Combustion Analysis of the Renewable Fuel HVO and RME with Hydrogen Addition in a Reciprocating Internal Combustion Engine
Abstract
1. Introduction
1.1. Hydrogen
1.2. HVO and RME
1.3. Combustion Analysis
2. Materials and Methods
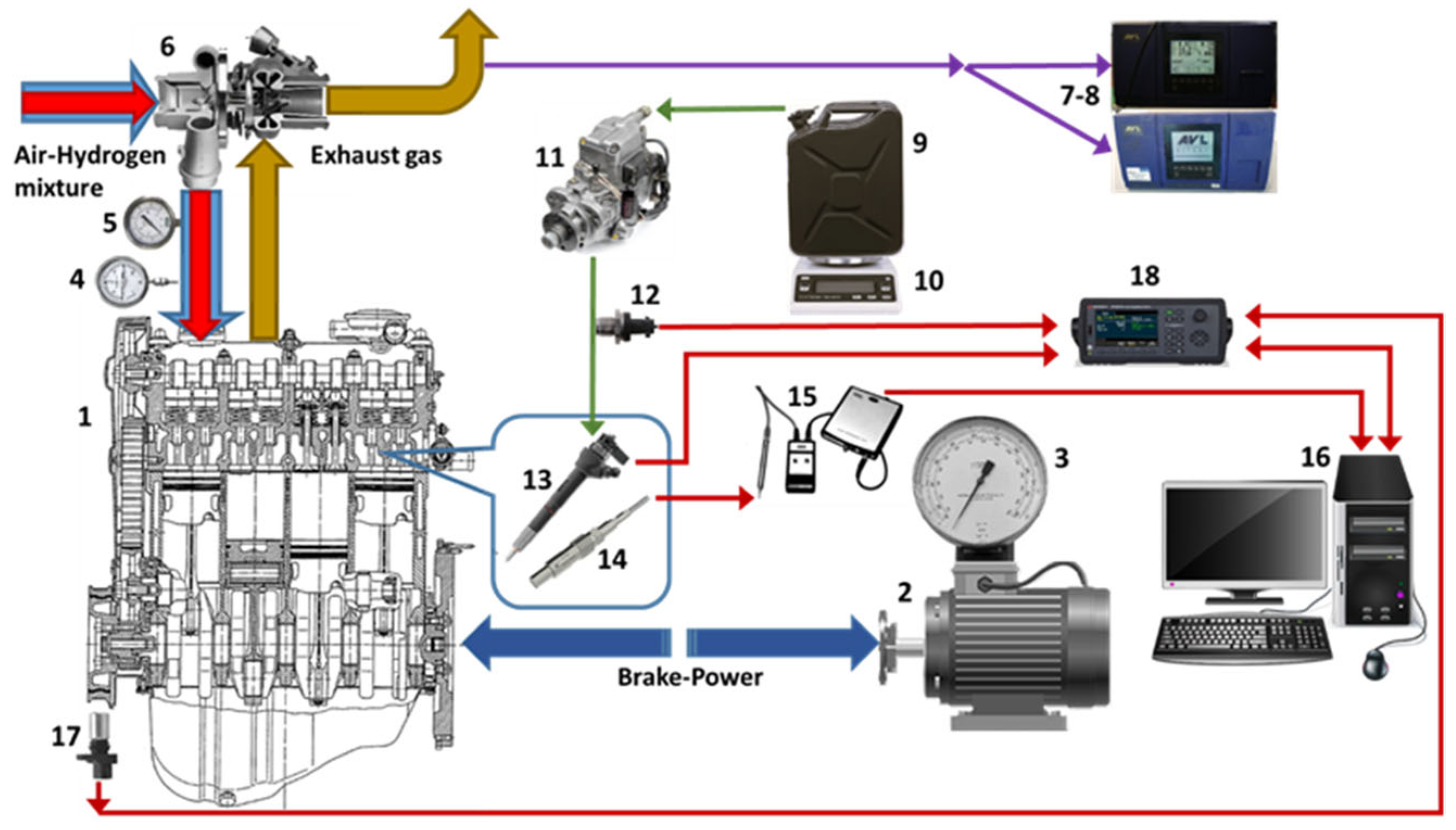
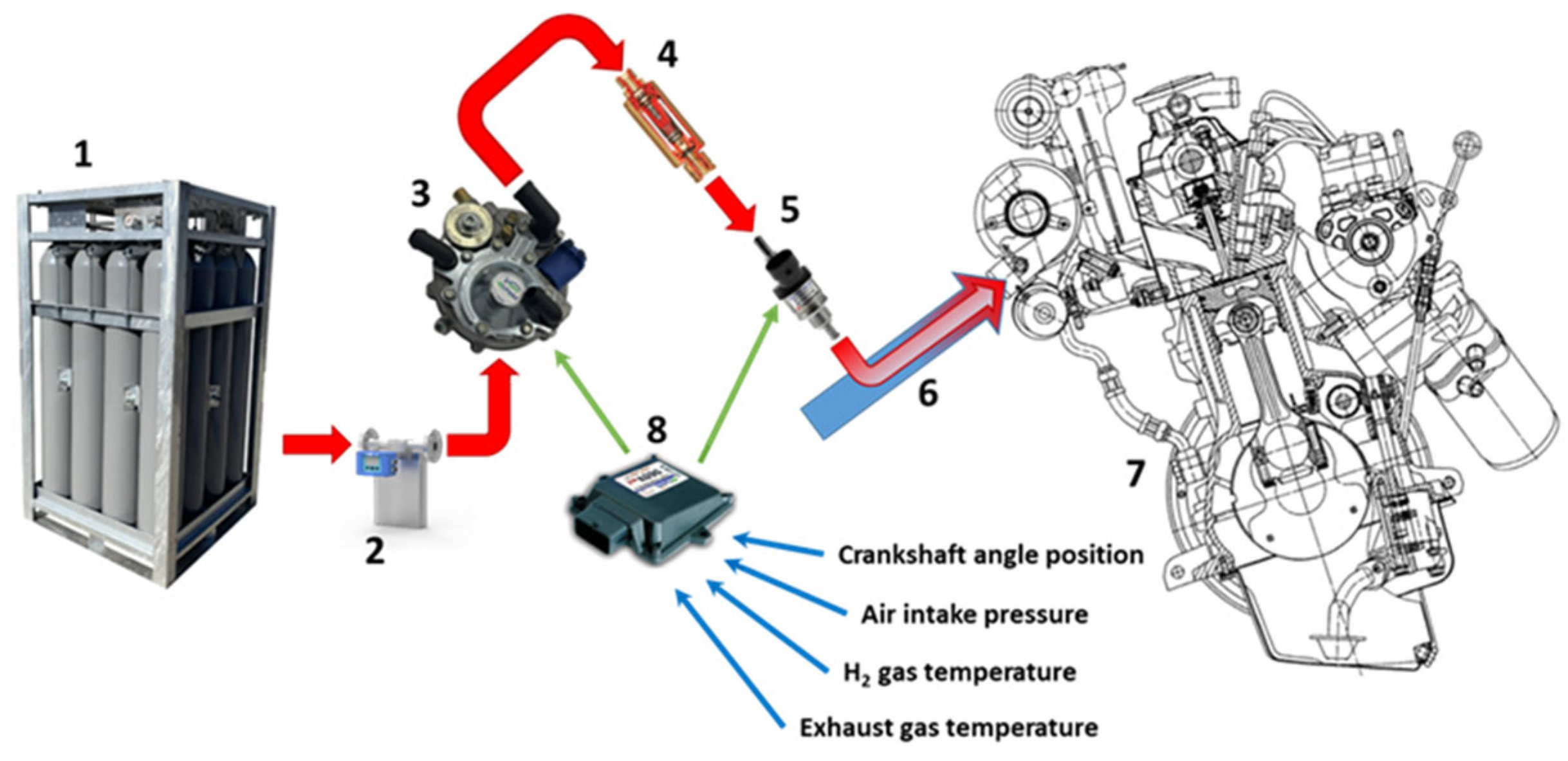
2.1. Experimental Setup
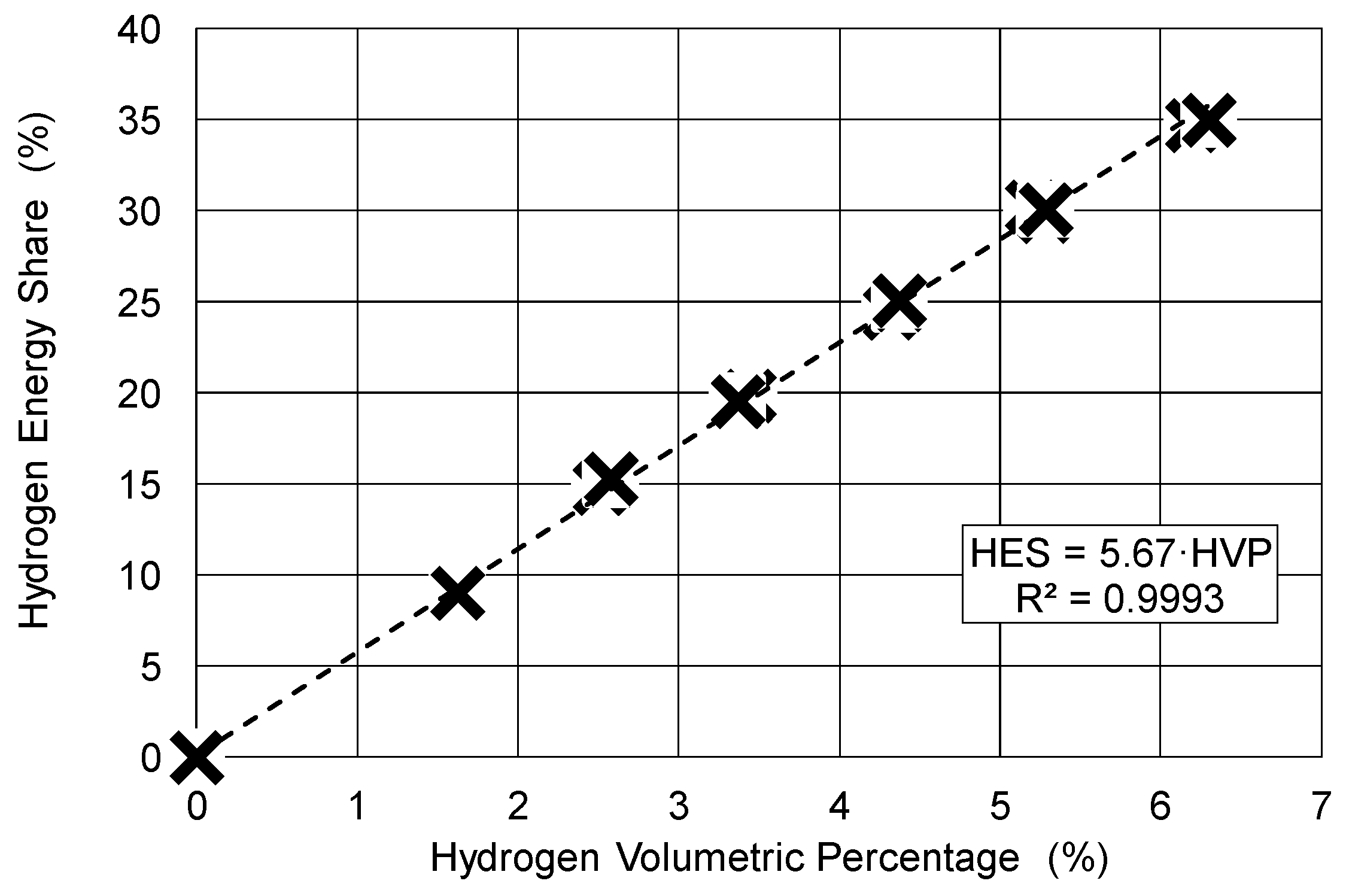
2.2. Methodology
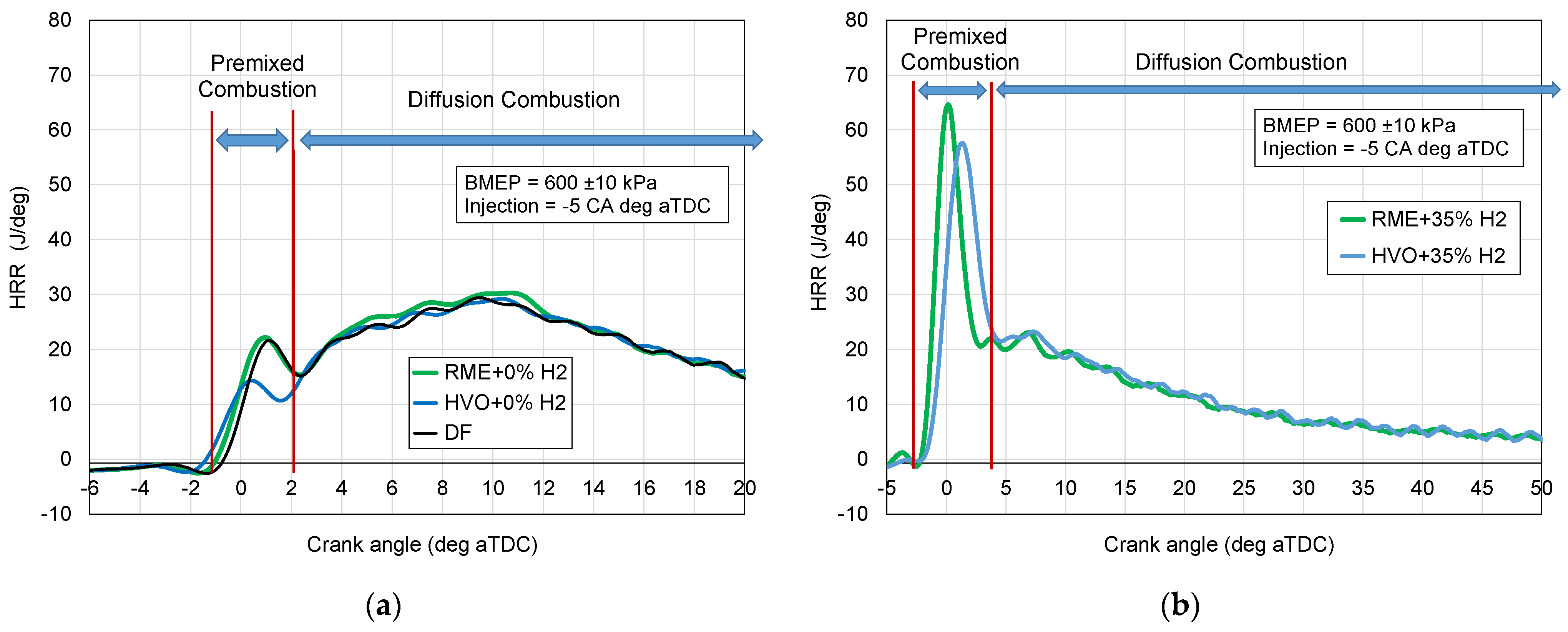
- Heat release rate (HRR) divided into premixed and diffusion combustion phases;
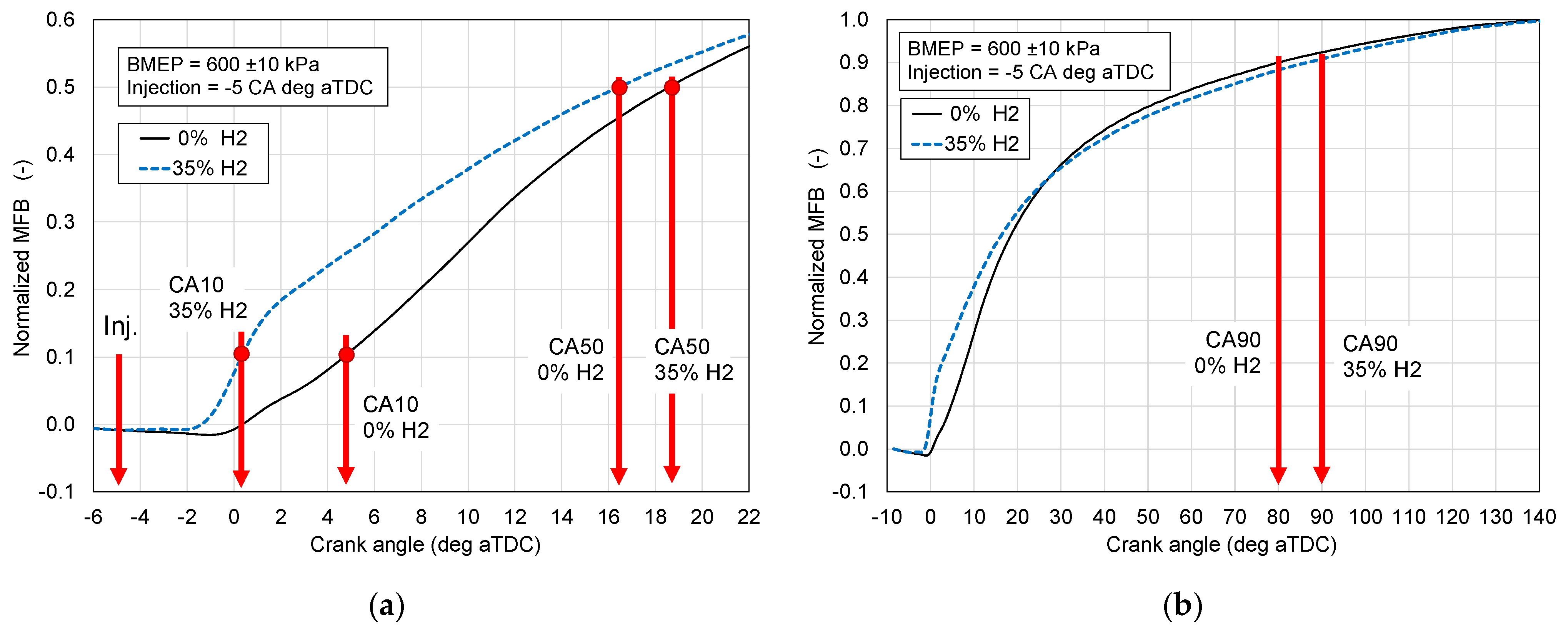
- The course of burning out the fuel, denoted mass fraction burnt (MFB), including the initial phase CA0–10, which is affected by the fuel self-ignition delay, and the main combustion phase denoted CA10–90.
Characteristics of Fuels Used for Investigation
2.3. Uncertainty Analysis
3. Results and Discussion
- Peak in-cylinder combustion pressure;
- Premixed combustion phase;
- Diffusion combustion phase;
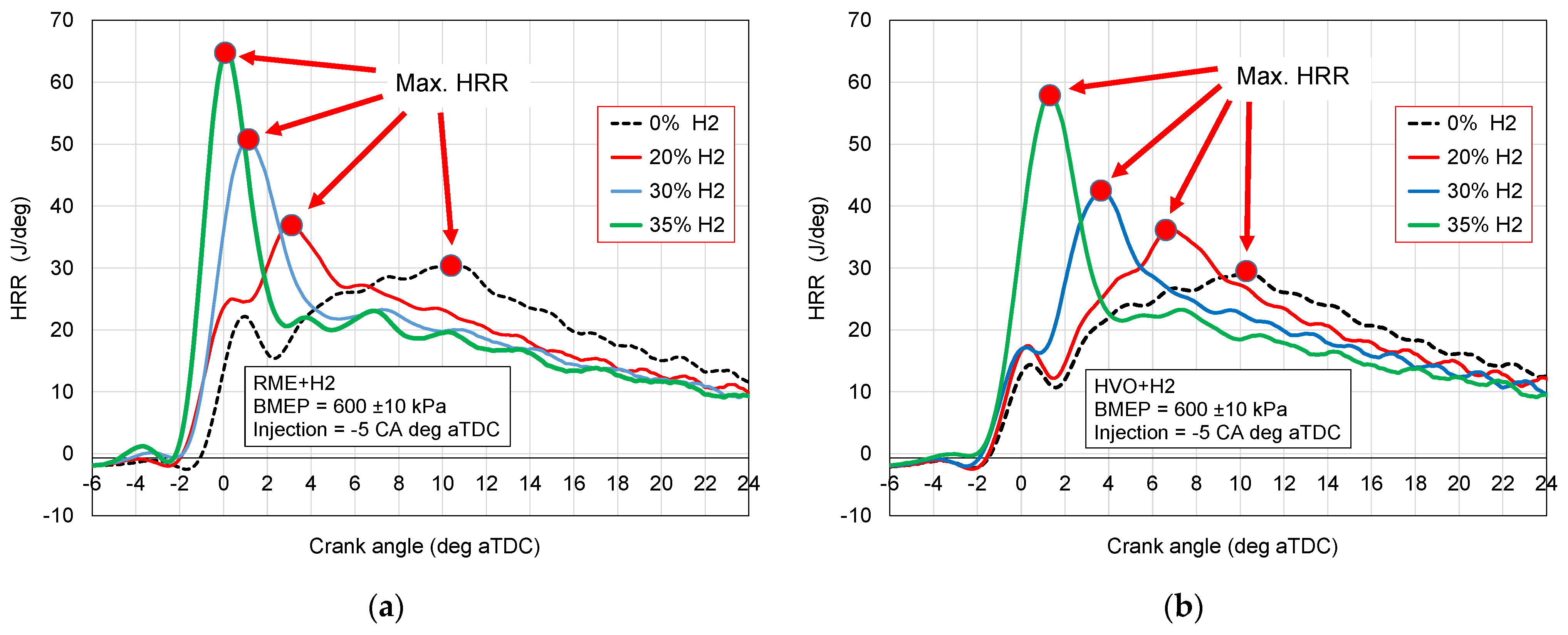
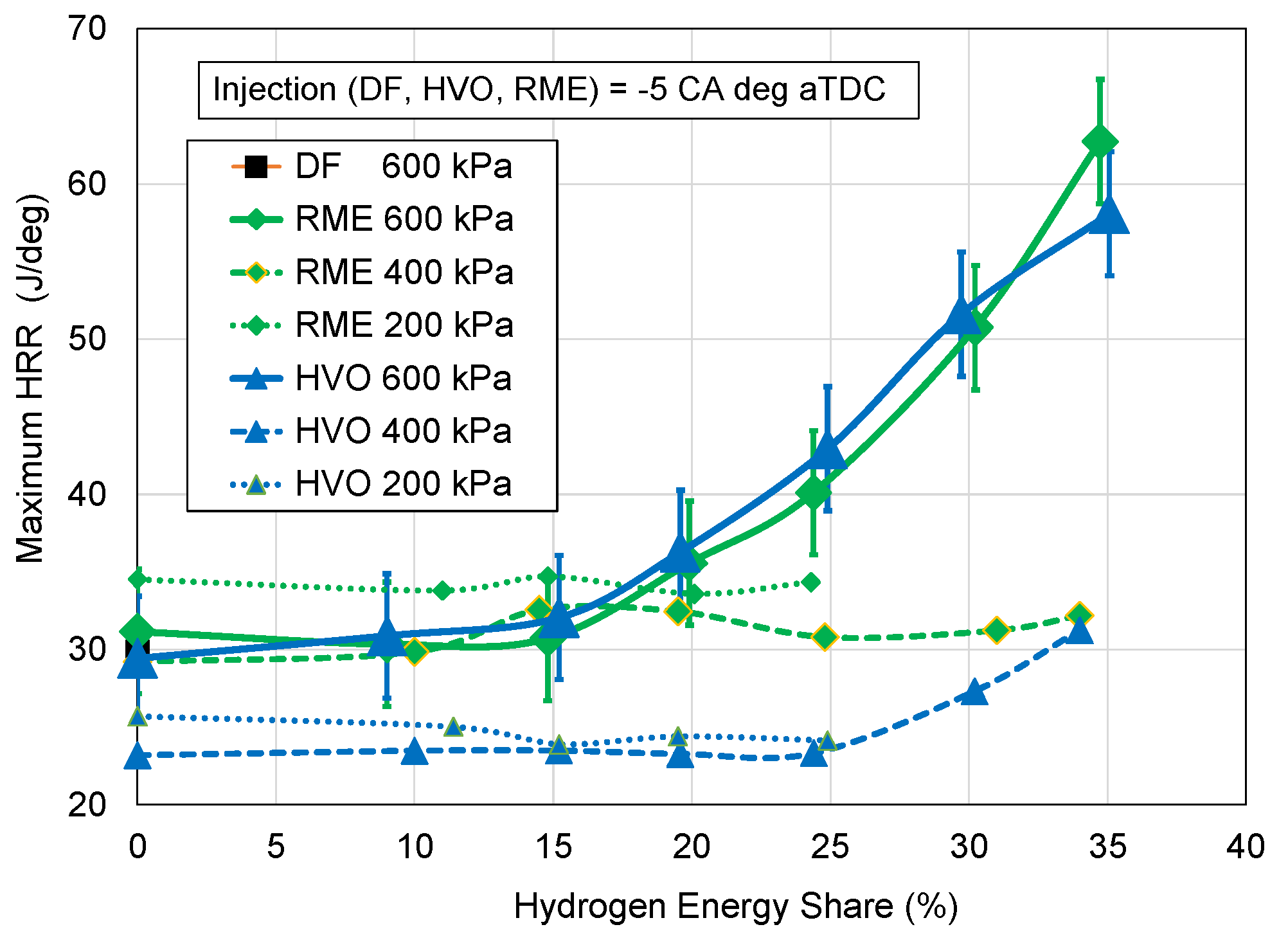
- Maximum HRR;
- First combustion phase denoted CA0–10;
- Main combustion phase CA10–90;
- Entire combustion duration CA0–90.
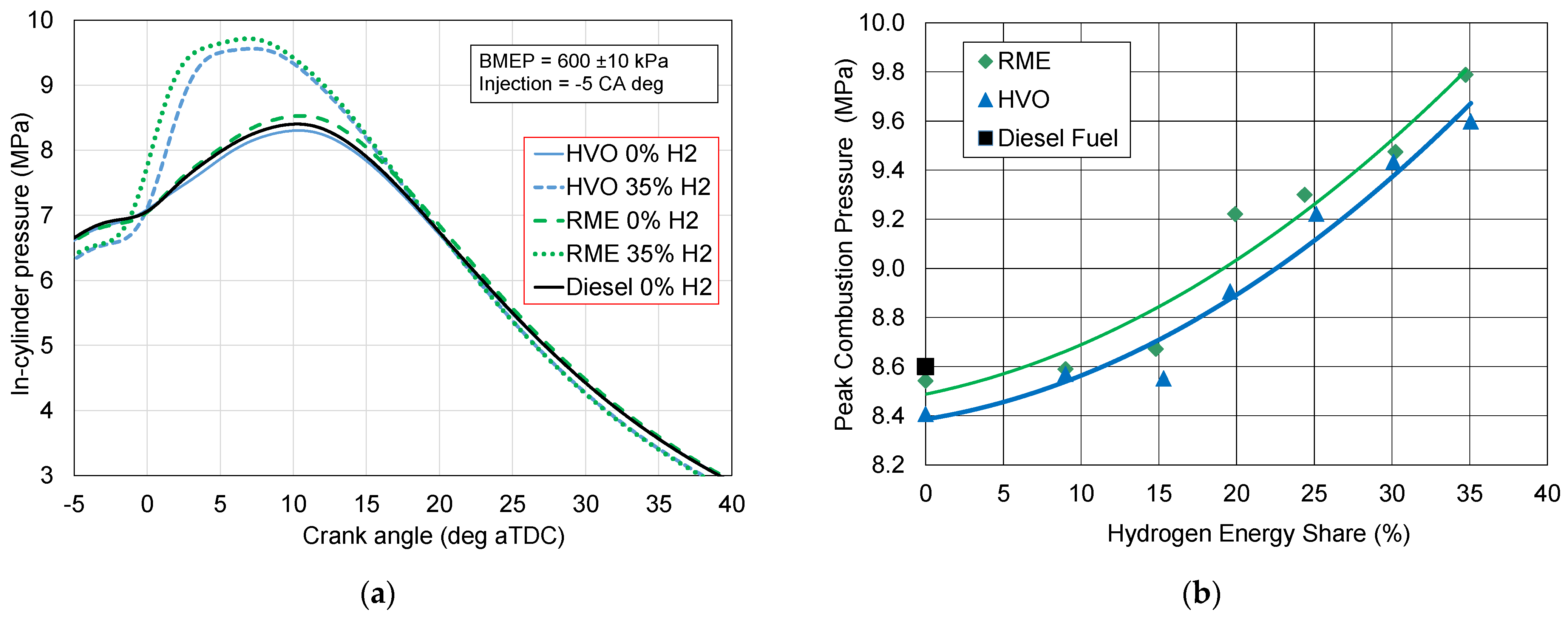
3.1. In-Cylinder Combustion Pressure
3.2. Heat Release Rate
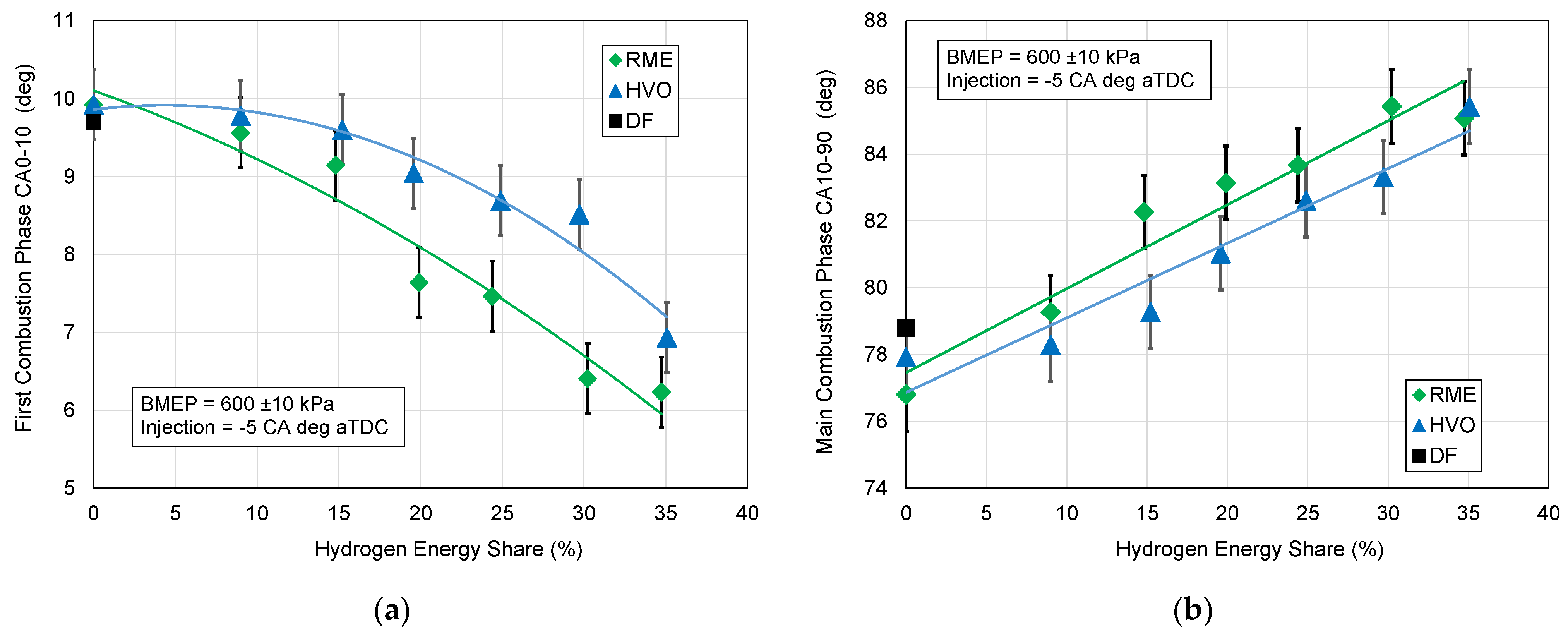
3.3. Combustion Phases
- The first combustion phase CA0–10, counted from the injection of liquid fuel to 10% heat released;
- The main combustion phase CA10–90, counted from 10 to 90% of heat released.
3.4. Discussion Summary
4. Conclusions
- Regular diesel fuel, RME, and HVO as the only fuels burn at nearly the same combustion rates;
- The addition of hydrogen as an additional fuel to RME and HVO by injecting it into the intake manifold affects the following parameters:
- −
- Increase in the in-cylinder peak combustion pressure;
- −
- Shortens first combustion phase CA0–10;
- −
- Lengthens main combustion phase CA10–90;
- The shortening of the CA0–10 phase for RME is greater than that one for HVO, which is probably due to the chemically bound oxygen in the RME fuel and this affects the longer CA10–90 phase for RME in comparison to that of HVO;
- As regards the amount of hydrogen added to the combustion of liquid fuel RME or HVO, the noticeable increase in the combustion rate is for hydrogen above 20% by energy content;
- Maximum of HRR at 35% hydrogen addition by energy is twice higher in comparison to combustion of sole RME and HVO.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSFC | Brake-specific fuel consumption |
| CA | Crank angle |
| CA0–10 | Combustion phase from ignition to 10% heat released |
| CA10–90 | Combustion phase from 10% to 90% heat released |
| CI | Compression-ignition |
| CN | Cetane number |
| CR | Compression ratio |
| DF | Diesel fuel |
| EGR | Exhaust gases recirculation |
| HRR | Heat release rate |
| HVO | Hydrotreated vegetable oil |
| IC | Internal combustion |
| LFL | Lower flammability limits |
| LHV | Lower heating value |
| MFB | Mass fraction burnt |
| NOx | Nitric oxides |
| RME | Rapeseed methyl ester |
| UHC | Unburned hydrocarbons |
References
- Cheng, Q.; Tuomo, H.; Kaario, O.; Martti, L. HVO, RME, and Diesel Fuel Combustion in an Optically Accessible Compression Ignition Engine. Energy Fuels 2019, 33, 2489–2501. [Google Scholar] [CrossRef]
- Novakovic, M.; Eriksson, A.; Gren, L.; Malmborg, V.; Shamun, S.; Karjalainen, P.; Svenningsson, B.; Tuner, M.; Verhelst, S.; Pagels, J. Fresh and Aged Organic Aerosol Emissions from Renewable Diesel-Like Fuels HVO and RME in a Heavy-Duty Compression Ignition Engine. SAE Int. J. Adv. Curr. Pr. Mobil. 2023, 6, 533–544. [Google Scholar] [CrossRef]
- Abifarin, J.K.; Abifarin, F.B. Carbon Emission Reduction and Hydrogen Production Maximization from Carbon Emission-Based Hydrogen Sources. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Lv, J.; Liu, Z.; Gao, S.; Hu, J.; Zhang, J.; Zhong, W.; Zhao, Z. Evaluation of Hydrogen Addition on Combustion and Emission Characteristics of Dual-Fuel Diesel Engines with Different Compression Ratios. Processes 2023, 11, 2675. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Grab-Rogaliński, K. Co-Combustion of Hydrogen with Diesel and Biodiesel (RME) in a Dual-Fuel Compression-Ignition Engine. Energies 2023, 16, 4892. [Google Scholar] [CrossRef]
- Dimitrov, E.; Peychev, M.; Tashev, A. Study of the Hydrogen Influence on the Combustion Parameters of Diesel Engine. Int. J. Hydrogen Energy 2025, 123, 219–230. [Google Scholar] [CrossRef]
- Rajak, U.; Apparao, K.C.; Verma, T.N.; Ağbulut, Ü. Enhancing Performance, and Combustion Efficiency, and Reducing Tailpipe Emissions of an Engine Fuelled with Hydrogen-Enriched Diesel and Ethanol Blends at Varying CRs Using RSM. Int. J. Hydrogen Energy 2024, 92, 1236–1247. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Ahmadi, R. Performance and Emissions Characteristics in the Combustion of Co-Fuel Diesel-Hydrogen in a Heavy Duty Engine. Appl. Energy 2017, 205, 911–925. [Google Scholar] [CrossRef]
- Tira, H.S.; Herreros, J.M.; Tsolakis, A.; Wyszynski, M.L. Influence of the Addition of LPG-Reformate and H2 on an Engine Dually Fuelled with LPG–Diesel, –RME and –GTL Fuels. Fuel 2014, 118, 73–82. [Google Scholar] [CrossRef]
- Szwaja, S.; Grab-Rogalinski, K. Hydrogen Combustion in a Compression Ignition Diesel Engine. Int. J. Hydrogen Energy 2009, 34, 4413–4421. [Google Scholar] [CrossRef]
- Žaglinskis, J.; Rimkus, A. Research on the Performance Parameters of a Compression-Ignition Engine Fueled by Blends of Diesel Fuel, Rapeseed Methyl Ester and Hydrotreated Vegetable Oil. Sustainability 2023, 15, 14690. [Google Scholar] [CrossRef]
- Sukjit, E.; Herreros, J.M.; Dearn, K.D.; Tsolakis, A.; Theinnoi, K. Effect of Hydrogen on Butanol–Biodiesel Blends in Compression Ignition Engines. Int. J. Hydrogen Energy 2013, 38, 1624–1635. [Google Scholar] [CrossRef]
- Di Blasio, G.; Ianniello, R.; Beatrice, C. Hydrotreated Vegetable Oil as Enabler for High-Efficient and Ultra-Low Emission Vehicles in the View of 2030 Targets. Fuel 2022, 310, 122206. [Google Scholar] [CrossRef]
- d’Ambrosio, S.; Mancarella, A.; Manelli, A. Utilization of Hydrotreated Vegetable Oil (HVO) in a Euro 6 Dual-Loop EGR Diesel Engine: Behavior as a Drop-In Fuel and Potentialities along Calibration Parameter Sweeps. Energies 2022, 15, 7202. [Google Scholar] [CrossRef]
- Liu, D.; Ghafourian, A.; Xu, H. Phenomenology of EGR in a Light Duty Diesel Engine Fuelled with Hydrogenated Vegetable Oil (HVO), Used Vegetable Oil Methyl Ester (UVOME) and Their Blends; SAE: Warrendale, PA, USA, 2013. [Google Scholar] [CrossRef]
- Hunicz, J.; Krzaczek, P.; Gęca, M.; Rybak, A.; Mikulski, M. Comparative Study of Combustion and Emissions of Diesel Engine Fuelled with FAME and HVO. Combust. Engines 2021, 184, 72–78. [Google Scholar] [CrossRef]
- Happonen, M.; Heikkilä, J.; Murtonen, T.; Lehto, K.; Sarjovaara, T.; Larmi, M.; Keskinen, J.; Virtanen, A. Reductions in Particulate and NOx Emissions by Diesel Engine Parameter Adjustments with HVO Fuel. Environ. Sci. Technol. 2012, 46, 6198–6204. [Google Scholar] [CrossRef]
- Wu, Y.; Ferns, J.; Li, H.; Andrews, G. Investigation of Combustion and Emission Performance of Hydrogenated Vegetable Oil (HVO) Diesel. SAE Int. J. Fuels Lubr. 2017, 10, 895–903. [Google Scholar] [CrossRef]
- Aatola, H.; Larmi, M.; Sarjovaara, T.; Mikkonen, S. Hydrotreated Vegetable Oil (HVO) as a Renewable Diesel Fuel: Trade-off between NOx, Particulate Emission, and Fuel Consumption of a Heavy Duty Engine. SAE Int. J. Engines 2008, 1, 1251–1262. [Google Scholar] [CrossRef]
- Orliński, P.; Sikora, M.; Bednarski, M.; Gis, M. The Influence of Powering a Compression Ignition Engine with HVO Fuel on the Specific Emissions of Selected Toxic Exhaust Components. Appl. Sci. 2024, 14, 5893. [Google Scholar] [CrossRef]
- Pinto, G.M.; de Souza, T.A.Z.; da Costa, R.B.R.; Roque, L.F.A.; Frez, G.V.; Coronado, C.J.R. Combustion, Performance and Emission Analyses of a CI Engine Operating with Renewable Diesel Fuels (HVO/FARNESANE) under Dual-Fuel Mode through Hydrogen Port Injection. Int. J. Hydrogen Energy 2023, 48, 19713–19732. [Google Scholar] [CrossRef]
- Pinto, G.M.; da Costa, R.B.R.; de Souza, T.A.Z.; Rosa, A.J.A.C.; Raats, O.O.; Roque, L.F.A.; Frez, G.V.; Coronado, C.J.R. Experimental Investigation of Performance and Emissions of a CI Engine Operating with HVO and Farnesane in Dual-Fuel Mode with Natural Gas and Biogas. Energy 2023, 277, 127648. [Google Scholar] [CrossRef]
- Pechout, M.; Kotek, M.; Jindra, P.; Macoun, D.; Hart, J.; Vojtisek-Lom, M. Comparison of Hydrogenated Vegetable Oil and Biodiesel Effects on Combustion, Unregulated and Regulated Gaseous Pollutants and DPF Regeneration Procedure in a Euro6 Car. Sci. Total Environ. 2019, 696, 133748. [Google Scholar] [CrossRef]
- Lionus Leo, G.M.; Jayabal, R.; Kathapillai, A.; Sekar, S. Performance and Emissions Optimization of a Dual-Fuel Diesel Engine Powered by Cashew Nut Shell Oil Biodiesel/Hydrogen Gas Using Response Surface Methodology. Fuel 2025, 384, 133960. [Google Scholar] [CrossRef]
- Rimkus, A.; Juknelevičius, R. RME Co-Combustion with Hydrogen in Compression Ignition Engine: Performance, Efficiency and Emissions. Moksl. Liet. Ateitis 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Heywood, J.B. Engine Combustion Modeling—An Overview. In Combustion Modeling in Reciprocating Engines; Springer: Boston, MA, USA, 1980; pp. 1–38. [Google Scholar]
- Caton, J.A. The Thermodynamics of Internal Combustion Engines: Examples of Insights. Inventions 2018, 3, 33. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill Book Company: New York, NY, USA, 1988. [Google Scholar]
- Arsie, I.; Frasci, E.; Irimescu, A.; Merola, S.S. Spark Timing Optimization through Co-Simulation Analysis in a Spark Ignition Engine. Energies 2024, 17, 3695. [Google Scholar] [CrossRef]
- Beccari, S.; Pipitone, E. A New Simple Function for Combustion and Cyclic Variation Modeling in Supercharged Spark Ignition Engines. Energies 2022, 15, 3796. [Google Scholar] [CrossRef]
- Caton, J.A. A Review of Investigations Using the Second Law of Thermodynamics to Study Internal-Combustion Engines. SAE Trans. 2000, 109, 1252–1266. [Google Scholar]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Kosmadakis, G.M.; Giakoumis, E.G.; Kyritsis, D.C. Exergy Analysis in Highly Hydrogen-Enriched Methane Fueled Spark-Ignition Engine at Diverse Equivalence Ratios via Two-Zone Quasi-Dimensional Modeling. Energies 2024, 17, 3964. [Google Scholar] [CrossRef]
- LST EN 15940:2023; Automotive Fuels. Paraffinic Diesel Fuel from Synthesis or Hydrotreatment. Requirements and Test Methods. British Standards Institution: London, UK, 2023. Available online: https://www.intertekinform.com/en-gb/standards/bs-en-15940-2023-289199_saig_bsi_bsi_3336124/ (accessed on 30 May 2025).
- EN 15940:2016; Automotive Fuels—Paraffinic Diesel Fuel from Synthesis or Hydrotreatment—Requirements and Test Methods. National Standards Authority of Ireland: Dublin, Ireland, 2020. Available online: https://www.intertekinform.com/en-gb/standards/i-s-en-15940-2016-879916_saig_nsai_nsai_2090618/ (accessed on 30 May 2025).
- EN 14214:2008; Liquid Petroleum Products—Fatty Acid Methyl Esters (Fame) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. British Standards Institution: London, UK, 2008. Available online: https://www.intertekinform.com/en-gb/standards/bs-en-14214-2008-237184_saig_bsi_bsi_554382/ (accessed on 30 May 2025).


| Number of cylinders | 4 |
| Bore diameter | 79.5 mm |
| Piston stroke | 95.5 mm |
| Displacement | 1896 cm3 |
| Compression ratio | 19.5 |
| Rated power | 66 kW |
| Rated speed | 4000 rpm |
| Peak torque | 180 Nm |
| Peak torque speed | 2000–2500 rpm |
| Length of connecting road | 150 mm |
| Intake valve opening | 16° bTDC |
| Intake valve closing | 25° aBDC |
| Exhaust valve opening | 28° bBDC |
| Exhaust valve closing | 19° aTDC |
| Voltage range | 8–15 V |
| Working pressure difference Δp | 0.95 bar |
| Max. output pressure | 2.2 bar |
| Test pressure | 45 bar |
| Voltage range | 12 V DC |
| Resistance | 1.3 Ohm |
| Max operating pressure | 3.5 bar |
| Maximum gas flow rate | 130 dm3/min |
| Opening time | 2.36 ms |
| Closing time | 1.2 ms |
| Pick current | 4 A |
| Hold current | 2 A |
| Working temperature range | −40…120 °C |
| Parameter/Quantity | Units | Data |
|---|---|---|
| Fuels | - | Diesel Fuel, HVO, RME Hydrogen from 0 to 35% (by energy) |
| Load as BMEP | kPa | 200, 400, 600 |
| Injection timing | CA deg aTDC | −5 |
| Speed | rpm | 1500 |
| Parameter | Unit | RME | HVO | DF |
|---|---|---|---|---|
| Elemental chemical composition (by mass) | C H O | 0.775 0.115 0.011 | 0.845 0.155 0 | 0.855 0.145 0 |
| Density at 15 °C and 1.01 bar | kg/m3 | 882 | 779.7 | 830.5 |
| LHV | MJ/kg | 36.8–37.4 | 44.04 | 42.95 |
| Auto-ign. temp. @ STP | °C | 342 | ~210 | 250 |
| Kin. viscosity @ 40 °C | mm2/s | 4.44 | 2.87 | 2.07 |
| Flash point | °C | 170 | 61 | 56 |
| Pour point | °C | −12 | – | −35–(−32) |
| Cloud point | °C | −3.3 | −34–(−5) | −22 |
| Iodine number | g I2/100 g | 111 | – | 6 |
| Total aromatics | % (wt.) | – | 0.3 | 24 |
| C/H ratio | (wt.) | 6.5 | 5.6 | 6.9 |
| Cetane number (CN) | - | 54.4 | 75–99 | 51.5 |
| Parameter | Measurement Range | Accuracy | |
|---|---|---|---|
| Exhaust gas analyzer AVL DiCom 4000 (AVL DiTEST, Graz, Austria) | NOx | 0–5000 ppm | 1 ppm |
| HC | 0–20,000 ppm | 1 ppm | |
| CO | 0–10% vol | 0.01% vol | |
| CO2 | 0–20% vol | 0.1% vol | |
| O2 | 0–25% vol | 0.01% vol | |
| Absorption (K-Value) | 0–99.99 m−1 | 0.01 m−1 | |
| Intake pressure sensor TP704-2BAI (CRN TECNOPART S.A., Barcelona, Spain | Pressure | 0–200 kPa | 0.2 kPa |
| Gauge Delta OHM HD 2304.0 (Axioma Measurement Systems, Vilnius, Lithuania) | Pressure | 0–200 kPa | 0.1 kPa |
| Piezo-ceramic sensor AVL GH13P (AVL List GmbH, Graz, Austria) | Pressure | 0–250 bar | ±0.09 pC/bar |
| K-type thermocouples | Temperature | 0–900 °C | 1.5 °C |
| Electronic scale SK–5000 (A&D Engineering Inc., San Jose, CA, USA) | Weight | 0–20 kG | 0.5% |
| Gas meter KG-0095-G06-94-10 (Sure Instrument Co., Ltd., Tianjin, China) | Flow rate | 0.01–3 kg/min | 0.5% |
| Mass flow meter RHEONIK RHM 015 | H2 flow rate | 0.004–0.6 kg/min | 0.1% |
| Parameter/Quantity | Units | Uncertainty |
|---|---|---|
| Peak Combustion Pressure | MPa | 0.19 |
| CA0–10 | CA deg | 1.12 |
| CA10–90 | CA deg | 1.25 |
| Maximum HRR | J/deg | 4.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwaja, S.; Pukalskas, S.; Juknelevicius, R.; Rimkus, A. Combustion Analysis of the Renewable Fuel HVO and RME with Hydrogen Addition in a Reciprocating Internal Combustion Engine. Energies 2025, 18, 3381. https://doi.org/10.3390/en18133381
Szwaja S, Pukalskas S, Juknelevicius R, Rimkus A. Combustion Analysis of the Renewable Fuel HVO and RME with Hydrogen Addition in a Reciprocating Internal Combustion Engine. Energies. 2025; 18(13):3381. https://doi.org/10.3390/en18133381
Chicago/Turabian StyleSzwaja, Stanislaw, Saugirdas Pukalskas, Romualdas Juknelevicius, and Alfredas Rimkus. 2025. "Combustion Analysis of the Renewable Fuel HVO and RME with Hydrogen Addition in a Reciprocating Internal Combustion Engine" Energies 18, no. 13: 3381. https://doi.org/10.3390/en18133381
APA StyleSzwaja, S., Pukalskas, S., Juknelevicius, R., & Rimkus, A. (2025). Combustion Analysis of the Renewable Fuel HVO and RME with Hydrogen Addition in a Reciprocating Internal Combustion Engine. Energies, 18(13), 3381. https://doi.org/10.3390/en18133381








