Study on the Characteristics of High-Temperature and High-Pressure Spray Flash Evaporation for Zero-Liquid Discharge of Desulfurization Wastewater
Abstract
1. Introduction
- (1)
- Develop a heat and mass transfer mathematical model tailored for superheated spray flash evaporation under high-temperature and high-pressure conditions, addressing the limitations of traditional DPMs;
- (2)
- Investigate the mechanisms of heat and mass transfer during the flash evaporation of desulfurization wastewater via numerical simulation;
- (3)
- Analyze the effects of critical parameters (e.g., feedwater temperature, pressure, spray angle, and mass flow rate) on evaporation efficiency;
- (4)
- Provide theoretical guidance for optimizing zero-liquid discharge (ZLD) processes in industrial applications.
2. Mathematical Models
2.1. Fundamental Governing Equations
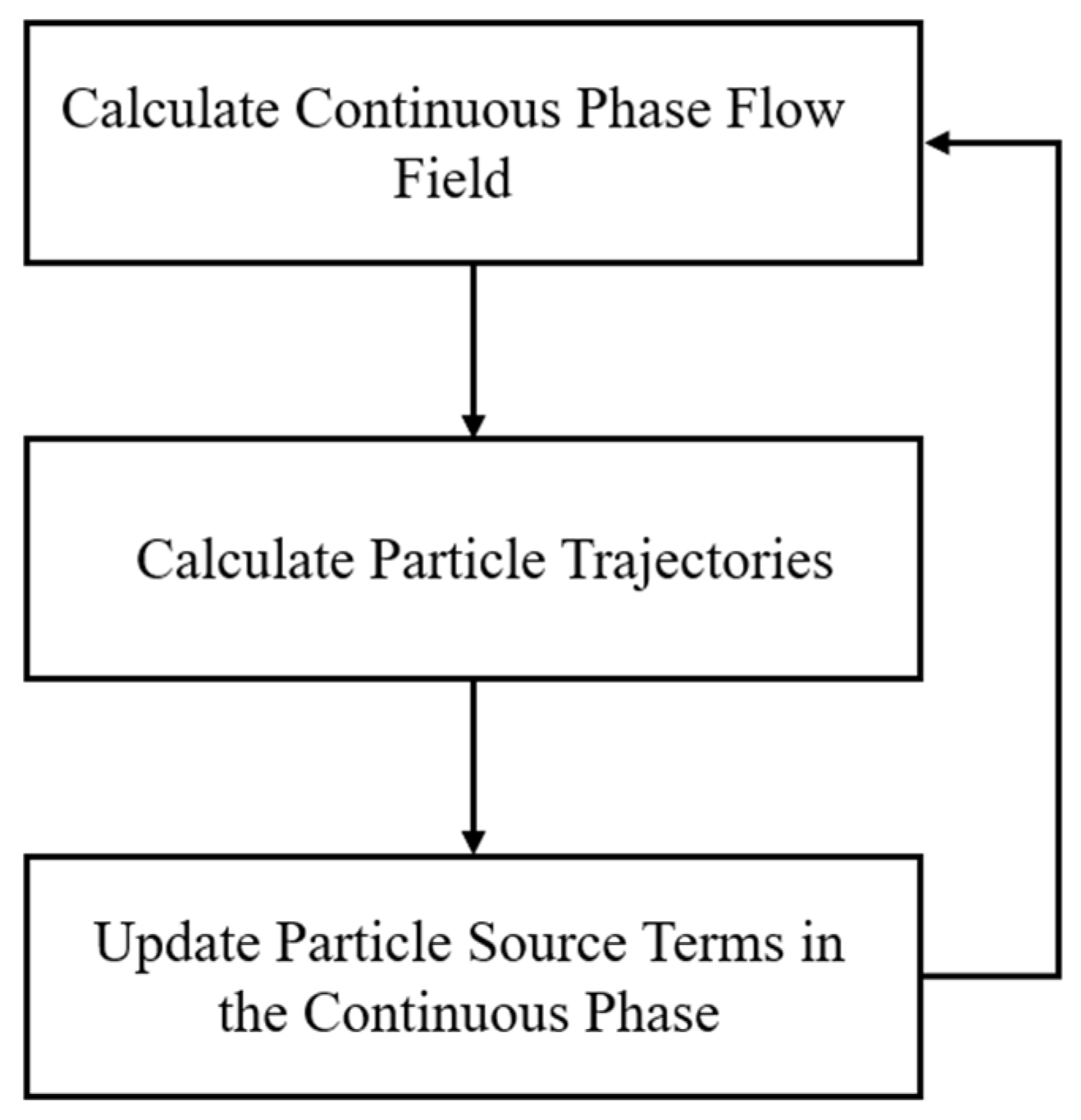
2.2. The Discrete Phase Model
2.2.1. Equation of Droplet Motion Trajectory
2.2.2. Mathematical Model for Heat and Mass Transfer in Flash Evaporation of Superheated Droplets
- (1)
- The droplet maintains a spherical shape throughout the flash evaporation process;
- (2)
- The droplet is divided into an interior and an outer surface, with the temperature gradient assumed to exist only in the radial direction perpendicular to the spherical surface;
- (3)
- Flash evaporation occurs exclusively at the surface of the spherical droplet, and the surface temperature is assumed to remain constant at the saturation temperature corresponding to the working pressure of the expansion chamber;
- (4)
- No heat exchange occurs between the spherical droplet and the surrounding environment;
- (5)
- Both the vapor phase and liquid phase resulting from flash evaporation are in a saturated state;
- (6)
- The pressure inside the expansion chamber is constant and maintained at the working pressure.
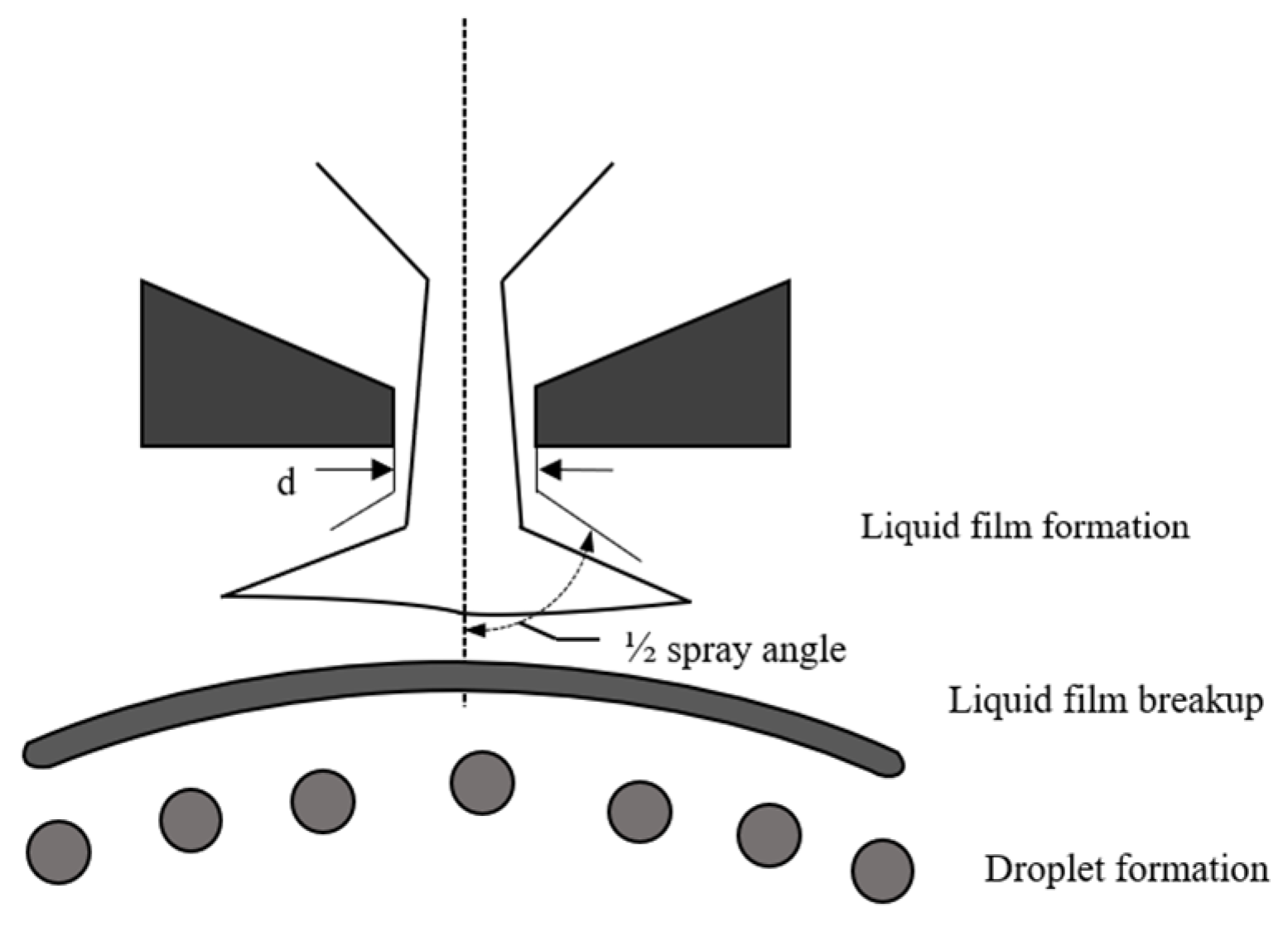
2.2.3. Atomization Model
3. Mesh Generation and Boundary Conditions
4. Results and Discussion
4.1. The Evolution of the Temperature Field Inside the Flash Tank
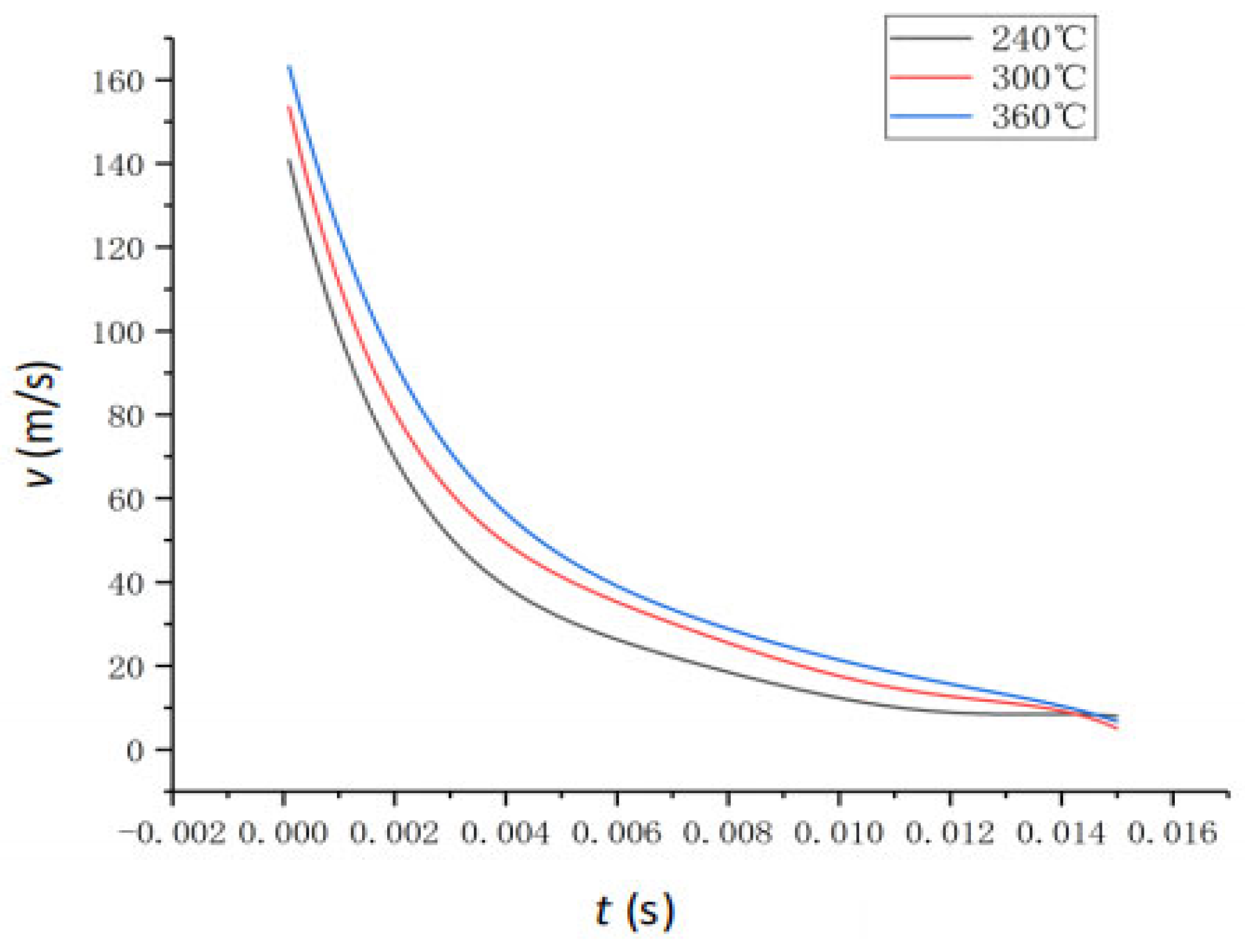
4.2. The Effect of Feedwater Temperature
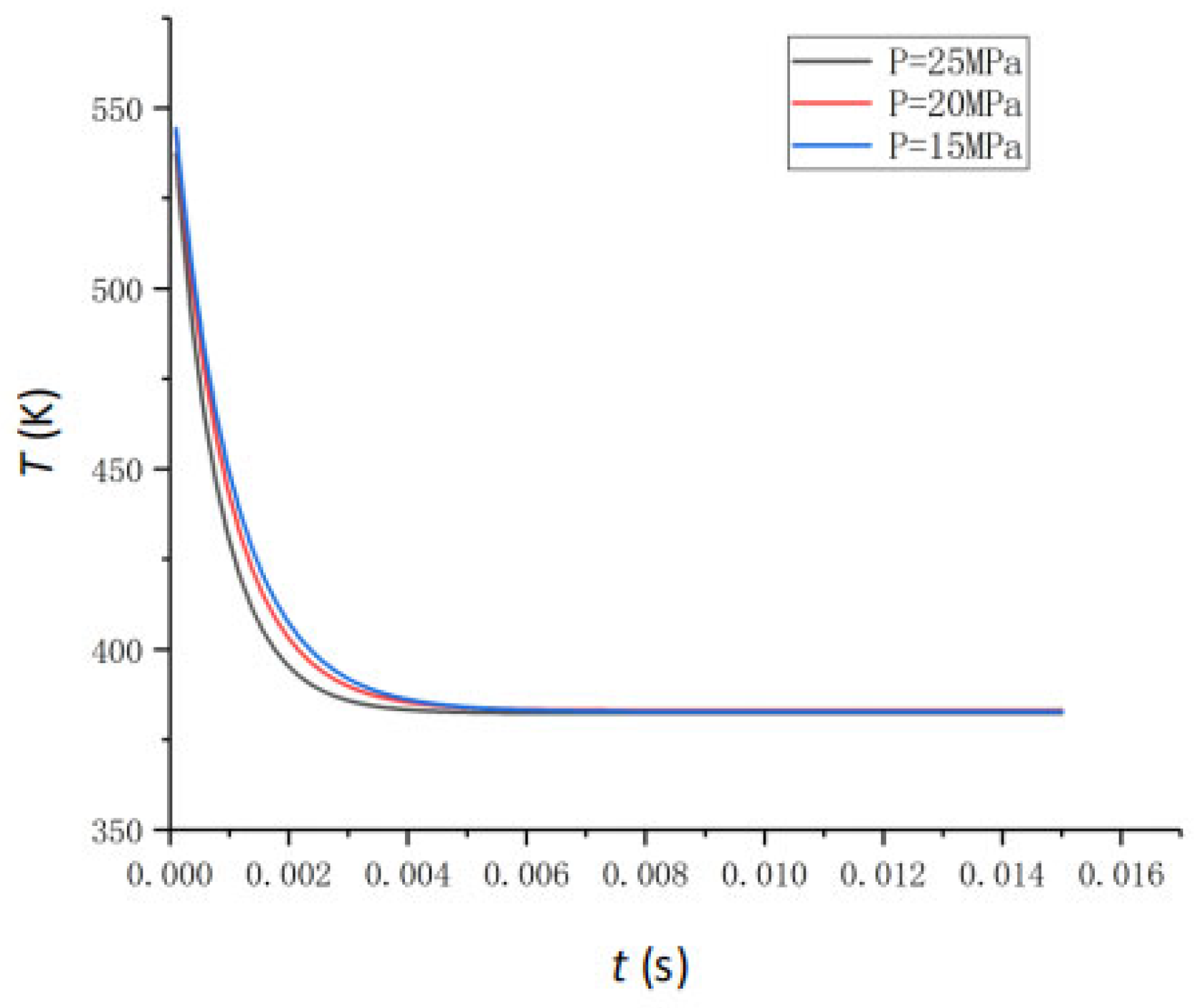
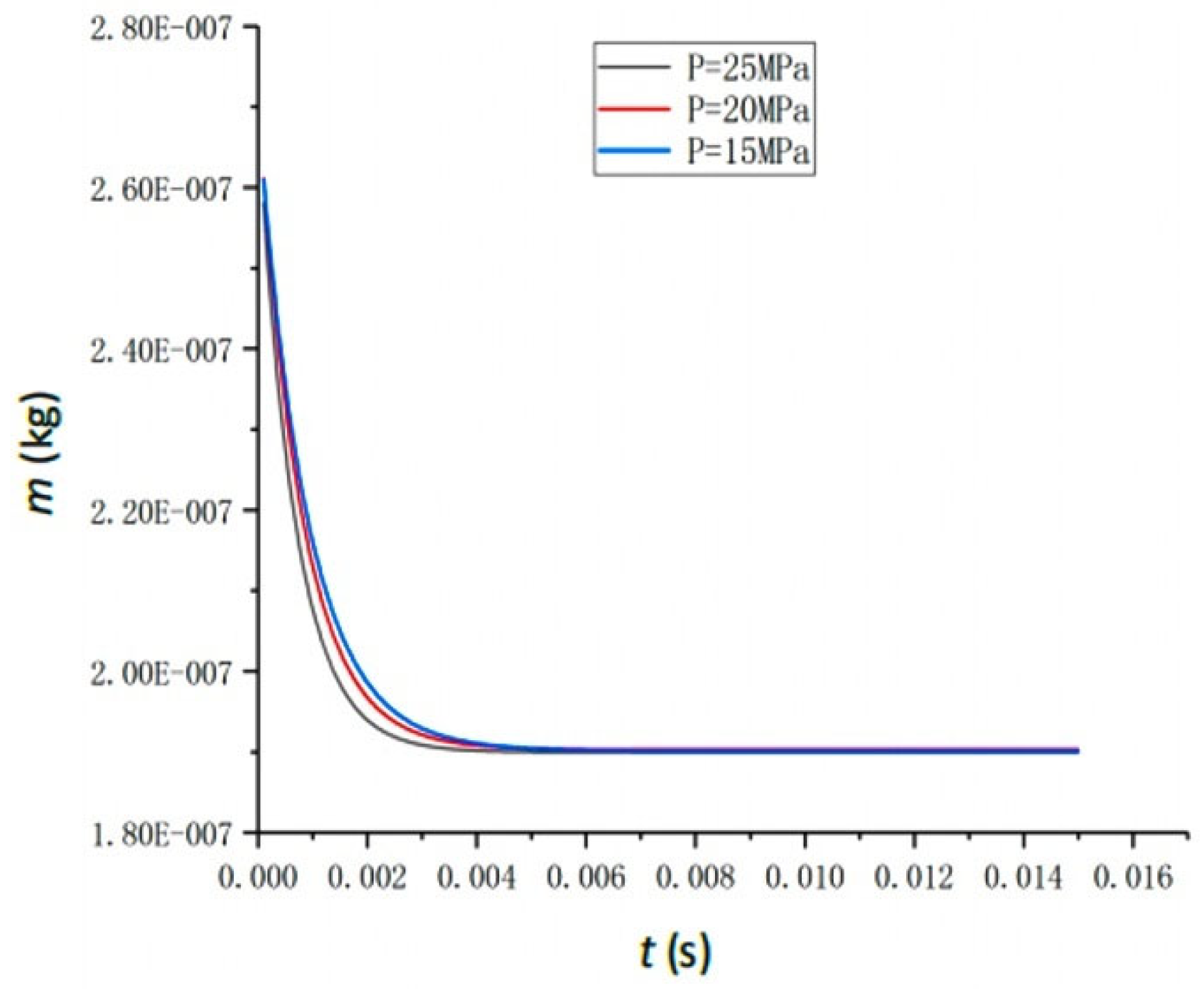
4.3. The Effect of Feedwater Pressure
4.4. The Effect of Nozzle Atomization Angle
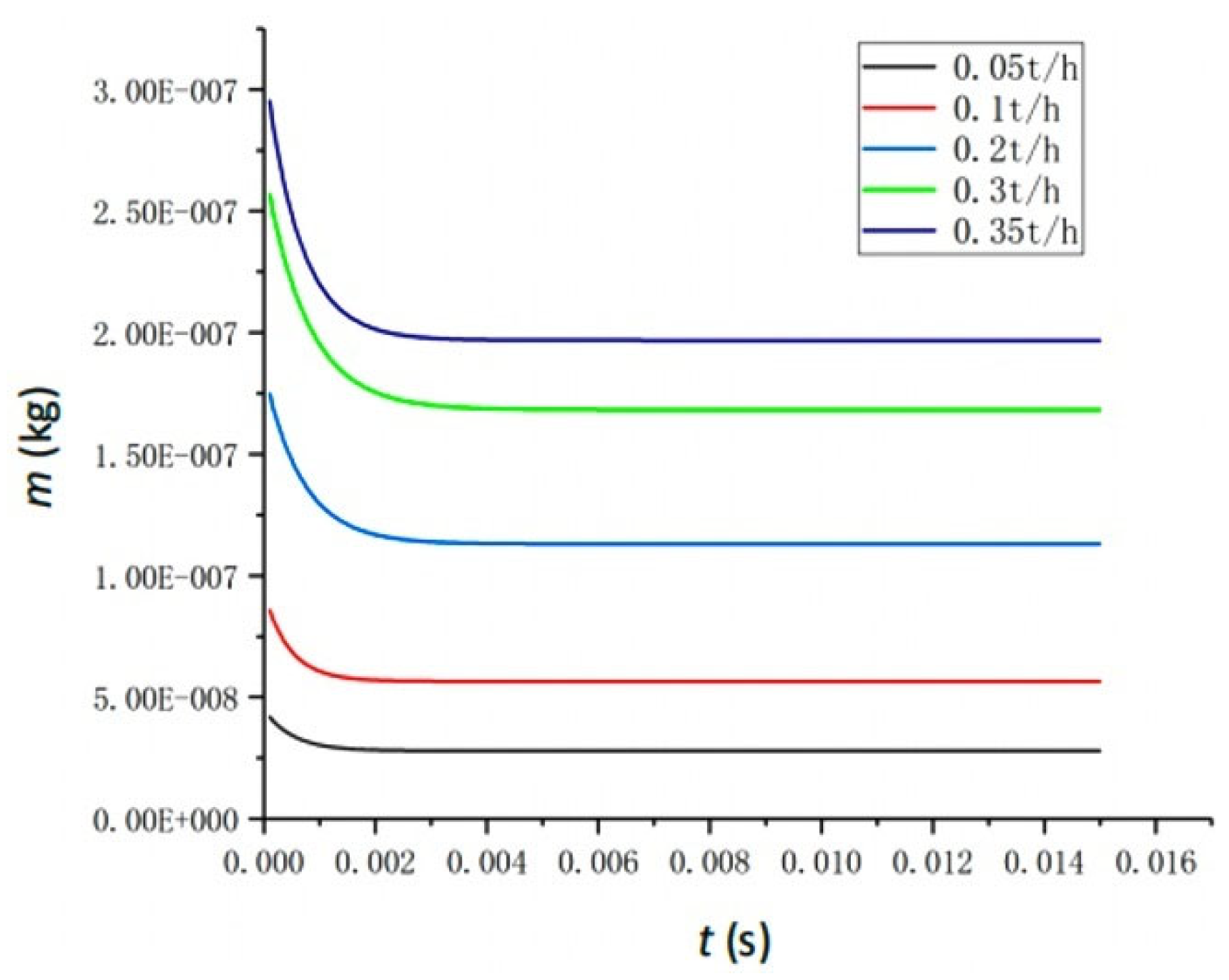
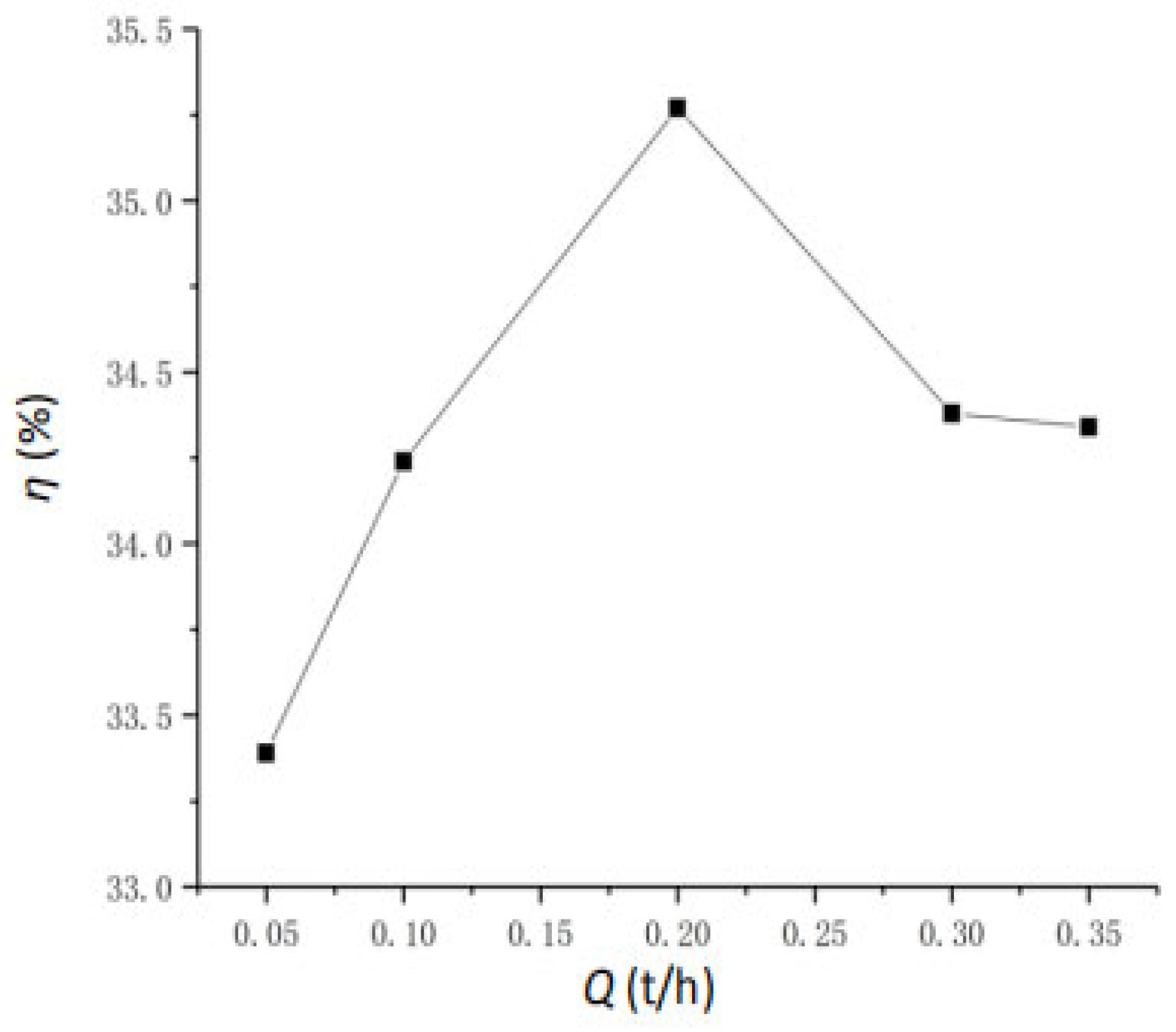
4.5. The Effect of Mass Flow Rate
5. Conclusions
- (1)
- Rapid Droplet Evaporation Driven by Internal Boiling: Superheated droplets (240–360 °C, 1–25 MPa) in a low-pressure (0.1 MPa) environment evaporate rapidly, reaching equilibrium in approximately 6 ms. Vaporization rates significantly increase with temperature, from 19.78% (240 °C) to 55.88% (360 °C). VOF simulations show that this process is dominated by internal boiling and droplet fragmentation, with internal bubble formation, expansion, and subsequent micro-explosions.
- (2)
- Key Parameter Impacts on Vaporization Rate: Feedwater temperature is the primary determinant of the vaporization rate. While increased feedwater pressure accelerates the process to equilibrium, it negligibly affects final water extraction efficiency (stable at ~33.93% for 300 °C feedwater across various pressures). The atomization angle shows minimal impact (<1% change) on the vaporization rate. An optimal feedwater flow rate of 0.2 t/h yields a peak vaporization rate of 42.6%; higher rates decrease efficiency due to fixed space limitations.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| dimensionless temperature | |
| turbulent kinetic energy | |
| turbulent dissipation rate | |
| density | |
| t | temperature |
| time | |
| thermal conductivity | |
| specific heat capacity | |
| the heat generated per unit time and per unit volume within the control element | |
| dimensionless mass transfer number | |
| the saturated vapor mass fraction at the droplet surface | |
| the vapor mass fraction in the surrounding environment | |
| mass | |
| time | |
| buoyancy force | |
| drag force | |
| gravitational acceleration | |
| the latent heat of vaporization under the current conditions | |
| the radius of the spherical droplet | |
| the overall heat transfer coefficient calculated at the outer surface of the droplet | |
| temperature | |
| the diameter of the spherical droplet | |
| the surface area of a spherical droplet | |
| the volume of a spherical droplet | |
| the average droplet mass | |
| the mass flow rate of the feedwater | |
| the number of droplet particles injected into the flash tank through the nozzle | |
| P | pressure |
| ZLD | zero-liquid discharge |
| DPM | discrete phase model |
| FGD | flue gas desulfurization |
| MED | multi-effect distillation |
| UDF | user-defined function |
References
- Li, Y.; Li, X.; Wang, L.; Li, Y. Limestone-gypsum wet flue gas desulfurization based on Cyber-Physical System. In Proceedings of the 2019 Chinese Control And Decision Conference (CCDC), Nanchang, China, 3–5 June 2019; pp. 473–477. [Google Scholar] [CrossRef]
- Kang, Q.; Yuan, Y. Diagnosis and Traceability Analysis of Slurry Foaming of Limestone-Gypsum Wet Flue-Gas Desulfurization (WFGD) System. Water Air Soil Pollut. 2023, 234, 108. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, S.; Li, B.; Li, H.; Ling, Z.; Zhang, G.; Liu, M. Current status of zero liquid discharge technology for desulfurization wastewater. Water 2024, 16, 900. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Johir, M.A.H.; Listowski, A.; Vigneswaran, S.; Kandasamy, J. Sustainable processes for treatment of waste water reverse osmosis concentrate to achieve zero waste discharge: A detailed study in water reclamation plant. Procedia Environ. Sci. 2016, 35, 930–939. [Google Scholar] [CrossRef]
- Aly, S.; Manzoor, H.; Simson, S.; Abotaleb, A.; Lawler, J.; Mabrouk, A.N. Pilot testing of a novel Multi Effect Distillation (MED) technology for seawater desalination. Desalination 2021, 519, 115221. [Google Scholar] [CrossRef]
- Deng, J.-J.; Pan, L.-M.; Chen, D.-Q.; Dong, Y.-Q.; Wang, C.-M.; Liu, H.; Kang, M.-Q. Numerical simulation and field test study of desulfurization wastewater evaporation treatment through flue gas. Water Sci. Technol. 2014, 70, 1285–1291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mansour, A.; Müller, N. A review of flash evaporation phenomena and resulting shock waves. Exp. Therm. Fluid Sci. 2019, 107, 146–168. [Google Scholar] [CrossRef]
- Mujumdar, A.S.; Alterman, D.S. Drying in the pharmaceutical and biotechnology fields. In Handbook of Downstream Processing; Springer: Dordrecht, The Netherlands, 1997; pp. 235–260. [Google Scholar] [CrossRef]
- Jiang, S.; Luo, W.; Peng, Q.; Wu, Z.; Li, H.; Li, H.; Yu, J. Effects of flash evaporation conditions on the quality of UHT milk by changing the dissolved oxygen content in milk. Foods 2022, 11, 2371. [Google Scholar] [CrossRef]
- Ma, W.; Zhai, S.; Zhang, P.; Xian, Y.; Zhang, L.; Shi, R.; Sheng, J.; Liu, B.; Wu, Z. Research progresses of flash evaporation in aerospace applications. Int. J. Aerosp. Eng. 2018, 2018, 3686802. [Google Scholar] [CrossRef]
- Goto, S.; Yamamoto, Y.; Sugi, T.; Yasunaga, T.; Ikegami, Y.; Nakamura, M. Construction of simulation model for spray flash desalination system. Electr. Eng. Jpn. 2010, 170, 9–17. [Google Scholar] [CrossRef]
- Miyatake, O.; Tomimura, T.; Ide, Y.; Fujii, T. An experimental study of spray flash evaporation. Desalination 1981, 36, 113–128. [Google Scholar] [CrossRef]
- Miyatake, O.; Tomimura, T.; Ide, Y.; Yuda, M.; Fujii, T. Effect of liquid temperature on spray flash evaporation. Desalination 1981, 37, 351–366. [Google Scholar] [CrossRef]
- Uehara, H. Experimental study of spray flash evaporation for desalination and otec. In Proceedings of the Joint Solar Engineering Conference, Washington, DC, USA, 4–8 April 1993; ASME: New York, NY, USA, 1993. [Google Scholar]
- Mutair, S.; Ikegami, Y. Experimental study on flash evaporation from superheated water jets: Influencing factors and formulation of correlation. Int. J. Heat Mass Transf. 2009, 52, 5643–5651. [Google Scholar] [CrossRef]
- Mutair, S.; Ikegami, Y. Experimental investigation on the characteristics of flash evaporation from superheated water jets for desalination. Desalination 2010, 251, 103–111. [Google Scholar] [CrossRef]
- Chen, Q.K.J.M.; Li, Y.; Chua, K.J. Experimental and mathematical study of the spray flash evaporation phenomena. Appl. Therm. Eng. 2018, 130, 598–610. [Google Scholar] [CrossRef]
- Duan, R.Q.; Jiang, S.Y.; Koshizuka, S.; Oka, Y.; Yamaguchi, A. Direct simulation of flashing liquid jets using the MPS method. Int. J. Heat Mass Transf. 2006, 49, 402–405. [Google Scholar] [CrossRef]
- Simões-Moreira, J.R.; Vieira, M.M.; Angelo, E. Highly expanded flashing liquid jets. J. Thermophys. Heat Transf. 2002, 16, 415–424. [Google Scholar] [CrossRef]
- Fathinia, F.; Al-Abdeli, Y.M.; Khiadani, M. Evaporation rates and temperature distributions in fine droplet flash evaporation sprays. Int. J. Therm. Sci. 2019, 145, 106037. [Google Scholar] [CrossRef]
- Price, C.; Hamzehloo, A.; Aleiferis, P.; Richardson, D. Numerical modelling of droplet breakup for flash-boiling fuel spray predictions. Int. J. Multiph. Flow 2020, 125, 103183. [Google Scholar] [CrossRef]
- Zahari, N.M.; Zawawi, M.H.; Sidek, L.M.; Mohamad, D.; Itam, Z.; Ramli, M.Z.; Syamsir, A.; Abas, A.; Rashid, M. Introduction of discrete phase model (DPM) in fluid flow: A review. AIP Conf. Proc. 2018, 2030, 040008. [Google Scholar] [CrossRef]
- Owen, O.I.; Jalil, J.M. Heterogeneous Flashing in Water Drops. Int. J. Multiph. Flow 1991, 17, 653–660. [Google Scholar] [CrossRef]
- Adachi, A.A.M.; McDonell, V.G.; Tanaka, D.; Senda, J.; Fujimoto, H. Characterization of Fuel Vapor Concentration Inside a Flash Boiling Spray; SAE Technical Paper 970871; SAE International: Warrendale, PA, USA, 1997. [Google Scholar] [CrossRef]
- Wang, G.C.; Lu, S.; Li, M.; Zhang, Y.; Sa, Z.; Liu, J.; Wang, H.; Wang, S. Study on the dust removal and temperature reduction coupling performances of magnetized water spray. Environ. Sci. Pollut. Res. 2022, 29, 6151–6165. [Google Scholar] [CrossRef] [PubMed]










| Model | Feedwater Temperature (°C) | Feedwater Pressure (MPa) | Nozzle Atomization Angle (°) | Mass Flow Rate (t/h) |
|---|---|---|---|---|
| 1 | 300 | 10 | 80 | 0.3 |
| 2 | 240 | 25 | 80 | 0.3 |
| 3 | 300 | 25 | 80 | 0.3 |
| 4 | 360 | 25 | 80 | 0.3 |
| 5 | 300 | 15 | 80 | 0.3 |
| 6 | 300 | 20 | 80 | 0.3 |
| 7 | 300 | 25 | 60 | 0.3 |
| 8 | 300 | 25 | 100 | 0.3 |
| 9 | 300 | 25 | 80 | 0.05 |
| 10 | 300 | 25 | 80 | 0.1 |
| 11 | 300 | 25 | 80 | 0.2 |
| 12 | 300 | 25 | 80 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, Z. Study on the Characteristics of High-Temperature and High-Pressure Spray Flash Evaporation for Zero-Liquid Discharge of Desulfurization Wastewater. Energies 2025, 18, 3180. https://doi.org/10.3390/en18123180
Zhang L, Liu Z. Study on the Characteristics of High-Temperature and High-Pressure Spray Flash Evaporation for Zero-Liquid Discharge of Desulfurization Wastewater. Energies. 2025; 18(12):3180. https://doi.org/10.3390/en18123180
Chicago/Turabian StyleZhang, Lanshui, and Zhong Liu. 2025. "Study on the Characteristics of High-Temperature and High-Pressure Spray Flash Evaporation for Zero-Liquid Discharge of Desulfurization Wastewater" Energies 18, no. 12: 3180. https://doi.org/10.3390/en18123180
APA StyleZhang, L., & Liu, Z. (2025). Study on the Characteristics of High-Temperature and High-Pressure Spray Flash Evaporation for Zero-Liquid Discharge of Desulfurization Wastewater. Energies, 18(12), 3180. https://doi.org/10.3390/en18123180






