Abstract
Straw biomass is a renewable but problematic fuel due to its high alkali and chlorine content, which can cause slagging and corrosion during combustion. To mitigate these issues, this study investigates the influence of aluminosilicate additives on the thermal behavior and combustion characteristics of straw biomass. Laboratory-scale testing is carried out using thermogravimetric analysis under atmospheric air, showing the TG, DTG, and DSC profiles of samples (kaolinite, halloysite, straw biomass, and straw biomass with 4 wt.% of halloysite). Additionally, the main combustion parameters, like the ignition temperature, the maximum peak temperature, the burnout temperature, and some combustion indexes, are presented. The results show the effect of a heating rate in the range of 5–20 °C/min. Moreover, in this study, two non-isothermal model methods (Kissinger and Ozawa) are used to estimate energy activation. While halloysite slightly affects the combustion indexes and marginally reduces energy activation, its overall influence does not significantly alter combustion efficiency. These findings support the potential and safe use of halloysite for the biomass combustion process.
1. Introduction
Straw biomass is an abundant, renewable energy source, but presents challenges during thermal conversion. One of the primary drawbacks of co-firing chlorine-rich biomass is the increased likelihood of high-temperature corrosion on boiler heat exchange surfaces. Notably, even biomass with a minimal or negligible chlorine content does not ensure full protection against corrosion. Elevated potassium levels—especially in lignocellulosic feedstocks—can intensify slagging, which, in turn, accelerates corrosive degradation [1,2,3].
One promising approach to improving biomass combustion behavior and mitigating drawbacks is the use of mineral additives, especially aluminosilicates. Alkali metals—particularly potassium—can be effectively captured using aluminosilicate-based materials such as kaolinite, halloysite, and bentonite [4,5,6,7]. These compounds promote the formation of high-temperature-stable minerals like kalsilite and leucite, which immobilize potassium and diminish the presence of low-melting eutectics. As a result, such additives reduce the likelihood of corrosive deposit accumulation on boiler components. Additionally, these materials (including kaolinite and halloysite) are known to interact with alkali species in biomass, potentially altering thermal degradation pathways and improving combustion stability [8,9,10,11,12].
Halloysite, a two-layered aluminosilicate, stands out due to its high specific surface area (65–85 m2/g) and significant porosity (60–70%). These properties enhance the adsorption of alkali vapors, facilitating the formation of stable potassium aluminosilicates such as kalsilite, leucite, and potassium mullite. With the chemical formula Al2Si2O5(OH)4·2H2O, halloysite contains interlayer water up to ~60 °C, which is gradually released upon heating. Between 450 °C and 600 °C, halloysite undergoes dehydroxylation, yielding a more reactive phase—metakaolinite—capable of interacting with potassium compounds. This reaction produces hydrogen chloride (HCl), which poses significantly less risk to boiler surfaces than either potassium chloride or molecular chlorine [12,13,14,15]. The loss of hydroxyl groups during this transformation creates active sites that can bind nearby cations, including potassium and heavy metals, leading to the formation of durable potassium–aluminosilicate structures with melting points often exceeding 1600 °C. These compounds significantly lower the likelihood of chlorine-induced corrosion across various boiler systems [9,11,12,14].
Kaolinite (Al2Si2O5(OH)4), another widely used aluminosilicate, typically forms plate-like particles. When heated between 450 °C and 600 °C, it transforms into metakaolinite, which also reacts with potassium species to form stable, high-melting-point phases such as kalsilite (KAlSiO4) and leucite (KAlSi2O6), with melting points above 1600 °C and 1500 °C, respectively. However, kaolinite generally exhibits a lower specific surface area (3–15 m2/g) and porosity (0–10%) compared to halloysite [9,16,17,18].
Halloysite and kaolinite are minerals from the group of layered aluminosilicates with a similar chemical composition, but they differ in their crystallographic structure, water content, and physicochemical properties [19,20]. A comparison of XRD and FTIR test results allows for identifying these materials and determines differences in their internal structures. Both XRD and FTIR analysis allow for the identification of key differences in aluminosilicates [21]. XRD analysis shows the main difference between the considered aluminosilicates in the form of the position of the dominant diffraction peak. For kaolinite and anhydrous halloysite, a characteristic peak corresponding to the interlayer plane can be seen (kaolinite 2θ = 12.4°, anhydrous halloysite 2θ = 12°). In contrast, for halloysite containing water, this peak appears at 2θ ≈ 11.8° [22]. The presence of water in the sample also affects the lower crystallinity of halloysite, which can be seen in its diffraction patterns in the form of broader and less intense peaks [23]. Similarly, differences can be observed for the discussed aluminosilicates through FTIR analysis. These differences are visible in the range of hydroxyl vibrations and the presence of water. For kaolinite, clear and well-separated bands can be observed for hydroxyl and internal Al-OH groups. In the case of halloysite, OH bands also can be observed, but they are weaker and wider (lower degree of structure order) [24]. Moreover, only for halloysite can a broad band be observed corresponding to physically bound water in the tubular structure [25].
Numerous studies have evaluated the role of aluminosilicates in the biomass combustion process [9,17,26,27]. For instance, Glass [9] analyzed different kaolinite and halloysite samples using differential thermal analysis and X-ray diffraction (XRD), assessing their crystallinity and impurity levels. These samples were heated up to 1350 °C and analyzed after quenching to determine their phase transformations. Wang et al. [17] studied the interaction of kaolin with potassium compounds (KCl, K2CO3, and K2SO4) under suspension-fired conditions, utilizing an entrained flow reactor and thermodynamic modeling. Their findings showed considerable potassium capture by kaolinite, particularly from KCl and K2CO3, and also revealed its capability to retain KOH. In lab-scale experiments, Hardy et al. [26] examined the effects of various aluminosilicates—kaolin, bentonite, halloysite, and lignite-derived fly ash—on chlorine corrosion. Mixtures of KCl and each additive were heated between 600 °C and 1000 °C. The results demonstrated that kaolin, halloysite, and bentonite effectively sequestered potassium into stable high-temperature aluminosilicates while promoting the volatilization of chlorine between 800 °C and 1000 °C. Fly ash also showed a notable efficacy in suppressing chlorine-induced damage. In another study, Wang et al. [27] evaluated the potassium-capturing performance of Al/Si-based additives in suspension-fired systems operating between 1100 °C and 1450 °C. Their results confirmed that kaolinite and coal-derived fly ash effectively immobilized gaseous KCl. However, their efficiency decreased as the KCl-to-additive mass ratio increased in the feed.
While aluminosilicates are commonly used to mitigate high-temperature corrosion in biomass systems, their effect on the fundamental thermal behavior and combustion kinetics of biomass fuels is not fully recognized. Research has focused on their role in ash transformation or deposit mitigation. Less attention has been paid to their impact on key combustion parameters such as ignition temperature, peak decomposition temperature, burnout behavior, and kinetic characteristics.
The presented research aims to evaluate the influence of aluminosilicates on the thermal degradation and combustion performance of straw biomass. In this article, the authors explain how the addition of aluminosilicates affects the energy activation, combustion indexes, and main combustion temperatures of straw biomass. The focus is on combustion characteristics and thermal stability to support the design of more efficient and stable biomass-based energy systems.
2. Experimental
2.1. Materials and Sample Preparation
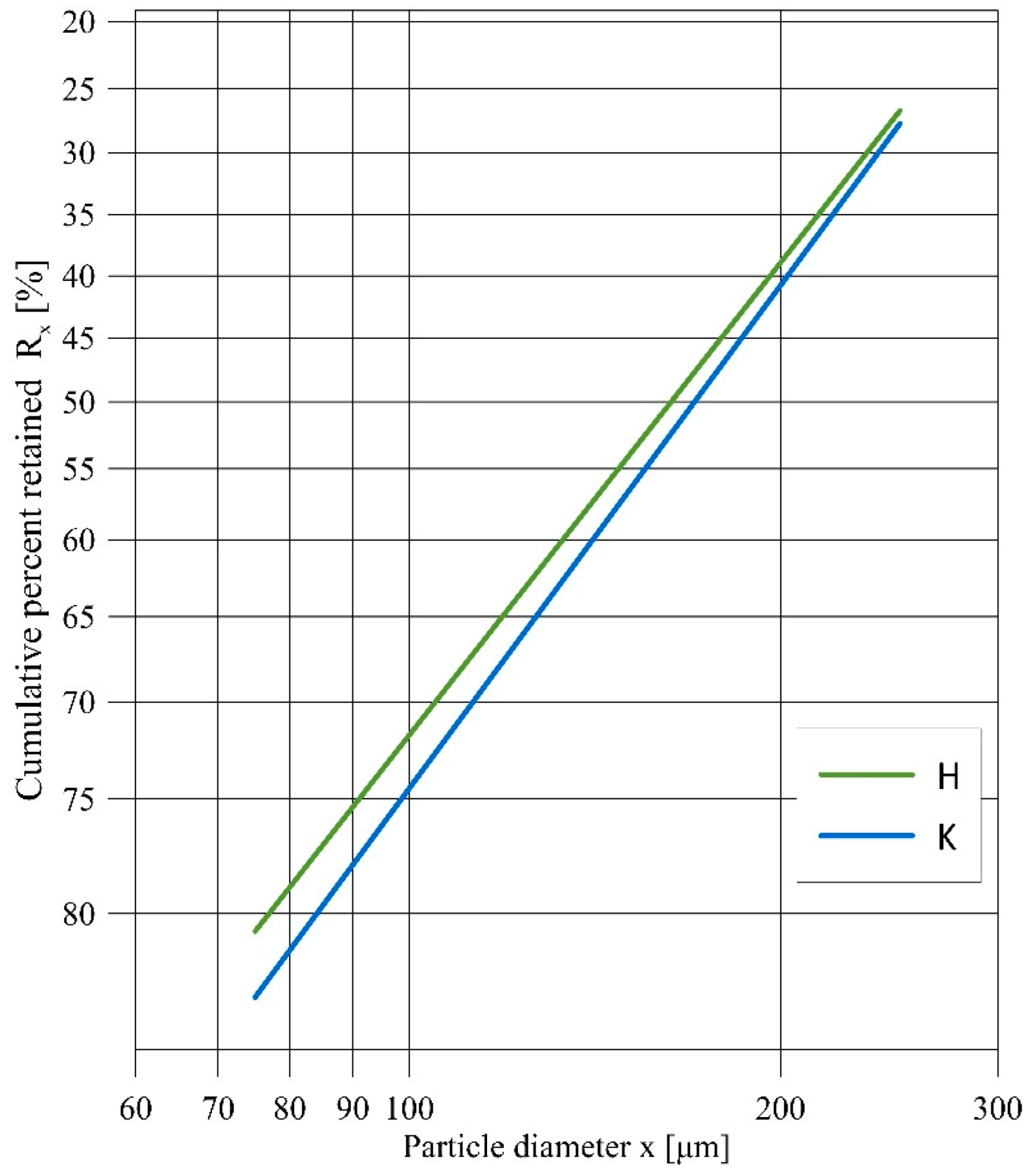
Two kinds of aluminasilicates (halloysite and kaolinite) were investigated during the experiments (Figure 1a,b). The halloysite (H) used in this study came from the DUNINO mine, which is located in southwestern Poland. As compared to its pure crystal form, the halloysite used in this study contained iron and other impurities. Additionally, pure kaolinite (K) was used in the study. Figure 2 shows the Rosin–Rammler particle size distribution for the H and K samples. Sieve analysis was performed using a Multiserw LPzE-2e laboratory shaker with a set of sieves. The obtained results show a similar particle size distribution of both samples, which is important for comparing further sample test results. The oxide compositions of these aluminasilicates and the straw biomass were determined according to the ICP-OES method and are shown in Table 1. Comparing the aluminosilicates, halloysite had a significantly higher content (often several times higher) of oxides than kaolinite. The greatest differences can be seen for Fe2O3 (17.30 wt.%, 0.910 wt.%) and TiO2 (2.39 wt.%, 0.68 wt.%). On the other hand, kaolinite had a few percent higher amount of SiO2 (47.30 wt.%, 36.90 wt.%) and Al2O3 (36.70 wt.%, 28.70 wt.%) than halloysite. Additionally, the straw biomass composition showed high K2O (8.53 wt.%) and Cl (0.086 wt.%) contents.

Figure 1.
(a) Halloysite sample (H) and (b) kaolinite sample (K).
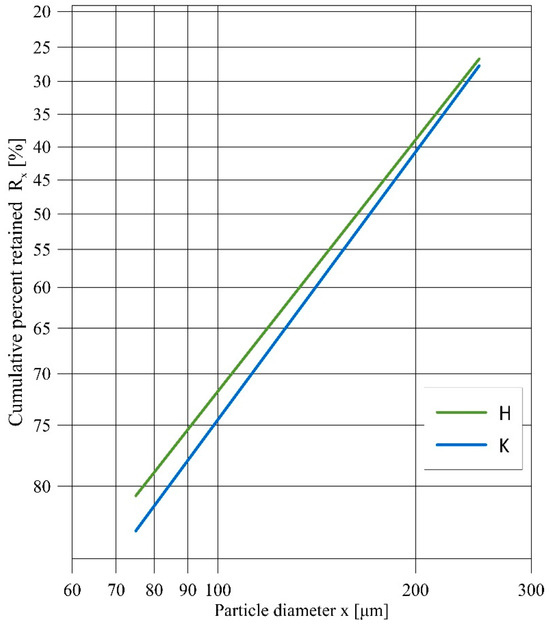
Figure 2.
Particle size distribution of H and K samples in a Rosin–Rammler coordinates.

Table 1.
Major oxide analysis of halloysite (K), kaolinite (K), and straw biomass (S) (in the dry state).
The basic fuel parameters for straw biomass (S0) are shown in Table 2. Analyses were carried out following the current PN-EN standards (PN-EN ISO 18134-2:2017-03 [28], PN-EN ISO 18122:2016-01 [29], PN-EN ISO 18123:2016-01 [30], PN-EN ISO 18125:2017-07 [31], and PN-EN ISO 16948:2015-07) [32]. Characteristic temperatures for the ash melting behavior were determined using a microscopic–photographic method according to CEN/TS 15370-1:2007 [33].

Table 2.
Fuel parameters (ar—as received, d—dry).
A sample of about 1000 g of straw biomass (Figure 3) was comminuted and sieved to obtain a particle size smaller than 200 μm. Next, it was divided into two parts. One of them was doped with 4 wt.% of halloysite.

Figure 3.
Straw biomass after initial crushing from the pellet form.
For samples which undergo chemical analysis (e.g., mixing before thermogravimetric analysis), it is recommended to mix the material for 5–15 min at a speed of 30–100 rpm. The mixing duration depends on the sample being mixed, e.g., the type, size, and number of mixed components [34]. The tested samples were separately mixed to obtain adequate homogenization for 10 min using a rotating horizontal drum. The mixing speed was 40 rpm to ensure the uniform mixing of the sample without excessively fragmenting or degrading it. It is important that the mixing is uniform and gentle to avoid changes in the sample structure before thermogravimetric analysis.
2.2. Methodology
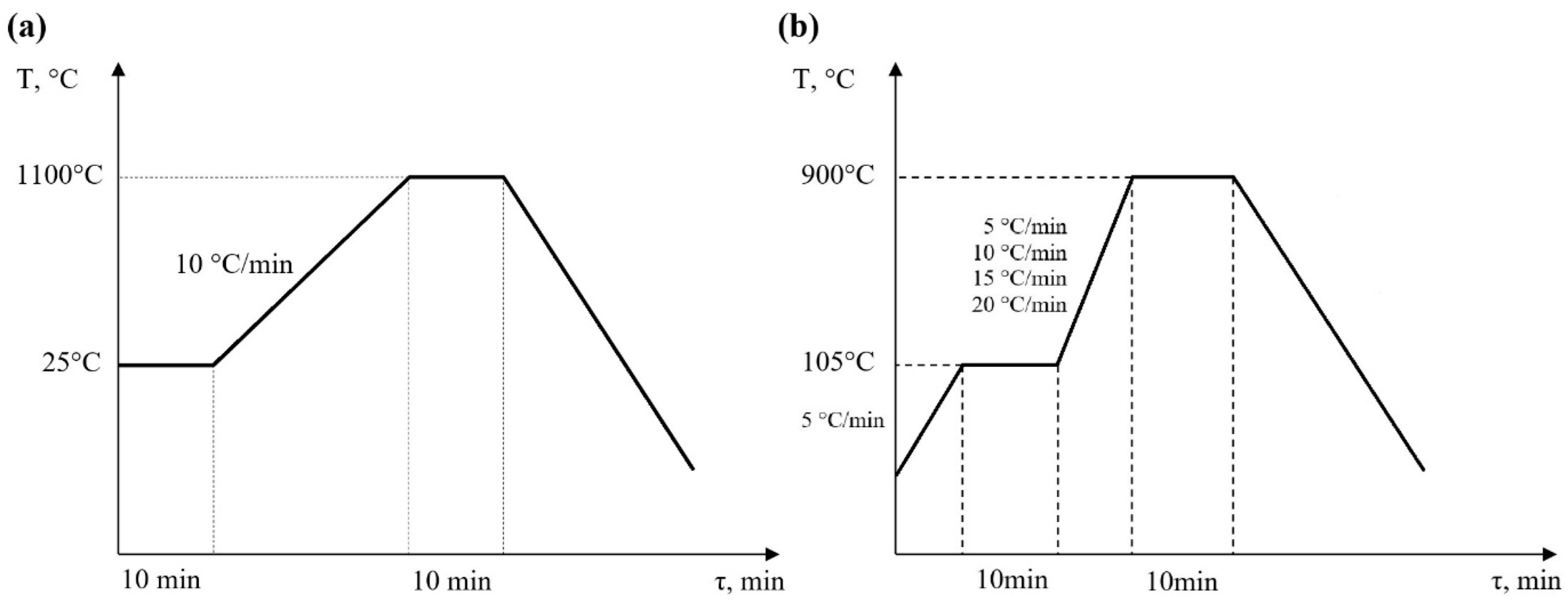
The heating procedures for all samples are shown in Figure 4a,b. For halloysite (H) and kaolinite (K), approximately 20 mg of samples was heated from ambient temperature (25 °C) to 1100 °C at a heating rate of 10 °C/min.
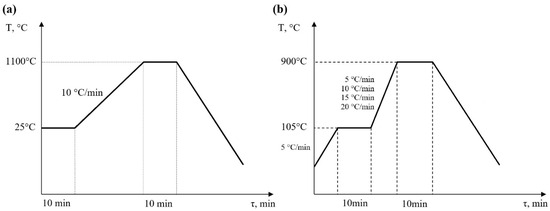
Figure 4.
Heating procedure diagram (a) for H and K samples and (b) for S0 and S + 4H samples.
The combustion temperature of biomass in boilers usually ranges from about 800 °C to 1000 °C. The optimum temperature depends on the type of biomass, boiler design, and combustion conditions, but generally, to ensure an effective combustion process and minimize the emission of harmful substances, the temperature should be maintained in this range. It is also important that the temperature is not too high, which can lead to the excessive wear of boiler components, or too low, which can cause incomplete combustion and the formation of soot or carbon oxides. According to this, the heating procedure for the biomass samples was different. Firstly, the samples of straw biomass (S0) and straw biomass with an addition of 4 wt.% of halloysite (S + 4H) were heated from 25 °C to 105 °C at a heating rate of 5 °C/min. Next, the samples were held for 10 min to complete the moisture vaporization process. Then, the samples were heated up to 900 °C at different heating rates (β) of 5, 10, 15, and 20 °C/min. Finally, they were held at this temperature for 10 min to finalize the burnout process. All experiments were carried out under non-isothermal conditions. Each sample was loaded into a 90 μL alumina crucible and examined under the same conditions, including the treatment temperature range and atmosphere composition. The entirety of the experiments was carried out in atmospheric air. The process was controlled by a TG/DSC SETARAM LABSYSTM analyser. The airflow was controlled according to the TGA analyser operation manual (a gas pressure valve adjusted to 1.5 bar) [35,36]. Additionally, a blank experiment was performed each time to calibrate the device.
3. Results and Discussion
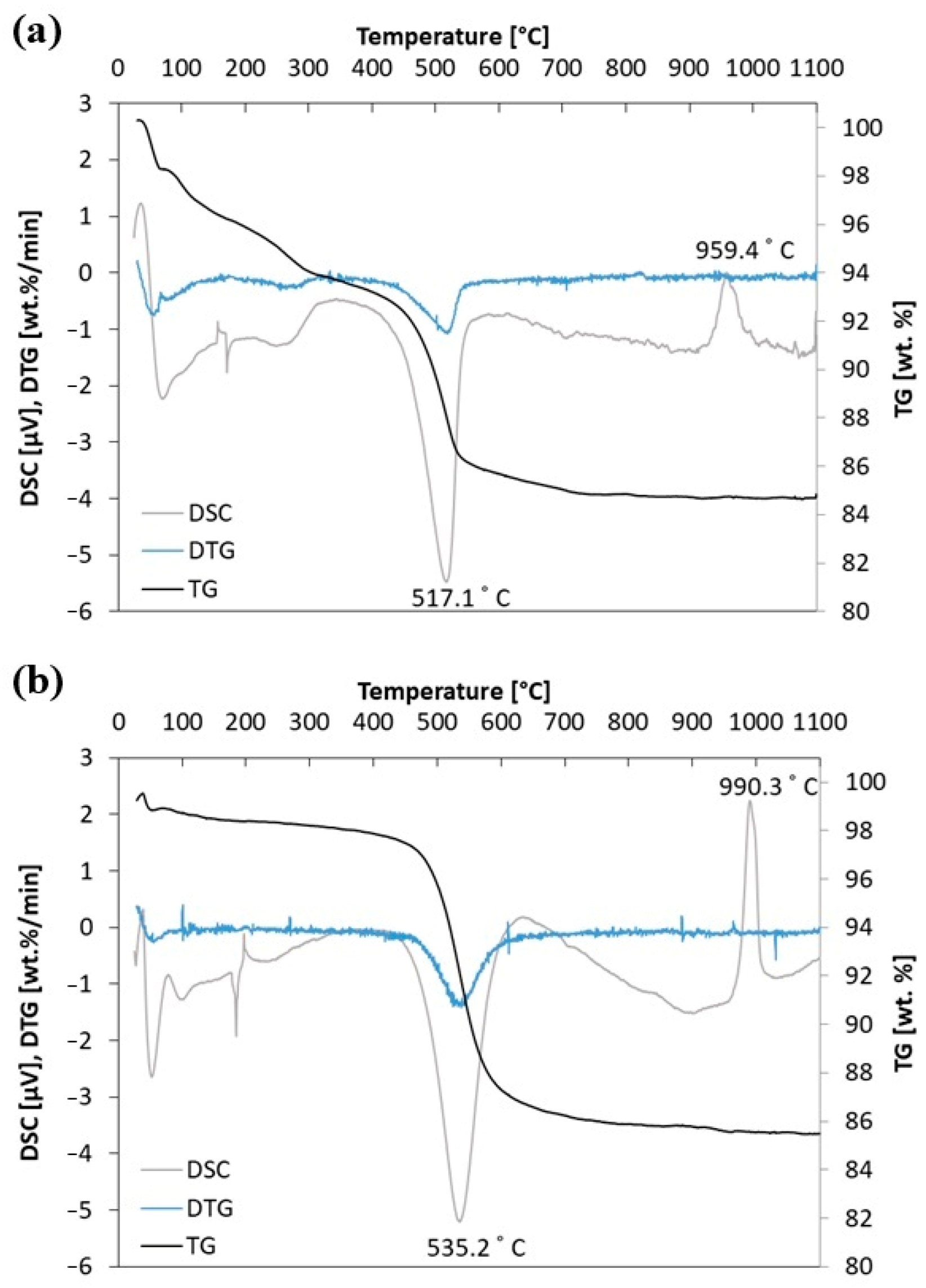
The first stage of research was thermoanalytical experiments on the halloysite (H) and kaolinite (K) samples. TG (thermogravimetry), DTG (derivative thermogravimetry), and DSC (differential scanning calorimetry) profiles for the tested samples are presented in Figure 5. The DSC and TG profiles for the aluminosilicates (halloysite—H and kaolinite—K) show that both aluminosilicates behave similarly under the influence of temperature in atmospheric air. Firstly, up to 400 °C, the mass of the sample is lost during the heating process (the elimination of absorbed water molecules on the external surfaces of particles). Next, the elimination of water molecules through dehydroxylation takes place. This is endothermic and observed as an intense endothermic peak in the differential scanning calorimetry (DSC) curve. This step is explained as a kinetically controlled recombination of alumina and silica to the metakaolinite structure. At a temperature of 517.1 °C for halloysite and 535.2 °C for kaolinite, they change to metakaolinite. Additionally, the thermal behavior of the high-temperature region is observed in the DSC curve as a slight endothermic peak immediately followed by an exothermic peak around 950–1000 °C. This is related to metakaolinite transformation to a spinel structure or Si-containing gamma-alumina (γ-Al2O3) and amorphous silica. No further endothermic and exothermic reactions are noted up to the temperature of 1100 °C [9,35].
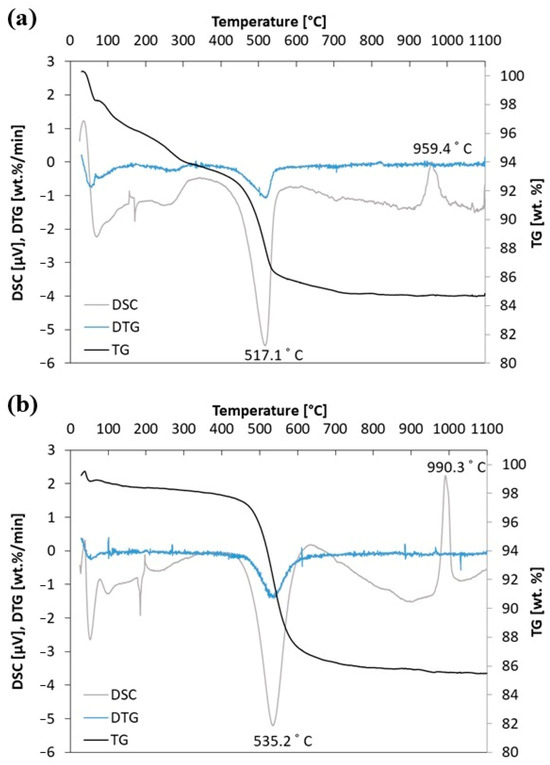
Figure 5.
(a) Thermal analysis for H sample and (b) thermal analysis for K sample.
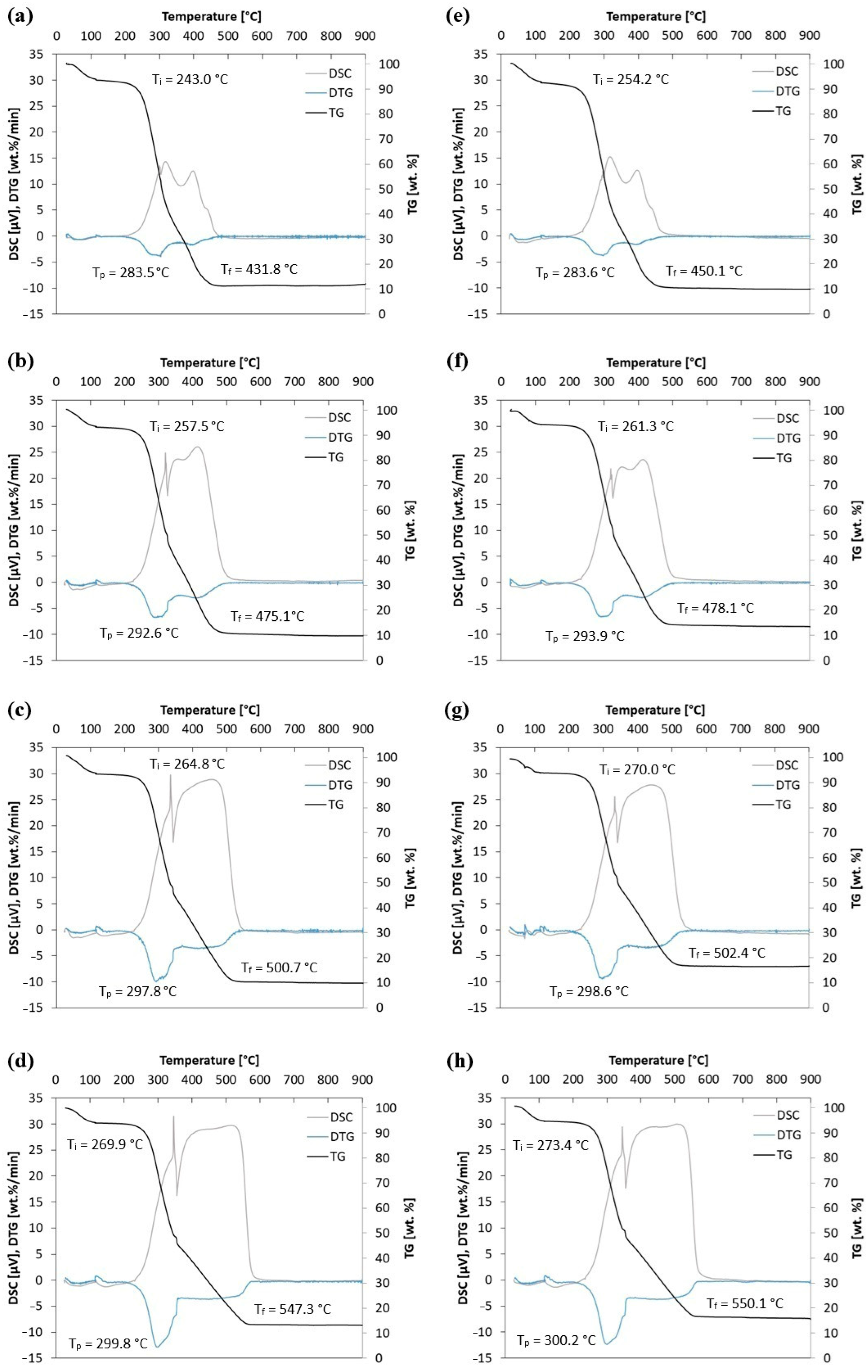
The combustion profiles for straw biomass without and with halloysite are presented in Figure 6, which show weight loss curves (TG), derivative thermogravimetric evolution curves (DTG), and differential scanning calorimeter curves (DSC) at heating rates of 5, 10, 15, and 20 °C/min. Additionally, ignition temperature (Ti), maximum peak temperature (Tp), and burnout temperature (Tf) are marked.
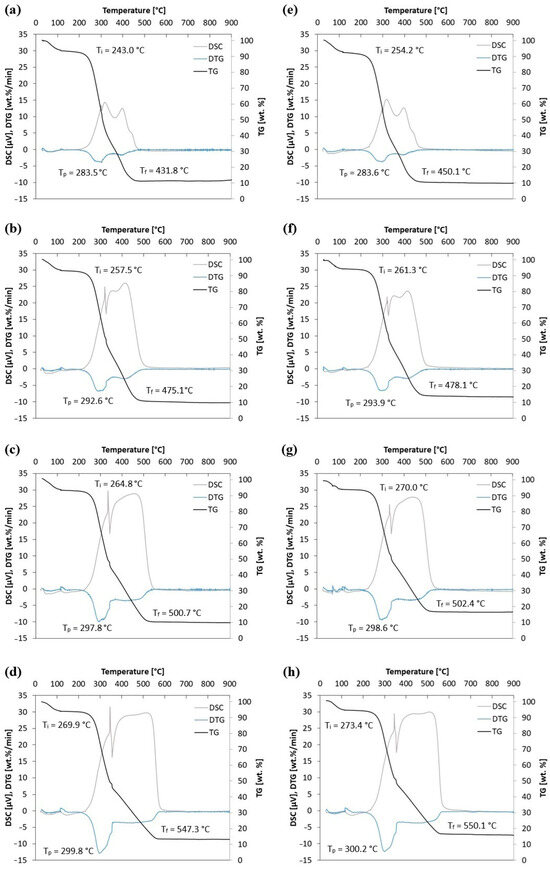
Figure 6.
TG, DTG and DSC curves for straw biomass (S0) at (a) 5 °C/min, (b) 10 °C/min, (c) 15 °C/min, and (d) 20 °C/min and straw biomass with 4 wt.% of halloysite (S + 4H) at (e) 5 °C/min, (f) 10 °C/min, (g) 15 °C/min, and (h) 20 °C/min.
The TGA curves show the percentage loss of sample mass as a function of temperature. The mass drops indicate the stages of thermal decomposition of biomass components. The TG curves show the gradual loss of sample mass due to drying, pyrolysis, and the oxidation of volatile and solid residues. With an increasing heating rate, a shift in thermal decomposition ranges towards higher temperatures is observed, which is typical for kinetically controlled processes. The DTG curve shows the rate of mass loss. The peaks on this curve correspond to the maximum rates of decomposition of individual components (hemicellulose, cellulose, and lignin). The DTG curve shows an increased maximum peak with an increase in the heating rate (5, 10, 15, and 20 °C/min). The ignition temperature is recorded at 243.0–273.4 °C, the maximum peak temperature at 283.5–300.2 °C, and the burnout temperature at 431.8–550.1 °C (variation depending on the sample type and heating rate). Additionally, it is noticed that with an increase in the heating rate, the characteristic temperatures of the combustion process (Ti, Tp, and Tf) increase. For example, for sample S0, with an increasing heating, rate the following Ti values are recorded: 243.0 °C, 257.5 °C, 264.8 °C, and 269.9 °C. A similar trend (increase) is observed for Tp and Tf.
The use of 4 wt.% halloysite causes a shift in characteristic temperatures and a change in TG, DTG, and DSC profiles, which suggests its influence on the oxidation mechanism of the material. This reflects the results obtained for biomass under atmospheric air at various heating rates presented in [35,36,37]. The addition of 4 wt.% of halloysite (S + 4H) affects the course of TG and DTG—shifts in the maximum decomposition peak (Tp) and burnout (Tf) temperatures can be observed (e.g., for 10 °C/min: S0: Tp = 292.6 °C, Tf = 475.1 °C; S + 4H: Tp = 293.9 °C, Tf = 478.1 °C), which may indicate an increased thermal stability of the mixture. The addition of halloysite affects the course of thermal processes—a shift in the burnout temperature (Tf) towards higher values is observed, which may indicate a barrier and stabilizing effect of the mineral additive. On the other hand, changes in the characteristic temperature values after adding halloysite (the difference between samples without and with halloysite, 0.1–18.3 °C) are perhaps not significant enough to expect such large changes in the combustion process. The smallest differences for different heating rates are noted for Tp (0.1–1.3 °C), while the largest differences for the heating rate of 5 °C/min are noted for Ti (11.3 °C) and Tf (18.3 °C). DSC curves additionally confirm changes in the energetic effects accompanying decomposition—in the presence of halloysite, differences in the intensity of exothermic effects are visible (e.g., clearly visible in comparing Figure 6b,f), which suggests a modification of the combustion mechanism. In addition, changes in the DSC profile suggest that halloysite modifies the mechanism of oxidation reactions, potentially through interactions with volatile decomposition products and the diffusion effect, limiting the access of oxygen to biomass particles. This effect may lead to delayed combustion and a greater thermal stability of the mixture, which are beneficial from the point of view of applications in energy systems.
During the straw biomass tests, different combustion indexes were calculated, such as the ignition index (Di), the burnout index (Df), the combustion index S, and the comprehensive combustion index Hf (Table 3). The higher the Di index, the easier ignition is [35,36,38,39]. The ignition index (Di) is in the range from 1.0 to 5.9 × 10−4 wt.%/min3 (value increases with an increasing heating rate β). When comparing the S0 sample with S + 4H, lower values are observed for the samples with halloysite addition. These results are also reflected in the trends obtained for the ignition temperature (Ti). This may suggest that the addition of halloysite slightly delays the ignition of the biomass. A lower Df index for biomass suggests a faster completion of the combustion process [34,35,36]. In the case of the tested biomass, the Df value increases from 0.8 × 10−5 wt.%/min4 at 5 °C/min to 9.9 × 10−5 wt.%/min4 at 20 °C/min. A higher heating rate means that the material is exposed to a higher temperature in a shorter time. In such conditions, the fuel decomposes faster. This phenomenon is typical for materials that react better in more intense thermal conditions, i.e., at higher heating rates. Increasing the heating rate (and, therefore, the temperature) can increase combustion efficiency because it allows for faster decomposition and combustion of the combustible material. Biomass with halloysite (S + 4H) has lower Df values, starting from 0.73 × 10−5 wt.%/min4 at 5 °C/min and increasing to 9.43 × 10−5 wt.%/min4 at 20 °C/min. The differences in the Df index values suggest that the sample with 4 wt.% halloysite addition may need more time and a higher treatment temperature to complete burnout at the same heating rate compared to pure biomass (S0). In the case of the S index, a higher value for biomass means more intensive combustion [34,35,36,37]. The S index value for pure biomass increases from 7.9 × 10−8 wt.%2/(min2 × °C3) at 5 °C/min to 41.2 × 10−8 wt.%2/(min2 × °C3) at 20 °C/min, indicating a significant improvement in combustion efficiency with an increasing heating rate. For biomass with 4 wt.% halloysite addition, the S index value increases from 6.6 × 10−8 wt.%2/(min2 × °C3) at 5 °C/min to 38.1 × 10−8 wt.%2/(min2 × °C3) at 20 °C/min, suggesting a smaller increase in combustion efficiency compared to pure biomass (S0). The S values are slightly lower for biomass with 4 wt.% of halloysite, which may suggest that the addition of halloysite does not improve combustion intensity. The higher the Hf index, the more efficient the combustion is [34,35,36,37]. The Hf index value for biomass (S0) remains relatively stable (from 1.4 × 103 to 1.1 × 103 °C) over the entire heating rate range, indicating a constant thermal characteristic of the material. Biomass with 4 wt.% of halloysite (S + 4H) shows a similar trend in values (from 1.4 × 103 °C at 5 °C/min to 1.1 × 103 °C at 20 °C/min). The trend of the main combustion indexes (Di, Df, S, and Hf) shows better combustion properties for straw biomass (S0) (higher values than those for biomass with halloysite addition). On the other hand, the values of the main combustion indexes do not differ significantly for a given heating rate (often less than a few percent), so it can be assumed that the addition of halloysite does not significantly affect the biomass combustion process.

Table 3.
Main combustion parameters.
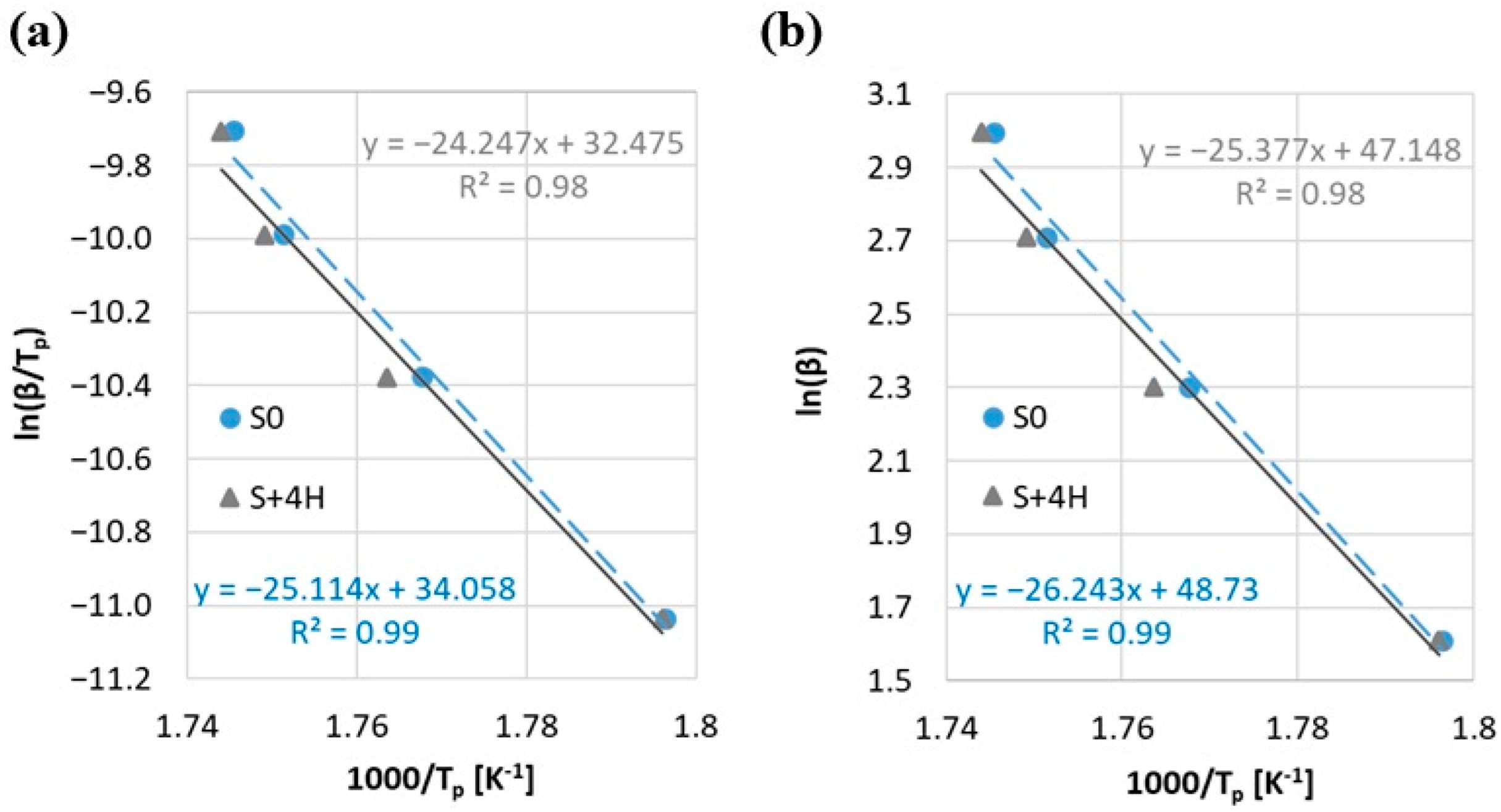
Additionally, the activation energy (Ea) was determined by using the Kissinger and the Ozawa methods (Figure 7, Table 3). The correlation coefficient R2 exhibits high values (ranging from 0.98 to 0.99) for both samples. This indicates a strong agreement between the fitted functions and the experimental data, confirming that both methods (Kissinger and Ozawa) yield dependable estimates of the activation energy. Despite slightly higher ignition temperatures (Ti) and decomposition completion (Tf) for the S + 4H sample compared to the reference sample (S0), a lower activation energy value is observed (for both methods decrease in value by less than 3.5%) (Table 3). Additionally, the higher the Di index, the easier ignition should be, and in the case of the S index, a higher value for the sample means more intensive combustion. The marginal reduction in activation energy (Ea) observed in Table 3 shows slightly different behavior. Taking into account the above, it can be stated that the activation energy does not directly correlate with these combustion indexes. This lack of a strong correlation can be explained by the fact that Ea reflects the overall reaction kinetics, while Di and S are more sensitive to specific stages such as ignition onset and volatile release. Additionally, this phenomenon may result from the synergistic effect of halloysite addition, which, on the one hand, acts as a physical barrier delaying the access of oxygen and the evaporation of volatile products, and on the other hand, can catalyze some stages of thermal decomposition. As a result, a shift in characteristic temperatures is observed, while the energy required to initiate chemical reactions at the microscopic level is reduced. On the other hand, such a small reduction in the energy activation may be caused by a measurement error. Due to the obtained results and the use of only the Kissinger and Ozawa methods (methods assuming a single reaction mechanism), one should rather lean towards the statistical error of the difference in results Ea (Δ < 3.5%). Due to the complex process of biomass combustion with aluminosilicates, other methods for determining the energy activation should be additionally considered for a comprehensive analysis. However, the above allows us to state that the 4 wt.% addition of halloysite does not negatively affect the combustion process of straw biomass.
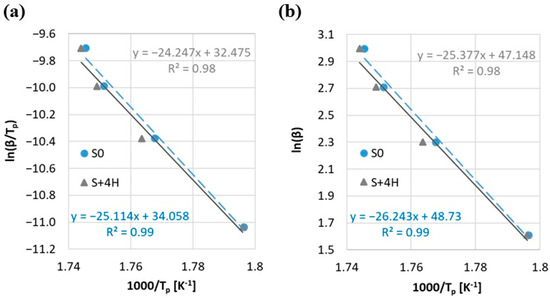
Figure 7.
Activation energy by (a) Kissinger method and (b) Ozawa method.
4. Conclusions
This study involved kinetic experiments on straw biomass valorized with aluminasilicates. The obtained results allow for the formulation of the following conclusions:
- −
- The DSC and TG profiles indicate that both additives—halloysite and kaolinite—exhibit comparable thermal behavior in atmospheric air.
- −
- The desorption of physically bound water from the outer surfaces of the additives occurs up to approximately 400 °C.
- −
- The phase transition of halloysite and kaolinite into metakaolinite takes place at 517.1 °C and 535.2 °C, respectively.
- −
- In the temperature range of 950–1000 °C, metakaolinite further transforms into a spinel phase or silicon-containing gamma-alumina (γ-Al2O3) along with amorphous silica.
- −
- The use of 4 wt.% halloysite causes a shift in characteristic temperatures and a change in TG, DTG, and DSC profiles, which suggests its influence on the oxidation mechanism of the material (shifts in the ignition (Ti), maximum decomposition peak (Tp), and burnout (Tf) temperatures are slightly visible).
- −
- In the case of straw biomass with 4 wt.% halloysite, slight increases in the Di, Df, and S indexes are observed. The Hf index remains at the same level.
- −
- The activation energies determined for both samples for a given method (Kissinger or Ozawa) are at a similar level, with a slight decrease for the sample with halloysite addition. For the Ozawa method, the obtained values are noted to be about 10% higher than those for the Kissinger method.
Author Contributions
J.W.: conceptualization, methodology, investigation, formal analysis, writing—original draft preparation, writing—review and editing. M.T.: supervision, writing—review and editing. S.K.: supervision, writing—review and editing, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The research presented in this work was performed within a project on “Process optimisation and valorisation of combustion by-products in the transition to a Circular Economy” (UPS-Plus) funded by The Foundation for Polish Science within the Team Teach Core Facility Programme (project ID: POIR.04.04.00-00-31B4/17-00) and partly funded by the Silesian University of Technology Statutory Research Fund: 08/050/BK_25/0383.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaniowski, W.; Taler, J.; Wang, X.; Kalemba-Rec, I.; Gajek, M.; Mlonka-Mędrala, A.; Nowak-Woźny, D.; Magdziarz, A. Investigation of biomass, RDF and coal ash-related problems: Impact on metallic heat exchanger surfaces of boilers. Fuel 2022, 326, 125122. [Google Scholar] [CrossRef]
- Míguez, J.L.; Porteiro, J.; Behrendt, F.; Blanco, D.; Patiño, D.; Dieguez-Alonso, A. Review of the use of additives to mitigate operational problems associated with the combustion of biomass with high content in ash-forming species. Renew. Sustain. Energy Rev. 2021, 141, 110502. [Google Scholar] [CrossRef]
- Wang, L.; Hustad, J.E.; Skreiberg, Ø.; Skjevrak, G.; Grønli, M. A critical review on additives to reduce ash related operation problems in biomass combustion applications. Energy Procedia 2012, 20, 20–29. [Google Scholar] [CrossRef]
- Dragutinovic, N.; Höfer, I.; Kaltschmitt, M. Effect of additives on thermochemical conversion of solid biofuel blends from wheat straw, corn stover, and corn cob. Biomass Convers. Biorefin. 2019, 9, 35–54. [Google Scholar] [CrossRef]
- Gollmer, C.; Höfer, I.; Kaltschmitt, M. Laboratory-scale additive content assessment for aluminum-silicate-based wood chip additivation. Renew. Energy 2021, 164, 1471–1484. [Google Scholar] [CrossRef]
- Iturria-Quintero, P.J.; Piloto-Rodríguez, R.; Rubio-González, A.; Lariot-Sánchez, C.A.; Carrazana-Díaz, O.; Rodríguez-Machín, L. Additives for the reduction of alkali chlorides during biomass combustion: A review. Rev. Cent. Azúcar 2024, 51, e1076. [Google Scholar]
- Mroczek, K.; Kalisz, S.; Pronobis, M.; Sołtys, J. The effect of halloysite additive on operation of boilers firing agricultural biomass. Fuel Process. Technol. 2011, 92, 845–855. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Glass, H.D. High-temperature phases from kaolinite and halloysite. Am. Mineral. J. Earth Planet. Mater. 1954, 39, 193–207. [Google Scholar]
- Muljadi, D.; Posner, A.M.; Quirk, J.P. The mechanism of phosphate adsorption by kaolinite, gibbsite, and Pseudoboehmite: Part I. The isotherms and the effect of pH on adsorption. J. Soil Sci. 1966, 17, 212–228. [Google Scholar] [CrossRef]
- Gądek, W. Badania nad Zastosowaniem Haloizytu Jako Dodatku Paliwowego do Biomasy. Doctoral Dissertation, Silesian University of Technology, Gliwice, Poland, 2019. [Google Scholar]
- Szydełko, A.; Ferens, W.; Rybak, W. The effect of mineral additives on the process of chlorine bonding during combustion and co-combustion of Solid Recovered Fuels. Waste Manag. 2020, 102, 624–634. [Google Scholar] [CrossRef]
- Plaza, P. The Development of a Slagging and Fouling Predictive Methodology for Large Scale Pulverised Boilers Fired with Coal/Biomass Blends. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2013. [Google Scholar]
- Sobieraj, J.; Gądek, W.; Jagodzińska, K.; Kalisz, S. Investigations of optimal additive dose for Cl-rich biomasses. Renew. Energy 2021, 163, 2008–2017. [Google Scholar] [CrossRef]
- Johnson, S.L.; Guggenheim, S.; Van Groos, A.K. Thermal stability of halloysite by high-pressure differential thermal analysis. Clays Clay Miner. 1990, 38, 477–484. [Google Scholar] [CrossRef]
- Tran, Q.K.; Steenari, B.-M.; Iisa, K.; Lindqvist, O. Capture of potassium and cadmium by kaolin in oxidizing and reducing atmospheres. Energy Fuels 2004, 18, 1870–1876. [Google Scholar] [CrossRef]
- Wang, G.; Jensen, P.A.; Wu, H.; Frandsen, F.J.; Sander, B.; Glarborg, P. Potassium capture by kaolin, part 2: K2CO3, KCl, and K2SO4. Energy Fuels 2018, 32, 3566–3578. [Google Scholar] [CrossRef]
- Heller-Kallai, L.; Lapides, I. Thermal reactions of kaolinite with potassium carbonate. J. Therm. Anal. Calorim. 2003, 71, 689–698. [Google Scholar] [CrossRef]
- Lutyński, M.; Sakiewicz, P.; Lutyńska, S. Characterization of diatomaceous earth and halloysite resources of Poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef]
- Dewi, R.; Agusnar, H.; Alfian, Z.; Tamrin. Characterization of technical kaolin using XRF, SEM, XRD, FTIR and its potentials as industrial raw materials. J. Phys. Conf. Ser. 2018, 1116, 042010. [Google Scholar] [CrossRef]
- Du Plessis, P.I.; Gazley, M.F.; Tay, S.L.; Trunfull, E.F.; Knorsch, M.; Branch, T.; Fourie, L.F. Quantification of kaolinite and halloysite using machine learning from FTIR, XRF, and brightness data. Minerals 2021, 11, 1350. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Appl. Clay Sci. 2005, 29, 1–23. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. Appl. Clay Sci. 2013, 74, 47–53. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Cheng, H.; Frost, R.L.; Yang, J.; Liu, Q.; He, J. Infrared and infrared emission spectroscopic study of typical Chinese kaolinite and halloysite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 1014–1020. [Google Scholar] [CrossRef]
- Hardy, T.; Kordylewski, W.; Mościcki, K. Chlorine corrosion hazard due to firing and co-firing of biomass in boilers. Arch. Spalania 2009, 9, 181–195. [Google Scholar]
- Wang, G.; Jensen, P.A.; Wu, H.; Frandsen, F.J.; Sander, B.; Glarborg, P. K-capture by al-si based Additives in an Entrained Flow Reactor. In Proceedings of the 26th International Conference on Impacts of Fuel Quality on Power Production, Prague, Czech Republic, 19–23 September 2016. [Google Scholar]
- PN-EN ISO 18134-2:2017-03; Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 2: Total Moisture—Simplified Method. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-EN ISO 18122:2016-01; Solid Biofuels—Determination of Ash Content. Polish Committee for Standardization: Warsaw, Poland, 2016.
- PN-EN ISO 18123:2016-01; Solid Biofuels—Determination of Volatile Matter Content. Polish Committee for Standardization: Warsaw, Poland, 2016.
- PN-EN ISO 18125:2017-07; Solid Biofuels—Determination of Calorific Value. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-EN ISO 16948:2015-07; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen. Polish Committee for Standardization: Warsaw, Poland, 2015.
- CEN/TS 15370-1:2007; Solid Biofuels—Method for the Determination of Ash Melting Behaviour—Part 1: Characteristic Temperatures Method. European Committee for Standardization: Brussels, Belgium, 2007.
- Brown, M.E. (Ed.) Introduction to Thermal Analysis: Techniques and Applications; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Wnorowska, J. Badania Wybranych Właściwości Paliw w celu Wypełnienia Założeń Gospodarki Obiegu Zamkniętego. Doctoral Dissertation, Silesian University of Technology, Gliwice, Poland, 2022. [Google Scholar]
- Wnorowska, J.; Ciukaj, S.; Kalisz, S. Thermogravimetric analysis of solid biofuels with additive under air atmosphere. Energies 2021, 14, 2257. [Google Scholar] [CrossRef]
- Mureddu, M.; Dessì, F.; Orsini, A.; Ferrara, F.; Pettinau, A. Air-and oxygen-blown characterization of coal and biomass by thermogravimetric analysis. Fuel 2018, 212, 626–637. [Google Scholar] [CrossRef]
- Bermejo, S.P.; Prado-Guerra, A.; Pérez, A.I.G.; Prieto, L.F.C. Study of quinoa plant residues as a way to produce energy through thermogravimetric analysis and indexes estimation. Renew. Energy 2020, 146, 2224–2233. [Google Scholar] [CrossRef]
- Yan, Y.F.; Zhang, Z.E. Influence of coal properties on the co-combustion characteristics of low-grade coal and city mud. Glob. NEST J. 2014, 16, 330–339. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).