Abstract
Ammonia is increasingly recognised as a promising carbon-free fuel and hydrogen carrier due to its high hydrogen content, ease of liquefaction, and existing global infrastructure. However, its direct utilisation in combustion systems poses significant challenges, including low flame speed, high ignition temperature, and the formation of nitrogen oxides (NOX). This review explores catalytic ammonia cracking as a viable method to enhance combustion through in situ hydrogen production. It evaluates traditional catalytic burner designs originally developed for hydrocarbon fuels and assesses their adaptability for ammonia-based applications. Special attention is given to ruthenium- and nickel-based catalysts supported on various oxides and nanostructured materials, evaluating their ammonia conversion efficiency, resistance to sintering, and thermal stability. The impact of the main operational parameters, including reaction temperature and gas hourly space velocity (GHSV), is also discussed. Strategies for combining partial ammonia cracking with stable combustion are studied, with practical issues such as catalyst degradation, NOX regulation, and system scalability. The analysis highlights recent advancements in structural catalyst support, which have potential for industrial-scale application. This review aims to provide future development of low-emission, high-efficiency catalytic burner systems and advance ammonia’s role in next-generation hydrogen energy technologies.
1. Introduction
Global warming is a critical challenge that demands proactive measures to lower greenhouse gas emissions, particularly by minimising hydrocarbon fuel usage [1]. One promising approach involves adopting alternative fuels such as ammonia (NH3) and hydrogen (H2), which have demonstrated significant potential in reducing CO2 emissions [2,3]. Recently, there has been a significant focus on alternatives that mitigate environmental impacts and promote sustainable energy. Ammonia stands out as a promising candidate in global research efforts aimed at developing sustainable energy systems without carbon dependency. It offers an attractive solution for hydrogen storage and transport while also serving as a clean fuel. Its significance arises from its zero carbon content, high hydrogen density, and well-developed global infrastructure, making it a viable alternative fuel for transportation, power generation, and energy storage [4,5].
Ammonia offers a high hydrogen content—approximately 17.6, positioning it as a promising hydrogen carrier for low-carbon energy systems. It can be liquefied under moderate conditions at −33 °C at atmospheric pressure or around 10 bar at room temperature, making logistics much simpler and lowering infrastructure needs compared to other types of hydrogen carriers [6]. Table 1 highlights a comparison of ammonia with other common hydrogen carriers based on key features. Furthermore, Figure 1 illustrates the NH3 value chain as a hydrogen carrier for long-distance NH3 maritime transport.

Table 1.
Comparative characteristics of hydrogen carriers [7,8,9].
Table 1.
Comparative characteristics of hydrogen carriers [7,8,9].
| Properties | Unit | Compressed Hydrogen | Liquid Hydrogen | Methanol | Liquid Ammonia |
|---|---|---|---|---|---|
| Storage Method | - | Compression | Liquefaction | Ambient | Liquefaction |
| Storage Temperature | °C | 25 | −252.9 | 25 | 25 |
| Storage Pressure | MPa | 69 | 0.1 | 0.1 | 0.99 |
| Hydrogen Content | wt.% | 100 | 100 | 12.5 | 17.8 |
| Density | kg/m3 | 39 | 70.8 | 792 | 600 |
| Explosive Limit in Air | %vol | 4–75 | 4–75 | 6.7–36 | 15–28 |
| Gravimetric Energy Density (LHV) | MJ/kg | 120 | 120 | 20.1 | 18.6 |
| Volumetric Energy Density (LHV) | MJ/L | 4.5 | 8.49 | 15.8 | 12.7 |
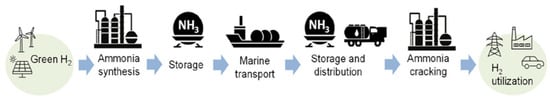
Figure 1.
NH3 value chain as a hydrogen carrier [10].
Ammonia has the potential to be a carbon-free fuel when it is synthesised from renewable energy, which is known as “green ammonia” [5]. From an environmental standpoint, the combustion of ammonia or its use in fuel cells produces no carbon dioxide (CO2). The shift from grey (fossil-based) to blue (with carbon capture) and ultimately green ammonia. That reflects the energy sector’s efforts to become more sustainable. In power applications, ammonia has exhibited strong potential as a fuel in solid oxide fuel cells (SOFCs) with high efficiency and has shown stable performance as a fuel [7]. Furthermore, ammonia can be directly employed in fuel cells or any hydrogen-carrying system, demonstrating its versatility in various energy systems. Although efficiencies are currently lower with direct use of ammonia in fuel cells than with hydrogen fuel cells, its advantages regarding transport and storage outweigh the disadvantages, especially over long distances.
Nevertheless, utilising ammonia directly as a fuel in combustion presents significant challenges. Ammonia’s narrow flammability, high ignition temperature, and low laminar flame speed make it challenging to ignite and sustain a strong flame [11,12,13,14]. The current research primarily addresses low combustion intensity and ignition difficulties through mixed fuel combustion, gas pretreatment technology, optimisation of combustion conditions, and catalytic cracking technology. Due to hydrogen’s significant reactivity and high laminar flame speed, it is considered an effective additive for enhancing ammonia combustion. Joo et al. [15] investigated the flame stability of premixed hydrogen/ammonia flames in a tube-type combustor at normal temperature and pressure. The results indicated that the stability limits expanded with the addition of more hydrogen to the ammonia blend. Zhang et al. [16] examined the combustion and emission characteristics of non-premixed ammonia/hydrogen flames in swirl combustion. The findings show that a 70:30 ratio of NH3 to H2 represents the optimal balance for achieving stable combustion, controlling NOX emissions, minimising NH3 slip, and maximising combustion efficiency in near-stoichiometric conditions. Despite the several benefits associated with co-combustion, it inevitably can lead to high NOX, particularly at elevated hydrogen ratios [17,18]. Therefore, optimising in situ hydrogen production from ammonia presents a promising approach to improve combustion efficiency while reducing exhaust emissions.
Partial cracking is a feasible strategy to improve ammonia combustion, as this technology offers substantial benefits in improving combustion intensity, decreasing ignition delay time, lowering emissions, and being compatible with the existing combustion equipment. Tong et al. [19] conducted a numerical study to examine the improvement of pure ammonia combustion efficiency by a catalytic pre-cracking method utilising a Ni/Al2O3 catalyst and found better ignition, higher flame temperature (by roughly 250 K), and improved flame uniformity. The combustion performance attained through catalytic pre-cracking was comparable to that of NH3–H2 co-combustion with around 40% hydrogen addition. Yu et al. [20] experimentally showed the catalytic efficacy of economical Ni-based systems for partial NH3 decomposition. They generated various Ni catalysts using diverse supports, determining γ-Al2O3 as the most efficacious substrate. Additional performance improvement was attained by doping the Al2O3 support with cerium, resulting in the Ni/Al0.5Ce0.5Ox catalyst exhibiting the greatest activity and stability. This review takes a dual perspective by examining both conventional catalytic burner designs initially created for hydrocarbon fuels and the changing catalyst specifications necessary for the direct production of hydrogen from ammonia via partial decomposition. The objective is to link previous design strategies with the current demand for cleaner, ammonia-based combustion technology. The discourse begins with fundamental principles and reaction mechanisms pertinent to catalytic ammonia decomposition, subsequently transitioning to an examination of traditional burner configurations that have been evaluated with ammonia and ammonia-blended fuels. It offers a comprehensive comparison of ruthenium- and nickel-based catalysts, emphasising the influence of support materials, promoters, temperature, and GHSV on their performance. The paper continues by examining the practical challenges of incorporating these catalysts into combustion systems and proposes avenues for further research. This review offers an innovative perspective by integrating catalyst development with burner design to support the use of ammonia as a viable, carbon-neutral energy carrier. Unlike earlier reviews that tend to address catalyst chemistry and burner configuration in isolation, this work brings these areas together, highlighting how advancements such as structured catalyst supports, modular reaction zones, and in situ hydrogen production can enhance combustion stability and reduce NOX emissions.
2. Fundamentals of Catalytic NH3 Decomposition
Improvements in catalyst research play a significant role in how efficiently energy is used during hydrogen production, whether it is through the thermal cracking of ammonia or using electrocatalytic methods. Better catalysts can make these processes more effective and less energy-intensive. The ammonia cracking is the inverse reaction of the ammonia synthesis process at equilibrium [21]. The following equation can represent NH3 decomposition into H2 and N2 [22].
2NH3 ⇌ N2 + 3H2 ΔH = 46.19 kJ mol−1
NH3 cracking is an endothermic reaction that requires high heat energy. It is important to acknowledge that in the absence of a catalyst, the temperature required for ammonia decomposition is significantly higher. This is due to the strong hydrogen bonds within ammonia molecules, which require significant amounts of energy to break down [23]. Therefore, ammonia decomposition without a catalyst is not practical due to the requirement for high temperatures, energy consumption, and a slow reaction rate. As a result, catalysts have been developed to improve the kinetics of NH3 decomposition and H2 production. The decomposition of ammonia over transition metal catalysts follows a well-recognised sequence of surface reactions. These individual steps have been extensively studied through the density functional theory (DFT) and supported by experimental investigations. The process involves the gradual removal of hydrogen atoms from ammonia molecules adsorbed on the catalyst surface, eventually leading to the formation and release of nitrogen and hydrogen gases. Each intermediate species binds to specific active sites on the surface, typically represented by (*). This mechanistic pathway, fundamental to hydrogen production from ammonia, can be described through the following series of elementary reactions. A visual representation of this mechanism is shown in Figure 2, which illustrates the adsorption of NH3, its progressive dehydrogenation on the catalyst surface, and the final desorption of H2 and N2 molecules within a structured catalytic reactor system [24,25].
NH3 + * ⇌ NH3*
NH3* + * ⇌ NH2* + H*
NH2* + * ⇌ NH* + H*
NH* + * ⇌ N* + H*
N* + N* ⇌ N2 + 2*
H* + H* ⇌ H2 + 2*
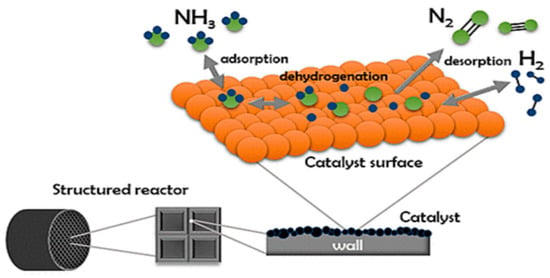
Figure 2.
Catalytic NH3 decomposition into N2 and H2 [26].
3. Traditional Catalytic Ammonia Burner
The experimental development of catalytic burners for ammonia combustion has been driven by the need to overcome ammonia’s low reactivity, high ignition temperature, and the challenge of managing NOX emissions. Contemporary research has largely focused on advanced reactor designs and catalyst formulations to facilitate stable and clean ammonia combustion. A prominent approach in experimental studies involves porous media burners, which are recognised for their ability to enhance flame stabilisation and reduce emissions. Researchers have investigated various configurations of porous burners. For instance, Chen et al. [27] utilised a porous media burner to study pure ammonia combustion and the effects of hydrogen addition. Their experimental setup was designed to facilitate the investigation of flame characteristics, temperature distribution, and NO formation. They found that the physical characteristics of porous materials significantly affect combustion performance. Similarly, Wang et al. [28] explored the dual-combustion of ammonia/methane in a silicon carbide (SiC) ceramic foam burner featuring a two-layer structure specifically designed for flame stabilisation under lean burn conditions. The results show that stable lean dual combustion of methane and ammonia is achievable in a two-layer ceramic foam burner, with the equivalence ratio lower limit reaching 0.5, even for pure ammonia under particular operating conditions. Dai et al. [29] developed a combined porous media burner, integrating pellets within an annular ceramic foam structure, to study lean and rich co-combustion of methane and ammonia, as can be seen in Figure 3. They found that ammonia combustion with methane improved hydrogen production in porous structures. Moreover, Nozari et al. [30] showed that adding hydrogen to an ammonia feed in a SiC porous-media burner significantly improves flame stability and combustion efficiency. Their experiments with premixed NH3–H2–air flames in an inert SiC block burner showed stable operation at ammonia concentrations as high as 90 vol% with combustion efficiencies above 95% and NOX emissions of 35 ppm under fuel-rich conditions. More advanced designs include the two-stage, rich–quench–lean burner design demonstrated by Vignat et al. [31] for pure ammonia combustion. This design uses open-cell ceramic forms with a pore-size-graded topology to achieve substantial improvements in both NOX and unburnt ammonia emissions compared to a single-stage design. Beyond burner geometry improvement, several experimental efforts have been directed towards identifying and characterising effective catalysts for ammonia combustion. Hinokuma et al. [32] conducted detailed studies on copper oxides (CuOx) supported on aluminium silicates and silicon oxides for catalytic ammonia combustion, aiming to lower ignition temperatures while suppressing N2O/NOX formation. Their experimental methodology involved preparing and optimising these catalysts to achieve high ammonia combustion activity and desirable N2 selectivity.
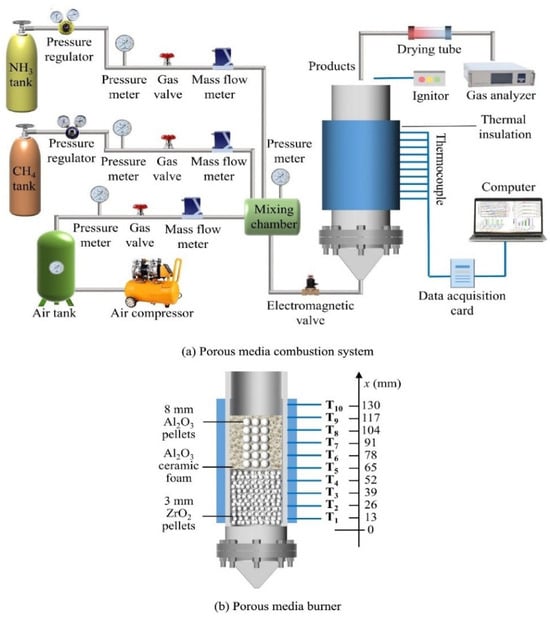
Figure 3.
Schematic diagram of the experimental setup that combined a porous media burner with pellets embedded in annular ceramic foam [29].
4. Catalysts for Ammonia Cracking in Burner Systems
Partial pre-cracking of ammonia through adsorption on the catalyst surface, releasing hydrogen in a sequential process, offers a promising approach to enhancing ammonia combustion by increasing the adiabatic flame temperature and laminar flow velocity. Thermodynamically, high ammonia conversion is achievable at high temperatures. However, the use of a catalyst in ammonia cracking makes the process more energy-efficient, practical, and economically viable by lowering the temperature and activation energy required for hydrogen production [33]. The rates of catalyst reactions and the activity of the catalyst can be improved by the nature of the active metal; the features of the supported material with excellent physical properties, surface area, particle size, and catalyst dispersion; and the effectiveness of the promoter material [21]. The support can stabilise the size and morphology of the metal particles, enhance the exposure of their active sites, and simultaneously influence the electrical structure of the supporting metals [34]. A basic catalyst surface can improve ammonia decomposition by providing active sites that support the adsorption of nitrogen species and promote the stepwise breaking of N–H bonds. In contrast, acidic surfaces lack sufficient electron density, which limits ammonia activation and leads to reduced catalytic efficiency [35]. Ruthenium-based catalyst is widely considered the standard because of their high activity and dependability among the other materials investigated [36]. Nonetheless, the high cost of Ru restricts its large-scale industrial utilisation. In contrast, non-noble metals like nickel, cobalt, and iron are more economical. Ni-based catalysts have attracted considerable interest due to their superior activity, ranking below Ru-based catalysts among non-noble metal alternatives [26].
4.1. Ruthenium-Based Catalysts Supported on Metal Oxide
Recently, researchers have been paying more attention to metal oxides as support for Ru-based catalysts in ammonia decomposition. Thanks to their high number of oxygen vacancies, active sites, and strong redox behaviour, these materials can attract and interact with reactant molecules more easily. This interaction helps change the molecules’ electronic structure, making it easier for the reaction to happen by lowering the energy needed to break down ammonia. Specific experiments have been studied and analysed on various metal oxides, including Al2O3, CeO2, MgO, La2O3, Pr6O11, ZrO2, and TiO2 [37,38,39,40]. These studies are exploring how well different metal oxides perform and how stable they are during ammonia decomposition. The goal is to build an advanced comprehension both in theory and through experiments, which can help guide the development of more effective and affordable catalysts in the future.
Researchers have studied the ammonia decomposition reaction of Ru on Al2O3 and found that Al2O3 exhibits a highly dispersed porous structure characterised by a significant specific surface area, notable porosity, distinctive surface activity, and stability, making it an excellent support material. The structured mesoporous and microporous arrangement of Al2O3 effectively prevents Ru particles from sintering while simultaneously enriching the reactant molecules and extending their reaction time with the catalysts, ultimately resulting in improved catalytic performance [41]. A study investigated ammonia catalytic cracking on a commercial Ru/Al2O3 catalyst at different operative pressures and temperatures. The results indicated that the catalyst had high activity and close to equilibrium conversion at various temperatures and pressures. The decomposition was always higher than 99% at 1 bar at 400 °C, while the conversion at 5 bar reached 96% at 400 °C and 99% at 500 °C [42].
Cerium oxide (CeO2) has emerged as an effective support material for ammonia decomposition catalysts, particularly at lower temperatures, owing to its excellent redox properties and high oxygen storage capacity. Surface oxygen vacancies facilitate strong metal–support interactions, especially with Ru nanoparticles, enhancing active site dispersion, improving catalytic activity, and promoting stability under reaction conditions relevant to partial ammonia cracking. Moreover, the Ru/CeO2 catalyst showed the ability to initiate activity at a temperature as low as 270 °C, which is significantly lower than the 350 °C required by other one-dimensional supports like carbon, alumina, and titanate [43]. The 1.0Ru/CeO2 catalyst achieves a hydrogen production rate of 814 at 300 °C, and the ammonia conversion reaches 33% at the same temperature. Additionally, the catalyst maintained nearly stable performance throughout a 168 h continuous operation at 450 °C with a (GHSV) of 72,000 , exhibiting remarkable stability [44].
MgO is a stable support characterised by strong Lewis basicity that improves the interaction between the metal and the support. However, the relatively low specific surface area of commercial MgO limits the dispersion of Ru. Researchers are dedicated to employing various preparation methods to achieve MgO supports characterised by large specific surface areas and abundant mesoporous structures. The Ru/MgO catalyst synthesised via the deposition–precipitation (DP) method exhibited smaller average Ru particle sizes and a lower apparent activation energy compared to the catalyst prepared through the impregnation (IM) technique. As shown in Figure 4, the Ru/MgO-DP with 5 wt% catalyst achieved an ammonia conversion of 23% at 400 °C and 91% at 500 °C. In contrast, the Ru/MgO-IM with 5 wt% catalyst demonstrated lower conversion rates of 10% at 400 °C and 42.8% at 500 °C [45].
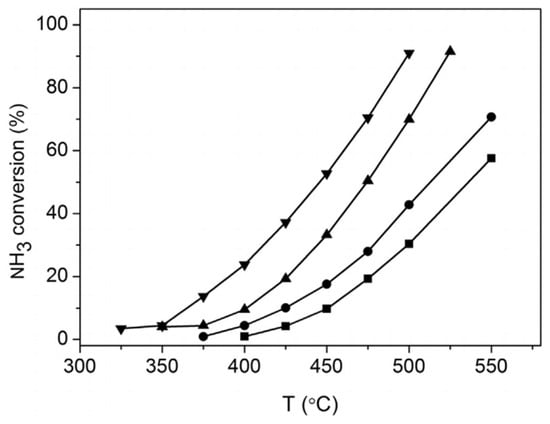
Figure 4.
NH3 conversion as a function of reaction temperature over Ru/MgO catalysts prepared from different methods: (■) 3% Ru/MgO-IM; (●) 5% Ru/MgO-IM; (▲) 3% Ru/MgO-DP; (▼) 5% Ru/MgO-DP [45].
La2O3 is characterised by notable chemical stability and elevated thermal conductivity. Utilising La2O3 as a support facilitates a significant interaction with Ru, effectively regulating its average particle size to 2.3 nm. Ruthenium-based catalysts supported on La2O3, prepared using the impregnation method followed by calcination at 700 °C, demonstrated notable activity in ammonia decomposition. At a temperature of 350 °C and GHSV of 18,000 mL·g−1 h−1, the catalyst achieved an ammonia conversion rate of 15%. This conversion increased to 35% when the temperature was raised to 400 °C. [46].
Besides the previously mentioned metal oxides, alternative supports such as carbon-based materials, hydrotalcite, silicon-based materials, and zeolites are utilised for synthesising Ru-based catalysts, enhancing their structural and electronic characteristics, as well as improving the activity and stability of NH3 decomposition [47].
Carbon-based materials have been widely investigated as supports for ruthenium-based catalysts in the decomposition of ammonia (NH3), with examples including carbon nanotubes (CNTs), carbon nanofibers (CNFs), activated carbon (AC), graphene, and other carbonaceous structures. These materials are characterised by their high specific surface area and excellent electrical conductivity, which facilitate the uniform dispersion of the active metal and enhance electron transfer between the metal and the support [48].
Porous materials like zeolites offer an interesting approach for supporting and stabilising active metal sites used in ammonia decomposition [49]. When ruthenium clusters are encapsulated within the zeolite structure, the catalyst’s activity and stability improve significantly, making it a strong candidate for hydrogen production from ammonia. For example, the 0.1Ru@NaY catalyst was able to convert 92.7% of ammonia and produce hydrogen at a rate of 690 at 500 °C, with a GHSV of 9000 . It also showed outstanding durability, maintaining stable performance over a 95 h test [50].
Impact of Promoters on Ru-Based Catalysts
Promoters play a vital role in ammonia decomposition, with different types affecting the production of hydrogen catalysts in specific ways. The selection and enhancement of promoters are dependent upon the requirements of the catalyst to achieve optimal efficacy. Alkali metal promoters, including Li, Na, K, and Cs, have a significant electron-donating capacity, enhancing the electron density of Ru and promoting the recombination of adsorbed nitrogen [51]. Potassium is the most extensively studied alkali metal promoter in ruthenium-based catalysts for ammonia decomposition. It has been reported that the (K-Ru4O8) catalyst supported on Al2O3 shows a 30–50% increase in NH3 conversion at 350 °C compared to the unpromoted Ru catalyst [52]. On the other hand, the promoters made of alkaline earth metals mostly consist of Ca, Mg, and Ba [53]. All three are primarily utilised to modify the support materials to increase the specific surface area and the catalyst’s basicity. These modifications help improve the dispersion of Ru and optimise its electronic environment, thereby enhancing overall catalytic performance The Ru nanoparticles, confined within ordered mesoporous alumina channels and magnesium oxide, demonstrate structural characteristics that provide uniform 2–3 nm Ru nanoparticles highly dispersed in mesoporous supports, displaying significant catalytic activity and stability [41]. La, Ce, and Y are the most extensively researched rare earth metal promoters that increase metal-support interaction and facilitate electron transport to the active metal Ru. It has been observed that the incorporation of La atoms into the Al2O3 support resulted in a transformation to the La-AlO3 phase, especially when the La content exceeded 10 mol%. The newly formed La-AlO3 species were suggested to exhibit strong interactions with Ru active sites, which subsequently led to reduced Ru sintering and enhanced catalyst stability [54]. Table 2 summarises the partial cracking of ammonia across different Ru-based catalyst supports. Subsequent research should concentrate on enhancing the combination and proportion of support and promoters to evaluate their effect on the long-term stability of catalysts in practical applications.

Table 2.
Summary of experimental studies on Ru-based catalysts for ammonia decomposition.
4.2. Ni-Based Catalysts
The high expense of Ru catalysts restricts their extensive industrial utilisation for ammonia decomposition [24]. Therefore, researchers are looking for other alternative catalysts, and they found that transition metals, including Ni, Fe, and Co, are more economical and have excellent catalytic effectiveness for ammonia decomposition [67,68]. Ni is widely recognised as an active metallic component that facilitates the catalytic decomposition of NH3 into H2. Due to its high activity and cost-effectiveness, it plays a crucial role in the development of metal-based catalysts for ammonia cracking applications. Nickel is far less costly than ruthenium, rendering it more suitable for commercial and industrial applications. When used alone, nickel exhibits limited activity and inadequate stability in ammonia conversion. This is mainly because nickel requires more energy to break the N–H bonds in ammonia, and it tends to sinter when exposed to high temperatures during the reaction. As a result, its surface area shrinks, leaving fewer active sites for the reaction to occur. On top of that, nitrogen-containing species can stick strongly to the nickel surface, blocking those sites and slowing down the process even more. Researchers have developed various supported catalysts exhibiting thermal stability and improved catalytic activity to address these challenges. Nevertheless, owing to the naturally reduced activity of Ni in comparison to noble metals such as Ru, Ni-based catalysts generally require significantly larger loading to achieve comparable activities [69]. The performance of Ni-based catalysts can vary depending on various critical parameters, including the size of the nickel particles, the nature of the support material, the incorporation of promoters, and the method of catalyst preparation. Examining these factors enables researchers to enhance the design of more efficient and stable Ni-based catalysts that perform effectively at reduced temperatures for ammonia decomposition.
The catalytic performance of Ni-based catalysts is affected by the particle size of the active nickel species, as smaller particles provide a higher surface area and more active sites for the reaction. Nanoparticles with a size of around 3.0 nm showed the optimal catalytic activity across various reaction temperatures. However, at temperatures above 400 °C, nickel particles tend to aggregate, resulting in increased particle sizes, leading to a reduction in the catalyst activity [70,71].
The selection of support material influences the catalytic effectiveness in NH3 decomposition, as well as the active phase employed. The use of support characterised by a large surface area, enhanced dispersion, and reduced metal particle sizes typically leads to a rise in catalytic activity. Additionally, the support could influence the electrical structure of the supported metal. The efficiency of Ni-based catalysts is greatly affected by the nature of the support and promoters [72].
4.2.1. Ni-Based Catalysts Supported
The high specific surface area of Al2O3 (approximately 200 m2/g) makes it one of the most common choices as a support, especially in Ni-based catalysts for ammonia decomposition, as it enhances the dispersion of Ni particles, therefore improving the ammonia decomposition reaction. The presence of an additional support and promoter has a significant effect on enhancing the catalytic activity and stability of Ni/Al2O3 catalysts. For example, the addition of promoters like cerium oxide (CeO2) has shown a notable enhancement in catalytic activity and stability. This improvement is due to larger pores in the catalyst, a more even spread of Ni, and a greater ability of Ni/Al2O3 to be reduced. The addition of CeO2 to the Ni/Al2O3 catalyst resulted in a 71.9% conversion of NH3 and a hydrogen formation rate of 24.1 at 773 K and a space velocity of 30,000 [73]. Moreover, the decomposition of ammonia over Ni/Al2O3 catalysts modified by rare-earth elements has been studied to clarify the influence of additions on catalytic activity. The incorporation of rare-earth elements accelerated the degradation reaction. Among the modified catalysts, the La-modified catalyst had the highest activity, achieving 30% conversion at 450 °C, in contrast to the unmodified Ni/Al2O3, which attained 15% conversion at the same temperature [74].
The efficacy of Ni/MgAl2O4-LDH as a catalyst for the decomposition of NH3 has been examined. The results demonstrated exceptional catalytic performance and enhanced durability during the long-term test at 600 °C. The better performance of Ni/MgAl2O4-LDH arises from the capacity of highly dispersed Ni on the MgAl2O4 support, promoting ammonia decomposition and mitigating H2 poisoning. The decomposition reaction commenced at 300 °C and achieved 18% at 350 °C [69].
Among the various support materials investigated, CeO2 has shown consistent performance benefits and is frequently used in studies evaluating ammonia decomposition under low-temperature and high-throughput conditions. On Ni catalysts, CeO2 has been examined with other metal oxide supports (Y2O3, CeO2, MgO, La2O3, Al2O3, and ZrO2). At 450 °C, the Ni/CeO2 catalyst showed superior activity compared to other supports, achieving a conversion rate of 28.6%, which is due to the formation of a reducible O-Ni-O-Ce superstructure at low temperatures [72]. Recently, researchers have employed 3D printing technology to rapidly and efficiently produce CeO2 supports for Ni-based catalysts in ammonia decomposition reactions. The efficacy of the 3D-printed catalysts is equivalent to that of conventional powder catalysts and demonstrates excellent stability. Furthermore, the decrease in Ni progressively activates the catalyst, resulting in an enhancement of the reaction performance [75].
Researchers have examined the potential of rare earth oxides to enhance the efficacy of nickel-based catalysts. The study examined the efficiency of ammonia decomposition using nickel catalysts based on several rare-earth oxides, including Al2O3, La2O3, Sm2O3, Gd2O3, and Y2O3. At 550 °C, Ni/Y2O3 exhibits the highest NH3 conversion, 40%, at 475 °C. This discovery highlights the exceptional efficacy of Ni/Y2O3 catalysts in decomposing NH3, indicating their promise in catalytic applications. Their findings indicated that Ni/Y2O3 exhibited reduced H2 atom adsorption on Ni particles in comparison to alternative catalysts. This demonstrates the efficacy of Y2O3 support in promoting H2 production via dehydrogenation and subsequent H2 desorption. Thus, Ni/Y2O3 catalysts are regarded as potential agents for the decomposition of NH3 [76].
SiO2, which is commonly found in nature, has become an important material in catalysis because of its strong resistance to heat and chemicals. When used as a support in ammonia decomposition, it provides a large surface area that helps spread out the nickel particles more evenly. This better distribution creates more active sites on the catalyst, which can significantly improve its performance. The Ni@SiO2 catalyst, synthesised via a simple sol–gel method, has a high surface area, small Ni particle size, and stable nickel silicate species, because of the strong interaction between nickel and silica species, which prevents the aggregation of nickel species under high-temperature reaction conditions. The catalytic activity of Ni@SiO2 catalysts with differing Ni concentrations has been examined at a GHSV of 30,000 finding that catalytic activity increases with higher Ni content [77].
Besides the commonly utilised Al2O3, CeO2, SiO2, and rare earth oxides, carbon materials, alkaline earth metal oxides, and perovskite oxides may also function as supports for Ni-based catalysts in ammonia decomposition processes. Carbon nanofibers (CNFs) are typically synthesised through the breakdown of carbon-containing gases over transition metal catalysts, particularly those based on nickel. Researchers found the resultant Ni nanoparticles at the tips or bases of CNFs. The Ni nanoparticles at the tips of carbon nanofibers often exhibit a higher number of exposed surfaces compared to those at the roots of carbon [78]. MgO is added to nitrogen-doped carbon nanofibers (NCFs) to form a combined support material, known as MgO-NCF, for nickel-based catalysts. This structure of Ni/MgO-NCFs takes advantage of the support’s strong acidity, good nickel distribution, and highly porous structure. This process generates a mesoporous architecture that improves the adsorption and desorption of reactants and facilitates the effective dissociation of products. Thus, the Ni/MgO-NCFs catalyst demonstrates enhanced catalytic performance and stability relative to Ni/NCFs and Ni/MgO catalysts in ammonia decomposition processes [79].
The perovskite-type oxides (ABO3) are exciting materials due to their remarkable properties, including high electron conductivity, high ion conductivity, and exceptional chemical stability across a broad temperature range. These characteristics have made them valuable as catalysts in various catalytic reactions [80]. The Ni/Co perovskite catalysts based on La have been investigated and employed as a catalyst for NH3 decomposition. La-Ni demonstrated notably appealing activity at a lower temperature in comparison to the La-Co catalyst, achieving 20% of conversion at 375 °C. The conversion of NH3 increased significantly in both Ni and Co catalysts. The combination of La, Ce, and Ni, i.e., the La-Ce-Ni catalyst, exhibited superior performance compared to all the other synthesised catalysts, attributed to its increased surface area, ease of reduction, and suitable basicity [81].
4.2.2. Influence of Promoters on Ni-Based Catalysts
The performance of Ni-based catalysts can be enhanced through the utilisation of various types of promoters. These metals can enhance the activity of Ni-based catalysts by modifying the morphology of Ni particles, increasing the basicity of supported materials, improving NH3 decomposition and N2 desorption, or optimising Ni dispersion and surface area. Adding rare earth, alkaline earth, or alkali metals to nickel-based catalysts has been shown to boost their performance in ammonia decomposition. These added elements can donate electrons, which helps change the shape and electronic properties of the catalyst, as well as how strongly nickel interacts with nitrogen atoms. As a result, the catalyst becomes more effective [82]. The addition of various promoters like K, Na, and Ba to examine the impact of acidic–basic structural modifications of Ni-based catalyst on ammonia cracking has been investigated. The findings indicated that adding alkali metals as promoters, specifically K and Na, to 15% Ni/Al2O3 led to stronger basic sites and a more balanced distribution of acidic and basic surface properties and improved hydrogen desorption from the surface of the catalyst, thereby achieving the highest ammonia decomposition [74].
4.3. Bimetallic Catalyst
Bimetallic catalysts have emerged as innovative materials in heterogeneous catalysis, offering enhanced activity, selectivity, and durability relative to monometallic catalysts. This improved performance is mostly due to the synergistic effect of the metal–metal interaction in bimetallic catalysts. Designing effective bimetallic catalysts for ammonia decomposition largely comes down to how strongly nitrogen atoms bind to the catalyst’s surface. This binding energy plays a crucial role in determining the reaction’s progress. Additionally, the arrangement of the two metals on the surface can alter the catalyst’s behaviour, directly influencing its performance and efficiency in ammonia cracking. Atoms are uniformly scattered throughout the surface, and inside the core comprises an alloy formation. Both metals are present in a pure phase composition within the bulk and on the surface [83].
A group of Ni and Ru and Ni-Ru supported on CeO2 and Al2O3 has been prepared for the catalytic ammonia cracking to produce hydrogen, and their performance was evaluated. The CeO2 showed better support than Al2O3 for this reaction, and the bimetallic system Ni-Ru/CeO2 does not allow for improved results compared to Ru/CeO2. However, the addition of Ru to Ni/Al2O3 and Ni/CeO2 showed a significant increase in ammonia decomposition, especially at low temperatures [84].
The catalytic performance of Ni/Al2O3 for NH3 decomposition has been examined using M-Ni/Al2O3 catalysts (M = Cu, Co, and Fe) to evaluate the impact of transition metal alloying. Among the three bimetallic catalysts, Fe-Ni/Al2O3 and Cu-Ni/Al2O3 exhibited poor catalytic activity, with ammonia conversions that were lower than those of the monometallic Ni/Al2O3. On the contrary, Co-Ni/Al2O3 demonstrated a conversion rate 50% higher than that of Ni/Al2O3, achieving 30% at 450 °C [85].
High-entropy alloys (HEAs), a class of novel materials including five or more elements in near-equiatomic proportions, have recently been recognised as a new paradigm across several applications. This distinctive composition provides an extensive and adjustable chemical space, facilitating the precise modification of surface electronic structures and adsorption energies of reaction intermediates. In ammonia decomposition, high-entropy alloys (HEAs) dynamically integrate the advantageous catalytic characteristics of various transition metals, potentially attaining activity comparable to ruthenium while substantially reducing material cost. Recent investigations indicate the effective application of HEA catalysts, consisting of Ni, Co, Fe, Mo, and Cr, for NH3 decomposition, demonstrating competitive hydrogen yields and superior durability relative to monometallic alternatives. Figure 5. Shows the atomic structure of a representative HEA nanoparticle catalyst composed of Fe, Co, Ni, Cu, and Mo atoms. The equimolar distribution of elements ensures structural stability and optimised electronic interactions at active sites, enhancing catalytic activity for ammonia decomposition [86]. Table 3 provides a comprehensive summary of the effects of nickel content on various support materials and promoters, as well as on bimetallic catalysts for partial cracking of ammonia.
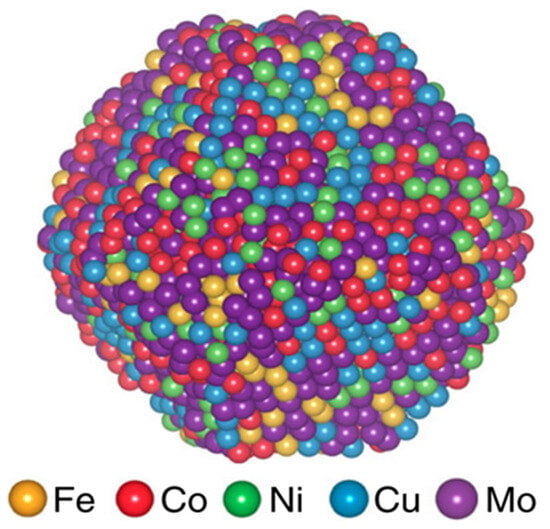
Figure 5.
Schematic atomic structure of a high-entropy alloy (HEA) nanoparticle composed of Ni, Co, Fe, Cu, and Mo atoms [86].

Table 3.
Summary of experimental studies on Ni-based catalysts for ammonia decomposition.
5. Burner Operational Factors Affecting Catalyst Performance
Operating parameters, including catalyst characteristics, physical properties, and reaction conditions, including reaction temperature and gas hourly space velocity, can significantly influence the performance of catalytic ammonia decomposition. Wenjuan Guo et al. [101] developed a machine learning model investigating the impacts of various parameters on catalytic ammonia decomposition, indicating that gas hourly space velocity and the reaction temperature are the two most significant factors influencing ammonia conversion and hydrogen production rate.
5.1. Hourly Space Velocity
GHSV is a crucial operational statistic in heterogeneous catalytic reactions. It is defined by the volumetric flow rate of input gas per unit volume or mass of catalyst per hour. In ammonia decomposition, GHSV directly indicates the residence time of reactant gases in the catalyst bed, influencing the rate of reaction, catalyst performance, and process efficiency. GHSV is expressed in units of h−1 (e.g., or ), reflecting the dynamic equilibrium between kinetic activity and reactor efficiency [102].
A higher GHSV leads to a reduction in residence time between reactant gases and active catalytic sites, causing a decrease in ammonia. In contrast, lower GHSV values increase contact time, potentially improving conversion while lowering the total hydrogen yield per hour and thus impacting overall productivity [103,104]. Therefore, optimising GHSV is crucial for achieving the desired conversion while maintaining acceptable hydrogen production rates, especially under practical operating conditions.
5.2. Reaction Temperature
Controlling the reaction temperature in the catalytic zone of the burner is essential for achieving partial ammonia cracking. Catalytic activity begins around 250–350 °C for Ru-based catalysts, and above 350 °C for Ni-based catalysts. Temperatures between 300 and 500 °C are generally optimal for generating sufficient hydrogen to support ignition and flame stabilisation, depending on the catalyst and burner design. In addition, high temperatures also accelerate sintering and thermal degradation of catalyst supports, especially in Ni-based systems.
6. Challenges and Future Research Directions
Despite advancements in catalytic ammonia decomposition and combustion, significant technical and scientific challenges remain that must be addressed for efficient in situ hydrogen production within burner-integrated systems. The challenges include catalyst degradation, operational constraints, and system-level integration challenges, all of which must be addressed to enable the complete implementation of ammonia-based low-carbon energy solutions. Catalyst deactivation is a major limitation in catalytic ammonia cracking systems due to sintering and poisoning. At high-temperature operation, support phase transformation and metal particle agglomeration occur, affecting catalytic activity and efficiency. Studies have indicated that the porosity, structure, and interaction between the metal and the catalyst’s support material are all affected by the sintering of active sites [105,106]. Current research should focus on developing thermally stable, sintering-resistant catalysts capable of sustaining high dispersion and activity under moderate conditions. The deactivation of catalysts due to poisoning by impurities such as sulphur, water vapour, or residual hydrocarbons in industrial-grade ammonia feedstocks presents a considerable durability challenge. Poisoning reduces catalytic activity by obstructing adsorption sites and changing the surface’s electrical or geometric structure.
Operating at elevated GHSV is crucial for the scalability of catalytic ammonia systems. Elevated GHSV values (>30,000 ) lead to less gas–catalyst residence durations, frequently decreasing ammonia conversion efficiency. Concurrent effective thermal regulation is essential to sustain reaction temperatures while preventing localised overheating. New designs for burner-integrated reactors, such as honeycomb monoliths, structured foams, and 3D-printed catalyst structures, offer potential for increasing contact time, reducing temperature differences, and lowering pressure drops [107,108]. Effective integration into combustion systems requires the precise control of in situ hydrogen generation through partial decomposition of ammonia. Over-cracking in the upstream can lead to near-complete decomposition, which is undesirable in staged combustion systems. This early decomposition of ammonia may result in insufficient unreacted fuel for downstream stages, potentially affecting flame stability and emissions control.
The scalable catalyst manufacturing and the strategic integration of the catalyst within the burner are considered among the challenges in catalytic ammonia decomposition systems. Together, these factors determine the effectiveness, durability, and economic viability of catalytic burners for in situ hydrogen generation. The catalyst positioning within the combustion system is critical for achieving a balance between hydrogen production and enough uncracked ammonia for downstream combustion. Placing the catalysts in the wrong locations can cause the complete cracking of ammonia before combustion, which reduces flame strength and leads to an increase in nitrogen oxide formation. In contrast, insufficient cracking results in poor ignition and stability.
In the future, multiple critical areas must be investigated to advance catalytic ammonia combustion toward practical use in real-world applications. A primary focus must be placed on the development of catalysts that are both effective and cost-efficient. Although ruthenium-based catalysts have excellent performance, they are costly and rare. The following studies need to investigate alternatives using nickel, cobalt, or iron, which are more available and economically viable, although they require enhancements in durability and activity. The utilisation of advanced support materials, such as porous ceramics or nanostructured oxides, can enhance the dispersion of active metals and their long-term performance. Structured catalyst designs, such as honeycomb monoliths and 3D-printed components, present interesting potential, particularly for compact and efficient burner systems, as they improve heat and mass transfer.
Beyond materials, it is crucial to comprehend the performance of these catalysts under real conditions, characterised by temperature variations, different fuels, and the presence of impurities like water or sulphur. Further research is required to optimally position the catalyst inside the combustion system to facilitate partial ammonia cracking while maintaining flame stability. This includes the study of staged combustion configurations and thermal regulation techniques. Comprehensive simulation tools that include reaction kinetics, heat transfer, and fluid dynamics will facilitate improved designs and enhance the prediction of performance at scale. Finally, techno-economic assessments will be crucial for the optimal applications of these systems, including power production, industrial heating, and transportation. Together, these directions provide a definitive framework for transforming ammonia-based combustion from a viable concept into a realistic, sustainable energy solution.
7. Conclusions
Catalytic ammonia combustion represents a promising pathway for zero-carbon energy generation and in situ hydrogen production. This review has examined historical catalytic burner configurations alongside emerging requirements for Ru- and Ni-based catalysts, which remain among the most effective due to their high catalytic activity and selectivity. However, their large-scale implementation is hindered by persistent issues such as sintering, deactivation under prolonged operation, and the high cost of noble metals. Adapting conventional burner architectures for ammonia combustion necessitates careful control of GHSV, temperature distribution, and catalyst positioning to enable efficient partial cracking and stable flame characteristics.
Recent developments in structured catalyst supports, 3D-printed monoliths, and modular reactor setups offer promising ways to build more robust and scalable systems. However, challenges remain, especially when scaling up from lab to industrial scale, where heat distribution and long-term stability are harder to control. There are also integration challenges with downstream technologies like solid oxide fuel cells (SOFCs), which are sensitive to leftover ammonia. In such cases, additional post-combustion cleanup may be necessary to keep emissions low.
Future research should prioritise the development of durable catalysts, scalable fabrication methods, and real-time diagnostic tools. Progress in these areas will be crucial for enabling the effective deployment of ammonia-based energy systems.
Author Contributions
Conceptualisation, K.A.S., E.N. and D.W.; methodology, K.A.S., E.N. and D.W.; formal analysis, K.A.S., E.N. and D.W.; investigation, K.A.S., E.N. and D.W.; data curation, K.A.S., E.N. and D.W.; writing—original draft preparation, K.A.S., E.N. and D.W.; writing—review and editing, K.A.S., E.N. and D.W.; supervision, D.W.; project administration, D.W.; funding acquisition, D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Engineering and Physical Sciences Research Council (EPSRC) through the Flex-Fund project: An Ultra-low NOX Emission Catalytic Burner fueled with Neat Ammonia (UNISON) from the UK-HyRes (UK Hub for Research Challenges in Hydrogen and Alternative Liquid Fuels) (grant number: EP/X038963/1). This work was also financially supported by the UK National Clean Maritime Research Hub (grant number: EP/Y024605/1).
Acknowledgments
The authors would like to thank the support provided by EPSRC (Engineering and Physical Sciences Research Council, United Kingdom) through the research projects (grant numbers: EP/X038963/1 and EP/Y024605/1). The authors would also like to thank the Ministry of Higher Education, Research and Innovation of the Sultanate of Oman for the Stipend Scholarship awarded to the first author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| GHSV | Gas hourly space velocity |
| SOFCs | Solid oxide fuel cells |
| NOX | Nitrogen oxides |
| DFT | Density functional theory |
| Wt.% | Weight percent |
| HEA | High-entropy alloy |
| IM | Impregnation |
| DP | Deposition–precipitation |
| AC | Activated carbon |
| CNFs | Carbon nanofibers |
| Cat | Catalyst |
| MWCNTs | Multi-walled carbon nanotubes |
References
- Zhai, L.; Liu, S.; Xiang, Z. Ammonia as a Carbon-Free Hydrogen Carrier for Fuel Cells: A Perspective. Ind. Chem. Mater. 2023, 1, 332–342. [Google Scholar] [CrossRef]
- Pinzón, M.; García-Carpintero, R.; de la Osa, A.R.; Romero, A.; Abad-Correa, D.; Sánchez, P. Ammonia as a Hydrogen Carrier: An Energy Approach. Energy Convers. Manag. 2024, 321, 118998. [Google Scholar] [CrossRef]
- Erdemir, D.; Dincer, I. A Quicker Route to Hydrogen Economy with Ammonia. Int. J. Hydrogen Energy 2024, 82, 1230–1237. [Google Scholar] [CrossRef]
- Rodriguez, G.D.M.; Carreño, A.P. Ammonia: A Clean Fuel for a Cleaner Future. In Proceedings of the 10th International Conference on Maritime Transport (MT’24), Barcelona, Spain, 5–7 June 2024. [Google Scholar]
- Kojima, Y.; Yamaguchi, M. Ammonia as a Hydrogen Energy Carrier. Int. J. Hydrogen Energy 2022, 47, 22832–22839. [Google Scholar] [CrossRef]
- Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Ammonia as an Effective Hydrogen Carrier and a Clean Fuel for Solid Oxide Fuel Cells. Energy Convers. Manag. 2021, 228, 113729. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P.; et al. Reversible Ammonia-Based and Liquid Organic Hydrogen Carriers for High-Density Hydrogen Storage: Recent Progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-Scale Storage of Hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Spatolisano, E.; Pellegrini, L.A.; de Angelis, A.R.; Cattaneo, S.; Roccaro, E. Ammonia as a Carbon-Free Energy Carrier: NH3 Cracking to H2. Ind. Eng. Chem. Res. 2023, 62, 10813–10827. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar Burning Velocity and Markstein Length of Ammonia/Air Premixed Flames at Various Pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hayakawa, A.; Kitagawa, Y.; Kunkuma Amila Somarathne, K.D.; Kudo, T.; Kobayashi, H. Laminar Burning Velocity and Markstein Length of Ammonia/Hydrogen/Air Premixed Flames at Elevated Pressures. Int. J. Hydrogen Energy 2015, 40, 9570–9578. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.I.; Kwon, O.C. Effects of Ammonia Substitution on Hydrogen/Air Flame Propagation and Emissions. Int. J. Hydrogen Energy 2010, 35, 11332–11341. [Google Scholar] [CrossRef]
- Joo, J.M.; Lee, S.; Kwon, O.C. Effects of Ammonia Substitution on Combustion Stability Limits and NO x Emissions of Premixed Hydrogen-Air Flames. Int. J. Hydrogen Energy 2012, 37, 6933–6941. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, G.; Wang, Z.; Wu, D.; Jangi, M.; Xu, H. Experimental Investigation on Combustion and Emission Characteristics of Non-Premixed Ammonia/Hydrogen Flame. Int. J. Hydrogen Energy 2024, 61, 25–38. [Google Scholar] [CrossRef]
- Ku, J.W.; Ahn, Y.J.; Kim, H.K.; Kim, Y.H.; Kwon, O.C. Propagation and Emissions of Premixed Methane-Ammonia/Air Flames. Energy 2020, 201, 117632. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, B.S. Catalytic Removal of Nitrogen Oxides (NO, NO2, N2O) from Ammonia-Fueled Combustion Exhaust: A Review of Applicable Technologies. Chem. Eng. J. 2023, 461, 141958. [Google Scholar] [CrossRef]
- Tong, C.; Chen, Z.; Cao, J.; Deng, Z.; Chan, S.H. Improvement of Pure Ammonia Combustion Performance Using the Catalytic Pre-Cracking Method. Int. J. Heat Mass Transf. 2025, 241, 126667. [Google Scholar] [CrossRef]
- Yu, M.; Sun, R.; Luo, G.; Wang, L.; Li, X.; Yao, H. Ammonia Partial Cracking over Low-Cost Ni Catalysts for Enhancing Combustion. Fuel 2024, 367, 131306. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.R.F.; Wu, G. Low-Temperature Ammonia Decomposition Catalysts for Hydrogen Generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, Q.; Zhao, D.; Cao, T.; Sha, H.; Zhang, C.; Song, H.; Da, Z. Ammonia as Hydrogen Carrier: Advances in Ammonia Decomposition Catalysts for Promising Hydrogen Production. Renew. Sustain. Energy Rev. 2022, 169, 112918. [Google Scholar] [CrossRef]
- Yin, S.F.; Zhang, Q.H.; Xu, B.Q.; Zhu, W.X.; Ng, C.F.; Au, C.T. Investigation on the Catalysis of COx-Free Hydrogen Generation from Ammonia. J. Catal. 2004, 224, 384–396. [Google Scholar] [CrossRef]
- Lucentini, I.; Garcia, X.; Vendrell, X.; Llorca, J. Review of the Decomposition of Ammonia to Generate Hydrogen. Ind. Eng. Chem. Res. 2021, 60, 18560–18611. [Google Scholar] [CrossRef]
- Lanzani, G.; Laasonen, K. NH3 Adsorption and Dissociation on a Nanosized Iron Cluster. Int. J. Hydrogen Energy 2010, 35, 6571–6577. [Google Scholar] [CrossRef]
- Ristig, S.; Poschmann, M.; Folke, J.; Gómez-Cápiro, O.; Chen, Z.; Sanchez-Bastardo, N.; Schlögl, R.; Heumann, S.; Ruland, H. Ammonia Decomposition in the Process Chain for a Renewable Hydrogen Supply. Chem. Ing. Tech. 2022, 94, 1413–1425. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Li, X.; Deng, L.; He, Z.; Huang, H.; Kobayashi, N. Study on Combustion Characteristics of Hydrogen Addition on Ammonia Flame at a Porous Burner. Energy 2023, 263, 125613. [Google Scholar] [CrossRef]
- Wang, G.; Huang, L.; Tu, H.; Zhou, H.; Chen, X.; Xu, J. Stable Lean Co-Combustion of Ammonia/Methane with Air in a Porous Burner. Appl. Therm. Eng. 2024, 248, 123092. [Google Scholar] [CrossRef]
- Dai, H.; Gao, X.; Liu, C.; Dai, H.; Zhang, L. Lean-Rich Combustion Characteristics of Methane and Ammonia in the Combined Porous Structures for Carbon Reduction and Alternative Fuel Development. Sci. Total Environ. 2024, 938, 173375. [Google Scholar] [CrossRef]
- Nozari, H.; Karaca, G.; Tuncer, O.; Karabeyoglu, A. Porous Medium Based Burner for Efficient and Clean Combustion of Ammonia–Hydrogen–Air Systems. Int. J. Hydrogen Energy 2017, 42, 14775–14785. [Google Scholar] [CrossRef]
- Vignat, G.; Zirwes, T.; Boigné, É.; Ihme, M. Experimental Demonstration of a Two-Stage Porous Media Burner for Low-Emission Ammonia Combustion. Proc. Combust. Inst. 2024, 40, 105491. [Google Scholar] [CrossRef]
- Hinokuma, S.; Kiritoshi, S.; Kawabata, Y.; Araki, K.; Matsuki, S.; Sato, T.; Machida, M. Catalytic Ammonia Combustion Properties and Operando Characterization of Copper Oxides Supported on Aluminum Silicates and Silicon Oxides. J. Catal. 2018, 361, 267–277. [Google Scholar] [CrossRef]
- Chen, C.; Wu, K.; Ren, H.; Zhou, C.; Luo, Y.; Lin, L.; Au, C.; Jiang, L. Ru-Based Catalysts for Ammonia Decomposition: A Mini-Review. Energy Fuels 2021, 35, 11693–11706. [Google Scholar] [CrossRef]
- Hill, A.K.; Torrente-Murciano, L. Low Temperature H2 Production from Ammonia Using Ruthenium-Based Catalysts: Synergetic Effect of Promoter and Support. Appl. Catal. B Environ. 2015, 172–173, 129–135. [Google Scholar] [CrossRef]
- Schüth, F.; Palkovits, R.; Schlögl, R.; Su, D.S. Ammonia as a Possible Element in an Energy Infrastructure: Catalysts for Ammonia Decomposition. Energy Environ. Sci. 2012, 5, 6278–6289. [Google Scholar] [CrossRef]
- Le, T.A.; Do, Q.C.; Kim, Y.; Kim, T.W.; Chae, H.J. A Review on the Recent Developments of Ruthenium and Nickel Catalysts for COx-Free H2 Generation by Ammonia Decomposition. Korean J. Chem. Eng. 2021, 38, 1087–1103. [Google Scholar] [CrossRef]
- Guan, B.; Chen, J.; Zhuang, Z.; Zhu, L.; Ma, Z.; Hu, X.; Zhu, C.; Zhao, S.; Shu, K.; Dang, H.; et al. Study on the Effect and Mechanism of Support Properties on Ru-Based Catalysts for Ammonia Decomposition. Energy Fuels 2025, 39, 2143–2150. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, F.; Qin, Y.; Zhu, Y.H.; Zhou, J.; Li, N. High Performance Ru Loaded MgO Nanoparticle Catalysts for the Hydrogenation of Pyrrole to Pyrrolidine. Catal. Lett. 2025, 155, 175. [Google Scholar] [CrossRef]
- Yan, W.; Wang, W.; Gu, Q.; Zhang, B.; Bi, G.; Zhuo, H.; Shang, X.; Xu, G.; Wang, F.; Zhang, T.; et al. Interfacial Evolution of Ru/TiO2 Catalysts in NH3 Decomposition. J. Energy Chem. 2025, 108, 47–56. [Google Scholar] [CrossRef]
- Felli, A.; Danielis, M.; Trovarelli, A.; Colussi, S. Parametric Study on Ru/CeO2 Ammonia Decomposition Catalysts for Hydrogen Production. ChemCatChem 2025, e00585. [Google Scholar] [CrossRef]
- Tan, H.; Li, K.; Sioud, S.; Cha, D.; Amad, M.H.; Hedhili, M.N.; Al-Talla, Z.A. Synthesis of Ru Nanoparticles Confined in Magnesium Oxide-Modified Mesoporous Alumina and Their Enhanced Catalytic Performance during Ammonia Decomposition. Catal. Commun. 2012, 26, 248–252. [Google Scholar] [CrossRef]
- Di Carlo, A.; Vecchione, L.; Del Prete, Z. Ammonia Decomposition over Commercial Ru/Al2O3 Catalyst: An Experimental Evaluation at Different Operative Pressures and Temperatures. Int. J. Hydrogen Energy 2014, 39, 808–814. [Google Scholar] [CrossRef]
- Hu, Z.; Mahin, J.; Datta, S.; Bell, T.E.; Torrente-Murciano, L. Ru-Based Catalysts for H2 Production from Ammonia: Effect of 1D Support. Top. Catal. 2019, 62, 1169–1177. [Google Scholar] [CrossRef]
- Hu, X.C.; Fu, X.P.; Wang, W.W.; Wang, X.; Wu, K.; Si, R.; Ma, C.; Jia, C.J.; Yan, C.H. Ceria-Supported Ruthenium Clusters Transforming from Isolated Single Atoms for Hydrogen Production via Decomposition of Ammonia. Appl. Catal. B Environ. 2020, 268, 118424. [Google Scholar] [CrossRef]
- Ju, X.; Liu, L.; Yu, P.; Guo, J.; Zhang, X.; He, T.; Wu, G.; Chen, P. Mesoporous Ru/MgO Prepared by a Deposition-Precipitation Method as Highly Active Catalyst for Producing COx-Free Hydrogen from Ammonia Decomposition. Appl. Catal. B Environ. 2017, 211, 167–175. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Yang, J.; Yan, Y.; Wang, D.; Hu, F.; Wang, X.; Zhang, R.; Feng, G. Ru/La 2 O 3 Catalyst for Ammonia Decomposition to Hydrogen. Appl. Surf. Sci. 2019, 476, 928–936. [Google Scholar] [CrossRef]
- Su, Z.; Guan, J.; Liu, Y.; Shi, D.; Wu, Q.; Chen, K.; Zhang, Y.; Li, H. Research Progress of Ruthenium-Based Catalysts for Hydrogen Production from Ammonia Decomposition. Int. J. Hydrogen Energy 2024, 51, 1019–1043. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.H.; Wang, S.; Ma, Q.; Rudolph, V.; Lu, G.Q. Effects of Nitrogen Doping on the Structure of Carbon Nanotubes (CNTs) and Activity of Ru/CNTs in Ammonia Decomposition. Chem. Eng. J. 2010, 156, 404–410. [Google Scholar] [CrossRef]
- Leung, K.C.; Hong, S.; Li, G.; Xing, Y.; Ng, B.K.Y.; Ho, P.L.; Ye, D.; Zhao, P.; Tan, E.; Safonova, O.; et al. Confined Ru Sites in a 13X Zeolite for Ultrahigh H2 Production from NH3 Decomposition. J. Am. Chem. Soc. 2023, 145, 14548–14561. [Google Scholar] [CrossRef]
- Gong, S.; Du, Z.; Tang, Y.; Chen, J. Encapsulation of Ru Nanoparticles within NaY Zeolite for Ammonia Decomposition. Int. J. Hydrogen Energy 2024, 88, 142–150. [Google Scholar] [CrossRef]
- Zhu, N.; Yang, F.; Hong, Y.; Liang, J. Hydrogen Production from Ammonia Decomposition: Advances in Ru- and Ni-Based Catalysts. Int. J. Hydrogen Energy 2025, 98, 1243–1261. [Google Scholar] [CrossRef]
- Pyrz, W.; Vijay, R.; Binz, J.; Lauterbach, J.; Buttrey, D.J. Characterization of K-Promoted Ru Catalysts for Ammonia Decomposition Discovered Using High-Throughput Experimentation. Top. Catal. 2008, 50, 180–191. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, Y.; Shen, X.; Cai, Z. Ruthenium Catalyst Supported on Ba Modified ZrO 2 for Ammonia Decomposition to CO x -Free Hydrogen. Int. J. Hydrogen Energy 2019, 44, 7300–7307. [Google Scholar] [CrossRef]
- Chung, D.B.; Kim, H.Y.; Jeon, M.; Lee, D.H.; Park, H.S.; Choi, S.H.; Nam, S.W.; Jang, S.C.; Park, J.H.; Lee, K.Y.; et al. Enhanced Ammonia Dehydrogenation over Ru/La(x)-Al2O3 (x = 0–50 Mol%): Structural and Electronic Effects of La Doping. Int. J. Hydrogen Energy 2017, 42, 1639–1647. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Ng, C.F.; Au, C.T. Nano Ru/CNTs: A Highly Active and Stable Catalyst for the Generation of COx-Free Hydrogen in Ammonia Decomposition. Appl. Catal. B Environ. 2004, 48, 237–241. [Google Scholar] [CrossRef]
- Armenise, S.; Cazaña, F.; Monzón, A.; García-Bordejé, E. In Situ Generation of COx-Free H2 by Catalytic Ammonia Decomposition over Ru-Al-Monoliths. Fuel 2018, 233, 851–859. [Google Scholar] [CrossRef]
- Furusawa, T.; Kuribara, H.; Kimura, K.; Sato, T.; Itoh, N. Development of a Cs-Ru/CeO2 Spherical Catalyst Prepared by Impregnation and Washing Processes for Low-Temperature Decomposition of NH3: Characterization and Kinetic Analysis Results. Ind. Eng. Chem. Res. 2020, 59, 18460–18470. [Google Scholar] [CrossRef]
- Yamazaki, K.; Matsumoto, M.; Ishikawa, M.; Sato, A. NH3 Decomposition over Ru/CeO2-PrOx Catalyst under High Space Velocity Conditions for an on-Site H2 Fueling Station. Appl. Catal. B Environ. 2023, 325, 122352. [Google Scholar] [CrossRef]
- Hu, X.C.; Wang, W.W.; Si, R.; Ma, C.; Jia, C.J. Hydrogen Production via Catalytic Decomposition of NH3 Using Promoted MgO-Supported Ruthenium Catalysts. Sci. China Chem. 2019, 62, 1625–1633. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Wang, S.J.; Ng, C.F.; Au, C.T. Magnesia–Carbon Nanotubes (MgO–CNTs) Nanocomposite: Novel Support of Ru Catalyst for the Generation of COx-Free Hydrogen from Ammonia. Catal. Lett. 2004, 96, 113–119. [Google Scholar] [CrossRef]
- Nagaoka, K.; Eboshi, T.; Abe, N.; Miyahara, S.I.; Honda, K.; Sato, K. Influence of Basic Dopants on the Activity of Ru/Pr6O11 for Hydrogen Production by Ammonia Decomposition. Int. J. Hydrogen Energy 2014, 39, 20731–20735. [Google Scholar] [CrossRef]
- Im, Y.; Muroyama, H.; Matsui, T.; Eguchi, K. Investigation on Catalytic Performance and Desorption Behaviors of Ruthenium Catalysts Supported on Rare-Earth Oxides for NH3 Decomposition. Int. J. Hydrogen Energy 2022, 47, 32543–32551. [Google Scholar] [CrossRef]
- Nagaoka, K.; Honda, K.; Ibuki, M.; Sato, K.; Takita, Y. Highly Active Cs2O/Ru/Pr6O11 as a Catalyst for Ammonia Decomposition. Chem. Lett. 2010, 39, 918–919. [Google Scholar] [CrossRef]
- Wang, F.; Deng, L.; Wu, Z.; Ji, K.; Chen, Q.; Jiang, X. The Dispersed SiO2 Microspheres Supported Ru Catalyst with Enhanced Activity for Ammonia Decomposition. Int. J. Hydrogen Energy 2021, 46, 20815–20824. [Google Scholar] [CrossRef]
- Duan, X.; Zhou, J.; Qian, G.; Li, P.; Zhou, X.; Chen, D. Carbon Nanofiber-Supported Ru Catalysts for Hydrogen Evolution by Ammonia Decomposition. Cuihua Xuebao/Chin. J. Catal. 2010, 31, 979–986. [Google Scholar] [CrossRef]
- Yao, L.H.; Li, Y.X.; Zhao, J.; Ji, W.J.; Au, C.T. Core-Shell Structured Nanoparticles (M@SiO2, Al2O3, MgO.; M = Fe, Co, Ni, Ru) and Their Application in COx-Free H2 Production via NH3 Decomposition. Catal. Today 2010, 158, 401–408. [Google Scholar] [CrossRef]
- Dong, X.; Zhu, S.; Li, H. Recent Research Progress of Catalysts for Thermocatalytic Ammonia Decomposition. Int. J. Hydrogen Energy 2025, 143, 286–306. [Google Scholar] [CrossRef]
- Li, T.; Zuo, J.; Zhao, Z.; Li, W.; Zhang, K.; Wang, Q.; Du, H.; Liu, H.; Aili, E.; Ye, Y. Hydrogen Production by Ammonia Decomposition: A Strategy to Enhance the Activity and Stability of Metal Catalysts. Int. J. Hydrogen Energy 2025, 97, 1153–1167. [Google Scholar] [CrossRef]
- Qiu, Y.; Fu, E.; Gong, F.; Xiao, R. Catalyst Support Effect on Ammonia Decomposition over Ni/MgAl2O4 towards Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 5044–5052. [Google Scholar] [CrossRef]
- Inokawa, H.; Ichikawa, T.; Miyaoka, H. Catalysis of Nickel Nanoparticles with High Thermal Stability for Ammonia Decomposition. Appl. Catal. A Gen. 2015, 491, 184–188. [Google Scholar] [CrossRef]
- Li, Y.; Wen, J.; Ali, A.M.; Duan, M.; Zhu, W.; Zhang, H.; Chen, C.; Li, Y. Size Structure-Catalytic Performance Correlation of Supported Ni/MCF-17 Catalysts for CO: X-Free Hydrogen Production. Chem. Commun. 2018, 54, 6364–6367. [Google Scholar] [CrossRef]
- Nakamura, I.; Fujitani, T. Role of Metal Oxide Supports in NH3 Decomposition over Ni Catalysts. Appl. Catal. A Gen. 2016, 524, 45–49. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, J.; Ge, Q.; Xu, H.; Li, W. Effects of CeO2 Addition on Ni/Al2O3 Catalysts for the Reaction of Ammonia Decomposition to Hydrogen. Appl. Catal. B Environ. 2008, 80, 98–105. [Google Scholar] [CrossRef]
- Okura, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Promotion Effect of Rare-Earth Elements on the Catalytic Decomposition of Ammonia over Ni/Al2O3 Catalyst. Appl. Catal. A Gen. 2015, 505, 77–85. [Google Scholar] [CrossRef]
- Lucentini, I.; Serrano, I.; Soler, L.; Divins, N.J.; Llorca, J. Ammonia Decomposition over 3D-Printed CeO2 Structures Loaded with Ni. Appl. Catal. A Gen. 2020, 591, 117382. [Google Scholar] [CrossRef]
- Okura, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Ammonia Decomposition over Nickel Catalysts Supported on Rare-Earth Oxides for the On-Site Generation of Hydrogen. ChemCatChem 2016, 8, 2988–2995. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Guo, Y.; Wang, Y. Highly Active and Stable Ni@SiO2 Catalyst for Ammonia Decomposition. Fuel 2024, 368, 131543. [Google Scholar] [CrossRef]
- Ji, J.; Duan, X.; Qian, G.; Zhou, X.; Chen, D.; Yuan, W. In Situ Production of Ni Catalysts at the Tips of Carbon Nanofibers and Application in Catalytic Ammonia Decomposition. Ind. Eng. Chem. Res. 2013, 52, 1854–1858. [Google Scholar] [CrossRef]
- Prabu, S.; Dharman, R.K.; Chiang, K.Y.; Oh, T.H. Highly Efficient Ni Nanoparticles Embedded on MgO and N-Doped Carbon Nanofibers for Efficient Ammonia Decomposition. J. Ind. Eng. Chem. 2023, 125, 402–409. [Google Scholar] [CrossRef]
- Amini, S.; Meshkani, F.; Rezaei, M. Catalytic Oxidation of CO over Nanocrystalline La1–XCexNiO3 Perovskite-Type Oxides. Chem. Eng. Technol. 2019, 42, 2443–2449. [Google Scholar] [CrossRef]
- Podila, S.; Driss, H.; Ali, A.M.; Al-Zahrani, A.A.; Daous, M.A. Influence of Ce Substitution in LaMO3 (M = Co/Ni) Perovskites for COx-Free Hydrogen Production from Ammonia Decomposition. Arab. J. Chem. 2022, 15, 103547. [Google Scholar] [CrossRef]
- Guo, X.Y.; Wang, J.H.; Zhang, Q.; Li, T.Z.; Dong, H.; Jia, C.J.; Li, C.; Zhang, Y.W. Alkaline Earth Metal Promoted Hydrogen Production from Ammonia Decomposition over Ni/La2O3-Based Catalysts. Appl. Catal. B Environ. 2024, 348, 123844. [Google Scholar] [CrossRef]
- Guo, W.; Vlachos, D.G. Patched bimetallic surfaces are active catalysts for ammonia decomposition. Nat. Commun. 2015, 6, 8619. [Google Scholar] [CrossRef]
- Lucentini, I.; Casanovas, A.; Llorca, J. Catalytic Ammonia Decomposition for Hydrogen Production on Ni, Ru and Ni[Sbnd]Ru Supported on CeO2. Int. J. Hydrogen Energy 2019, 44, 12693–12707. [Google Scholar] [CrossRef]
- Fu, E.; Qiu, Y.; Lu, H.; Wang, S.; Liu, L.; Feng, H.; Yang, Y.; Wu, Z.; Xie, Y.; Gong, F.; et al. Enhanced NH3 Decomposition for H2 Production over Bimetallic M(M=Co, Fe, Cu)Ni/Al2O3. Fuel Process. Technol. 2021, 221, 106945. [Google Scholar] [CrossRef]
- Xie, P.; Yao, Y.; Huang, Z.; Liu, Z.; Zhang, J.; Li, T.; Wang, G.; Shahbazian-Yassar, R.; Hu, L.; Wang, C. Highly Efficient Decomposition of Ammonia Using High-Entropy Alloy Catalysts. Nat. Commun. 2019, 10, 4011. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, Y.; Long, Z.; Zhao, S.; Wang, Y.; Zhang, W. One-Pot Synthesis of Supported Ni@Al2O3 Catalysts with Uniform Small-Sized Ni for Hydrogen Generation via Ammonia Decomposition. Int. J. Hydrogen Energy 2021, 46, 4045–4054. [Google Scholar] [CrossRef]
- Al-Shafei, E.N.; Albahar, M.Z.; Albashrayi, R.; Aljishi, M.; Alasseel, A.; Tanimu, G.; Aitani, A. The Effect of Acidic–Basic Structural Modification of Nickel-Based Catalyst for Ammonia Decomposition for Hydrogen Generation. Mol. Catal. 2023, 550, 113581. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Liu, S.; Li, S.; Liu, G. Ni-CeO2 Nanocomposite with Enhanced Metal-Support Interaction for Effective Ammonia Decomposition to Hydrogen. Chem. Eng. J. 2023, 473, 145371. [Google Scholar] [CrossRef]
- Chen, C.; Fan, X.; Zhou, C.; Lin, L.; Luo, Y.; Au, C.; Cai, G.; Wang, X.; Jiang, L. Hydrogen Production from Ammonia Decomposition over Ni/CeO2 Catalyst: Effect of CeO2 Morphology. J. Rare Earths 2023, 41, 1014–1021. [Google Scholar] [CrossRef]
- He, H.; Jiang, H.; Yang, F.; Liu, J.; Zhang, W.; Jin, M.; Li, Z. Bimetallic NixCo10-x/CeO2 as Highly Active Catalysts to Enhance Mid-Temperature Ammonia Decomposition: Kinetics and Synergies. Int. J. Hydrogen Energy 2023, 48, 5030–5041. [Google Scholar] [CrossRef]
- Hu, X.C.; Wang, W.W.; Jin, Z.; Wang, X.; Si, R.; Jia, C.J. Transition Metal Nanoparticles Supported La-Promoted MgO as Catalysts for Hydrogen Production via Catalytic Decomposition of Ammonia. J. Energy Chem. 2019, 38, 41–49. [Google Scholar] [CrossRef]
- Sima, D.; Wu, H.; Tian, K.; Xie, S.; Foo, J.J.; Li, S.; Wang, D.; Ye, Y.; Zheng, Z.; Liu, Y.Q. Enhanced Low Temperature Catalytic Activity of Ni/Al–Ce0.8Zr0.2O2 for Hydrogen Production from Ammonia Decomposition. Int. J. Hydrogen Energy 2020, 45, 9342–9352. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.D.; Jung, U.; Park, Y.; Lee, K.B.; Koo, K.Y. Clean Hydrogen Production from Ammonia Decomposition over Zeolite 13X-Supported Ni Catalysts. Sustain. Energy Fuels 2024, 8, 896–904. [Google Scholar] [CrossRef]
- Wan, Z.; Tao, Y.; You, H.; Zhang, X.; Shao, J. Na-ZSM-5 Zeolite Nanocrystals Supported Nickel Nanoparticles for Efficient Hydrogen Production from Ammonia Decomposition. ChemCatChem 2021, 13, 3027–3036. [Google Scholar] [CrossRef]
- Zhang, H.; Alhamed, Y.A.; Kojima, Y.; Al-Zahrani, A.A.; Miyaoka, H.; Petrov, L.A. Structure and Catalytic Properties of Ni/MWCNTs and Ni/AC Catalysts for Hydrogen Production via Ammonia Decomposition. Int. J. Hydrogen Energy 2014, 39, 277–287. [Google Scholar] [CrossRef]
- Wu, Z.W.; Li, X.; Qin, Y.H.; Deng, L.; Wang, C.W.; Jiang, X. Ammonia Decomposition over SiO2-Supported Ni–Co Bimetallic Catalyst for COx-Free Hydrogen Generation. Int. J. Hydrogen Energy 2020, 45, 15263–15269. [Google Scholar] [CrossRef]
- Simonsen, S.B.; Chakraborty, D.; Chorkendorff, I.; Dahl, S. Alloyed Ni-Fe Nanoparticles as Catalysts for NH3 Decomposition. Appl. Catal. A Gen. 2012, 447–448, 22–31. [Google Scholar] [CrossRef]
- Hu, R.; Yuan, Y.; Wang, W.; Zhang, T.; Gu, Y.; Yan, A.; Wang, Z. Metal-Organic Framework-Derived FeNi@NC Catalyst for Highly Efficient Hydrogen Generation via Ammonia Decomposition. Mater. Sci. Eng. B 2025, 314, 118051. [Google Scholar] [CrossRef]
- Ajeebi, A.M.; Ali, A.; AlAmoudi, O.M.; Sanhoob, M.A.; Hossain, M.M.; Alghamdi, H.S.; Usman, M.; Zahir, M.H.; Shaikh, M.N. Alumina-Supported Bimetallic Catalysts with Ruthenium and CoNi for Enhanced Ammonia Decomposition. Sustain. Energy Fuels 2025, 9, 2396–2409. [Google Scholar] [CrossRef]
- Guo, W.; Shafizadeh, A.; Shahbeik, H.; Rafiee, S.; Motamedi, S.; Ghafarian Nia, S.A.; Nadian, M.H.; Li, F.; Pan, J.; Tabatabaei, M.; et al. Machine Learning for Predicting Catalytic Ammonia Decomposition: An Approach for Catalyst Design and Performance Prediction. J. Energy Storage 2024, 89, 111688. [Google Scholar] [CrossRef]
- Chen, W.H.; Chou, W.S.; Chein, R.Y.; Hoang, A.T.; Juan, J.C. Multiple-Objective Optimization on Ammonia Decomposition Using Membrane Reactor. Int. J. Hydrogen Energy 2024, 52, 1002–1017. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, Y.; Kim, H.W.; Lee, S.U.; Kim, J.R.; Kim, T.W.; Lee, Y.J.; Chae, H.J. Ru-Supported Lanthania-Ceria Composite as an Efficient Catalyst for COx-Free H2 Production from Ammonia Decomposition. Appl. Catal. B Environ. 2021, 285, 119831. [Google Scholar] [CrossRef]
- Gómez-García, M.A.; Pitchon, V.; Kiennemann, A. Pollution by nitrogen oxides: An approach to NOx abatement by using sorbing catalytic materials. Environ. Int. 2005, 31, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Alavi, S.M.; Rezaei, M. Hydrogen Production from COx-Free Thermocatalytic Decomposition of Methane over the Mesoporous Iron Aluminate Spinel (FeAl2O4) Nanopowder Supported Nickel Catalysts. Int. J. Hydrogen Energy 2022, 47, 18370–18383. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Zhao, X.; Karakaya, C.; Qian, M.; Zou, R.; Zhang, W.; Lu, Z.; Maiti, D.; Samanta, A.; Wan, W.; Liu, X.; et al. 3D printing synthesis of catalysts. Mater. Today Sustain. 2024, 26, 100746. [Google Scholar] [CrossRef]
- Kovacev, N.; Li, S.; Li, W.; Zeraati-Rezaei, S.; Tsolakis, A.; Essa, K. Additive Manufacturing of Novel Hybrid Monolithic Ceramic Substrates. Aerospace 2022, 9, 255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).