Enhanced Charge Transport in Inverted Perovskite Solar Cells via Electrodeposited La-Modified NiOx Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical Synthesis of La-Modified NiOx Layers
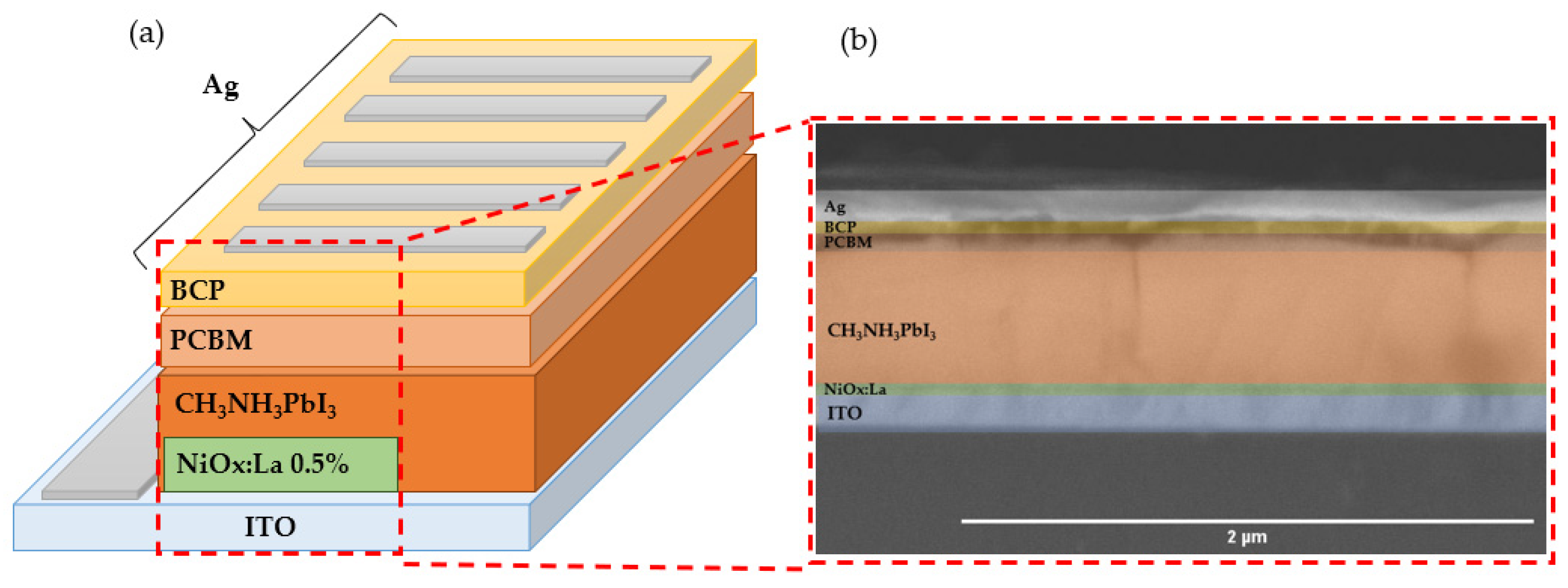
2.2. Inverted Perovskite Solar Cell Fabrication
2.3. Characterization Methods
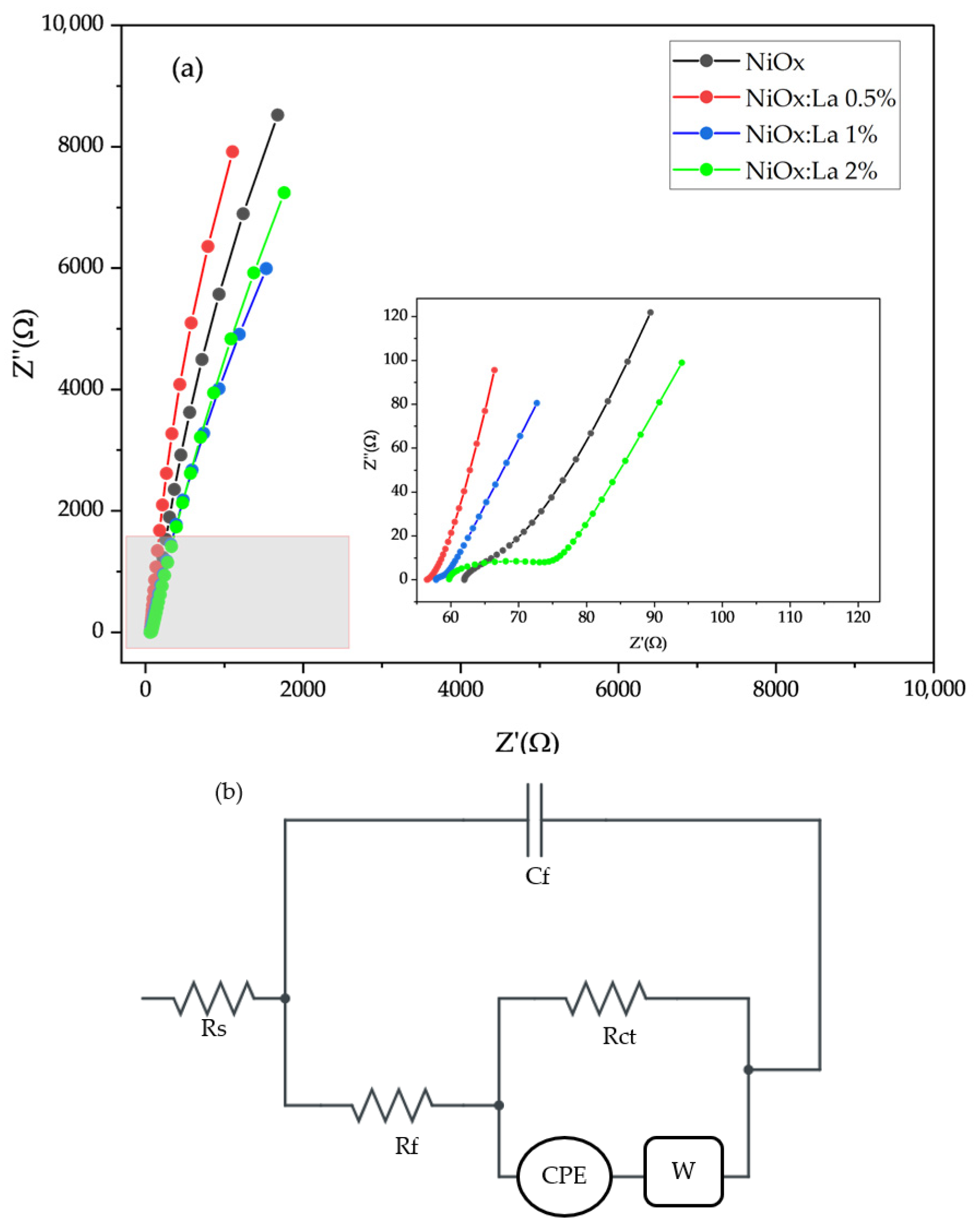
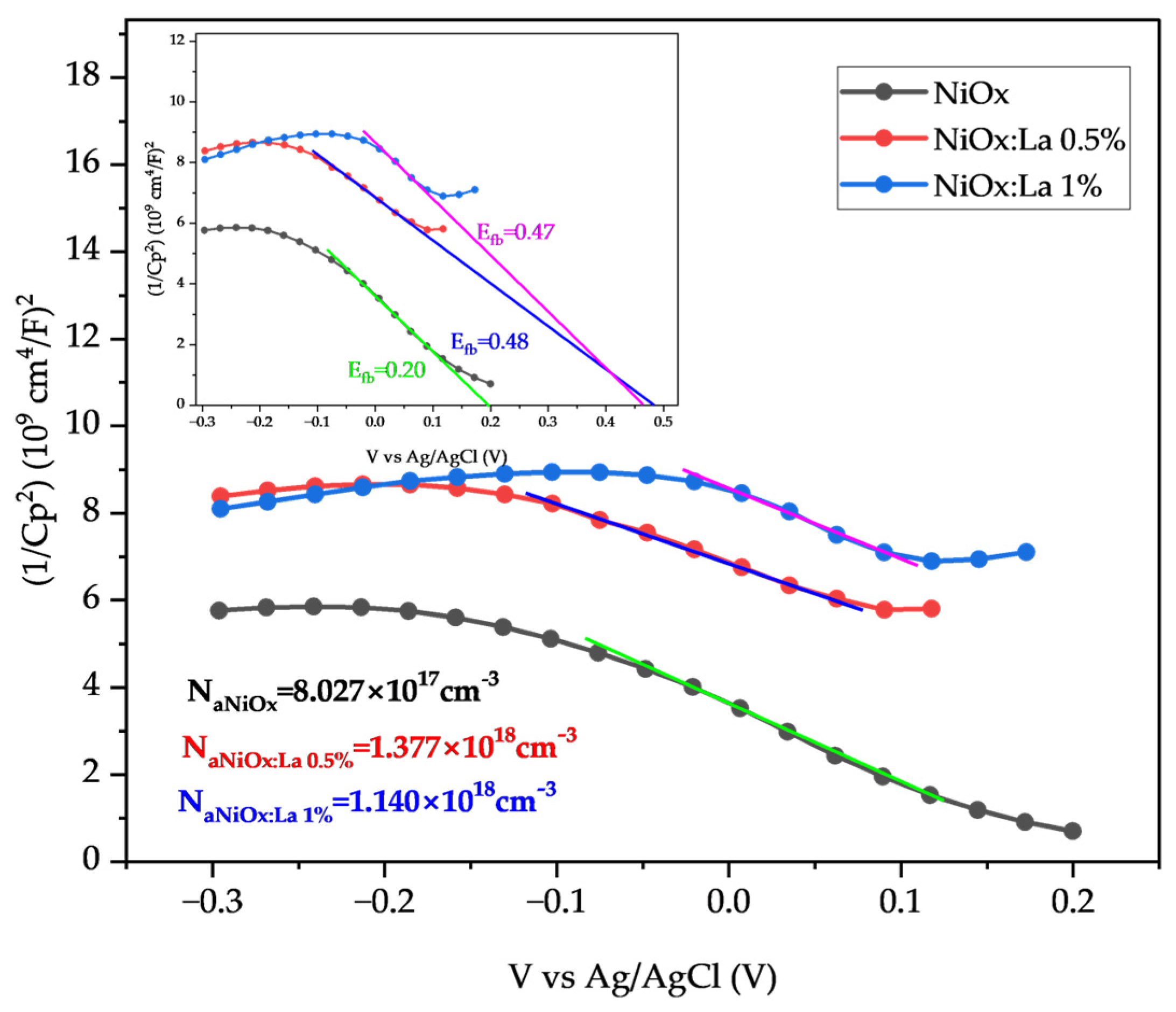
2.3.1. Electrochemical Characterization of La-Modified NiOx
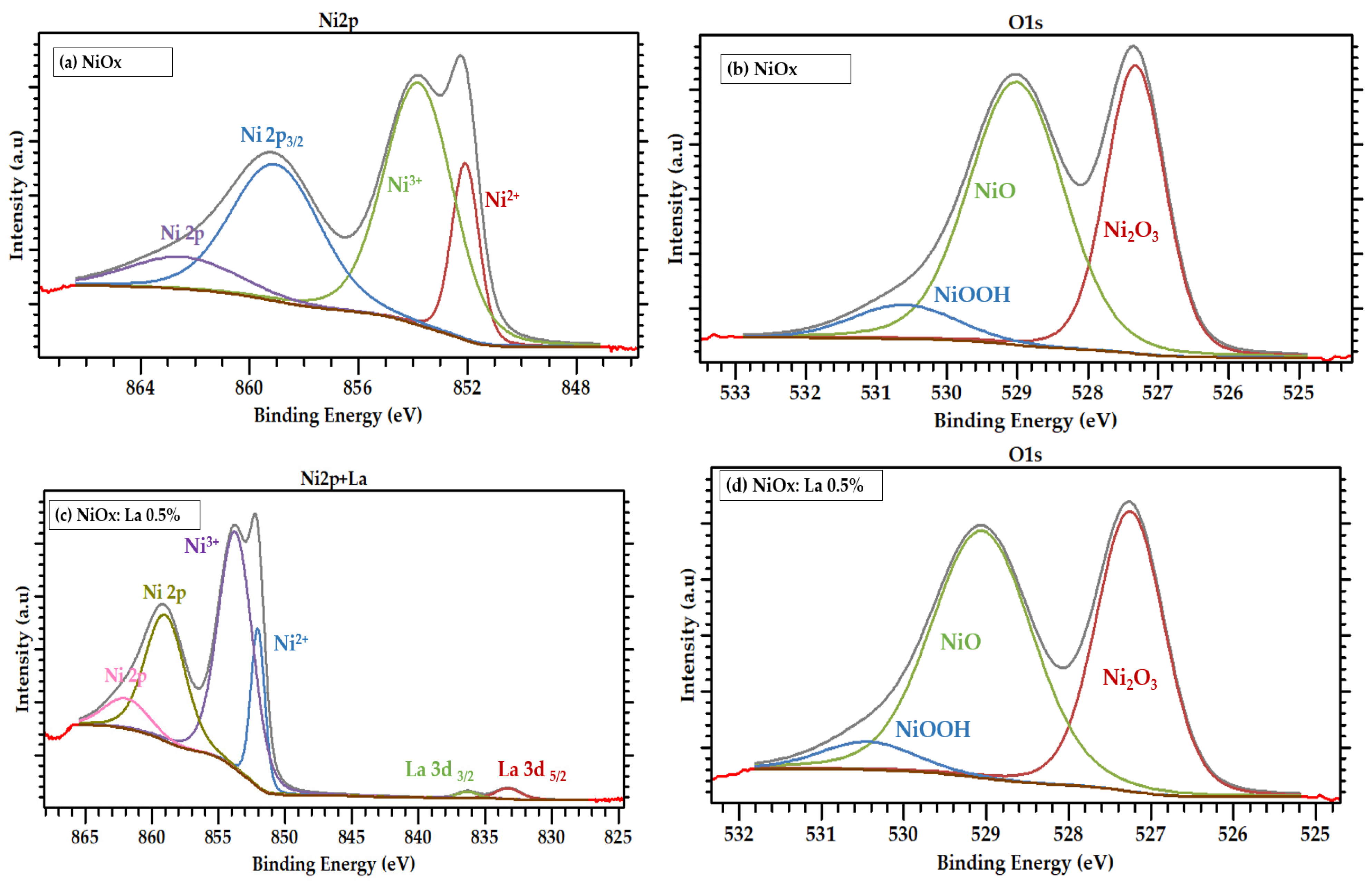
2.3.2. Physicochemical Characterization of La-Modified NiOx
2.3.3. Photovoltaic Characterization of Inverted Perovskite Solar Cells (IPSC)
3. Results and Discussions
3.1. Electrochemical Synthesis and Chemical Characterization of La-Modified NiOx
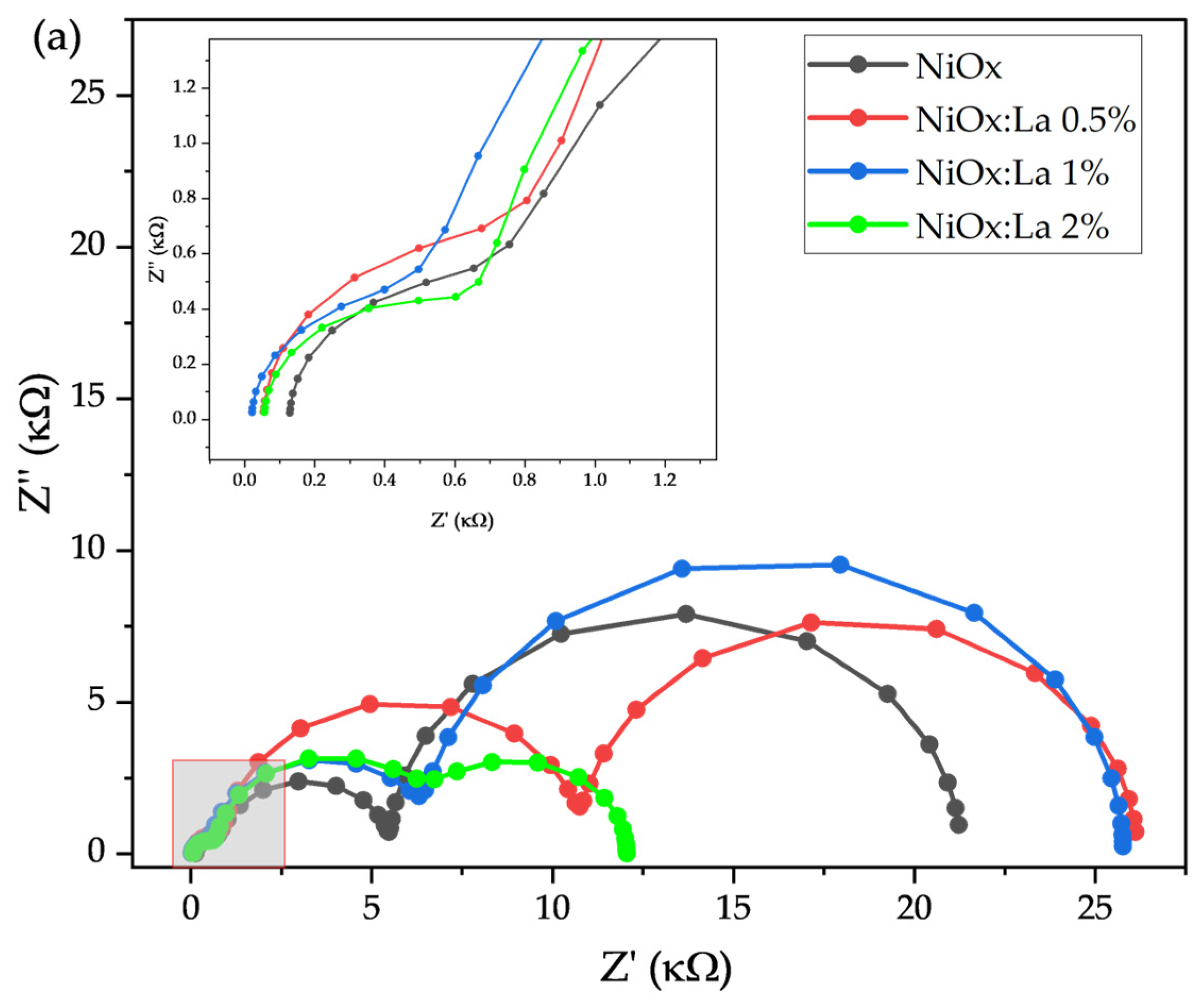
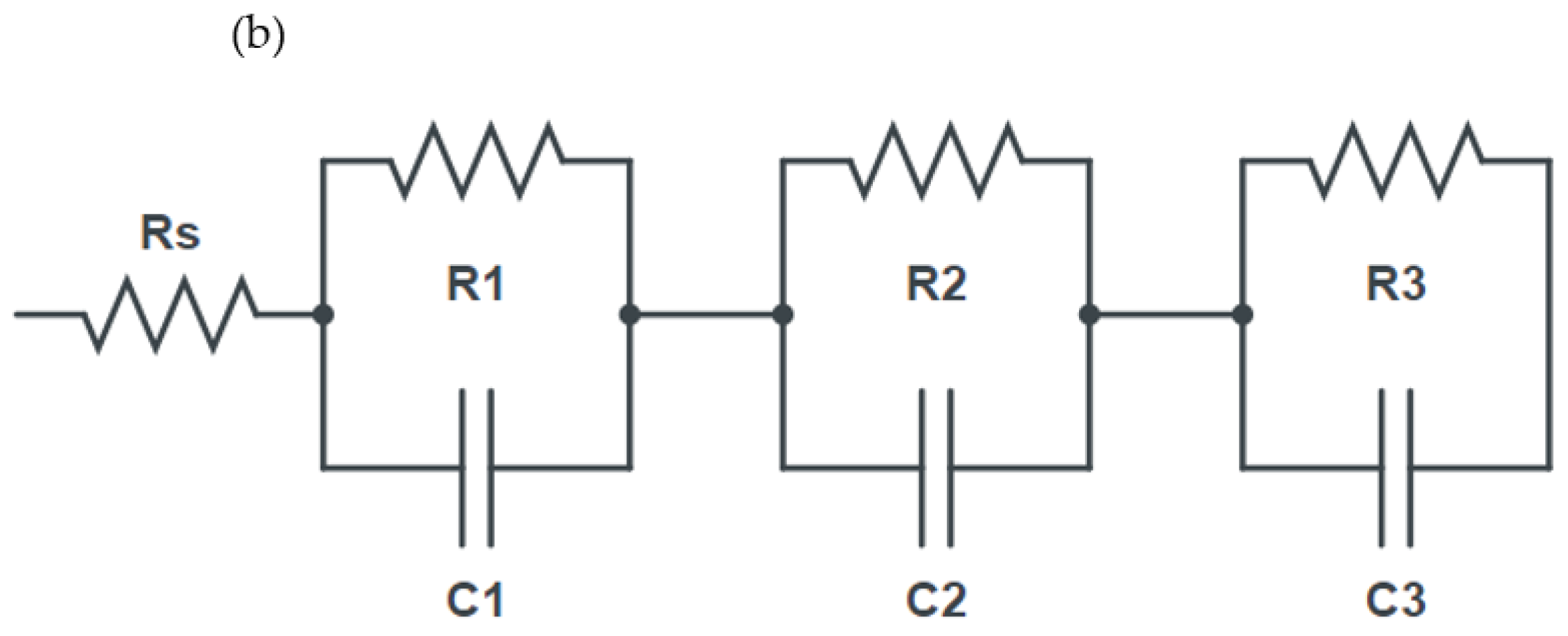
3.2. Electrochemical Characterization of La-Modified NiOx (HTL)
3.3. Photovoltaic Performance of Inverted Perovskite Solar Cells (IPSC)
3.4. Physical, Morphological, and Optoelectronic Characterization of PVK/HTL
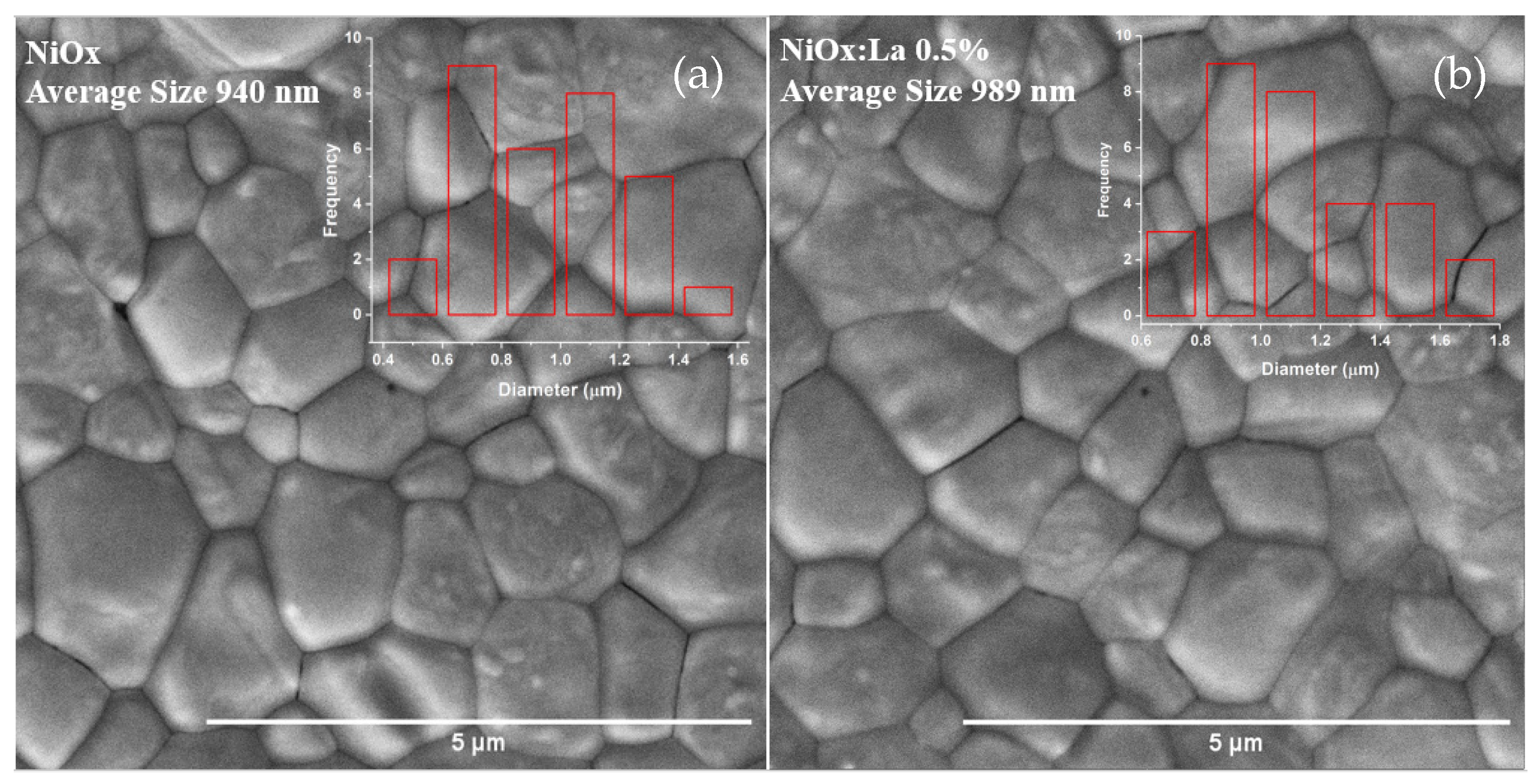
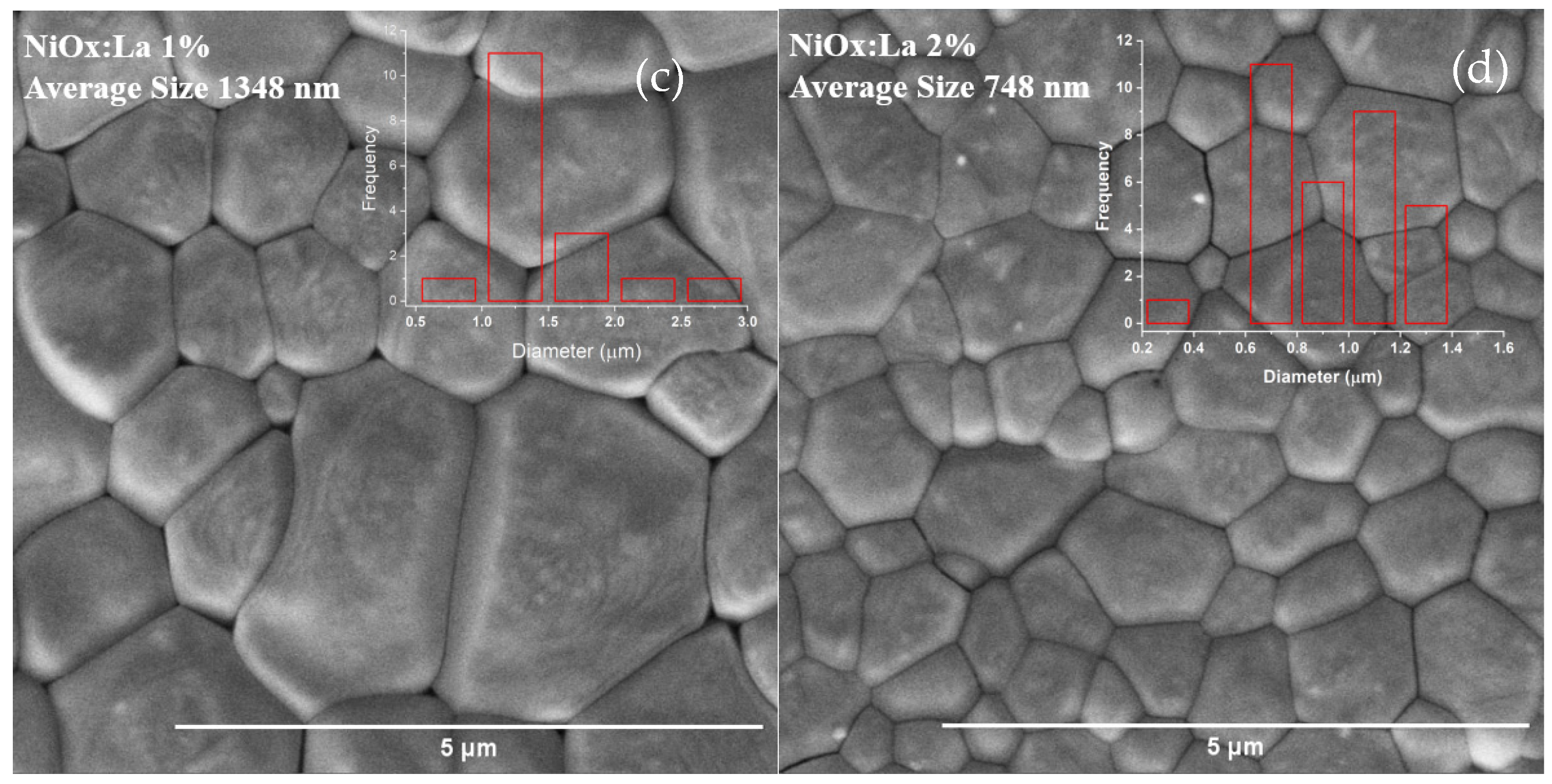
3.4.1. SEM Characterization
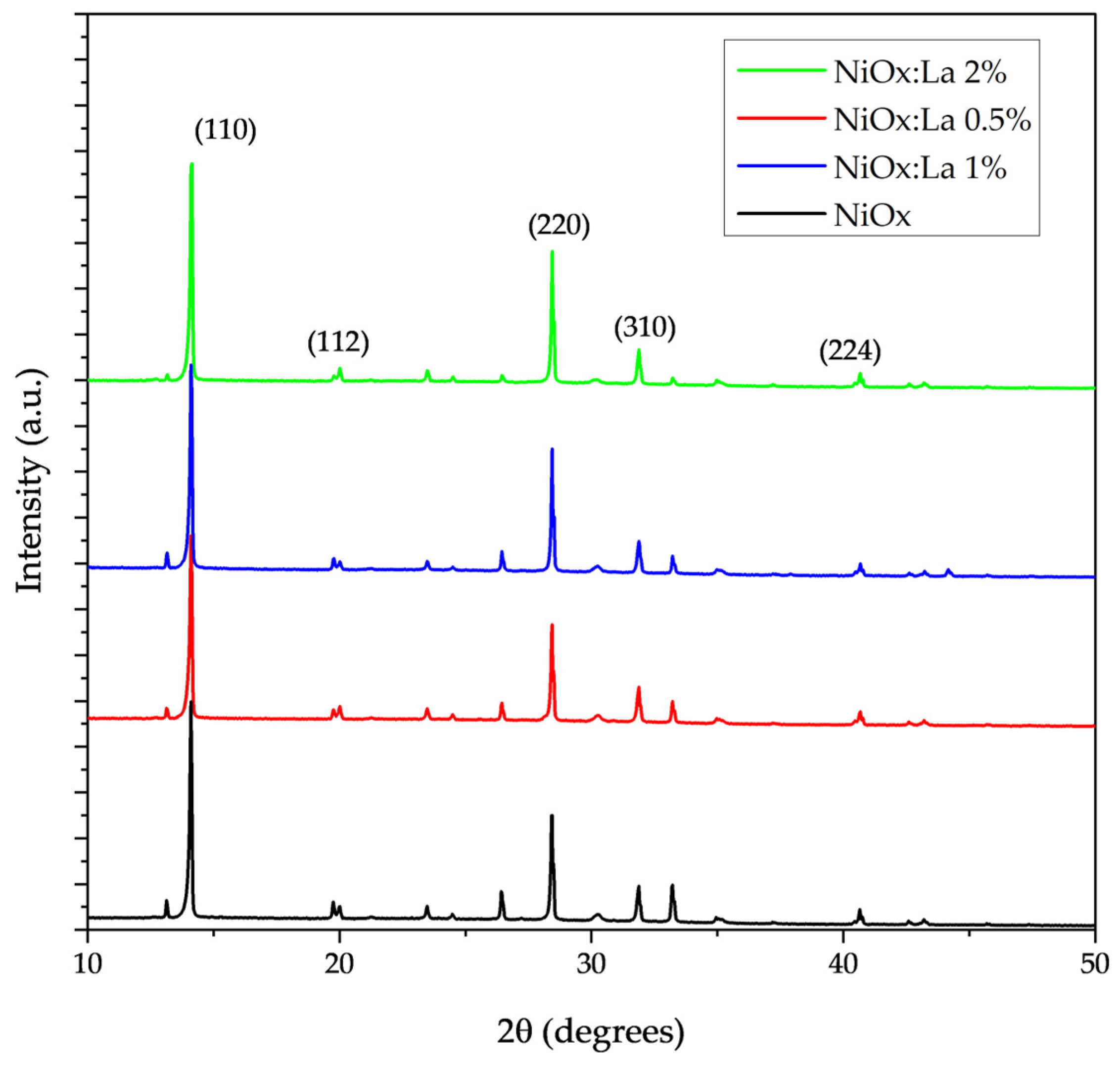
3.4.2. XRD Characterization
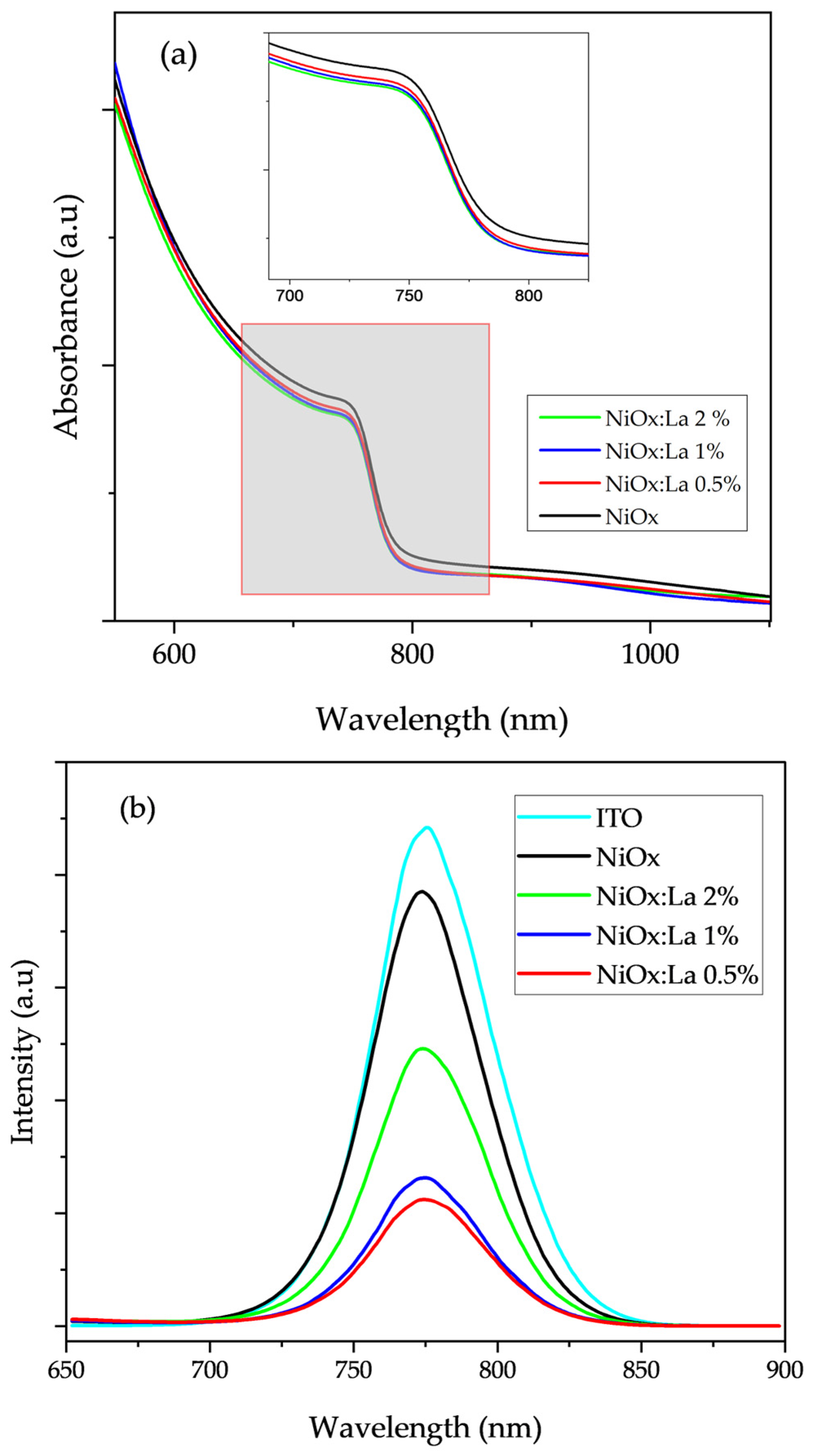
3.4.3. Optical Characterization
3.5. Stability
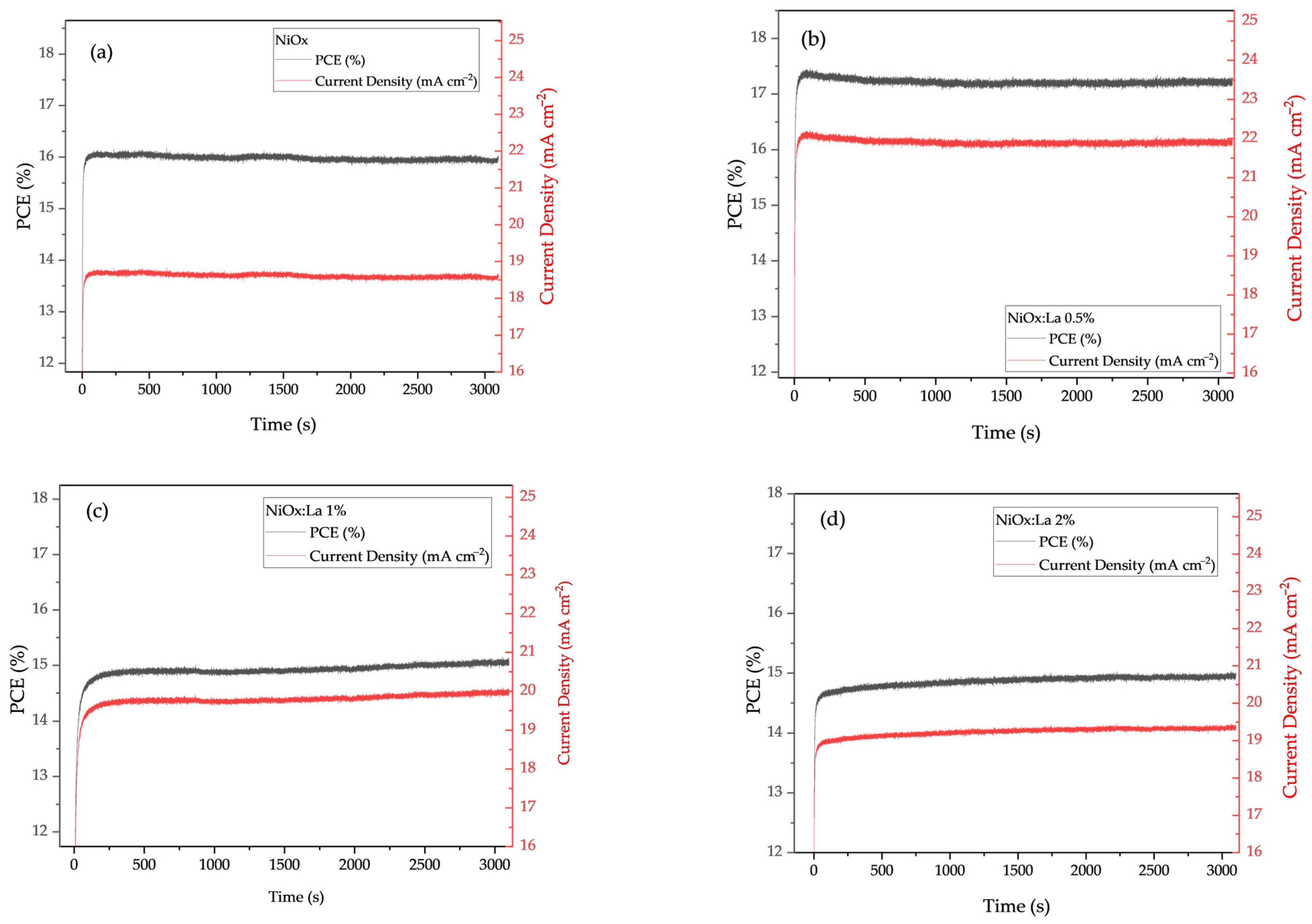
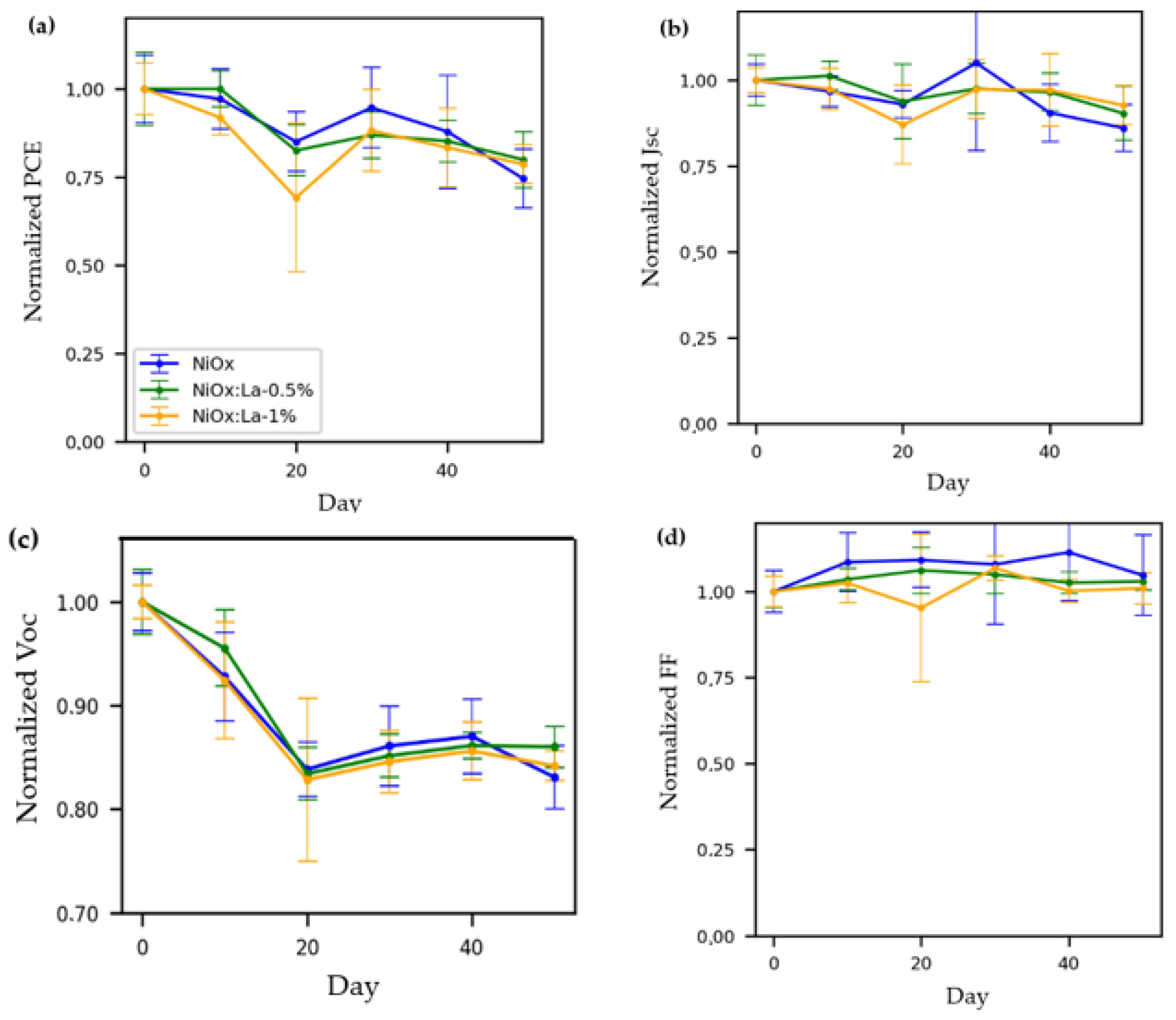
3.5.1. Stability of Cell Photovoltaic Parameters
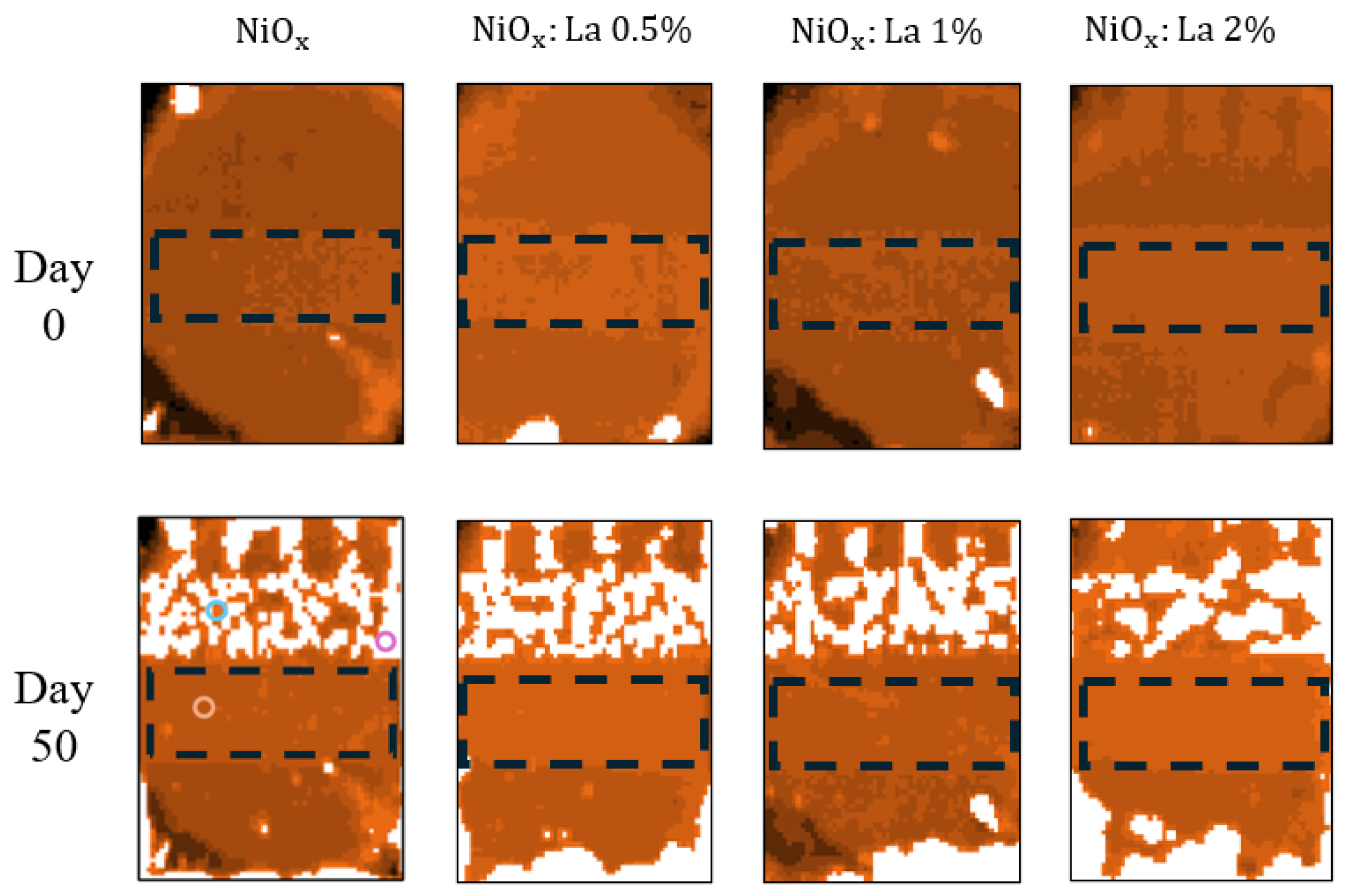
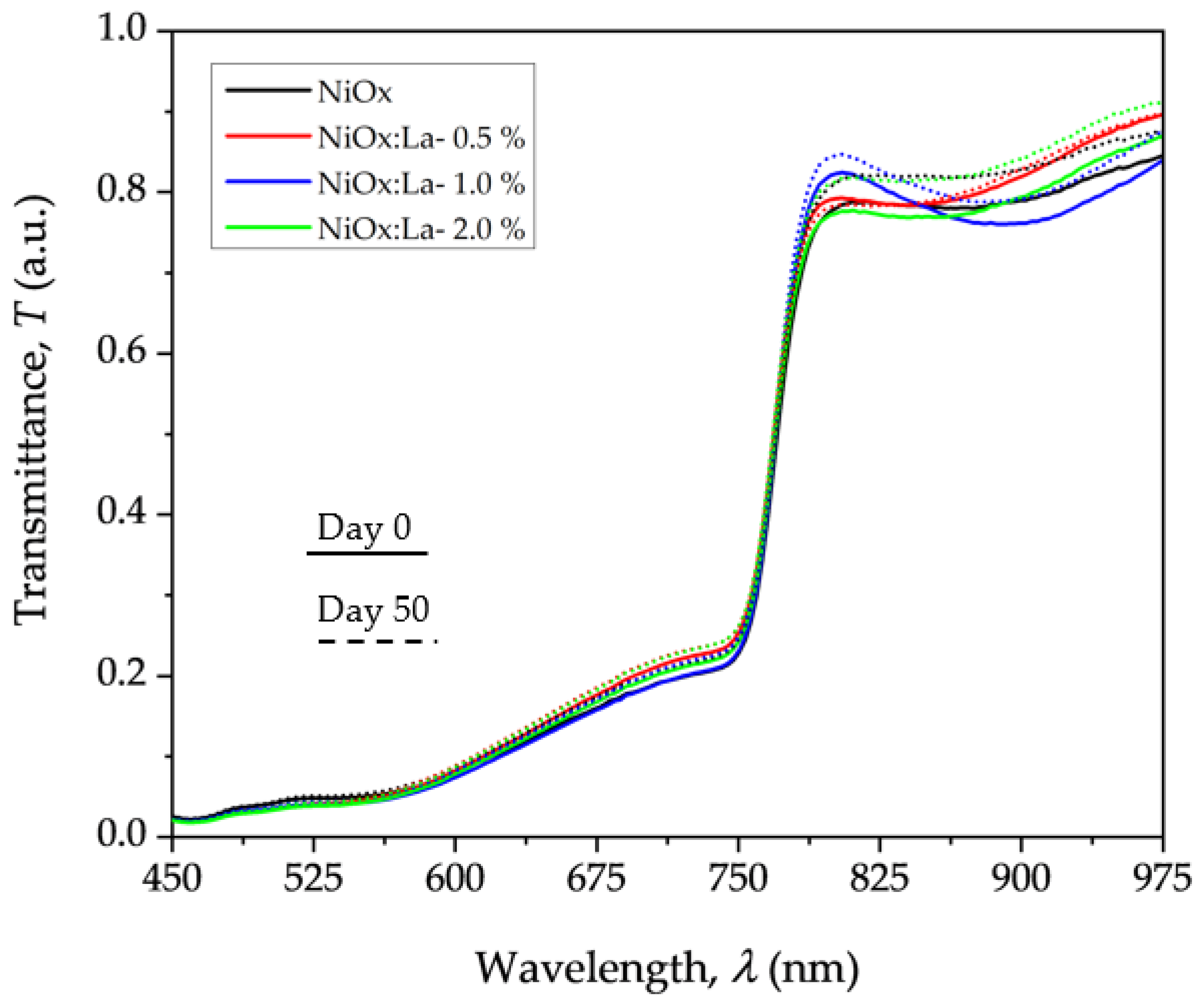
3.5.2. Stability of PVK in ITO/HTL/PVK
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hu, W.; Liu, J.; Han, C.; Gao, Q.; Mei, A.; Zhou, Y.; Guo, F.; Han, H. Achievements, challenges, and future prospects for industrialization of perovskite solar cells. Light Sci. Appl. 2024, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, N.; Chandra Babu Naidu, K. A review on perovskite solar cells (PSCs), materials and applications. J. Mater. 2021, 7, 940–956. [Google Scholar] [CrossRef]
- Vivo, P.; Salunke, J.K.; Priimagi, A. Hole-transporting materials for printable perovskite solar cells. Materials 2017, 10, 1087. [Google Scholar] [CrossRef]
- Cai, X.; Hu, T.; Hou, H.; Zhu, P.; Liu, R.; Peng, J.; Luo, W.; Yu, H. A review for nickel oxide hole transport layer and its application in halide perovskite solar cells. Mater. Today Sustain. 2023, 23, 100438. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y.; Yan, F.; Wang, L.; Wang, K.; Xing, Y.; Dong, Q.; Ma, T. A dual functional additive for the HTM layer in perovskite solar cells. Chem. Commun. 2014, 50, 5020–5022. [Google Scholar] [CrossRef]
- Song, Z.; Watthage, S.C.; Phillips, A.B.; Heben, M.J. Pathways toward high-performance perovskite solar cells: Review of recent advances in organo-metal halide perovskites for photovoltaic applications. J. Photonics Energy 2016, 6, 022001. [Google Scholar] [CrossRef]
- Saha, N.; Brunetti, G.; Armenise, M.N.; Di Carlo, A.; Ciminelli, C. Modeling Highly Efficient Homojunction Perovskite Solar Cells With Graphene-TiO2 Nanocomposite as the Electron Transport Layer. IEEE J. Photovoltaics 2023, 13, 705–710. [Google Scholar] [CrossRef]
- Agresti, A.; Pazniak, A.; Pescetelli, S.; Di Vito, A.; Rossi, D.; Pecchia, A.; Auf der Maur, M.; Liedl, A.; Larciprete, R.; Kuznetsov, D.V.; et al. Titanium-carbide MXenes for work function and interface engineering in perovskite solar cells. Nat. Mater. 2019, 18, 1228–1234. [Google Scholar] [CrossRef]
- Pitchaiya, S.; Natarajan, M.; Santhanam, A.; Asokan, V.; Yuvapragasam, A.; Madurai Ramakrishnan, V.; Palanisamy, S.E.; Sundaram, S.; Velauthapillai, D. A review on the classification of organic/inorganic/carbonaceous hole transporting materials for perovskite solar cell application. Arab. J. Chem. 2020, 13, 2526–2557. [Google Scholar] [CrossRef]
- Kaya, I.C.; Akin, S.; Sonmezoglu, S. Future Perspectives of Perovskite Solar Cells: Metal Oxide-Based Inorganic Hole-Transporting Materials; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128206287. [Google Scholar]
- Park, H.; Chaurasiya, R.; Jeong, B.H.; Sakthivel, P.; Park, H.J. Nickel Oxide for Perovskite Photovoltaic Cells. Adv. Photonics Res. 2021, 2, 2000178. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Chen, C.; Wu, Y.; Bi, W.; Song, Z.; Zhuang, X.; Yang, S.; Zhu, S.; Song, H. Rare earth ions doped NiOx hole transport layer for efficient and stable inverted perovskite solar cells. J. Power Sources 2019, 444, 227267. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, D.; Yang, P.; Yang, L.; Qin, L.; Huang, Y.; Zhao, X. Sol-gel-processed yttrium-doped NiO as hole transport layer in inverted perovskite solar cells for enhanced performance. Appl. Surf. Sci. 2018, 441, 258–264. [Google Scholar] [CrossRef]
- Teo, S.; Guo, Z.; Xu, Z.; Zhang, C.; Kamata, Y.; Hayase, S.; Ma, T. The Role of Lanthanum in a Nickel Oxide-Based Inverted Perovskite Solar Cell for Efficiency and Stability Improvement. ChemSusChem 2019, 12, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Chappidi, V.R.; Nayak, S.K.; Kayesh, M.E.; Karim, M.A.; He, Y.; Islam, A.; Raavi, S.S.K. Elucidating the improved properties of defect engineered lanthanum-doped nickel oxide as hole-transport layer in triple-cation perovskite solar cells. Sol. Energy 2024, 281, 112888. [Google Scholar] [CrossRef]
- Park, I.J.; Kang, G.; Park, M.A.; Kim, J.S.; Seo, W.; Kim, D.H.; Zhu, K.; Park, T.; Kim, J.Y. Highly efficient and uniform 1 cm2 perovskite solar cells with an electrochemically deposited NiOx hole-extraction layer. Energy Mater. 2017, 10, 2660–2667. [Google Scholar] [CrossRef]
- Bogachuk, D.; Baretzky, C.; Eckert, J.; Yang, B.; Suo, J.; Dangudubiyyam, U.K.; Loukeris, G.; Mohammadzadeh, H.; Kluska, S.; Kohlstädt, M.; et al. Rethinking Electrochemical Deposition of Nickel Oxide for Photovoltaic Applications. Sol. RRL 2024, 8, 2300750. [Google Scholar] [CrossRef]
- Shojaie, A.F.; Loghmani, M.H. La3+ and Zr4+ co-doped anatase nano TiO2 by sol-microwave method. Chem. Eng. J. 2010, 157, 263–269. [Google Scholar] [CrossRef]
- González-Hernández, M.; Reyes, S.E.; Erazo, E.A.; Ortiz, P.; Cortés, M.T. Enhanced inverted perovskite solar cells via indium doped nickel oxide as hole transport layer. Sol. Energy 2024, 276, 112657. [Google Scholar] [CrossRef]
- Imran, M.; Coskun, H.; Khan, N.A.; Ouyang, J. Role of annealing temperature of nickel oxide (NiOx) as hole transport layer in work function alignment with perovskite. Appl. Phys. A 2021, 127, 117. [Google Scholar] [CrossRef]
- Blume, A.R.; Calvet, W.; Ghafari, A.; Mayer, T.; Knop-Gericke, A.; Schlögl, R. Structural and chemical properties of NiOx thin films: The role of oxygen vacancies in NiOOH formation in a H2O atmosphere. Phys. Chem. Chem. Phys. 2023, 25, 25552–25565. [Google Scholar] [CrossRef]
- Sharma, R.; Yadav, K. Effect of lattice defects on the structural and optical properties of Ni1 − XAgXO (where X = 0.0, 0.01, 0.03, 0.05, 0.10 and 0.15) nanoparticles. Appl. Phys. A 2018, 124, 88. [Google Scholar] [CrossRef]
- Geng, X.; Lahem, D.; Zhang, C.; Li, C.J.; Olivier, M.G.; Debliquy, M. Visible light enhanced black NiO sensors for ppb-level NO2 detection at room temperature. Ceram. Int. 2019, 45, 4253–4261. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, C.; Chiu, F.; Yang, S. Efficient and stable perovskite solar cells using manganese-doped nickel oxide as the hole transport layer. RSC Adv. 2022, 12, 22984–22995. [Google Scholar] [CrossRef]
- Fingerle, M.; Tengeler, S.; Calvet, W.; Jaegermann, W.; Mayer, T. Sputtered Nickel Oxide Thin Films on n-Si(100)/SiO2 Surfaces for Photo-Electrochemical Oxygen Evolution Reaction (OER): Impact of Deposition Temperature on OER Performance and on Composition before and after OER. J. Electrochem. Soc. 2020, 167, 136514. [Google Scholar] [CrossRef]
- Cole, R.J.; Macdonald, B.F.; Weightman, P. Relative core level shifts in XPS: A theoretical study. J. Electron Spectrosc. Relat. Phenom. 2002, 125, 147–152. [Google Scholar] [CrossRef]
- Akalin, S.A.; Erol, M.; Uzunbayir, B.; Oguzlar, S.; Yildirim, S.; Gokdemir Choi, F.P.; Gunes, S.; Yilmazer Menda, U.D.; Mendes, M.J. Physically-Deposited Hole Transporters in Perovskite PV: NiOx Improved with Li/Mg Doping. Adv. Mater. Technol. 2024, 9, 2301760. [Google Scholar] [CrossRef]
- Grządziel, L.; Krzywiecki, M.; Szwajca, A.; Sarfraz, A.; Genchev, G.; Erbe, A. Detection of intra-band gap defects states in spin-coated sol-gel SnOx nanolayers by photoelectron spectroscopies. J. Phys. D Appl. Phys. 2018, 51, 315301. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Abfalterer, A.; Shamsi, J.; Kubicki, D.J.; Savory, C.N.; Xiao, J.; Divitini, G.; Li, W.; Macpherson, S.; Gałkowski, K.; Macmanus-Driscoll, J.L.; et al. Colloidal Synthesis and Optical Properties of Perovskite-Inspired Cesium Zirconium Halide Nanocrystals. ACS Mater. Lett. 2020, 2, 1644–1652. [Google Scholar] [CrossRef]
- Sahoo, L.; Behera, S.A.; Achary, P.G.R.; Parida, S.K. Impedance spectroscopy and optical properties of lanthanum-modified Bi2FeMnO6 for NTC thermistor applications. Mater. Adv. 2025, 6, 1455–1467. [Google Scholar] [CrossRef]
- Malavekar, D.B.; Magdum, V.V.; Khot, S.D.; Kim, J.H.; Lokhande, C.D. Doping of rare earth elements: Towards enhancing the electrochemical performance of pseudocapacitive materials. J. Alloys Compd. 2023, 960, 170601. [Google Scholar] [CrossRef]
- Han, D.; Jing, X.; Wang, J.; Yang, P.; Song, D.; Liu, J. Porous lanthanum doped NiO microspheres for supercapacitor application. J. Electroanal. Chem. 2012, 682, 37–44. [Google Scholar] [CrossRef]
- Iskandar, F.; Abdillah, O.B.; Stavila, E.; Aimon, A.H. The influence of copper addition on the electrical conductivity and charge transfer resistance of reduced graphene oxide (rGO). N. J. Chem. 2018, 42, 16362–16371. [Google Scholar] [CrossRef]
- Soltani-Ahmadi, M.; Muhler, H. Impedance Spectroscopy. In Electrochemical Impedance Spectroscopy; Wiley-Interscience: Hoboken, NJ, USA, 2020; ISBN 9780470041406. [Google Scholar]
- Randviir, E.P.; Banks, C.E. Electrochemical Impedance Spectroscopy—An Overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Digdaya, I.A.; Rodriguez, P.P.; Ma, M.; Adhyaksa, G.W.P.; Garnett, E.C.; Smets, A.H.M.; Smith, W.A. Engineering the kinetics and interfacial energetics of Ni/Ni-Mo catalyzed amorphous silicon carbide photocathodes in alkaline media. J. Mater. Chem. A 2016, 4, 6842–6852. [Google Scholar] [CrossRef]
- Saki, Z.; Sveinbjörnsson, K.; Boschloo, G.; Taghavinia, N. The Effect of Lithium Doping in Solution-Processed Nickel Oxide Films for Perovskite Solar Cells. ChemPhysChem 2019, 20, 3322–3327. [Google Scholar] [CrossRef] [PubMed]
- Natu, G.; Hasin, P.; Huang, Z.; Ji, Z.; He, M.; Wu, Y. Valence band-edge engineering of nickel oxide nanoparticles via cobalt doping for application in p-type dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2012, 4, 5922–5929. [Google Scholar] [CrossRef]
- Bagade, S.S.; Barik, S.B.; Malik, M.M.; Patel, P.K. Impact of band alignment at interfaces in perovskite-based solar cell devices. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Crovetto, A.; Hansen, O. What is the band alignment of Cu2ZnSn(S,Se)4 solar cells? Sol. Energy Mater. Sol. Cells 2017, 169, 177–194. [Google Scholar] [CrossRef]
- Latrous, A.R.; Mahamdi, R.; Touafek, N.; Pasquinelli, M. Conduction Band Offset Effect on the Cu2ZnSnS4 Solar Cells Performance. Ann. Chim.-Sci. Mater. 2021, 45, 431–437. [Google Scholar] [CrossRef]
- Khenkin, M.V.; Katz, E.A.; Abate, A.; Bardizza, G.; Berry, J.J.; Brabec, C.; Brunetti, F.; Bulović, V.; Burlingame, Q.; Di Carlo, A.; et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 2020, 5, 35–49. [Google Scholar] [CrossRef]
- Contreras-Bernal, L.; Ramos-Terrón, S.; Riquelme, A.; Boix, P.P.; Idígoras, J.; Mora-Seró, I.; Anta, J.A. Impedance analysis of perovskite solar cells: A case study. J. Mater. Chem. A 2019, 7, 12191–12200. [Google Scholar] [CrossRef]
- Hörantner, M.T.; Nayak, P.K.; Mukhopadhyay, S.; Wojciechowski, K.; Beck, C.; McMeekin, D.; Kamino, B.; Eperon, G.E.; Snaith, H.J. Shunt-Blocking Layers for Semitransparent Perovskite Solar Cells. Adv. Mater. Interfaces 2016, 3, 1500837. [Google Scholar] [CrossRef]
- Von Hauff, E.; Klotz, D. Impedance spectroscopy for perovskite solar cells: Characterisation, analysis, and diagnosis. J. Mater. Chem. C 2022, 10, 742–761. [Google Scholar] [CrossRef]
- Ghahremanirad, E.; Almora, O.; Suresh, S.; Drew, A.A.; Chowdhury, T.H.; Uhl, A.R. Beyond Protocols: Understanding the Electrical Behavior of Perovskite Solar Cells by Impedance Spectroscopy. Adv. Energy Mater. 2023, 13, 2204370. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mishra, A.; Abdulrahim, S.M.; Touati, F. Electrical equivalent circuit (EEC) based impedance spectroscopy analysis of HTM free perovskite solar cells. J. Electroanal. Chem. 2020, 871, 114294. [Google Scholar] [CrossRef]
- Muscarella, L.A.; Hutter, E.M.; Sanchez, S.; Dieleman, C.D.; Savenije, T.J.; Hagfeldt, A.; Saliba, M.; Ehrler, B. Crystal Orientation and Grain Size: Do They Determine Optoelectronic Properties of MAPbI3 Perovskite? J. Phys. Chem. Lett. 2019, 10, 6010–6018. [Google Scholar] [CrossRef]
- Gedamu, D.; Asuo, I.M.; Benetti, D.; Basti, M.; Ka, I.; Cloutier, S.G.; Rosei, F.; Nechache, R. Solvent-Antisolvent Ambient Processed Large Grain Size Perovskite Thin Films for High-Performance Solar Cells. Sci. Rep. 2018, 8, 12885. [Google Scholar] [CrossRef]
- Zheng, X.; Deng, Y.; Chen, B.; Wei, H.; Xiao, X.; Fang, Y.; Lin, Y.; Yu, Z.; Liu, Y.; Wang, Q.; et al. Dual Functions of Crystallization Control and Defect Passivation Enabled by Sulfonic Zwitterions for Stable and Efficient Perovskite Solar Cells. Adv. Mater. 2018, 30, 1803428. [Google Scholar] [CrossRef]
- Marand, Z.R.; Kermanpur, A.; Karimzadeh, F.; Barea, E.M.; Hassanabadi, E.; Anaraki, E.H.; Julián-López, B.; Masi, S.; Mora-Seró, I. Structural and electrical investigation of cobalt-doped NiOx/perovskite interface for efficient inverted solar cells. Nanomaterials 2020, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, Y.; Tu, B.; Liu, F.; Djurišić, A.B.; He, Z. Inverted planar organic-inorganic hybrid perovskite solar cells with NiOx hole-transport layers as light-in window. Appl. Surf. Sci. 2018, 451, 325–332. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, J.; Gan, X.; Yuan, H.; Yu, L.; Lu, C.; Sun, H.; Hu, Z.; Zhu, Y. Pb and Li co-doped NiOx for efficient inverted planar perovskite solar cells. J. Colloid Interface Sci. 2020, 559, 29–38. [Google Scholar] [CrossRef]
- Du, T.; Macdonald, T.J.; Yang, R.X.; Li, M.; Jiang, Z.; Mohan, L.; Xu, W.; Su, Z.; Gao, X.; Whiteley, R.; et al. Additive-Free Low-Temperature Crystallization of Stable-FAPbI3 Perovskite. Adv. Mater. 2022, 34, 2107850. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.Z.; Feng, X.Y.; Djurišić, A.B.; Chan, W.K.; He, Z.B. Cesium Doped NiOx as an Efficient Hole Extraction Layer for Inverted Planar Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1700722. [Google Scholar] [CrossRef]
- Da Silva, E.L.; Skelton, J.M.; Parker, S.C.; Walsh, A. Phase stability and transformations in the halide perovskite CsSnI3. Phys. Rev. B 2015, 91, 144107. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, Z.; Su, J.; Zhang, J.; Chang, J.; Hao, Y. A Review on Energy Band-Gap Engineering for Perovskite Photovoltaics. Sol. RRL 2019, 3, 1900304. [Google Scholar] [CrossRef]
- Shalan, A.E.; Oshikiri, T.; Narra, S.; Elshanawany, M.M.; Ueno, K.; Wu, H.P.; Nakamura, K.; Shi, X.; Diau, E.W.G.; Misawa, H. Cobalt Oxide (CoOx) as an Efficient Hole-Extracting Layer for High-Performance Inverted Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 33592–33600. [Google Scholar] [CrossRef]
- Reyes, S.E.; González-Hernández, M.; Cortés, M.T.; Ortiz, P. Effect of NH4F surface treatment of electrodeposited NiOx on the properties and reproducibility of inverted perovskite solar cells. Surf. Interfaces 2025, 56, 105654. [Google Scholar] [CrossRef]
- Roose, B. Ion migration drives self-passivation in perovskite solar cells and is enhanced by light soaking. RSC Adv. 2021, 11, 12095–12101. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.; Bae, S.; Cho, K.; Chung, T.; Mundt, L.E.; Lee, S.; Park, S.; Park, H.; Schubert, M.C.; et al. UV Degradation and Recovery of Perovskite Solar Cells. Sci. Rep. 2016, 6, 38150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, D.; Hu, Y.; Mei, A.; Han, H. Degradation pathways in perovskite solar cells and how to meet international standards. Commun. Mater. 2022, 3, 58. [Google Scholar] [CrossRef]
- Stoddard, R.J.; Dunlap-Shohl, W.A.; Qiao, H.; Meng, Y.; Kau, W.F.; Hillhouse, H.W. Forecasting the Decay of Hybrid Perovskite Performance Using Optical Transmittance or Reflected Dark-Field Imaging. ACS Energy Lett. 2020, 5, 946–954. [Google Scholar] [CrossRef]
- Besleaga, C.; Abramiuc, L.E.; Stancu, V.; Tomulescu, A.G.; Sima, M.; Trinca, L.; Plugaru, N.; Pintilie, L.; Nemnes, G.A.; Iliescu, M.; et al. Iodine Migration and Degradation of Perovskite Solar Cells Enhanced by Metallic Electrodes. J. Phys. Chem. Lett. 2016, 7, 5168–5175. [Google Scholar] [CrossRef] [PubMed]
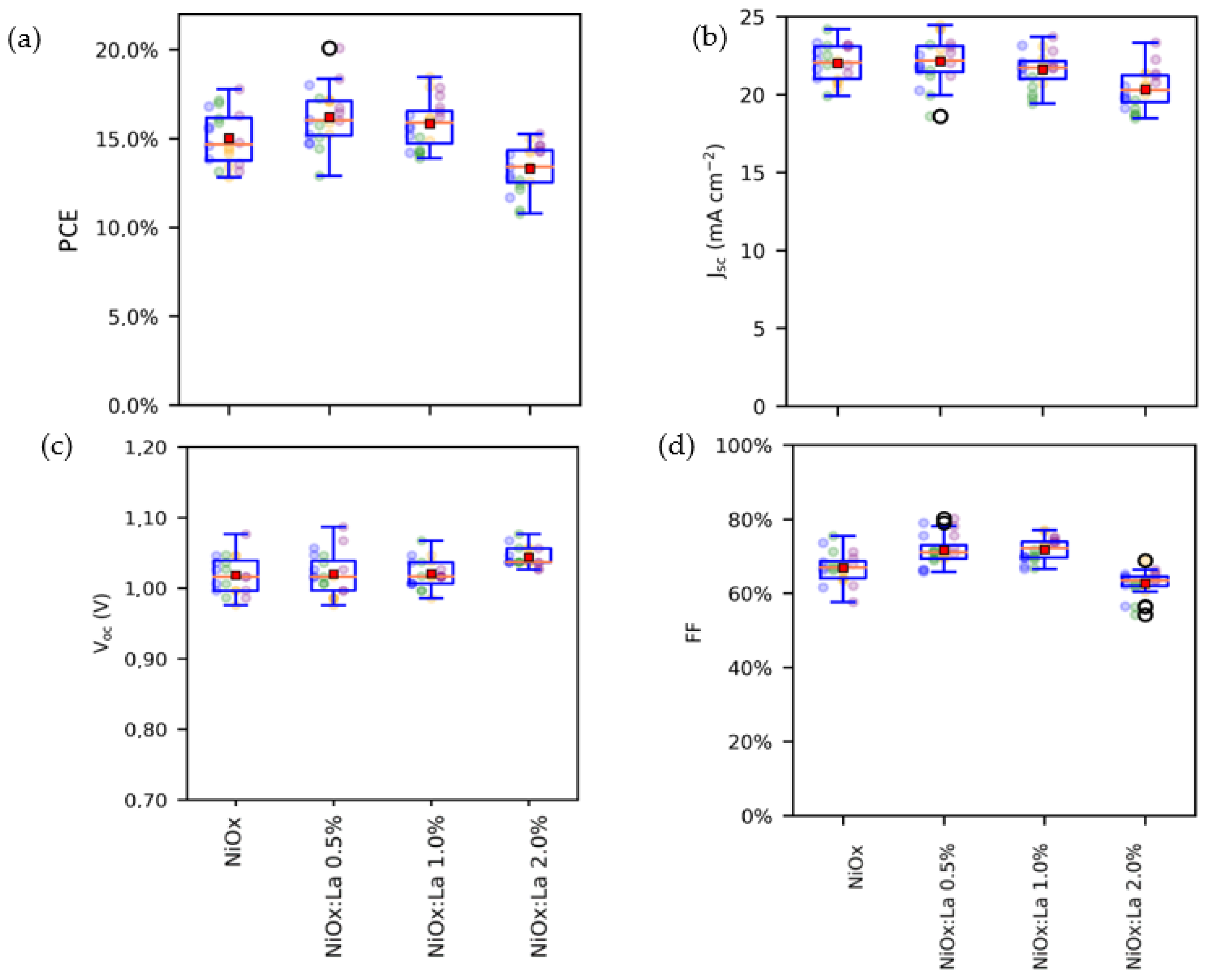
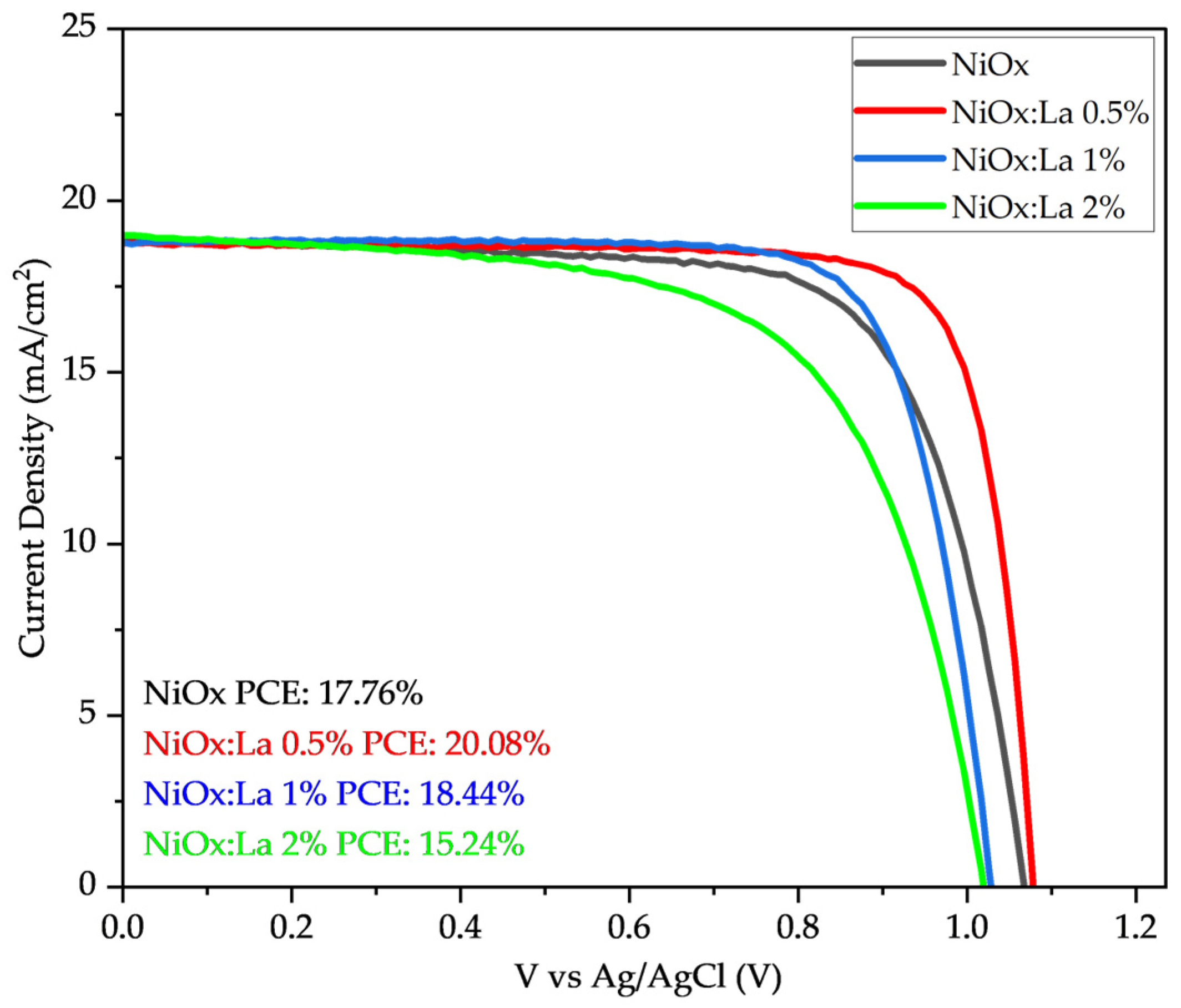
| HTL | PCE (%) | Voc (V) | Jsc (mA cm−2) | FF (%) |
|---|---|---|---|---|
| NiOx | 17.76 | 1.076 | 23.18 | 71.14 |
| NiOx:La 0.5% | 20.08 | 1.086 | 23.06 | 80.12 |
| NiOx:La 1% | 18.44 | 1.036 | 23.08 | 77.06 |
| NiOx:La 2% | 15.24 | 1.026 | 23.33 | 63.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aristizábal-Duarte, L.; González-Hernández, M.; Reyes, S.E.; Ramírez-Rincón, J.A.; Ortiz, P.; Cortés, M.T. Enhanced Charge Transport in Inverted Perovskite Solar Cells via Electrodeposited La-Modified NiOx Layers. Energies 2025, 18, 3590. https://doi.org/10.3390/en18143590
Aristizábal-Duarte L, González-Hernández M, Reyes SE, Ramírez-Rincón JA, Ortiz P, Cortés MT. Enhanced Charge Transport in Inverted Perovskite Solar Cells via Electrodeposited La-Modified NiOx Layers. Energies. 2025; 18(14):3590. https://doi.org/10.3390/en18143590
Chicago/Turabian StyleAristizábal-Duarte, Lina, Martín González-Hernández, Sergio E. Reyes, J. A. Ramírez-Rincón, Pablo Ortiz, and María T. Cortés. 2025. "Enhanced Charge Transport in Inverted Perovskite Solar Cells via Electrodeposited La-Modified NiOx Layers" Energies 18, no. 14: 3590. https://doi.org/10.3390/en18143590
APA StyleAristizábal-Duarte, L., González-Hernández, M., Reyes, S. E., Ramírez-Rincón, J. A., Ortiz, P., & Cortés, M. T. (2025). Enhanced Charge Transport in Inverted Perovskite Solar Cells via Electrodeposited La-Modified NiOx Layers. Energies, 18(14), 3590. https://doi.org/10.3390/en18143590







