Numerical Modelling of 1d Isothermal Lithium-Ion Battery with Varied Electrolyte and Electrode Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical and Chemical Properties
2.2. Numerical Method
- A diffusion term, , which accounts for Li-ion movement due to concentration gradients.
- A migration term, , which accounts for Li-ion movement due to the electric field.
- A conductive term, (), representing the movement of charged species under an electric potential.
- A concentration gradient term, , which accounts for the variation of ionic conductivity with concentration, and with being the initial concentration of that species at the beginning of the simulation.
- : Current per unit volume (A/m3), critical for porous electrodes with high surface area.
- : Specific surface area (m2/m3), representing the reactive surface area per unit volume.
- : Local current density (A/m2): current per unit surface area at the reaction sites.
- : normal current density (scalar value representing current per unit area perpendicular to the surface).
- : surface area factor (may represent specific surface area or a scaling parameter).
3. Results
3.1. Validation of the Experimental Results
3.2. Different Electrolyte Material Effects on Simulation Results
- Group 1 has two Li-ion batteries having different solvents in polymer electrolytes.
- Group 2 has four Li-ion batteries having different solvents in liquid electrolytes.
- Polymeric electrolyte consisting of LiPF6 salt dissolved in a 1:2 EC:DMC (by volume) solvent mixture, combined with a PVDF-HFP polymer matrix for Li-ion batteries. Its main properties include a diffusion coefficient of 7.5 × 10−11 m2/s, a transport number of 0.363, an electrolyte salt concentration of 1000 mol/m3, and an electrolyte conductivity of 1000 mol/m3.
- Polymeric electrolyte with LiPF6 salt but in a 2:1 EC:DMC (by volume) solvent with the same PVDF-HFP polymer structure, exhibiting identical material properties.
- Liquid electrolyte based on LiPF6 dissolved in a 1:1 EC:DEC solvent system (liquid electrolyte), with an electrolyte salt concentration of 1000 mol/m3.
- Liquid electrolyte with LiPF6 in a 1:1 EC:DMC mixture (liquid Li-ion battery).
- Liquid electrolyte with LiPF6 in a 3:7 EC:EMC solvent system with a salt concentration of 1200 mol/m3.
- Liquid electrolyte with LiPF6 in a 0.1 PC:0.27 EC:0.63 EMC mixture, also with an electrolyte salt concentration of 1200 mol/m3.
- 1200 s: captures the mid-discharge phase (within the first 2000 s), offering insight into the concentration profile during active discharge before nearing completion.
- 1800 s: represents the late discharge phase, revealing how concentration changes as the battery approaches full depletion.
- 2200 s: occurs during the rest period after discharge (300 s), observing the post-discharge electrolyte relaxation.
- 2400 s: marks the start of the charge phase, providing a baseline for concentration changes as charging begins.
- 3000 s: reflects the late charge phase (2000 s), showing how lithium ions redistribute as charging nears completion.
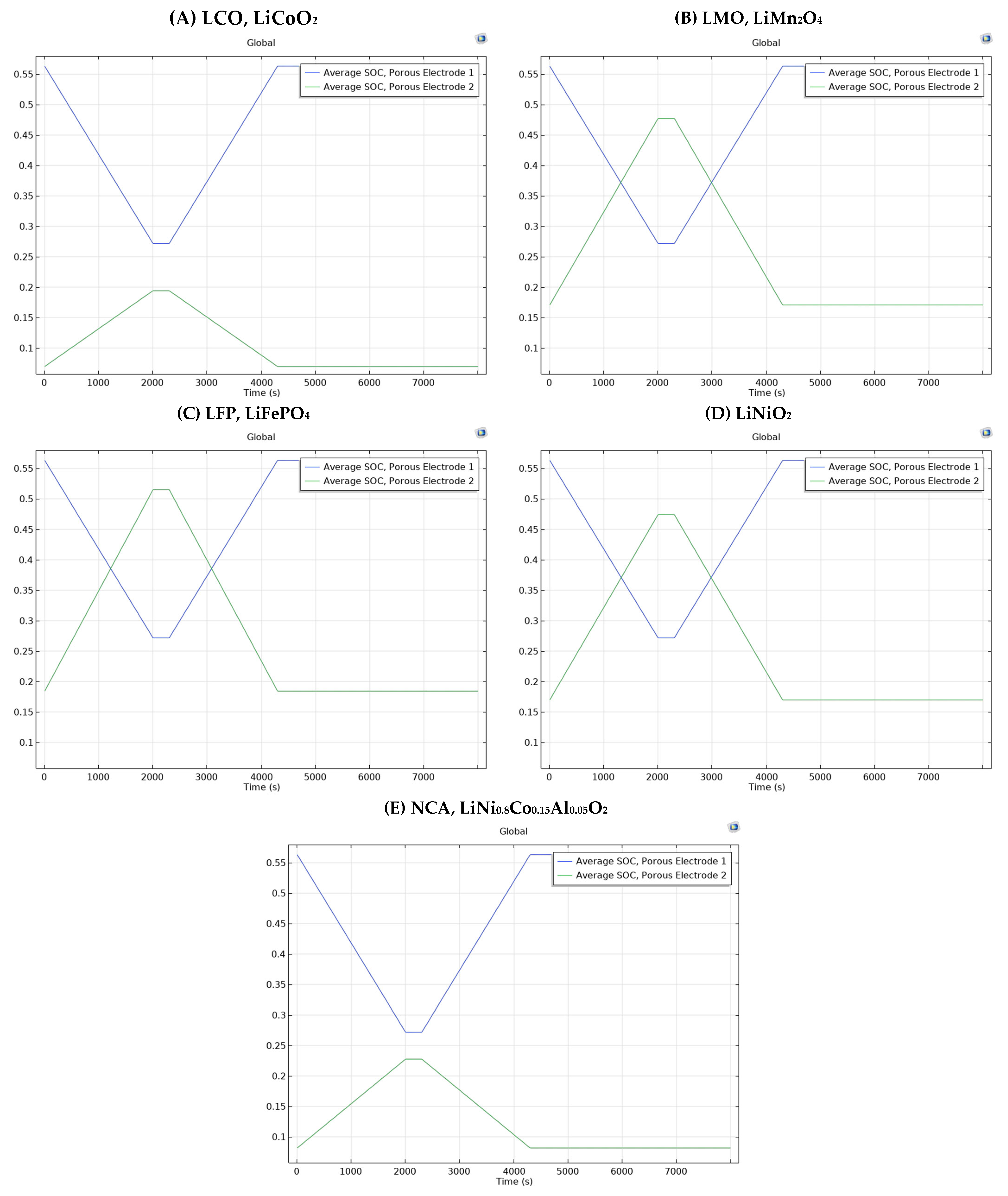
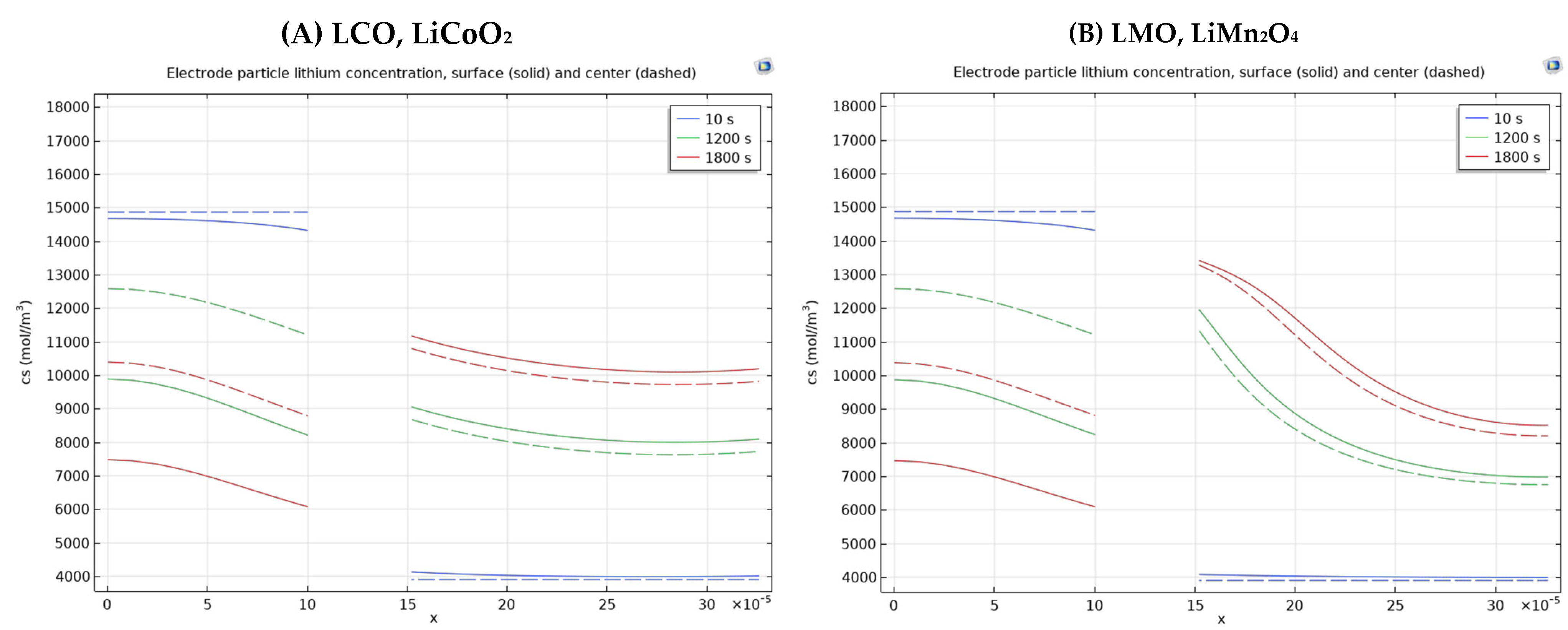
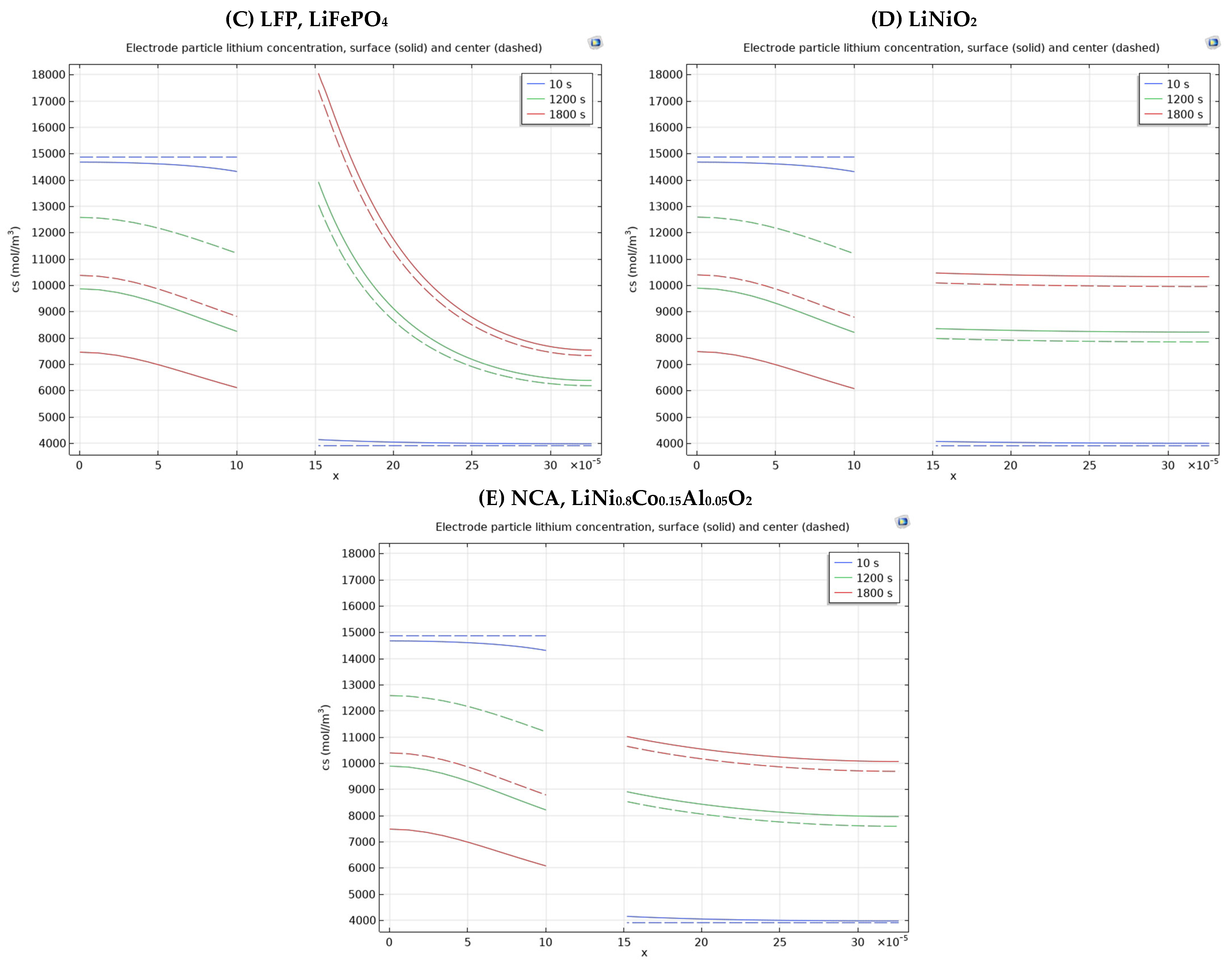
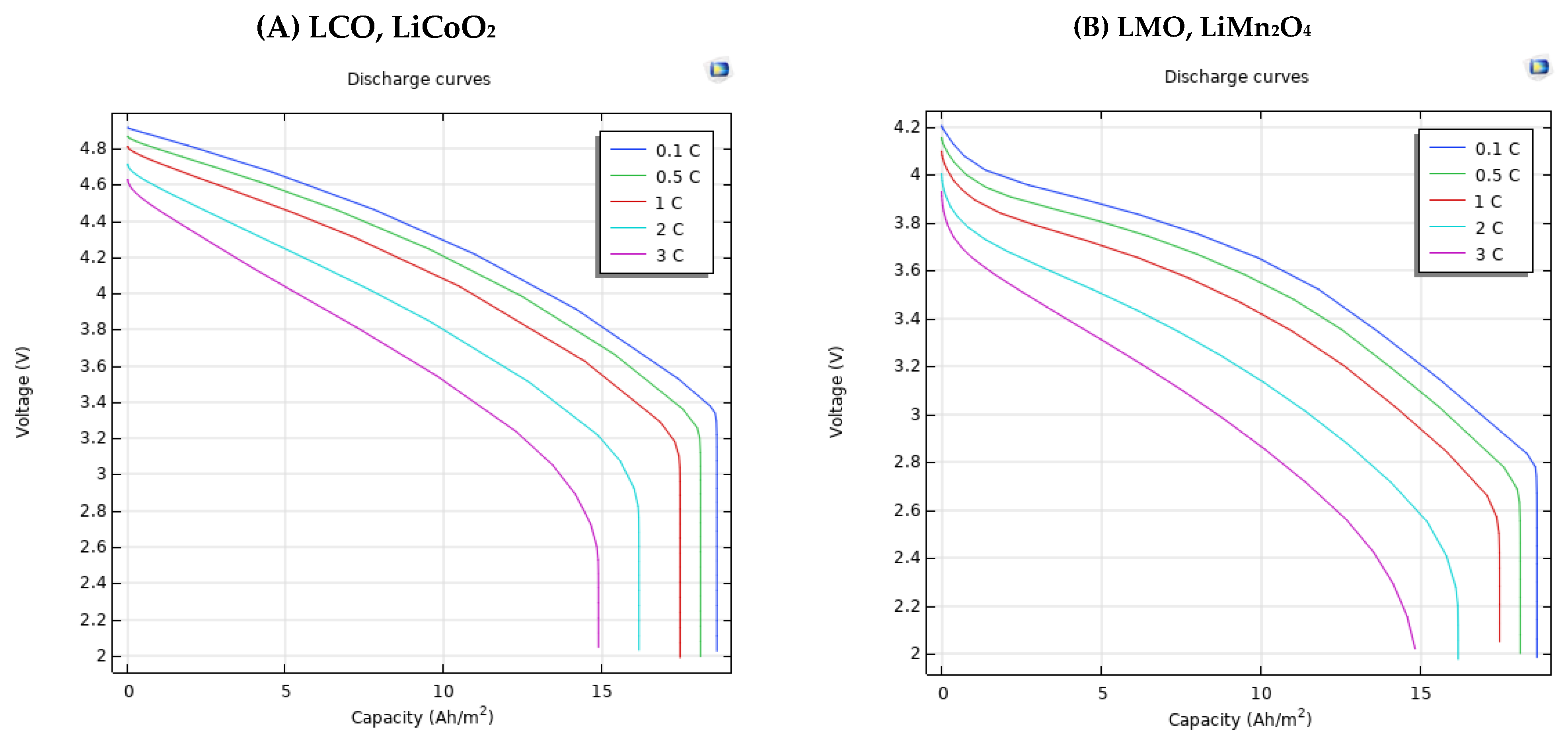
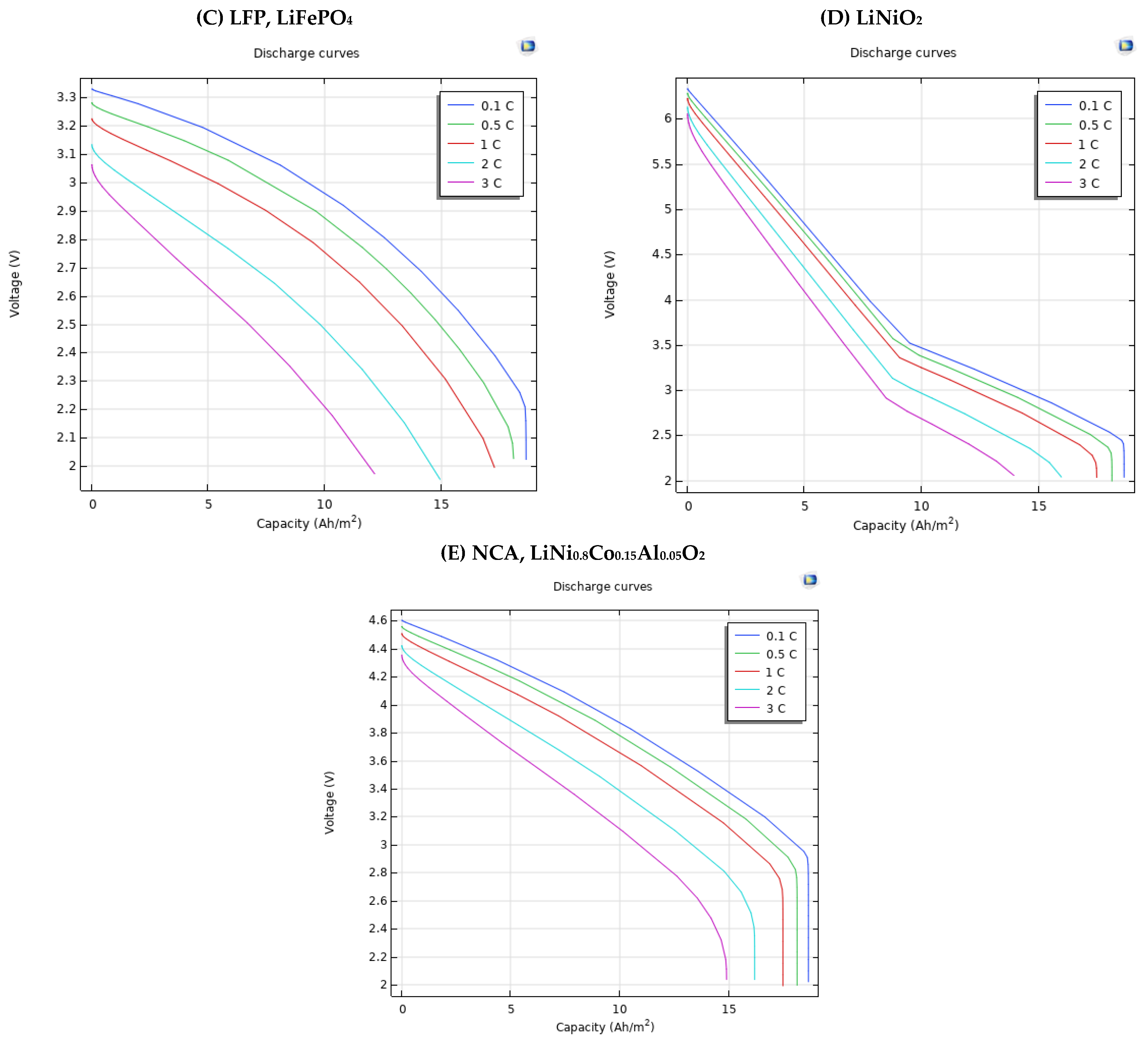
3.3. Different Electrode Material Effects on Simulation Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Li-ion | Lithium-ion |
| LFP | Lithium Iron Phosphate |
| RES | Renewable Energy Source |
| DMC | Dimethyl Carbonate |
| DEC | Diethyl Carbonate |
| EMC | Ethyl Methyl Carbonate |
| EC | Ethylene Carbonate |
| PC | Propylene Carbonate |
| PEO | Polyethylene Oxide |
| PVDF | Polyvinylidene Fluoride |
| HFP | Hexafluoropropylene |
| PMMA | Polymethyl Methacrylate |
| PAN | Polyacrylonitrile |
| PVC | Polyvinyl Chloride |
| SEI | Solid Electrolyte Interphase |
| SPE | Solid Polymer Electrolyte |
| PVA | Polyvinyl Alcohol |
| PS | Polystyrene |
| EV | Electric Vehicle |
| Fe | Iron |
| Al | Aluminum |
| Co | Cobalt |
| Ni | Nickel |
| Mn | Manganese |
| LCO | Lithium Cobalt Oxide |
| LMO | Lithium Manganese Oxide |
| NCA | Lithium Nickel Cobalt Aluminum Oxide |
| SEM | Scanning Electron Microscope |
| DCR | Direct Current Resistance |
| AESOC | Average Electrode State of Charge |
Appendix A
| Electrode Material Type | Electrical Conductivity | Reference Concentration | Maximum-Minimum Electrode SOC | Poisson’s Ratio | Young’s Modulus | Density | Diffusion Coefficient | Some Other Properties | |
|---|---|---|---|---|---|---|---|---|---|
| LCO, LiCoO2 | 1.13 mS/cm | 56250 mol/m3 | 1 0.43 | 0.24 | 191 GPa | 5000 kg/m3 | 5 × 10−13 m2/s | Coefficient of Thermal Expansion | |
| 1.3 × 10−5 1/K | |||||||||
| LMO, LiMn2O4 | 3.8 S/m | 22860 mol/m3 | 0.995 0.175 | 0.26 | 194 GPa | 4140 kg/m3 | |||
| LFP, LiFePO4 | 91 S/m | 21190 mol/m3 | 0.90 0.01 | 0.3 | 117.8 GPa | 3600 kg/m3 | 3.2 × 10−13 m2/s | Thermal Conductivity | Heat Capacity at Constant Pressure |
| 1 W/(m × K) | 881 J/(kg × K) | ||||||||
| LiNiO2 | 100 S/m | 23000 mol/m3 | 1 0.45 | 4650 kg/m3 | Temperature Derivative of Equilibrium Potential | ||||
| 0 V/K | |||||||||
| NCA, LiNi0.8Co0.15Al0.05O2 | 91 S/m | 48000 mol/m3 | 1 0.25 | 4740 kg/m3 | 1.5 × 10−15 m2/s | ||||
References
- Kaya, A.F.; Acir, A.; Kaya, E. Numerical investigation of wind-lens combinations for improving aerodynamic performance of an elliptical-bladed Savonius wind turbine. J. Braz. Soc. Mech. Sci. Eng. 2023, 45, 309. [Google Scholar] [CrossRef]
- Available online: https://www.turcomoney.com/batarya-teknolojisi-gelecegi-sekillendirmede-belirleyici-olacak.html (accessed on 1 March 2025).
- Available online: https://www.pilsitesi.com/blog/icerik/pil-nedir (accessed on 5 March 2025).
- Silberberg, M. Chemistry: The Molecular Nature of Matter and Change; McGraw-Hill Higher Education: New York, NY, USA, 2014. [Google Scholar]
- Li, A.; Yuen, A.C.Y.; Wang, W.; De Cachinho Cordeiro, I.M.; Wang, C.; Chen, T.B.Y.; Zhang, J.; Chan, Q.N.; Yeoh, G.H. A review on lithium-ion battery separators towards enhanced safety performances and modelling approaches. Molecules 2021, 26, 478. [Google Scholar] [CrossRef]
- Available online: https://evreporter.com/understanding-memory-effect-in-lithium-ion-batteries/ (accessed on 2 February 2025).
- Lithium Salts And Solvents Effects On Electrolyte Wetting. Available online: https://iestbattery.com/effect-of-lithium-salts-on-the-electrolyte-wetting/ (accessed on 24 March 2025).
- Baskakova, Y.V.; Yarmolenko, O.V.; Efimov, O.N. Polymer gel electrolytes for lithium batteries. Russ. Chem. Rev. 2012, 81, 367–380. [Google Scholar] [CrossRef]
- Different Electrolytes between Li-ion and Li-Po Batteries—Lithium ion Battery Manufacturer and Supplier in China-DNK Power. Available online: https://www.dnkpower.com/different-electrolytes-li-ion-vs-lipo/ (accessed on 17 January 2025).
- Esd, A. Material and Energy Flows in the Materials Production, Assembly, and End-of-Life Stages of the Automotive Lithium-Ion Battery Life Cycle Energy Systems Division. n.d. Available online: www.anl.gov (accessed on 15 January 2025).
- O’leary, B. High-Volume Manufacturing of LiPF 6, A Critical Lithium-Ion Battery Material; Honeywell International Inc.: Buffalo, NY, USA, 2011. [Google Scholar]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. In Chemistry of Materials; ACS Publications: Washington, DC, USA, 2010; Volume 22, pp. 587–603. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, S.; Zou, X.; Deng, Z.; Xu, Y.; Cao, Z.; Kang, Y.; Deng, Y.; Shi, Q.; Xu, K.; et al. How electrolyte additives work in Li-ion batteries. Energy Storage Mater. 2019, 20, 208–215. [Google Scholar] [CrossRef]
- Ball, R. Electrolytes for Lithium and Lithium-Ion Batteries. Johns. Matthey Technol. Rev. 2015, 59, 30–33. [Google Scholar] [CrossRef]
- Yang, S.; Li, S.; Meng, Y.; Yu, M.; Liu, J.; Li, B. Corrosion inhibition of aluminum current collector with molybdate conversion coating in commercial LiPF6-esters electrolytes. Corros. Sci. 2021, 190, 109632. [Google Scholar] [CrossRef]
- Walker, C.W.; Cox, J.D.; Salomon, M. Conductivity and electrochemical stability of electrolytes containing organic solvent mixtures with Lithium tris(Trifluoromethanesulfonyl)methide. J. Electrochem. Soc. 1996, 143, L80–L82. [Google Scholar] [CrossRef]
- Kanamura, K.; Okagawa, T.; Takehara, Z.-I. Electrochemical oxidation of propylene carbonate (containing various salts) on aluminium electrodes. J. Power Sources 1995, 57, 119–123. [Google Scholar] [CrossRef]
- Sashmitha, K.; Rani, M.U. A comprehensive review of polymer electrolyte for lithium-ion battery. In Polymer Bulletin; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2023; Volume 80, pp. 89–135. [Google Scholar] [CrossRef]
- Ramasekhara Reddy, M.; Srinivas, D. Comparative Performance Analysis of Different Cathode materials of Solid State Lithium ion Battery. Int. J. Recent Innov. Trends Comput. Commun. 2023, 11, 465–478. [Google Scholar] [CrossRef]
- Available online: https://batteryuniversity.com/articles/article/types_of_lit (accessed on 27 January 2025).
- Shim, J.; Striebel, K.A. Effect of electrode density on cycle performance and irreversible capacity loss for natural graphite anode in lithium-ion batteries. J. Power Sources 2003, 119, 934–937. [Google Scholar] [CrossRef]
- Choi, J.; Son, B.; Ryou, M.H.; Kim, S.H.; Ko, J.M.; Lee, Y.M. Effect of LiCoO2 cathode density and thickness on electrochemical performance of lithium-ion batteries. J. Electrochem. Sci. Technol. 2013, 4, 27–33. [Google Scholar] [CrossRef]
- Smekens, J.; Gopalakrishnan, R.; Van den Steen, N.; Omar, N.; Hegazy, O.; Hubin, A.; Van Mierlo, J. Influence of electrode density on the performance of Li-ion batteries: Experimental and simulation results. Energies 2016, 9, 104. [Google Scholar] [CrossRef]
- Chabot, V.; Farhad, S.; Chen, Z.; Fung, A.S.; Yu, A.; Hamdullahpur, F. Effect of electrode physical and chemical properties on lithium-ion battery performance. Int. J. Energy Res. 2013, 37, 1723–1736. [Google Scholar] [CrossRef]
- Ye, Y.; Shi, Y.; Cai, N.; Lee, J.; He, X. Electro-thermal modeling and experimental validation for lithium ion battery. J. Power Sources 2012, 199, 227–238. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- 1D Isothermal Lithium Ion Battery. COMSOL Inc. Available online: https://www.comsol.com/model/1d-isothermal-lithium-ion-battery-686 (accessed on 17 February 2025).
- Lu, J. Development of Fast One-Dimensional Model for Prediction of Coupled Electrochemical-Thermal Behavior of Lithium-Ion Batteries. Doctoral Dissertation, The Ohio State University, Columbus, OH, USA, 2013. [Google Scholar]
- Huber, I.S. Using COMSOL to Model a 1-D Lithium-Ion Battery; College of William and Mary, Department of Physics: Williamsburg, VA, USA, 2017. [Google Scholar]
- Linden, D. Handbook of Batteries; McGraw-Hill: New York, NY, USA, 1995. [Google Scholar]
- Kaya, E.; Reina, L.; d’Adamo, A. Investigating the Impact of Varied C-Rates on Lithium-Ion Batteries: A 1D Simulation Study. J. Phys. Conf. Ser. 2024, 2893, 012050. [Google Scholar] [CrossRef]
- Doyle, M.; Newman, J.; Gozdz, A.S.; Schmutz, C.N.; Tarascon, J.M. Comparison of modeling predictions with experimental data from plastic lithium ion cells. J. Electrochem. Soc. 1996, 143, 1890. [Google Scholar] [CrossRef]
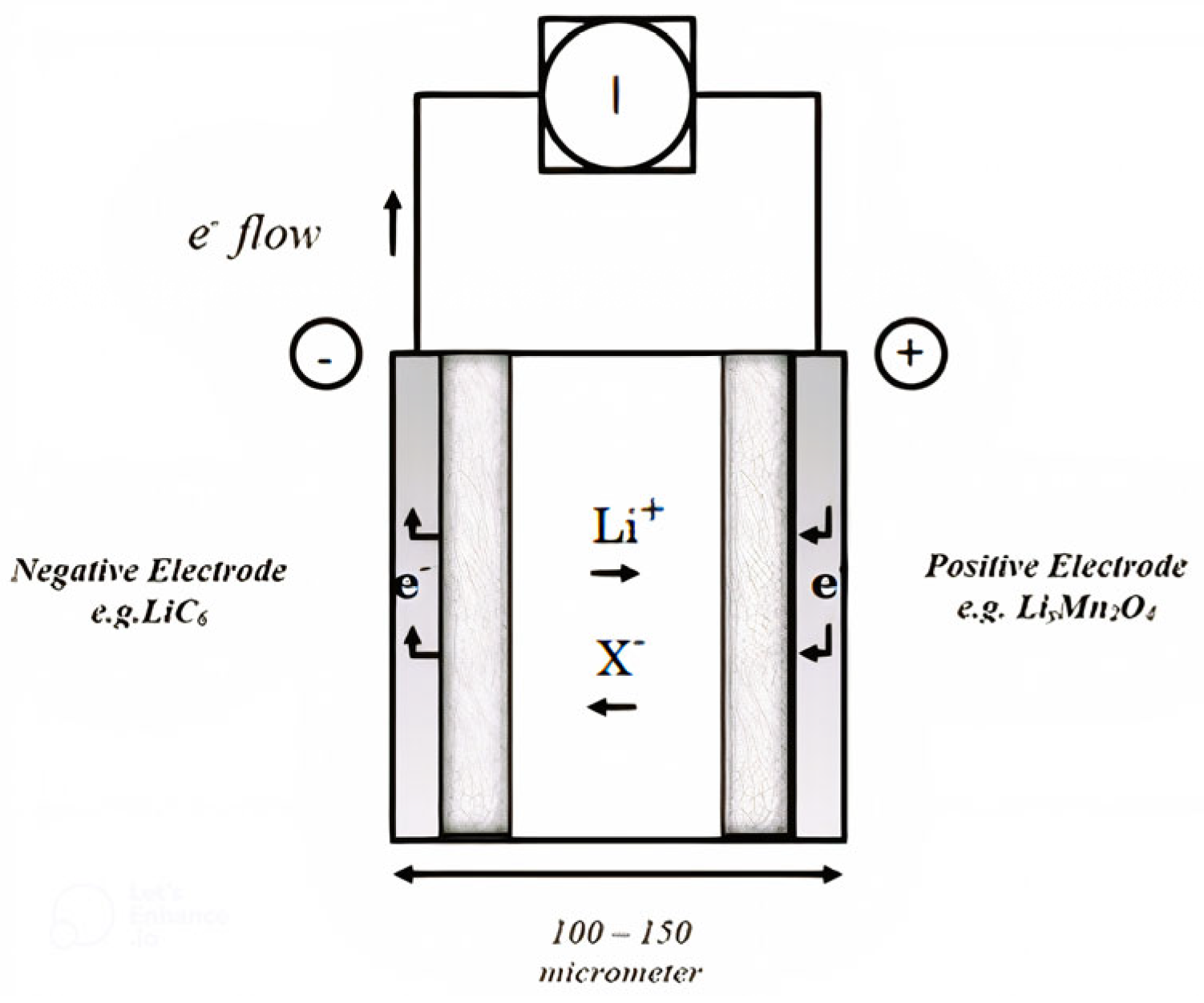
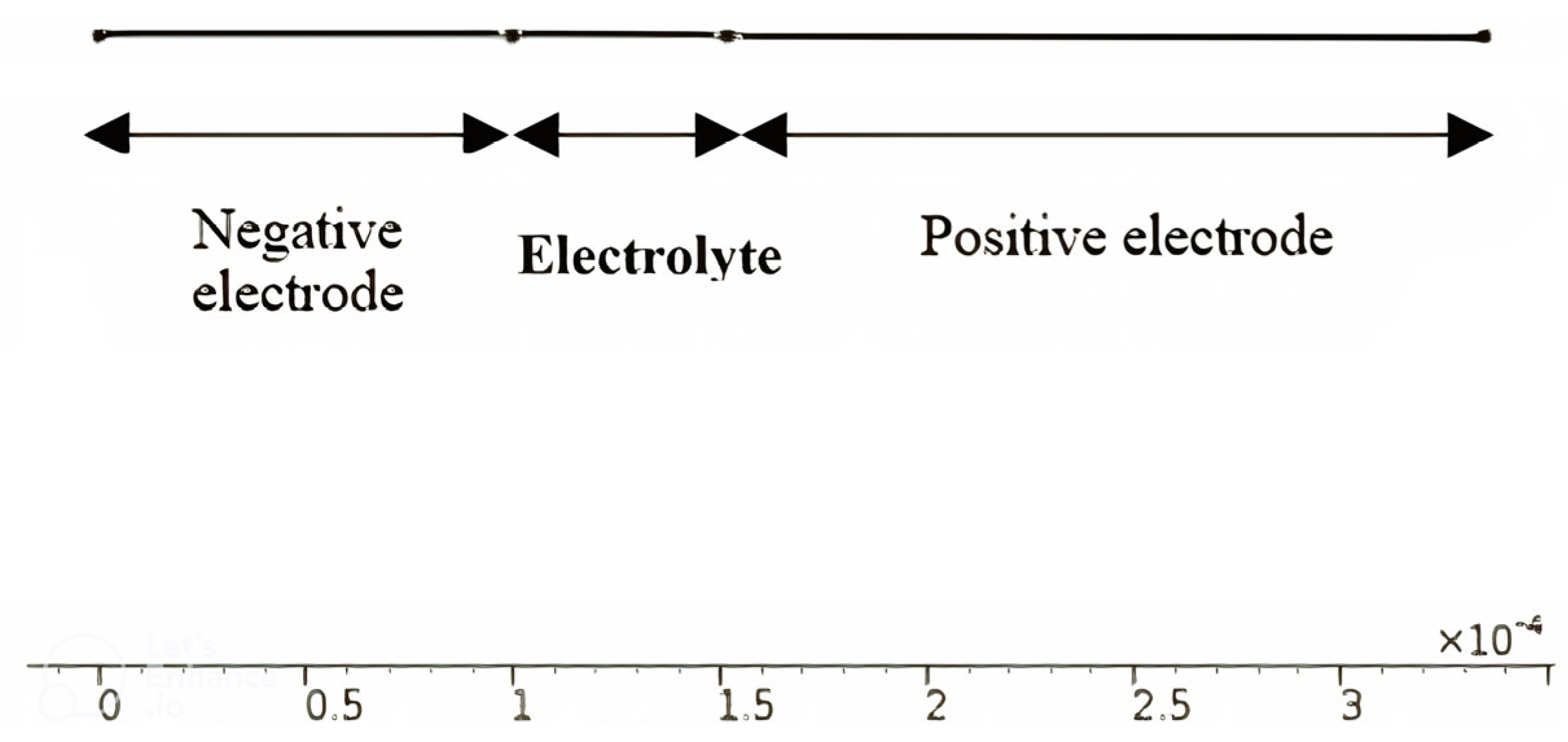
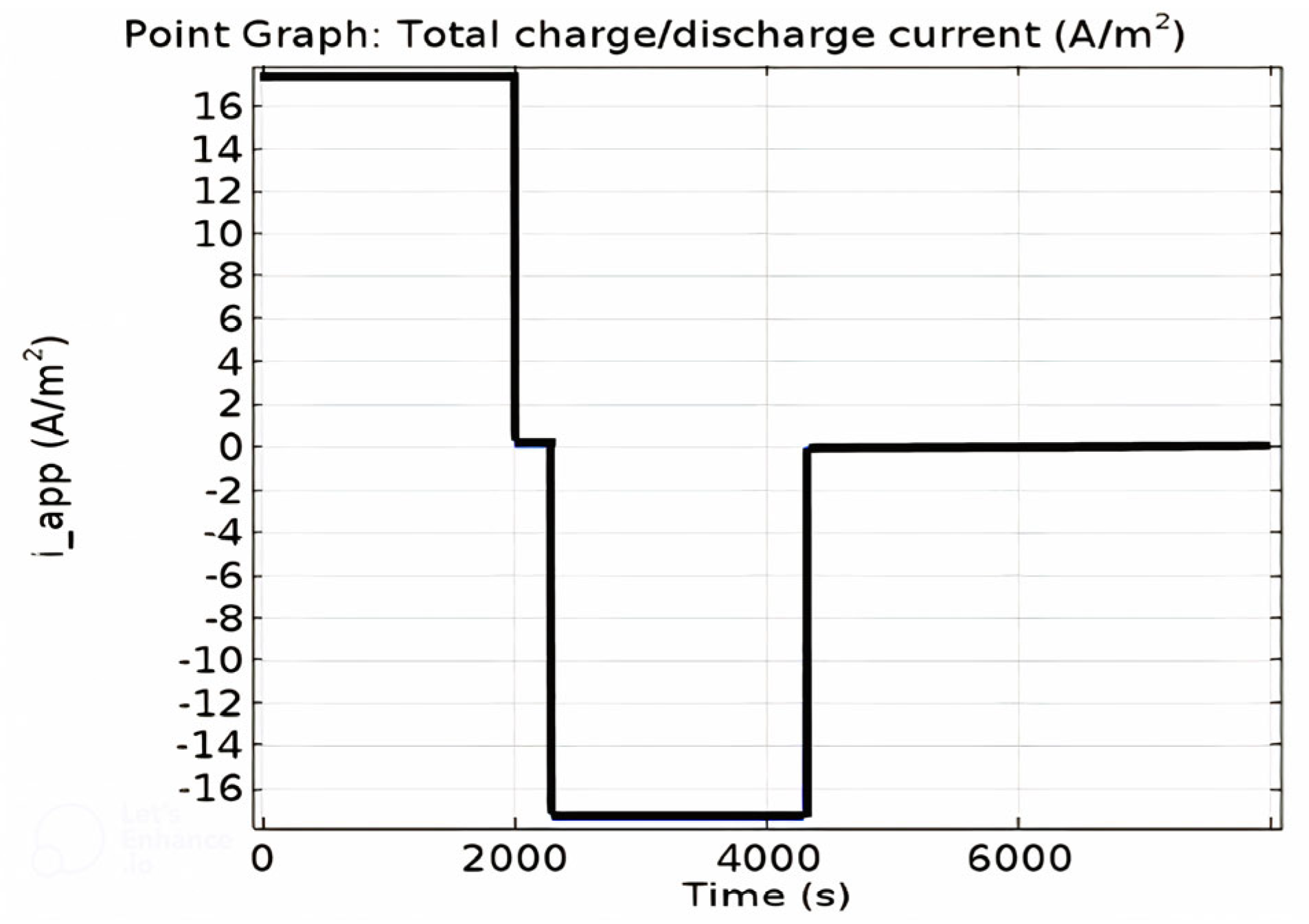
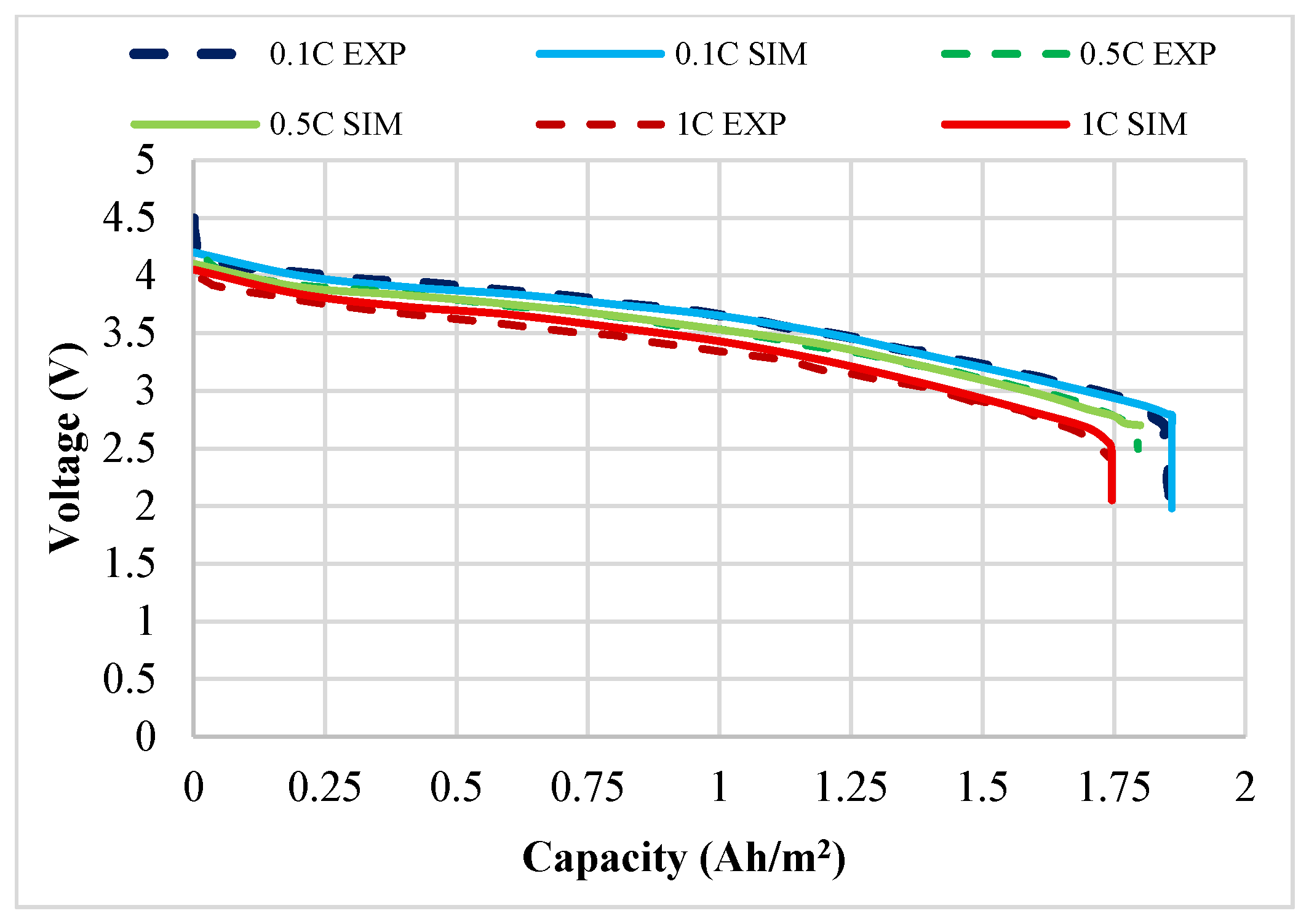
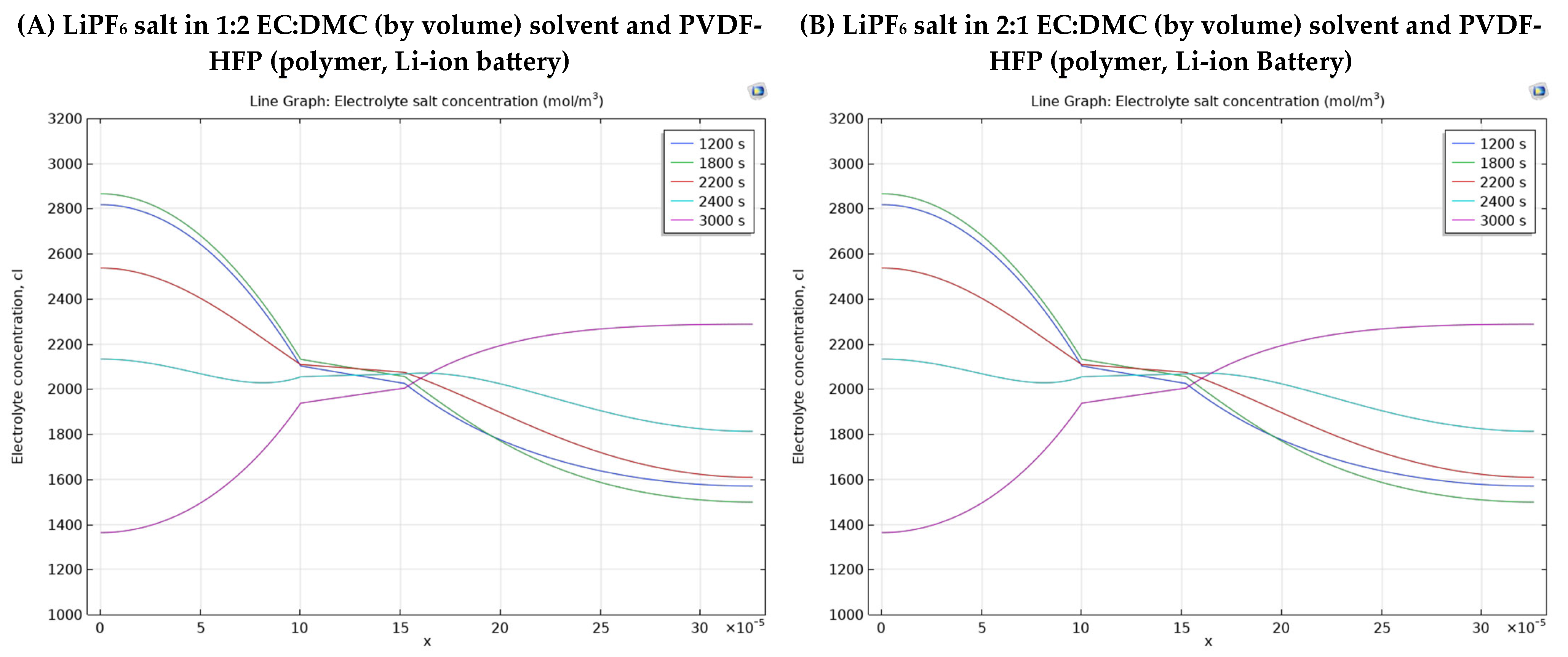
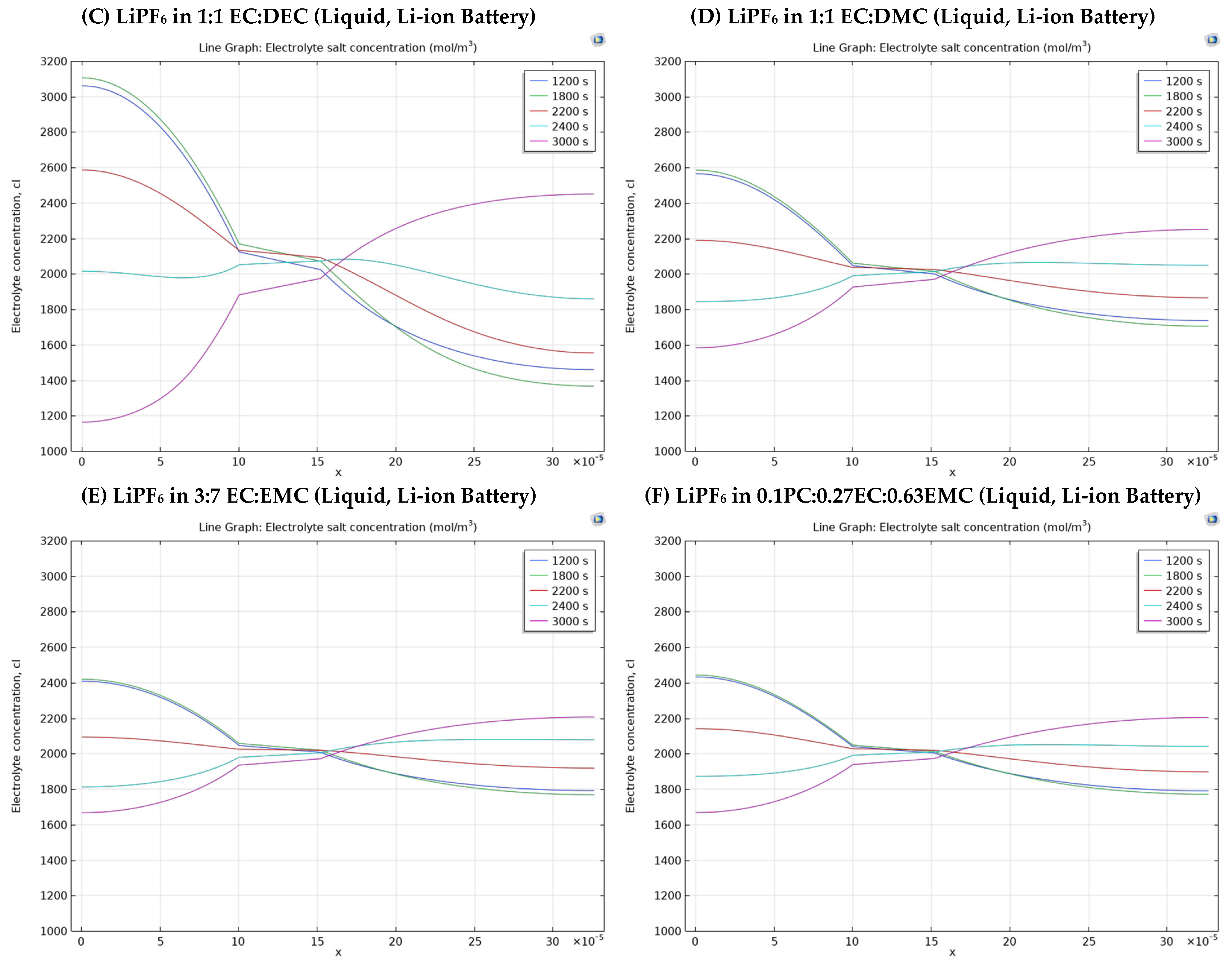
| Electrolyte Material Types | Average Electrolyte Salt Concentration Range of Negative Electrode [0–10] × 10−5 m | Average Electrolyte Salt Concentration Range of Electrolyte [10–15.2] × 10−5 m | Average Electrolyte Salt Concentration Range of Positive Electrode [15.2–32.6] × 10−5 m |
|---|---|---|---|
| LiPF6 salt in 1:2 EC:DMC (by volume) solvent and PVDF-HFP (polymer, Li-ion battery) | 2680 mol/m3–1495 mol/m3 Difference = 1185 mol/m3 | 2095 mol/m3–1970 mol/m3 Difference = 125 mol/m3 | 2265 mol/m3–1586 mol/m3 Difference = 679 mol/m3 |
| LiPF6 salt in 2:1 EC:DMC (by volume) solvent and PVDF-HFP (polymer, Li-ion battery) | 2680 mol/m3–1515 mol/m3 Difference = 1165 mol/m3 | 2095 mol/m3–1980 mol/m3 Difference = 115 mol/m3 | 2245 mol/m3–1583 mol/m3 Difference = 662 mol/m3 |
| LiPF6 in 1:1 EC:DEC (liquid, Li-ion battery) | 2870 mol/m3–1297 mol/m3 Difference = 1573 mol/m3 | 2122 mol/m3–1927 mol/m3 Difference = 195 mol/m3 | 2395 mol/m3–1465 mol/m3 Difference = 930 mol/m3 |
| LiPF6 in 1:1 EC:DMC (liquid, Li-ion battery) | 2436 mol/m3–1659 mol/m3 Difference = 777 mol/m3 | 2037 mol/m3–1947 mol/m3 Difference = 90 mol/m3 | 2208 mol/m3–1754 mol/m3 Difference = 454 mol/m3 |
| LiPF6 in 3:7 EC:EMC (liquid, Li-ion battery) | 2330 mol/m3–1725 mol/m3 Difference = 605 mol/m3 | 2040 mol/m3–1953 mol/m3 Difference = 87 mol/m3 | 2171 mol/m3–1807 mol/m3 Difference = 364 mol/m3 |
| LiPF6 in 0.1PC:0.27EC:0.63EMC (iquid, Li-ion battery) | 2333 mol/m3–1729 mol/m3 Difference = 604 mol/m3 | 2031 mol/m3–1955 mol/m3 Difference = 76 mol/m3 | 2167 mol/m3–1810 mol/m3 Difference = 357 mol/m3 |
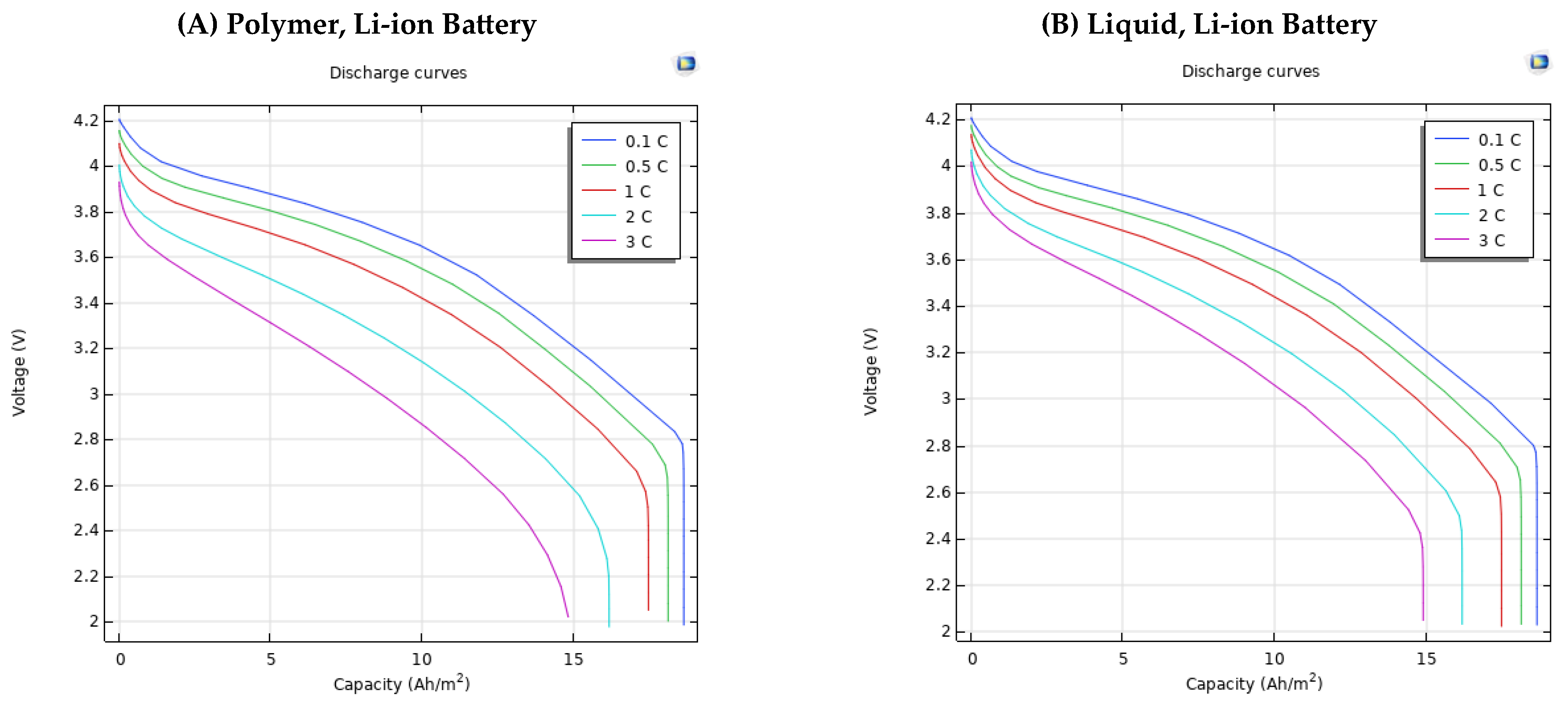
| Electrolyte Material Type | Capacity | (Ah/m2) | |||
|---|---|---|---|---|---|
| C-Rate | 0 | 5 | 10 | 15 | |
| Polymer, Li-ion Battery | 0.1 | 4.2 V | 3.875 V | 3.65 V | 3.2 V |
| 0.5 | 4.155 V | 3.8 V | 3.55 V | 3.09 V | |
| 1 | 4.099 V | 3.7 V | 3.419 V | 2.93 V | |
| 2 | 4 V | 3.5 V | 3.14 V | 2.58 V | |
| 3 | 3.93 V | 3.31 V | 2.86 V | - | |
| liquid, Li-ion battery | 0.1 | 4.21 V | 3.87 V | 3.65 V | 3.2 V |
| 0.5 | 4.17 V | 3.82 V | 3.57 V | 3.1 V | |
| 1 | 4.14 V | 3.74 V | 3.47 V | 2.98 V | |
| 2 | 4.08 V | 3.6 V | 3.28 V | 2.7 V | |
| 3 | 4.02 V | 3.47 V | 3.08 V | - |
| Positive Electrode Material Type | Time (s) | |||
|---|---|---|---|---|
| 0 | 2000 | 4000 | ||
| LCO, LiCoO2 | Negative Electrode (Porous Electrode 1) | 0.56 | 0.27 | 0.519 |
| Positive Electrode (Porous Electrode 2) | 0.069 | 0.19 | 0.087 | |
| LMO, LiMn2O4 | Negative Electrode (Porous Electrode 1) | 0.56 | 0.27 | 0.519 |
| Positive Electrode (Porous Electrode 2) | 0.17 | 0.47 | 0.21 | |
| LFP, LiFePO4 | Negative Electrode (Porous Electrode 1) | 0.56 | 0.27 | 0.519 |
| Positive Electrode (Porous Electrode 2) | 0.18 | 0.51 | 0.23 | |
| LiNiO2 | Negative Electrode (Porous Electrode 1) | 0.56 | 0.27 | 0.519 |
| Positive Electrode (Porous Electrode 2) | 0.16 | 0.47 | 0.21 | |
| NCA, LiNi0.8Co0.15Al0.05O2 | Negative Electrode (Porous Electrode 1) | 0.56 | 0.27 | 0.519 |
| Positive Electrode (Porous Electrode 2) | 0.08 | 0.22 | 0.1 | |
| Positive Electrode Material Type | Difference of Electrode Particle Lithium Concentration of the Positive Electrode Surface Two Chosen Points [(16–30) × 10−5 m] According to Times | ||
|---|---|---|---|
| 10 | 1200 | 1800 | |
| LCO, LiCoO2 | 4105 mol/m3–3991 mol/m3 = 114 mol/m3 | 8918 mol/m3–8011 mol/m3 = 907 mol/m3 | 11034 mol/m3–10108 mol/m3 = 926 mol/m3 |
| LMO, LiMn2O4 | 4069 mol/m3–3992 mol/m3 = 77 mol/m3 | 11341 mol/m3–7023 mol/m3 = 4318 mol/m3 | 13227 mol/m3–8604 mol/m3 = 4623 mol/m3 |
| LFP, LiFePO4 | 4110 mol/m3–3975 mol/m3 = 135 mol/m3 | 12742 mol/m3–6465 mol/m3 = 6277 mol/m3 | 16787 mol/m3–7665 mol/m3 = 9122 mol/m3 |
| LiNiO2 | 4059 mol/m3–3997 mol/m3 = 62 mol/m3 | 8337 mol/m3–8222 mol/m3 = 115 mol/m3 | 10449 mol/m3–10326 mol/m3 = 123 mol/m3 |
| NCA, LiNi0.8Co0.15Al0.05O2 | 4122 mol/m3–3971 mol/m3 = 151 mol/m3 | 8810 mol/m3–7979 mol/m3 = 831 mol/m3 | 10920 mol/m3–10081 mol/m3 = 839 mol/m3 |
| Positive Electrode Material Type | Capacity | (Ah/m2) | |||
|---|---|---|---|---|---|
| C-Rate | 0 | 5 | 10 | 15 | |
| LCO, LiCoO2 | 0.1 | 4.91 V | 4.64 V | 4.29 V | 3.81 V |
| 0.5 | 4.87 V | 4.56 V | 4.2 V | 3.7 V | |
| 1 | 4.81 V | 4.46 V | 4.07 V | 3.55 V | |
| 2 | 4.71 V | 4.24 V | 3.79 V | 3.19 V | |
| 3 | 4.63 V | 4.03 V | 3.51 V | - | |
| LMO, LiMn2O4 | 0.1 | 4.2 V | 3.875 V | 3.65 V | 3.2 V |
| 0.5 | 4.155 V | 3.8 V | 3.55 V | 3.09 V | |
| 1 | 4.099 V | 3.7 V | 3.419 V | 2.93 V | |
| 2 | 4 V | 3.5 V | 3.14 V | 2.58 V | |
| 3 | 3.93 V | 3.31 V | 2.86 V | - | |
| LFP, LiFePO4 | 0.1 | 3.32 V | 3.18 V | 2.96 V | 2.61 V |
| 0.5 | 3.28 V | 3.1 V | 2.87 V | 2.49 V | |
| 1 | 3.22 V | 3.01 V | 2.75 V | 2.32 V | |
| 2 | 3.13 V | 2.81 V | 2.48 V | - | |
| 3 | 3.06 V | 2.62 V | 2.2 V | - | |
| LiNiO2 | 0.1 | 6.33 V | 4.83 V | 3.46 V | 2.92 V |
| 0.5 | 6.28 V | 4.74 V | 3.37 V | 2.79 V | |
| 1 | 6.22 V | 4.61 V | 3.24 V | 2.64 V | |
| 2 | 6.13 V | 4.34 V | 2.96 V | 2.28 V | |
| 3 | 6.05 V | 4.08 V | 2.68 V | - | |
| NCA, LiNi0.8Co0.15Al0.05O2 | 0.1 | 4.6 V | 4.27 V | 3.87 V | 3.37 V |
| 0.5 | 4.56 V | 4.19 V | 3.77 V | 3.26 V | |
| 1 | 4.5 V | 4.09 V | 3.65 V | 3.12 V | |
| 2 | 4.42 V | 3.88 V | 3.38 V | 2.76 V | |
| 3 | 4.35 V | 3.68 V | 3.11 V | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, E.; d’Adamo, A. Numerical Modelling of 1d Isothermal Lithium-Ion Battery with Varied Electrolyte and Electrode Materials. Energies 2025, 18, 3288. https://doi.org/10.3390/en18133288
Kaya E, d’Adamo A. Numerical Modelling of 1d Isothermal Lithium-Ion Battery with Varied Electrolyte and Electrode Materials. Energies. 2025; 18(13):3288. https://doi.org/10.3390/en18133288
Chicago/Turabian StyleKaya, Elif, and Alessandro d’Adamo. 2025. "Numerical Modelling of 1d Isothermal Lithium-Ion Battery with Varied Electrolyte and Electrode Materials" Energies 18, no. 13: 3288. https://doi.org/10.3390/en18133288
APA StyleKaya, E., & d’Adamo, A. (2025). Numerical Modelling of 1d Isothermal Lithium-Ion Battery with Varied Electrolyte and Electrode Materials. Energies, 18(13), 3288. https://doi.org/10.3390/en18133288







