Comparison of the Characteristics of Hydrochar and Torrefied-Char of Traditional Chinese Medicine Residues
Abstract
1. Introduction
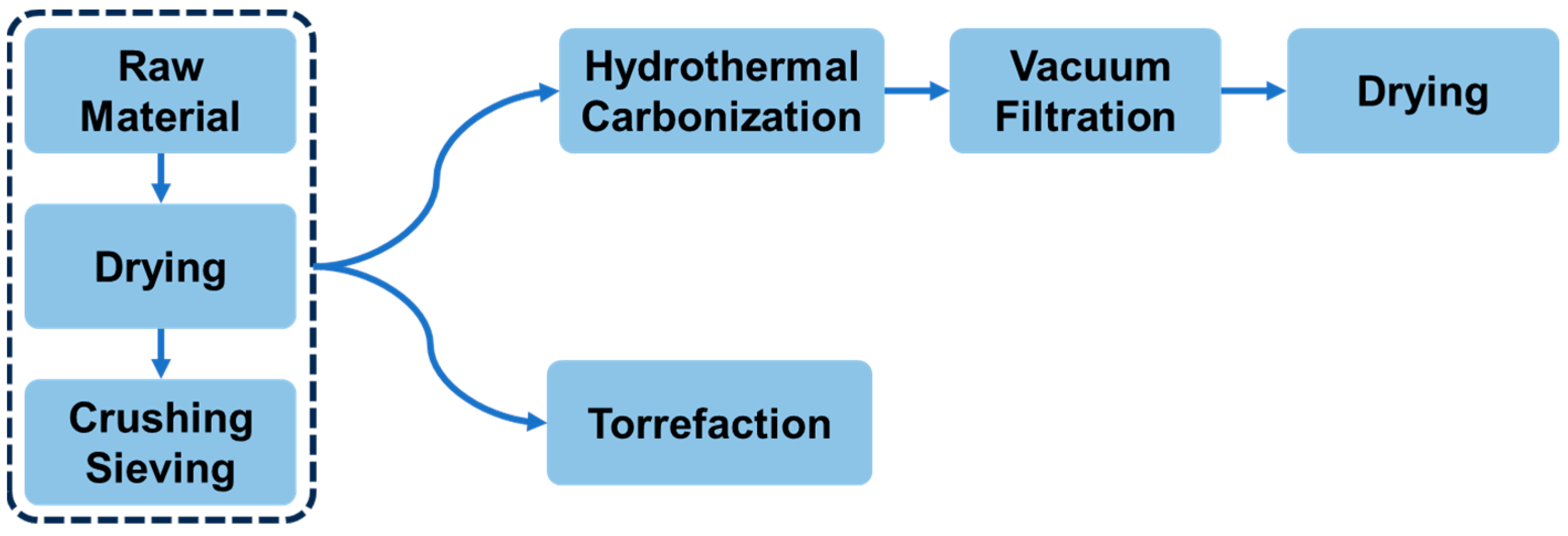
2. Materials and Methods
Characterization of Hydrochar and Torrefied-Char
3. Results and Discussion
3.1. Biochar Yield Analysis
3.2. Biochar HHV Analysis
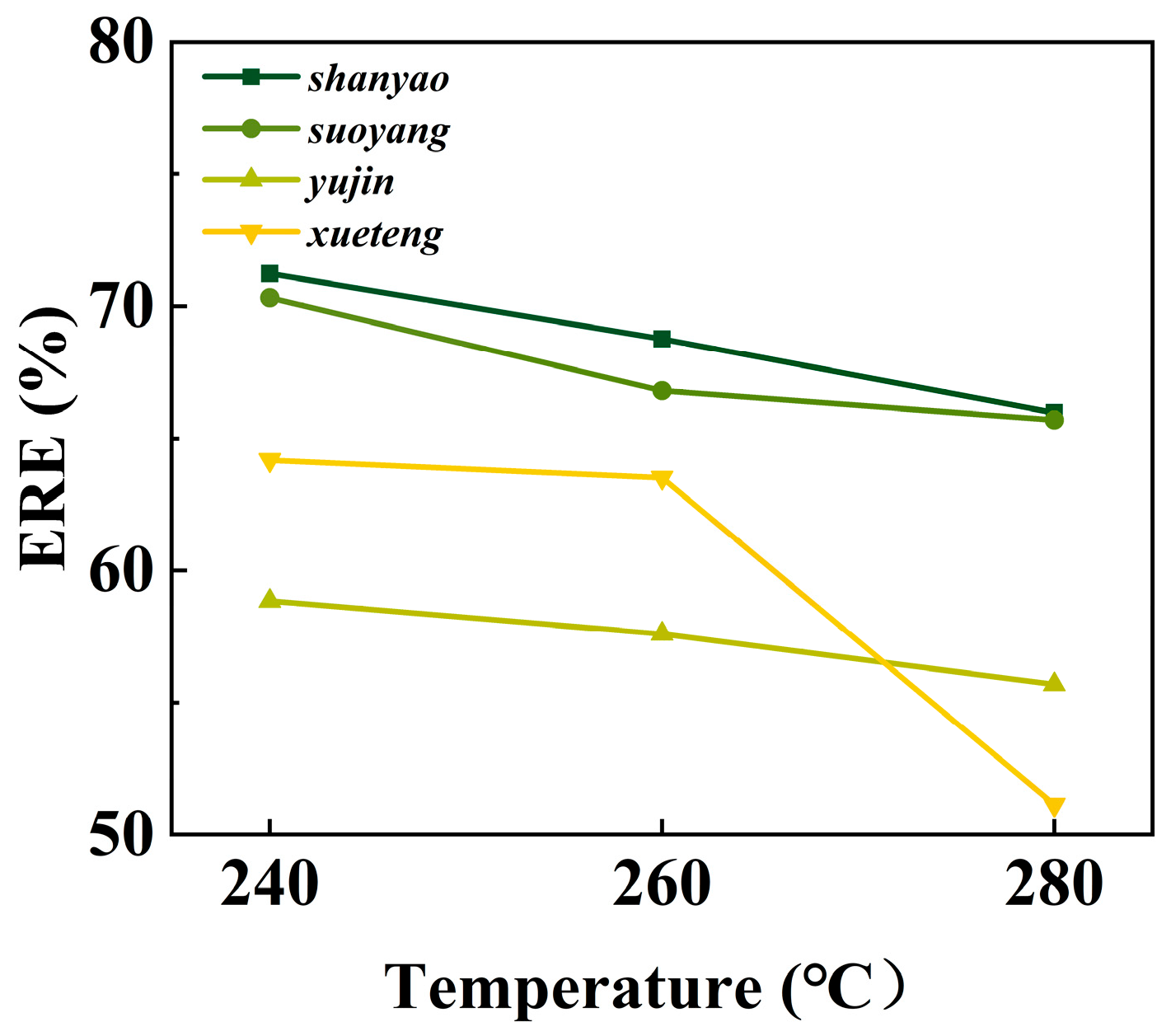
3.3. Biochar ERE Analysis
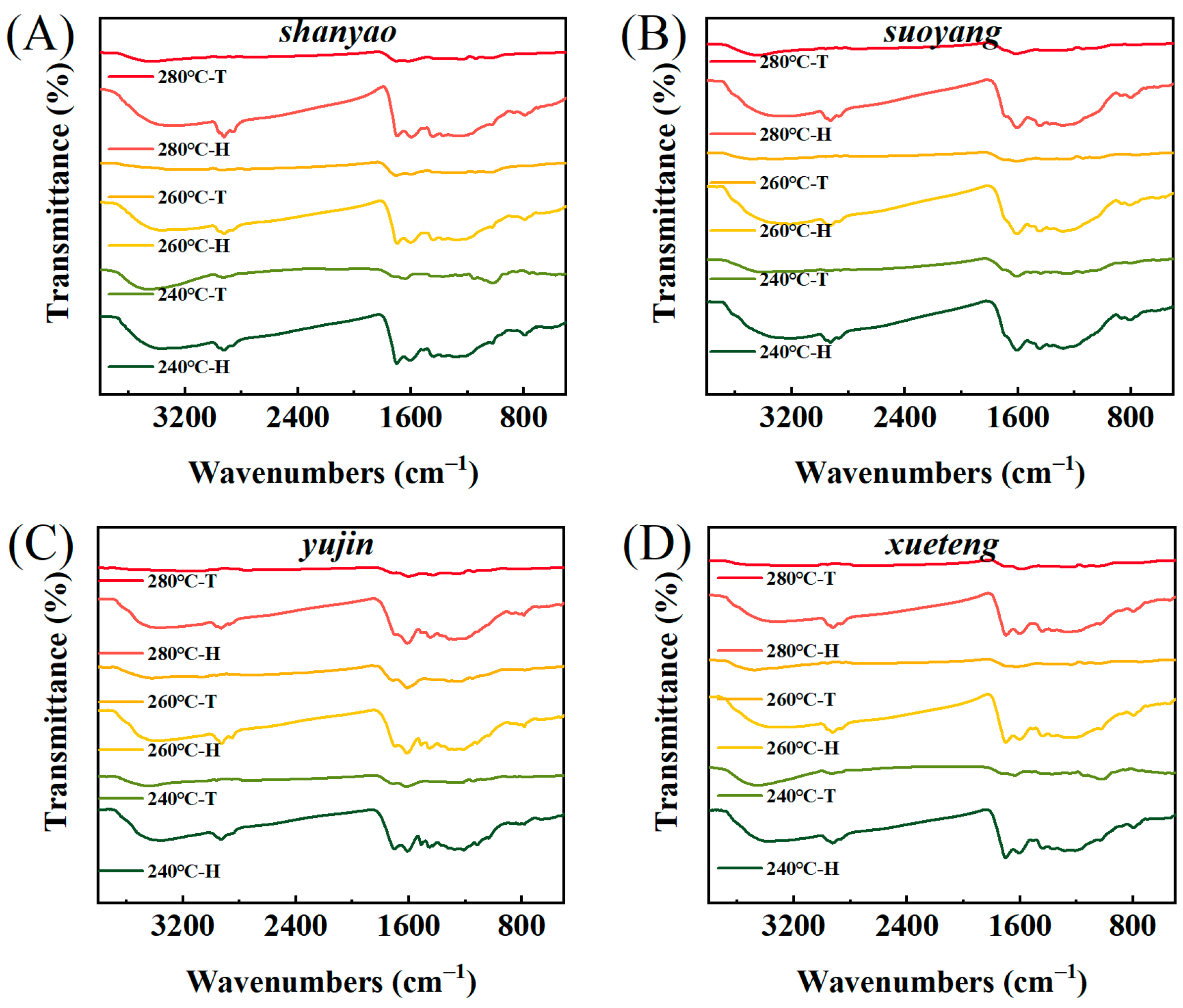
3.4. Biochar FT-IR Analysis
3.5. Biochar XRD Analysis
3.6. Biochar SEM Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| IEA | International Energy Agency |
| HTC | Hydrothermal carbonization |
| TCM | traditional Chinese medicine |
| TCMRs | traditional Chinese medicine residues |
| FT-IR | Fourier-transform infrared spectroscopy |
| XRD | X-ray diffraction |
| SEM | scanning electron microscopy |
| HCY | hydrochar yield |
| TCY | torrefied-char yield |
| ERE | energy recovery efficiency |
| HHV | highest heating value |
References
- Belaïd, F.; Al-Sarihi, A.; Al-Mestneer, R. Balancing climate mitigation and energy security goals amid converging global energy crises: The role of green investments. Renew. Energy 2023, 205, 534–542. [Google Scholar] [CrossRef]
- IEA. Global Energy Review 2025. 2025. Available online: https://www.iea.org/reports/global-energy-review-2025 (accessed on 25 April 2025).
- Wang, B.; Wang, J.; Dong, K.; Dong, X. Is the digital economy conducive to the development of renewable energy in Asia? Energy Policy 2023, 173, 113381. [Google Scholar] [CrossRef]
- Kumar, J.A.; Sathish, S.; Prabu, D.; Renita, A.A.; Saravanan, A.; Deivayanai, V.C.; Anish, M.; Jayaprabakar, J.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Agricultural waste biomass for sustainable bioenergy production: Feedstock, characterization and pre-treatment methodologies. Chemosphere 2023, 331, 138680. [Google Scholar] [CrossRef] [PubMed]
- Kalak, T. Potential Use of Industrial Biomass Waste as a Sustainable Energy Source in the Future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakajima, L. Sustainable development goals for advanced materials provided by industrial wastes and biomass sources. Curr. Opin. Green Sustain. Chem. 2021, 28, 100439. [Google Scholar] [CrossRef]
- Wu, X.W.; Dai, D.; Li, N.; Zheng, H.X.; Wang, C.X.; Lin, W.X.; Liu, L.Y.; Zhang, Z.; Rinklebe, J.; Lin, C.S.K.; et al. Recycling traditional Chinese medicine residues: A review. Environ. Chem. Lett. 2025, 23, 977–997. [Google Scholar] [CrossRef]
- Zhu, S.; Ke, J.; Li, X.; Xu, X.; Liu, Y.; Guo, R.; Chen, J. Selective hydrolysis of traditional Chinese medicine residue into reducing sugars catalysed by sulfonated carbon catalyst and application of hydrolysate. Chem. Eng. J. 2024, 497, 154586. [Google Scholar] [CrossRef]
- Jia, L.; Yu, G.; Qin, Z.; Wang, G.; Ruan, D.; Tu, J. The performance analysis of supercapacitors utilizing chemically activated carbon derived from Chinese medicine residues. Biomass Convers. Biorefinery 2024, 15, 13783–13796. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Z.; Zhang, M.; Su, H.; Zhao, T.; Feng, W.; Zhang, Z. Exploring the Potential of Biochar Derived from Chinese Herbal Medicine Residue for Efficient Removal of Norfloxacin. Molecules 2024, 29, 2063. [Google Scholar] [CrossRef]
- Li, L.; Xie, Y.; Chen, K.; Zhou, J.; Wang, M.; Wang, W.; Zhang, Z.; Lu, F.; Du, Y.; Feng, Y. Adsorption Characteristics of Ball Milling-Modified Chinese Medicine Residue Biochar Toward Quercetin. ACS Omega 2024, 9, 11658–11670. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J. Removal of chlortetracycline from water by Bacillus cereus immobilized on Chinese medicine residues biochar. Environ. Technol. Innov. 2021, 24, 101930. [Google Scholar] [CrossRef]
- Yang, C.; Xia, P.; Zhao, L.; Huang, R.; Wang, K.; Yang, H.; Yao, Y. Hydrothermal carbonization of Chinese medicine residue from licorice: Effects of pore and chemical structures on chromium migration. Ecotoxicol. Environ. Saf. 2024, 284, 116928. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, C. Comprehensive utilization of Chinese medicine residues for industry and environment protection: Turning waste into treasure. J. Clean. Prod. 2021, 279, 123856. [Google Scholar] [CrossRef]
- Fan, R.; Chen, L.; Xue, B. Transforming biomass into engineered biochar materials forhigh-performance supercapacitors: Recent advances, challenges, and prospects. Energy Environ. Prot. 2024, 38, 13–24. [Google Scholar] [CrossRef]
- Kong, T.; Wang, H.; Zhang, W. Research on effects of loading methods on magnetic biochar properties. Energy Environ. Prot. 2024, 38, 157–165. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, X.; Chen, H. Advances in biochar carbonization regulation and remediation of soil heavy metals. Energy Environ. Prot. 2024, 38, 14–23. [Google Scholar] [CrossRef]
- Zhang, Y.; He, M.; Wang, L.; Yan, J.; Ma, B.; Zhu, X.; Ok, Y.S.; Mechtcherine, V.; Tsang, D.C.W. Biochar as construction materials for achieving carbon neutrality. Biochar 2022, 4, 59. [Google Scholar] [CrossRef]
- Khosravi, A.; Zheng, H.; Liu, Q.; Hashemi, M.; Tang, Y.; Xing, B. Production and characterization of hydrochars and their application in soil improvement and environmental remediation. Chem. Eng. J. 2022, 430, 133142. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, M.; Jiang, E.; Wang, D.; Zhang, K.; Ren, Y.; Jiang, Y. Pyrolysis of Torrefied Biomass. Trends Biotechnol. 2018, 36, 1287–1298. [Google Scholar] [CrossRef]
- Islam, M.T.; Sultana, A.I.; Chambers, C.; Saha, S.; Saha, N.; Kirtania, K.; Reza, M.T. Recent Progress on Emerging Applications of Hydrochar. Energies 2022, 15, 9340. [Google Scholar] [CrossRef]
- Jiang, H.; Deng, F.; Luo, Y.; Xie, Z.; Chen, Y.; Zhou, P.; Liu, X.; Li, D. Hydrothermal carbonization of corn straw in biogas slurry. J. Clean. Prod. 2022, 353, 131682. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Zhong, H. Remediation of mercury-contaminated soils and sediments using biochar: A critical review. Biochar 2021, 3, 23–35. [Google Scholar] [CrossRef]
- Patel, A.K.; Katiyar, R.; Chen, C.W.; Singhania, R.R.; Awasthi, M.K.; Bhatia, S.; Bhaskar, T.; Dong, C.D. Antibiotic bioremediation by new generation biochar: Recent updates. Bioresour. Technol. 2022, 358, 127384. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, L.; Zhang, X.; Zhao, C.; Sun, J.; Li, G.; Shi, H. Advances and prospects of multifunctional biochar-based materials from organic solid waste of traditional Chinese medicine: A review. Biomass Bioenergy 2024, 187, 107296. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, C.; Tang, Z.; Chu, T.; Zheng, C.; Han, Q.; Chen, H.; Tan, Y. Biochar derived from traditional Chinese medicine residues: An efficient adsorbent for heavy metal Pb(II). Arab. J. Chem. 2024, 17, 105606. [Google Scholar] [CrossRef]
- Wei, L.; Huang, Y.; Huang, L.; Li, Y.; Huang, Q.; Xu, G.; Muller, K.; Wang, H.; Ok, Y.S.; Liu, Z. The ratio of H/C is a useful parameter to predict adsorption of the herbicide metolachlor to biochars. Environ. Res. 2020, 184, 109324. [Google Scholar] [CrossRef]
- Luo, Y.; Mi, T.; Huang, F.; Kuang, Y.; Liu, Y.; Liu, Y.; Zheng, C.; Zhou, X.; Xin, S.; Liu, X. Hydrothermal conversion behavior of Chinese medicine residues and pyrolysis and combustion characteristics of hydrochars. Fuel 2025, 384, 133776. [Google Scholar] [CrossRef]
- Ercan, B.; Alper, K.; Ucar, S.; Karagoz, S. Comparative studies of hydrochars and biochars produced from lignocellulosic biomass via hydrothermal carbonization, torrefaction and pyrolysis. J. Energy Inst. 2023, 109, 101298. [Google Scholar] [CrossRef]
- Beskov, S.D. Technochimicheskie Rascheti; Vishaya sckola Chimiya: Moscow, Russia, 1962. [Google Scholar]
- Krysanova, K.; Krylova, A.; Zaichenko, V. Properties of biochar obtained by hydrothermal carbonization and torrefaction of peat. Fuel 2019, 256, 115929. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Chen, D.Y.; Gao, A.J.; Cen, K.H.; Zhang, J.; Cao, X.B.; Ma, Z.Q. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood. J. Anal. Appl. Pyrolysis 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Khiari, B.; Ghouma, I.; Ferjani, A.I.; Azzaz, A.A.; Jellali, S.; Limousy, L.; Jeguirim, M. Kenaf stems: Thermal characterization and conversion for biofuel and biochar production. Fuel 2020, 262, 116654. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Cui, D.; Wang, X.; Wu, D.; Bai, J.; Xu, F.; Wang, Z.; Zhang, J. Analysis of fuel properties of hydrochar derived from food waste and biomass: Evaluating varied mixing techniques pre/post-hydrothermal carbonization. J. Clean. Prod. 2023, 430, 139660. [Google Scholar] [CrossRef]
- Yu, S.; Dong, X.; Zhao, P.; Luo, Z.; Sun, Z.; Yang, X.; Li, Q.; Wang, L.; Zhang, Y.; Zhou, H. Decoupled temperature and pressure hydrothermal synthesis of carbon sub-micron spheres from cellulose. Nat. Commun. 2022, 13, 3616. [Google Scholar] [CrossRef]
- Yu, S.; Yang, X.; Li, Q.; Zhang, Y.; Zhou, H. Breaking the temperature limit of hydrothermal carbonization of lignocellulosic biomass by decoupling temperature and pressure. Green Energy Environ. 2023, 8, 1216–1227. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant. Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Lim, S.R.; Kim, G.H.; Um, B.H. Hydrothermal Carbonization and Torrefaction of Kenaf, Rice Husk, Corncob, and Wood Chip: Characteristics and Differences of Hydrochar and Torrefied Char. BioEnergy Res. 2024, 17, 1816–1831. [Google Scholar] [CrossRef]
- Ramos-Carmona, S.; Martínez, J.D.; Pérez, J.F. Torrefaction of patula pine under air conditions: A chemical and structural characterization. Ind. Crops Prod. 2018, 118, 302–310. [Google Scholar] [CrossRef]
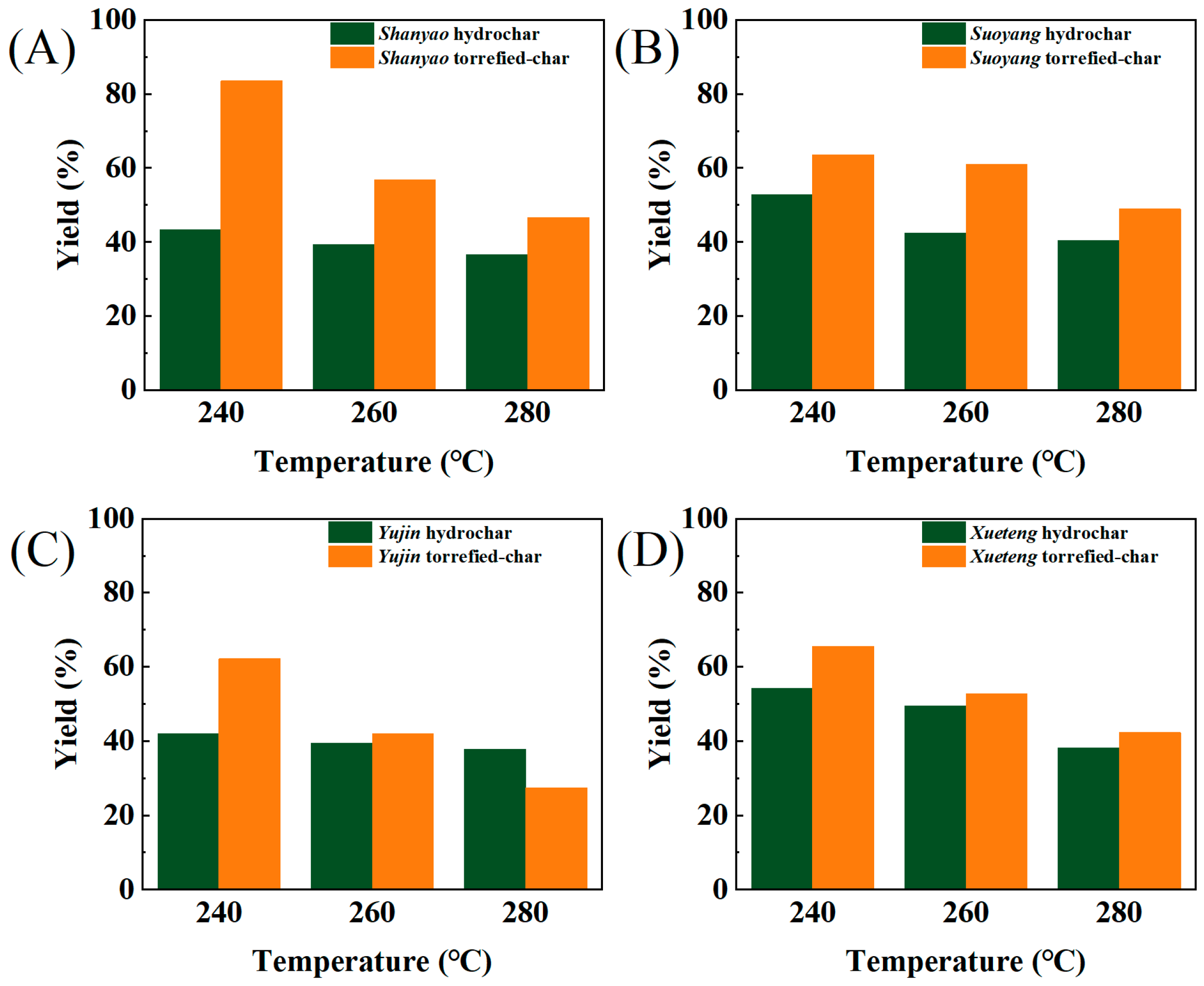

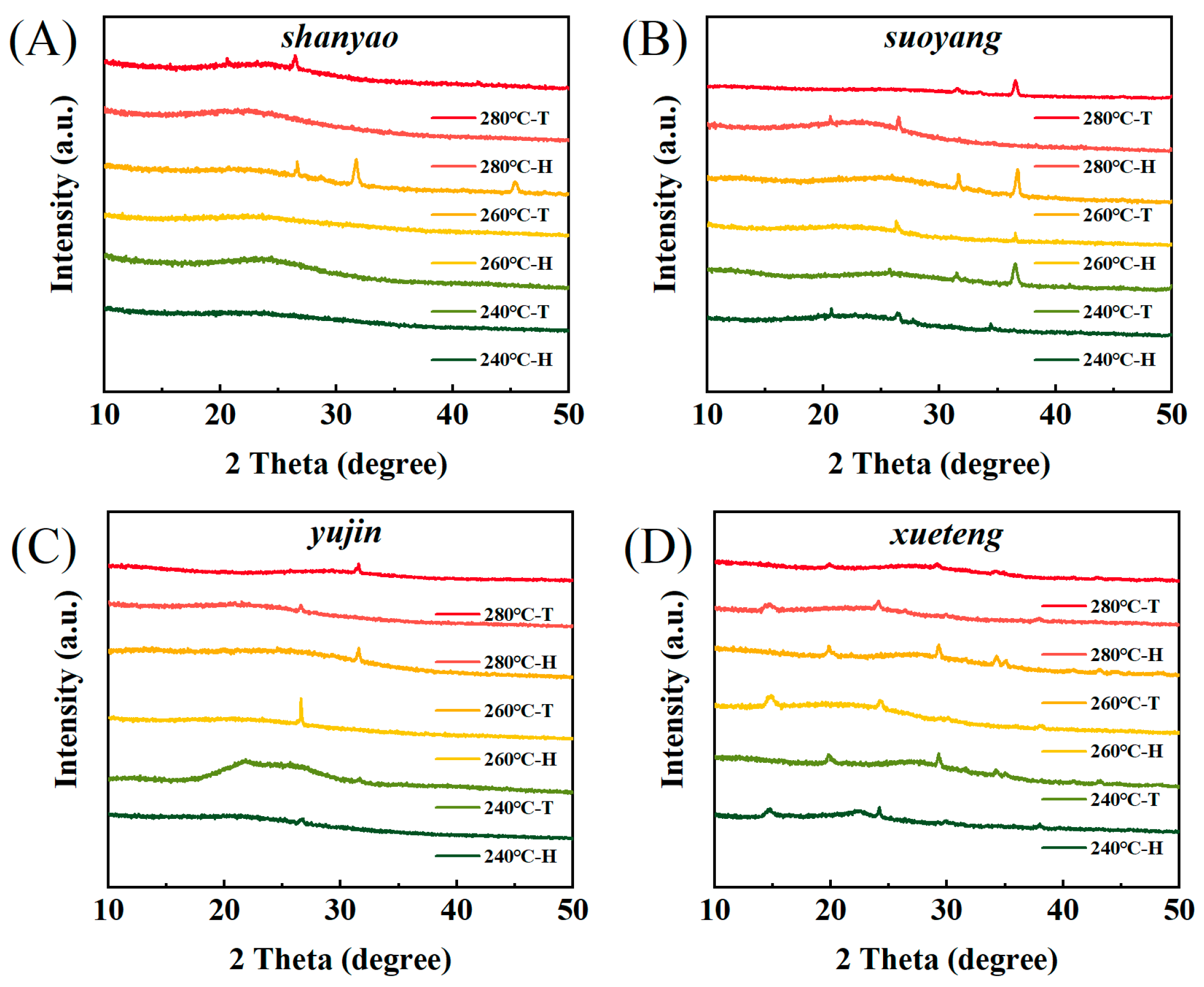
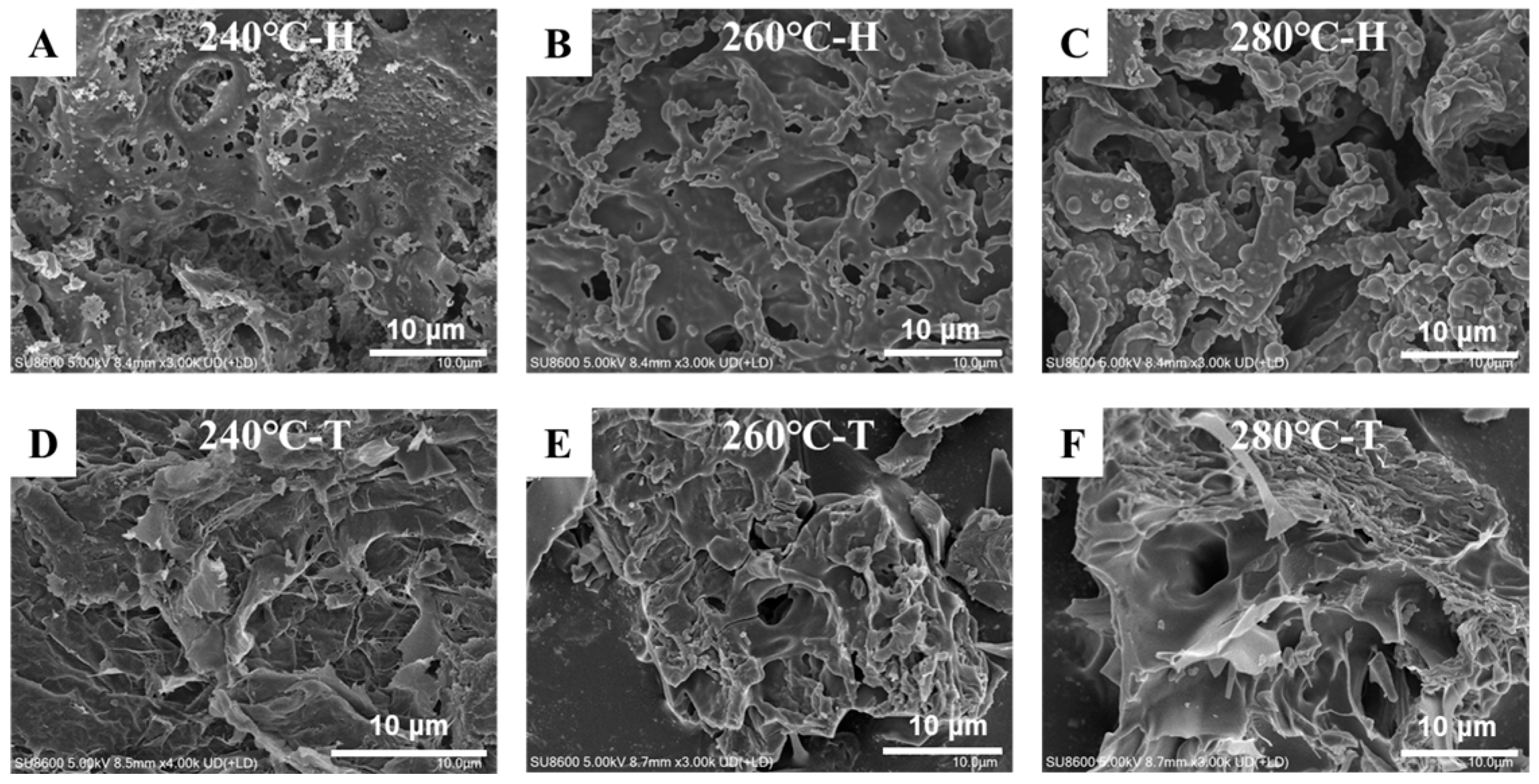


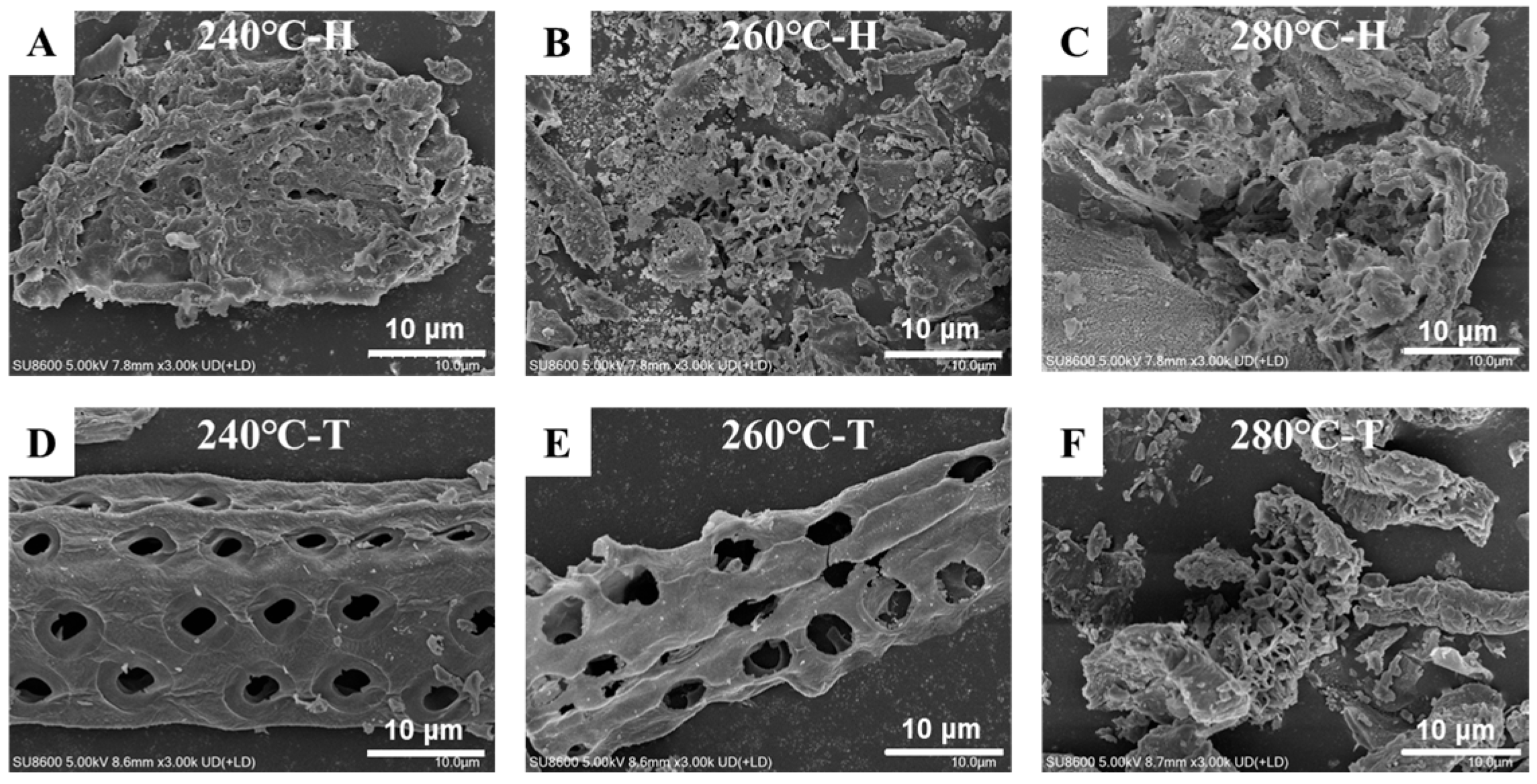
| Shanyao | C | H | N | S | O | A | HHV | Yield | ERE |
|---|---|---|---|---|---|---|---|---|---|
| raw | 40.81 | 7.16 | 1.42 | 0.17 | 47.27 | 3.17 | / | / | / |
| 240-H | 70.63 | 5.5 | 2.39 | 0.12 | 16.54 | 4.82 | 29.06 | 43.37% | 71.23% |
| 260-H | 74.37 | 5.35 | 2.7 | 0.41 | 8.86 | 8.31 | 31.01 | 39.24% | 68.77% |
| 280-H | 74.24 | 5.68 | 2.88 | 0.41 | 3.91 | 13.15 | 31.89 | 36.61% | 65.98% |
| 240-T | 48.185 | 6.17 | 1.68 | 0.185 | 40.07 | 3.71 | 19.74 | 83.50% | / |
| 260-T | 65.14 | 4.83 | 2.65 | 0.12 | 21.60 | 5.66 | 25.81 | 56.78% | / |
| 280-T | 66.005 | 4.79 | 3.125 | 0.06 | 19.57 | 6.45 | 26.27 | 46.69% | / |
| Suoyang | C | H | N | S | O | A | HHV | Yield | ERE |
|---|---|---|---|---|---|---|---|---|---|
| raw | 44.89 | 5.37 | 2.07 | 0.14 | 39.08 | 8.45 | / | / | / |
| 240-H | 58.69 | 5.16 | 3.1 | 0.10 | 25.47 | 7.48 | 23.61 | 52.77% | 70.32% |
| 260-H | 66.44 | 5.64 | 2.91 | 0.20 | 15.88 | 8.93 | 27.90 | 47.43% | 66.81% |
| 280-H | 69.48 | 5.05 | 2.59 | 0.18 | 10.06 | 12.64 | 28.82 | 40.40% | 65.71% |
| 240-T | 56.76 | 4.16 | 2.63 | 0.19 | 33.24 | 3.04 | 20.86 | 63.56% | / |
| 260-T | 57.16 | 4.22 | 2.67 | 0.15 | 32.25 | 3.57 | 21.17 | 61.06% | / |
| 280-T | 59.65 | 4.52 | 2.79 | 0.16 | 29.26 | 3.63 | 22.73 | 48.94% | / |
| Yujin | C | H | N | S | O | A | HHV | Yield | ERE |
|---|---|---|---|---|---|---|---|---|---|
| raw | 46.21 | 6.53 | 0.86 | 0.12 | 38.69 | 7.59 | / | / | / |
| 240-H | 68.19 | 4.83 | 1.19 | 0.15 | 15.29 | 10.35 | 27.53 | 42.03% | 58.85% |
| 260-H | 69.61 | 5.1 | 1.41 | 0.12 | 12.12 | 11.64 | 28.70 | 41.78% | 57.61% |
| 280-H | 70.42 | 4.92 | 1.57 | 0.09 | 10.48 | 12.52 | 28.92 | 37.87% | 55.69% |
| 240-T | 46.28 | 6.09 | 0.70 | 0.02 | 46.19 | 0.73 | 18.30 | 62.16% | / |
| 260-T | 67.99 | 2.79 | 2.65 | 0.12 | 25.40 | 1.14 | 23.47 | 42.06% | / |
| 280-T | 66.01 | 4.79 | 3.13 | 0.06 | 29.21 | 1.31 | 30.14 | 27.43% | / |
| Xueteng | C | H | N | S | O | A | HHV | Yield | ERE |
|---|---|---|---|---|---|---|---|---|---|
| raw | 51.69 | 5.97 | 0.79 | 0.05 | 28.38 | 28.38 | / | / | / |
| 240-H | 64.17 | 5.02 | 1.02 | 0.04 | 19.3 | 10.45 | 25.96 | 54.22% | 64.18% |
| 260-H | 69.35 | 4.76 | 1.34 | 0.04 | 11.74 | 12.77 | 28.21 | 49.38% | 63.52% |
| 280-H | 72.51 | 4.59 | 1.26 | 0.04 | 8.36 | 13.24 | 29.44 | 38.11% | 51.15% |
| 240-T | 55.37 | 3.19 | 1.71 | 0.02 | 34.59 | 5.13 | 19.01 | 65.55% | / |
| 260-T | 63.64 | 2.60 | 1.85 | 0.06 | 22.05 | 6.25 | 22.44 | 52.77% | / |
| 280-T | 67.94 | 2.66 | 1.76 | 0.06 | 24.39 | 9.39 | 23.71 | 42.30% | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Ren, W.; Wu, S.; Xiao, R. Comparison of the Characteristics of Hydrochar and Torrefied-Char of Traditional Chinese Medicine Residues. Energies 2025, 18, 3646. https://doi.org/10.3390/en18143646
Xu Z, Ren W, Wu S, Xiao R. Comparison of the Characteristics of Hydrochar and Torrefied-Char of Traditional Chinese Medicine Residues. Energies. 2025; 18(14):3646. https://doi.org/10.3390/en18143646
Chicago/Turabian StyleXu, Zhiqiang, Wenyu Ren, Shiliang Wu, and Rui Xiao. 2025. "Comparison of the Characteristics of Hydrochar and Torrefied-Char of Traditional Chinese Medicine Residues" Energies 18, no. 14: 3646. https://doi.org/10.3390/en18143646
APA StyleXu, Z., Ren, W., Wu, S., & Xiao, R. (2025). Comparison of the Characteristics of Hydrochar and Torrefied-Char of Traditional Chinese Medicine Residues. Energies, 18(14), 3646. https://doi.org/10.3390/en18143646





