1. Introduction
The development of alternative energy sources is crucial to realizing the goals of the Paris Agreement. Renewable energy sources such as photovoltaics and windmills generate fluctuating energy, requiring storage to prevent waste. Surplus energy could be used for hydrogen production, which can power fuel cells, boilers, and combustion engines [
1]. Such a solution could significantly reduce greenhouse gas emissions. The United Nations Industrial Development Organization (UNIDO) highlights hydrogen’s potential in achieving climate neutrality [
2]. Consequently, the hydrogen market is expected to grow and become a key energy carrier in the European Union [
3,
4].
However, an essential aspect related to the use of hydrogen technologies alongside hydrogen production is its storage. Currently, hydrogen can be stored in tanks, using physical adsorption or chemical methods such as hydrogen bonding to metallic hydride or ammonia. Ammonia is considered a potential hydrogen carrier in many documents and strategies, for example, in the Hydrogen Strategy for a Climate-Neutral Europe in 2020 [
5]. Therefore, a straightforward and effective technique for ammonia decomposition must be developed to release H
2 stored in it. The two main methods for decomposing ammonia are thermal and catalytic [
6,
7,
8,
9,
10,
11,
12]. Other methods for NH
3 decomposition are plasma (homogeneous) and plasma–catalytic processes [
13,
14,
15,
16,
17]. The disadvantage of processes using plasma is high energy consumption, mainly due to the power supply systems’ low efficiency. It is mainly caused either by the use of generators based on vacuum-tube technology, applying a simplistic approach by using the available mains with transformers, or poor availability of reactors and power systems, which can operate at frequencies above 10 kHz at atmospheric pressure [
18]. Furthermore, systems operated at high frequency are most often RF electrodeless discharges or Dielectric Barrier Discharge (DBD) reactors with low-pressure systems in which it is difficult to achieve high productivity [
19].
Publications discussing the influence of plasma generator frequency typically concentrate on barrier discharges. For example, research about the influence of different frequencies by Chau Xu et al. reported a slight influence of DBD discharge frequencies in the range of 20–50 kHz on the methane conversion process [
20]. Lotfalipour et al. have applied the nanosecond pulsed plasma DBD with a frequency of 8–22 kHz. The authors observed an increase in methane conversion with the increase of frequency [
21].
In the case of ammonia decomposition, Andersen et al. conducted the research on dielectric barrier discharge in the frequency range of 1–4 kHz. It was found that throughout the residence time, a higher frequency results in more micro-discharges. Therefore, a larger number of molecules can potentially interact with the micro-discharges and dissociate [
22].
Several papers are available about the effect of frequency on gliding discharge plasma processes. Młotek et al. investigated the influence of two different low frequencies: 23 Hz and 50 Hz, on the toluene conversion. It was discovered that frequency significantly influences process results [
23]. Higher frequencies in the range of 10–30 kHz were tested in the CH
4-CO
2 reforming process. Frequencies between 10 and 20 kHz significantly improved conversion efficiency and enhanced the stability and continuity of the gliding arc, resulting in increased energy density and overall reaction performance. However, at frequencies above 20 kHz, a decline in conversion efficiency was observed, likely due to a reduction in the discharge volume, despite the increased energy density in the arc region [
24].
Nevertheless, there are not many papers reporting on the influence of the driving frequency of electric discharge plasma on the ammonia decomposition, which differs by 2–4 orders of magnitude in the same reaction system. This kind of system was studied in H
2S decomposition [
25]. The frequency of the applied electric field is a critical parameter that influences the key plasma characteristics, including electric field distribution, electron energies, and electron density. The high-frequency field can induce electron velocities that significantly exceed their thermal velocity, with the distance traveled by an electron in one period often far surpassing the Debye radius [
26]. Moreover, Sretenović et al. observed that high-frequency electric fields have a stabilizing effect on plasma. This stabilization is attributed to the rapid oscillations induced by higher-frequency fields, which mimic the effects of thermal motion and effectively constrain the conditions required for instabilities to arise [
27].
This paper investigates how the ammonia decomposition process in the plasma and plasma–catalytic systems is affected when the driving frequency of a gliding discharge is changed from 50 Hz up to 20 kHz using the same plasma reactor. The study’s innovation is the application of two extreme frequencies—50 Hz and 20 kHz, which differ by 4 orders of magnitude—in the same reaction system and an analysis of the impact of these power supply systems on the ammonia decomposition process in a plasma–catalytic system.
2. Experimental
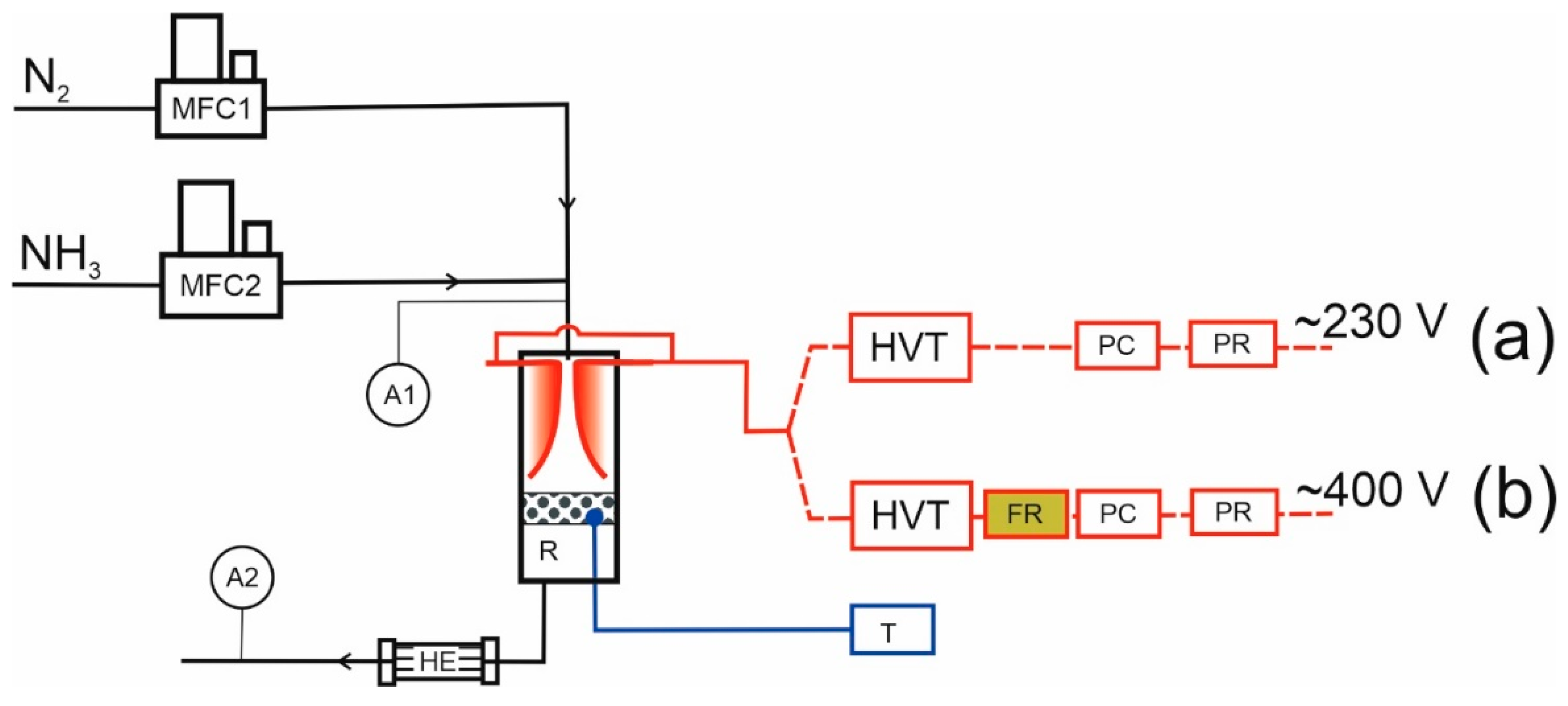
This study was conducted at high ammonia concentrations (50–100%). The tests were carried out using a Warsaw University of Technology (WUT) (Warsaw, Poland) thyristor power supply of 50 Hz AC and 4–6 kV obtained with a Fart Resinblock 2000 high-voltage transformer and TruPlasma Bipolar 4020 G2.1 HV power supply (hereinafter referred to as TH Bipolar HV), delivered by TRUMPF Huettinger sp. z o. o., Zielonka, Poland. The discharge power of the WUT power supply was measured with an energymeter, Orno OR-WE-512 (Zakręt, Poland).
Table 1 depicts the most important parameters of both power supplies. It should be noted that the TH Bipolar HV could achieve higher operating power; nonetheless, due to electrode heating caused by excessive power, the maximum discharge power was limited to 600 W.
Nitrogen (99,999%) and ammonia (99.85%) gases were supplied by Multax (Babice, Poland). Gas streams were regulated by Bronkhorst mass flow controllers (Ruurlo, The Netherlands). The gas streams were mixed before entering the reactor, and the total gas flow rate was 180 Nl/h. The tests were conducted in the range of ammonia concentrations of 50–100% by volume. The reactor volume was about 0.4 dm3, the catalyst bed volume was about 30 cm3, and it was located directly below the electrodes, approximately 7 mm from their end. Gas samples for chromatographic analysis were taken downstream of the reactor after the gas was cooled. The gas was analyzed by chromatography using a Chrompack CP 9002 chromatograph (Delft, Netherlands) with a TCD detector and a ThermoScientific (Waltham, MA, USA) Trace 1600 with a TCD detector and Rtx-Volatile Amine column (Bellefonte, PA, USA). The gas temperature was measured with a thermocouple K inside the catalyst bed. The temperature was in the range of 160–400 °C.
The apparatus scheme is shown in
Figure 1.
2.1. The Progress of the Ammonia Decomposition Process Was Calculated Using the Formulas and Definitions Provided Below
—outlet hydrogen flow rate, Nl/h
—inlet ammonia flow rate, Nl/h
—outlet ammonia flow rate, Nl/h
—discharge power, W
2.2. Catalysts Preparation
The Co/γ-Al2O3 catalyst was prepared using the dry impregnation method using cobalt nitrate solution. Subsequently, the catalyst was dried at 90 °C for 18 h. After drying, it was calcined at 500 °C for 5 h in an air atmosphere and reduced in hydrogen at 400 °C for 24 h.
The Co/Ce/Ba catalyst was prepared using the coprecipitation method with potassium carbonate as the precipitant. Appropriate amounts of a mixture of Co(NO
3)
2·6H
2O and Ce(NO
3)
3·6H
2O were dissolved in distilled water and warmed to approximately 90 °C. Then, a warm (90 °C) solution of K
2CO
3 was slowly added. The obtained precipitate, i.e., mixtures of cobalt carbonate and cerium carbonate, was filtered and washed until the pH was ∼7. The materials were then dried and calcined at 500 °C overnight. The barium promoter was added by the dry impregnation method. After impregnation, the catalyst was dried and calcinated at 450 °C. Similar catalysts were prepared by Raróg-Pilecka [
28,
29]. The specific surface area of catalysts is given in
Table 2.
3. Results
3.1. Electrical Measurements
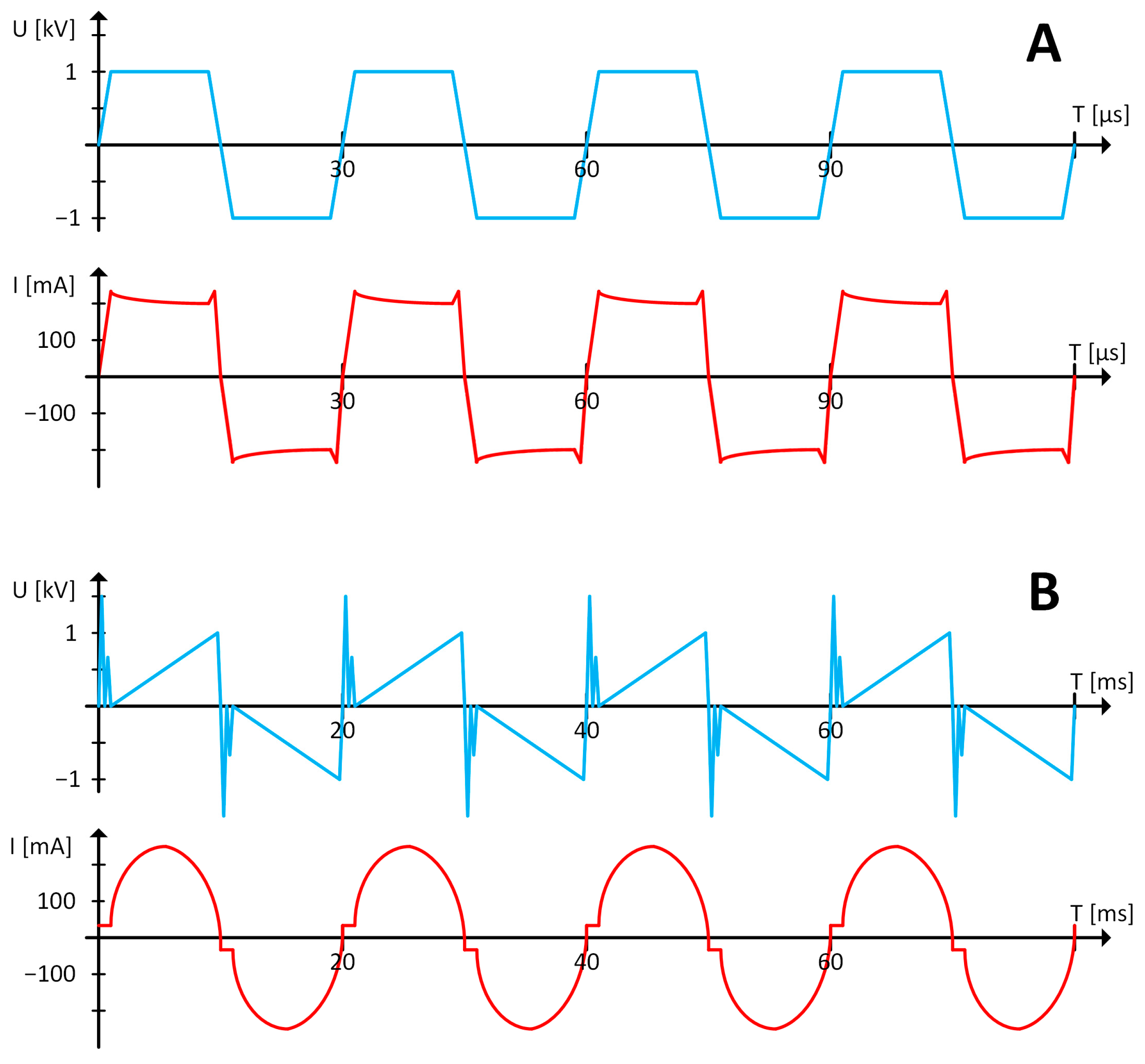
Figure 2 presents the current and voltage waveforms collected from two types of power supplies. The operation of voltage in the TH Bipolar HV power supply and a thyristor power supply is comparable; both waveforms are rectangular, and their shape is based on the discharge column. The high-frequency operation and constant energy supply to the discharge column in the TH Bipolar HV power supply (
Figure 2A) prevent it from going out. On the other hand, in a thyristor power supply, the arc is extinguished when the current flowing to the discharge column crosses zero every half-cycle.
Consequently, a gliding discharge in a thyristor power supply can last only 20 ms, corresponding to the main’s frequency. Due to the discharge being quenched by the absence of current flow every 20 ms (
Figure 2B), the gliding discharge powered by the thyristor power supply has a reduced duration time. There is no possibility of maintaining the discharge column longer, regardless of the voltage and current applied; the discharge column will extinguish due to a change in the polarity of the applied voltage. In contrast, due to the control technique, the transition in the TH Bipolar HV power supply is not longer than 100–200 ns; consequently, the discharge column is not quenched by altering the current’s polarization. The highest voltage that can be supplied by the TH Bipolar HV power supply or the critical arc length (
, limit the gliding discharge’s duration and the path it takes on the electrodes [
30].
3.2. Ammonia Decomposition Measurements
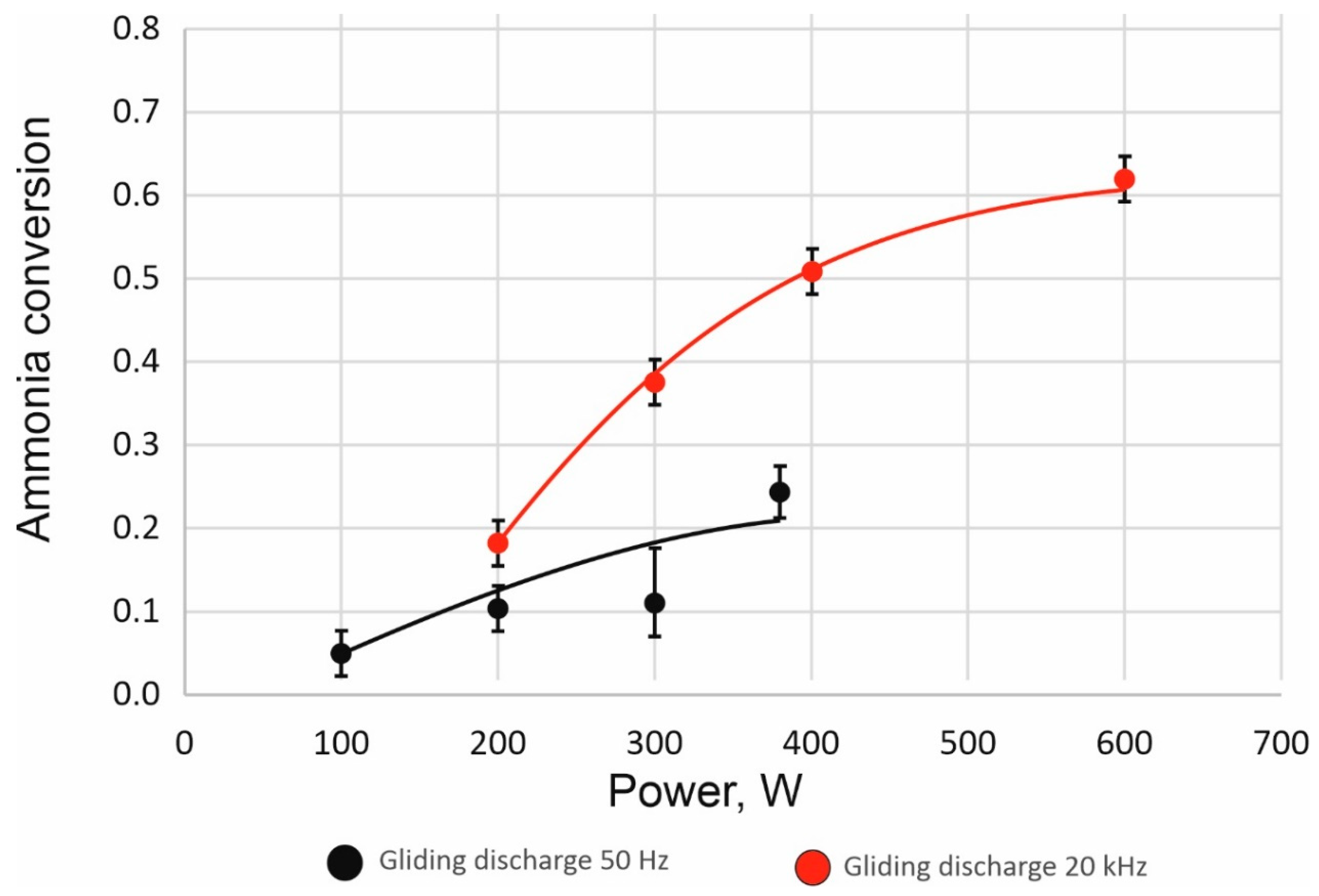
The ammonia conversion increases with the rise of the discharge power. In addition, application of the high-frequency plasma generator results in higher values of ammonia conversion throughout the entire range of applied power (
Figure 3). With discharge power close to 400 W, with a thyristor power supply and TH Bipolar HV power supply, ammonia conversion was 0.21 and 0.5, respectively. Due to the limitation of the thyristor power supply, it was not possible to achieve higher discharge power than 400 W. The highest ammonia conversion was approx. 0.6 for the Co/Ce/Ba coprecipitated catalyst at a discharge power of 600 W and a frequency of 20 kHz with the TH Bipolar HV power supply.
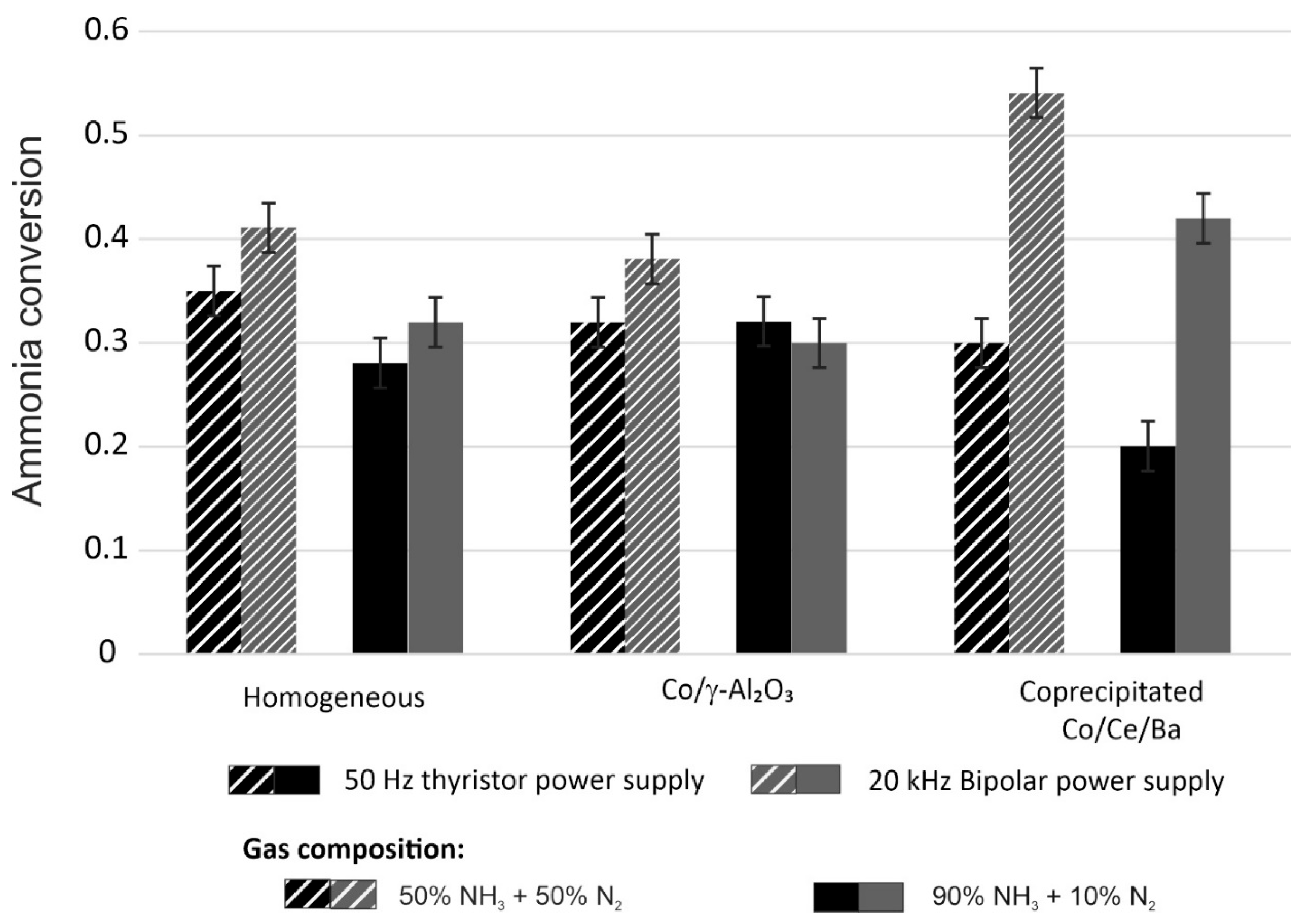
The influence of the power supply in both the plasma and plasma–catalytic systems on the conversion of ammonia was investigated. With the high-frequency power source, ammonia conversion increased in both homogeneous and plasma–catalytic (GD+coprecipitated) systems. As depicted in
Figure 4, the change in the power supply system had a significant impact on the ammonia decomposition conducted in a plasma–catalytic system with coprecipitated catalyst. The use of a high-frequency power supply and gas containing 50% NH
3 resulted in an increase in ammonia conversion by 24 percentage points.
The homogeneous system shows relatively lower ammonia conversion efficiency compared to the plasma–catalytic systems. However, its performance improves with the use of the 20 kHz TH Bipolar HV power supply. The supported Co/γ-Al2O3 catalyst demonstrates moderate activity: higher conversion has been measured at 20 kHz and the 50% NH3 + 50% N2 gas mixture, whereas the use of higher ammonia concentration (90% NH3 + 10% N2) slightly reduces its efficiency. The coprecipitated Co/Ce/Ba catalyst achieves the highest conversion, particularly under the 20 kHz TH Bipolar HV power supply and in the 50% NH3 + 50% N2 gas composition, reaching a maximum conversion of approximately 0.55. These results confirm that catalytic performance is improved with increased frequency at lower ammonia concentrations, with the coprecipitated system being the most responsive to these factors.
It is highlighted that the homogeneous system can match or slightly outperform plasma–catalytic systems under specific low-frequency conditions. Moreover, the results show greater potential when high-frequency power is applied under adjusted gas compositions.
As shown above, using a 20 kHz TH Bipolar HV in both systems, homogeneous and plasma-catalytic, results in a decrease in energy consumption. In the case of the coprecipitated catalyst, this change is 125 kJ/molH
2. The positive influence of using higher frequency discharge is observed in a higher hydrogen molar fraction obtained in both systems, as summarized in
Table 3.
The relationship between the molar fraction of hydrogen and specific energy is presented in
Figure 5. The curves have an increasing character, and the use of TH Bipolar HV results in higher hydrogen concentration in the post-reaction gas throughout the entire range of applied energies.
4. Discussion
There is limited literature on the impact of different frequencies on chemical processes occurring in gliding discharge. Studies on H2S have shown that lower frequencies are more favorable. This may be due to the more than twofold lower enthalpy of H2S formation (−20.15 kJ/mol) compared to NH3 (−45.65 kJ/mol).
In the case of H2S, lower frequencies resulted in fewer high-energy electrons while still providing sufficient energy to decompose hydrogen sulfide. Higher energy inputs lead to the formation of greater amounts of H radicals, which readily react with HS radicals, regenerating hydrogen sulfide. For ammonia, however, breaking the N-H bonds requires more energy; thus, the energy supplied at lower frequencies may be insufficient for effective decomposition.
High-frequency plasma tends to have higher electron energies due to the rapid acceleration provided by the oscillating electric field. Oscillations in the electron density and temperature are influenced by discharge frequency, affecting vibrational excitation efficiency [
31]. This results in electrons with sufficient energy to initiate and sustain various chemical reactions.
The high-frequency plasma (20 kHz) applied in this study enhances the excitation of nitrogen molecules to higher vibrational states, which is essential for improving the efficiency of chemical processes, such as ammonia synthesis. Vibrationally excited states of N
2 molecules reduce the dissociation barrier, without influencing the subsequent hydrogenation reactions and ammonia desorption [
32,
33]. This enhancement is attributed to the interaction of electrons with the oscillating electric field, which provides more energy to the electrons.
As a result, electrons collide more frequently with nitrogen molecules, increasing the population of vibrationally excited N2. The rate of vibrational excitation of N2 is about 106–107 times larger than the rate of electronic excitation in atmospheric pressure plasmas.
Higher frequencies impart more energy to electrons in shorter bursts, leading to higher overall electron temperatures and densities. These conditions reduce the time that electrons spend in a low-energy state, decreasing the possibility of recombination with ions and therefore maintaining higher ion densities. Additionally, high-frequency power sustains elevated vibrational levels by enhancing vibrational-to-vibrational relaxation during the gliding arc discharge cycle [
34].
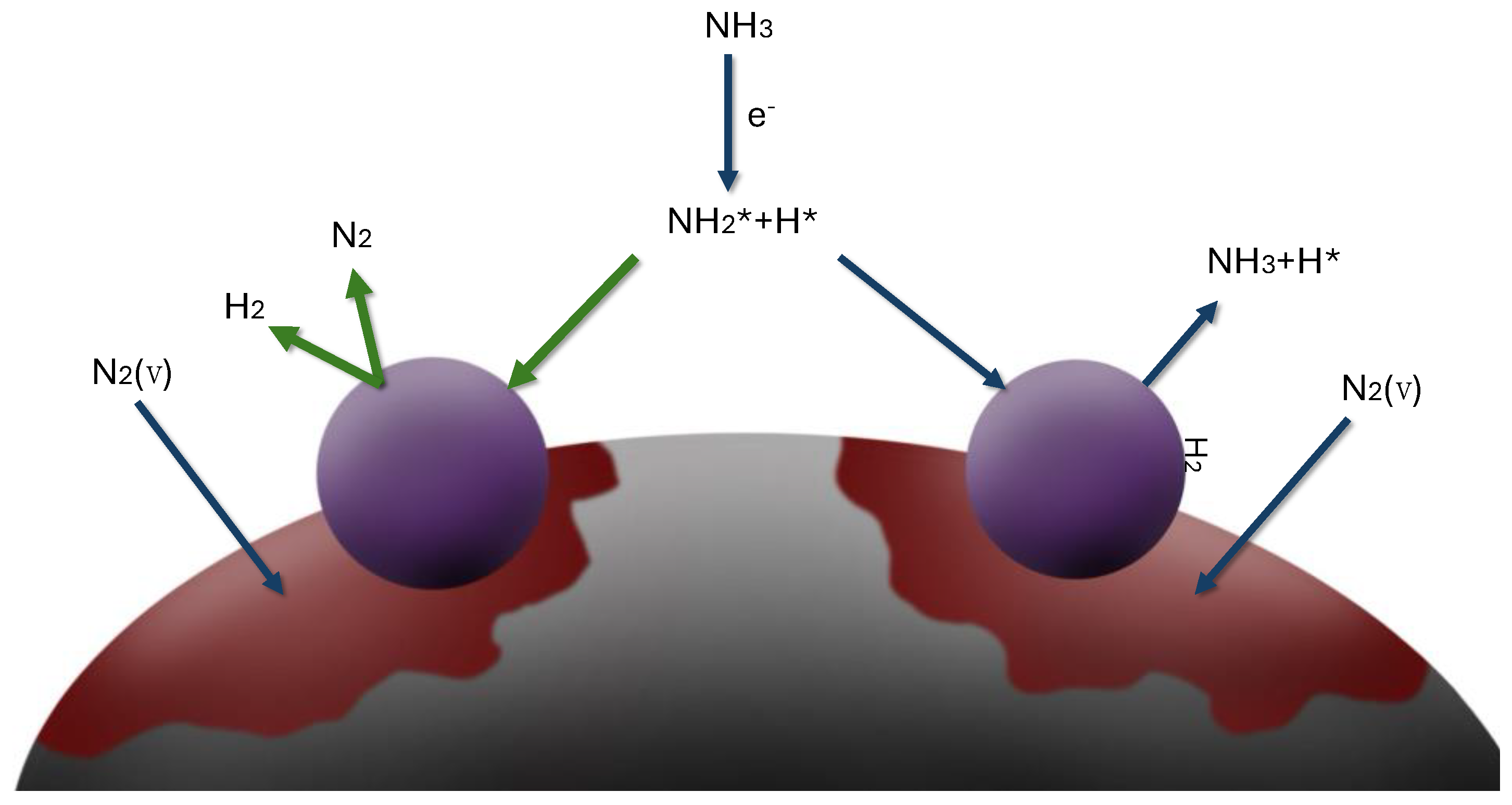
On the other hand, low-frequency plasma 50 Hz has lower electron densities and energy [
29,
31]. Due to the slower oscillation of the electric field, the energy transfer to electrons is less efficient. Consequently, the collisions between electrons and nitrogen molecules are less energetic, resulting in a lower rate of vibrational excitation. Despite the absence of chemical activity in the ammonia decomposition process, the vibrationally excited nitrogen with a coprecipitated catalyst strikes the catalyst’s surface. It can excite the active centers that take part in the ammonia decomposition reaction path (
Figure 6).
At 20 kHz, the rapid oscillation of the electric field leads to more frequent acceleration and collisions of electrons with neutral atoms. This frequent interaction increases the excitation rate, thereby increasing plasma density. Energy is distributed more fairly throughout the plasma volume. As a result, the plasma becomes more uniform since the energy is less likely to localize. Higher frequency fields can help stabilize the plasma by reducing the formation of large-scale instabilities and gradients [
26].
According to the results, at 50 Hz, slower oscillations result in less frequent acceleration of electrons, leading to lower ionization rate and hence lower plasma density compared to higher frequencies. The longer intervals between oscillations give more time for recombination processes to occur, which can reduce the overall density of free electrons and ions. Slower oscillations can result in more localized energy deposition, leading to less uniform plasma. Regions near the electrodes might have higher densities compared to the bulk plasma. This can lead to some non-uniformities in the density and temperature of the plasma.
5. Conclusions
This work demonstrates that the application of higher-frequency, 20 kHz, gliding discharge results in a significantly higher population of vibrationally excited nitrogen molecules compared to low-frequency (50 Hz) plasma. The use of the TH Bipolar HV power supply resulted in higher ammonia conversion compared to the thyristor-powered system. As a result, the productivity of ammonia decomposition increased by around 30%, and the energy consumption required to produce hydrogen was reduced by nearly 36% when using a coprecipitated catalyst. This difference is primarily due to the enhanced electron density and energy distribution in high-frequency plasmas, which facilitates more effective energy transfer to nitrogen molecules. This leads to activation of the catalyst surface and increasing ammonia conversion. These findings underscore the importance of selecting appropriate plasma electric process parameters for specific applications to achieve desired outcomes in terms of overall process efficiency.
Gliding arc stands as a promising option for ammonia decomposition for a larger-scale operation because it combines non-equilibrium plasma characteristics with relatively high energy densities. It provides enough energy to activate ammonia effectively, without reaching extreme temperatures or material degradation seen in thermal arcs. Compared to DBD or corona discharges, which tend to have lower energy densities, gliding arcs are more robust and can handle larger gas flow rates under atmospheric conditions. Their design makes them easier to integrate into modular systems powered by renewable energy, an essential advantage for future on-site hydrogen production.
Energy consumption values in kJ/molNH
3 for plasma–catalytic systems are reported by F. Van Steenweghen et al. [
35], ranging from 157 to 157,000 kJ/molNH
3. In this study, an energy consumption of 465 kJ/molNH
3 was achieved for the homogeneous system using a 20 kHz power supply. In comparison, 330 kJ/molNH
3 was obtained for the plasma-catalytic system with a co-precipitated catalyst (
Table 3).
Author Contributions
Conceptualization, M.P. and M.M.; Methodology, M.M.; Validation, M.W.; Formal analysis, M.P. and M.M.; Investigation, M.P. and M.W.; Resources, W.G.; Data curation, M.P., M.W. and M.M.; Writing—original draft, M.P., M.W. and M.M.; Writing—review & editing, M.P., W.G., K.K. and M.M.; Supervision, W.G. and M.M.; Project administration, K.K.; Funding acquisition, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by Trumpf Huettinger, Marecka 47, 05-220 Zielonka, Poland.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
This work was supported by the Warsaw University of Technology. The Authors are deeply grateful to the Trumpf Huettinger sp. z.o.o for their support and for providing the TruePlasma Bipolar HV essential for conducting this research.
Conflicts of Interest
Author Michalina Perron, Mateusz Wiosna and Wojciech Gajewski were employed by the company TRUMPF Huettinger. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hanada, N.; Hino, S.; Ichikawa, T.; Suzuki, H.; Takai, K.; Kojima, Y. Hydrogen generation by electrolysis of liquid ammonia. Chem. Commun. 2010, 46, 7775–7777. [Google Scholar] [CrossRef] [PubMed]
- Kupecki, J.; Skrzypkiewicz, M.; Błesznowski, M. Proceedings of the 24th Session of the Conference of the Parties to the United Nations Framework Convention on Climate Change (UNFCCC)—COP24, 2–14 December 2018. Available online: https://www.unido.org/sites/default/files/files/2019-05/REPORT_Towards%20Hydrogen%20Societies_final.pdf (accessed on 5 July 2025).
- Polish Hydrogen strategy until 2023 with an outlook until 2040; Warszawa, Październik 2021. Available online: https://www.gov.pl/attachment/06213bb3-64d3-4ca8-afbe-2e50dadfa2dc (accessed on 5 July 2025).
- Andruszkiewicz, M. Wodór jako element dekarbonizacji gospodarki w świetle strategii wodorowej Unii Europejskiej i Polski. Nowa Energ. 2021, 3, 54–58. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions a Hydrogen Strategy for a Climate-Neutral Europe; COM/2020/301 final; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Ju, X.; Liu, L.; Yu, P.; Guo, J.; Zhang, X.; He, T.; Wu, G.; Chen, P. Mesoporous Ru/MgO prepared by a deposition-precipitation method as highly active catalyst for producing COx-free hydrogen from ammonia decomposition. Appl. Catal. B Environ. 2017, 211, 167–175. [Google Scholar] [CrossRef]
- Ren, S.; Huang, F.; Zheng, J.; Chen, S.; Zhang, H. Ruthenium supported on nitrogen-doped ordered mesoporous carbon as highly active catalyst for NH3 decomposition to H2. Int. J. Hydrogen Energy 2017, 42, 5105–5113. [Google Scholar] [CrossRef]
- Okura, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Ammonia Decomposition over Nickel Catalysts Supported on Rate-Earth Oxides for the On-Site Generation of Hydrogen. ChemCatChem 2016, 8, 2988–2995. [Google Scholar] [CrossRef]
- Czekajło, Ł.; Lendzion-Bieluń, Z. Effect of preparation conditions and promoters on the structure and activity of the ammonia decomposition reaction catalyst based on nanocrystalline cobalt. Chem. Eng. J. 2016, 289, 254–260. [Google Scholar] [CrossRef]
- Jedynak, A.; Kowalczyk, Z.; Szmigiel, D.; Raróg, W.; Zieliński, J. Ammonia decomposition over the carbon-based iron catalyst promoted with potassium. Appl. Catal. A Gen. 2002, 237, 223–226. [Google Scholar] [CrossRef]
- Lucentini, I.; Garcia, X.; Vendrell, X.; Llorca, J. Review of the Decomposition of Ammonia to Generate Hydrogen. Ind. Eng. Chem. Res. 2021, 60, 18560–18611. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Miura, T.; Shizuya, K.; Wakazono, S.; Tokunaga, K.; Kambara, S. Hydrogen Production System Combined with a Catalytic Reactor and a Plasma Membrane Reactor from Ammonia. Int. J. Hydrogen Energy 2019, 44, 9987–9993. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, L.; Guo, Y.; Sun, S.; Guo, H. Plasma-Assisted Ammonia Decomposition over Fe–Ni Alloy Catalysts for COx-Free Hydrogen. AIChE J. 2018, 65, 691–701. [Google Scholar] [CrossRef]
- Akiyama, M.; Aihara, K.; Sawaguchi, T.; Matsukata, M.; Iwamoto, M. Ammonia Decomposition to Clean Hydrogen Using Non-Thermal Atmospheric-Pressure Plasma. Int. J. Hydrogen Energy 2018, 43, 14493–14497. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Zhao, Y.; Zhang, R.; Zhang, J.; Guo, H. NH3 Decomposition for H2 Generation: Effects of Cheap Metals and Supports on Plasma-Catalyst Synergy. ACS Catal. 2015, 5, 4167–4174. [Google Scholar] [CrossRef]
- Kambara, S.; Hayakawa, Y.; Inoue, Y.; Miura, T. Hydrogen Production from Ammonia Using Plasma Membrane Reactor. J. Sustain. Dev. Energy Water Environ. Syst. 2016, 4, 193–202. [Google Scholar] [CrossRef]
- Dong, G.; Zhou, Y.; Ming, P.; Wu, Z.; Chen, H.; Huang, Y.; Li, L. Kinetics-Based analysis of the gliding arc plasma assisted ammonia decomposition process towards vehicle on-board applications. Chem. Eng. J. 2025, 505, 159443. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Recent developments in non-thermal catalytic DBD plasma reactor for dry reforming of methane. Energy Convers. Manag. 2019, 183, 529–560. [Google Scholar] [CrossRef]
- Xu, C.; Tu, X. Plasma-assisted methane conversion in an atmospheric pressure dielectric barrier discharge reactor. J. Energy Chem. 2013, 22, 420–425. [Google Scholar] [CrossRef]
- Lotfalipour, R.; Ghorbanzadeh, A.M.; Mahdian, A. Methane conversion by repetitive nanosecond pulsed plasma. J. Phys. D Appl. Phys. 2014, 47, 365201. [Google Scholar] [CrossRef]
- Andersen, J.A.; Veer, K.V.; Christensen, J.M.; Østberg, M.; Bogaerts, A.; Jensen, A.D. Ammonia decomposition in a dielectric barrier discharge plasma: Insights from experiments and kinetic modeling. Chem. Eng. Sci. 2023, 271, 118550. [Google Scholar] [CrossRef]
- Mlotek, M.; Reda, E.; Reszke, E.; Ulejczyk, B.; Krawczyk, K. A gliding discharge reactor supplied by a ferro-resonance system for liquid toluene decomposition. Chem. Eng. Res. Des. 2016, 111, 277–283. [Google Scholar] [CrossRef]
- Song, L.; Liang, T.; Liu, C.; Li, X. Experimental investigation of hydrogen production by CH4-CO2 reforming using rotating gliding arc discharge plasma. Int. J. Hydrogen Energy 2019, 44, 29450–29459. [Google Scholar] [CrossRef]
- Dalaine, V.; Cormier, J.M.; Pellerin, S.; Lefaucheux, P. H2S destruction in 50 Hz and 25kHz gliding arc reactors. J. Appl. Phys. 1998, 84, 1215–1221. [Google Scholar] [CrossRef]
- Aliev, Y.M.; Silin, V.P. Plasma oscillations in a high-frequency electric field. J. Exptl. Theoret. Phys. (U.S.S.R) 1965, 48, 901–912. [Google Scholar]
- Sretenović, G.B.; Krstić, I.B.; Kovacević, V.V.; Obradović, B.M.; Kuraica, M.M. Spectroscopic measurement of electric field in atmospheric-pressure plasma jet operating in bullet mode. Appl. Phys. Lett. 2011, 99, 161502. [Google Scholar] [CrossRef]
- Karolewska, M.; Truszkiewicz, E.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. Ammonia synthesis over cobalt catalysts doped with cerium and barium. Effect of the ceria loading. Appl. Catal. A Gen. 2012, 445–446, 280–286. [Google Scholar] [CrossRef]
- Tarka, A.; Zybert, M.; Ronduda, H.; Patkowski, W.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. On optimal barium promoter content in a cobalt catalyst for ammonia synthesis. Catalysts 2022, 12, 199. [Google Scholar] [CrossRef]
- Fridman, A.; Kennedy, L.A. Plasma Physics and Engineering, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 547. [Google Scholar]
- Wang, Y.; Shi, J.; Li, C.; Feng, C.; Ding, H. Effect of Nitrogen Addition on Electron Density and Temperature of Cascaded Arc Argon Discharge Plasma Diagnosed by Laser Thomson Scattering. IEEE Trans. Plasma Sci. 2019, 47, 1909–1916. [Google Scholar] [CrossRef]
- Yamazaki, M.; Sasaki, K. Efficient vibrational excitation of molecular nitrogen in low-pressure plasma with ultralow electron temperature. Plasma Sources Sci. Technol. 2022, 31, 094004. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Kim, H.-H.; Lefferts, L. Vibrationally Excited Activation of N2 in Plasma-Enhanced Catalytic Ammonia Synthesis: A Kinetic Analysis. ACS Sustain. Chem. Eng. 2019, 7, 17515–17522. [Google Scholar] [CrossRef]
- Wang, W.; Patil, B.; Heijkers, S.; Hessel, V.; Bogaerts, A. Nitrogen Fixation by Gliding Arc Plasma: Better Insight by Chemical Kinetics Modelling. ChemSusChem 2017, 10, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Van Steenweghen, F.; Verschueren, A.; Fedirchyk, I.; Martens, J.A.; Bogaerts, A.; Hollevoet, L. Reversed Plasma Catalysis Process Design for Efficient Ammonia Decomposition. ACS Sustain. Chem. Eng. 2025, 13, 737–743. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).