Bioaugmentation with Electroactive Microbes—A Promising Strategy for Improving Process Performances of Microbial Electrochemical Technologies
Abstract
1. Introduction
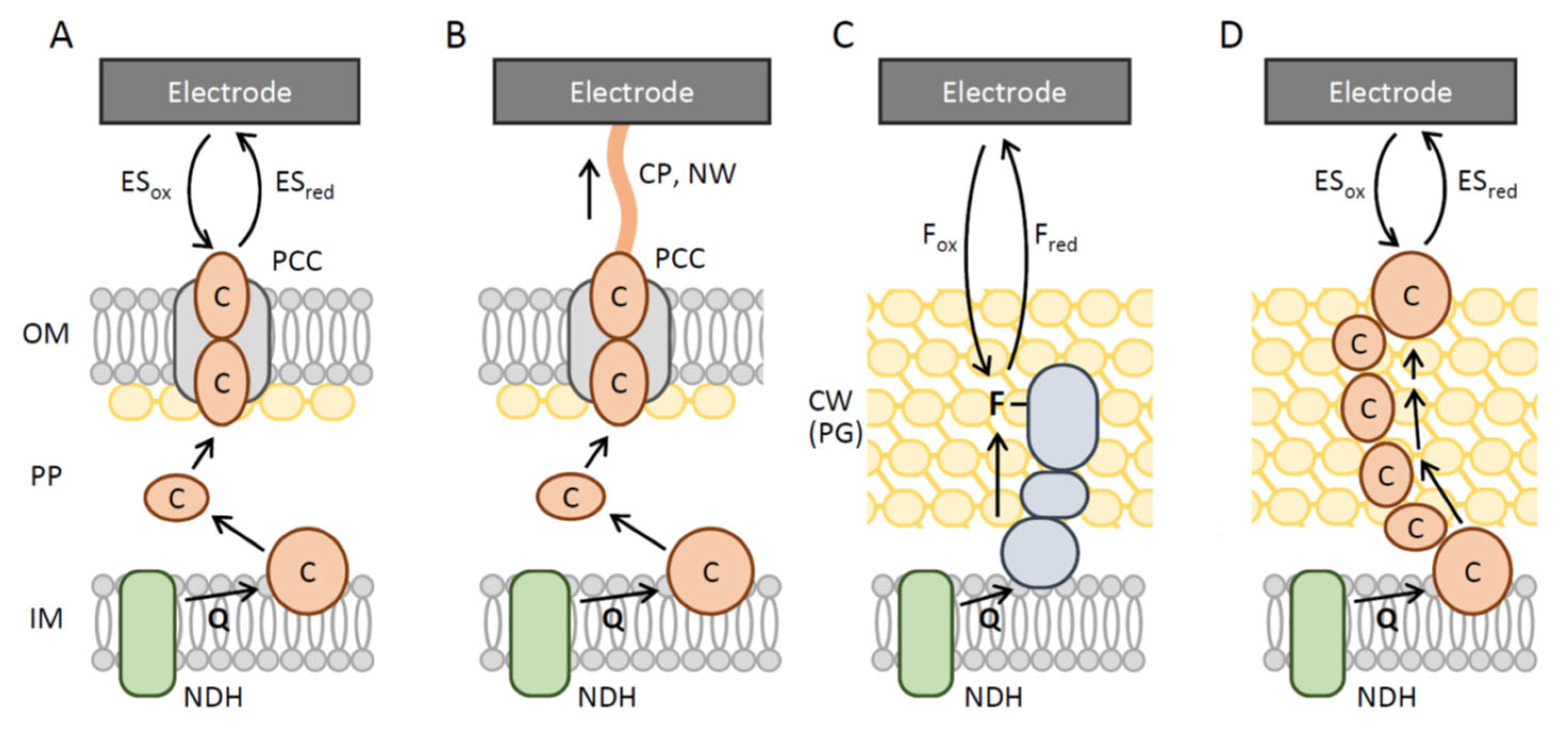
2. EAMs as Possible BA Agents
| Strain | Taxon | Habitat | Electrochemical Activity 1 | Description | Reference |
|---|---|---|---|---|---|
| ZH1 | Pseudomonas aeruginosa | Palm oil mill sludge | 451 mW m−2, 65 μA cm−2 | Power generation from oil sludge | [54] |
| 39E | Thermoanaerobacter pseudethanolicus | Hotspring | 0.6 mA cm−2 | Current generation from sugars at 60 °C | [55] |
| Z7 | Citrobacter freundii | Aerobic sewage sludge | 205 mW m−2, 0.1 mA cm−2 | Current generation from sugars using mediators | [56] |
| D-8 | Geobacter sulfurreducens | Rice paddy soil | 1.1 mA cm−2 | Grow on ethanol, glycerol and sugars | [57] |
| MK2 | Stenotrophomonas maltophilia | Groundwater/sludge | 27 μA cm−2 | Petrotrophic exoelectrogen | [58] |
| WE1-13 | Delftia | Deep aquifer | 0.4 μA cm−2 | Continental subsurface environments | [59] |
| WE2-4 | Azonexus | Deep aquifer | 350 nA cm−2 | Continental subsurface environments | [59] |
| SCS5 | Aeromonas jandaei | Wastewater sludge | 7 μA cm−2 | Isolated from acetate-fed MFC | [60] |
| WTL | Desulfuromonas soudanensis | Deep subsurface brine | 58 μA cm−2 | Halophilic iron-reducing bacterium | [61] |
| 6 | Clostridium amylolyticum | Soil | 29 mW m−2, 20 μA cm−2 | Fourteen other EAB were also isolated. | [62] |
| LAR-1 | Citrobacter braakii | River sediment | 610 mW/m−2, 0.2 mA cm−2 | Current generation in acetate-fed MFC | [63] |
| LZ-1 | Citrobacter freundii | Domestic sewage | 865 μA cm−2 | Facultative anaerobe | [64] |
| COM1 | Pyrococcus furiosus | Volcanic marine sediment | 0.2 mA cm−2 | Current generation at 90°C | [65] |
| JhA | Anaerosinus | Coastal sediment | 4 μA cm−2 | Coastal gold mining site | [66] |
| R6 | Clostridium sporogenes | Cu-contaminated soil | 25 mW m−2, 20 μA cm−2 | Resistant to 10 mg L−1 Cu2+ | [67] |
| RNV-4 | Dietzia | Intertidal zone sediment | 30 μA cm−2 | Aerobic growth in nutrient-rich media | [68] |
| KVM11 | Citrobacter | Groundwater/sludge | 36 μA cm−2 | Petrotrophic exoelectrogen | [69] |
| EB-1 | Mycobacterium fortuitum | Polluted river sediment | 0.84 W m−2, 0.2 mA cm−2 | Denitrifying bacterium | [70] |
| H | Bacillus cereus | Anaerobic digester | 32 mW m−2, 25 μA cm−2 | Isolated from Cr(VI)-reducing MFC | [71] |
| CL-1 | Geobacter sulfurreducens | Paddy soil | 1.0 mA cm−2 | Current generation from acetate and ethanol | [72] |
| ND-2 | Citrobacters | Rice paddy soil | 20 μA cm−2 | Isolated by electrode-plate cultivation | [73] |
| RPFA-12G | Geobacter sulfurreducens | Rice paddy soil | 0.2 mA cm−2 | Isolated by electrode-plate cultivation | [73] |
| AEDII12DO | Ferroglobus placidus | Hydrothermal system | 68 μA cm−2 | Archaeon, current generation at 85°C | [34] |
| 234 | Geoglobus ahangari | Hydrothermal system | 57 μA cm−2 | Archaeon, current generation at 80°C | [34] |
| 10403S | Listeria monocytogenes | Rabbit | 20 μA cm−2 | Use of flavin for EET | [42] |
| LLD-1 | Bacillus megaterium | Activated sludge | 40 μA cm−2 | Use of flavin for EET | [74] |
| S05 | Klebsiella quasipneumoniae | MBR treating wastewater | 0.07 mA cm−2 | Causative agent of membrane fouling | [75] |
| DIF1 | Bacillus cereus | MFC | 10 μA cm−2 | Use of flavin for EET | [76] |
| DIF2 | Rhodococcus ruber | MFC | 15 μA cm−2 | Use of flavin for EET | [76] |
| Gut-S1 | Enterococcus avium | Human feces | 120 nA cm−2 | Current generation from glucose | [77] |
| MCC 3673 | Kluyvera georgiana | Lake sediment | 39 μW cm−2 | Power generation from oil cakes | [78] |
| E8 | Pseudomonas protegens | Activated sludge | 70 mW m−2, 80 μA cm−2 | Potential for acid mine-drainage treatment | [79] |
| G7K4R3 | Clostridium cochlearium | Mouse gut | 0.53 mA cm−2 | Current generation from glucose | [80] |
| JSUX1 | Cystobasidium slooffiae | Activated sludge | 21 μW cm−2 | Eukaryote, current generation from Xylose | [35] |
| OR-1 | Shewanella algae | Costal sediment | 0.45 mA cm−2 | This strain generates current from acetate. | [53] |
| MA-72 | Paenibacillus dendritiformis | Freshwater sediment | 0.5 μA cm−2 | Current generation in peptone meat broth | [81] |
| EG | Alicyclobacillus hesperidum | Sewage treatment plant | 188 mW m−2, 45 μA cm−2 | Current generation under acidic conditions | [82] |
| YM18 | Geobacter sulfurreducens | River sediment | 1.1 mA cm−2 | YM18 generates more current than KN400 | [83] |
| NIT-T3 | Desulfuromonas versatilis | Coastal sand | 0.2 mA cm−2 | Isolated from graphene-reducing enrichment | [84] |
| GY3 | Lysinibacillus varians | Freshwater sediment | 7.5 μA cm−2 | Filamentous Gram-positive EAB | [85] |
| Isolate 1 | Klebsiella pneumoniae | Tropical lake sediment | 129 mW m−2, 0.07 mA cm−2 | Isolated using MnO2 plates | [86] |
| Isolate 2 | Agrobacterium salinitolerans | Tropical lake sediment | 51 mW m−2, 0.03 mA cm−2 | Isolated using MnO2 plates | [86] |
| Isolate 3 | Serratia nematodiphila | Tropical lake sediment | 23 mW m−2, 0.02 mA cm−2 | Isolated using MnO2 plates | [86] |
| Isolate 4 | Kosakonia oryzendophytica | Tropical lake sediment | 80 mW m−2, 0.05 mA cm−2 | Isolated using MnO2 plates | [86] |
| Isolate 5 | Enterobacter cloacae | Tropical lake sediment | 81 mW m−2, 0.04 mA cm−2 | Isolated using MnO2 plates | [86] |
| S116 | Pseudomonas stutzeri | Marine sludge | 765 mW m−2 | Marine sulfur-oxidizing bacterium | [87] |
| SAP-1 | Geoalkalibacter halelectricus | Lake sediment | 0.5 mA cm−2 | Current generation under haloalkaline conditions | [88] |
| CS-1 | Dechloromonas agitata | River sediment | −30 μA cm−2 | Preferentially generate cathodic current | [89] |
| CS-2 | Clostridium magenotii | River sediment | −10 μA cm−2 | Preferentially generate cathodic current | [89] |
| SHE10 | Shinella zoogloeoides | Sweet potato root | 78 mW m−2, 22 µA cm−2 | Plant endophytic EAB | [90] |
| EB1 | Paenibacillus lautus | Activated sludge | 1.6 μA cm−2 | Current generation from starch | [91] |
| DWW1 | Enterococcus faecalis | Dairy wastewater | 144 mW m−2, 26 µA cm−2 | Current generation from dairy wastewater | [92] |
| NTE-D12 | Cellulomonas | Soil | 0.9 μA cm−2 | Possibly represent a novel species | [93] |
| PBH03 | Pseudomonas aeruginosa | Anaerobic sludge | 9 µA cm−2 | Isolated using WO3 nanorod probes | [94] |
| NCIMB8826 | Lactiplantibacillus plantarum | Saliva | 20 μA cm−2 | Current generation in the presence of flavin | [95] |
| YoMME | Paenibacillus profundus | Freshwater sediment | 20 μA cm−2 | Current generation from peptone | [96] |
| A1 | Lysinibacillus sphaericus | Poultry dropping | 4 μA cm−2 | Current generation from poultry wastewater | [97] |
| AKS46 | Paraclostridium | Solid waste disposal site | 63 μA cm−2 | May have multiple electron-transfer routes | [98] |
| NIT-SL11 | Geotalea uranireducens | Municipal sewage | 0.7 mA cm−2 | Graphene oxide-reducing bacterium | [99] |
| 620C | Pseudomonas citronellolis | Drilling waste | 1 mW m−2, 3 μA cm−2 | Use of pyocyanin as an electron shuttle | [100] |
| SQ-1 | Klebsiella variicola | Activated sludge | 0.6 mA cm−2 | Facultative anaerobe | [101] |
| ADMFC1 | Eubacteriales | Anaerobic digester | 0.6 mA cm−2 | Possibly represent a novel family | [102] |
| ADMFC2 | Sulfurospirillaceae | Anaerobic digester | 0.2 mA cm−2 | Possibly represent a novel genus | [102] |
| ADMFC3 | Geovibrio | Anaerobic digester | 0.4 mA cm−2 | Possibly represent a novel species | [102] |
| 2Se | Brevundimonas diminuta | Activated sludge | 102 mW m−2, 52 µA cm−2 | Selenite reduction | [103] |
| AMB-1 | Magnetospirillum magneticum | Water pond | 27 µW m−2, 0.16 µA cm−2 | Magnetotactic bacterium | [104] |
| MSR-1 | Magnetospirillum gryphiswaldense | Wetland | 11 µW m−2, 0.11 µA cm−2 | Magnetotactic bacterium | [104] |
| KCf2 | Bacillus altitudinis | Aquaculture sediment | 67 mW m−2, 33 µA cm−2 | Current generation from glucose | [105] |
| YH-1 | Micrococcus | Metal-contaminated soil | 8.3 µA cm−2 | Halotolerant bacterium | [106] |
| RH-1 | Gordonia | Metal-contaminated soil | 2.2 µA cm−2 | Halotolerant bacterium | [106] |
| CH-1 | Stutzerimonas | Metal-contaminated soil | 1.7 µA cm−2 | Halotolerant bacterium | [106] |
| 60473 | Geobacter sulfurreducens | Lakeshore mud | 1.5 mA cm−2 | Useful in bioaugmentation | [23] |
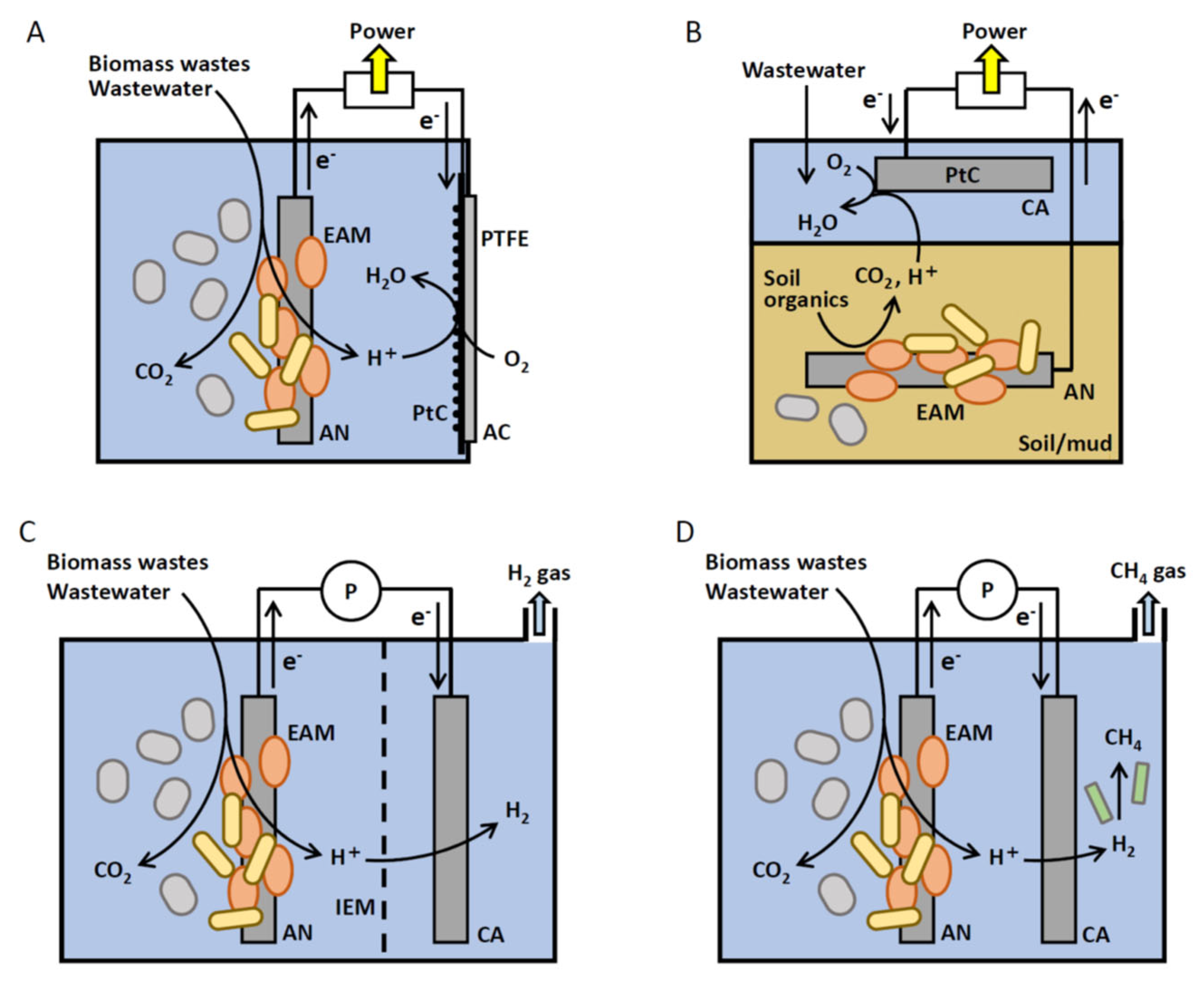
3. MET Processes and Their Performances
4. BA for Improving MET Processes
| Reactor Type 1 | Substrate | BA Agent | Electrochemical Performance 2 | Description | Reference | |
|---|---|---|---|---|---|---|
| Non-Bioaugmented | Bioaugmented | |||||
| MFC | ||||||
| DC | LB medium | Pseudomonas chlororaphis PCL1391 | 49 μA | 83 μA | Positive effects were observed only when the BA agent was contained in a slow-release tube. No power measuremet. | [137] |
| SC | Glucose | P. aeruginosa (P), Escherichia coli (E) | 45 mW m−2 | P: 230 mW m−2 E: 58 mW m−2 | BAs with EAB (P) and non-EAB (E) were compared in relatively short time periods (~60 h). | [134] |
| SC | Fermented molasses | Pseudomonas aeruginosa IIT BT SS1 | 255 mW m−2 | 281 mW m−2 | BA shortened start-up time of MFC. | [135] |
| CW | Textile dye wastewater | Electroactive bacterial community DC5 | 177 mW m−2 | 198 mW m−2 | BA improved dye degradation and COD removal. | [138] |
| SC | Wastewater | Rumen microbes | 634 mW·m−2 | 825 mW·m−2 | The BA agent improved cathode performance. | [139] |
| CW | Root exudates | G. sulfurreducens DL-1 | 2 mW·m−2 | Day 5: 91 mW·m−2 Day 30: 18 mW·m−2 | The BA agent was injected to carbon-fibers anodes in sediment using sterile syringes. | [136] |
| CW | Haloxyfop-P-methyl (HM) and soil organics | Myrothecium verrucaria(fungus) | 5.7 mW·m−2 | 11.7 mW·m−2 | BA slightly improved HM degradation. | [140] |
| SC | Food waste | Geobacter sulfurreducens 60473 | 168 mW m−2 | 1760 mW m−2 | Positive effects were observed even one month after BA, when Geobacter shared 40% of anode bacteria. | [141] |
| MEC | ||||||
| CW | Sediment organics | Acidimicrobiaceae sp. A6 | 3 mA m−2 | 7 mA m−2 | Current increased after constructed wetland microcosm MECs were augmented with A6. | [142] |
| SC | Acetate | G. sulfurreducens PCA | 0.012 mA cm−2 | 0.064 mA cm−2 | Positive effects were observed only when the BA agent was encapsulated around anodes. | [143] |
| DC | Starch and yeast extract | G. sulfurreducens YM18 | 3.6 mA | 13.3 mA | BA MEC generated sixfold more hydrogen than non-bioaugmented MEC. | [22] |
| SC | Acetate | G. sulfurreducens PCA | 0.03 mA cm−2 | 0.27 mA cm−2 | An anode was put in a dialysis bag, in which the BA agent was introduced along with graphite. | [144] |
| SC | Starch and yeast extract | G. sulfurreducens PCA, YM18, 60473 | 0.020 mA cm−2 | PCA: 0.014 mA cm−2 YM18: 1.0 mA cm−2 60473: 1.6 mA cm−2 | BA agents largely affect the effectiveness of BA. Hydrophobic cells of 60473 facilitate the colonization on anodes. | [23] |
5. Factors That Need to Be Considered for Successful BA
6. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | Bioaugmentation |
| EAM | Electroactive microbe |
| MET | Microbial electrochemical technology |
| EET | Extracellular electron transfer |
| MFC | Microbial fuel cell |
| MEC | Microbial electrolysis cell |
| EC | Electrochemical cell |
| PCC | Porin/cytochrome complex |
| IEM | Ion-exchange membranes |
| PTFE | Polytetrafluoroethylene |
| PtC | Platinum/carbon catalyst |
| AD | Anaerobic digester |
| 3D | Three-dimensional |
References
- Abas, N.; Kalair, A.; Khan, N. Review of Fossil Fuels and Future Energy Technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Al-Ghussain, L. Global Warming: Review on Driving Forces and Mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Srirangan, K.; Akawi, L.; Moo-Young, M.; Chou, C.P. Towards Sustainable Production of Clean Energy Carriers from Biomass Resources. Appl. Energy 2012, 100, 172–186. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Bonilla-Petriciolet, A.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Emerging Technologies for Biofuel Production: A Critical Review on Recent Progress, Challenges and Perspectives. J. Environ. Manag. 2021, 290, 112627. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Baredar, P.; Shukla, A. A Review on Biomass Energy Resources, Potential, Conversion and Policy in India. Renew. Sustain. Energy Rev. 2015, 45, 530–539. [Google Scholar] [CrossRef]
- Subbarao, P.M.; D’Silva, T.C.; Adlak, K.; Kumar, S.; Chandra, R.; Vijay, V.K. Anaerobic Digestion as a Sustainable Technology for Efficiently Utilizing Biomass in the Context of Carbon Neutrality and Circular Economy. Environ. Res. 2023, 234, 116286. [Google Scholar] [CrossRef]
- Hyun, C.T.; Ranjan, D.B. A Multi-Perspective Review on Microbial Electrochemical Technologies for Food Waste Valorization. Bioresour. Technol. 2021, 342, 125950. [Google Scholar] [CrossRef]
- Harnisch, F.; Schröder, U. From MFC to MXC: Chemical and Biological Cathodes and Their Potential for Microbial Bioelectrochemical Systems. Chem. Soc. Rev. 2010, 39, 4433–4448. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. A Comprehensive Review of Microbial Electrochemical Systems as a Platform Technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial Fuel Cell (MFC) Power Performance Improvement through Enhanced Microbial Electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327. [Google Scholar] [CrossRef]
- Javed, M.M.; Nisar, M.A.; Ahmad, M.U.; Yasmeen, N.; Zahoor, S. Microbial Fuel Cells as an Alternative Energy Source: Current Status. Biotechnol. Genet. Eng. Rev. 2018, 34, 216–242. [Google Scholar] [CrossRef]
- Cai, T.; Meng, L.; Chen, G.; Xi, Y.; Jiang, N.; Song, J.; Zheng, S.; Liu, Y.; Zhen, G.; Huang, M. Application of Advanced Anodes in Microbial Fuel Cells for Power Generation: A Review. Chemosphere 2020, 248, 125985. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Mier, A.A.; Olvera-Vargas, H.; Mejía-López, M.; Longoria, A.; Verea, L.; Sebastian, P.J.; Arias, D.M. A Review of Recent Advances in Electrode Materials for Emerging Bioelectrochemical Systems: From Biofilm-Bearing Anodes to Specialized Cathodes. Chemosphere 2021, 283, 131138. [Google Scholar] [CrossRef]
- Agrahari, R.; Bayar, B.; Abubackar, H.N.; Giri, B.S.; Rene, E.R.; Rani, R. Advances in the Development of Electrode Materials for Improving the Reactor Kinetics in Microbial Fuel Cells. Chemosphere 2022, 290, 133184. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive Microorganisms in Bioelectrochemical Systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E. Electromicrobiology: The Ecophysiology of Phylogenetically Diverse Electroactive Microorganisms. Nat. Rev. Microbiol. 2022, 20, 5–19. [Google Scholar] [CrossRef]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.E. Overview of Electroactive Microorganisms and Electron Transfer Mechanisms in Microbial Electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Wade, M.J.; Sloan, W.T.; Quince, C.; Curtis, T.P. Temperature, Inocula and Substrate: Contrasting Electroactive Consortia, Diversity and Performance in Microbial Fuel Cells. Bioelectrochemistry 2018, 119, 43–50. [Google Scholar] [CrossRef]
- Flayac, C.; Trably, E.; Bernet, N. Microbial Anodic Consortia Fed with Fermentable Substrates in Microbial Electrolysis Cells: Significance of Microbial Structures. Bioelectrochemistry 2018, 123, 219–226. [Google Scholar] [CrossRef]
- Salar-Garcia, M.J.; Obata, O.; Kurt, H.; Chandran, K.; Greenman, J.; Ieropoulos, I.A. Impact of Inoculum Type on the Microbial Community and Power Performance of Urine-Fed Microbial Fuel Cells. Microorganisms 2020, 8, 1921. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, I.; Harada, T.; Jomori, S.; Kouzuma, A.; Watanabe, K. Bioaugmentation of Microbial Electrolysis Cells with Geobacter sulfurreducens YM18 for Enhanced Hydrogen Production from Starch. Bioresour. Technol. 2023, 386, 129508. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Yamada, Y.; Toda, M.; Takamatsu, Y.; Tomita, K.; Inoue, K.; Kouzuma, A.; Watanabe, K. Geobacter sulfurreducens Strain 60473, a Potent Bioaugmentation Agent for Improving the Performances of Bioelectrochemical Systems. J. Biosci. Bioeng. 2025, 139, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.O.; Fufeyin, P.T.; Okoro, S.E.; Ehinomen, I. Bioremediation, Biostimulation and Bioaugmentation: A Review. Int. J. Environ. Bioremediation Biodegrad. 2015, 3, 28–39. [Google Scholar] [CrossRef]
- Chettri, D.; Verma, A.K.; Verma, A.K. Bioaugmentation: An Approach to Biological Treatment of Pollutants. Biodegradation 2024, 35, 117–135. [Google Scholar] [CrossRef]
- Nzila, A.; Razzak, S.A.; Zhu, J. Bioaugmentation: An Emerging Strategy of Industrial Wastewater Treatment for Reuse and Discharge. Int. J. Environ. Res. Public Health 2016, 13, 846. [Google Scholar] [CrossRef]
- Nzila, A. Mini Review: Update on Bioaugmentation in Anaerobic Processes for Biogas Production. Anaerobe 2017, 46, 3–12. [Google Scholar] [CrossRef]
- Li, C.; Lü, F.; Peng, W.; He, P.; Zhang, H. Efficacy of Bioaugmentation with Nondomesticated Mixed Microbial Consortia under Ammonia Inhibition in Anaerobic Digestion. Bioresour. Technol. 2024, 391, 129954. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, H.J.; Hyun, M.S.; Park, D.H. Direct Electrode Reaction of Fe(III)-Reducing Bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 1999, 9, 127–131. [Google Scholar]
- Bond, D.R.; Lovley, D.R. Electricity Production by Geobacter sulfurreducens Attached to Electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial Phenazine Production Enhances Electron Transfer in Biofuel Cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Ellington, A.D. Optimization of the Biological Component of a Bioelectrochemical Cell. Bioelectrochemistry 2007, 70, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.W.; May, H.D. Electrochemical Evidence of Direct Electrode Reduction by a Thermophilic Gram-Positive Bacterium, Thermincola ferriacetica. Energy Environ. Sci. 2009, 2, 699–705. [Google Scholar] [CrossRef]
- Yilmazel, Y.D.; Zhu, X.; Kim, K.Y.; Holmes, D.E.; Logan, B.E. Electrical Current Generation in Microbial Electrolysis Cells by Hyperthermophilic Archaea Ferroglobus placidus and Geoglobus ahangari. Bioelectrochemistry 2018, 119, 142–149. [Google Scholar] [CrossRef]
- Moradian, J.M.; Xu, Z.A.; Shi, Y.T.; Fang, Z.; Yong, Y.C. Efficient Biohydrogen and Bioelectricity Production from Xylose by Microbial Fuel Cell with Newly Isolated Yeast Cystobasidium slooffiae. Int. J. Energy Res. 2020, 44, 325–333. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, V. Recent Trends in Upgrading the Performance of Yeast as Electrode Biocatalyst in Microbial Fuel Cells. Chemosphere 2021, 284, 131383. [Google Scholar] [CrossRef]
- Verma, M.; Singh, V.; Mishra, V. Moving Towards the Enhancement of Extracellular Electron Transfer in Electrogens. World J. Microbiol. Biotechnol. 2023, 39, 130. [Google Scholar] [CrossRef]
- Burton, J.A.J.; Edwards, M.J.; Richardson, D.J.; Clarke, T.A. Electron Transport Across Bacterial Cell Envelopes. Annu. Rev. Biochem. 2025, in press. [Google Scholar] [CrossRef]
- Paquete, C.M. Electroactivity Across the Cell Wall of Gram-Positive Bacteria. Comput. Struct. Biotechnol. J. 2020, 18, 3796–3802. [Google Scholar] [CrossRef]
- Carlson, H.K.; Iavarone, A.T.; Gorur, A.; Yeo, B.S.; Tran, R.; Melnyk, R.A.; Mathies, R.A.; Auer, M.; Coates, J.D. Surface Multiheme c-Type Cytochromes from Thermincola potens and Implications for Respiratory Metal Reduction by Gram-Positive Bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 1702–1707. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kasai, T.; Hirose, A.; Watanabe, K. Catabolic and Regulatory Systems in Shewanella oneidensis MR-1 Involved in Electricity Generation in Microbial Fuel Cells. Front. Microbiol. 2015, 6, 609. [Google Scholar] [CrossRef] [PubMed]
- Light, S.H.; Su, L.; Rivera-Lugo, R.; Cornejo, J.A.; Louie, A.; Iavarone, A.T.; Ajo-Franklin, C.M.; Portnoy, D.A. A Flavin-Based Extracellular Electron Transfer Mechanism in Diverse Gram-Positive Bacteria. Nature 2018, 562, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Wrighton, K.C.; Thrash, J.C.; Melnyk, R.A.; Bigi, J.P.; Byrne-Bailey, K.G.; Remis, J.P.; Schichnes, D.; Auer, M.; Chang, C.J.; Coates, J.D. Evidence for Direct Electron Transfer by a Gram-Positive Bacterium Isolated from a Microbial Fuel Cell. Appl. Environ. Microbiol. 2011, 77, 7633–7639. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, J.K.; Romine, M.F.; Beliaev, A.S.; Auchtung, J.M.; Driscoll, M.E.; Gardner, T.S.; Nealson, K.H.; Osterman, A.L.; Pinchuk, G.; Reed, J.L.; et al. Towards Environmental Systems Biology of Shewanella. Nat. Rev. Microbiol. 2008, 6, 592–603. [Google Scholar] [CrossRef]
- Hirose, A.; Kouzuma, A.; Watanabe, K. Towards Development of Electrogenetics Using Electrochemically Active Bacteria. Biotechnol. Adv. 2019, 37, 107351. [Google Scholar] [CrossRef]
- Ikeda, S.; Takamatsu, Y.; Tsuchiya, M.; Suga, K.; Tanaka, Y.; Kouzuma, A.; Watanabe, K. Shewanella oneidensis MR-1 as a Bacterial Platform for Electro-Biotechnology. Essays Biochem. 2021, 65, 355–364. [Google Scholar] [CrossRef]
- Chen, S.; Ding, Y. A Bibliography Study of Shewanella oneidensis Biofilm. FEMS Microbiol. Ecol. 2023, 99, fiad124. [Google Scholar] [CrossRef]
- Venkateswaran, K.; Moser, D.P.; Dollhopf, M.E.; Lies, D.P.; Saffarini, D.A.; MacGregor, B.J.; Ringelberg, D.B.; White, D.C.; Nishijima, M.; Sano, H.; et al. Polyphasic Taxonomy of the Genus Shewanella and Description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 1999, 49, 705–724. [Google Scholar] [CrossRef]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella Secretes Flavins That Mediate Extracellular Electron Transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef]
- Newman, D.K.; Kolter, R. A Role for Excreted Quinones in Extracellular Electron Transfer. Nature 2000, 405, 94–97. [Google Scholar] [CrossRef]
- Edwards, M.J.; Hall, A.; Shi, L.; Fredrickson, J.K.; Zachara, J.M.; Butt, J.N.; Richardson, D.J.; Clarke, T.A. The Crystal Structure of the Extracellular 11-Heme Cytochrome UndA Reveals a Conserved 10-Heme Motif and Defined Binding Site for Soluble Iron Chelates. Structure 2012, 20, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Hirose, A.; Kasai, T.; Aoki, M.; Umemura, T.; Watanabe, K.; Kouzuma, A. Electrochemically Active Bacteria Sense Electrode Potentials for Regulating Catabolic Pathways. Nat. Commun. 2018, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Inohana, Y.; Katsuya, S.; Koga, R.; Kouzuma, A.; Watanabe, K. Shewanella algae Relatives Capable of Generating Electricity from Acetate Contribute to Coastal-Sediment Microbial Fuel Cells Treating Complex Organic Matter. Microbes Environ. 2020, 35, ME19161. [Google Scholar] [CrossRef]
- Nor, M.H.; Mubarak, M.F.; Elmi, H.S.; Ibrahim, N.; Wahab, M.F.; Ibrahim, Z. Bioelectricity Generation in Microbial Fuel Cell Using Natural Microflora and Isolated Pure Culture Bacteria from Anaerobic Palm Oil Mill Effluent Sludge. Bioresour. Technol. 2015, 190, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Lusk, B.G.; Khan, Q.F.; Parameswaran, P.; Hameed, A.; Ali, N.; Rittmann, B.E.; Torres, C.I. Characterization of Electrical Current-Generation Capabilities from Thermophilic Bacterium Thermoanaerobacter pseudethanolicus Using Xylose, Glucose, Cellobiose, or Acetate with Fixed Anode Potentials. Environ. Sci. Technol. 2015, 49, 14725–14731. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, N.; Cao, Y.; Peng, Y.; Wu, P.; Dong, W. Exoelectrogenic Bacterium Phylogenetically Related to Citrobacter freundii, Isolated from Anodic Biofilm of a Microbial Fuel Cell. Appl. Biochem. Biotechnol. 2015, 175, 1879–1891. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Liu, Y. A Geobacter Strain Isolated from Rice Paddy Soil with Higher Bioelectricity Generation Capability in Comparison to Geobacter sulfurreducens PCA. RSC Adv. 2015, 5, 43978–43989. [Google Scholar] [CrossRef]
- Venkidusamy, K.; Megharaj, M. Identification of Electrode Respiring, Hydrocarbonoclastic Bacterial Strain Stenotrophomonas maltophilia MK2 Highlights the Untapped Potential for Environmental Bioremediation. Front. Microbiol. 2016, 7, 1965. [Google Scholar] [CrossRef]
- Jangir, Y.; French, S.; Momper, L.M.; Moser, D.P.; Amend, J.P.; El-Naggar, M.Y. Isolation and Characterization of Electrochemically Active Subsurface Delftia and Azonexus Species. Front. Microbiol. 2016, 7, 756. [Google Scholar] [CrossRef]
- Sharma, S.C.; Feng, C.; Li, J.; Hu, A.; Wang, H.; Qin, D.; Yu, C.P. Electrochemical Characterization of a Novel Exoelectrogenic Bacterium Strain SCS5, Isolated from a Mediator-Less Microbial Fuel Cell and Phylogenetically Related to Aeromonas jandaei. Microbes Environ. 2016, 31, 213–225. [Google Scholar] [CrossRef]
- Badalamenti, J.P.; Summers, Z.M.; Chan, C.H.; Gralnick, J.A.; Bond, D.R. Isolation and Genomic Characterization of Desulfuromonas soudanensis WTL, a Metal- and Electrode-Respiring Bacterium from Anoxic Deep Subsurface Brine. Front. Microbiol. 2016, 7, 913. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.B.; Zhong, W.H.; Han, C.; Deng, H. Characterization of Electricity Generated by Soil in Microbial Fuel Cells and the Isolation of Soil Source Exoelectrogenic Bacteria. Front. Microbiol. 2016, 7, 1776. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lee, D.J.; Wang, A.; Ren, N.; Su, A.; Lai, J.Y. Isolation of Fe(III)-Reducing Bacterium, Citrobacter sp. LAR-1, for Startup of Microbial Fuel Cell. Int. J. Hydrogen Energy 2016, 41, 4498–4503. [Google Scholar] [CrossRef]
- Zhou, L.; Deng, D.; Zhang, Y.; Zhou, W.; Jiang, Y.; Liu, Y. Isolation of a Facultative Anaerobic Exoelectrogenic Strain LZ-1 and Probing Electron Transfer Mechanism In Situ by Linking UV/Vis Spectroscopy and Electrochemistry. Biosens. Bioelectron. 2017, 90, 264–268. [Google Scholar] [CrossRef]
- Sekar, N.; Wu, C.H.; Adams, M.W.W.; Ramasamy, R.P. Electricity Generation by Pyrococcus furiosus in Microbial Fuel Cells Operated at 90 °C. Biotechnol. Bioeng. 2017, 114, 1419–1427. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, B.; Li, Y.; Liu, F.; Wang, O. Electrochemically Active Iron (III)-Reducing Bacteria in Coastal Riverine Sediments. J. Basic Microbiol. 2017, 57, 1045–1054. [Google Scholar] [CrossRef]
- Deng, H.; Xue, H.; Zhong, W. A Novel Exoelectrogenic Bacterium Phylogenetically Related to Clostridium sporogenes Isolated from Copper Contaminated Soil. Electroanalysis 2017, 29, 1294–1300. [Google Scholar] [CrossRef]
- Sacco, N.J.; Bonetto, M.C.; Cortón, E. Isolation and Characterization of a Novel Electrogenic Bacterium, Dietzia sp. RNV-4. PLoS ONE 2017, 12, e0169955. [Google Scholar] [CrossRef]
- Venkidusamy, K.; Hari, A.R.; Megharaj, M. Petrophilic, Fe(III)-Reducing Exoelectrogen Citrobacter sp. KVM11, Isolated from Hydrocarbon-Fed Microbial Electrochemical Remediation Systems. Front. Microbiol. 2018, 9, 349. [Google Scholar] [CrossRef]
- Jin, X.; Guo, F.; Liu, Z.; Liu, Y.; Liu, H. Enhancing the Electricity Generation and Nitrate Removal of Microbial Fuel Cells with a Novel Denitrifying Exoelectrogenic Strain EB-1. Front. Microbiol. 2018, 9, 2633. [Google Scholar] [CrossRef]
- Wu, X.; Ren, X.; Owens, G.; Brunetti, G.; Zhou, J.; Yong, X.; Wei, P.; Jia, H. A Facultative Electroactive Chromium(VI)-Reducing Bacterium Aerobically Isolated from a Biocathode Microbial Fuel Cell. Front. Microbiol. 2018, 9, 2883. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, Y.; Liu, Z.; Ma, Y.; Kang, J.; Liu, Y. Isolation and Characterization of an Exoelectrogenic Strain CL-1 from Soil and Electron Transfer Mechanism by Linking Electrochemistry and Spectroscopy. Electrochim. Acta 2018, 292, 982–989. [Google Scholar] [CrossRef]
- Ueoka, N.; Kouzuma, A.; Watanabe, K. Electrode Plate-Culture Methods for Colony Isolation of Exoelectrogens from Anode Microbiomes. Bioelectrochemistry 2018, 124, 1–6. [Google Scholar] [CrossRef] [PubMed]
- You, L.X.; Liu, L.D.; Xiao, Y.; Dai, Y.F.; Chen, B.L.; Jiang, Y.X.; Zhao, F. Flavins Mediate Extracellular Electron Transfer in Gram-Positive Bacillus megaterium Strain LLD-1. Bioelectrochemistry 2018, 119, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, S.; Papry, R.I.; Miyake, H.; Narita, Y.; Okabe, S. Membrane Fouling Potentials of an Exoelectrogenic Fouling-Causing Bacterium Cultured with Different External Electron Acceptors. Front. Microbiol. 2019, 9, 3284. [Google Scholar] [CrossRef]
- Tian, T.; Fan, X.; Feng, M.; Su, L.; Zhang, W.; Chi, H.; Fu, D. Flavin-Mediated Extracellular Electron Transfer in Gram-Positive Bacteria Bacillus cereus DIF1 and Rhodococcus ruber DIF2. RSC Adv. 2019, 9, 40903–40909. [Google Scholar] [CrossRef]
- Naradasu, D.; Miran, W.; Sakamoto, M.; Okamoto, A. Isolation and Characterization of Human Gut Bacteria Capable of Extracellular Electron Transport by Electrochemical Techniques. Front. Microbiol. 2019, 9, 3267. [Google Scholar] [CrossRef]
- Thapa, B.S.; Chandra, T.S. Kluyvera georgiana MCC 3673: A Novel Electrogen Enriched in Microbial Fuel Cell Fed with Oilseed Cake. Curr. Microbiol. 2019, 76, 650–657. [Google Scholar] [CrossRef]
- Ai, C.; Hou, S.; Yan, Z.; Zheng, X.; Amanze, C.; Chai, L.; Qiu, G.; Zeng, W. Recovery of Metals from Acid Mine Drainage by Bioelectrochemical System Inoculated with a Novel Exoelectrogen, Pseudomonas sp. E8. Microorganisms 2019, 8, 41. [Google Scholar] [CrossRef]
- Schwab, L.; Rago, L.; Koch, C.; Harnisch, F. Identification of Clostridium cochlearium as an Electroactive Microorganism from the Mouse Gut Microbiome. Bioelectrochemistry 2019, 130, 107334. [Google Scholar] [CrossRef]
- Hubenova, Y.; Hubenova, E.; Mitov, M. Electroactivity of the Gram-Positive Bacterium Paenibacillus dendritiformis MA-72. Bioelectrochemistry 2020, 136, 107632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Xia, T.; Wang, X. Characterization of a New Electrochemically Active Bacterium Phylogenetically Related to Alicyclobacillus hesperidum and Its Electrochemical Performance in Microbial Fuel Cell. Biosens. Bioelectron. 2021, 175, 112865. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Ogura, Y.; Ishigami, K.; Kawano, Y.; Nagamine, M.; Hayashi, T.; Inoue, K. Unexpected Genomic Features of High Current Density-Producing Geobacter sulfurreducens Strain YM18. FEMS Microbiol. Lett. 2021, 368, fnab119. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yoshida, N.; Ishii, S.; Meng, L. Isolation and Polyphasic Characterization of Desulfuromonas versatilis sp. nov., an Electrogenic Bacteria Capable of Versatile Metabolism Isolated from a Graphene Oxide-Reducing Enrichment Culture. Microorganisms 2021, 9, 1953. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Gan, C.; Klausen, L.H.; Bonné, R.; Kong, G.; Luo, D.; Meert, M.; Zhu, C.; Sun, G.; et al. Long-Distance Electron Transfer in a Filamentous Gram-Positive Bacterium. Nat. Commun. 2021, 12, 1709. [Google Scholar] [CrossRef]
- Nazeer, Z.; Fernando, E.Y. A novel Growth and Isolation Medium for Exoelectrogenic Bacteria. Enzym. Microb. Technol. 2022, 155, 109995. [Google Scholar] [CrossRef]
- Li, P.; Yuan, W.; Huang, Y.; Zhang, C.; Ni, C.; Lin, Q.; Zhu, Z.; Wang, J. Complete Genome Sequence of Pseudomonas stutzeri S116 Owning Bifunctional Catalysis Provides Insights into Affecting Performance of Microbial Fuel Cells. BMC Microbiol. 2022, 22, 137. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, R.; Sundharam, S.S.; Chaudhary, S.; Krishnamurthi, S.; Patil, S.A. Geoalkalibacter halelectricus SAP-1 sp. nov. Possessing Extracellular Electron Transfer and Mineral-Reducing Capabilities from a Haloalkaline Environment. Environ. Microbiol. 2022, 24, 5066–5081. [Google Scholar] [CrossRef]
- Torres-Rojas, F.; Muñoz, D.; Pía Canales, C.; Vargas, I.T. Bioprospecting for Electrochemically Active Perchlorate-Reducing Microorganisms. Bioelectrochemistry 2022, 147, 108171. [Google Scholar] [CrossRef]
- Ling, L.; Luo, H.; Li, Z.; Yang, C.; Pang, M.; Tu, Y.; Cheng, W.; Jiang, K.; Lu, L. Isolation, Identification and Characteristic Analysis of Plant Endophyte Electrogenic Bacteria Shinella zoogloeoides SHE10. Curr. Microbiol. 2022, 79, 268. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Zhen, S.H.; Chao, S.L.; Wu, J.; Cheng, L.; Li, S.W.; Xiao, X.; Zhou, X. Electrochemistry of Newly Isolated Gram-Positive Bacteria Paenibacillus lautus with Starch as Sole Carbon Source. Electrochim. Acta 2022, 411, 140068. [Google Scholar] [CrossRef]
- Parihar, P.S.; Keshavkant, S.; Jadhav, S.K. Electrogenic Potential of Enterococcus faecalis DWW1 Isolated from the Anodic Biofilm of a Dairy Wastewater Fed Dual Chambered Microbial Fuel Cell. J. Water Process Eng. 2022, 45, 102503. [Google Scholar] [CrossRef]
- Ihara, S.; Wakai, S.; Maehara, T.; Okamoto, A. Electrochemical Enrichment and Isolation of Electrogenic Bacteria from 0.22 µm Filtrate. Microorganisms 2022, 10, 2051. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, H.; Mutyala, S.; Kim, M.; Eun Song, Y.; Li, S.; Jang, M.; Oh, S.E.; Kim, J.R. Colorimetric Isolation of a Novel Electrochemically Active Pseudomonas Strain Using Tungsten Nanorods for Bioelectrochemical Applications. Bioelectrochemistry 2022, 146, 108136. [Google Scholar] [CrossRef]
- Tejedor-Sanz, S.; Stevens, E.T.; Li, S.; Finnegan, P.; Nelson, J.; Knoesen, A.; Light, S.H.; Ajo-Franklin, C.M.; Marco, M.L. Extracellular Electron Transfer Increases Fermentation in Lactic Acid Bacteria via a Hybrid Metabolism. eLife 2022, 11, e70684. [Google Scholar] [CrossRef]
- Hubenova, Y.; Borisov, G.; Slavcheva, E.; Mitov, M. Gram-Positive Bacteria Covered Bioanode in a Membrane-Electrode Assembly for Use in Bioelectrochemical Systems. Bioelectrochemistry 2022, 144, 108011. [Google Scholar] [CrossRef]
- Temirbekova, A.; Tekebayeva, Z.; Temirkhanov, A.; Yevneyeva, D.; Sadykov, A.; Meiramkulova, K.; Mkilima, T.; Abzhalelov, A. Isolation and Characterization of Bacteria with High Electroactive Potential from Poultry Wastewater. Biology 2023, 12, 623. [Google Scholar] [CrossRef]
- Basu, A.; Manna, S.; Sil, A.K. A New Electro-Active Bacterium, Paraclostridium sp. AKS46, Converts Waste Efficiently into Electricity in Microbial Fuel Cell. Chem. Eng. J. 2023, 475, 145626. [Google Scholar] [CrossRef]
- Xie, L.; Yoshida, N.; Meng, L. Polyphasic Characterization of Geotalea uranireducens NIT-SL11 Newly Isolated from a Complex of Sewage Sludge and Microbially Reduced Graphene Oxide. Microorganisms 2023, 11, 349. [Google Scholar] [CrossRef]
- Varnava, C.K.; Persianis, P.; Ieropoulos, I.; Tsipa, A. Electricity Generation and Real Oily Wastewater Treatment by Pseudomonas citronellolis 620C in a Microbial Fuel Cell: Pyocyanin Production as Electron Shuttle. Bioprocess Biosyst. Eng. 2024, 47, 903–917. [Google Scholar] [CrossRef]
- Zhou, L.; Tang, T.; Deng, D.; Wang, Y.; Pei, D. Isolation and Electrochemical Analysis of a Facultative Anaerobic Electrogenic Strain Klebsiella sp. SQ-1. Pol. J. Microbiol. 2024, 73, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Yoshizu, D.; Shimizu, S.; Tsuchiya, M.; Tomita, K.; Kouzuma, A.; Watanabe, K. Isolation of Electrochemically Active Bacteria from an Anaerobic Digester Treating Food Waste and Their Characterization. Microorganisms 2024, 12, 1645. [Google Scholar] [CrossRef] [PubMed]
- Sakr, E.A.; Khater, D.Z.; El Khatib, K.M. Electroactive Brevundimonas diminuta Consortium Mediated Selenite Bioreduction, Biogenesis of Selenium Nanoparticles and Bio-Electricity Generation. J. Nanobiotechnol. 2024, 22, 352. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.; Su, Q.; Jensen, M.M.; Zhang, Y. Electroactivity of the Magnetotactic Bacteria Magnetospirillum magneticum AMB-1 and Magnetospirillum gryphiswaldense MSR-1. Front. Environ. Sci. Eng. 2024, 18, 48. [Google Scholar] [CrossRef]
- Indriyani, Y.A.; Rusmana, I.; Anwar, S.; Djajakirana, G.; Santosa, D.A. Bioelectrochemical Assessment of a Novel Electrogenic Bacillus altitudinis AC11.2 for Electricity Generation in Microbial Fuel Cell (MFC) System. J. Appl. Electrochem. 2024, 54, 977–997. [Google Scholar] [CrossRef]
- Mukherjee, D.; Doyle, L.E. Electrochemical Enrichment of a Community of Weak Electricigens and Characterisation of Three Halotolerant Electroactive Isolates: Micrococcus sp. YH-1, Gordonia sp. RH-1 and Stutzerimonas sp. CH-1. Electrochim. Acta 2025, 510, 145350. [Google Scholar] [CrossRef]
- Caccavo, F., Jr.; Lonergan, D.J.; Lovley, D.R.; Davis, M.; Stolz, J.F.; McInerney, M.J. Geobacter sulfurreducens sp. nov., a Hydrogen- and Acetate-Oxidizing Dissimilatory Metal-Reducing Microorganism. Appl. Environ. Microbiol. 1994, 60, 3752–3759. [Google Scholar] [CrossRef]
- Lovley, D.R.; Ueki, T.; Zhang, T.; Malvankar, N.S.; Shrestha, P.M.; Flanagan, K.A.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.E.; et al. Geobacter: The Microbe Electric’s Physiology, Ecology, and Practical Applications. Adv. Microb. Physiol. 2011, 59, 1–100. [Google Scholar] [CrossRef]
- Ueki, T. Cytochromes in Extracellular Electron Transfer in Geobacter. Appl. Environ. Microbiol. 2021, 87, e03109-20. [Google Scholar] [CrossRef]
- Lovley, D.R.; Walker, D.J.F. Geobacter Protein Nanowires. Front. Microbiol. 2019, 10, 2078. [Google Scholar] [CrossRef]
- Liu, X.; Walker, D.J.F.; Nonnenmann, S.S.; Sun, D.; Lovley, D.R. Direct Observation of Electrically Conductive Pili Emanating from Geobacter sulfurreducens. mBio 2021, 12, e02209-21. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. Influence of Anode Potentials on Current Generation and Extracellular Electron Transfer Paths of Geobacter Species. Int. J. Mol. Sci. 2017, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Nevin, K.P.; Kim, B.C.; Franks, A.E.; Klimes, A.; Tender, L.M.; Lovley, D.R. Selection of a Variant of Geobacter sulfurreducens with Enhanced Capacity for Current Production in Microbial Fuel Cells. Biosens. Bioelectron. 2009, 24, 3498–3503. [Google Scholar] [CrossRef] [PubMed]
- Ravindhiran, R.; Sivarajan, K.; Sekar, J.N.; Murugesan, R.; Dhandapani, K. Listeria monocytogenes, an Emerging Pathogen: A Comprehensive Overview on Listeriosis, Virulence Determinants, Detection, and Anti-Listerial Interventions. Microb. Ecol. 2023, 86, 2231–2251. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. Biomed. Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Parameswaran, P.; Bry, T.; Popat, S.C.; Lusk, B.G.; Rittmann, B.E.; Torres, C.I. Kinetic, Electrochemical, and Microscopic Characterization of the Thermophilic, Anode-Respiring Bacterium Thermincola ferriacetica. Environ. Sci. Technol. 2013, 47, 4934–4940. [Google Scholar] [CrossRef]
- Faustino, M.M.; Fonseca, B.M.; Costa, N.L.; Lousa, D.; Louro, R.O.; Paquete, C.M. Crossing the Wall: Characterization of the Multiheme Cytochromes Involved in the Extracellular Electron Transfer Pathway of Thermincola ferriacetica. Microorganisms 2021, 9, 293. [Google Scholar] [CrossRef]
- Yan, X.; Bu, J.; Chen, X.; Zhu, M.J. Comparative Genomic Analysis Reveals Electron Transfer Pathways of Thermoanaerobacterium thermosaccharolyticum: Insights into Thermophilic Electroactive Bacteria. Sci. Total Environ. 2023, 905, 167294. [Google Scholar] [CrossRef]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of Electricity During Wastewater Treatment Using a Single Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, J.; Wang, L.; Ding, L.; Xu, K.; Ren, H. Stable Operation of Microbial Fuel Cells at Low Temperatures (5–10 °C) with Light Exposure and Its Anodic Microbial Analysis. Bioprocess Biosyst. Eng. 2014, 37, 819–827. [Google Scholar] [CrossRef]
- Call, D.; Logan, B.E. Hydrogen Production in a Single Chamber Microbial Electrolysis Cell Lacking a Membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. Evaluation of Catalysts and Membranes for High Yield Biohydrogen Production via Electrohydrogenesis in Microbial Electrolysis Cells (MECs). Water Sci. Technol. 2008, 58, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Fujinawa, K.; Nagoya, M.; Kouzuma, A.; Watanabe, K. Conductive Carbon Nanoparticles Inhibit Methanogens and Stabilize Hydrogen Production in Microbial Electrolysis Cells. Appl. Microbiol. Biotechnol. 2019, 103, 6385–6392. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Li, W.; Tang, L.; Li, W.; Lv, S.; Xing, D. Suppressing Methane Production to Boost High-Purity Hydrogen Production in Microbial Electrolysis Cells. Environ. Sci. Technol. 2022, 56, 11931–11951. [Google Scholar] [CrossRef]
- Yu, Z.; Leng, X.; Zhao, S.; Ji, J.; Zhou, T.; Khan, A.; Kakde, A.; Liu, P.; Li, X. A Review on the Applications of Microbial Electrolysis Cells in Anaerobic Digestion. Bioresour. Technol. 2018, 255, 340–348. [Google Scholar] [CrossRef]
- Ruiz, Y.; Baeza, J.A.; Guisasola, A. Enhanced Performance of Bioelectrochemical Hydrogen Production using a pH Control Strategy. ChemSusChem 2015, 8, 389–397. [Google Scholar] [CrossRef]
- Sun, G.; Thygesen, A.; Meyer, A.S. Acetate Is a Superior Substrate for Microbial Fuel Cell Initiation Preceding Bioethanol Effluent Utilization. Appl. Microbiol. Biotechnol. 2015, 99, 4905–4915. [Google Scholar] [CrossRef]
- Lu, L.; Ren, Z.J. Microbial Electrolysis Cells for Waste Biorefinery: A State of the Art Review. Bioresour. Technol. 2016, 215, 254–264. [Google Scholar] [CrossRef]
- Christgen, B.; Spurr, M.; Milner, E.M.; Izadi, P.; McCann, C.; Yu, E.; Curtis, T.; Scott, K.; Head, I.M. Does Pre-Enrichment of Anodes with Acetate to Select for Geobacter spp. Enhance Performance of Microbial Fuel Cells When Switched to More Complex Substrates? Front. Microbiol. 2023, 14, 1199286. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kasai, T.; Nakagawa, G.; Yamamuro, A.; Abe, T.; Watanabe, K. Comparative Metagenomics of Anode-Associated Microbiomes Developed in Rice Paddy-Field Microbial Fuel Cells. PLoS ONE 2013, 8, e77443. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Kalil, M.S.; Abdeshahian, P.; Hamid, A.A. A Review of the Substrates Used in Microbial Electrolysis Cells (MECs) for Producing Sustainable and Clean Hydrogen Gas. Renew. Energy 2014, 71, 466–472. [Google Scholar] [CrossRef]
- Blanchet, E.; Desmond, E.; Erable, B.; Bridier, A.; Bouchez, T.; Bergel, A. Comparison of Synthetic Medium and Wastewater Used as Dilution Medium to Design Scalable Microbial Anodes: Application to Food Waste Treatment. Bioresour. Technol. 2015, 185, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.D.; Kiely, P.D.; Call, D.F.; Rismani-Yazdi, H.; Bibby, K.; Peccia, J.; Regan, J.M.; Logan, B.E. Convergent Development of Anodic Bacterial Communities in Microbial Fuel Cells. ISME J. 2012, 6, 2002–2013. [Google Scholar] [CrossRef] [PubMed]
- Raghavulu, S.V.; Modestra, J.A.; Amulya, K.; Reddy, C.N.; Venkata Mohan, S. Relative Effect of Bioaugmentation with Electrochemically Active and Non-Active Bacteria on Bioelectrogenesis in Microbial Fuel Cell. Bioresour. Technol. 2013, 146, 696–703. [Google Scholar] [CrossRef]
- Pandit, S.; Khilari, S.; Roy, S.; Ghangrekar, M.M.; Pradhan, D.; Das, D. Reduction of Start-Up Time Through Bioaugmentation Process in Microbial Fuel Cells Using an Isolate from Dark Fermentative Spent Media Fed Anode. Water Sci. Technol. 2015, 72, 106–115. [Google Scholar] [CrossRef]
- Guadarrama-Pérez, O.; Guevara-Pérez, A.C.; Guadarrama-Pérez, H.V.; Bustos-Terrones, V.; Hernández-Romano, J.; Guillén-Garcés, A.; Moeller-Chávez, E. Bioelectricity Production from the Anodic Inoculation of Geobacter sulfurreducens DL-1 Bacteria in Constructed Wetlands-Microbial Fuel Cells. Bioelectrochemistry 2023, 154, 108537. [Google Scholar] [CrossRef]
- Pham, T.H.; Boon, N.; De Maeyer, K.; Höfte, M.; Rabaey, K.; Verstraete, W. Use of Pseudomonas Species Producing Phenazine-Based Metabolites in the Anodes of Microbial Fuel Cells to Improve Electricity Generation. Appl. Microbiol. Biotechnol. 2008, 80, 985–993. [Google Scholar] [CrossRef]
- Patel, D.; Bapodra, S.L.; Madamwar, D.; Desai, C. Electroactive Bacterial Community Augmentation Enhances the Performance of a Pilot Scale Constructed Wetland Microbial Fuel Cell for Treatment of Textile Dye Wastewater. Bioresour. Technol. 2021, 332, 125088. [Google Scholar] [CrossRef]
- Vargas, I.T.; Tapia, N.; Regan, J.M. Rumen Inoculum Enhances Cathode Performance in Single-Chamber Air-Cathode Microbial Fuel Cells. Materials 2022, 15, 379. [Google Scholar] [CrossRef]
- Hao, D.C.; Wang, F.; Li, C.; Wang, Y.; Xue, J.; Xiao, P.G. Fungal Bioaugmentation Enhanced Herbicide Removal via Soil Microbial Fuel Cell: Taking Myrothecium verrucaria and Haloxyfop-P as an Example. Sci. Total Environ. 2025, 958, 178012. [Google Scholar] [CrossRef]
- Harada, T.; Toda, M.; Yamada, Y.; Tomita, K.; Kouzuma, A.; Watanabe, K. Bioaugmentation of Microbial Fuel Cells with Geobacter sulfurreducens Strain 60473 for Boosting Power Outputs from Food Wastes. Biosci. Biotechnol. Biochem. 2025, 89, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Urigüen, M.; Shuai, W.; Jaffé, P.R. Electrode Colonization by the Feammox Bacterium Acidimicrobiaceae sp. Strain A6. Appl. Environ. Microbiol. 2018, 84, e02029-18. [Google Scholar] [CrossRef] [PubMed]
- Dubrovin, I.A.; Ouaknin Hirsch, L.; Rozenfeld, S.; Gandu, B.; Menashe, O.; Schechter, A.; Cahan, R. Hydrogen Production in Microbial Electrolysis Cells Based on Bacterial Anodes Encapsulated in a Small Bioreactor Platform. Microorganisms 2022, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Dubrovin, I.A.; Hirsch, L.O.; Chiliveru, A.; Jukanti, A.; Rozenfeld, S.; Schechter, A.; Cahan, R. Microbial Electrolysis Cells Based on a Bacterial Anode Encapsulated with a Dialysis Bag Including Graphite Particles. Microorganisms 2024, 12, 1486. [Google Scholar] [CrossRef]
- Zafar, H.; Peleato, N.; Roberts, D. A Review of the Role of Pre-Treatment on the Treatment of Food Waste Using Microbial Fuel Cells. Environ. Technol. Rev. 2022, 11, 72–90. [Google Scholar] [CrossRef]
- Raja, V.; Dutta, S.; Murugesan, P. Electricity Production Using Food Waste: A Review. Environ. Chem. Lett. 2023, 21, 839–864. [Google Scholar] [CrossRef]
- Bonanni, P.S.; Schrott, G.D.; Busalmen, J.P. A Long Way to the Electrode: How Do Geobacter Cells Transport Their Electrons? Biochem. Soc. Trans. 2012, 40, 1274–1279. [Google Scholar] [CrossRef]
- Zhao, J.; Li, F.; Cao, Y.; Zhang, X.; Chen, T.; Song, H.; Wang, Z. Microbial Extracellular Electron Transfer and Strategies for Engineering Electroactive Microorganisms. Biotechnol. Adv. 2021, 53, 107682. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Han, X.; Shan, Y.; Li, F.; Shi, L. Biofilm Biology and Engineering of Geobacter and Shewanella spp. for Energy Applications. Front. Bioeng. Biotechnol. 2021, 9, 786416. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic Digestion of Food Waste—Challenges and Opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujikawa, R.; Hagiwara, M.; Tomita, K.; Watanabe, K. Bioaugmentation with Electroactive Microbes—A Promising Strategy for Improving Process Performances of Microbial Electrochemical Technologies. Energies 2025, 18, 3164. https://doi.org/10.3390/en18123164
Fujikawa R, Hagiwara M, Tomita K, Watanabe K. Bioaugmentation with Electroactive Microbes—A Promising Strategy for Improving Process Performances of Microbial Electrochemical Technologies. Energies. 2025; 18(12):3164. https://doi.org/10.3390/en18123164
Chicago/Turabian StyleFujikawa, Riku, Manami Hagiwara, Keisuke Tomita, and Kazuya Watanabe. 2025. "Bioaugmentation with Electroactive Microbes—A Promising Strategy for Improving Process Performances of Microbial Electrochemical Technologies" Energies 18, no. 12: 3164. https://doi.org/10.3390/en18123164
APA StyleFujikawa, R., Hagiwara, M., Tomita, K., & Watanabe, K. (2025). Bioaugmentation with Electroactive Microbes—A Promising Strategy for Improving Process Performances of Microbial Electrochemical Technologies. Energies, 18(12), 3164. https://doi.org/10.3390/en18123164






