Effect of Humidity on the Energy and CO2 Separation Characteristics of Membranes in Direct Air Capture Technology
Abstract
1. Introduction
1.1. Importance of DAC Installation Site Selection
- Climatic conditions—primarily temperature and humidity of the air;
- Access to low-emission electricity and/or heat sources;
- Existing infrastructure enabling CO2 transport, storage, or utilization;
- Local and regional legal and political conditions, including subsidies and other forms of financial support.
1.2. Membrane Separation and the Impact of Local Climatic Conditions
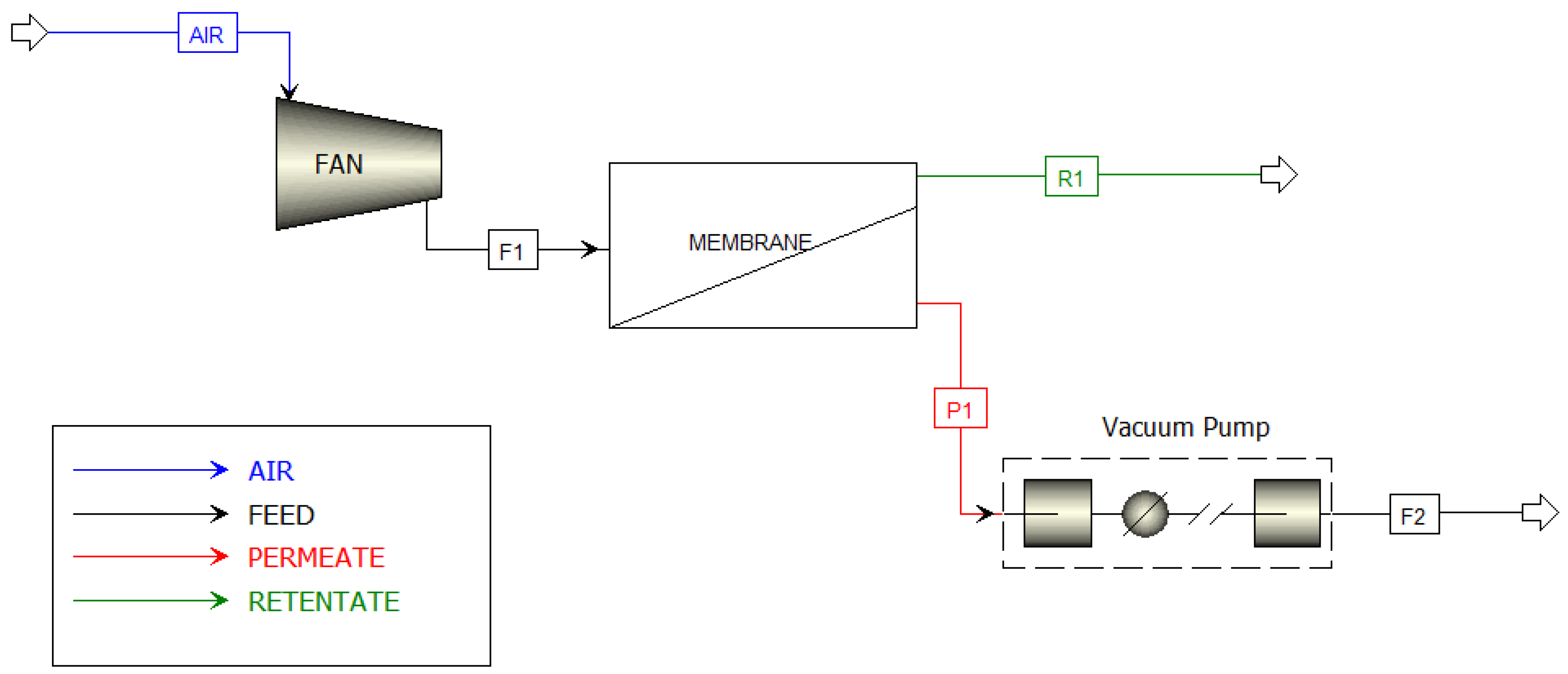
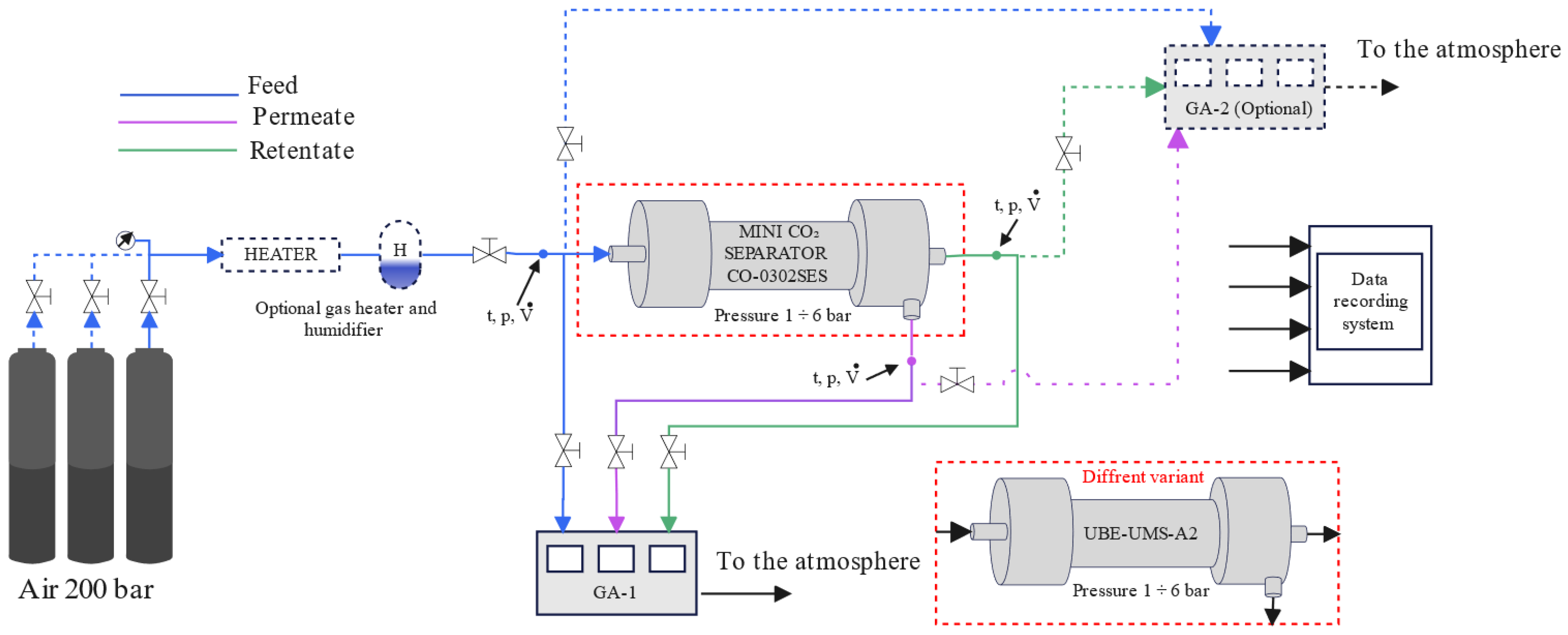
2. Materials and Methods
- The membrane module is built with cross-flow capillaries;
- The process is carried out under isothermal conditions;
- The values of gas permeance coefficients are constant throughout the operating range;
- Atmospheric air is treated as a semi-ideal gas.
3. Results and Discussion
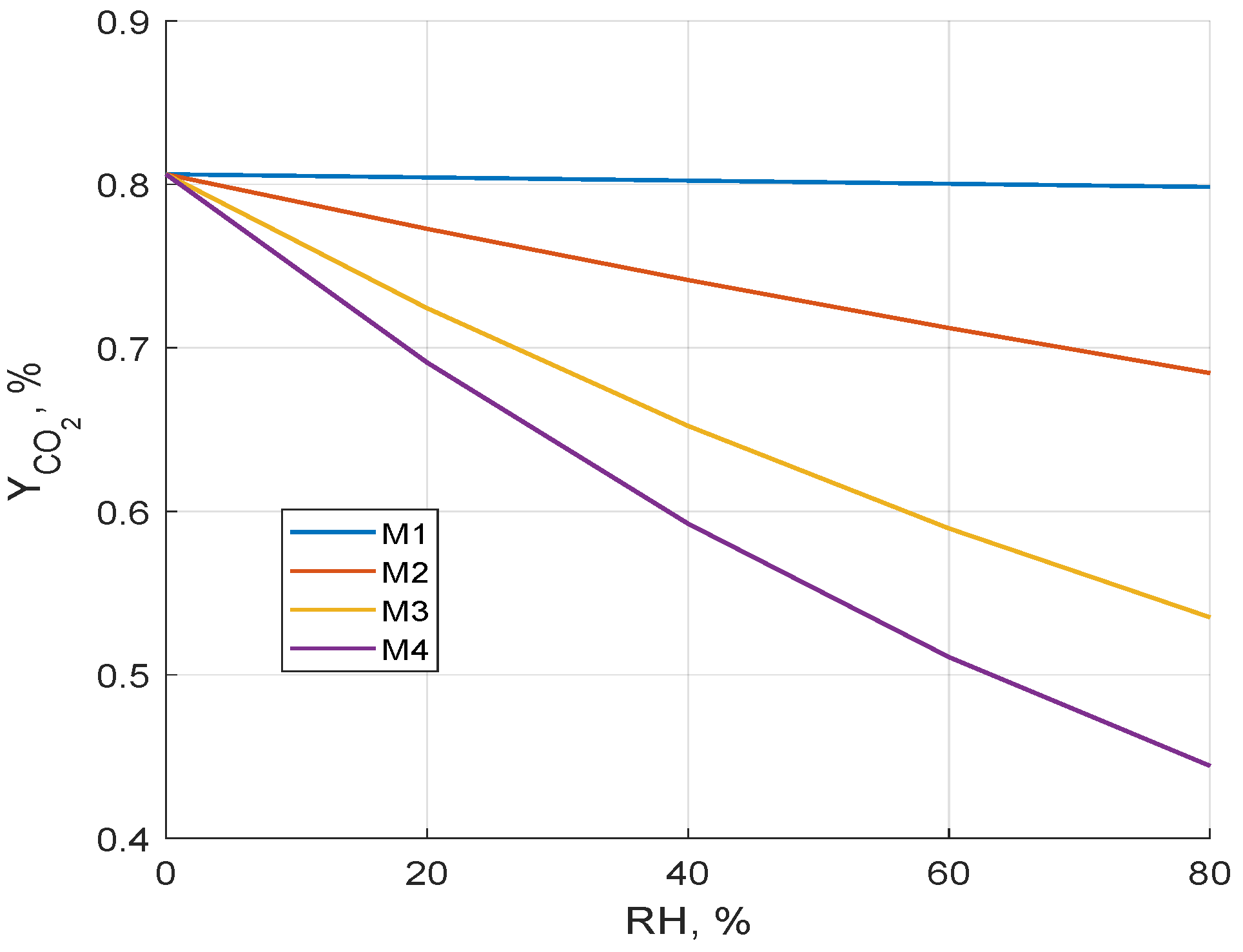
3.1. Membrane Selectivity and the Influence of Relative Humidity on CO2 Capture Efficiency
- Membrane M1 represents a commonly used assumption in the literature of constant selectivity of the membrane toward CO2 relative to other air components. Its separation parameters correspond to the minimal limiting values reported by Fujikawa et al. [28].
- Membrane M2 corresponds to a scenario where the permeance of water vapor (H2O) is 0.5 times the permeance of CO2.
- Membrane M3 assumes that the permeance of H2O is twice as high as that of CO2.
- Membrane M4 considers the case where the permeance of H2O is five times higher than that of CO2.
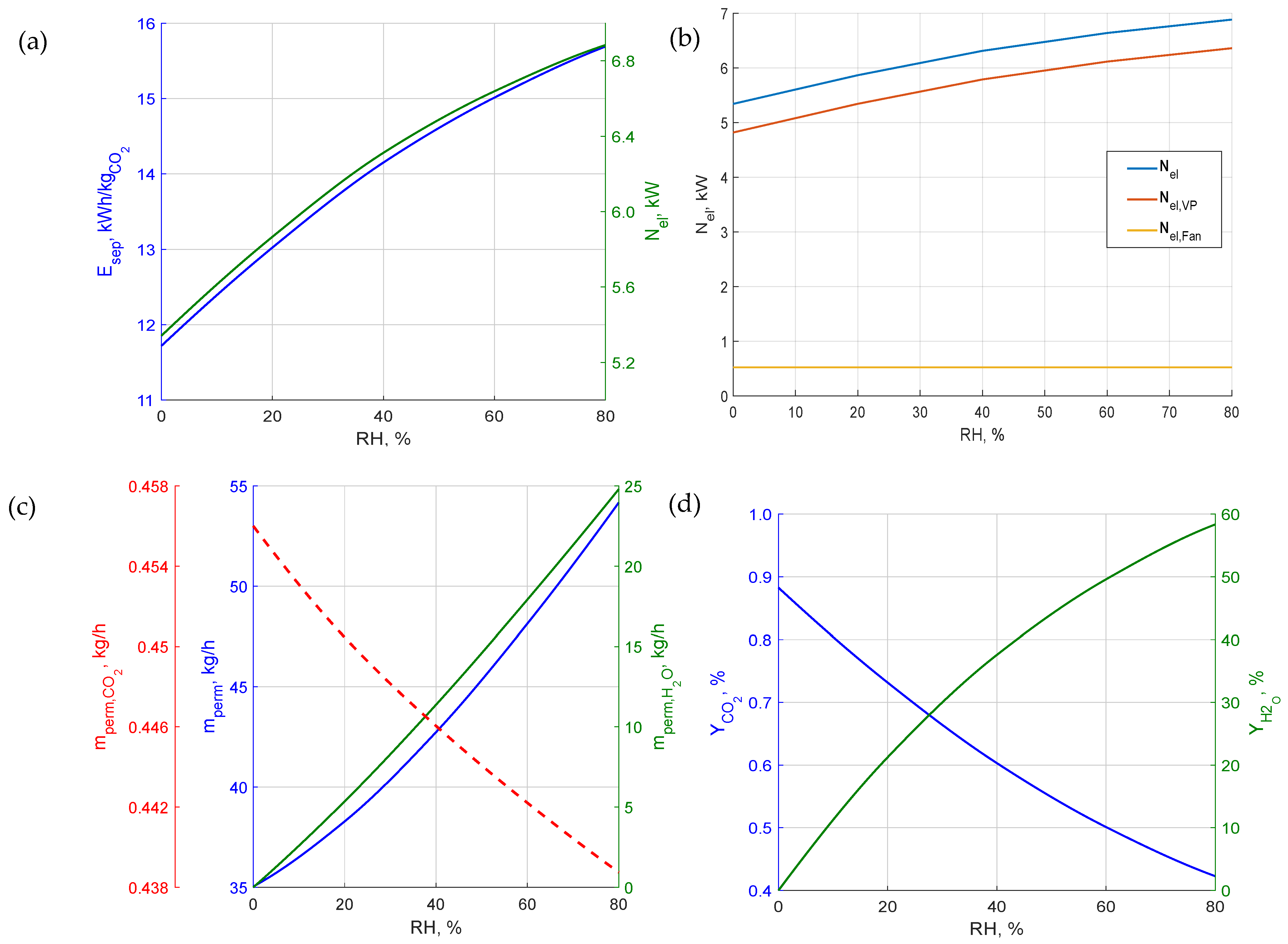
3.2. Analyses for the PolyActiveTM Membrane
4. Opportunities for Dehumidification with DAC Technologies
5. Perspective
6. Conclusions
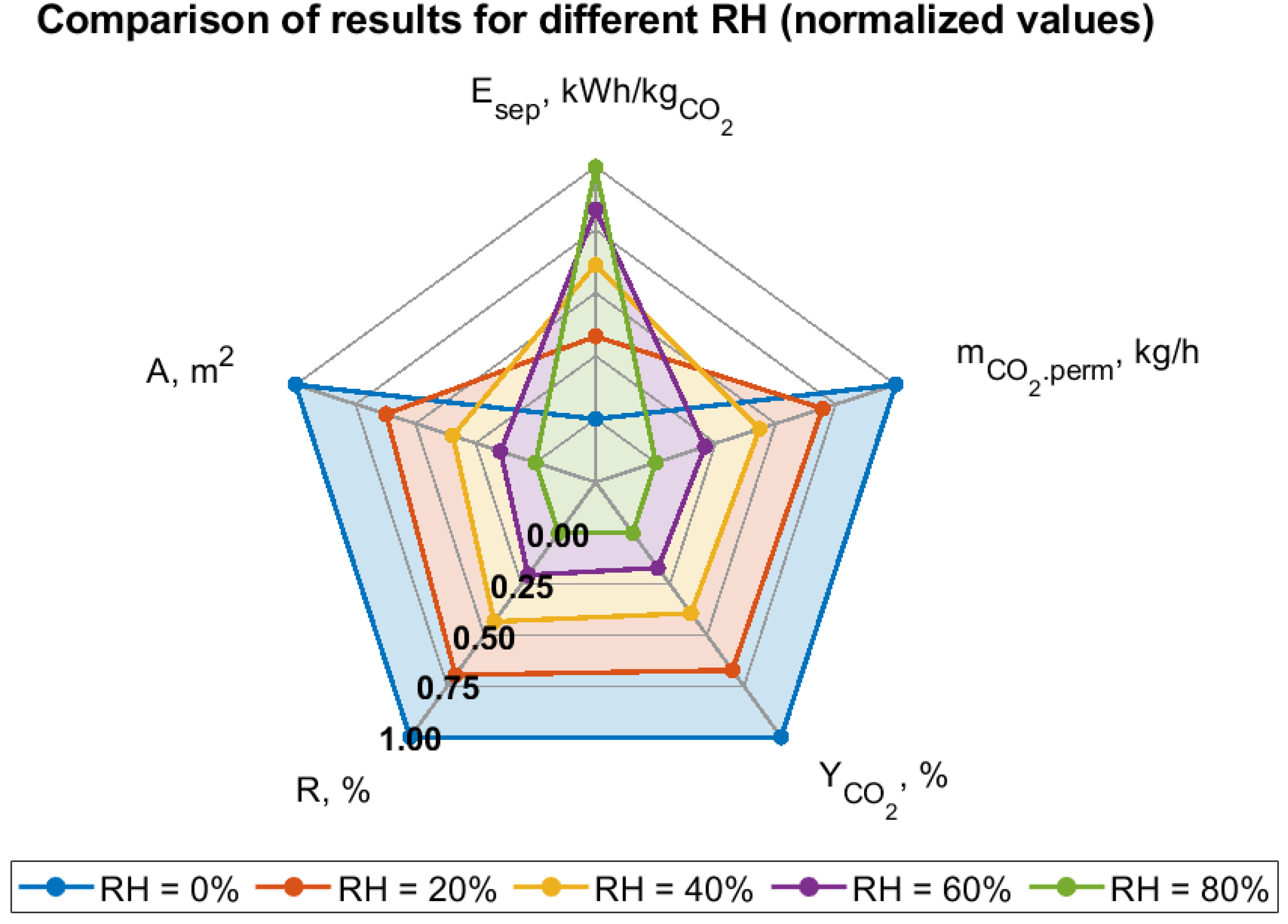
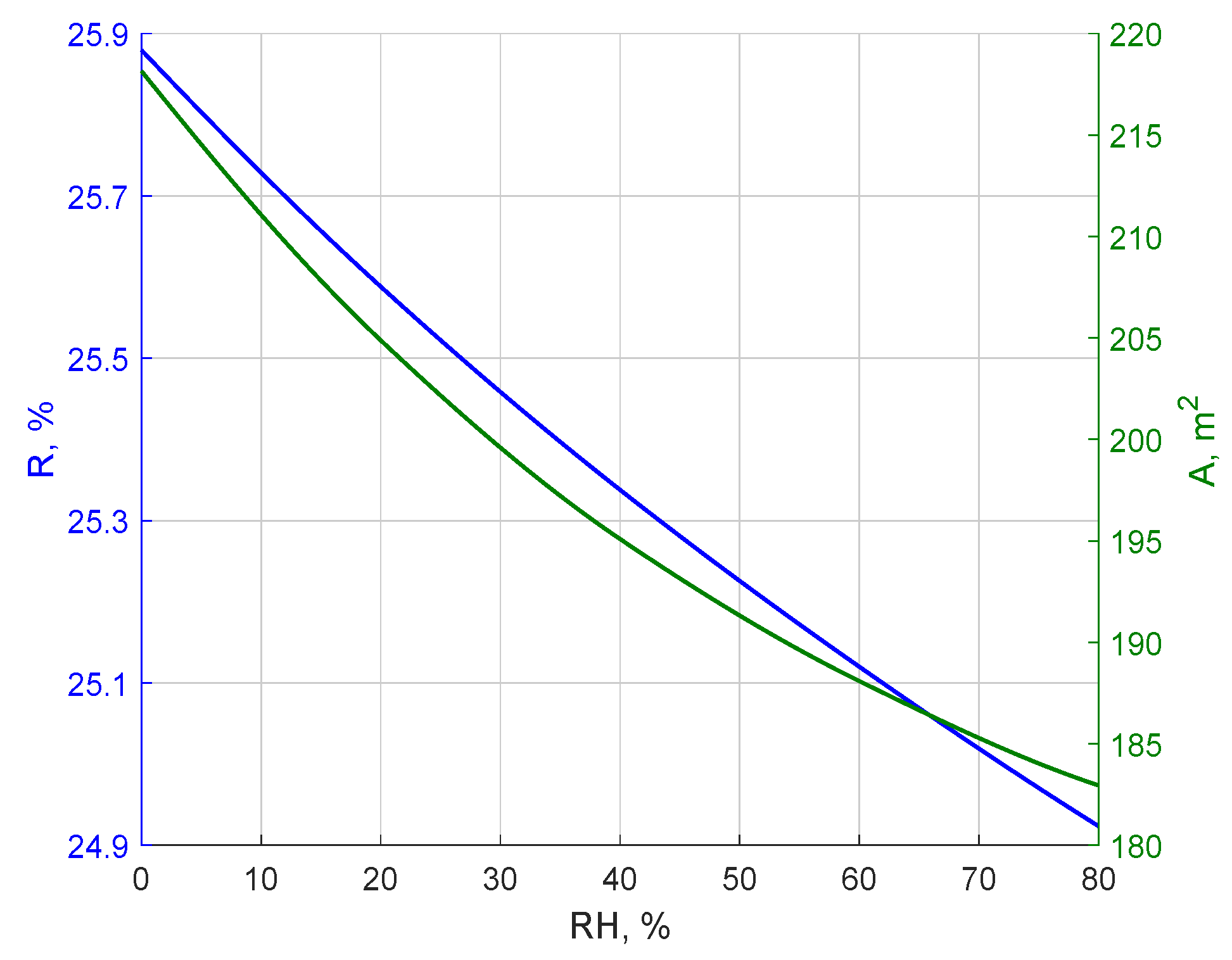
- An increase in relative air humidity leads to the deterioration of all key system performance parameters, such as CO2 purity in the permeate, recovery rate, captured CO2 mass flow, process energy intensity, and electrical power demand. Therefore, the location of m-DAC installations should consider regions with the lowest possible average annual relative humidity. The sensitivity of m-DAC technology to humidity may be significantly higher compared to alternative sorption-based CO2 capture methods, due to the possibility of rapid membrane degradation. However, this aspect requires long-term experimental studies for a full evaluation.
- Analyses performed for different assumptions regarding selectivity toward water vapor show that even a twofold lower permeance of H2O relative to CO2 can significantly increase the process’s energy intensity and reduce product purity. Consequently, separation parameters related to water vapor should be considered as important as those related to CO2.
- The effect of relative humidity on the energy performance of the process is a significant barrier that needs to be overcome, such as through the development of membranes with high separation properties, to ensure that m-DAC technology is competitive with other alternative CO2 capture methods. High energy intensity, limited separation efficiency at low CO2 concentrations, and high sensitivity to gas moisture indicate the need to consider m-DAC systems as purification units in hybrid configurations. Purification can be used in processes where CO2 content is in the range of 10–14%, which corresponds to flue gas from coal-fired power plants. Such processes have already been successfully demonstrated using membranes outside the laboratory scale in pilot studies.
- To mitigate the negative impact of moisture, it is recommended to use air drying systems before introducing air into the membrane separation system or to apply permeate dehydration before further processing in subsequent stages. However, air drying systems can significantly increase both capital and operational expenditures, which may pose a substantial barrier to the implementation of stand-alone m-DAC units. Newly developed membranes dedicated to CO2 separation should additionally exhibit the lowest possible water vapor permeance—close to that of nitrogen—to reduce undesirable moisture transport effects.
- Existing data from the literature on newly developed membranes with very high CO2 permeance (above 10,000 GPU) are often incomplete and do not include key information regarding selectivity toward H2O and O2. The lack of such data significantly limits their practical use in m-DAC applications and complicates energy and environmental analyses for other researchers. Moreover, conducting a reliable economic assessment is currently not feasible because thorough analyses require supplementing knowledge about membrane selectivity to estimate the actual separation capabilities of membranes under varying operating conditions, as well as estimating production costs—tasks that primarily rest with the membrane developers. The lack of data prevents a clear assessment of the competitiveness of m-DAC technology compared to other CO2 capture methods under varying humidity conditions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAC | Direct Air Capture |
| m-DAC | Membrane-based Direct Air Capture |
| RES | Renewable Energy Sources |
| CCU | Carbon Capture and Utilization |
| CCS | Carbon Capture and Storage |
| IEA | International Energy Agency |
| L-DAC | Liquid-based Direct Air Capture |
| S-DAC | Solid-based Direct Air Capture |
| LCOC | Levelized Cost of Capture |
| RH | Relative Humidity |
| GPU | Gas Permeation Unit |
| m-DAC-M | Membrane-based Direct Air Capture and a Methanation process |
| HVAC | Heating, Ventilation, Air Conditioning |
| NASA | National Aeronautics and Space Administration |
References
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Millar, M.A.; Yu, Z.; Yu, J. An Assessment of the Impacts of Heat Electrification on the Electric Grid in the UK. Energy Rep. 2022, 8, 14934–14946. [Google Scholar] [CrossRef]
- Tamor, M.A.; Stechel, E.B. Electrification of Transportation Means a Lot More than a Lot More Electric Vehicles. iScience 2022, 25, 104376. [Google Scholar] [CrossRef] [PubMed]
- Boa Morte, I.B.; Araújo, O.d.Q.F.; Morgado, C.R.V.; de Medeiros, J.L. Electrification and Decarbonization: A Critical Review of Interconnected Sectors, Policies, and Sustainable Development Goals. Energy Storage Sav. 2023, 2, 615–630. [Google Scholar] [CrossRef]
- IEA. CO2 Emissions in 2023, IEA, Paris. 2024. Available online: https://www.iea.org/reports/co2-emissions-in-2023 (accessed on 21 March 2024).
- IEA—Direct Air Capture—Energy System. Available online: https://www.iea.org/energy-system/carbon-capture-utilisation-and-storage/direct-air-capture (accessed on 25 March 2024).
- Bui, M.; Fajardy, M.; Mac Dowell, N. Bio-Energy with CCS (BECCS) Performance Evaluation: Efficiency Enhancement and Emissions Reduction. Appl. Energy 2017, 195, 289–302. [Google Scholar] [CrossRef]
- Greig, C.; Uden, S. The Value of CCUS in Transitions to Net-Zero Emissions. Electr. J. 2021, 34, 107004. [Google Scholar] [CrossRef]
- Popielak, P.; Majchrzak-Kucęba, I.; Wawrzyńczak, D. Climate Change Mitigation with CCUS—A Case Study with Benchmarking for Selected Countries in Adapting the European Union’s Green Deal. Int. J. Greenh. Gas Control 2024, 132, 104057. [Google Scholar] [CrossRef]
- Bouaboula, H.; Chaouki, J.; Belmabkhout, Y.; Zaabout, A. Comparative Review of Direct Air Capture Technologies: From Technical, Commercial, Economic, and Environmental Aspects. Chem. Eng. J. 2024, 484, 149411. [Google Scholar] [CrossRef]
- Joppa, L.; Luers, A.; Willmott, E.; Friedmann, S.J.; Hamburg, S.P.; Broze, R. Microsoft’s Million-Tonne CO2-Removal Purchase—Lessons for Net Zero. Nature 2021, 597, 629–632. [Google Scholar] [CrossRef]
- Microsoft Inks Another Deal to Capture and Store Its Carbon Emissions Underground—The Verge. Available online: https://www.theverge.com/2023/3/22/23651587/microsoft-climate-tech-startup-carboncapture-wyoming (accessed on 25 March 2024).
- Heirloom Opens First, U.S. Direct Air Capture Plant—The New York Times. Available online: https://www.nytimes.com/2023/11/09/climate/direct-air-capture-carbon.html (accessed on 25 March 2024).
- World’s First Large-Scale Direct Air Capture Plant to Use Siemens Energy Equipment. Available online: https://www.siemens-energy.com/global/en/home/press-releases/worlds-first-large-scale-direct-air-capture-plant-use-siemens-energy-equipment.html (accessed on 21 December 2024).
- Amazon Supports World’s Largest Deployment of DAC Tech. Available online: https://www.aboutamazon.com/news/sustainability/amazon-direct-air-capture-investment-fights-climate-change (accessed on 21 December 2024).
- Aramco, Siemens Energy Launch Direct Air Capture Project. Available online: https://www.cnbc.com/2023/10/09/aramco-siemens-energy-launch-direct-air-capture-project-.html (accessed on 21 December 2024).
- Kotowicz, J.; Niesporek, K.; Baszczeńska, O. Advancements and Challenges in Direct Air Capture Technologies: Energy Intensity, Novel Methods, Economics, and Location Strategies. Energies 2025, 18, 496. [Google Scholar] [CrossRef]
- Brooks, B.G.J.; Geissler, C.H.; An, K.; McCoy, S.T.; Middleton, R.S.; Ogland-Hand, J.D. The Performance of Solvent-Based Direct Air Capture across Geospatial and Temporal Climate Regimes. Front. Clim. 2024, 6, 1394728. [Google Scholar] [CrossRef]
- An, K.; Farooqui, A.; McCoy, S.T. The Impact of Climate on Solvent-Based Direct Air Capture Systems. Appl. Energy 2022, 325, 119895. [Google Scholar] [CrossRef]
- Cai, X.; Coletti, M.A.; Sholl, D.S.; Allen-Dumas, M.R. Assessing Impacts of Atmospheric Conditions on Efficiency and Siting of Large-Scale Direct Air Capture Facilities. JACS Au 2024, 4, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Schellevis, H.M.; de la Combé, J.D.; Brilman, D.W.F. Optimizing Direct Air Capture under Varying Weather Conditions. Energy Adv. 2024, 3, 1678–1687. [Google Scholar] [CrossRef]
- Leonzio, G.; Fennell, P.S.; Shah, N. Modelling and Analysis of Direct Air Capture Systems in Different Locations. Chem. Eng. Trans. 2022, 96, 1–6. [Google Scholar] [CrossRef]
- Krull, L.F.; Baum, C.M.; Sovacool, B.K. A Geographic Analysis and Techno-Economic Assessment of Renewable Heat Sources for Low-Temperature Direct Air Capture in Europe. Energy Convers. Manag. 2025, 323, 119186. [Google Scholar] [CrossRef]
- Boerst, J.; Pena Cabra, I.; Sharma, S.; Zaremsky, C.; Iyengar, A.K.S. Strategic Siting of Direct Air Capture Facilities in the United States. Energies 2024, 17, 3755. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- Sendi, M.; Bui, M.; Mac Dowell, N.; Fennell, P. Geospatial Analysis of Regional Climate Impacts to Accelerate Cost-Efficient Direct Air Capture Deployment. One Earth 2022, 5, 1153–1164. [Google Scholar] [CrossRef]
- Fujikawa, S.; Selyanchyn, R. Direct Air Capture by Membranes. MRS Bull. 2022, 47, 416–423. [Google Scholar] [CrossRef]
- Fujikawa, S.; Selyanchyn, R.; Kunitake, T. A New Strategy for Membrane-Based Direct Air Capture. Polym. J. 2020, 53, 111–119. [Google Scholar] [CrossRef]
- Castel, C.; Bounaceur, R.; Favre, E. Membrane Processes for Direct Carbon Dioxide Capture From Air: Possibilities and Limitations. Front. Chem. Eng. 2021, 3, 668867. [Google Scholar] [CrossRef]
- Ignatusha, P.; Lin, H.; Kapuscinsky, N.; Scoles, L.; Ma, W.; Patarachao, B.; Du, N. Membrane Separation Technology in Direct Air Capture. Membranes 2024, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Zamidi Ahmad, M.; Malankowska, M.; Coronas, J. A New Relevant Membrane Application: CO2 Direct Air Capture (DAC). Chem. Eng. J. 2022, 446, 137047. [Google Scholar] [CrossRef]
- Niesporek, K.; Kotowicz, J.; Baszczeńska, O.; Maj, I. Performance Characteristics and Optimization of a Single-Stage Direct Air Capture Membrane System in Terms of Process Energy Intensity. Energies 2024, 17, 2046. [Google Scholar] [CrossRef]
- Gama, V.; Dantas, B.; Sanyal, O.; Lima, F.V. Process Operability Analysis of Membrane-Based Direct Air Capture for Low-Purity CO2 Production. ACS Eng. Au 2024, 4, 394–404. [Google Scholar] [CrossRef]
- Installation of M-DAC® Technology in Tokyo to Capture and Utilize Carbon Dioxide from the Atmosphere| News Room|Sojitz Corporation. Available online: https://www.sojitz.com/en/news/article/topics-20241017.html (accessed on 21 May 2025).
- Tsuji, T.; Sorai, M.; Shiga, M.; Fujikawa, S.; Kunitake, T. Geological Storage of CO2–N2–O2 Mixtures Produced by Membrane-Based Direct Air Capture (DAC). Greenh. Gases Sci. Technol. 2021, 11, 610–618. [Google Scholar] [CrossRef]
- Miroshnichenko, D.; Shalygin, M.; Bazhenov, S. Simulation of the Membrane Process of CO2 Capture from Flue Gas via Commercial Membranes While Accounting for the Presence of Water Vapor. Membranes 2023, 13, 692. [Google Scholar] [CrossRef]
- Remiorz, L.; Wiciak, G.; Grzywnowicz, K. Novel Concept of Supporting the Membrane Separation of CO2 in Power Plants by Thermoacoustic Dehumidification. Energy 2019, 189, 116191. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Ferrari, M.C.; Brandani, S. Humidity Impact on the Gas Permeability of PIM-1 Membrane for Post-Combustion Application. Energy Procedia 2014, 63, 194–201. [Google Scholar] [CrossRef]
- Wiciak, G. The Influence of the Moisture Content in Gaseous CO2/N2 Mixture on Selected Parameters of CO2 Separation in a Capillary Polymeric Membrane. Desalination Water Treat. 2018, 128, 314–323. [Google Scholar] [CrossRef]
- Wade, J.L.; Lopez Marques, H.; Wang, W.; Flory, J.; Freeman, B. Moisture-Driven CO2 Pump for Direct Air Capture. J. Membr. Sci. 2023, 685, 121954. [Google Scholar] [CrossRef]
- Kotowicz, J.; Chmielniak, T.; Janusz-Szymańska, K. The Influence of Membrane CO2 Separation on the Efficiency of a Coal-Fired Power Plant. Energy 2010, 35, 841–850. [Google Scholar] [CrossRef]
- Kotowicz, J.; Janusz-Szymańska, K.; Wiciak, G. Technologie Membranowe Wychwytu Dwutlenku Węgla Ze Spalin Dla Nadkrytycznego Bloku Węglowego; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2015; ISBN 978-83-7880-270-9. [Google Scholar]
- Fujikawa, S.; Ariyoshi, M.; Selyanchyn, R.; Kunitake, T. Ultra-Fast, Selective CO2 Permeation by Free-Standing Siloxane Nanomembranes. Chem. Lett. 2019, 48, 1351–1354. [Google Scholar] [CrossRef]
- Yoo, M.J.; Kim, K.H.; Lee, J.H.; Kim, T.W.; Chung, C.W.; Cho, Y.H.; Park, H.B. Ultrathin Gutter Layer for High-Performance Thin-Film Composite Membranes for CO2 Separation. J. Membr. Sci. 2018, 566, 336–345. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Z.; Sheng, M.; Qiao, Z.; Wang, J. Scaling up of Defect-Free Flat Membrane with Ultra-High Gas Permeance Used for Intermediate Layer of Multi-Layer Composite Membrane and Oxygen Enrichment. Sep. Purif. Technol. 2020, 239, 116580. [Google Scholar] [CrossRef]
- Ying, Y.; Yang, Z.; Shi, D.; Peh, S.B.; Wang, Y.; Yu, X.; Yang, H.; Chai, K.; Zhao, D. Ultrathin Covalent Organic Framework Film as Membrane Gutter Layer for High-Permeance CO2 Capture. J. Membr. Sci. 2021, 632, 119384. [Google Scholar] [CrossRef]
- Pohlmann, J.; Bram, M.; Wilkner, K.; Brinkmann, T. Pilot Scale Separation of CO2 from Power Plant Flue Gases by Membrane Technology. Int. J. Greenh. Gas Control 2016, 53, 56–64. [Google Scholar] [CrossRef]
- Brinkmann, T.; Lillepärg, J.; Notzke, H.; Pohlmann, J.; Shishatskiy, S.; Wind, J.; Wolff, T. Development of CO2 Selective Poly(Ethylene Oxide)-Based Membranes: From Laboratory to Pilot Plant Scale. Engineering 2017, 3, 485–493. [Google Scholar] [CrossRef]
- Li, L.; Miyazaki, S.; Wu, Z.; Toyao, T.; Selyanchyn, R.; Maeno, Z.; Fujikawa, S.; Shimizu, K. Continuous Direct Air Capture and Methanation Using Combined System of Membrane-Based CO2 Capture and Ni-Ca Based Dual Functional Materials. Appl. Catal. B 2023, 339, 123151. [Google Scholar] [CrossRef]
- Knox, J.C.; Watson, D.W.; Giesy, T.J.; Cmarik, G.E.; Miller, L.A. Investigation of Desiccants and CO2 Sorbents for Exploration Systems 2016–2017. In Proceedings of the 47th International Conference on Environmental Systems (ICES-2017-188), Charleston, SC, USA, 16–20 July 2017. [Google Scholar]
- Behnke, K.; Weber, S. Air Drying Device of a DAC System, DAC System and Method for Operating the Same 2023. Available online: https://patents.google.com/patent/DE102023119768A1/en (accessed on 23 June 2025).
- Ranjan, M.; Herzog, H.J. Feasibility of Air Capture. Energy Procedia 2011, 4, 2869–2876. [Google Scholar] [CrossRef]
- What Are Safe Levels of CO and CO2 in Rooms? | Kane International Limited. Available online: https://www.kane.co.uk/knowledge-centre/what-are-safe-levels-of-co-and-co2-in-rooms (accessed on 23 June 2025).
- Mini CO2 Separator CO-0302SES. Available online: https://ube.es/mini-co2-separator-co-0302ses-optimized-performance-and-technical-specifications-for-co2-removal/ (accessed on 15 May 2025).

| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| Atmospheric air composition | - | % | |
| (RH = 0%): | |||
| - N2 | 78.08 | ||
| - O2 | 20.95 | ||
| - Ar | 0.93 | ||
| - CO2 | 0.04 | ||
| Atmospheric pressure | 1.013 | bar | |
| Air temperature | 20 | °C | |
| Isentropic efficiency of auxiliary equipment (pumps, compressors, fans) | 90 | % | |
| Mechanical efficiency of auxiliary equipment | 99 | % | |
| CO2 concentration in the retentate | 300 | ppm |
| Membrane | ||
|---|---|---|
| N2, Ar, O2 | H2O | |
| M1 | ||
| M2 | 30 | 2.0 |
| M3 | 30 | 0.5 |
| M4 | 30 | 0.2 |
| Permeance of CO2 LCO2 = This value expressed in GPU LCO2 = 10,000 GPU | ||
| Membrane | Selectivity | ||
|---|---|---|---|
| N2, Ar | O2 | H2O | |
| PolyActiveTM | 45.9 | 16.8 | 0.10 |
| Permeance of CO2 LCO2 = or This value expressed in GPU LCO2 = 1481 GPU | |||
| Parameter | Value | Units | ||||
|---|---|---|---|---|---|---|
| 0 | 20 | 40 | 60 | 80 | % | |
| 1.30 | 1.37 | 1.42 | 1.46 | 1.49 | % | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niesporek, K.; Wiciak, G.; Kotowicz, J.; Baszczeńska, O. Effect of Humidity on the Energy and CO2 Separation Characteristics of Membranes in Direct Air Capture Technology. Energies 2025, 18, 3422. https://doi.org/10.3390/en18133422
Niesporek K, Wiciak G, Kotowicz J, Baszczeńska O. Effect of Humidity on the Energy and CO2 Separation Characteristics of Membranes in Direct Air Capture Technology. Energies. 2025; 18(13):3422. https://doi.org/10.3390/en18133422
Chicago/Turabian StyleNiesporek, Kamil, Grzegorz Wiciak, Janusz Kotowicz, and Oliwia Baszczeńska. 2025. "Effect of Humidity on the Energy and CO2 Separation Characteristics of Membranes in Direct Air Capture Technology" Energies 18, no. 13: 3422. https://doi.org/10.3390/en18133422
APA StyleNiesporek, K., Wiciak, G., Kotowicz, J., & Baszczeńska, O. (2025). Effect of Humidity on the Energy and CO2 Separation Characteristics of Membranes in Direct Air Capture Technology. Energies, 18(13), 3422. https://doi.org/10.3390/en18133422







