Progress in Materials and Metal Substrates for Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Materials for Solid Oxide Fuel Cells
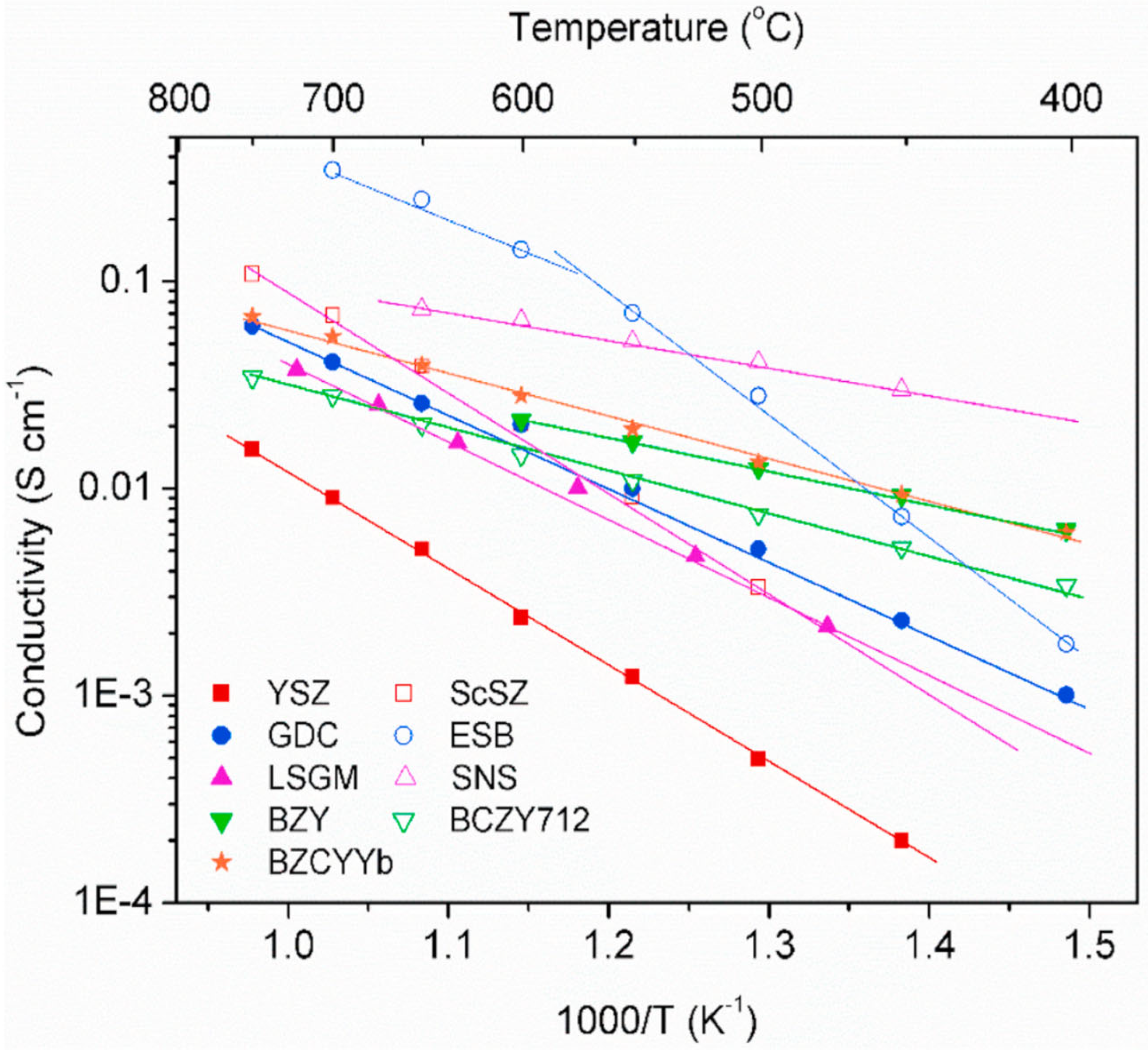
2.1. Electrolyte Materials of SOFCs
- Stability: The electrolyte must be chemically and morphologically stable under dual atmospheres, i.e., H2 and O2.
- Conductivity: The electrolyte must have appropriate ionic conductivity. To minimize ohmic losses, the ionic conductivity must be as high as possible. The electrolyte must also have negligible electronic conductivity to avoid voltage loss.
- Compatibility: The electrolyte must be chemically compatible with other battery components during manufacturing as well as during operation.
- Thermal expansion: The thermal expansion of the electrolyte must be similar to that of the other battery components to avoid cracking and delamination during manufacturing and operation, including thermal cycling.
- Porosity: The electrolyte must be dense to prevent cross-linking of the reactants and to maximize conductivity.
2.2. Cathode Materials of SOFCs
- High electronic conductivity (more than 100 S/cm);
- High oxygen ion conductivity;
- High catalytic activity for oxygen molecular adsorption and dissociation and for oxygen reduction;
- A TEC that matches those of the other cell components;
- Sufficient porosity to transport gases to the reaction site.
2.3. Anode Materials of SOFCs
- Sufficiently high electronic conductivity (>100 S/cm) in a reducing environment at the operating temperature;
- Oxygen ion conductivity (enhanced mixed conductivity);
- High catalytic activity for hydrogen molecules or fuel adsorption and dissociation, and electrochemical oxidation of fuel;
- TECs matching those of the other cell components;
- Sufficient porosity to enable gas to move to the reaction site.
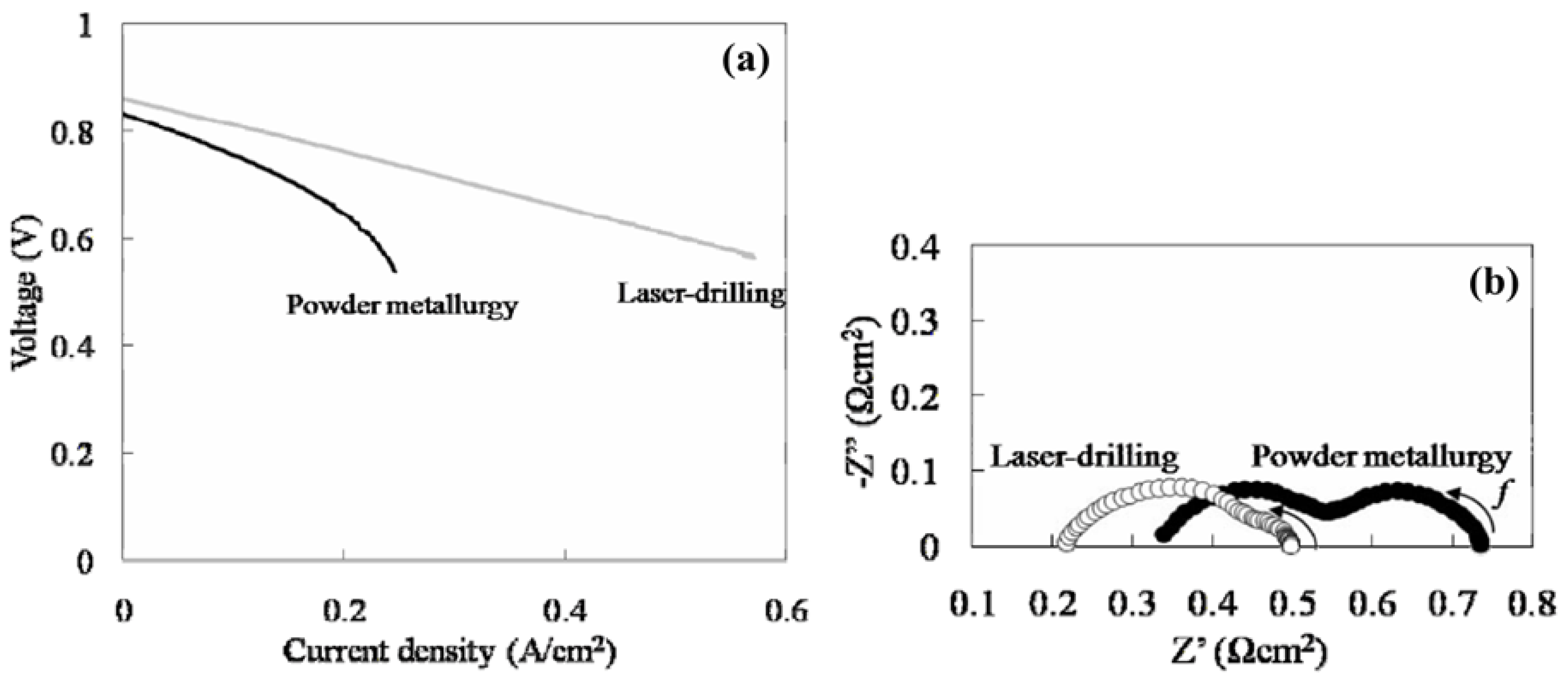
3. Metal-Supported Solid Oxide Fuel Cells
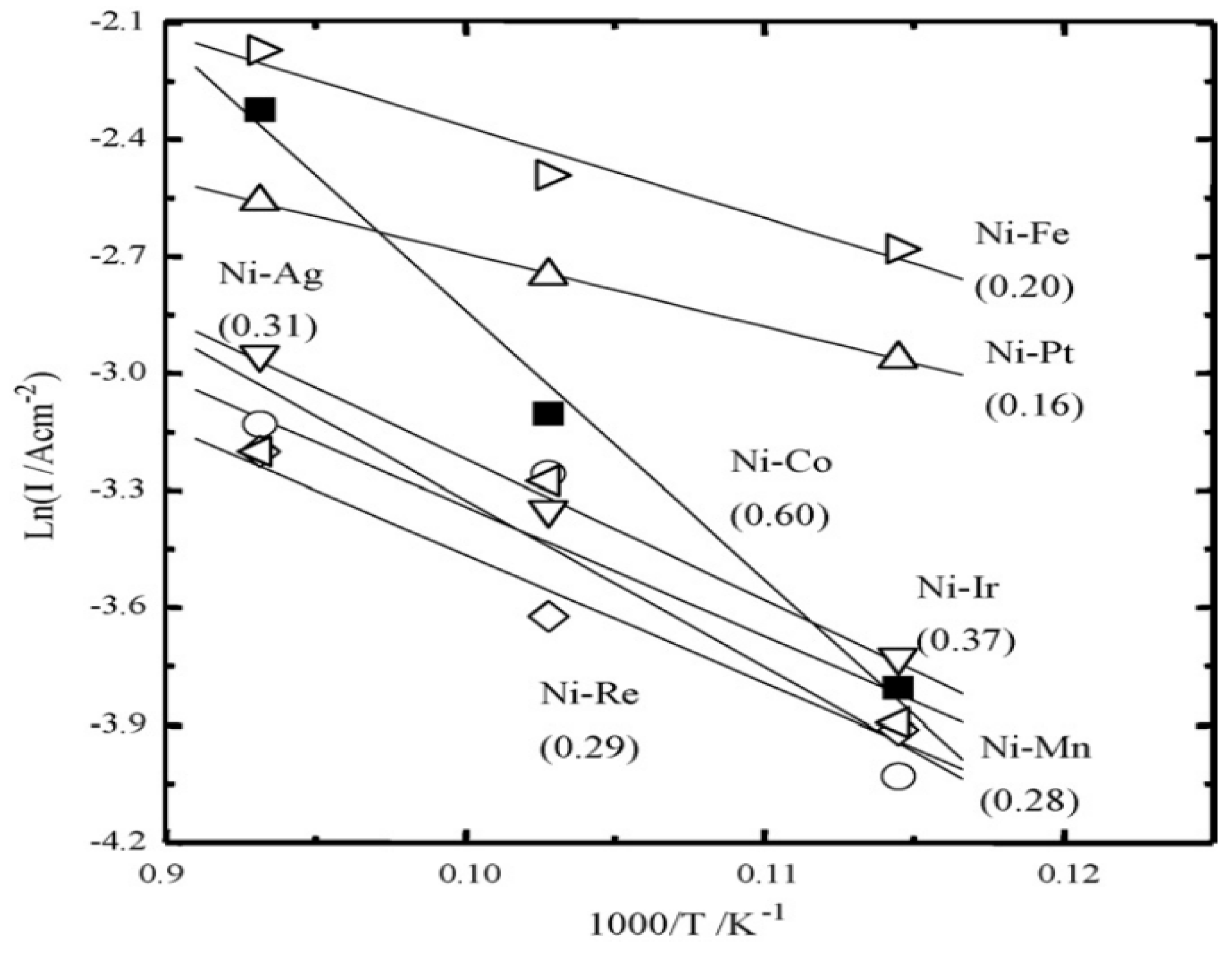
3.1. Ni-Based Metal Substrate

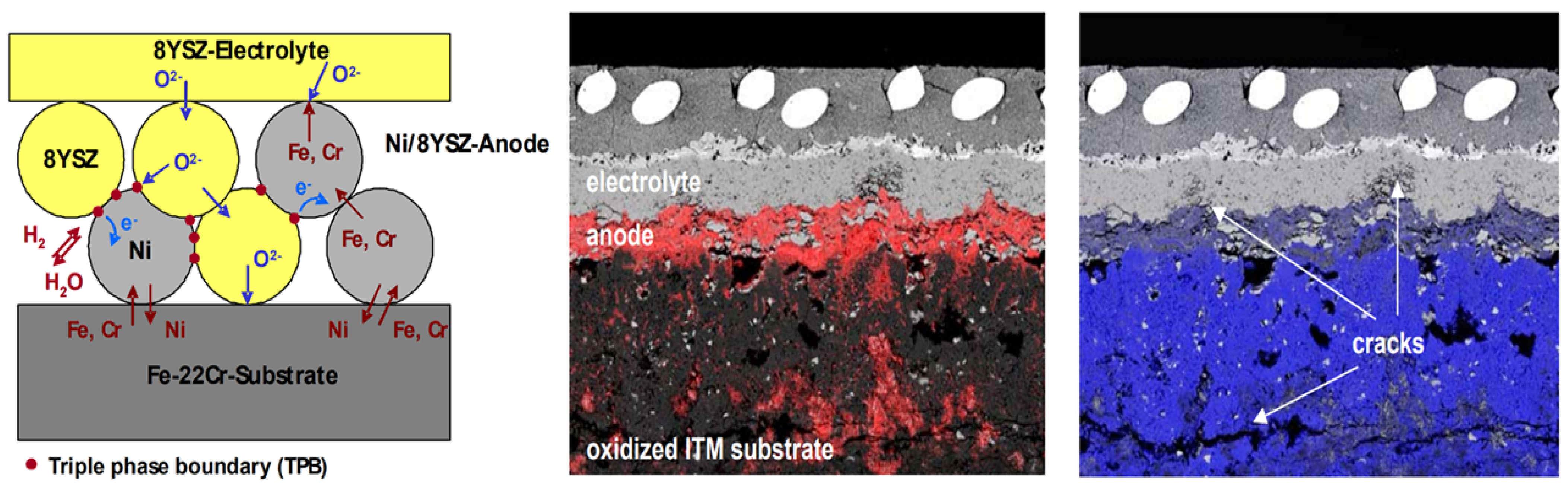
3.2. Fe-Based Metal Substrate
4. Conclusions
- Research has been conducted to improve the ionic conductivity of electrolyte materials (e.g., triple-doped Bi2O3 oxygen ion conductors and doped BaZr(Ce,Y)O3 proton-conducting materials) by doping with multi-heterogeneous elements, helping lower the operating temperature of SOFCs by increasing the ion conductivity of the electrolyte, even at low temperatures.
- To maintain and enhance the catalytic properties of Ni-based anodes and prevent reactions with electrolytes (especially LSGM electrolytes), various ceramic-based anode catalysts have been studied, and layered perovskite anode materials have shown the best catalytic properties among ceramic anode materials.
- The low oxygen reduction reaction at low temperatures has a significant impact on the degradation of SOFC performance. Therefore, many researchers have investigated cathode materials, and various transition metals are being used. Cobalt-based materials have received attention because they provide mixed conductivity and a higher oxygen vacancy concentration than other cathode materials at low temperatures. However, the high TEC and reduced stability resulting from the sintering characteristics are issues that need to be resolved. Recently, Ruddlesden–Popper structures and layered perovskite structured cathode materials without Co have been studied extensively, and they have shown excellent mixed conductivity and stability.
- The development of Ni-based metal substrates using heterogeneous elements helps obtain TEC values similar to those of conventional electrolytes, thereby reducing thermal stress and improving structural stability during high-temperature operation. It also helps improve the catalytic properties and pore formation, contributing to improved SOFC performance. In addition, the introduction of a functional layer (between the electrolyte and metal substrate) to suppress reactivity with electrolyte materials and increase the reaction area leads to excellent power generation characteristics, even at low temperatures. Fe-based metal substrates have TECs similar to those of electrolyte materials and have been used in many studies to manufacture MS-SOFCs; however, there are difficulties in forming sufficient pores to increase fuel utilization. In batch manufacturing processes, such as tape casting, the use of pore-forming agents to secure pores and the introduction of a side reaction barrier layer by cation diffusion have improved performance and stability, and large-area cell and stack research is being conducted.
Funding
Conflicts of Interest
References
- Dyer, C.K. Replacing the battery in portable electronics. Sci. Am. 1999, 281, 88–93. [Google Scholar] [CrossRef]
- Song, C. Fuel processing for low-temperature and high-temperature fuel cells: Challenges, and opportunities for sustainable development in the 21st century. Catal. Today 2002, 77, 17–49. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 764–774. [Google Scholar] [CrossRef]
- Tsai, T.; Perry, E.; Barnett, S. Low-temperature solid-oxide fuel cells utilizing thin bilayer electrolytes. J. Electrochem. Soc. 1997, 144, L130–L132. [Google Scholar] [CrossRef]
- Kim, J.W.; Virkar, A.V.; Fung, K.Z.; Mehta, K.; Singhal, S.C. Low Temperature High Performance Anode Supported Solid Oxide Fuel Cells. J. Electrochem. Soc. 1999, 146, 69–78. [Google Scholar] [CrossRef]
- Ishihara, T.; Shimose, K.; Kudo, T.; Nishiguchi, H.; Akbay, T.; Takita, Y. Preparation of Yttria-Stabilized Zirconia Thin Films on Strontium-Doped LaMnO3 Cathode Substrates via Electrophoretic Deposition for Solid Oxide Fuel Cells. J. Am. Ceram. Soc. 2000, 83, 1921–1927. [Google Scholar] [CrossRef]
- Kim, S.D.; Hyun, S.H.; Moon, J.; Kim, J.-H.; Song, R.H. Fabrication and characterization of anode-supported electrolyte thin films for intermediate temperature solid oxide fuel cells. J. Power Sources 2005, 139, 67–72. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor. J. Am. Chem. Soc. 1994, 116, 3801–3803. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Takita Effects of rare earth cations doped for La site on the oxide ionic conductivity of LaGaO3-based perovskite type oxide. Solid State Ion. 1995, 79, 147–151. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Fini, D.; Ciacchi, F.T.; Munnings, C.; Kimpton, J.A.; Drennan, J. Structural and microstructural stability of ceria—Gadolinia electrolyte exposed to reducing environments of high temperature fuel cells. J. Mater. Chem. A 2013, 1, 10768–10782. [Google Scholar] [CrossRef]
- Yu, H.; Jeong, I.; Jang, S.; Kim, D.; Im, H.N.; Lee, C.W.; Wachsman, E.D.; Lee, K.T. Temperature reduction of solid oxide electrochemical cells using triple-doped bismuth oxide. Adv. Mater. 2024, 36, 2470040. [Google Scholar] [CrossRef]
- Takahashi, T.; Iwahara, H.; Nagai, Y. High oxide ion conductivity of sintered Bi2O3 containing SrO, CaO or La2O3. J. Appl. Electrochem. 1972, 2, 97–104. [Google Scholar] [CrossRef]
- Ni, M.; Shao, Z. Fuel cells that operate at 300 ° to 500 °C. Science 2020, 369, 138–139. [Google Scholar] [CrossRef]
- Takahashi, T.; Iwahara, H. Solid-state ionics: Protonic conduction in perovskite type oxide solid solutions. Rev. Chim. Miner. 1980, 17, 243–253. [Google Scholar]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3–4, 359–363. [Google Scholar] [CrossRef]
- Iwahara, H.; Esaka, T.; Uchida, H.; Yamauchi, T.; Ogaki, K. High temperature type protonic conductor based on SrCeO3 and its application to the extraction of hydrogen gas. Solid State Ion. 1986, 18–19, 1003–1007. [Google Scholar] [CrossRef]
- Gao, J.; Meng, Y.; Duffy, J.H.; Brinkman, K.S. Low-Temperature Protonic Ceramic Fuel Cells through Interfacial Engineering of Nanocrystalline BaCe0.7Zr0.1Y0.1Yb0.1O3−δ Electrolytes. Adv. Energ. Sust. Res. 2021, 2, 2100098. [Google Scholar] [CrossRef]
- Hu, D.; Kim, J.; Niu, H.; Daniels, L.M.; Manning, T.D.; Chen, R.; Liu, B.; Feetham, R.; Claridge, J.B.; Rosseinsky, M.J. High-performance protonic ceramic fuel cell cathode using protophilic mixed ion and electron conducting material. J. Mater. Chem. A 2022, 10, 2559–2566. [Google Scholar] [CrossRef]
- Kreuer, K.; Adams, S.; Münch, W.; Fuchs, A.; Klock, U.; Maier, J. Maier; Proton conducting alkaline earth zirconates and titanates for high drain electrochemical applications. Solid State Ion. 2001, 145, 295–306. [Google Scholar] [CrossRef]
- Zvonareva, I.; Fu, X.-Z.; Medvedev, D.; Shao, Z. Electrochemistry and energy conversion features of protonic ceramic cells with mixed ionic-electronic electrolytes. Energ. Environ. Sci. 2022, 15, 439–465. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Noh, T.; Kim, J.; Lee, H. Structural change and electrical conductivity according to Sr content in Cu-doped LSM (La1-xSrxMn0.8Cu0.2O3). J. Korean Cryst. Growth C 2012, 22, 78–83. [Google Scholar] [CrossRef]
- Yamamoto, O.; Takeda, Y.; Kanno, R.; Noda, M. Perovskite-type oxides as oxygen electrodes for high temperature oxide fuel cells. Solid State Ion. 1987, 22, 241–246. [Google Scholar] [CrossRef]
- Teraoka, Y.; Zhang, H.M.; Okamoto, K.; Yamazoe, N. Mixed Ionic-Electronic Conductivity of Lal−xSrxCol−yFey03−δ Perovskite-type Oxides. Mater. Res. Bull. 1988, 23, 51–58. [Google Scholar] [CrossRef]
- Tai, L.W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and electrical properties of La1−xSrxCo1−yFeY03. Part 1. The system La0.8Sr0.2Co1-yFey03. Solid State Ion. 1995, 76, 259–271. [Google Scholar] [CrossRef]
- Skinner, S.J. Recent advances in Perovskite-type materials for solid oxide fuel cell cathodes. Int. J. Inorg. Mater. 2001, 3, 113–121. [Google Scholar] [CrossRef]
- Shao, Z.; Haile, S.M.; Ahn, J.; Ronney, P.D.; Zhan, Z.; Barnett, S.A. A thermally self-sustained micro solid-oxide fuel-cell stack with high power density. Nature 2005, 435, 795–798. [Google Scholar] [CrossRef]
- Tietz, F.; Arulraj, I.; Zahid, M.; Stover, D. Electrical conductivity and thermal expansion of La0.8Sr0.2(Mn,Fe,Co)O3−δ perovskites. Solid State Ion. 2006, 177, 1753–1756. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Jia, L.; Naden, A.; Chen, J.; Chi, B.; Pu, J.; Irvine, J.T.; Li, J. A novel electrode with multifunction and regeneration for highly efficient and stable symmetrical solid oxide cell. J. Power Source 2020, 475, 228620. [Google Scholar] [CrossRef]
- Beznosikov, B.V.; Aleksandrov, K.S. Perovskite-like crystals of the Ruddlesden-Popper series. Crystallogr. Rep. 2000, 45, 792–798. [Google Scholar] [CrossRef]
- Torres-Garibay, C.; Kovar, D. Perovskite-related intergrowth cathode materials with thin YSZ electrolytes for intermediate temperature solid oxide fuel cells. J. Power Sources 2009, 192, 396–399. [Google Scholar] [CrossRef]
- Amow, G.; Davidson, I.; Skinner, S. A comparative study of the Ruddlesden-Popper series, Lan+1NinO3n+1 (n = 1, 2 and 3), for solid-oxide fuel-cell cathode applications. Solid State Ion. 2006, 177, 1205–1210. [Google Scholar] [CrossRef]
- Chaianansutcharit, S.; Hosoi, K.; Hyodo, J.; Ju, Y.-W.; Ishihara, T. Ruddlesden Popper oxides of LnSr3Fe3O10−δ(Ln = La, Pr, Nd, Sm, Eu, and Gd) as active cathodes for low temperature solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 12357–12366. [Google Scholar] [CrossRef]
- Hyodo, J.; Tominaga, K.; Ju, Y.-W.; Ida, S.; Ishihara, T. Electrical conductivity and oxygen diffusivity in Cu- and Ga-doped Pr2NiO4. Solid State Ion. 2014, 256, 5–10. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered A cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Choi, S.; Yoo, S.; Kim, J.; Park, S.; Jun, A.; Sengodan, S.; Kim, J.; Shin, J.; Jeong, H.Y.; Choi, Y.; et al. Highly efficient and robust cathode materials for low-temperature solid oxide fuel cells: PrBa0.5Sr0.5Co2−xFexO5+δ. Sci. Rep. 2013, 3, 2426–2431. [Google Scholar] [CrossRef]
- Lee, W.; Han, J.W.; Chen, Y.; Cai, Z.; Yildiz, B. Cation Size Mismatch and Charge Interactions Drive Dopant Segregation at the Surfaces of Manganite Perovskites. J. Am. Chem. Soc. 2013, 135, 7909–7925. [Google Scholar] [CrossRef]
- Yoo, S.; Jun, A.; Ju, Y.; Odkhuu, D.; Hyodo, J.; Jeong, H.Y.; Park, N.; Shin, J.; Ishihara, T.; Kim, G. Development of Double-Perovskite Compounds as Cathode Materials for Low-Temperature Solid Oxide Fuel Cells. Angew. Chem. 2014, 126, 13280–13283. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electr. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Li, G.; Gou, Y.; Cheng, X.; Bai, Z.; Ren, R.; Xu, C.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Enhanced Electrochemical Performance of the Fe-Based Layered Perovskite Oxygen Electrode for Reversible Solid Oxide Cells. ACS Appl. Mater. Interfaces 2021, 13, 34282–34291. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Shi, Y.; Wei, T.; Li, Z.P.; An, D.M.; Hu, J.W.; Zhao, W.H.; Zhang, W.; Ji, Z.H.; Wang, J.H. Novel YBaCo3.2Ga0.8O7+δ as a cathode material and performance optimization for IT-SOFCs. Int. J. Hydrogen Energy 2014, 39, 10710–10717. [Google Scholar] [CrossRef]
- Ye, F.M.; Zhou, Q.J.; Xu, K.; Zhang, Z.Y.; Han, X.; Yang, L.; Xu, J.; Xu, H.Y.; Wu, K.J.; Guan, Y.J. Phase stability and electrochemical performance of Y0.5Ca0.5−xInxBaCo3.2Ga0.8O7+δ (x = 0 and 0.1) as cathodes for intermediate temperature solid oxide fuel cells. J. Alloys Compd. 2016, 680, 163–168. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Yang, X.; Bu, F.; Yang, F.; Wang, M.; Li, Y. Basic properties of low thermal expansion coefficient (Y0.5Ca0.5)1−xInxBaCo3ZnO7+δ (x = 0, 0.1, 0.2, 0.3) solid solutions for solid oxide fuel cell cathode materials. Mater. Res. Bull. 2022, 156, 112001–112007. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, X.; Wang, Y.; Ma, Y.; Yang, H. A thermal-expansion offset to cobalt-based cathode materials for solid oxide fuel cells. Next Energy 2024, 5, 100168. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Huang, Y.-H. Alternative anode materials for solid oxide fuel cells. J. Power Source 2007, 173, 1151–1160. [Google Scholar] [CrossRef]
- Mogensen, M.; Skaarup, S. Kinetic and geometric aspects of solid oxide fuel cell electrodes. Solid State Ion. 1996, 86–88, 1151–1160. [Google Scholar] [CrossRef]
- Iwanschitz, B.; Sfeir, J.; Mai, A.; SchütZe, M. Degradation of SOFC anodes upon redox cycling: A comparison between Ni/YSZ and Ni/CGO. J. Electrochem. Soc. 2010, 157, B269–B278. [Google Scholar] [CrossRef]
- Liu, M.; Lynch, M.E.; Blinn, K.; Alamgir, F.M.; Choi, Y. Rational SOFC material design: New advances and tools. Mater. Today 2011, 14, 534–546. [Google Scholar] [CrossRef]
- Slater, P.R.; Fagg, D.P.; Irvine, J.T.S. Synthesis and electrical characterisation of doped perovskite titanates as potential anode materials for solid oxide fuel cells. J. Mater. Chem. 1997, 7, 2495–2498. [Google Scholar] [CrossRef]
- Sengodan, S.; Yeo, H.J.; Shin, J.; Kim, G. Assessment of perovskite-type La0.8Sr0.2ScxMn1−xO3−δ oxides as an anode for intermediate temperature solid oxide fuel cells using hydrocarbon. J. Power Sources 2011, 196, 3083–3088. [Google Scholar] [CrossRef]
- Corre, G.; Kim, G.; Cassidy, M.; Vohs, J.M.; Gorte, R.J.; Irvine, J.T.S. Activation and ripening of impregnated manganese containing perovskite SOFC electrodes under redox cycling. Chem. Mater. 2009, 21, 1077–1084. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T.S. A redox-stable efficient anode for solid-oxide fuel cells. Nat. Mater. 2003, 2, 320–323. [Google Scholar] [CrossRef]
- Shin, T.H.; Ida, S.; Ishihara, T. Doped CeO2–LaFeO3 composite oxide as an active anode for direct hydrocarbon-type solid oxide fuel cells. J. Am. Chem. Soc. 2011, 48, 19399–19407. [Google Scholar] [CrossRef]
- Peng, X.; Tian, Y.; Liu, Y.; Wang, W.; Jia, L.; Pu, J.; Chi, B.; Li, J. An efficient symmetrical solid oxide electrolysis cell with LSFM-based electrodes for direct electrolysis of pure CO2. J. CO2 Util. 2020, 36, 18–24. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Z.; Jin, C.; Xiao, G.; Chen, F.; Han, M. Sulfur-tolerant redox-reversible anode material for direct hydrocarbon solid oxide fuel cells. Adv. Mater. 2012, 24, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Dass, R.I.; Xing, Z.-L.; Goodenough, J.B. Double perovskites as anode materials for solid-oxide fuel cells. Science 2006, 312, 254–257. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; He, Q.; He, T. Double-perovskites A2FeMoO6−δ (A = Ca, Ba) as anodes for solid oxide fuel cells. J. Power Sources 2010, 195, 6356–6366. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.-W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.S.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef]
- Sengodan, S.; Ju, Y.-W.; Kwon, O.; Jun, A.; Jeong, H.Y.; Ishihara, T.; Shin, J.; Kim, G. Self-Decorated MnO Nanoparticles on Double Perovskite Solid Oxide Fuel Cell Anode by in Situ Exsolution. ACS Sustain. Chem. Eng. 2017, 5, 9207–9213. [Google Scholar] [CrossRef]
- Williams, K.R.; Smith, J.G. Fuel Cell with Solid State Electrolytes. US3 1969, 464, 861. [Google Scholar]
- Okuo, T.; Kaga, Y.; Momma, A. New Tubular Type SOFC Using Metallic System Components. Denkikagaku 1996, 64, 555–561. [Google Scholar] [CrossRef]
- Momma, A.; Kaga, Y.; Fujii, K.; Hohjyo, K.; Kanazawa, M.; Okuo, T. High Potential Performance of Tubular Type SOFC using Metallic System Components. Proc. Electrochem. Soc. 1997, 1997, 311–320. [Google Scholar] [CrossRef]
- Mineshige, A.; Inaba, M.; Ogumi, Z.; Takahashi, T.; Kawagoe, T.; Tasaka, A.; Kikuchi, K. Preparation of Yttria-Stabilized Zirconia Microtube by Electrochemical Vapor Deposition. J. Am. Ceram. Soc. 1995, 78, 3157–3159. [Google Scholar] [CrossRef]
- Mineshige, A.; Fukushima, K.; Okada, S.; Kikuchi, T.; Kobune, M.; Yazawa, T.; Kikuchi, K.; Inaba, M.; Ogumi, Z. Porous metal tubular support for solid oxide fuel cell design. Electrochem. Solid-State Lett. 2006, 9, A427–A429. [Google Scholar] [CrossRef]
- Hwang, C.; Tsai, C.-H.; Lo, C.-H.; Sun, C.-H. Plasma sprayed metal supported YSZ/Ni–LSGM–LSCF ITSOFC with nanostructured anode. J. Power Sources 2008, 180, 132–142. [Google Scholar] [CrossRef]
- Hwang, C.-S.; Tsai, C.-H.; Yu, J.-F.; Chang, C.-L.; Lin, J.-M.; Shiu, Y.-H.; Cheng, S.-W. High performance metal-supported intermediate temperature solid oxide fuel cells fabricated by atmospheric plasma spraying. J. Power Sources 2011, 196, 1932–1939. [Google Scholar] [CrossRef]
- Cho, H.J.; Choi, G.M. Fabrication and characterization of Ni-supported solid oxide fuel cell. Solid State Ion. 2009, 180, 792–795. [Google Scholar] [CrossRef]
- Ishihara, T.; Yan, J.; Shinagawa, M.; Matsumoto, H. Ni–Fe bimetallic anode as an active anode for intermediate temperature SOFC using LaGaO3 based electrolyte film. Electrochim. Acta 2006, 52, 1645–1650. [Google Scholar] [CrossRef]
- Ju, Y.-W.; Eto, H.; Inagaki, T.; Ida, S.; Ishihara, T. Preparation of Ni–Fe bimetallic porous anode support for solid oxide fuel cells using LaGaO3 based electrolyte film with high power density. J. Power Sources 2010, 195, 6294–6300. [Google Scholar] [CrossRef]
- Ishihara, T.; Kusaba, H.; Kim, H.H.; Kang, B.S. Preparation of La0.9Sr0.1Ga0.8Mg0.2O3 Film by Pulse Laser Deposition (PLD) Method on Porous Ni–Fe Metal Substrate for CO2 Electrolysis. ISIJ Int. 2019, 59, 613–618. [Google Scholar] [CrossRef]
- Ju, Y.-W.; Eto, H.; Inagaki, T.; Ida, S.; Ishihara, T. Improvement in Thermal Cycling Durability of SOFCs Using LaGaO3-Based Electrolyte by Inserting Convex Sm0.5Sr0.5CoO3 Interlayer. Electrochem. Solid-State Lett. 2010, 13, B139–B141. [Google Scholar] [CrossRef]
- Ju, Y.-W.; Ida, S.; Ishihara, T. A Ce(Mn,Fe)O2 dense nanofilm as an improved active anode for metal-supported solid oxide fuel cells. RSC Adv. 2013, 3, 10508–10515. [Google Scholar] [CrossRef]
- Ju, Y.; Hyodo, J.; Inoishi, A.; Ida, S.; Tohei, T.; So, Y.; Ikuhara, Y.; Ishihara, T. Double columnar structure with a nanogradient composite for increased oxygen diffusivity and reduction activity. Adv. Energy Mater. 2014, 4, 1400783. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.; Jia, L.; Yan, D.; Pu, J.; Chi, B.; Jian, L. High performance Ni–Fe alloy supported SOFCs fabricated by low cost tape casting-screen printing-cofiring process. Int. J. Hydrogen Energy 2014, 39, 19747–19752. [Google Scholar] [CrossRef]
- Park, H.C.; Virkar, A.V. Bimetallic (Ni–Fe) anode-supported solid oxide fuel cells with gadolinia-doped ceria electrolyte. J. Power Sources 2009, 186, 133–137. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, G.M. Ceria Film Supported on Ni-Fe Metal Film. ECS Trans. 2009, 25, 727–730. [Google Scholar] [CrossRef]
- Wang, M.; Li, N.; Wang, Z.; Chen, C.; Zhan, Z. Electrochemical performance and redox stability of solid oxide fuel cells supported on dual-layered anodes of Ni-YSZ cermet and Ni–Fe alloy. Int. J. Hydrogen Energy 2022, 47, 5453–5461. [Google Scholar] [CrossRef]
- Kim, H.Y.; Watanabe, M.; Song, J.T.; Inada, M.; Ishihara, T. BaZr(Ce,Y)O3-Pr-Doped CeO2 Double Columnar for the Cathodic Functional Layer of Ni–Fe Metal-Supported Protonic Ceramic Fuel Cells. ACS Appl. Energy Mater. 2024, 7, 7945–7955. [Google Scholar] [CrossRef]
- Kim, H.Y.; Watanabe, M.; Song, J.T.; Inada, M.; Ishihara, T. A high power metal-supported protonic ceramic fuel cell using increased proton conductivity in the cathode functional layer of La1−xSrxScO3 (LSSc, x = 0.1–0.25). J. Mater. Chem. A 2025, 13, 1172–1180. [Google Scholar] [CrossRef]
- Vafaeenezhad, S.; Sandhu, N.K.; Hanifi, A.R.; Etsell, T.H.; Sarkar, P. Development of proton conducting fuel cells using nickel metal support. J. Power Sources 2019, 435, 226763. [Google Scholar] [CrossRef]
- Wang, R.; Lau, G.Y.; Ding, D.; Zhu, T.; Tucker, M.C. Approaches for co-sintering metal-supported proton-conducting solid oxide cells with Ba(Zr,Ce,Y,Yb)O3−δ electrolyte. Int. J. Hydrogen Energy 2019, 44, 13768–13776. [Google Scholar] [CrossRef]
- Babar, Z.U.; Hanif, M.B.; Gao, J.T.; Li, C.J.; Li, C.X. Sintering behavior of BaCe0.7Zr0.1Y0.2O3−δ electrolyte at 1150 °C with the utilization of CuO and Bi2O3 as sintering aids and its electrical performance. Int. J. Hydrogen Energy 2022, 47, 7403–7414. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Usoltsev, V.V.; Fedorova, Y.E.; Sobyanin, V.A.; Kalinin, P.V.; Arzhannikov, A.V.; Vlasov, A.Y.; Korobeinikov, M.V.; Bryazgin, A.A.; Salanov, A.N.; et al. Design of medium–temperature solid oxide fuel cells on porous supports of deformation strengthened Ni–Al alloy. Russ. J. Electrochem. 2011, 47, 488–493. [Google Scholar] [CrossRef]
- Yang, S.-F.; Hwang, C.-S.; Tsai, C.-H.; Chang, C.-L.; Wu, M.-H.; Fu, C.-Y.; Lee, R.-Y. Fabrication of 100 Centimeter Square Metal-Supported Solid Oxide Fuel Cell Using Thermal Plasma Technique. ECS Trans. 2017, 78, 2021–2028. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hwang, C.S.; Chang, C.L.; Wu, S.H.; Lin, H.H.; Shiu, W.H.; Lin, J.K.; Yang, S.F.; Fu, C.Y.; Yang, C.S. Performances of Plasma Sprayed Metal-supported Solid Oxide Fuel Cell and Stack. Fuel Cells 2018, 18, 680–823. [Google Scholar] [CrossRef]
- Villarreal, I.; Jacobson, C.; Leming, A.; Matus, Y.; Visco, S.; De Jonghe, L. Metal-Supported Solid Oxide Fuel Cells. Electrochem. Solid-State Lett. 2003, 6, A178–A179. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Li, N.; Xiong, Y.; Jing, H. Effect of Sigma Phase Precipitation on the Pitting Corrosion Mechanism of Duplex Stainless Steels. Int. J. Electrochem. Sci. 2018, 13, 9868–9887. [Google Scholar]
- Xie, Y.; Neagu, R.; Hsu, C.-S.; Zhang, X.; Decès-Petit, C. Spray pyrolysis deposition of electrolyte and anode for metal-supported solid oxide fuel cell. J. Electrochem. Soc. 2008, 155, B407–B410. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhou, Y.; Wang, S.; Liu, X.; Meng, X.; Wen, T. Nanostructure electrodes for metal-supported solid oxide fuel cells. ECS Trans. 2013, 57, 925–931. [Google Scholar] [CrossRef]
- Zhou, Y.; Xin, X.; Li, J.; Ye, X.; Xia, C.; Wang, S.; Zhan, Z. Performance and degradation of metal-supported solid oxide fuel cells with impregnated electrodes. Int. J. Hydrogen Energy 2014, 39, 2279–2285. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, D.; Yuan, C.; Liu, M.; Chen, T.; Wang, S.; Zhan, Z. Infiltrated SmBa0.5Sr0.5Co2O5+δ cathodes for metal–supported solid oxide fuel cells. Electrochim. Acta 2014, 149, 231–236. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, S.J.; Choi, G.M. Y0.08Sr0.88TiO3-CeO2 Composite as a Diffusion Barrier Layer of STS-Supported SOFC. ECS Trans. 2013, 57, 897–903. [Google Scholar] [CrossRef]
- Dayaghi, A.M.; Kim, K.J.; Kim, S.; Park, J.; Kim, S.J.; Park, B.H.; Choi, G.M. Stainless steel-supported solid oxide fuel cell with La0.2Sr0.8Ti0.9Ni0.1O3-δ/yttria-stabilized zirconia composite anode. J. Power Sources 2016, 324, 288–293. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Cheng, Y.; Shen, F.; Wang, R.; Hu, B.; Tucker, M.C. Scaleup and manufacturability of symmetric-structured metal-supported solid oxide fuel cells. J. Power Sources 2021, 489, 229439. [Google Scholar] [CrossRef]
- Hu, B.; Lau, G.; Song, D.; Fukuyama, Y.; Tucker, M.C. Optimization of metal-supported solid oxide fuel cells with a focus on mass transport. J. Power Sources 2023, 555, 232402. [Google Scholar] [CrossRef]
- Jang, Y.-H.; Lee, S.; Shin, H.Y.; Bae, J. Development and evaluation of a 3-cell stack of metal-based solid oxide fuel cells fabricated via a sinter-joining method for auxiliary power unit applications. Int. J. Hydrogen Energy 2018, 43, 16215–16229. [Google Scholar] [CrossRef]
- Lee, K.; Kang, J.; Lee, J.; Lee, S.; Bae, J. Evaluation of metal-supported solid oxide fuel cells (MS-SOFCs) fabricated at low temperature (~1000 °C) using wet chemical coating processes and a catalyst wet impregnation method. Int. J. Hydrogen Energy 2018, 43, 3786–3796. [Google Scholar] [CrossRef]
- Fernández-González, R.; Hernández, E.; Savvin, S.; Núñez, P.; Makradi, A.; Sabaté, N.; Esquivel, J.; Ruiz-Morales, J. A novel microstructured metal-supported solid oxide fuel cell. J. Power Sources 2014, 272, 233–238. [Google Scholar] [CrossRef]
- Brandner, M.; Bram, M.; Froitzheim, J.; Buchkremer, H.; Stöver, D. Electrically Conductive Diffusion barrier layers for Metal-Supported SOFC. Solid State Ion. 2008, 179, 1501–1504. [Google Scholar] [CrossRef]
- Oishi, N.; Yoo, Y. Fabrication of Cerium Oxide Based SOFC Having a Porous Stainless Steel Support. ECS Trans. 2009, 25, 739–744. [Google Scholar] [CrossRef]
- Franco, T.; Schibinger, K.; Ilhan, Z.; Schiller, G.; Venskutonis, A. Ceramic Diffusion Barrier Layers for Metal Supported SOFCs. ECS Trans. 2007, 7, 771–780. [Google Scholar] [CrossRef]
- Garcia-Fresnillo, L.; Shemet, V.; Chyrkin, A.; de Haart, L.; Quadakkers, W. Long-term behaviour of solid oxide fuel cell interconnect materials in contact with Ni-mesh during exposure in simulated anode gas at 700 and 800 °C. J. Power Sources 2014, 271, 213–222. [Google Scholar] [CrossRef]
- Szabo, P.; Arnold, J.; Franco, T.; Gindrat, M.; Refke, A.; Zagst, A.; Ansar, A. Progress in the Metal Supported Solid Oxide Fuel Cells and Stacks for APU. ECS Trans. 2009, 25, 175–185. [Google Scholar] [CrossRef]
- Ansar, A.; Szabo, P.; Arnold, J.; Ilhan, Z.; Soysal, D.; Costa, R.; Zagst, A.; Gindrat, M.; Franco, T. Metal Supported Solid Oxide Fuel Cells and Stacks for Auxiliary Power Units—Progress, Challenges and Lessons Learned. ECS Trans. 2011, 35, 147–155. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Y.; Liu, M.; Yuan, C.; Ye, X.; Zhan, Z.; Wang, S. High performance solid oxide electrolysis cell with impregnated electrodes. Electrochem. Commun. 2015, 54, 23–27. [Google Scholar] [CrossRef]
- Wang, R.; Dogdibegovic, E.; Lau, G.Y.; Tucker, M.C. Metal-Supported Solid Oxide Electrolysis Cell with Significantly Enhanced Catalysis. Energy Technol. 2019, 7, 1801154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, Y.-W. Progress in Materials and Metal Substrates for Solid Oxide Fuel Cells. Energies 2025, 18, 3379. https://doi.org/10.3390/en18133379
Ju Y-W. Progress in Materials and Metal Substrates for Solid Oxide Fuel Cells. Energies. 2025; 18(13):3379. https://doi.org/10.3390/en18133379
Chicago/Turabian StyleJu, Young-Wan. 2025. "Progress in Materials and Metal Substrates for Solid Oxide Fuel Cells" Energies 18, no. 13: 3379. https://doi.org/10.3390/en18133379
APA StyleJu, Y.-W. (2025). Progress in Materials and Metal Substrates for Solid Oxide Fuel Cells. Energies, 18(13), 3379. https://doi.org/10.3390/en18133379




