, and material pyrolysis.
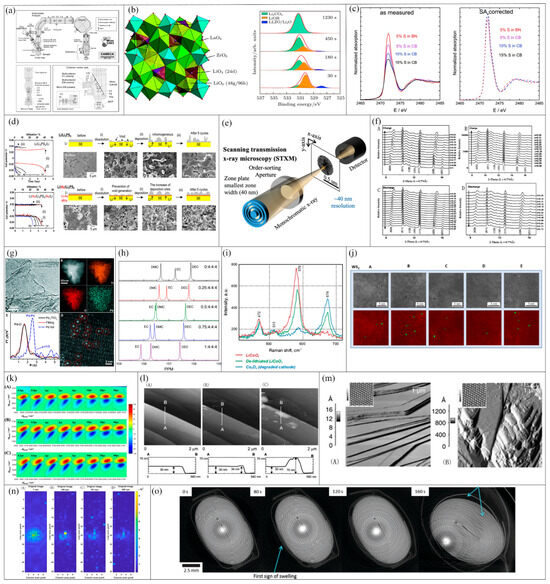

Figure 7.
Example of laboratory diagnosis. (a) IMS-1280 schematic diagram [35]. (b) Li7La3Zr2O12(c-LLZO) schematic diagram of crystal structure (left) and energy spectrum variation in LLZO micro powder surface at different exposure times in air (right) [36]. (c) Sulfur k-edge XANES spectra (left) and same spectra after self-absorption (SA) correction (right) were measured on samples with different concentrations of sulfur added to carbon black (CB) and boron nitride (BN [37]. (d) SEM image and schematic diagram of morphology change on lithium metal surface in constant current cycling test. (e) STXM diagrammatic sketch [38]. (f) The variation laws in XRD spectra of LixMn0.25Fe0.75PO4 with lithium content x during the first cycle (0.05C charge–discharge (A,B)) and the second cycle (0.5 C charge–discharge (C,D)). (g): (A) representative TEM image of Pd1/TiO2 (B) STEM-EDS elemental mapping of a single-Pd1/TiO2 nanosheet (C) FT-EXAFS spectra of Pd1/TiO2 and its adjacent atoms; (D) high-resolution HAADF-STEM image of Pd1/TiO2 [39]. (h) NMR spectrum is shown with different molar ratios LiPF6’s carbonyl zone 13C. (i) Raman spectra of the three cathode materials. (j) Top row: AC-STEM images of ion irradiated two-dimensional WS2 thin sheets containing nanopores (dark areas in the image) (A–E); bottom row: artificially identified nanopores are bright green [40]. (k) Two-dimensional infrared spectra of the C≡N stretching of SCN-anion probes in LiTFSI aqueous solutions with concentrations (A) 1, (B) 10, and (C) 18 mol/kg. (l) STM images of the anode graphite before and after CV (2 × 2 μm) and its height distribution in 1 m LiClO4/TFPC (A) before cycling, (B) after one cycle between 2.8 to 0 V, and (C) after three cycles in 1 m LiClO4/TFP [41]. (m) 10 × 10 µm2 AFMFigure: (A) HOPG and (B) composite electrode [42]. (n) UGW diagrams of lithium batteries at different ages [43]: (A) the first cycle, (B) the 100th cycle, (C) the 151st cycle, and (D) the 200th cycle. (o) CT images of LiCoO2 battery during charging at 0 s,80 s,120 s, and 160 s.
4. Specific Diagnostic Protocols for Each Aging Mechanism
The aging mechanisms of batteries vary significantly at different stages of aging. In the early stage, the dominant mechanism is LLI caused by the growth and thickening of SEI/CEI films. This is partly due to the formation of SEI/CEI films during the battery’s pre-manufacturing process and partly because particles in the electrolyte react with the SEI/CEI film during subsequent use, leading to further thickening. As the battery continues to be used, the primary aging mechanism shifts from LLI to LAM, with LAM being predominant in the negative electrode. The main factors contributing to LAM include electrode metal dissolution, disordered electrode particle structure, and changes in electrode composition.
At the electrode interfaces, aging induces distinct yet interrelated degradation processes. The anode SEI progressively thickens due to continuous reduction reactions and mechanical stress, primarily consuming active lithium-ions and increasing interfacial impedance. Conversely, the cathode CEI thickens through oxidation reactions and transition metal dissolution, which predominantly compromise cathode structural integrity and charge transport kinetics. These parallel but interconnected mechanisms synergistically accelerate overall battery degradation.
Although these factors, which account for a significant portion of the aging mechanism, have been identified and are subject to relatively comprehensive implementable diagnostic methods, mechanisms such as electrolyte decomposition, lithium dendrite formation, graphite spalling, and particle microcracking, which account for a smaller proportion, have a minimal impact on the external characteristic curves diagnosed practically. Moreover, the trends reflected in these curves are often the result of multiple mechanisms working together, so they can only be observed using laboratory diagnostic techniques.
4.1. Diagnostic Methods Dominated by LLI
4.1.1. Electrolyte Decomposition
The electrolyte plays an important role in ion transport and charge balance in batteries. It is usually a mixture of organic solvent and LiPF6. Although this ratio has been optimized for safety, it is still not stable enough. For example, when HF is present, the contaminated LiPF6 will attack the electrode material as a corrosive medium, leading to material dissolution [44]. In recent decades, due to the relatively minor role of electrolyte decomposition in aging mechanisms, most research has focused on the decomposition mechanisms and stability of electrolytes [45,46]. For instance, a thermosensitive smart polymer aqueous two-phase flotation system, combined with HPLC, can be used to simultaneously separate, enrich, and detect trace amounts of ciprofloxacin and lomefloxacin in food samples. In battery analysis, for the detection and analysis of trace substances in batteries, reference can be made to their separation and detection techniques to examine impurities or degradation products within the battery, thereby understanding its aging condition [47]. At the beginning of the 21st century, Gnanaraj and Zinigrad et al. used an accelerated calorimeter to verify that when the temperature rises to around 70 °C, the SEI/CEI film will decompose, and pyruvic acid (C3H4O3) from the solvent in the electrolyte will directly contact lithium, producing gas. Gas production leads to direct contact between the negative electrode and air, reacting with oxygen, which also causes secondary damage to the battery [48]. Wang et al. used a C80 calorimeter to study the decomposition of LiPF6 when the temperature reached 170 °C [49]. In the following decade, research on electrolyte decomposition products and electrolyte materials sprang up like mushrooms after rain. The focus was primarily on new types of electrolyte materials, electrolyte leakage and stability, and the chemical recycling of these materials [50,51,52,53]. Similar to how external factors influence physical processes in such studies, this research also examines how different environmental conditions or treatment methods affect the charging and discharging processes or the aging of batteries. Due to the limitations of the “one-too-many” mechanism of macro-characteristic curves, to accurately determine whether the electrolyte decomposes during aging, it is usually necessary to combine other analytical methods, such as energy spectrum analysis, to determine the chemical composition and structure of the decomposition products and assess whether the electrolyte has decomposed and the degree of decomposition.
4.1.2. SEI/CEI Film Thickens
The formation of the SEI/CEI film is due to the reaction between the anode material and lithium-ions in the electrolyte during the initial use of the battery, resulting in the creation of a stable film. This film primarily consists of dissolved substances from the electrolyte, decomposition products of the electrolyte, and reduction products of the anode material. The SEI/CEI film protects the anode from reacting with the electrolyte, promotes the conduction of lithium-ions within the battery, and prevents particles from moving freely inside the battery. However, as the battery cycles, the film continuously reacts with active lithium, increasing its thickness and consuming active lithium, which reduces the battery’s capacity. This is a significant cause of battery aging [54].
Li et al. used EIS to preliminarily estimate the impedance of the SEI/CEI film [11]. The EIS diagnostic results are described using a Nyquist diagram, which intuitively illustrates the impedance characteristics of the battery’s SEI/CEI film. For example, the resistance (RSEI/CEI) of the film can indicate the quality and stability of the SEI/CEI film. If the SEI/CEI film is in good condition, RSEI/CEI should be low, as shown in Figure 8a. After an initial assessment using EIS, further verification can be conducted by observing the SEI/CEI film of aged batteries with SEM. There will be certain differences between the SEI/CEI films of new and aged batteries: the SEI/CEI film of a new battery typically has a relatively uniform and smooth surface, presenting a more regular structure. In contrast, the SEI/CEI film of an aged battery may exhibit an uneven, rough, or granular surface, possibly with cracks or pores, as shown in Figure 8b.
4.1.3. Lithium Dendrite Phenomenon
During the battery charging process, some lithium-ions are not fully embedded into the anode material but gradually accumulate on its surface. This leads to an increasing number of lithium-ions on the anode material until capacity is exceeded, resulting in free lithium metal deposits on the anode surface. This phenomenon is known as lithium plating, and the deposited lithium is referred to as lithium dendrites, as shown in Figure 8c [55,56,57]. Overcharging can exacerbate this deposition. Here, we can refer to the development of a recyclable non-ligand biphase extraction method for determining the presence of lead in food samples. In terms of detecting heavy metal impurities in batteries, their detection methods can be referenced to measure the heavy metal content in batteries, thereby assessing the quality and safety of the battery [58]. Regarding the specific reasons for lithium plating, there are currently two perspectives. The first perspective is from the potential angle, which holds that when the overpotential of the anode relative to metallic lithium/lithium-ions is less than 0 V, lithium plating will occur. The second perspective focuses on concentration, suggesting that due to the lower solid-phase diffusion coefficient of the anode, lithium plating will happen when the concentration of diffused lithium-ions reaches the level of saturation [59,60,61,62,63].
In previous studies, researchers have tried methods such as Coulomb efficiency, electrochemical calorimetry, and three electrodes to determine dendrite precipitation [64,65,66]. However, these methods not only require expensive equipment but also yield inaccurate results. This section primarily introduces the differential voltage analysis (DVA) method, which has a simple operation process and can preliminarily diagnose lithium plating on batteries through non-destructive means. It is sufficient to obtain the relationship curve between battery voltage and charge capacity. Subsequently, the data obtained should be processed with first and second derivatives, as shown in Figure 8d. It is evident that when constant current charging is performed at 2 C and 1.5 C, there are distinct characteristic peaks in the first derivative at approximately 22 mAh and 40 mAh, respectively. In the second derivative plot, there are noticeable decreases at these two points, indicating lithium plating. To further observe whether dendrite formation has occurred, in situ optical microscopy or other electron microscopy techniques can be used for observation.
4.2. A Diagnostic Method Dominated by LAM
4.2.1. Graphite Flaking
Graphite, due to its excellent electrical conductivity and chemical stability, is often used as an additive in negative-electrode materials to enhance the conductivity of batteries and the reaction rate for intercalation/deintercalation. A decade ago, to increase energy density, researchers began exploring and using silicon materials as battery anodes. Silicon has a higher theoretical capacity, but it tends to expand and contract in volume during charging and discharging, leading to structural damage. Therefore, researchers have addressed the issue of volume expansion in silicon anode materials to some extent by synthesizing nanostructures and coating protective layers, thereby achieving higher energy density [67].
Although researchers have made considerable achievements in the research of graphite anodes [68]; however, with the continuous disembedding of particles during use, thry still suffer varying degrees of damage (volume change is usually about 12%) and even fall off [69]. Sethuraman et al. provide a good summary of the causes of graphite structural disorder and even fracture [70]; based on the Daumas–Hérold model, relevant diagrams have been poduced for lithium intercalation and deintercalation within a certain voltage platform region, as shown in Figure 8e. This demonstrates the state of the anode graphite under normal conditions and during different stages of charging (Stage 1–Stage 4—where gray stripes represent anode graphite and blue spherical particles represent Li+), and the phenomenon of graphite exfoliation under a certain number of charge–discharge cycles has been observed using Raman spectroscopy (ID/IG is the peak ratio of D to G peaks, with a higher value indicating more defects in that area).
4.2.2. Electrode Particle Microcrack
The phrase microcracks in electrode particles refers to the phenomenon of microcracks being in the particles in the electrode during the use of the battery. They can be divided into intergranular cracks and intragranular cracks, which will hurt the performance and life of the battery. Thomas et al. conducted thorough research on the microstructure of cracks in the positive-electrode particles of nickel-rich NMC811 lithium-ion batteries [71]. High-resolution CT technology was used to observe the particles before and after cycling, as shown in Figure 8g. The green box contains the observation results of the battery in its factory condition, while the red box shows the results after multiple cycles of charging to 4.5 V and discharging to 3 V. This figure shows that the particles already had multiple cracks before cycling, with lengths reaching 8 μm, and many cracks even penetrated to the surface of the particle. This is due to defects in the preparation process, such as coating and imprinting techniques. Comparing these with the images from after cycling, it was found that the phenomena were more severe and the cracks were more pronounced.
4.2.3. Metal Dissolves
The ions such as manganese (Mn2+), nickel (Ni+), and cobalt (Co2+) in cathode materials are known as transition metal ions. During charging, these transition metals are oxidized to higher oxidation states, releasing lithium-ions. In the discharge process, they are reduced back to lower oxidation states and reabsorbed. Theoretically, only Li+ should move back and forth between the positive and negative electrodes. However, with increasing cycle counts, the presence of these metal components can often be detected in the electrolyte, SEI/CEI film, or even the carbon anode [72]. Similar sensors can be used for diagnosis, drawing on models that detect the height of a pole using ultrasonic sensors throughout the entire growth cycle of wheat. Due to the chemical reactions during charging and discharging [73], the HF produced can corrode the electrodes, exposing transition metals on the electrode surface and leading to the loss of active materials. At this point, we can draw on research on the inhibitory effect of lemongrass oil encapsulated in liposomes on the growth of Listeria monocytogenes in cheese [74]. In battery research, methods similar to those used for studying antimicrobial effects can be applied to investigate the inhibitory effects of additives or protective materials on internal chemical reactions within the battery, thereby delaying battery aging [75,76]. Chen et al. [77] studied the relationship between Mn content in the anode and battery capacity loss using an ICP atomic spectrophotometer. The results showed that when 100 ppm Mn2+ was added, the battery capacity loss was about 63.3%, while the loss was only 10.6% without the addition of Mn2+. In addition, Mn2+ would dissolve into the SEI/CEI film and capture Li+ during the deintercalation process [78]. James et al. [79] carried out a series of experiments to study the phenomenon of capacity loss caused by transition metal dissolution, and the relationship between Mn2+ dissolution and capacity loss was quantified. Combined with ICP-MS, the following conclusions were obtained: each deposition of Mn2+ on SEI/CEI film captured an additional 0~102 Li+, which was consistent with the analysis results of previous scholars.
Although it has been confirmed that the dissolution of Mn2+ is the primary mechanism leading to Li+ loss due to transition metal dissolution, relatively few studies have been conducted using implementable diagnostics. Therefore, more specific experimental methods are needed to accurately determine the extent of transition metal dissolution. The dissolution of Mn2+ was observed using fluorescence spectroscopy to measure the Mn content in the SEI/CEI film and on the anode, as shown in Figure 8f [80,81,82].
4.2.4. The Structure Is Disordered
Different cathode materials have different structural and performance characteristics. At present, the three main cathode materials have a layered structure [83,84], Olivine structure [85], and Spinel structure [86]. Researchers are also constantly looking for more cathode materials, such as LiCo2/3Ni1/6Mn1/6O2 [87], to improve the performance of the battery.
Previous studies were performed on the diagnosis of structural disorder [88,89,90,91,92]. One very effective method is to use neutron diffraction to observe the structural changes of battery cathode materials during charging and discharging processes. As shown in Figure 8h, the left image shows the diffraction pattern of the battery at the 5th minute during cycling, while the right image displays the diffraction patterns corresponding to different phase transitions. If the crystal structure changes, the intensity will vary, even if the diffraction angle 2θ remains unchanged. By comparing these with the diffraction patterns of newly charged and discharged batteries, one can diagnose whether the structure has become disordered.
4.2.5. Electrode Composition Changed
Electrode composition change refers to the phenomenon that the active material in the electrode changes during the use of the battery [93]. This change primarily occurs in the cathode material. During charging and discharging, lithium-ions intercalate and deintercalate between the cathode material and the electrolyte, altering the composition or structure of the cathode material. The self-assembly technology of nanomaterials can be utilized to create highly ordered electrode structures, which may help to improve battery performance and extend its lifespan [94]. Additionally, nanomaterials can be used to develop highly sensitive sensors to monitor chemical and physical changes associated with battery aging [95]. These changes are more pronounced at high temperatures [96]. As the battery continues to be recycled, the active material in the cathode material will decay [97]. This is due to the fragmentation of active material particles, structural damage, and surface deactivation. Additionally, lithium-ions in the cathode material may be dissolved by transition metals and undergo other chemical reactions. In such cases, studies used color sensor arrays, sensory analysis, and gas chromatography techniques to identify honey [98]. In battery aging diagnosis, when it is necessary to classify and differentiate multiple battery samples, this multi-technology approach can be referenced, combining the advantages of various detection methods to more accurately determine the aging state and type of batteries [99,100]. The differential thermal analyzer can be used to observe whether the electrode composition changes. The differential thermal analysis (DTA) spectrum and the schematic diagram of the electrode image under SEM observation are shown in Figure 8i.
4.2.6. The Adhesive Decomposed
A binder is a material used to fix and connect various components inside a battery. It serves functions such as securing electrode materials, providing electronic conductive pathways, offering ion transport channels, protecting electrode materials, and enhancing battery performance. In addition to these capabilities, binders must also resist electrolyte corrosion and withstand electrochemical corrosion caused by charging, discharging, and side reactions. Currently, for lithium cobalt oxide, ternary, and iron phosphate batteries, the main binder used is PVDF (Poly (vinylidene fluoride)), with NMP (N-Methyl-2-pyrrolidone) as the solvent. For graphite anodes, the primary binder is CMC + SBR (carboxymethyl cellulose + styrene-butadiene rubber), with water as the solvent. Of course, different types of batteries and battery systems require different types and performance levels of binders. Furthermore, with the development of battery technology, new binders and synthesis techniques continue to emerge, such as PDADMAC (Poly (diallyl dimethylammonium chloride)), styrene, and mixed solvents comprising methyl acrylate [101,102,103,104]. Research has also explored the synergistic antibacterial effects of cold nitrogen plasma and clove oil on E. coli O157:H7 biofilms on lettuce. In battery aging studies, similar approaches can be applied to explore synergistic effects between different diagnostic techniques or treatment methods, thereby improving the accuracy and comprehensiveness of battery aging diagnostics [105].
Using SEM, it is relatively simple to observe whether the binder decomposes. It is necessary to adjust the parameters of the SEM instrument, such as acceleration voltage, magnification, and probe current, to obtain clear images [106,107]. Due to the different materials, the decomposition mechanisms of various binders also vary. For example, PVDF undergoes chemical reactions with O2 in the air during preparation, forming K(HF2), among other products. By observing and analyzing the characteristic peaks in the XRD diffraction patterns, one can determine whether the binder has decomposed. If the binder decomposes or degrades, it results in a weakening or disappearance of peak shapes and intensities, or the appearance of new peaks. Comparing the experimental XRD diffraction patterns with known standard samples can help to assess the degree of binder decomposition [108].
4.2.7. Collective Fluid Corrosion
The current collector needs to carry the active materials of both positive- and negative-electrodes and can gather and output the current generated by these materials. However, in practical applications, different materials have different defects; for example, aluminum foil is prone to corrosion, making it commonly used as the material for positive-electrode current collectors. Copper, on the other hand, is resistant to corrosion but can oxidize at higher potentials, making it suitable as the material for negative-electrode current collectors. Corrosion generally occurs at the interface between the positive- and negative-electrode current collectors and the electrolyte, as lithium battery electrolytes typically contain corrosive substances such as fluorides and acidic materials [109,110]. Research has utilized a polyoxymethylene lauryl ether–salt biphasic system combined with high-performance liquid chromatography to separate, concentrate, and determine trace amounts of chloramphenicol in shrimp from different waters. In battery diagnostics, to detect impurities or harmful substances in batteries, reference can be made to their separation and detection techniques, allowing the analysis of pollutants or additives in the battery [111].
Observing whether the positive- and negative-electrode collectors have corroded or the degree of corrosion is relatively simple. If the corrosion is severe, it is easy to detect from macroscopic images that the collector has changed color or appears uneven on the surface. Specifically, SEM can be used to observe the corrosion of aluminum foil collectors; corroded collectors will show pits or holes, as well as oxides or corrosion products [112]. These voids or products will present different shapes and colors. At present, advanced collector materials, anti-corrosion coatings, and optimized battery design processes are the main approaches to deal with this situation [113].
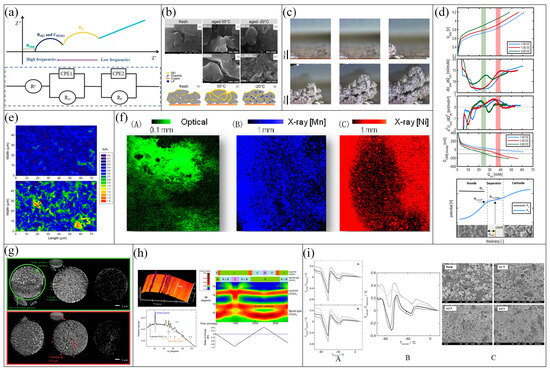

Figure 8.
Example diagram of specific aging mechanism diagnosis. (a) Typical electrochemical impedance spectroscopy principle diagram (top) and equivalent circuit model for lithium-ion batteries (bottom). (b) SEM images of anode aging: (A) is a new battery, (C) is at 50% depth of discharge (DOD), (D) is using AP2 at 100% DOD at 55 °C (F) is at 50% DOD, (G) is using AP2 100% DOD at −20 °C, and (B,E,H) show anode conditions of new and old batteries at 55 °C and −20 °C, respectively. (c) In situ optical snapshot of lithium dendrite growth during plating. (d) Principle of differential voltage analysis, from top to bottom: terminal voltage during charge rate testing, first derivative of charging voltage, second derivative of charging voltage, individual anode potentials, schematic diagrams of solid and electrolyte potentials. (e) Raman spectra of original graphite electrode (top) and electrode cycling between 1 and 0.18 vs. Li/Li+ (bottom) [114]; (f) A is optical fluorescence image, and B and C are X-ray fluorescence (XRF) spectra of Mn and Ni element distribution [115]; (g) Study of microstructure of primary and secondary particles in NMC811 under waveguide X-ray imaging technology; uncharged original secondary particles (top) and aged secondary particles that were charged five times to 4.5 V before disassembly imaging and n discharged to 3 V (bottom); (h) Neutron diffraction patterns during charge–discharge cycles; (i) Differential thermal analysis spectra of the electrolyte for cycling and storage batteries at 40 °C from March to December (A), (B) the storage batteries at 55 °C from March to December, SEM images of new batteries and those under different charge cut-off voltages (C) [116].
5. Diagnostic Process and Development Trend
When receiving samples of aged batteries, inspectors do not know in advance the proportion of aging mechanisms. They usually first determine whether the current aging state is early or midway/late through basic charge–discharge curves, then diagnose the specific mechanism that needs to be diagnosed. In this section, we summarize a routine process for diagnosing the dominant aging mechanisms in batteries and offer insights into future diagnostic trends.
5.1. Routine Diagnostic Process
The specific diagnostic methods for various aging mechanisms of batteries have been detailed in the previous section. For specific aging battery samples, in-depth research can be conducted by combining implementable and microdiagnostic techniques. This section will provide a detailed explanation of how to conduct an aging diagnosis on a complete battery aging sample, as shown in Figure 9.
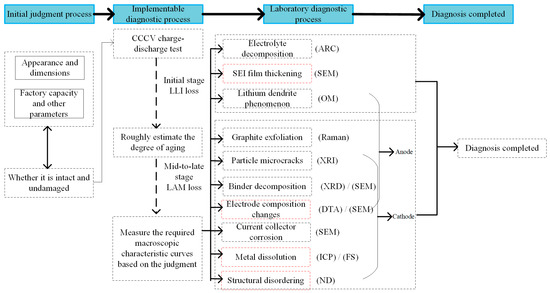
Figure 9.
Specific aging mechanism diagnostic flow chart.
First, by reviewing the factory parameters of the battery, information such as voltage, internal resistance, mass, and size can be obtained. Comparing these with the original dimensions can help to determine if deformation has occurred and the extent of this deformation, which can provide an initial assessment of aging. If the battery deforms, the likelihood of safety issues is higher, often due to improper usage conditions like overcharging or deep cycling. According to the basic working principles of batteries discussed in Section 1, overcharging can cause lithium-ions to penetrate through the separator into the graphite anode in large quantities or even entirely, leading to a collapse of the grid structure in the positive-electrode material. This not only damages the original structure but also increases the risk of lithium plating on the anode, which accelerates the growth of lithium dendrites. In severe cases, this can puncture the separator, cause internal short circuits, and lead to electrolyte decomposition and gas production, resulting in battery swelling. Deep cycling has similar effects, causing changes in the volume of the SEI/CEI film covering the surface of the anode material and creating cracks that allow reactions between the inner layer and the electrolyte, ultimately leading to SEI/CEI film failure and safety issues with the battery. If no deformation occurs, this indicates normal aging.
Next, it is necessary to conduct routine charge–discharge tests on aged samples, properly connect the battery’s charge–discharge equipment, and select appropriate charge–discharge values and rates based on previously queried factory parameters. The test will record parameters such as current, voltage, and temperature throughout the complete charging process of the battery. These parameters are typically collected over time. After obtaining these parameters, data processing is performed to derive information about the capacity state. In experiments, the discharge process is generally used to calculate the battery’s capacity state. The calculated capacity state is then compared with the factory parameters to roughly determine whether the current sample is in the early or mid–late stages of aging. The aging mechanisms differ significantly between different aging periods. In the early stage, capacity loss is mainly caused by LLI, while in the mid–late stages, it is due to LAM.
After determining the aging process, it is necessary to select appropriate testing methods based on requirements to obtain the required macroscopic characteristic curves, such as using impedance spectra to determine if the SEI/CEI film has thickened, or differential voltage to detect lithium dendrite formation. Since the trend of the curves and various characteristics are influenced by multiple mechanisms, it is necessary to combine laboratory diagnostic techniques to determine the proportion of aging caused by specific mechanisms. For example, it is necessary to use an accelerated calorimeter to study whether the electrolyte has undergone compositional changes or decomposition and use a scanning electron microscope to observe the thickness of the SEI/CEI coating on the outer layer of the anode graphite. It is necessary choose the appropriate equipment based on the mechanism to be observed, disassemble the sample before observation, and polish it according to the requirements of the observation equipment to prepare it for observation. It is necessary place the processed sample on the sample holder for observation to obtain results, which requires operators to have sufficient safety awareness. If conditions permit, new battery samples can be tested using the same procedure to compare the observed results and determine whether the aging mechanism measured is the primary cause of aging.
5.2. Trend in Development
The previous text introduced the aging mechanism of batteries and conventional diagnostic methods. While conventional diagnostic methods can identify specific aging mechanisms, implementable diagnosis requires a large amount of data and takes too long, while microdiagnosis necessitates disassembling the battery for detailed observation using specialized equipment. This approach is not suitable for diagnosing operational batteries. Moreover, with the increasing production volume of batteries, there is an urgent need to develop diagnostic methods that are rapid, accurate, and non-destructive to meet market application needs. For this reason, we outline the future trends in diagnostics from four perspectives, as shown in Figure 10.
Figure 10.
Disadvantages of aging conventional diagnosis and future trends.
5.2.1. Machine Learning
As the variety of batteries increases and their application scope broadens, conducting routine diagnostics for each battery would be a massive task. Machine learning, with its powerful data processing capabilities and clear input and output, has gradually been adopted in battery diagnostics. Scholars, both domestically and internationally, have conducted extensive research on integrating machine learning with battery diagnostic technologies. The battery is excited using a pulse current, and the response voltage and resistance are used as characteristic inputs to establish a support vector machine model to quickly estimate the state of capacity [117]. U. Yayan et al. used the current, voltage, measured current, measured voltage, and temperature in constant current charging as characteristic inputs to establish a neural network model to quickly predict the capacity state [118]. One study [119] used the voltage/current difference at fixed time intervals during constant current/voltage processes, the time difference between two predefined voltages, and other five characteristics as inputs to achieve the goal of rapidly estimating battery capacity status. Overall, the key to achieving this goal in these studies lies in selecting health factors with high correlation to capacity status as model input features and packaging these inputs along with the current capacity status into training data to train models for estimating battery capacity. Establishing a mathematical relationship between input features and battery capacity is sufficient. Common estimation algorithms include neural networks, support vector machines, Gaussian regression, and more. Additionally, insights can be drawn from using microbial antagonists to control the fungi that produce ochratoxin A in grape berries [120,121,122]. In research on the aging diagnosis of lithium-ion batteries, one can refer to the review approach of biological control methods to summarize and analyze the development of battery aging diagnosis technologies, examining the advantages and disadvantages of different techniques [123]. Model training is complete, and subsequent estimation can be repeated by repeating the above steps or calling the trained model.
5.2.2. New Sensor Technology
With the rapid development of sensor technology, physical and chemical changes in batteries during storage or operation can potentially be directly measured using sensors. In cases where appropriate sensors are available, the non-destructive testing of battery conditions can be performed using these sensors. Compared to other technologies, sensors offer a simple operation process, direct information acquisition, and precise results. For example, some researchers have used magnetometers to measure the causes of inconsistent capacity in battery packs. The principle is that when there is inconsistency in capacity, it indicates that the aging rate of cells within the pack is inconsistent, and their internal resistance also differs. Therefore, during charging and discharging processes, the internal current will also show inconsistencies, leading to inconsistent induced magnetic fields due to the current [124,125]. Here, research can be conducted on how CO2 injection in solid foods enhances the impact of low-frequency ultrasound on the immersion freezing process. In battery research, methods similar to those used to study the influence of external factors on physical processes can be applied to investigate the effects of different environmental factors or treatment methods on the charging and discharging processes or aging of batteries [126]. In addition, during the operation of the battery, the positive- and negative-electrode materials of the battery will have a slight expansion or contraction, and the battery shell will have corresponding changes, and the external temperature will also rise or fall. Using high-precision sensors such as optical fiber sensors to measure these parameters can also accurately obtain aging-related information.
5.2.3. Cross-Disciplinary Methods for Studying Battery Aging Mechanisms
These interdisciplinary approaches primarily aim to reveal fundamental degradation processes rather than develop diagnostic tools. For example, we can refer to the effects of low-frequency alternating magnetic fields (LF-AMF) on the water fermentation of basidiomycete Antrodia camphorata (A. camphorata) mechanism analysis in terms of magnetic fields and environment. Alternatively, we can study the impact of highland barley germination times (24, 48 and 72 h) on thermodynamics, adhesion, fermentation, and dynamic rheology study batteries under different conditions under different conditions using various methods. We can also consider combining colorimetric sensor arrays (CSA) with chemical measurement techniques to evaluate the aroma of tea characterization. In addition, we can draw inspiration from mechanical peeling and texture preservation after peeling to perform additional treatments on batteries or refer to cell-based electrochemical taste sensors and the potential uses of wheat bran in food for more in-depth analysis of battery structures and better data acquisition. Similarly, we can use ultrasonic and homogenization tools for battery testing and, during data acquisition, refer to the latest advancements and preservation techniques for extending the shelf life of fresh wet noodles to protect the battery and ensure accurate data [127,128,129,130,131,132,133,134].
In addition, the characteristics of ratio sensors, such as better sensitivity, repeatability, and stability than single sensors, can be used to select some more accurate sensor mechanisms for investigation [115]. At the same time, methods combining NIR spectroscopy with multivariate calibration can be used to rapidly and simultaneously quantify phenolic compounds in peanut (Arachis hypogaea L.) seeds. In aging studies, various methods are also combined, utilizing spectral knowledge. Additionally, methods based on self-assembled polytrimesidine nanocomposites for electrochemical sensors to measure nitrite levels in food are referenced. Certain electrochemical sensors are used to evaluate battery parameters, and studies on the mechanical properties and microstructure of transglutaminase-crosslinked whey protein and potato protein hydrolysate composites are referenced at the microstructure level [135,136,137,138].
Finally, we can draw on the in vitro and in situ characterization of the restorative spirit nourishment bacteria from refrigerated chickens to modulate the degradation processes of batteries of batteries, or refer to studies on the effects of ultrasonic curing at different frequencies on the mass transfer of sodium chloride (NaCl) in pig muscles and its potential mechanisms, as well as the effective degradation of soy protein B3 subunits in soy sauce by ultrasonic-assisted propyl endopeptidase and its main mechanisms, degradation pathways [139,140,141].
5.2.4. Vehicle–Cloud Data Fusion
Traditional diagnostic methods have a single data processing approach. If the measurement process is interfered with, the accuracy of the diagnosis will significantly decrease. Given the increasing production of batteries, to achieve the goal of a simple estimation process and strong interference resistance, vehicle–cloud integration is a good choice. Cloud data has advantages such as high security, data sharing, and strong interference resistance. Cloud computing also provides storage functions for data. For batteries of the same type or operating conditions, historical data can be called upon for estimation and comparison, greatly improving diagnostic accuracy. Additionally, when combining Kennard–Stone (KS) sample selection with Soft Independent Modeling by Class Analogy (SIMCA), the GC-IMS system achieves high classification accuracy for edible plant oils [142]. This chemometrics approach shows potential for adaptation to battery state classification in cloud platforms, particularly for distinguishing aging patterns across vehicle fleets.
The architecture of vehicle–cloud integration includes the terminal layer, network layer, platform layer, and application layer. The terminal layer is responsible for collecting initial information, encrypting transmitted data, and executing commands. Initial information includes external data directly related to the vehicle, such as mileage and charging parameters. After obtaining this initial information, it is processed and transmitted through communication methods like 5G networks, high-speed fiber optics, and mobile networks (network layer) to the platform layer. The platform layer is the core part of the vehicle–cloud data fusion architecture and serves as the “brain” of the next-generation BMS. It is responsible for cleaning and storing cloud data. After cleaning and processing the data, it can estimate battery capacity status and predict remaining life. When receiving cleaned and processed information and control commands, it executes functions such as balancing control, fault reporting and warning, status display, and traffic control based on modules installed in the vehicle equipment. It then transmits the acquired data back to the cloud. Through this repetitive cycle, a closed-loop data system is ultimately formed, enabling full-cycle state monitoring and the health management of the battery.
6. Summary
The aging diagnosis of lithium-ion batteries is of great significance for the safe use of batteries throughout their life cycle. This paper reviews the aging mechanism of batteries and the corresponding diagnosis methods and puts forward the prospect of future diagnosis trends. While our current review focuses primarily on characterization methods, we emphasize that a more comprehensive understanding of battery aging requires the integration of material characterization with electrochemical analysis, and an approach we hope to explore in future publications to more fully address these electrochemical relationships.
It is noteworthy that battery aging mechanisms are not solely determined by operational conditions but are also profoundly influenced by manufacturing processes. Recent studies [143] have demonstrated how critical manufacturing parameters, including slurry rheology, calendaring density, and drying conditions, fundamentally affect aging behavior. Variations in electrode uniformity during production can create localized aging hotspots [144], which are detectable through advanced differential voltage analysis techniques. These findings underscore the importance of developing manufacturing-aware diagnostic approaches to better understand and predict battery aging patterns.
Currently, widely adopted diagnostic methods can be divided into implementable and laboratory diagnostic techniques. Implementable diagnostic techniques measure battery curves and compare them with factory curves to intuitively assess the degree of battery aging. However, since battery aging is typically the result of multiple aging mechanisms acting in concert, the detailed characteristics of the curve changes are influenced by various aging mechanisms. Therefore, it is necessary to combine laboratory diagnostic techniques for specific observations. To this end, optical microscopes, energy-dispersive spectrometers, and other microscopic testing equipment are used to observe the impact of a particular aging mechanism on the entire aging process. Ideally, these characterization methods should be correlated with electrochemical measurements to establish complete structure–property relationships. By integrating implementable and laboratory diagnostic methods, a highly universal routine diagnostic procedure for battery aging can be systematically summarized.
With the popularization of the digital twin technology, the new generation of battery diagnosis technology is gradually developing towards machine learning, using new sensor technology and vehicle–cloud data fusion. It is hoped that this paper can serve as a beginning of future battery diagnosis technology, which needs to be supplemented and improved, particularly through the combined use of advanced characterization tools and electrochemical analysis techniques to provide a more holistic understanding of battery degradation mechanisms.
Author Contributions
Conceptualization, T.W. and L.P.; methodology, T.W., H.W., X.S., C.L. and W.W.; software, Y.X. and W.W.; formal analysis, T.W., H.W., X.S. and C.L.; investigation, T.W. and H.W.; resources, W.W. and H.L.; writing–original draft, T.W. and H.W.; writing—review and editing, X.S. and C.L.; validation, T.W., H.W., X.S., C.L., Y.X. and H.L.; visualization, T.W., H.W., X.S., L.P. and C.L.; supervision, T.W., L.P., Y.X. and H.L.; funding acquisition, W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by the authors.
Conflicts of Interest
Author Wanlin Wang was employed by the company Farasis Energy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ouyang, Y.; Guo, J. Carbon capture and storage investment strategy towards the dual carbon goals. J. Asian Econ. 2022, 82, 101527. [Google Scholar] [CrossRef]
- Jiang, B.; Raza, M.Y. Research on China’s renewable energy policies under the dual carbon goals: A political discourse analysis. Energy Strategy Rev. 2023, 48, 101118. [Google Scholar] [CrossRef]
- Menyhart, J. Electric Vehicles and Energy Communities: Vehicle-to-Grid Opportunities and a Sustainable Future. Energies 2025, 18, 854. [Google Scholar] [CrossRef]
- Rufino Júnior, C.A.; Sanseverino, E.R.; Gallo, P.; Amaral, M.M.; Koch, D.; Kotak, Y.; Diel, S.; Walter, G.; Schweiger, H.-G.; Zanin, H. Unraveling the degradation mechanisms of lithium-ion batteries. Energies 2024, 17, 3372. [Google Scholar] [CrossRef]
- Delmas, C.; Maccario, M.; Croguennec, L.; Le Cras, F.; Weill, F. Lithium deintercalation in LiFePO4 nanoparticles via a domino-cascade model. Nat. Mater. 2008, 7, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Uher, C. High pressure properties of graphite and its intercalation compounds. Adv. Phys. 1984, 33, 469–566. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zhu, Z.; Zheng, F.; Gao, R. Food-derived collagen peptides: Safety, metabolism, and anti-skin-aging effects. Curr. Opin. Food Sci. 2023, 51, 101012. [Google Scholar] [CrossRef]
- Shouket, S.; Khan, J.; Batool, R.; Sarwar, A.; Aziz, T.; Alhomrani, M.; Alamri, A.S.; Sameeh, M.Y.; Filimban, F.Z. Enhancement of shelf-life of food items via immobilized enzyme nanoparticles on varied supports: A sustainable approach towards food safety and sustainability. Food Res. Int. 2023, 169, 112940. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, H.; Fang, W.; Huang, X.; Shi, J.; Zou, X. Effects of variety and pulsed electric field on the quality of fresh-cut apples. Agriculture 2023, 13, 929. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Xin, Y.; Yang, Q.; Dhanasekaran, S.; Zhang, X.; Zhang, H. Aureobasidium pullulans S2 controls tomato gray mold and produces volatile organic compounds and biofilms. Postharvest Biol. Technol. 2023, 204, 112450. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, Y.; Wang, J.; He, H.; Peng, S.; Pecht, M. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium-ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Shi, Y.; Li, W.; Hu, X.; Zhang, X.; Huang, X.; Li, Z.; Zhai, X.; Shen, T.; Shi, J.; He, Y. A novel sustainable biomass-based fluorescent probe for sensitive detection of salicylic acid in rice. Food Chem. 2024, 434, 137260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, F.; Shi, T.; Xiong, Z.; Gao, R.; Yuan, L. Suanyu fermentation strains screening, process optimization and the effect of thermal processing methods on its flavor. Food Res. Int. 2023, 173, 113296. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Boateng, I.D.; Chen, S.; Yang, X.-M.; Soetanto, D.A.; Liu, W. Pulsed light irradiation improves degradation of ginkgolic acids and retainment of ginkgo flavonoids and terpene trilactones in Ginkgo biloba leaves. Ind. Crops Prod. 2023, 204, 117297. [Google Scholar] [CrossRef]
- Shi, J.; Liang, J.; Pu, J.; Li, Z.; Zou, X. Nondestructive detection of the bioactive components and nutritional value in restructured functional foods. Curr. Opin. Food Sci. 2023, 50, 100986. [Google Scholar] [CrossRef]
- Saha, B.; Goebel, K. Battery Data Set. In NASA Ames Prognostics Data Repository; NASA Ames Research Center: Mountain View, CA, USA, 2007. [Google Scholar]
- Adam, A.; Knobbe, E.; Wandt, J.; Kwade, A. Application of the differential charging voltage analysis to determine the onset of lithium-plating during fast charging of lithium-ion cells. J. Power Sources 2021, 495, 229794. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Ouyang, M.; Lu, L.; Li, J.; He, X. Using probability density function to evaluate the state of health of lithium-ion batteries. J. Power Sources 2013, 232, 209–218. [Google Scholar] [CrossRef]
- Meddings, N.; Heinrich, M.; Overney, F.; Lee, J.-S.; Ruiz, V.; Napolitano, E.; Seitz, S.; Hinds, G.; Raccichini, R.; Gaberšček, M. Application of electrochemical impedance spectroscopy to commercial Li-ion cells: A review. J. Power Sources 2020, 480, 228742. [Google Scholar] [CrossRef]
- Bruj, O.; Calborean, A. Electrochemical impedance spectroscopy investigation on the charge-discharge cycle life performance of lithium-ion batteries. Energies 2025, 18, 1324. [Google Scholar] [CrossRef]
- Khan, S.R.; Sharma, B.; Chawla, P.A.; Bhatia, R. Inductively coupled plasma optical emission spectrometry (ICP-OES): A powerful analytical technique for elemental analysis. Food Anal. Methods 2022, 15, 666–688. [Google Scholar] [CrossRef]
- Qin, W.-C.; Qiu, B.-J.; Xue, X.-Y.; Chen, C.; Xu, Z.-F.; Zhou, Q.-Q. Droplet deposition and control effect of insecticides sprayed with an unmanned aerial vehicle against plant hoppers. Crop Prot. 2016, 85, 79–88. [Google Scholar] [CrossRef]
- Limbeck, A.; Galler, P.; Bonta, M.; Bauer, G.; Nischkauer, W.; Vanhaecke, F. Recent advances in quantitative LA-ICP-MS analysis: Challenges and solutions in the life sciences and environmental chemistry. Anal. Bioanal. Chem. 2015, 407, 6593–6617. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, E.A.; Rizzoli, S.O. Novel secondary ion mass spectrometry methods for the examination of metabolic effects at the cellular and subcellular levels. Front. Behav. Neurosci. 2020, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Khulal, U.; Zhao, J.; Hu, W.; Chen, Q. Nondestructive quantifying total volatile basic nitrogen (TVB-N) content in chicken using hyperspectral imaging (HSI) technique combined with different data dimension reduction algorithms. Food Chem. 2016, 197, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Ferry, M.; Miserque, F. Radio-oxidation of electric cable models: Ageing evaluation at the atomic scale. Energies 2022, 15, 1631. [Google Scholar] [CrossRef]
- Yu, L.; Li, M.; Wen, J.; Amine, K.; Lu, J. (S)TEM-EELS as an advanced characterization technique for lithium-ion batteries. Mater. Chem. Front. 2021, 5, 5186–5193. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, W.; Peng, Y.; Chen, Q.; Ouyang, Q.; Zhao, J. Color compensation and comparison of shortwave near infrared and long wave near infrared spectroscopy for determination of soluble solids content of ‘Fuji’ apple. Postharvest Biol. Technol. 2016, 115, 81–90. [Google Scholar] [CrossRef]
- Samigullin, R.R.; Bobyleva, Z.V.; Zakharkin, M.V.; Zharikova, E.V.; Rozova, M.G.; Drozhzhin, O.A.; Antipov, E.V. On the thermal stability of selected electrode materials and electrolytes for Na-ion batteries. Energies 2024, 17, 3970. [Google Scholar] [CrossRef]
- Li, B.; Zhao, L.; Chen, H.; Sun, D.; Deng, B.; Li, J.; Liu, Y.; Wang, F. Inactivation of lipase and lipoxygenase of wheat germ with temperature-controlled short wave infrared radiation and its effect on storage stability and quality of wheat germ oil. PLoS ONE 2016, 11, e0167330. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Markovsky, B.; Rodkin, A.; Cojocaru, M.; Levi, E.; Kim, H.-J. An analysis of rechargeable lithium-ion batteries after prolonged cycling. Electrochim. Acta 2002, 47, 1899–1911. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Hu, X.; Shi, J. Study on the diffusion and optimization of sucrose in gaido seak based on finite element analysis and hyperspectral imaging technology. Foods 2024, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Xu, X.; Tang, Y. Recent progress of magnetic field application in lithium-based batteries. Nano Energy 2022, 92, 106703. [Google Scholar] [CrossRef]
- Kita, N.T.; Ushikubo, T.; Fu, B.; Valley, J.W. High precision SIMS oxygen isotope analysis and the effect of sample topography. Chem. Geol. 2009, 264, 43–57. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Yang, M.-Z.; She, F.-Q.; Gong, L.; Zhang, X.-Q.; Chen, J.; Song, S.-Q.; Xie, F.-Y. Application of X-ray photoelectron spectroscopy to study interfaces for solid-state lithium ion battery. Acta Phys. Sin. 2021, 70, 178801. [Google Scholar] [CrossRef]
- Patel, M.U.; Arčon, I.; Aquilanti, G.; Stievano, L.; Mali, G.; Dominko, R. X-ray absorption near-edge structure and nuclear magnetic resonance study of the lithium-sulfur battery and its components. ChemPhysChem 2014, 15, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.-J.; Kim, K.; Jeoun, Y.; Yu, S.-H.; Kim, C.; Sung, Y.-E.; Cairns, E.J. Understandings about functionalized porous carbon via scanning transmission x-ray microscopy (STXM) for high sulfur utilization in lithium-sulfur batteries. Nano Energy 2022, 100, 107446. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, Y.; Qin, R.; Mo, S.; Chen, G.; Gu, L.; Chevrier, D.M.; Zhang, P.; Guo, Q.; Zang, D. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 2016, 352, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Balan, A.; Das, P.M.; Thiruraman, J.P.; Drndić, M. Computer vision AC-STEM automated image analysis for 2D nanopore applications. Ultramicroscopy 2021, 231, 113249. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Kawatate, Y.; Funabiki, A.; Jeong, S.-K.; Abe, T.; Ogumi, Z. STM study on graphite/electrolyte interface in lithium-ion batteries: Solid electrolyte interface formation in trifluoropropylene carbonate solution. Electrochim. Acta 1999, 45, 99–105. [Google Scholar] [CrossRef]
- Leroy, S.; Blanchard, F.; Dedryvère, R.; Martinez, H.; Carré, B.; Lemordant, D.; Gonbeau, D. Surface film formation on a graphite electrode in Li-ion batteries: AFM and XPS study. Surf. Interface Anal. 2005, 37, 773–781. [Google Scholar] [CrossRef]
- Li, X.; Wu, C.; Fu, C.; Zheng, S.; Tian, J. State characterization of lithium-ion battery based on ultrasonic guided wave scanning. Energies 2022, 15, 6027. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Y.; Yang, J.; Li, R.; Liang, G.; Sun, X. Nature of LiFePO4 aging process: Roles of impurity phases. J. Power Sources 2013, 238, 454–463. [Google Scholar] [CrossRef]
- Kraft, V.; Grützke, M.; Weber, W.; Winter, M.; Nowak, S. Ion chromatography electrospray ionization mass spectrometry method development and investigation of lithium hexafluorophosphate-based organic electrolytes and their thermal decomposition products. J. Chromatogr. A 2014, 1354, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zinigrad, E.; Larush-Asraf, L.; Gnanaraj, J.S.; Gottlieb, H.E.; Sprecher, M.; Aurbach, D. Calorimetric studies of the thermal stability of electrolyte solutions based on alkyl carbonates and the effect of the contact with lithium. J. Power Sources 2005, 146, 176–179. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, B.; Yu, M.; Han, J.; Wang, Y.; Tan, Z.; Yan, Y. Simultaneous separation/enrichment and detection of trace ciprofloxacin and lomefloxacin in food samples using thermosensitive smart polymers aqueous two-phase flotation system combined with HPLC. Food Chem. 2016, 210, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gnanaraj, J.S.; Zinigrad, E.; Asraf, L.; Gottlieb, H.E.; Sprecher, M.; Schmidt, M.; Geissler, W.; Aurbach, D. A detailed investigation of the thermal reactions of LiPF6 solution in organic carbonates using ARC and DSC. J. Electrochem. Soc. 2003, 150, A1533. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Lu, S.; Yao, X.; Chen, C. Study on the kinetics properties of lithium hexafluorophosphate thermal decomposition reaction. Solid State Ion. 2006, 177, 137–140. [Google Scholar] [CrossRef]
- Borodin, O.; Ren, X.; Vatamanu, J.; von Wald Cresce, A.; Knap, J.; Xu, K. Modeling insight into battery electrolyte electrochemical stability and interfacial structure. Acc. Chem. Res. 2017, 50, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Grützke, M.; Kraft, V.; Weber, W.; Wendt, C.; Friesen, A.; Klamor, S.; Winter, M.; Nowak, S. Supercritical carbon dioxide extraction of lithium-ion battery electrolytes. J. Supercrit. Fluids 2014, 94, 216–222. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, L.; Yu, Y.; Sun, J. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 2019, 55, 93–114. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Hu, J.; Zhang, P.; Zhang, L.; Lao, L. Investigation on calendar experiment and failure mechanism of lithium-ion battery electrolyte leakage. J. Energy Storage 2022, 54, 105286. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Aguesse, F.; Manalastas, W.; Buannic, L.; Lopez del Amo, J.M.; Singh, G.; Llordés, A.; Kilner, J. Investigating the dendritic growth during full cell cycling of garnet electrolyte in direct contact with Li metal. ACS Appl. Mater. Interfaces 2017, 9, 3808–3816. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Albertus, P.; Newman, J. Two-dimensional modeling of lithium deposition during cell charging. J. Electrochem. Soc. 2009, 156, A390. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, R.; Wang, S.; Chen, X.; Zhao, C.; Kuzmina, E.; Karaseva, E.; Kolosnitsyn, V.; Zhang, Q. The dynamic evolution of aggregated lithium dendrites in lithium metal batteries. Chin. J. Chem. Eng. 2021, 37, 137–143. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Liu, Y.; Wang, L.; Ni, L.; Tang, X. Recyclable non-ligand dual cloud point extraction method for determination of lead in food samples. Food Chem. 2016, 190, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Li, J.; Brushett, F.R.; Bazant, M.Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 2016, 9, 3221–3229. [Google Scholar] [CrossRef]
- Jana, A.; García, R.E. Lithium dendrite growth mechanisms in liquid electrolytes. Nano Energy 2017, 41, 552–565. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Tatsuma, T.; Taguchi, M.; Oyama, N. Inhibition effect of covalently cross-linked gel electrolytes on lithium dendrite formation. Electrochim. Acta 2001, 46, 1201–1205. [Google Scholar] [CrossRef]
- Zheng, G.; Lee, S.W.; Liang, Z.; Lee, H.-W.; Yan, K.; Yao, H.; Wang, H.; Li, W.; Chu, S.; Cui, Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C.; Stevens, D.A.; Dahn, J.R. In-situ detection of lithium plating using high precision coulometry. J. Electrochem. Soc. 2015, 162, A959. [Google Scholar] [CrossRef]
- Downie, L.E.; Krause, L.J.; Burns, J.C.; Jensen, L.D.; Chevrier, V.L.; Dahn, J.R. In situ detection of lithium plating on graphite electrodes by electrochemical calorimetry. J. Electrochem. Soc. 2013, 160, A588. [Google Scholar] [CrossRef]
- Paul, P.P.; McShane, E.J.; Colclasure, A.M.; Balsara, N.; Brown, D.E.; Cao, C.; Chen, B.-R.; Chinnam, P.R.; Cui, Y.; Dufek, E.J. A review of existing and emerging methods for lithium detection and characterization in Li-ion and Li-metal batteries. Adv. Energy Mater. 2021, 11, 2100372. [Google Scholar] [CrossRef]
- Li, Y.; Yan, K.; Lee, H.-W.; Lu, Z.; Liu, N.; Cui, Y. Growth of conformal graphene cages on micrometre-sized silicon particles as stable battery anodes. Nat. Energy 2016, 1, 15029. [Google Scholar] [CrossRef]
- Andersen, H.L.; Djuandhi, L.; Mittal, U.; Sharma, N. Strategies for the analysis of graphite electrode function. Adv. Energy Mater. 2021, 11, 2102693. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Riahi, A.R.; Alpas, A.T. A transmission electron microscopy study of crack formation and propagation in electrochemically cycled graphite electrode in lithium-ion cells. J. Power Sources 2011, 196, 8719–8727. [Google Scholar] [CrossRef]
- Gavilán-Arriazu, E.M.; Pinto, O.A.; de Mishima, B.A.L.; Barraco, D.E.; Oviedo, O.A.; Leiva, E.P.M. The kinetic origin of the Daumas-Hérold model for the Li-ion/graphite intercalation system. Electrochem. Commun. 2018, 93, 133–137. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Wade, A.; Tan, C.; Parker, J.E.; Matras, D.; Leach, A.S.; Robinson, J.B.; Llewellyn, A.; Dimitrijevic, A.; Jervis, R. Identifying the origins of microstructural defects such as cracking within Ni-rich NMC811 cathode particles for lithium-ion batteries. Adv. Energy Mater. 2020, 10, 2002655. [Google Scholar] [CrossRef]
- Lee, Y.K. Effect of transition metal ions on solid electrolyte interphase layer on the graphite electrode in lithium ion battery. J. Power Sources 2021, 484, 229270. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Pan, X.; Wang, X.; Zhao, X.; Gao, Y.; Yang, S.; Zhai, C. Model for detecting boom height based on an ultrasonic sensor for the whole growth cycle of wheat. Agriculture 2023, 14, 21. [Google Scholar] [CrossRef]
- Cui, H.-Y.; Wu, J.; Lin, L. Inhibitory effect of liposome-entrapped lemongrass oil on the growth of Listeria monocytogenes in cheese. J. Dairy Sci. 2016, 99, 6097–6104. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Markovsky, B.; Salitra, G.; Markevich, E.; Talyossef, Y.; Koltypin, M.; Nazar, L.; Ellis, B.; Kovacheva, D. Review on electrode-electrolyte solution interactions, related to cathode materials for Li-ion batteries. J. Power Sources 2007, 165, 491–499. [Google Scholar] [CrossRef]
- Sahore, R.; O’Hanlon, D.C.; Tornheim, A.; Lee, C.-W.; Garcia, J.C.; Iddir, H.; Balasubramanian, M.; Bloom, I. Revisiting the mechanism behind transition-metal dissolution from delithiated LiNixMnyCozO2 (NMC) cathodes. J. Electrochem. Soc. 2020, 167, 020513. [Google Scholar] [CrossRef]
- Chen, H.; Ma, T.; Zeng, Y.; Guo, X.; Qiu, X. Mechanism of capacity fading caused by Mn(II) deposition on anodes for spinel lithium manganese oxide cell. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2017, 32, 1–10. [Google Scholar] [CrossRef]
- Zhan, C.; Lu, J.; Kropf, A.J.; Wu, T.; Jansen, A.N.; Sun, Y.-K.; Qiu, X.; Amine, K. Mn(II) deposition on anodes and its effects on capacity fade in spinel lithium manganate-carbon systems. Nat. Commun. 2013, 4, 2437. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Shkrob, I.A.; Abraham, D.P. Transition metal dissolution, ion migration, electrocatalytic reduction and capacity loss in lithium-ion full cells. J. Electrochem. Soc. 2017, 164, A389. [Google Scholar] [CrossRef]
- Gowda, S.R.; Gallagher, K.G.; Croy, J.R.; Bettge, M.; Thackeray, M.M.; Balasubramanian, M. Oxidation state of cross-over manganese species on the graphite electrode of lithium-ion cells. Phys. Chem. Chem. Phys. 2014, 16, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- Shkrob, I.A.; Kropf, A.J.; Marin, T.W.; Li, Y.; Poluektov, O.G.; Niklas, J.; Abraham, D.P. Manganese in graphite anode and capacity fade in Li ion batteries. J. Phys. Chem. C 2014, 118, 24335–24348. [Google Scholar] [CrossRef]
- Terada, Y.; Nishiwaki, Y.; Nakai, I.; Nishikawa, F. Study of Mn dissolution from LiMn2O4 spinel electrodes using in situ total reflection X-ray fluorescence analysis and fluorescence XAFS technique. J. Power Sources 2001, 97, 420–422. [Google Scholar] [CrossRef]
- Banks, C.E. Recent development of LiNixCoyMnzO2: Impact of micro/nano structures for imparting improvements in lithium batteries. Trans. Nonferrous Met. Soc. China 2013, 23, 108–119. [Google Scholar]
- Shao-Horn, Y.; Croguennec, L.; Delmas, C.; Nelson, E.C.; O’Keefe, M.A. Atomic resolution of lithium ions in LiCoO2. Nat. Mater. 2003, 2, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Hojo, T.; Nishio, T.; Sadamura, H.; Oyama, N.; Moriyoshi, C.; Kuroiwa, Y. MEM charge density study of olivine LiMPO4 and MPO4 (M = Mn, Fe) as cathode materials for lithium-ion batteries. J. Phys. Chem. C 2013, 117, 2608–2615. [Google Scholar] [CrossRef]
- Kim, J.C.; Moore, C.J.; Kang, B.; Hautier, G.; Jain, A.; Ceder, G. Synthesis and electrochemical properties of monoclinic LiMnBO3 as a Li intercalation material. J. Electrochem. Soc. 2011, 158, A309. [Google Scholar] [CrossRef]
- Key, B.; Bhattacharyya, R.; Morcrette, M.; Seznec, V.; Tarascon, J.-M.; Grey, C.P. Real-time NMR investigations of structural changes in silicon electrodes for lithium-ion batteries. J. Am. Chem. Soc. 2009, 131, 9239–9249. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Yagi, I. In situ surface-enhanced Raman scattering spectroscopic study of pyridine adsorbed on gold electrode surfaces comprised of plasmonic crystal structures. J. Phys. Chem. C 2008, 112, 17603–17610. [Google Scholar] [CrossRef]
- Ren, D.; Xie, L.; Wang, L.; He, X. A practical approach to predict volume deformation of lithium-ion batteries from crystal structure changes of electrode materials. Int. J. Energy Res. 2021, 45, 7732–7740. [Google Scholar] [CrossRef]
- Xu, B.; Fell, C.R.; Chi, M.; Meng, Y.S. Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: A joint experimental and theoretical study. Energy Environ. Sci. 2011, 4, 2223–2233. [Google Scholar] [CrossRef]
- Zhou, Y.-N.; Yue, J.-L.; Hu, E.; Li, H.; Gu, L.; Nam, K.-W.; Bak, S.-M.; Yu, X.; Liu, J.; Bai, J. High-rate charging induced intermediate phases and structural changes of layer-structured cathode for lithium-ion batteries. Adv. Energy Mater. 2016, 6, 1600597. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y. Suppressing the phase transition of the layered Ni-rich oxide cathode during high-voltage cycling by introducing low-content Li2MnO3. ACS Appl. Mater. Interfaces 2016, 8, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, Y.; Shi, J.; Zhang, W.; Zhang, X.; Hang, X.; Li, Z.; Zou, X. Convenient self-assembled PDADMAC/PSS/Au@Ag NRs filter paper for swift SERS evaluation of non-systemic pesticides on fruit and vegetable surfaces. Food Chem. 2023, 424, 136232. [Google Scholar] [CrossRef] [PubMed]
- Boesenberg, U.; Meirer, F.; Liu, Y.; Shukla, A.K.; Dell’Anna, R.; Tyliszczak, T.; Chen, G.; Andrews, J.C.; Richardson, T.J.; Kostecki, R. Mesoscale phase distribution in single particles of LiFePO4 following lithium deintercalation. Chem. Mater. 2013, 25, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Kitao, H.; Fujihara, T.; Takeda, K.; Nakanishi, N.; Nohma, T. High-temperature storage performance of Li-ion batteries using a mixture of Li-Mn spinel and Li-Ni-Co-Mn oxide as a positive electrode material. Electrochem. Solid-State Lett. 2004, 8, A87. [Google Scholar] [CrossRef]
- Monnier, J.; Chen, H.; Joiret, S.; Bourgon, J.; Latroche, M. Identification of a new pseudo-binary hydroxide during calendar corrosion of (La,Mg)2Ni7-type hydrogen storage alloys for Nickel-Metal Hydride batteries. J. Power Sources 2014, 266, 162–169. [Google Scholar] [CrossRef]
- Tahir, H.E.; Zou, X.; Huang, X.; Shi, J.; Mariod, A.A. Discrimination of honeys using colorimetric sensor arrays, sensory analysis and gas chromatography techniques. Food Chem. 2016, 206, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zheng, J.; Zhang, H.; Jin, L.; Yang, D.; Lv, H.; Shen, C.; Shellikeri, A.; Zheng, Y.; Gong, R. Electrode materials, electrolytes, and challenges in nonaqueous lithium-ion capacitors. Adv. Mater. 2018, 30, 1705670. [Google Scholar] [CrossRef] [PubMed]
- Sayers, C.N.; Armstrong, N.R. X-ray photoelectron spectroscopy of TiO2 and other titanate electrodes and various standard titanium oxide materials: Surface compositional changes of the TiO2 electrode during photoelectrolysis. Surf. Sci. 1978, 77, 301–320. [Google Scholar] [CrossRef]
- Higgins, T.M.; Park, S.-H.; King, P.J.; Zhang, C.; McEvoy, N.; Berner, N.C.; Daly, D.; Shmeliov, A.; Khan, U.; Duesberg, G. A commercial conducting polymer as both binder and conductive additive for silicon nanoparticle-based lithium-ion battery negative electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.O.; El Kazzi, M.; Jämstorp Berg, E.; Pérez Villar, S.; Novák, P.; Villevieille, C. Understanding the interaction of the carbonates and binder in Na-ion batteries: A combined bulk and surface study. Chem. Mater. 2015, 27, 1210–1216. [Google Scholar] [CrossRef]
- Younesi, R.; Hahlin, M.; Treskow, M.; Scheers, J.; Johansson, P.; Edström, K. Ether based electrolyte, LiB(CN)4 salt and binder degradation in the Li-O2 battery studied by hard X-ray photoelectron spectroscopy (HAXPES). J. Phys. Chem. C 2012, 116, 18597–18604. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Evaluation on a water-based binder for the graphite anode of Li-ion batteries. J. Power Sources 2004, 138, 226–231. [Google Scholar] [CrossRef]
- Cui, H.-Y.; Ma, C.; Lin, L. Synergistic antibacterial efficacy of cold nitrogen plasma and clove oil against Escherichia coli O157:H7 biofilms on lettuce. Food Control 2016, 66, 8–16. [Google Scholar] [CrossRef]
- Chou, S.-L.; Pan, Y.; Wang, J.-Z.; Liu, H.-K.; Dou, S.-X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-L.; Wang, J.-Z.; Wexler, D.; Konstantinov, K.; Zhong, C.; Liu, H.-K.; Dou, S.-X. High-surface-area α-Fe2O3/carbon nanocomposite: One-step synthesis and its highly reversible and enhanced high-rate lithium storage properties. J. Mater. Chem. 2010, 20, 2092–2098. [Google Scholar] [CrossRef][Green Version]
- Nasybulin, E.; Xu, W.; Engelhard, M.H.; Nie, Z.; Li, X.S.; Zhang, J.-G. Stability of polymer binders in Li-O2 batteries. J. Power Sources 2013, 243, 899–907. [Google Scholar] [CrossRef]
- Yoon, E.; Lee, J.; Byun, S.; Kim, D.; Yoon, T. Passivation failure of Al current collector in LiPF6-based electrolytes for lithium-ion batteries. Adv. Funct. Mater. 2022, 32, 2200026. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A review of current collectors for lithium-ion batteries. J. Power Sources 2021, 485, 229321. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, H.; Li, C.; Han, J.; Tan, Z.; Yan, Y. Separation, concentration and determination of trace chloramphenicol in shrimp from different waters by using polyoxyethylene lauryl ether-salt aqueous two-phase system coupled with high-performance liquid chromatography. Food Chem. 2016, 192, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.-T.; Sasaki, Y.; Sakurada, S.; Sun, Y.-K.; Yashiro, H. Electrochemical behavior of current collectors for lithium batteries in non-aqueous alkyl carbonate solution and surface analysis by ToF-SIMS. Electrochim. Acta 2009, 55, 288–297. [Google Scholar] [CrossRef]
- Li, D.; Hu, H.; Chen, B.; Lai, W.-Y. Advanced current collector materials for high-performance lithium metal anodes. Small 2022, 18, 2200010. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, V.A.; Hardwick, L.J.; Srinivasan, V.; Kostecki, R. Surface structural disordering in graphite upon lithium intercalation/deintercalation. J. Power Sources 2010, 195, 3655–3660. [Google Scholar] [CrossRef]
- Jarry, A.; Gottis, S.; Yu, Y.-S.; Roque-Rosell, J.; Kim, C.; Cabana, J.; Kerr, J.; Kostecki, R. The formation mechanism of fluorescent metal complexes at the LiXNi0.5Mn1.5O4-δ/carbonate ester electrolyte interface. J. Am. Chem. Soc. 2015, 137, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sun, Q.; Liu, G.; Song, X.; Battaglia, V.S. Correlation between dissolution behavior and electrochemical cycling performance for LiNi1/3Co1/3Mn1/3O2-based cells. J. Power Sources 2012, 207, 134–140. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Wang, H. A new method for fast state of charge estimation using retired battery parameters. J. Energy Storage 2022, 55, 105621. [Google Scholar] [CrossRef]
- Yayan, U.; Arslan, A.T.; Yücel, H. A novel method for SoH prediction of batteries based on stacked LSTM with quick charge data. Appl. Artif. Intell. 2021, 35, 421–439. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, B.J. Online capacity estimation of lithium-ion batteries based on novel feature extraction and adaptive multi-kernel relevance vector machine. Energies 2015, 8, 12439–12457. [Google Scholar] [CrossRef]
- Klass, V.; Behm, M.; Lindbergh, G. A support vector machine-based state-of-health estimation method for lithium-ion batteries under electric vehicle operation. J. Power Sources 2014, 270, 262–272. [Google Scholar] [CrossRef]
- Pan, H.; Lü, Z.; Wang, H.; Wei, H.; Chen, L. Novel battery state-of-health online estimation method using multiple health indicators and an extreme learning machine. Energy 2018, 160, 466–477. [Google Scholar] [CrossRef]
- Tagade, P.; Hariharan, K.S.; Ramachandran, S.; Khandelwal, A.; Naha, A.; Kolake, S.M.; Han, S.H. Deep Gaussian process regression for lithium-ion battery health prognosis and degradation mode diagnosis. J. Power Sources 2020, 445, 227281. [Google Scholar] [CrossRef]
- Zhang, H.; Apaliya, M.T.; Mahunu, G.K.; Chen, L.; Li, W. Control of ochratoxin A-producing fungi in grape berry by microbial antagonists: A review. Trends Food Sci. Technol. 2016, 51, 88–97. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Wang, T.; Zhang, C.; Feng, T.; Tian, C. Three-dimensional interventional photoacoustic imaging for biopsy needle guidance with a linear array transducer. J. Biophotonics 2019, 12, e201900212. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, K.; Mao, L.; He, Q.; Wu, Q.; Li, Z. Evaluation of lithium-ion battery pack capacity consistency using one-dimensional magnetic field scanning. IEEE Trans. Instrum. Meas. 2022, 71, 1–10. [Google Scholar] [CrossRef]
- Xu, B.-G.; Zhang, M.; Bhandari, B.; Sun, J.; Gao, Z. Infusion of CO2 in a solid food: A novel method to enhance the low-frequency ultrasound effect on immersion freezing process. Innov. Food Sci. Emerg. Technol. 2016, 35, 194–203. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, L.; Guo, Y.; Zhao, Y.; Betchem, G.; Yolandani, Y.; Ma, H. Enhancing submerged fermentation of Antrodia camphorata by low-frequency alternating magnetic field. Innov. Food Sci. Emerg. Technol. 2023, 86, 103382. [Google Scholar] [CrossRef]
- Waleed, A.; Fadhl, J.A.; Abdullah, A.B.; Al-Adeeb, A.; Mahdi, A.A.; Al-Maqtari, Q.A.; Mushtaq, B.S.; Fan, M.; Li, Y.; Qian, H. Effect of highland barley germination on thermomechanical, rheological, and microstructural properties of wheat-oat composite flour dough. Food Biosci. 2023, 53, 102521. [Google Scholar]
- Liu, S.; Rong, Y.; Chen, Q.; Ouyang, Q. Colorimetric sensor array combined with chemometric methods for the assessment of aroma produced during the drying of tencha. Food Chem. 2024, 432, 137190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.; Zhou, C.; Mustapha, A.T.; Wahia, H. Advances in peeling techniques for tomato: A comprehensive review. Food Rev. Int. 2024, 40, 212–229. [Google Scholar] [CrossRef]
- Hao, M.; Li, Z.; Huang, X.; Wang, Y.; Wei, X.; Zou, X.; Shi, J.; Huang, Z.; Yin, L.; Gao, L. A cell-based electrochemical taste sensor for detection of hydroxy-α-sanshool. Food Chem. 2023, 418, 135941. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mense, A.L.; Brewer, L.R.; Shi, Y.-C. Wheat bran layers: Composition, structure, fractionation, and potential uses in foods. Crit. Rev. Food Sci. Nutr. 2024, 64, 6636–6659. [Google Scholar] [CrossRef] [PubMed]
- Qayum, A.; Rashid, A.; Liang, Q.; Wu, Y.; Cheng, Y.; Kang, L.; Liu, Y.; Zhou, C.; Hussain, M.; Ren, X. Ultrasonic and homogenization: An overview of the preparation of an edible protein-polysaccharide complex emulsion. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4242–4281. [Google Scholar] [CrossRef] [PubMed]
- Obadi, M.; Li, Y.; Xu, B. Recent advances in extending the shelf life of fresh wet noodles: Influencing factors and preservation technologies. J. Food Sci. 2023, 88, 3626–3648. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Hu, X.; Zhang, X.; Li, W.; Guo, Z.; Huang, X.; Li, Z.; Zhang, R.; Shen, T.; Zou, X. Ratiometric sensing for ultratrace tetracycline using electrochemically active metal-organic frameworks as response signals. J. Agric. Food Chem. 2023, 71, 7584–7592. [Google Scholar] [CrossRef] [PubMed]
- Haruna, S.A.; Ivane, N.M.A.; Adade, S.Y.-S.S.; Luo, X.; Geng, W.; Zareef, M.; Jargbah, J.; Li, H.; Chen, Q. Rapid and simultaneous quantification of phenolic compounds in peanut (Arachis hypogaea L.) seeds using NIR spectroscopy coupled with multivariate calibration. J. Food Compos. Anal. 2023, 123, 105516. [Google Scholar] [CrossRef]
- Han, E.; Li, L.; Gao, T.; Pan, Y.; Cai, J. Nitrite determination in food using electrochemical sensor based on self-assembled MWCNTs/AuNPs/poly-melamine nanocomposite. Food Chem. 2024, 437, 137773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Cheng, Y. Mechanical properties, microstructure, and in vitro digestion of transglutaminase-crosslinked whey protein and potato protein hydrolysate composite gels. Foods 2023, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Sun, Z.; Wang, D.; Liu, F.; Lin, L. In vitro and in situ characterization of psychrotrophic spoilage bacteria recovered from chilled chicken. Foods 2022, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, X.; Guo, Y.; Chen, Z.; Ma, H. Evaluation of ultrasonic-assisted pickling with different frequencies on NaCl transport, impedance properties, and microstructure in pork. Food Chem. 2024, 430, 137003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shan, P.; Zhang, Z.-H.; He, R.; Xing, L.; Liu, J.; He, D.; Ma, H.; Wang, Z.; Gao, X. Efficient degradation of soybean protein B3 subunit in soy sauce by ultrasound-assisted prolyl endopeptidase and its primary mechanism. Food Chem. 2023, 429, 136972. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Chen, X.; Lu, D.; Chen, B. Discrimination of different edible vegetable oils based on GC-IMS and SIMCA. CyTA-J. Food 2023, 21, 49–56. [Google Scholar] [CrossRef]
- Tao, R.; Gu, Y.; Du, Z.; Lyu, X.; Li, J. Advanced electrode processing for lithium-ion battery manufacturing. Nat. Rev. Clean Technol. 2025, 1, 116–131. [Google Scholar] [CrossRef]
- Muck, N.; David, C.; Knöri, T. Integrating fiber sensing for spatially resolved temperature measurement in fuel cells. Energies 2024, 17, 16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).