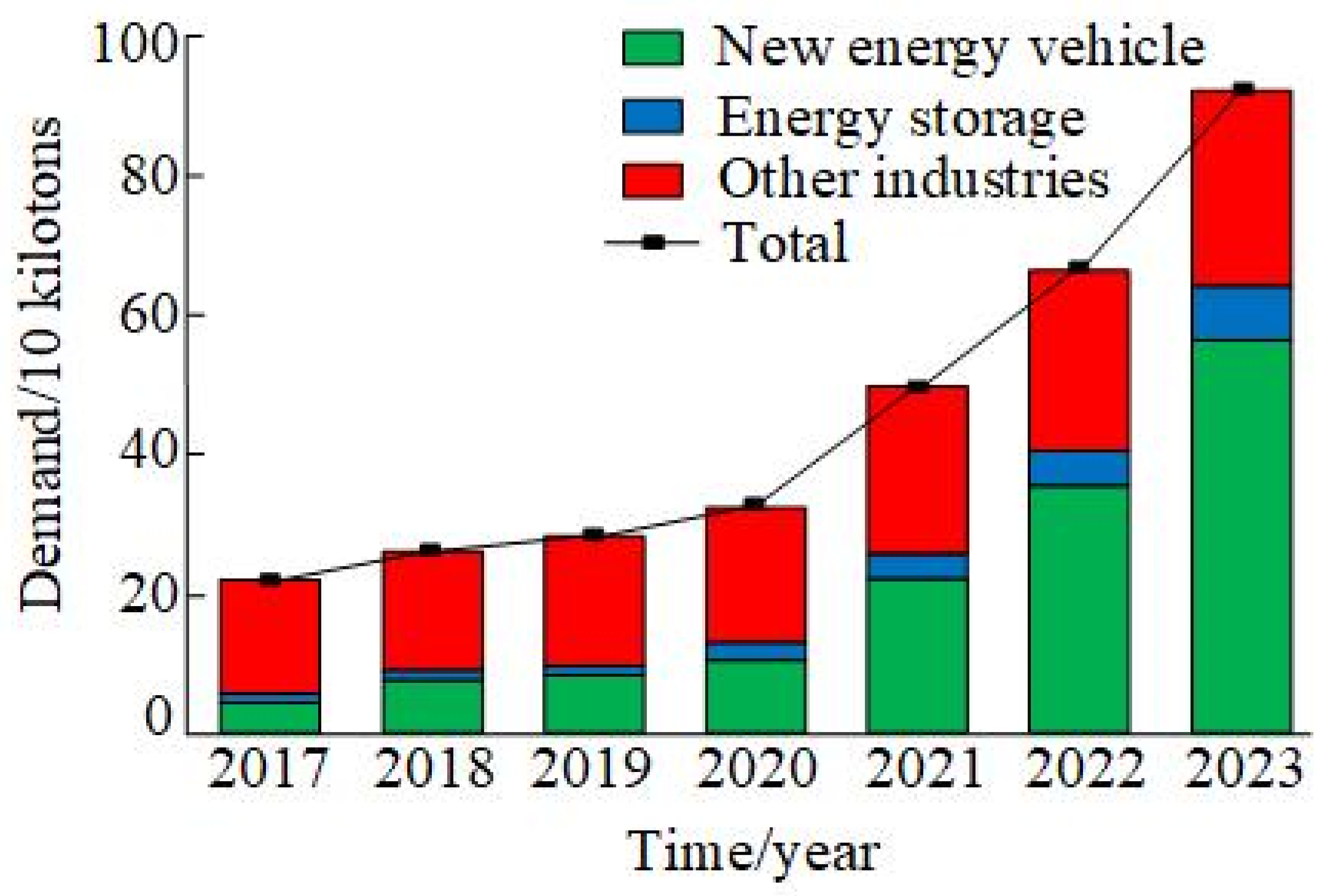
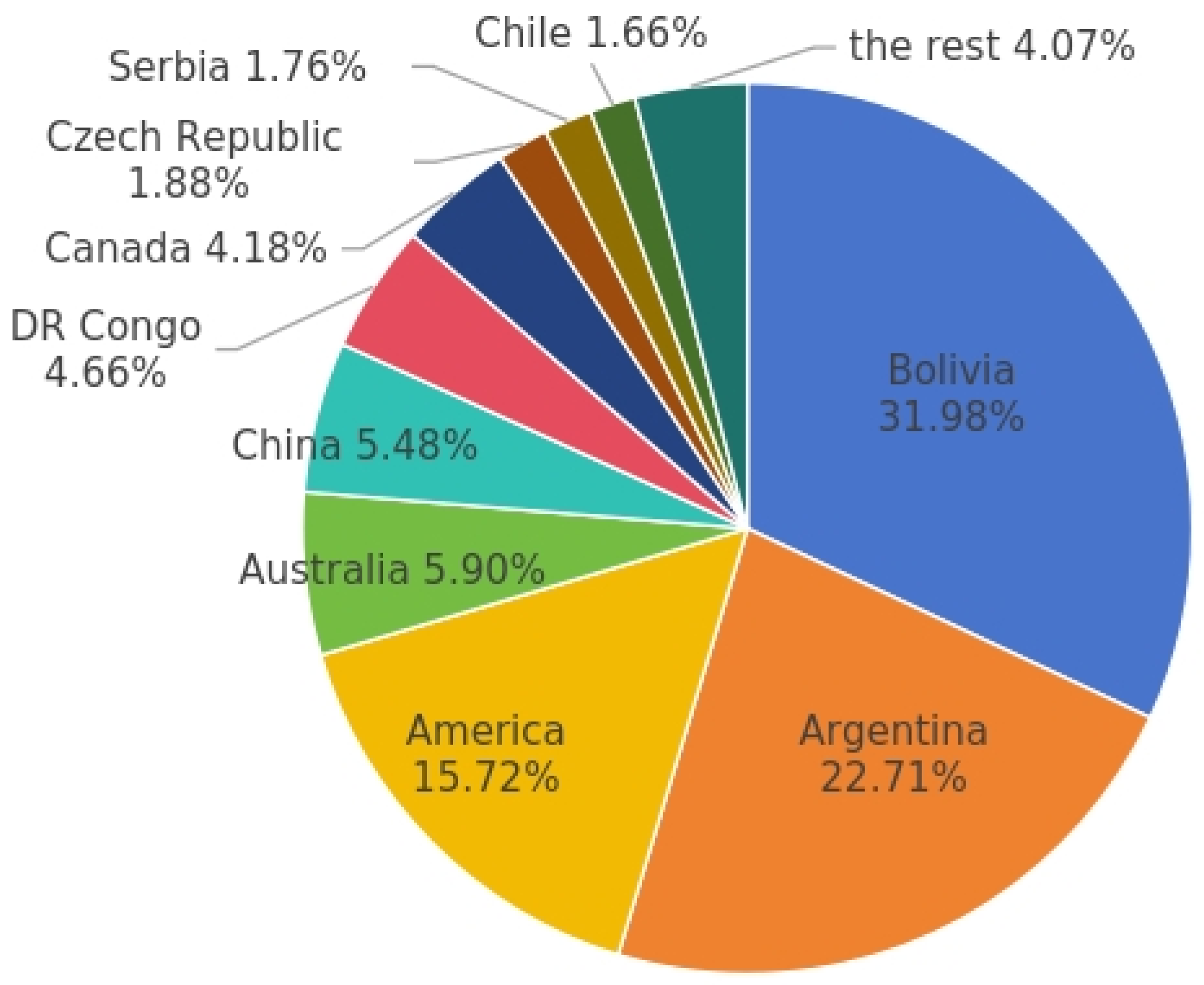
Geothermal Lithium Extraction Technology: Research Status and Prospects
Abstract
1. Introduction
2. Global Distribution and Genesis Characteristics of Lithium Resources
2.1. Distribution of Lithium Resources
2.2. Genetic Characteristics of Geothermal Brine Lithium Resources
3. Advantages of Geothermal Lithium Extraction
4. Main Methods of Lithium Resource Extraction
4.1. Evaporation–Precipitation Method
4.2. Chemical Precipitation Method
4.3. Adsorption Method
4.4. Solvent Extraction Method
4.5. Electrochemical Method
4.6. Membrane Separation Method
- Reverse Osmosis Technology
- b.
- Nanofiltration Technology
- c.
- Forward Osmosis Membrane Technology
5. Development Trends in Geothermal Lithium Extraction
5.1. Membrane Technology as a Key Development Direction
5.2. Industrialization Trends in Geothermal Lithium Extraction
5.3. Geothermal Lithium Extraction Promotes Comprehensive Utilization of Geothermal Resources
5.4. Challenges and Opportunity in Geothermal Lithium Extraction
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency (IEA). World Energy Outlook 2023. Available online: https://www.iea.org/reports/world-energy-outlook-2023 (accessed on 5 January 2025).
- Grand View Research. Lithium-Ion Battery Market Report. 2023. Available online: https://www.grandviewresearch.com/industry-analysis/lithium-ion-battery-market (accessed on 5 January 2025).
- Chen, H.L. The energy revolution will lead to a long term shortage of lithium carbonate. Int. Aid 2021, 30, 163–165. [Google Scholar]
- Zhu, R.S.; Cao, J.; Liu, T.R.; Li, Y.W.; Gao, F.; Hu, X.S. Research progress of lithium extraction technology and industrialization of unconventional brines in global. Inorg. Chem. Ind. 2023, 55, 1–11. [Google Scholar]
- Bloomberg, N.E.F. Electric Vehicle Outlook 2023. Available online: https://about.bnef.com/electric-vehicle-outlook (accessed on 10 January 2025).
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Gao, T.; Fan, N.; Dai, T. Multiple new paths of extracting lithium from brine: Technology, resources, environment and cost. Acta Geol. Sin. 2024, 98, 1310–1331. [Google Scholar]
- Zhao, X.; Zhang, Q.; Wu, H.H.; Hao, X.C.; Wang, L.; Huang, X.P. Extraction of Lithium from Salt Lake Brine. Process Chem. 2017, 29, 796–808. [Google Scholar]
- Stringfellow, W.T.; Dobson, P.E. Technology for the Recovery of Lithium from Geothermal Brines. Energies 2021, 14, 6805. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries 2023; Geological Survey: Reston, VA, USA, 2023.
- Lin, H.Y.; Yu, X.P.; Li, M.L.; Ji, D.; Deng, T. Synthesis of polyporous ion-sieve and its application for selective recovery of lithium from geothermal water. ACS Appl. Mater. Interfaces 2019, 11, 26364–26372. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.Y.; Liu, X.W.; Cao, L.; Lai, Z. Continuous electrical pumping membrane process for seawater lithium mining. Energy Environ. Sci. 2021, 14, 3152–3159. [Google Scholar] [CrossRef]
- Grosjean, C.; Miranda, P.H.; Perrin, M.; Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energy Rev. 2012, 16, 1735–1744. [Google Scholar] [CrossRef]
- Wang, D.H.; Fu, X.F. Breakthrough in lithium ore prospecting in the periphery of Jiajika, Sichuan. Rock Miner. Anal. 2013, 32, 987. [Google Scholar]
- Ding, T.; Zheng, M.P.; Zhang, X.F.; Wu, Q.; Zhang, X.Y. Development of lithium extraction technology and industrialization in brines of salt lake. Sci. Technol. Rev. 2020, 38, 16–23. [Google Scholar]
- Wang, Q.S.; Yuan, C.H.; Xu, H. Analysis of the global lithium resource distribution and potential. China Min. Mag. 2015, 2, 10–17. [Google Scholar]
- Bowell, R.J.; Lagos, L.; Hoyos, D.L.; Declercg, J. Classification and characteristics of natural lithium resources. Elements 2020, 16, 259–264. [Google Scholar] [CrossRef]
- Han, J.H.; Nie, Z.; Fang, C.H.; Wu, Q.; Cao, Q.; Wang, Y.S.; Bu, L.Z.; Yu, J.J. Analysis of existing circumstance of supply and demand on China′s lithium resources. Inorg. Chem. Ind. 2021, 53, 61–66. [Google Scholar]
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine with high magnesium/lithium ratio. CIESC J. 2021, 72, 2905–2921. [Google Scholar]
- Wu, X.S.; Wang, D.H.; Yang, T.T.; Yao, X.; Zhang, H.; Shao, M.J.; Zhang, W.; Liu, D. Innovation and disruptive technologies in the lithium mining industry under the carbon neutrality goal. Multipurp. Util. Miner. Resour. 2022, 2, 16–23. [Google Scholar]
- Luo, A.M.; Cheng, F.; Li, H.G.; Yang, J.Y. Research progress on lithium extraction from salt lake brine. Ind. Mater. Process. 2018, 5, 66–72. [Google Scholar]
- Zhou, Z.Y.; Luo, L.; Jin, D. Discussion on lithium resources of high temperature geothermal water in Southern Xizang and its technical and economic efficiency of extraction and utilization. Multipurp. Util. Miner. Resour. 2024, 45, 85–91. [Google Scholar]
- Li, N.N.; Tao, C.; Xiong, P.; Kong, Y.L.; Li, X.Y.; Zhao, Y.Q. Research development and hotspots of geothermal energy based on bibliometrics. Chin. J. Environ. Eng. 2024, 18, 2746–2753. [Google Scholar]
- Liu, X.F.; Zheng, J.P.; Qi, W. Sources of Ore-Forming Materials of the Superlarge B and Li Deposit in Zabuye Salt Lake, Tibet, China. Acta Geol. Sin. 2007, 81, 7. [Google Scholar]
- Zhan, D.P.; Yu, J.Q.; Gao, C.L.; Zhang, L.S.; Cheng, A.Y. Hydrogeochemical conditions and lithium brine formation in the four salt lakes of Qaidam Basin. Lake Sci. 2010, 22, 783–792. [Google Scholar]
- Brown, R.C.; Green, A.J. The Economic Viability of Geothermal Lithium Extraction: A Case Study Analysis. J. Energy Econ. 2022, 15, 15–28. [Google Scholar]
- Lund, J.W.; Boyd, T.L. Direct utilization of geothermal energy 2015 worldwide review. Geothermics 2016, 60, 66–93. [Google Scholar] [CrossRef]
- Sanjuan, B.; Millot, R.; Innocent, C.; Dezayes, C.; Brach, M. Major geochemical characteristics of geothermal brines from the Upper Rhine Graben granitic basement with constraints on temperature and circulation. Chem. Geol. 2016, 428, 27–47. [Google Scholar] [CrossRef]
- Liu, C.L.; Yu, X.C.; Yuan, X.Y.; Li, R.Q.; Yao, F.; Shen, L.J.; Li, Q.; Zhao, Y.Y. Characteristics, distribution regularity and formation model of brine-type Li deposits in salt lakes in the world. Acta Geol. Sin. 2021, 95, 2009–2029. [Google Scholar]
- Huang, S.Y.; Wang, J.; Wang, J.Y. On the classification of geothermal zones and models of geothermal fields. Hydrogeol. Eng. Geol. 1983, 5, 5–11. [Google Scholar]
- Warren, I. Technoeconomic Analysis of Lithium Extraction from Geothermal Brines; NREL/TP-5700-79178; National Renewable Energy Laboratory: Golden, CO, USA, 2021. [Google Scholar]
- Pramanik, B.K.; Nghiem, L.D.; Hai, F. Extraction of strategically important elements from brines: Constraints and opportunities. Water Res. 2020, 168, 115149. [Google Scholar] [CrossRef]
- Tang, X.; Yang, G. Selective Precipitation of Lithium from High-Magnesium Brines Using Aluminum Chloride. Hydrometallurgy 2019, 189, 105142. [Google Scholar]
- Ding, W.J.; Zhang, J.Y.; Liu, Y.; Guo, Y.; Deng, T.; Yu, X. Synthesis of granulated H4Mn5O12/chitosan with improved stability by a novel cross-linking strategy for lithium adsorption from aqueous solutions. Chem. Eng. J. 2021, 426, 131689. [Google Scholar] [CrossRef]
- Wang, S.L.; Chen, X.; Zhang, Y.; Zhang, Y.; Zheng, S.L. Lithium adsorption from brine by iron-doped titanium lithium ion sieves. Particuology 2018, 41, 40–47. [Google Scholar] [CrossRef]
- Wang, H.S.; Cui, J.J.; Li, M.L.; Guo, Y.F.; Deng, T.L.; Yu, X.P. Selective recovery of lithium from geothermal water by EGDE cross-linked spherical CTS/LMO. Chem. Eng. J. 2020, 389, 124410. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.; Iwuozor, K.; Akpomie, K.G.; Conradie, J.; Adegoke, K.; Oyedotun, K. Utilisation of adsorption as a resource recovery technique for lithium in geothermal water. J. Mol. Liq. 2022, 365, 120107. [Google Scholar] [CrossRef]
- Chen, S.; Gao, D.; Yu, X.; Guo, Y.; Deng, T. Thermokinetics of lithium extraction with the novel extraction systems(Tri-isobutyl phosphate+Ionic liquid+Kerosene). J. Chem. Thermodyn. 2018, 123, 79–85. [Google Scholar] [CrossRef]
- Yu, X.P.; Fan, X.B.; Guo, Y.F.; Deng, T.L. Recovery of lithium from underground brine by multistage centrifugal extraction using tri-isobutyl phosphate. Sep. Purif. Technol. 2019, 211, 790–798. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Ji, Z.Y.; Zhang, Y.G.; Guo, Z.Y.; Zhao, Y.; Liu, J.; Yuan, J.S. Study on lithium extraction from brines based on LiMn2O4/Li1−xMn2O4 by electrochemical method. Electrochim. Acta 2017, 252, 350–361. [Google Scholar] [CrossRef]
- Battistel, A.; Palagonia, M.S.; Brogioli, D.; Mantia, F.L.; Trócoli, R. Electrochemical methods for lithium recovery: A comprehensive and critical review. Adv. Mater. 2020, 32, 1905440. [Google Scholar] [CrossRef] [PubMed]
- Palagonia, M.S.; Brogioli, D.; Mantia, F.L. Lithium recovery from diluted brine by means of electrochemical ion exchange in a flow-through-electrodes cell. Desalination 2020, 475, 114192. [Google Scholar] [CrossRef]
- Jia, H.; He, L.H.; Xu, W.H.; Liu, X.H. Research progress of lithium recovery from salt lake brines with membrane separation method and electrochemical-adsorptive. Rare Met. Cem. Carbides 2017, 45, 6. [Google Scholar]
- Zhang, M.; Liu, S. Membrane Separation Technologies for Lithium Recovery from Geothermal Resources: A Critical Review. Sep. Purif. Technol. 2021, 260, 45–56. [Google Scholar]
- Smith, J.A.; Johnson, L.B. Recent Advances in Geothermal Lithium Extraction Technologies. J. Sustain. Energy Resour. 2020, 45, 45–60. [Google Scholar]
- Victoria, F.; Fernando, B.C.; Galli, C.L. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar]
- Jin, J.B.; Zheng, Y.R.; Wu, X.N.; Fu, S.; Cao, K.; Meng, H.H.; Bai, G.Z. Study on development situation of key technology for lithium extraction from salt lake. Mod. Chem. Ind. 2024, 44, 6–12. [Google Scholar]
- Liu, D.F.; Sun, S.Y.; Yu, J.G. Research and development on technique of lithium recovery from salt lake brine. CIESC J. 2018, 69, 141–155. [Google Scholar]
- Bukowsky, H.; Uhlemann, E.; Steinborn, D. The recovery of pure lithium chloride from brine containing higher contents of calcium chloride and magnesium chloride. Hydrometallurgy 1991, 27, 317–325. [Google Scholar] [CrossRef]
- Berthold, C.E.; Baker, D.H. Lithium recovery from geothermal fluids. In Lithium Resources and Requirements by the Year; U.S. Government Publishing Office: Washington, DC, USA, 1976. [Google Scholar]
- Schultze, L.E.; Bauer, D.J. Recovering Lithium Chloride from a Geothermal Brine; Department of the Interior/Bureau of Mines: Reno, NV, USA, 1984. [Google Scholar]
- Yoshinaga, T.; Kawano, K.; Imoto, H. Basic study on lithium recovery from lithium containing solution. Bull. Chem. Soc. Jpn. 1986, 59, 1207–1213. [Google Scholar] [CrossRef]
- Pan, L.; Li, L.T.; Tang, Y.C. Research on Li+ enriching technology in oilfield brine with high Ca2+. Inorg. Chem. Ind. 2019, 51, 42–44. [Google Scholar]
- Han, W.F.; Wang, H.B.; Guo, Z.; Zheng, C.Z.; Niu, L. Study on lithium extraction process by adsorption-membrane coupling method in high Mg2+/Li+ ratio salt lake. Nonferrous Met. Extr. Metall. 2024, 8, 54–61. [Google Scholar]
- Xiao, X.L.; Dai, Z.F.; Zhu, Z.H.; Ma, P.H. Extracting lithium from brines by absorption method. J. Salt Lake Res. 2005, 13, 66–69. [Google Scholar]
- Noerochim, L.; Sapputra, G.P.A.; Widodo, A. Lithium manganese oxide (LiMn2O4) nanoparticles synthesized by hydrothermal method as adsorbent of lithium recovery process from geothermal fluid of Lumpur Sidoarjo. In Proceedings of the International Conference on Advanced Materials Science & Technology, Tainan, Taiwan, 12–13 November 2016; AIP Publishing LLC: Melville, NY, USA, 2016. [Google Scholar]
- Qian, F.; Guo, M.; Qian, Z.Q.; Liu, Z. Highly lithium adsorption capacities of H1.6Mn1.6O4 ion-sieve by ordered array structure. Chem. Sel. 2019, 4, 10157–10163. [Google Scholar] [CrossRef]
- Moazeni, M.; Hajpour, H.; Askari, M.; Nusheh, M. Hydrothermalsynthesis and characterization of titanium dioxide nanotubes asnovel lithium adsorbents. Mater. Res. Bull. 2015, 61, 70–75. [Google Scholar] [CrossRef]
- Chen, S.; Hu, J.; Guo, Y.; Deng, T. Titanium-based ion sieve with enhanced post-separation ability for high performance lithium recovery from geothermal water. Chem. Eng. J. 2021, 410, 128320. [Google Scholar] [CrossRef]
- Lee, Y.J.; Cha, J.H.; Jung, D.Y. Selective lithium adsorption of silicon oxide coated lithium aluminum layered double hydroxide nanocrystals and their regeneration. Chem. Asian J. 2021, 16, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Lin, S.; Yu, J.G. Li*adsorption performance and mechanism using lithium/aluminum layered double hydroxides in low grade brines. Desalination 2021, 505, 114983. [Google Scholar] [CrossRef]
- Zhong, H. Property of H2TiO3 type ion exchangers and extraction of lithium from brine of natural gas wells. Chin. J. Appl. Chem. 2000, 17, 307–309. [Google Scholar]
- Huang, H.; Liu, Y.P.; Song, X.P.; Jia, G.B. Research progress on magnesium/lithium separation and lithium extraction technology from salt lake brines. Technol. Dev. Chem. Ind. 2024, 53, 40–44. [Google Scholar]
- Wisniewska, M.; Fialkowska, G.; Ostolska, I.; Franus, W.; Nosal-Wiercinska, A.; Tomaszewska, B.; Goscianska, J.; Wojcik, G. Investigations of the possibility of lithium acquisition from geothermal water using natural and synthetic zeolites applying poly (acrylic acid). J. Clean. Prod. 2018, 195, 821–830. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, Y.L.; Huo, J.J.; Sun, Y.X.; Dong, S.D.; He, X.; Xu, Q.; Ma, L.X.; Zhou, Y.; Hai, C.X. Research progress of aluminum adsorbents in lithium extraction from salt lakes. CIESC J. 2023, 74, 4777–4791. [Google Scholar]
- Li, C.; Xiao, J.L.; Sun, S.Y.; Song, X.F.; Yu, J.G. Preparation and lithium adsorption evaluation for spherical ion-sieve granulated by agarose. CIESC J. 2014, 65, 220–226. [Google Scholar]
- Mceachern, P.M.; Wong, N.; Andric, M. Method and Apparatus for the Treatment of Water with the Recovery of Metals. U.S. Patent 20200299805A1, 24 September 2020. [Google Scholar]
- Zhang, L.; Li, L.; Rui, H.; Shi, D.; Song, X. Lithium recovery from effluent of spent lithium battery recycling process using solvent extraction. J. Hazard. Mater. 2020, 398, 122840. [Google Scholar] [CrossRef]
- Zhu, H.F.; Gao, J.; Guo, Y.F.; Deng, T.L. Progresses on Solvent Extraction for Lithium Recovery from Brines. Guangdong Trace Elem. Sci. 2010, 17, 25–30. [Google Scholar]
- Xiang, W.; Liang, S.K.; Zhou, Z.Y.; Qin, W.; Fei, W. Extraction of lithium from salt lake brine containing borate anion and high concentration of magnesium. Hydrometallurgy 2016, 166, 9–15. [Google Scholar] [CrossRef]
- Ma, L.B.; Lu, S.Y.; Wu, H.H.; Sun, K.R.; Luo, B.J.; Wang, L.; Wang, Y.Q.; Zhang, Q.; Huang, X.P. Progress in electrochemical extraction of lithium from seawater. J. Salt Lake Res. 2024, 32, 115–124. [Google Scholar] [CrossRef]
- Sun, S.; Yu, X.; Li, M.; Ji, D.; Deng, T. Green recovery of lithium from geothermal water based on a novel lithium iron phosphate electrochemical technique. J. Clean. Prod. 2019, 247, 119178. [Google Scholar] [CrossRef]
- Zavahir, S.; Elmakki, T.; Gulied, M.; Ahmad, Z.; Al-Sulaiti, L.; Shon, H.K.; Chen, Y.; Park, H.; Batchelor, B.; Han, D.S. A review on lithium recovery using electrochemical capturing systems. Desalination 2021, 500, 31. [Google Scholar] [CrossRef]
- Zheng, H. Research progress of working electrode in electrochemical extraction of lithium from brine. Batteries 2022, 8, 225. [Google Scholar] [CrossRef]
- Baudino, L.; Santos, C.; Pirri, C.; Mantia, F.L.; Lamberti, A. Recent advances in the lithium recovery from water resources: From passive to electrochemical methods. Adv. Sci. 2022, 9, 2201380. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Mantia, F.L. Recent advances in reactor design and control for lithium recovery by means of electrochemical ion pumping. Curr. Opin. Electrochem. 2022, 35, 101089. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, C.Y.; Kim, S.; Hatton, T.A.; Mo, H.L.; Waite, D.T. Lithium recovery using electrochemical technologies: Advances and challenges. Water Res. 2022, 8, 118822. [Google Scholar] [CrossRef]
- Kanoh, H.; Ooi, K.; Miyai, Y.; Katoh, S. Electrochemical recovery of lithium ions in the aqueous phase. Sep. Sci. Technol. 1993, 28, 643–651. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, S.X.; Du, Y.M.; Li, C.; Bao, J.; He, P.; Zhou, H. A low-cost anodic catalyst of transition metal oxides for lithium extraction from seawater. Chem. Commun. 2020, 56, 6396–6399. [Google Scholar] [CrossRef]
- Wang, L.; Arnold, S.; Ren, P.; Wang, Q.S.; Jin, J.; Wen, Z.Y.; Presser, V. Redox flow battery for continuous and energy-effective lithium recovery from aqueous solution. ACS Energy Lett. 2022, 7, 3539–3544. [Google Scholar] [CrossRef]
- Razmjou, A.; Asadnia, M.; Hosseini, E.; Korayem, A.H.; Chen, V. Design principles of ion selective nanostructured membranes for the extraction of lithium ions. Nat. Commun. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.L.; Yang, S.M.; Zhang, S.C.; Shen, C.G.; Ma, X.P. Research of lithium recovery in lithium extracted tail solution from salt lake by nanofiltration and reverse osmosis combined technology. Inorg. Chem. Ind. 2019, 51, 53–57. [Google Scholar]
- Somrani, A.; Hamzaoui, A.H.; Pontie, M. Study on lithium separation from salt lake brines by nanofiltration (NF) and low pressure reverse osmosis (LPRO). Desalination 2013, 317, 184–192. [Google Scholar] [CrossRef]
- Wen, X.; Ma, P.; Zhu, C.; He, Q.; Deng, X. Preliminary study on recovering lithium chloride from lithium-containing waters by nanofiltration. Sep. Purif. Technol. 2006, 49, 230–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Sun, W.; Hu, Y.; Tang, H. Membrane technologies for Li+/Mg2+ separation from saltlake brines and seawater: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 7–23. [Google Scholar] [CrossRef]
- Kang, W.Q.; Shi, L.J.; Zhao, Y.J.; Zhang, D.Y.; Zhang, H.T.; Wang, M. Preliminary test of separation of Mg2+/Li+ in salt lake brine by nanofiltration. Inorg. Chem. Ind. 2014, 46, 22–24. [Google Scholar]
- Wang, Y.M.; Yang, G.S.; Li, X.C. Basic principles of forward osmosis and its engineering applications. Technol. Dev. Chem. Ind. 2012, 41, 4. [Google Scholar]
- Jin, H.Y.; Huang, Y.B.; Yu, P. Forward osmosis technology and its applications. Ind. Water Wastewater 2014, 45, 5. [Google Scholar]
- Hu, Q.H.; Zou, H.; Jiang, Y.; Peng, B.; Liu, D.; Liang, J. Key technologies and application progress of forward osmosis membrane separation. Membr. Sci. Technol. 2014, 34, 7. [Google Scholar]
- Sun, N.; Luo, J.; He, R.R.; Zhao, S.W.; Xu, S.S.; Chen, J.R.; Dou, P.J.; He, T. Low carbon salt-lake lithium extraction: High permselective nanofiltration membranes and forward osmosis membranes. J. Salt Lake 2023, 31, 63–70. [Google Scholar]
- Clutter, T.J. Mining economic benefits from geothermal brine. GeoHeat. Cent. Bull. 2000, 21, 1–3. [Google Scholar]
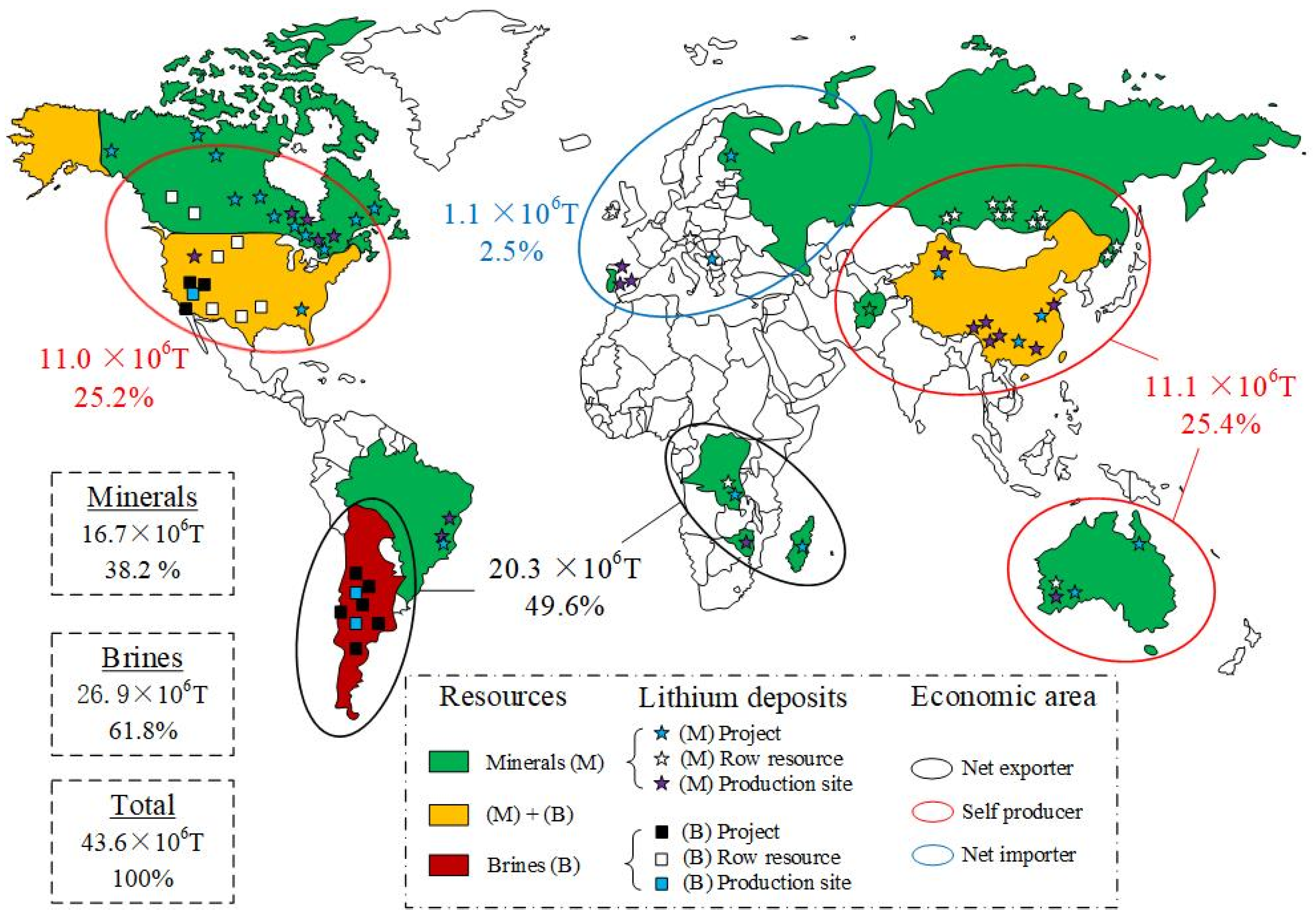

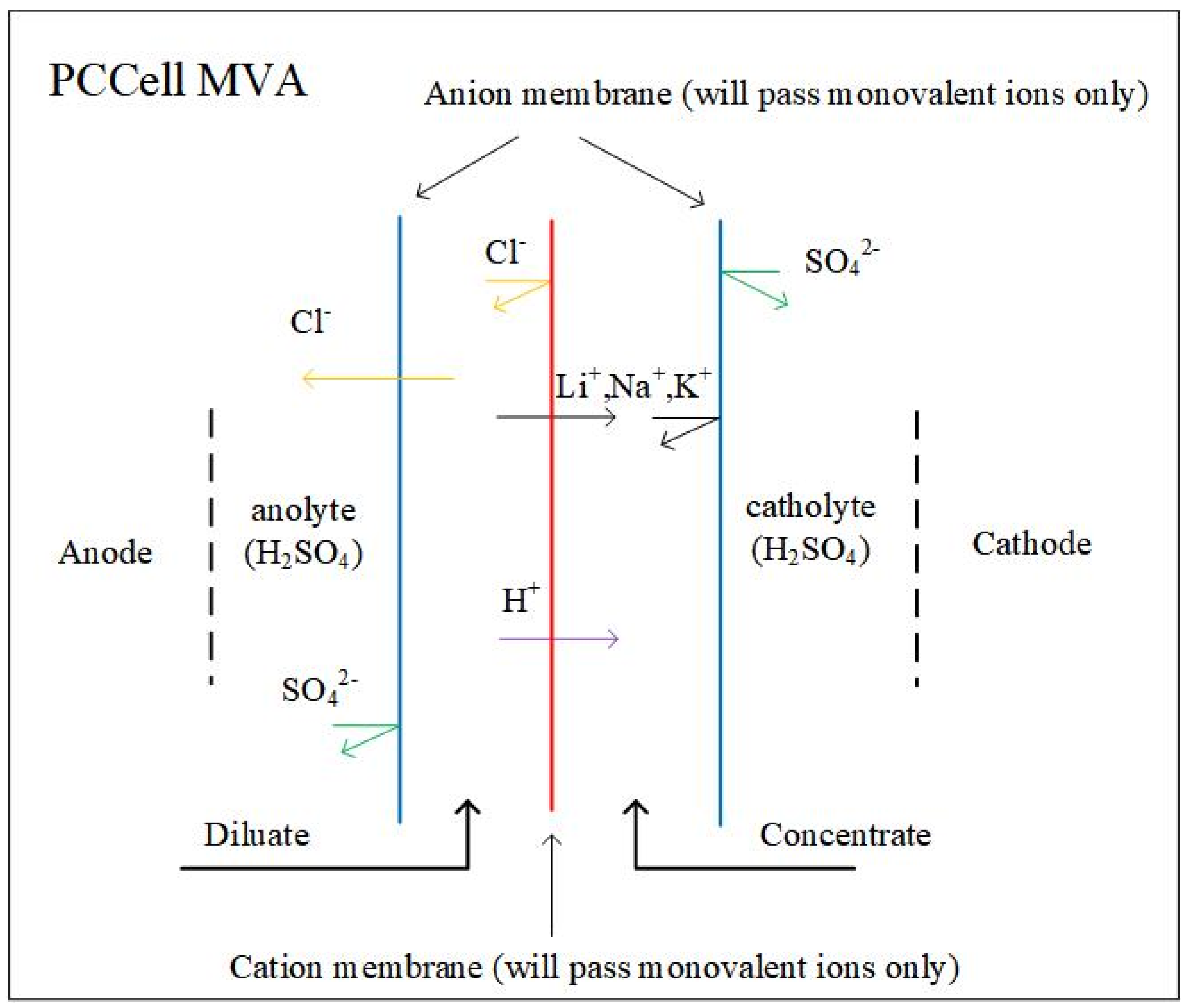
| Brine Type | Country (Region) | Region Name | ρ (Li+) | ρ (Na+) | ρ (K+) | ρ (Mg2+) | ρ (Ca2+) | ρ (B3+) | ρ (SiO2) | ρ (Cl−) | ρ (Br−) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Geothermal brine | United States (California) | Salton Sea | 202 | 49,249 | 14,467 | 109 | 25,684 | 298 | 342 | 142,015 | 91 |
| France (Alsace) | Upper Rhine Graben | 173 | 28,140 | 3195 | 131 | 7225 | 40.8 | 201 | 58,559 | 216 | |
| China (Tibet Autonomous Region) | Lithium-rich hot springs | 79.9 | 24,900 | 2160 | 850 | 2870 | — | — | 46,700 | — |
| Comparison Aspect | Geothermal Brine Extraction | Salt-Lake Brine Evaporation | Hard-Rock Ore Mining |
|---|---|---|---|
| Water consumption | 85% lower (recycled via reinjection) | 2000 m3/t Li (open evaporation) | 15–20 m3/t Li (processing) |
| Energy intensity | 0.8–1.2 kWh/kg Li (combined with geothermal power) | 45–60 kWh/kg Li (solar evaporation) | 250–300 kWh/kg Li (smelting) |
| CO2 emissions | 0.3–0.5 t/t Li (closed-loop system) | 1.2–1.5 t/t Li (chemical processing) | 3.5–4.2 t/t Li (mining + smelting) |
| Resource recovery | 92% Li + coproduction of B, K, and Rb | 75–80% Li (impurity losses) | 60–65% Li (ore waste) |
| Byproduct recovery | Multi-element (B, K, and SiO2) | Limited | Limited |
| Method | Geothermal Brine Adaptation | Salt-Lake Brine Application | Unique Characteristics for Geothermal Brine |
|---|---|---|---|
| Evaporation–precipitation | Waste heat integration from geothermal power | Solar evaporation ponds | Reduced energy cost via waste-heat utilization |
| Chemical precipitation | Aluminates preferred over carbonates due to high Mg/Li ratios | Carbonate precipitation at ambient temperature | Higher reaction rates at 80–120 °C |
| Adsorption | Thermally stable adsorbents (e.g., LiMn2O4) | Ambient-temperature adsorbents (e.g., zeolites) | Resistance to 150 °C brine conditions |
| Solvent extraction | High-temperature solvents like tri-isobutyl phosphate (TBP) maintain solubility at 100 °C | Organophosphates are commonly used for lithium extraction from salt-lake brines | Solvent degradation at 120 °C requires continuous regeneration, increasing operational complexity |
| Electrochemical method | Electrodialysis with bipolar membranes operates mainly at 80 °C | Operating under low temperatures and low pressures, a concentration of 20 g per liter can be achieved at a pressure of only 0.1 megapascals | High conductivity brines may cause ohmic losses |
| Membrane separation | Forward osmosis with thermal regeneration | Reverse osmosis at 25–40 °C | FO membrane tolerance to 90 °C operating temperature |
| Raw Material Source | Year | Chemical Reagent | pH | Lithium Recovery Rate/% | Product and Purity |
|---|---|---|---|---|---|
| Salton geothermal brine | 1976 | AlCl3, CaO | 7.5 | 98 | LiOH, — |
| Salton geothermal brine | 1984 | AlCl3, CaO | 7.5 | 89 | LiCl, 99.9% |
| Hatchobaru geothermal brine | 1986 | NaAlO2 | 11.5 | 98–99 | — |
| Brine in the Nan Yishan Oilfield in Qinghai, China | 2006 | CaO, Na2SO4, Na2CO3 | 10 | 56.26 | Li2CO3, 98.31% |
| Brine in a certain oilfield | 2019 | CCl4, Na2SO4, Na2CO3 | 6.35–6.81 | - | Li2CO3, 98.34% |
| Raw Material Source | Year | Adsorbent Name | Principle | pH | Adsorption Time/h | Maximum Adsorption Capacity |
|---|---|---|---|---|---|---|
| Kuala Lumpur Sidoarjo geothermal brine | 2016 | LiMnO2 | Ion exchange, physical adsorption | — | — | 68.35 mg/g |
| Kuala Lumpur Sidoarjo geothermal brine | 2019 | H1.6Mn1.6O4 | Ion exchange, physical adsorption | 12 | 19.0 | 43.80 mg/g |
| Sichuan Weiyuan gas-field water, China | 2000 | Li2TiO3 | Ion exchange, physical adsorption | 9 | 240 | 25.34 mg/g |
| Simulated lithium-containing water sample | 2015 | Li4Ti5O12 | Ion exchange, physical adsorption | 9.17 | 120 | 39.43 mg/g |
| Geothermal brine | 2021 | Li2TiO3 | Ion exchange, physical adsorption | 12 | 6 | 12.29 mg/g |
| Mixed lithium salt solution | 2021 | LixAl2−LDH@SiO2 | Ion exchange, physical adsorption | — | — | 18.00 mg/L |
| Chaerhan salt-lake brine | 2018 | Li/Al−LDH5 | Ion exchange, physical adsorption | — | 2 | 7.27 mg/g |
| A certain geothermal brine in Tibet, China | 2019 | PVC−HTO | Ion exchange, physical adsorption | 12 | 12 | 11.35 mg/g |
| Geothermal brine | 2020 | granular H4Mn2O12/chitosan | Ion exchange, physical adsorption | 12 | 24 | 8.98 mg/g |
| Rabka Zdroj geothermal brine in Poland | 2018 | natural clinoptilolite | Complexation | 5.5 | 3 | 5.00 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Wang, F.; Wang, R.; Shang, Y.; Li, F.; Li, M.; Wang, T. Geothermal Lithium Extraction Technology: Research Status and Prospects. Energies 2025, 18, 3146. https://doi.org/10.3390/en18123146
Zhang B, Wang F, Wang R, Shang Y, Li F, Li M, Wang T. Geothermal Lithium Extraction Technology: Research Status and Prospects. Energies. 2025; 18(12):3146. https://doi.org/10.3390/en18123146
Chicago/Turabian StyleZhang, Bo, Feng Wang, Ronggang Wang, Yuhan Shang, Feng Li, Mengjiao Li, and Tao Wang. 2025. "Geothermal Lithium Extraction Technology: Research Status and Prospects" Energies 18, no. 12: 3146. https://doi.org/10.3390/en18123146
APA StyleZhang, B., Wang, F., Wang, R., Shang, Y., Li, F., Li, M., & Wang, T. (2025). Geothermal Lithium Extraction Technology: Research Status and Prospects. Energies, 18(12), 3146. https://doi.org/10.3390/en18123146