Characterization of the Aqueous Phase from Pyrolysis of Açaí Seeds and Fibers (Euterpe oleracea Mart.)
Abstract
Nomenclature
| Symbolism | Variable Description | Unit |
| mass of the material before the drying process | g | |
| mass of the material after the drying process | g | |
| mass of the component (seed or fiber) | g | |
| total mass before the component separation process | g | |
| biomass before calcination at 900 °C | g | |
| biomass after calcination at 900 °C | g | |
| biomass before calcination at 575 °C | g | |
| biomass after calcination at 575 °C | g | |
| AC | ash content | % |
| VC | volatile content | % |
| operating temperature of the pyrolysis process | °C | |
| temperature around the reactor before the pyrolysis process | °C | |
| operating rate | °C/min | |
| mass of the pyrolysis product (bio-oil, aqueous phase, or biochar) | g | |
| biomass used in the pyrolysis process | g | |
| mass of biochar obtained after the pyrolysis process | g | |
| mass of aqueous phase obtained after the pyrolysis process | g | |
| mass of bio-oil obtained after the pyrolysis process | g | |
| volume of titrant used in the titration of the analyte | mL | |
| volume of titrant used in the titration of the blank | mL | |
| normality of the titrant solution | n°Eqg/L | |
| titrant solution correction factor | - |
1. Introduction
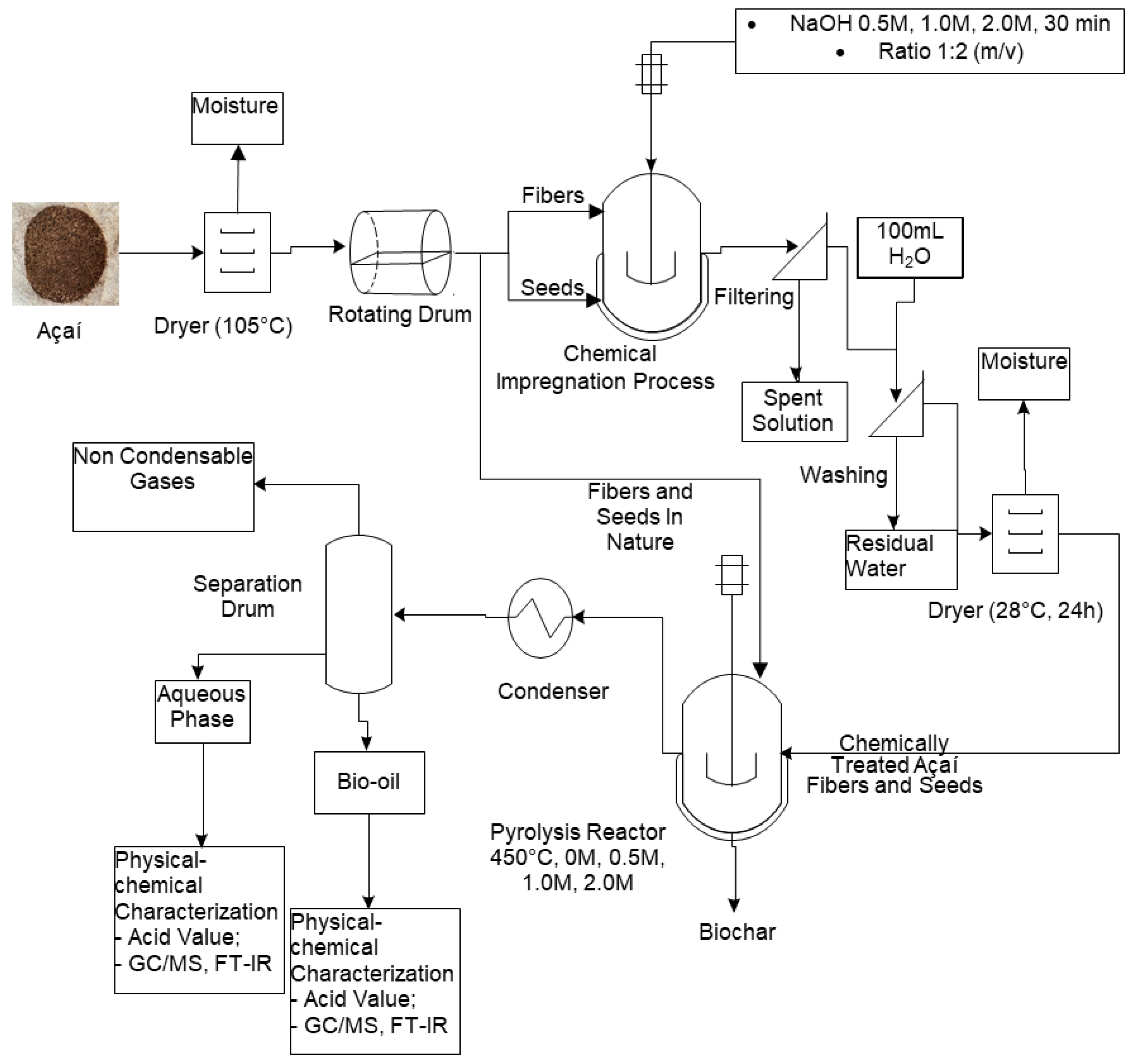
2. Materials and Methods
2.1. Methodology
2.2. Collection, Drying, and Mechanical Separation of the Fibers
2.3. Determination of the Volatile, Ash, and Fixed Carbon Contents of the Seeds and Fibers
2.3.1. Volatile Content
2.3.2. Ash Content
2.3.3. Fixed Carbon Content
2.4. Chemical Impregnation
2.5. Laboratory-Scale Pyrolysis
2.5.1. Description of the Experimental Apparatus
2.5.2. Pyrolysis Experiments
2.6. Physicochemical Characterization of the Aqueous Fractions and Bio-Oils Obtained
2.6.1. Acidity Index
2.6.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.6.3. Gas Chromatography Coupled with Mass Spectrometry (GC-MS)
3. Results and Discussion
3.1. Moisture and Mechanical Separation of Fibers
3.2. Ash Content, Volatile, and Fixed Carbon
3.3. Chemical Impregnation
3.4. Laboratory-Scale Pyrolysis
3.5. Physicochemical Characterization of the Obtained Liquid Products
3.5.1. Acidity
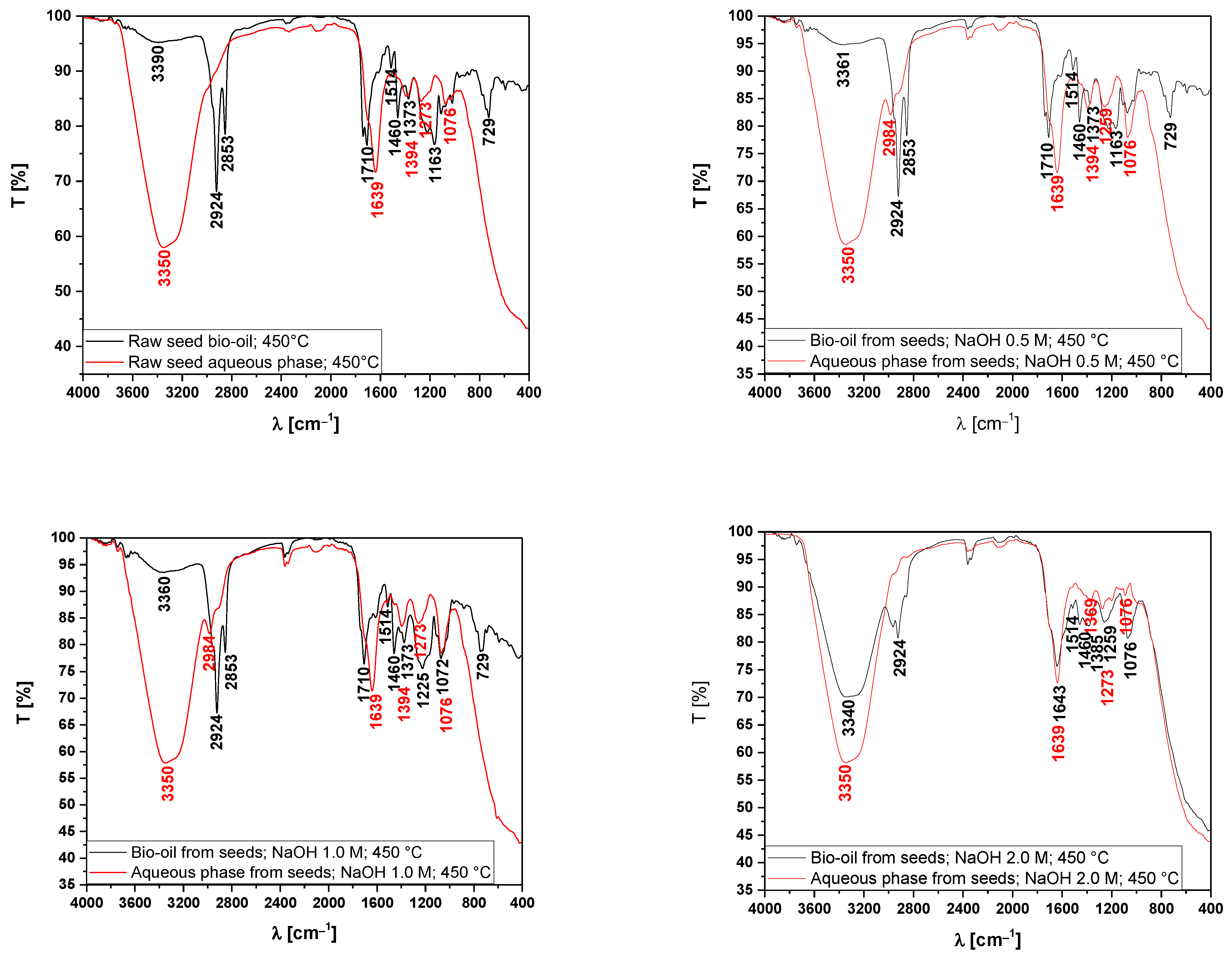
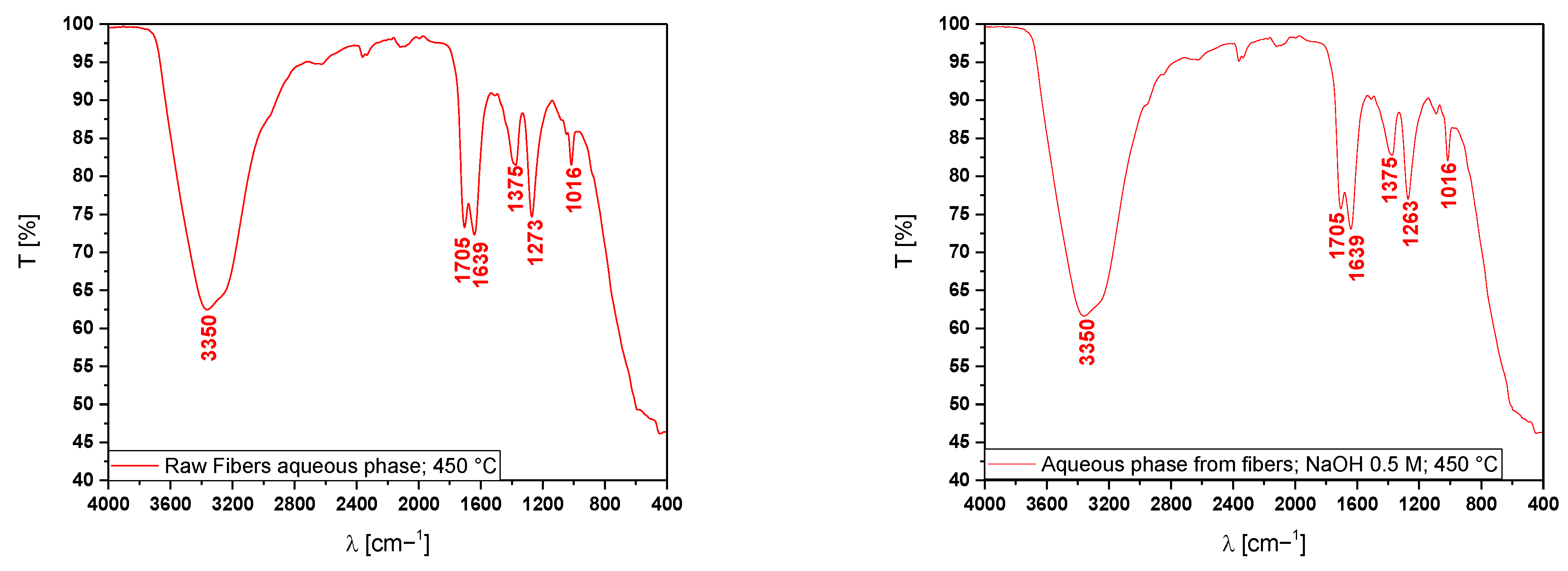
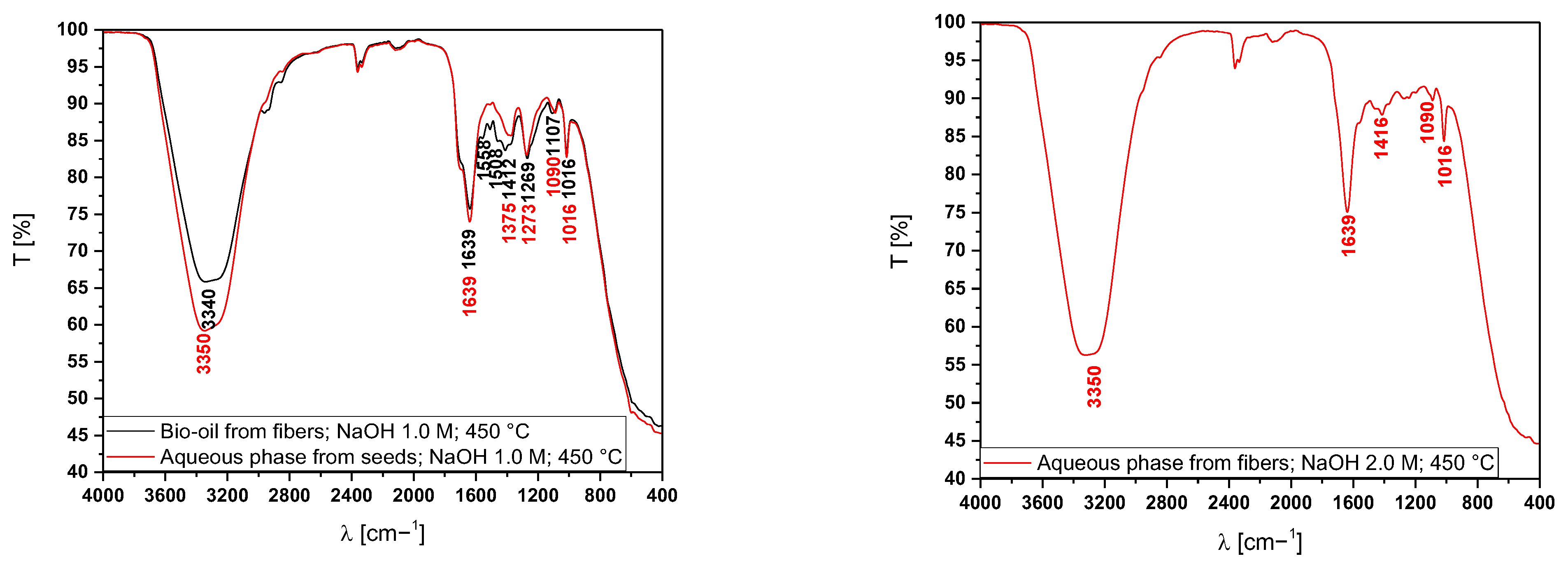
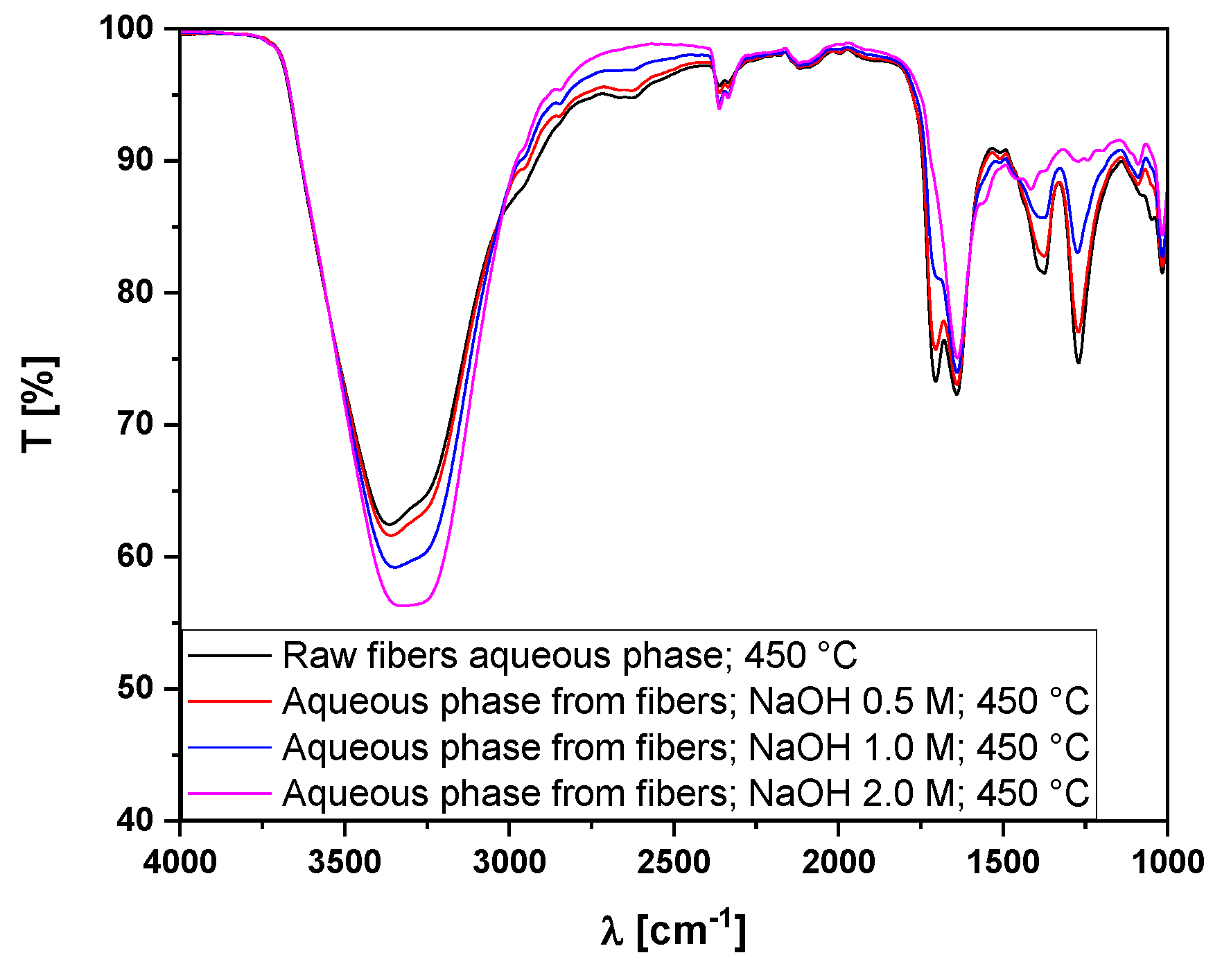
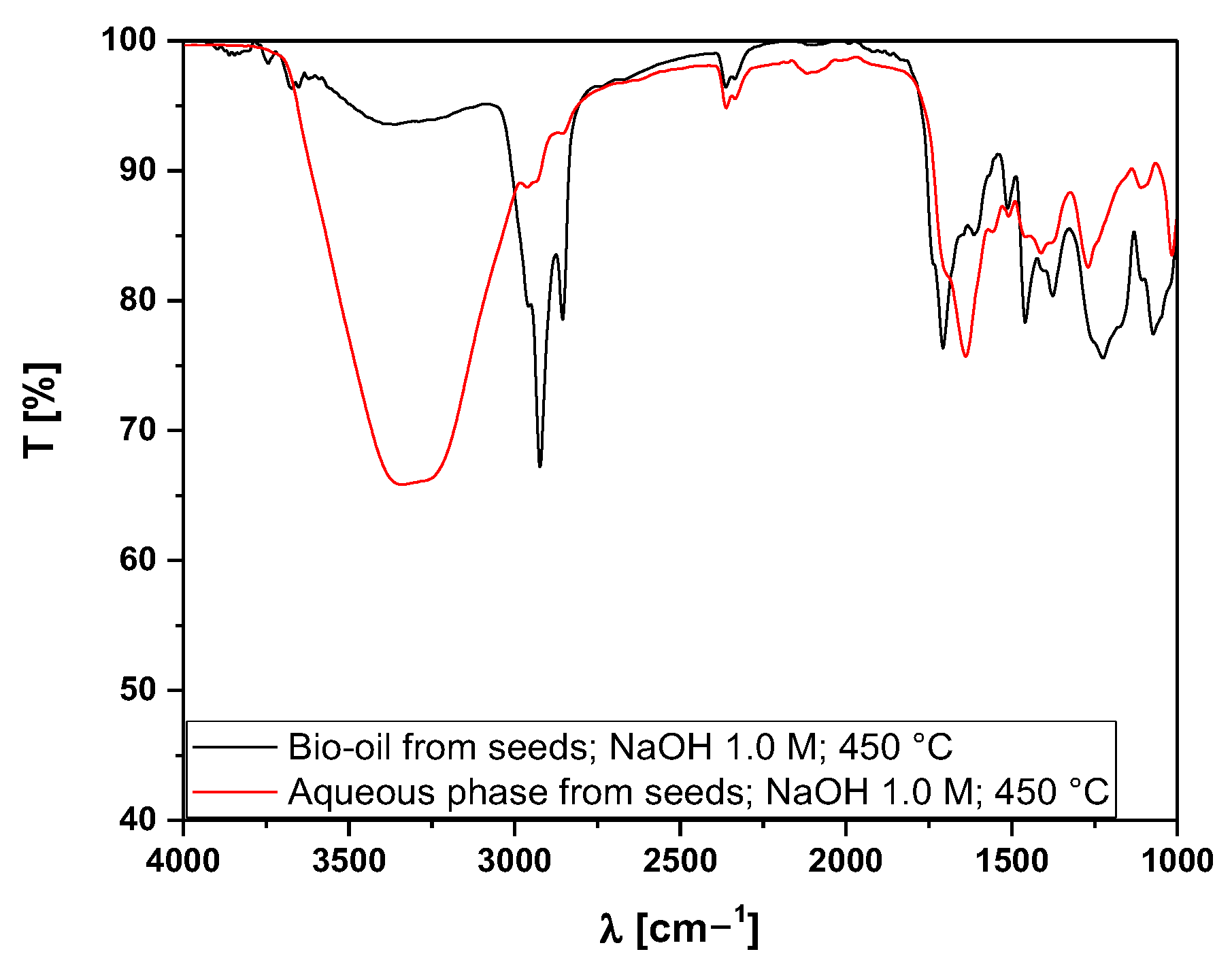
3.5.2. Fourier-Transform Infrared Spectroscopy
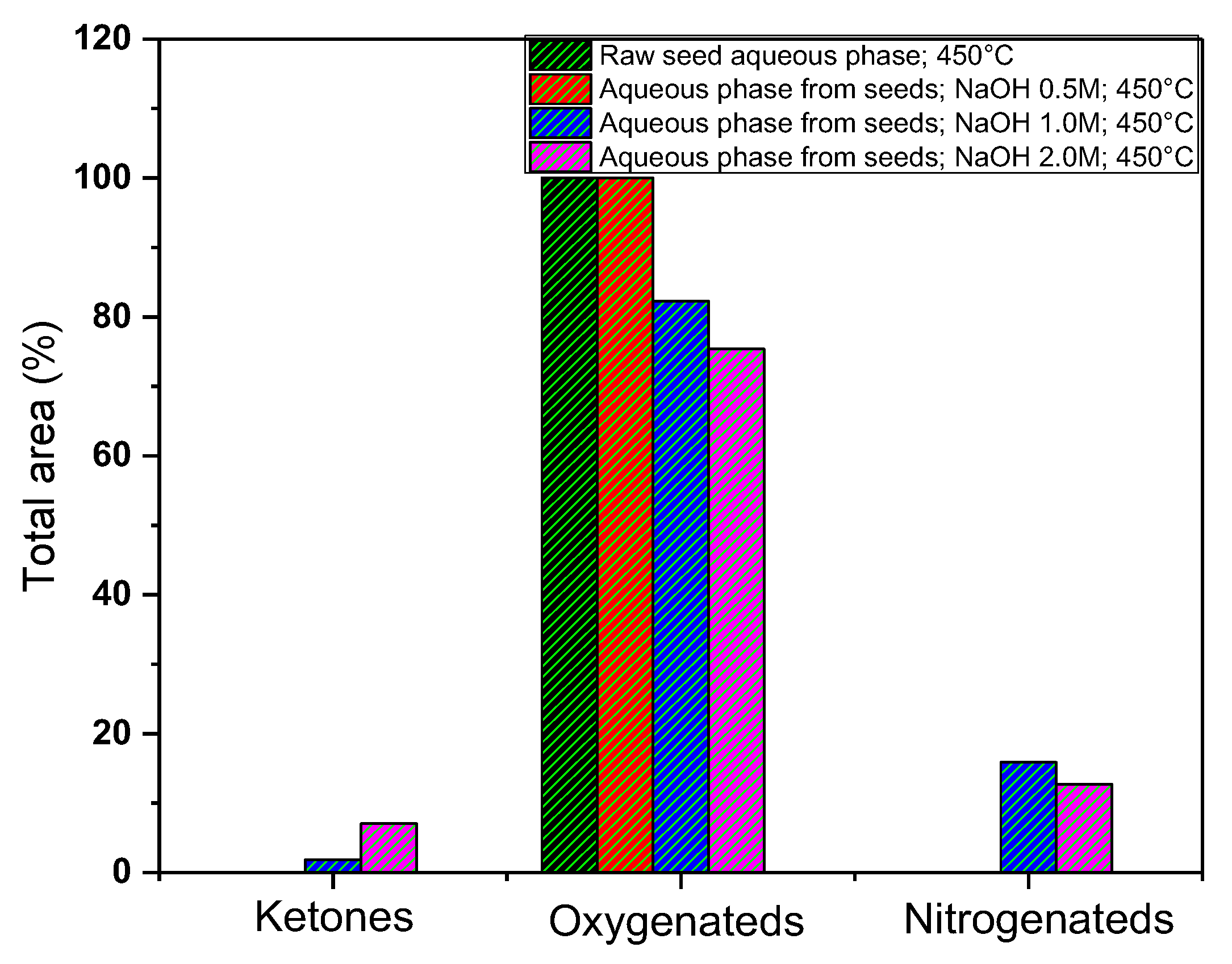
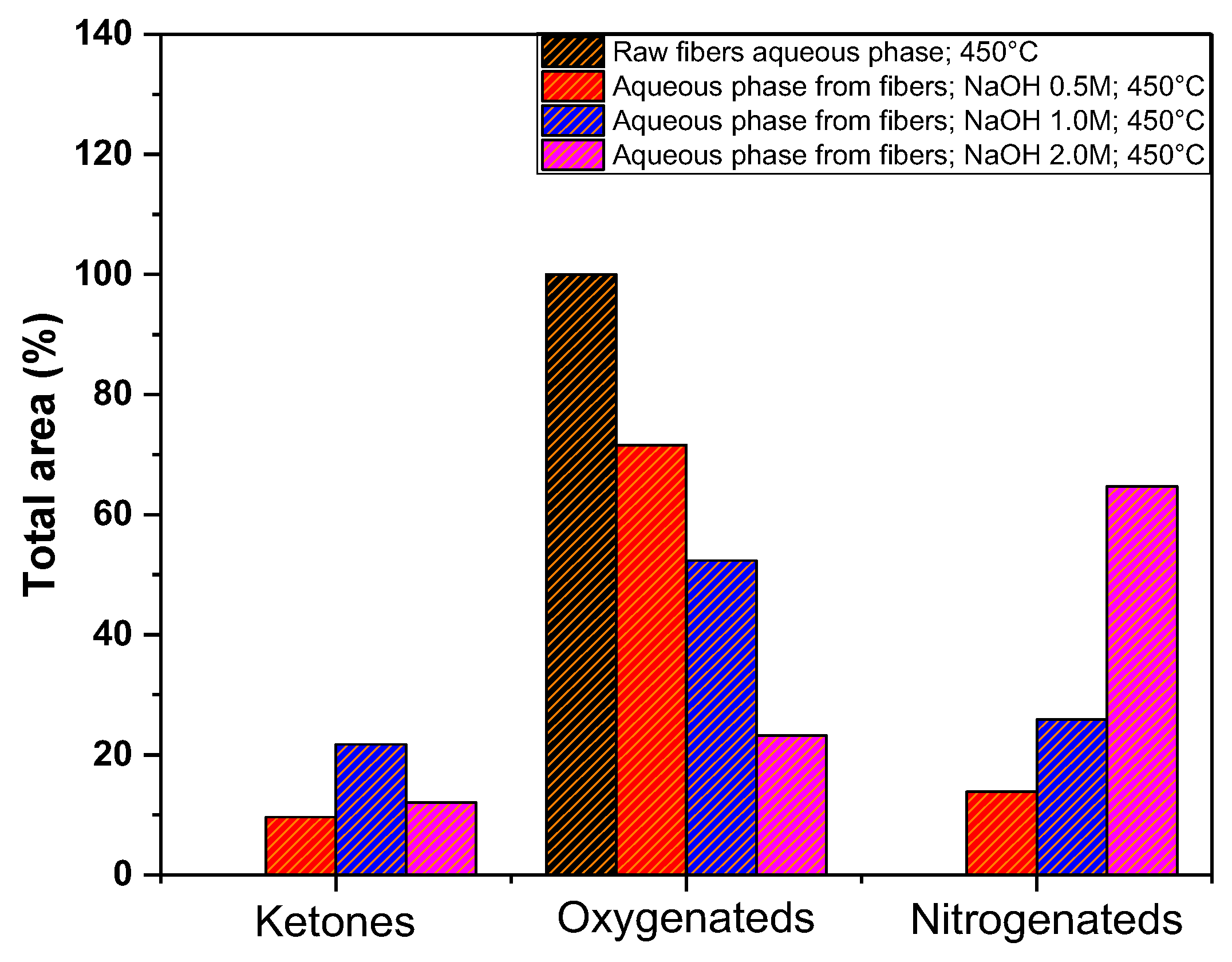
3.5.3. Gas Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gama, M.M.; Ribeiro, G.D.; Fernandes, C.F.; Medeiros, I.M. Açaí (Euterpe spp.): Características, formação de mudas e plantio para produção de frutos. Circ. Técnica 2005, 80. Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/859446 (accessed on 5 May 2024).
- Freitas, D.G.; Carvalhaes, M.A.; Bezerra, V.S. Boas Práticas na Cadeia de Produção de Açaí. 2021. Available online: https://www.embrapa.br/inteligencia-estrategica-para-pequenos-negocios/boas-praticas-na-cadeia-de-producao-do-acai (accessed on 5 May 2024).
- Oliveira, M.d.S.P.d.; Mattietto, R.d.A.; Domingues, A.F.N.; Carvalho, A.V.; Oliveira, N.P.d.; Farias Neto, J.T.d. Euterpe oleracea e E. Precatoria: Açaí. In Espécies Nativas da flora Brasileira de valor Econômico atual ou Potencial: Plantas para o futuro: Região Norte; MMA: Brasília, Brazil, 2022; Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1144331 (accessed on 5 May 2024).
- Pompeu, D.; Silva, E.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Sabbe, S.; Verbeke, W.; Deliza, R.; Matta, V.; Van Damme, P. Effect of a health claim and personal characteristics on consumer acceptance of fruit juices with different concentrations of açaí (Euterpe oleracea Mart.). Appetite 2009, 53, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Domingues, A.F.N.; Mattietto, R.A.; Oliveira, M.S.P. Teor de Lipídeos em Caroços de Euterpe oleracea Mart. Bol. Pesqui. Desenvolv. 2017, 115, 1–18. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1062268/1/BOLETIMPD115Ainfo.pdf (accessed on 5 May 2024).
- Ilyushin, Y.V.; Fetisov, V. Experience of virtual commissioning of a process control system for the production of high-paraffin oil. Sci. Rep. 2022, 12, 18415. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.A. Biocombustível na Aviação: Progressos e Desafios. Monografia. Bachelor’s Thesis, Universidade do Sul de Santa Catarina, Palhoça, Brazil, 2016. Available online: https://repositorio.animaeducacao.com.br/handle/ANIMA/8216 (accessed on 6 May 2024).
- de Castro, D.A.R.; da Silva Ribeiro, H.J.; Ferreira, C.C.; de Andrade Cordeiro, M.; Guerreiro, L.H.; Pereira, A.M.; Dos Santos, W.G.; Santos, M.C.; de Carvalho, F.B.; Junior, J.O.; et al. Fractional Distillation of Bio-Oil Produced by Pyrolysis of Açaí (Euterpe oleracea) Seeds. In Fractionation; Al-Haj Ibrahim, H., Ed.; Intechopen: London, UK, 2019; ISBN 978-1-78984-965-3. [Google Scholar]
- Valois, F.P.; Valdez, G.D.; Bezerra, K.C.A.; Bremer, S.J.; Bernar, L.P.; Paz, S.P.A.; Santos, M.C.; Feio, W.P.; Silva, R.M.P.; Mendonça, N.M.; et al. Effect of temperature and molarity on the bio-oil yield and quality by pyrolysis of Açaí seeds (Euterpe oleraceae, Mart.) activated with KOH. Preprints 2023, 2023052128. [Google Scholar] [CrossRef]
- Valdez, D.G.; Valois, F.P.; Bremer, S.J.; Bezerra, K.C.A.; Hamoy Guerreiro, L.H.; Santos, M.C.; Bernar, L.P.; Feio, W.P.; Moreira, L.G.S.; Mendonça, N.M.; et al. Improving the Bio-Oil Quality of Residual Biomass Pyrolysis by Chemical Activation: Effect of Alkalis and Acid Pre-Treatment. Energies 2023, 16, 3162. [Google Scholar] [CrossRef]
- Guerreiro, L.H.H.; Baia, A.C.F.; Assunção, F.P.D.C.; Rodrigues, G.D.O.; e Oliveira, R.L.; Junior, S.D.; Pereira, A.M.; de Sousa, E.M.P.; Machado, N.T.; de Castro, D.A.R.; et al. Investigation of the Adsorption Process of Biochar Açaí (Euterpea olerácea Mart.) Seeds Produced by Pyrolysis. Energies 2022, 15, 6234. [Google Scholar] [CrossRef]
- de Castro, D.R.; Ribeiro, H.D.S.; Guerreiro, L.H.; Bernar, L.P.; Bremer, S.J.; Santo, M.C.; Almeida, H.D.S.; Duvoisin, S.; Borges, L.P.; Machado, N.T. Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis. Energies 2021, 14, 3713. [Google Scholar] [CrossRef]
- Serrão, A.C.M.; Silva, C.M.S.; Assunção, F.P.C.; Ribeiro, H.J.S.; Santos, M.C.; Almeida, H.S.; Duvoisin, S., Jr.; Borges, L.E.P.; Castro, D.A.R.; Machado, N.T. Análise do processo de pirólise de sementes de Açaí (Euterpe oleracea, Mart): Influência da temperatura no rendimento dos produtos de reação e nas propriedades físico-químicas do BioÓleo. Braz. J. Dev. 2021, 7, 18200–18220. [Google Scholar] [CrossRef]
- Sousa, J.L.; Guerreiro, L.H.H.; Bernar, L.P.; Ribeiro, H.J.S.; Oliveira, R.L.; Santos, M.C.; Almeida, H.S.; Duvoisin, S., Jr.; Borges, L.E.P.; Castro, D.A.R.; et al. Análise da composição química do Bio-Óleo produzido via pirólise de sementes de Açaí (Euterpe oleracea, Mart). Braz. J. Dev. 2021, 7, 15549–15565. [Google Scholar] [CrossRef]
- Corrêa, F.S.; Bernar, L.P.; Ferreira, C.C.; Assunção, F.P.C.; Pereira, L.M.; Almeida, H.S.; Duvoisin, S., Jr.; Borges, L.E.P.; Castro, D.A.R.; Machado, N.T. Purificação do Bio-Óleo produzido via pirólise de sementes de Açaí (Euterpe oleracea Mart). Braz. J. Dev. 2021, 7, 18260–18277. [Google Scholar] [CrossRef]
- Feitoza, U.S.; Thue, P.S.; Lima, E.C.; Reis, G.S.; Rabiee, N.; Alencar, W.S.; Mello, B.L.; Dehmani, Y.; Rinklebe, J.; Dias, S.L. Use of Biochar Prepared from the Açaí Seed as Adsorbent for the Uptake of Catechol from Synthetic Effluents. Molecules 2022, 27, 7550. [Google Scholar] [CrossRef] [PubMed]
- Lucena, W.M.; Assunção, F.P.C.; Castro, D.A.R.; Guerreiro, L.H.H.; Machado, N.T. Estudo do processo de produção de biochar via pirólise da semente de açaí visando à remediação do solo. Revista DAE 2024, 72, 1–17. [Google Scholar] [CrossRef]
- Valois, F.P.; Bezerra, K.C.A.; Assunção, F.P.C.; Bernar, L.P.; Paz, S.P.A.; Santos, M.C.; Feio, W.P.; Silva, R.M.P.; Mendonça, N.M.; Castro, D.A.R.; et al. Improving the Antioxidant Activity, Yield, and Hydrocarbon Content of Bio-Oil from the Pyrolysis of Açaí Seeds by Chemical Activation: Effect of Temperature and Molarity. Catalysts 2024, 14, 44. [Google Scholar] [CrossRef]
- ASTM D3175-07; Standard Test Method for Volatile Matter in the Analysis of Coal and Coke. American Society for Testing and Materials: West Conshohocken, PA, USA, 1993.
- ASTM E1755; Standard Test Method for Ash in Biomass. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ASTM D3172-89; Standard Practice for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Method. American Society for Testing and Materials: West Conshohocken, PA, USA, 1989.
- Castro, D.A.R. Estudo do Processo de Pirólise de Sementes de açaí (Euterpe oleracea Mart.) Para Produção de Biocombustíveis. Ph.D. Thesis, Universidade Federal do Pará, Belém-Pa, Brazil, 2019. [Google Scholar]
- Cortez, L.A.B.; Lora, E.E.S.; Ayarza, J.A.C. Biomassa no Brasil e no Mundo: Biomassa Para Energia. UNICAMP, 2008, 15–29. Available online: https://editoraunicamp.com.br/catalogo/?id=1848 (accessed on 7 May 2024).
- Silva, M.G.; Numazawa, S.; Araujo, M.M.; Nagaishi, T.Y.R.; Galvão, G.R. Carvão de resíduos de indústria madeireira de três espécies florestais exploradas no município de Paragominas, PA. Acta Amaz. 2007, 37, 61–70. [Google Scholar] [CrossRef]
- Seye, O.; Souza, R.C.R.; Bacellar, A.A.; Morais, M.R. Caracterização do caroço de açaí como insumo para geração de eletricidade via gaseificação. In Proceedings of the Congresso Internacional Sobre Geração Distribuída e Energia no Meio Rural, Fortaleza, Brazil, 23–26 September 2008. [Google Scholar]
- Santos, M.M.; Pasolini, F.S.; Costa, A.P.O. Caracterização físico-química do caroço e da fibra do açaí (Euterpe oleracea Mart.) via métodos clássicos e instrumentais. Bras. J. Prod. Eng. 2023, 9, 144–160. [Google Scholar] [CrossRef]
- Mesquita, A.L. Estudos de Processos de Extração e Caracterização de FIBRAS do Fruto de açaí (Euterpe oleracea Mart.) Da Amazônia Para Produção de Ecopainel de Partículas de Média Densidade. Ph.D. Thesis, Universidade Federal do Pará, Belém-Pa, Brazil, 2013. [Google Scholar]
- Silva, T.F. Caroço de Açaí: Uma Alternativa Bioenergética. Master’s Thesis, Universidade de Brasília, Brasília, Brazil, 2021. [Google Scholar]
- Nagaishi, T.Y.R. Açaí (Euterpe oleracea Mart): Extrativismo, Características, Energia e Renda em uma Comunidade na Ilha de Marajó/PA. Master’s Thesis, Universidade Federal Rural da Amazônia, Belém, Brazil, 2007. [Google Scholar]
- Leão, R.M. Tratamento Superficial de Fibra de coco e Aplicação em Materiais Compósitos Como Reforço do Polipropileno. Master’s Thesis, Universidade de Brasília, Brasília, Brazil, 2012. [Google Scholar]
- Smith, B. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1999; 264p, ISBN 0-8493-2463-7. [Google Scholar]





| Publications | Experimental Matrix | Impregnating Agent | Pyrolysis Temperature | Biomass Comminution | Featured Products |
|---|---|---|---|---|---|
| [10] | Seeds | KOH 0.5 M, 1.0 M, 2.0 M | 350 °C, 400 °C, 450 °C | Yes | Bio-oil, aqueous phase, biochar |
| [11] | Seeds | HCl, KOH | 350 °C, 450 °C | Yes | Bio-oil, aqueous phase, biochar |
| [12] | Seeds | NaOH 2 M | 400 °C, 450 °C | Yes | Biochar |
| [13] | Seeds | - | 450 °C | Yes | Bio-oil |
| [14] | Seeds | - | 350 °C, 400 °C, 450 °C | Yes | Bio-oil |
| [15] | Seeds | - | 350 °C, 400 °C, 450 °C | Yes | Bio-oil |
| [16] | Seeds | - | 450 °C | Yes | Bio-oil |
| [17] | Seeds | ZnCl2 | 650 °C | Yes | Biochar |
| [18] | Seeds | - | 400 °C, 450 °C | Yes | Biochar |
| [19] | Seeds | KOH 0.5 M, 1.0 M, 2.0 M | 350 °C, 400 °C, 450 °C | Yes | Bio-oil, aqueous phase |
| Data | Drying | Separation-Seeds | Separation-Fibers |
|---|---|---|---|
| Initial mass (kg) | 7.35 | 1.35 | 1.35 |
| Final mass (kg) | 4.80 | 1.10 | 0.20 |
| Yield (%) | 65.31 | 81.48 | 14.54 |
| Moisture (%) | 34.69 | - | - |
| Data | Seeds | Fibers |
|---|---|---|
| Ash content (%) | 2.77 | 10.92 |
| Volatile content (%) | 77.39 | 77.69 |
| Fixed carbon (%) | 19.84 | 11.39 |
| Material | NaOH (mol/L) | Initial Mass (g) | Final Mass (g) | Mass Loss (%) |
|---|---|---|---|---|
| Fiber | 0.5 | 57.500 | 53.853 | 6.343 |
| Fiber | 1.0 | 57.500 | 53.729 | 6.558 |
| Fiber | 2.0 | 57.500 | 54.160 | 5.809 |
| Seed | 0.5 | 57.500 | 55.187 | 4.023 |
| Seed | 1.0 | 57.500 | 55.558 | 3.377 |
| Seed | 2.0 | 57.500 | 55.086 | 4.198 |
| Process Parameters | 450 °C | ||
|---|---|---|---|
| NaOH 0.5 M | NaOH 1.0 M | NaOH 2.0 M | |
| Mass of açaí fibers (g) | 20.003 | 20.007 | 20.007 |
| Cracking time (min) | 70 | 70 | 70 |
| Initial cracking temperature (°C) | 285 | 278 | 218 |
| Mass of solid (coke) (g) | 7.519 | 7.967 | 7.841 |
| Mass of liquid (bio-oil) (g) | 0.303 | 0.491 | 0.741 |
| Mass of H2O (g) | 6.089 | 4.843 | 4.242 |
| Mass of gas (g) | 6.092 | 6.706 | 7.183 |
| Bio-oil yield (wt.%) | 1.51 | 2.45 | 3.70 |
| Aqueous phase yield (wt.%) | 30.44 | 24.21 | 21.20 |
| Biochar yield (wt.%) | 37.59 | 39.82 | 39.19 |
| Gas yield (wt.%) | 30.46 | 33.52 | 35.90 |
| Acidity H2O (mg KOH/g) | 148.2 | 81.7 | 19 |
| Acidity bio-oil (mg KOH/g) | 130.7 | 76.4 | 26.4 |
| Process Parameters | 450 °C | ||
|---|---|---|---|
| NaOH 0.5 M | NaOH 1.0 M | NaOH 2.0 M | |
| Mass of açaí seeds (g) | 20.008 | 20.74 | 20.066 |
| Cracking time (min) | 70 | 70 | 70 |
| Initial cracking temperature (°C) | 255 | 270 | 280 |
| Mass of solid (coke) (g) | 6.710 | 5.450 | 6.696 |
| Mass of liquid (bio-oil) (g) | 0.910 | 1.202 | 1.322 |
| Mass of H2O (g) | 8.264 | 8.107 | 7.519 |
| Mass of gas (g) | 4.124 | 5.315 | 4.529 |
| Bio-oil yield (wt.%) | 4.55 | 5.99 | 6.59 |
| Aqueous phase yield (wt.%) | 41.30 | 40.39 | 37.47 |
| Biochar yield (wt.%) | 33.54 | 27.15 | 33.37 |
| Gas yield (wt.%) | 20.61 | 26.48 | 22.57 |
| Acidity H2O (mg KOH/g) | 77.4 | 73.6 | 68 |
| Acidity bio-oil (mg KOH/g) | 47.3 | 23.1 | 18.4 |
| Process Parameters | 450 °C | |
|---|---|---|
| Fibers | Seeds | |
| Mass of açaí seeds (g) | 20.000 | 20.034 |
| Cracking time (min) | 70 | 70 |
| Initial cracking temperature (°C) | 300 | 290 |
| Mass of solid (coke) (g) | 9.134 | 6.836 |
| Mass of liquid (bio-oil) (g) | 0.245 | 0.663 |
| Mass of H2O (g) | 6.747 | 8.155 |
| Mass of gas (g) | 3.874 | 4.380 |
| Bio-oil yield (wt.%) | 1.23 | 3.31 |
| Aqueous phase yield (wt.%) | 33.74 | 40.71 |
| Biochar yield (wt.%) | 45.67 | 34.12 |
| Gas yield (wt.%) | 19.37 | 21.86 |
| Acidity H2O (mg KOH/g) | 184.3 | 78.4 |
| Acidity bio-oil (mg KOH/g) | 202.6 | 71.5 |
| RT | Compounds | S-2.0 M |
|---|---|---|
| 4041 | 2-Furanmethanol | 3.17 |
| 6689 | Phenol | 3.24 |
| 22845 | Dodecanoic acid | 4.46 |
| 27619 | Tetradecanoic acid | 8.91 |
| 32124 | n-Hexadecanoic acid | 3.93 |
| RT | Compounds | S-IN | S-0.5 M | S-1.0 M | S-2.0 M |
|---|---|---|---|---|---|
| 3.742 | Furfural | 18.52 | 15.47 | ||
| 3.746 | 3,5-Dimethylpyrazole | 15.88 | 12.74 | ||
| 5.119 | Butyrolactone | 6.4 | 9.57 | 12.55 | |
| 20.820 | beta-D-Glucopyranose, 1,6-anhydro- | 55.43 | 65.53 | 59.04 | 52.34 |
| IR | Compounds | F-IN | F-0.5 M | F-1.0 M | F-2.0 M |
|---|---|---|---|---|---|
| 3.134 | Oxazole, 4,5-dihydro-2,4,4-trimethyl- | 5.01 | 16.76 | 48.31 | |
| 6.702 | Phenol | 33.08 | 37.28 | 28.19 | 14.86 |
| 9.613 | Phenol, 2-methoxy- | 11.76 | 26.11 | 15.86 | 8.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, E.M.; Bezerra, K.C.A.; Silva, R.M.P.; Martins, G.A.d.C.; de Assis, G.X.; Ferreira, R.B.P.; Bernar, L.P.; Mendonça, N.M.; Tavares Dias, C.G.B.; Castro, D.A.R.d.; et al. Characterization of the Aqueous Phase from Pyrolysis of Açaí Seeds and Fibers (Euterpe oleracea Mart.). Energies 2025, 18, 3820. https://doi.org/10.3390/en18143820
de Sousa EM, Bezerra KCA, Silva RMP, Martins GAdC, de Assis GX, Ferreira RBP, Bernar LP, Mendonça NM, Tavares Dias CGB, Castro DARd, et al. Characterization of the Aqueous Phase from Pyrolysis of Açaí Seeds and Fibers (Euterpe oleracea Mart.). Energies. 2025; 18(14):3820. https://doi.org/10.3390/en18143820
Chicago/Turabian Stylede Sousa, Erick Monteiro, Kelly Christina Alves Bezerra, Renan Marcelo Pereira Silva, Gabriel Arthur da Costa Martins, Gabriel Xavier de Assis, Raise Brenda Pinheiro Ferreira, Lucas Pinto Bernar, Neyson Martins Mendonça, Carmen Gilda Barroso Tavares Dias, Douglas Alberto Rocha de Castro, and et al. 2025. "Characterization of the Aqueous Phase from Pyrolysis of Açaí Seeds and Fibers (Euterpe oleracea Mart.)" Energies 18, no. 14: 3820. https://doi.org/10.3390/en18143820
APA Stylede Sousa, E. M., Bezerra, K. C. A., Silva, R. M. P., Martins, G. A. d. C., de Assis, G. X., Ferreira, R. B. P., Bernar, L. P., Mendonça, N. M., Tavares Dias, C. G. B., Castro, D. A. R. d., Rodrigues, G. d. O., Junior, S. D., Monteiro, M. C., & Machado, N. T. (2025). Characterization of the Aqueous Phase from Pyrolysis of Açaí Seeds and Fibers (Euterpe oleracea Mart.). Energies, 18(14), 3820. https://doi.org/10.3390/en18143820






