Effect of Mesopore Structural Parameters in Alumina Supports on Catalytic Hydrodeoxygenation of Guaiacol to Cycloalkanes via Ni-Supported Al2O3 Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.2.1. Preparation of Meso-Al2O3 with Different Mesoporous Sizes
2.2.2. Preparation of Meso-Al2O3-F with Different Mesoporous Volumes
2.2.3. Preparation of Ni-Supported Al2O3 Catalysts
2.3. Catalyst Characterization
2.4. Catalyst Test
3. Results and Discussion
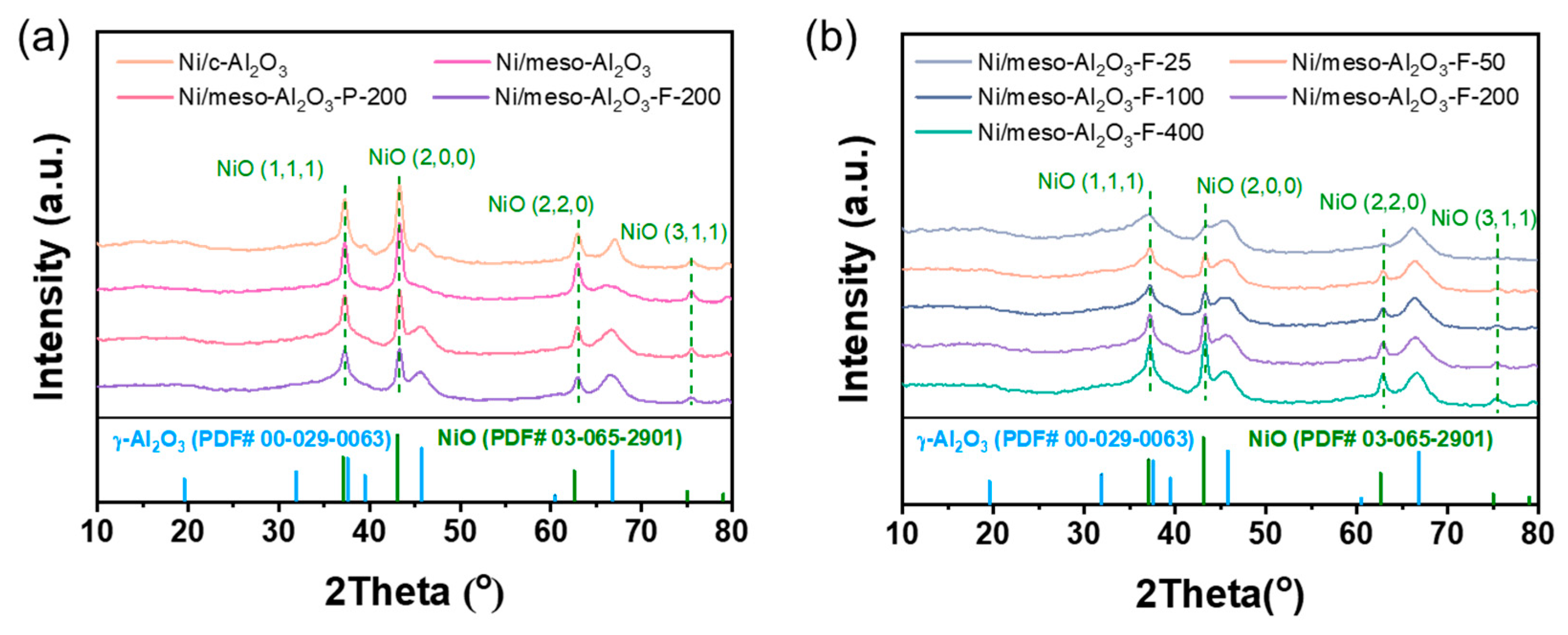
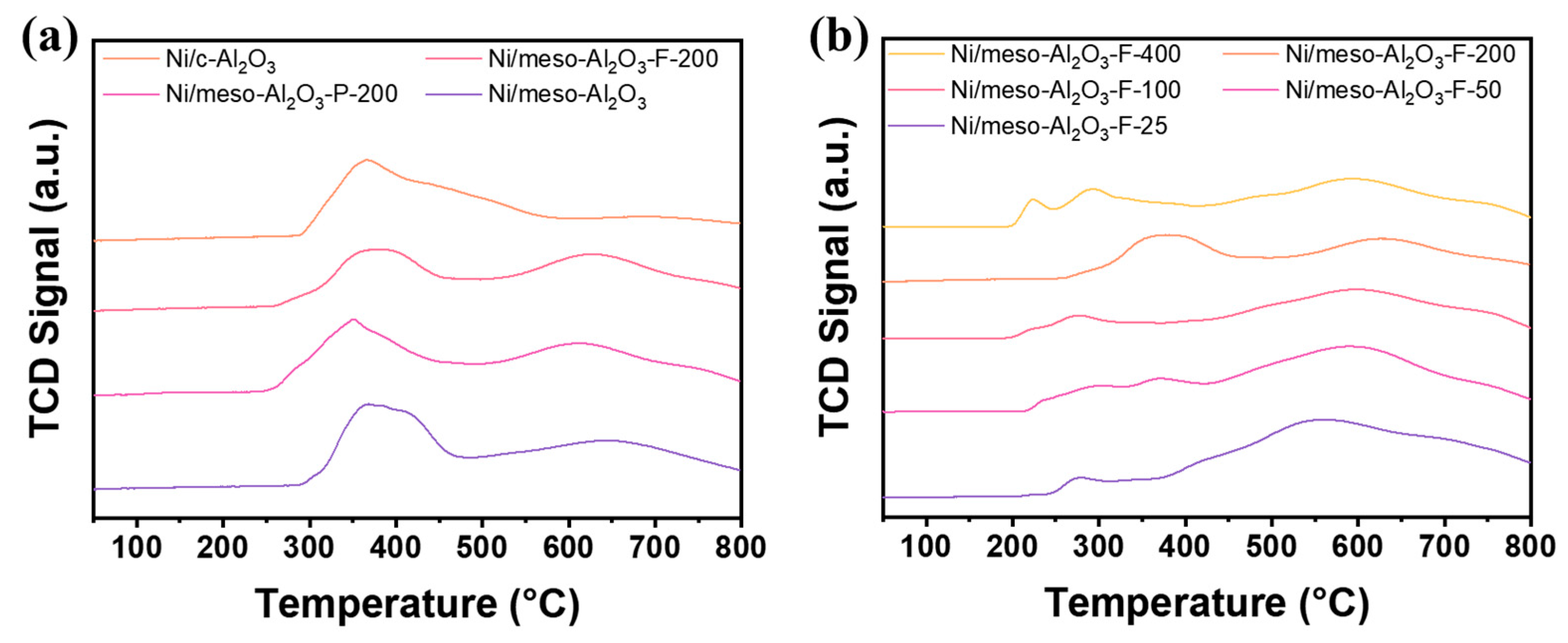
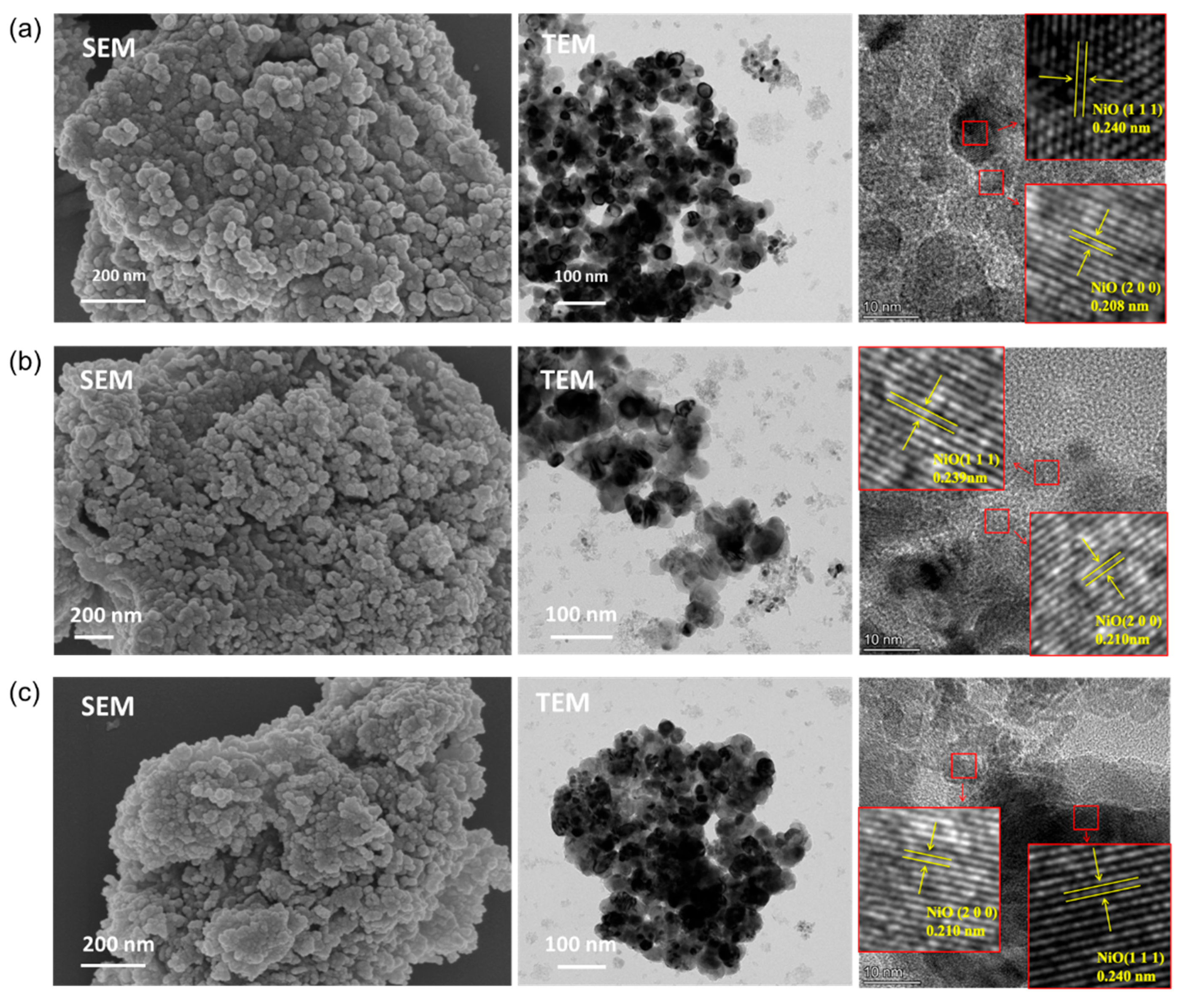
3.1. Textural Properties of the Catalysts
3.2. HDO of Guaiacol over Ni-Based Al2O3 Catalysts
3.2.1. Effect of Al2O3 Support’s Mesoporous Size
3.2.2. Effect of Al2O3 Support’s Mesoporous Volume
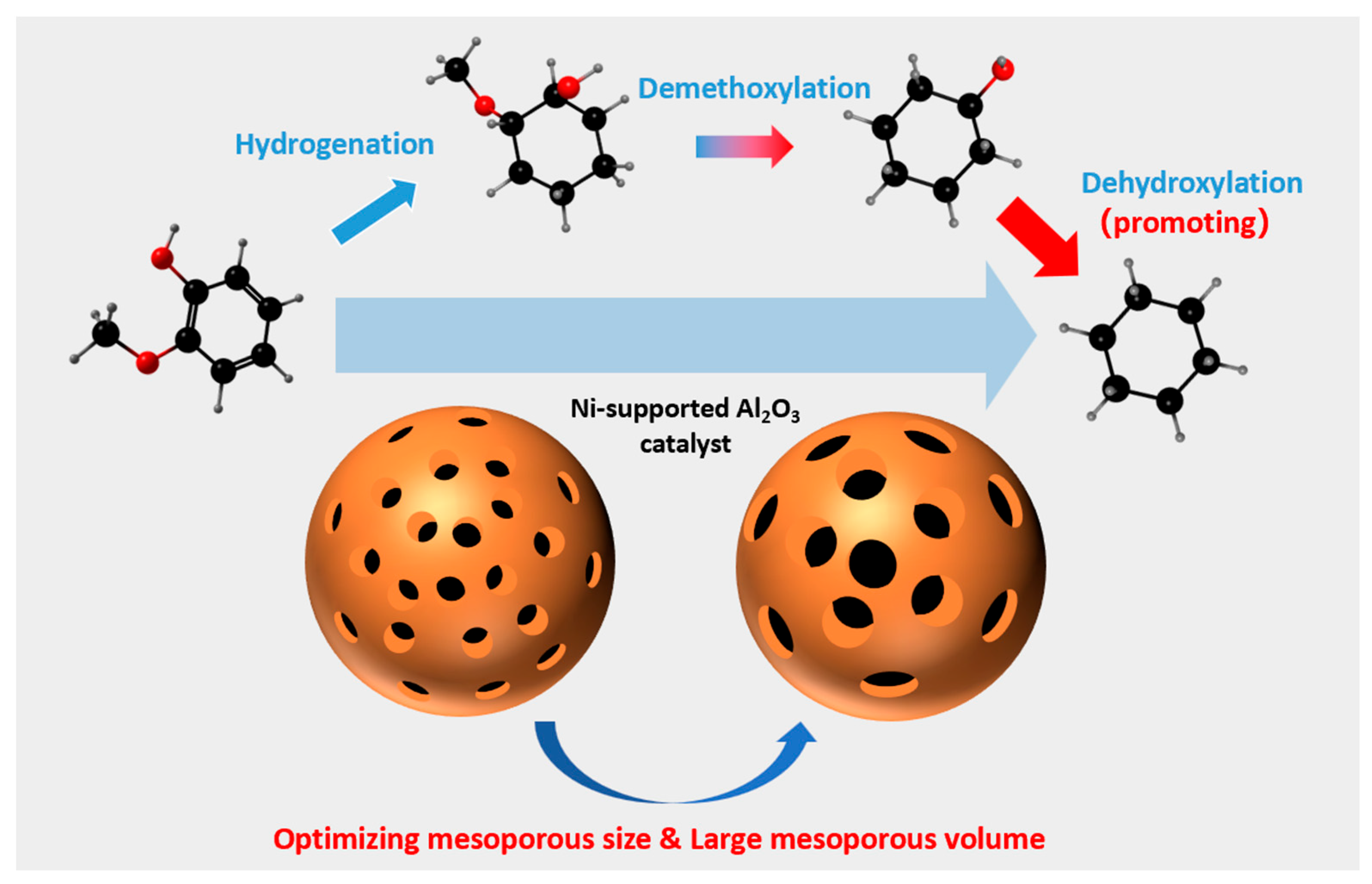
3.3. Mechanism for HDO of Guaiacol over the Ni-Supported Al2O3 Catalyst
3.4. HDO of Other Phenols and Lignin-Derived Bio-Oil over the Ni-Supported Al2O3 Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sust. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Paraschiv, L.S.; Paraschiv, S. Contribution of renewable energy (hydro, wind, solar and biomass) to decarbonization and transformation of the electricity generation sector for sustainable development. Energy Rep. 2023, 9, 535–544. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; You, S.; Park, Y. Bioenergy generation from thermochemical conversion of lignocellulosic biomass-based integrated renewable energy systems. Renew. Sustain. Energy Rev. 2023, 178, 113240. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Chen, W.; Xia, M.; Chen, Y.; Chen, H.; Zeng, K.; Yang, H. Biomass pyrolysis mechanism for carbon-based high-value products. Proc. Combust. Inst. 2023, 39, 3157–3181. [Google Scholar] [CrossRef]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N.J. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, X. Development of different pretreatments and related technologies for efficient biomass conversion of lignocellulose. Int. J. Biol. Macromol. 2022, 202, 256–268. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Conv. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Cai, W.; Wang, X.; Zhu, Z.; Kumar, R.; Amaniampong, P.N.; Zhao, J.; Hu, Z. Synergetic effects in the co-pyrolysis of lignocellulosic biomass and plastic waste for renewable fuels and chemicals. Fuel 2023, 353, 129210. [Google Scholar] [CrossRef]
- Shu, R.; Li, R.; Lin, B.; Wang, C.; Cheng, Z.; Chen, Y. A review on the catalytic hydrodeoxygenation of lignin-derived phenolic compounds and the conversion of raw lignin to hydrocarbon liquid fuels. Biomass Bioenergy 2020, 132, 105432. [Google Scholar] [CrossRef]
- Liang, J.; Shan, G.; Sun, Y. Catalytic fast pyrolysis of lignocellulosic biomass: Critical role of zeolite catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110707. [Google Scholar] [CrossRef]
- Chen, J.; Huang, J.; Chen, L.; Ma, L.; Wang, T.; Zakai, U.I. Hydrodeoxygenation of Phenol and Derivatives over an Ionic Liquid-Like Copolymer Stabilized Nanocatalyst in Aqueous Media. ChemCatChem 2013, 5, 1598–1605. [Google Scholar] [CrossRef]
- Feng, J.; Hse, C.; Yang, Z.; Wang, K.; Jiang, J.; Xu, J. Liquid phase in situ hydrodeoxygenation of biomass-derived phenolic compounds to hydrocarbons over bifunctional catalysts. Appl. Catal. A-Gen. 2017, 542, 163–173. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Lu, P.; Wang, X.; Liu, Y. Fabricating Pt0-acid synergy in Pt/Beta phenol hydrodeoxygenation catalysts via a surface modification strategy of Beta molecular sieves. Int. J. Hydrogen Energy 2024, 51, 860–868. [Google Scholar] [CrossRef]
- Ouedraogo, A.S.; Bhoi, P.R. Recent progress of metals supported catalysts for hydrodeoxygenation of biomass derived pyrolysis oil. Clean. Prod. 2020, 253, 119957. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Muthukumarappan, K.; Kharel, P.R.; Boakye, E. Hydrocarbon bio-oil production from pyrolysis bio-oil using non-sulfide Ni-Zn/Al2O3 catalyst. Fuel Process. Technol. 2017, 162, 78–86. [Google Scholar] [CrossRef]
- Liu, J.; Xiang, M.; Wu, D. Enhanced Phenol Hydrodeoxygenation Over a Ni Catalyst Supported on a Mixed Mesoporous ZSM-5 Zeolite and Al2O3. Catal. Lett. 2017, 147, 2498–2507. [Google Scholar] [CrossRef]
- Xue, H.; Xu, J.; Gong, X.; Hu, R. Performance of a Ni-Cu-Co/Al2O3 catalyst on in-situ hydrodeoxygenation of bio-derived phenol. Catalysts 2019, 9, 952. [Google Scholar] [CrossRef]
- Arun, N.; Nanda, S.; Hu, Y.; Dalai, A.K. Hydrodeoxygenation of oleic acid using γ-Al2O3 supported transition metallic catalyst systems: Insight into the development of novel FeCu/γ-Al2O3 catalyst. Mol. Catal. 2022, 523, 111526. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, X.; Zhang, Q.; Wang, T.; Ma, L. Hydrodeoxygenation of Anisole over Ni/α-Al2O3 Catalyst. Chin. J. Chem. Phys. 2016, 29, 617–622. [Google Scholar] [CrossRef]
- Popov, A.; Kondratieva, E.; Goupil, J.M.; Mariey, L.; Bazin, P.; Gilson, J.; Maugé, F. Bio-oils Hydrodeoxygenation: Adsorption of Phenolic Molecules on Oxidic Catalyst Supports. J. Phys. Chem. C 2010, 114, 15661–15670. [Google Scholar] [CrossRef]
- Shu, R.; Xu, Y.; Ma, L.; Zhang, Q.; Chen, P.; Wang, T. Synergistic effects of highly active Ni and acid site on the hydrodeoxygenation of syringol. Catal. Commun. 2017, 91, 1–5. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Q.; Lin, X.; Liu, W.; Qiu, S.; Shu, R. Enhanced hydrodeoxygenation of lignin-derived phenolic compounds by regulating support composition of Ni/Al2O3-C catalyst. Fuel 2025, 380, 133117. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, C.; Wen, C.; Liang, Z.; Tian, Z.; Jiang, Q.; Liao, Y.; Jiang, X.; Chen, L.; Liu, Q.; Ma, L.; et al. Fabrication of a sinter-resistant Fe-MFI zeolite dragonfruit-like catalyst for syngas to aromatics conversion. J. Energy Chem. 2023, 77, 70–79. [Google Scholar] [CrossRef]
- Pakharukova, V.P.; Yatsenko, D.A.; Gerasimov, E.Y.; Tsybulya, S.V. A study of γ-Al2O3 from the viewpoint of 3D nanostructure. J. Solid State Chem. 2021, 302, 122425. [Google Scholar] [CrossRef]
- Prins, R. On the structure of γ-Al2O3. J. Catal. 2020, 392, 336–346. [Google Scholar] [CrossRef]
- Jbara, A.S.; Othaman, Z.; Ati, A.A.; Saeed, M.A. Characterization of γ- Al2O3 nanopowders synthesized by Co-precipitation method. Mater. Chem. Phys. 2017, 188, 24–29. [Google Scholar] [CrossRef]
- Chen, S.; Pan, X.; Miao, C.; Xie, H.; Zhou, G.; Jiao, Z.; Zhang, X. Study of Catalytic Hydrodeoxygenation Performance for the Ni/KIT-6 Catalysts. J. Saudi Chem. Soc. 2018, 22, 614–627. [Google Scholar] [CrossRef]
- Huang, X.-J.; Mo, W.-L.; Zou, M.; Ma, F.-Y.; Ren, T.-Z.; Zhao, J.-Z.; Zhao, T.-S. Effect of MgO Promoter on the Structure, Performance and Carbon Deposition of Ni-Al2O3 Catalyst for CO Methanation Based-on Slurry-Bed Reactor. ChemistrySelect 2022, 7, e202103647. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, F.; Dong, B.; Cao, X.; Shang, Y.; Xu, X. Degradation of Ofloxacin by High-Efficiency Almond Shell-Based Biochar via Peroxymonosulfate Activation: A Study of the Effect of Pore Structure. Chem. Eng. J. 2023, 474, 145757. [Google Scholar] [CrossRef]
- Tao, J.; Liu, L.; Zhu, P.; Zhai, K.; Ma, Q.; Zhang, D.; Ma, J.; Zhai, Y.; Liu, Y.; Zhang, R. Dual-Template Synthesis of Cage-like Ni-Based Catalyst for Hydrotreatment of Bio-Oil. J. Porous Mater. 2019, 26, 819–828. [Google Scholar] [CrossRef]
- Li, G.; Hao, H.; Jin, P.; Wang, M.; Yu, Y.; Zhang, C. Dry reforming of methane over Ni/Al2O3 catalysts: Support morphological effect on the coke resistance. Fuel 2024, 362, 130855. [Google Scholar] [CrossRef]
- Kim, B.-H.; Yang, E.-H.; Moon, D.J.; Kim, S.W. Ni/MgO-MgAl2O4 Catalysts with Bimodal Pore Structure for Steam-CO2-Reforming of Methane. J. Nanosci. Nanotechnol. 2015, 15, 5959–5962. [Google Scholar] [CrossRef]
- Peters, J.E.; Carpenter, J.R.; Dayton, D.C. Anisole and Guaiacol Hydrodeoxygenation Reaction Pathways over Selected Catalysts. Energy Fuels 2015, 29, 909–916. [Google Scholar] [CrossRef]
- Kong, X.; Liu, C.; Han, Y.; Lei, M.; Fan, Y.; Li, M.; Xiao, R. Cu/CuMgAlOx-Catalyzed Guaiacol Hydrodeoxygenation in Supercritical Methanol: A Modeling Mechanistic Insight for Lignin-Derivatives Upgrading. Energy Fuels 2021, 35, 1511–1522. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, S.; Wang, S.; He, Y.; Liu, Y.; Wang, J.; Fan, W.; Lv, Y. Low Temperature Hydrodeoxygenation of Guaiacol into Cyclohexane over Ni/SiO2 Catalyst Combined with Hβ Zeolite. RSC Adv. 2019, 9, 3868–3876. [Google Scholar] [CrossRef]
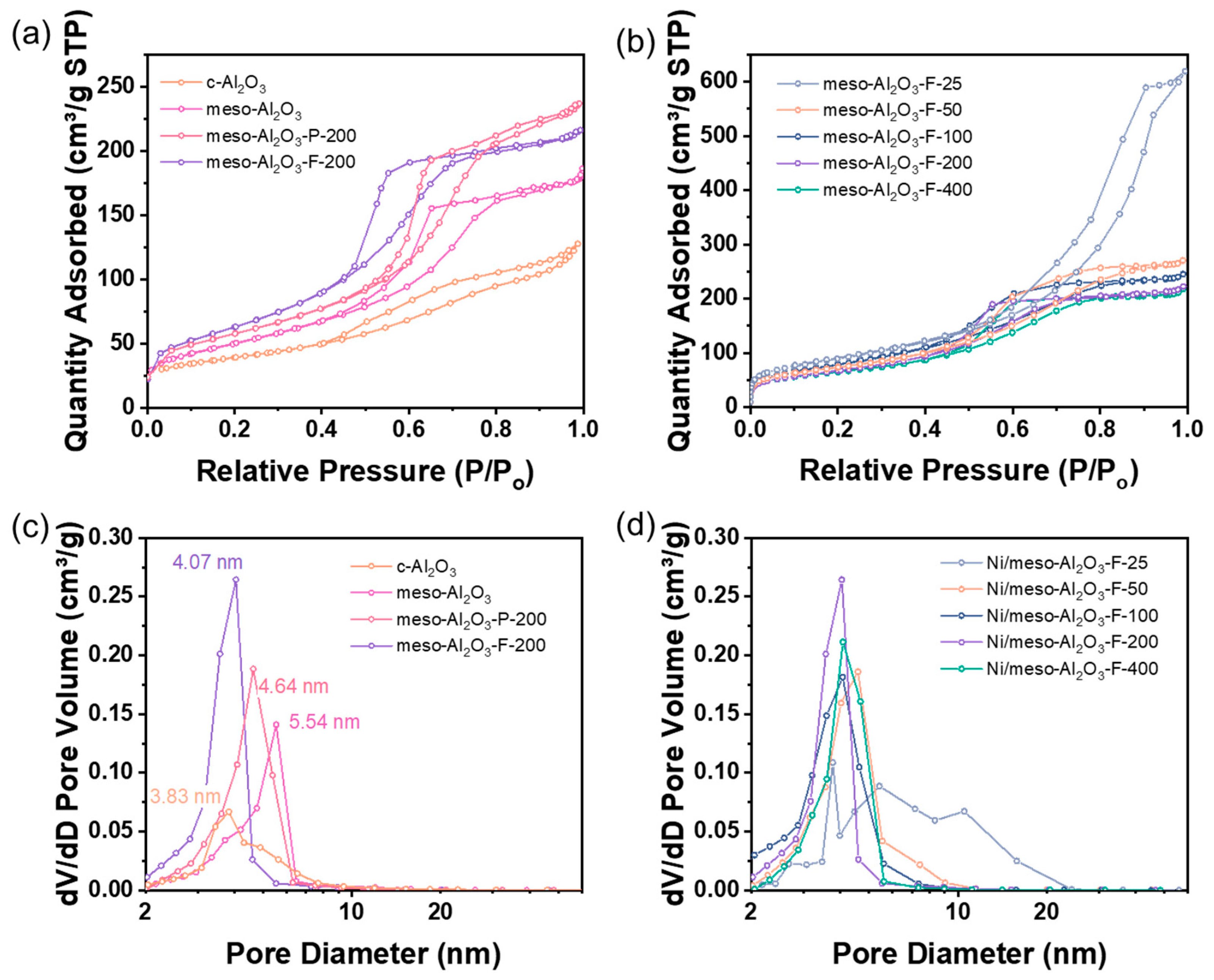
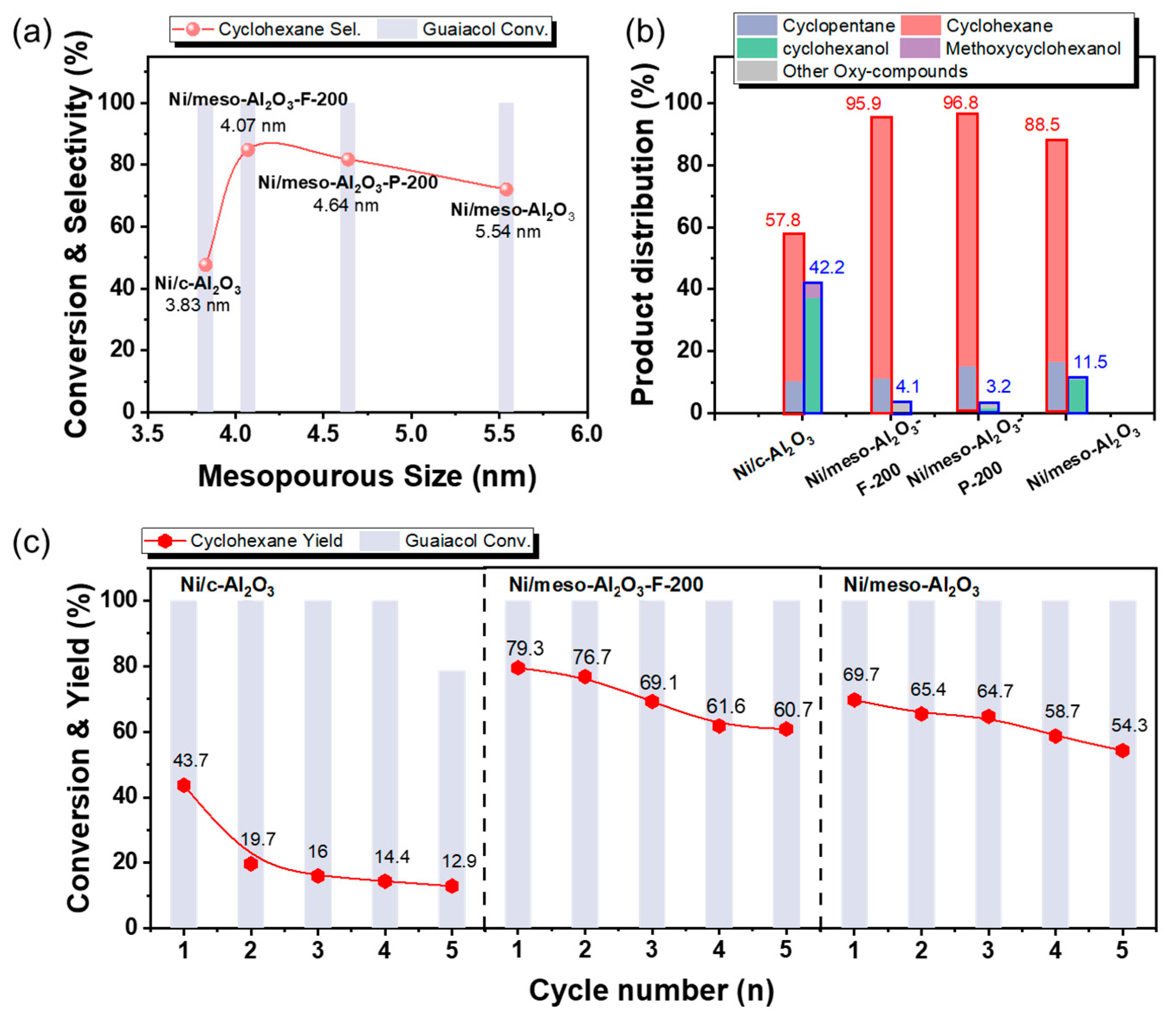
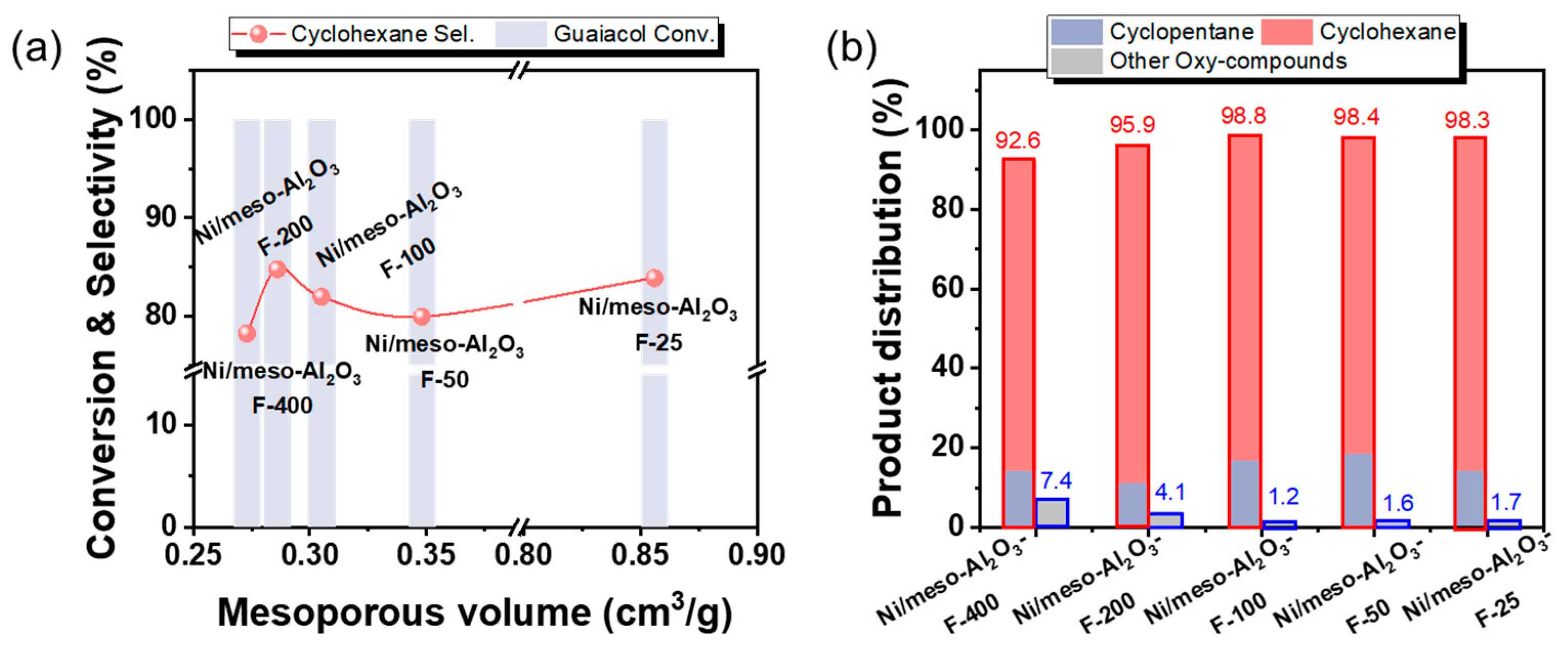
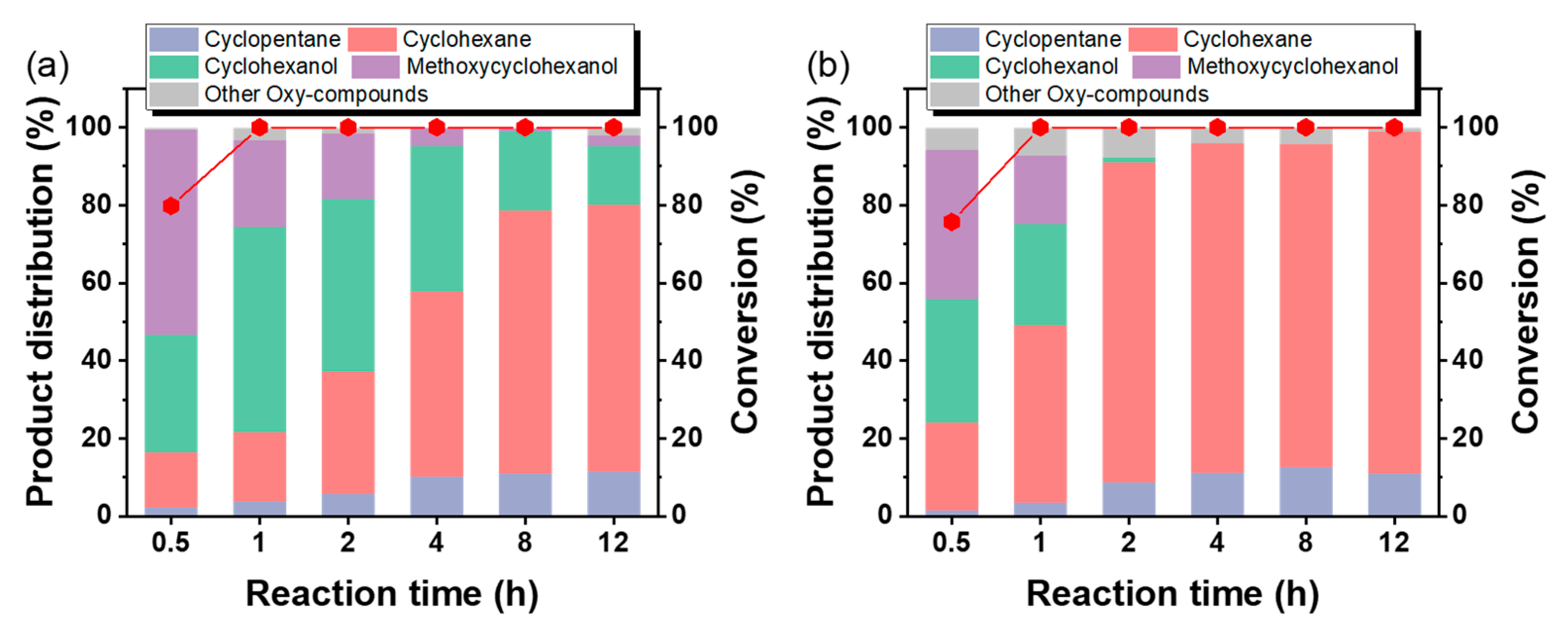
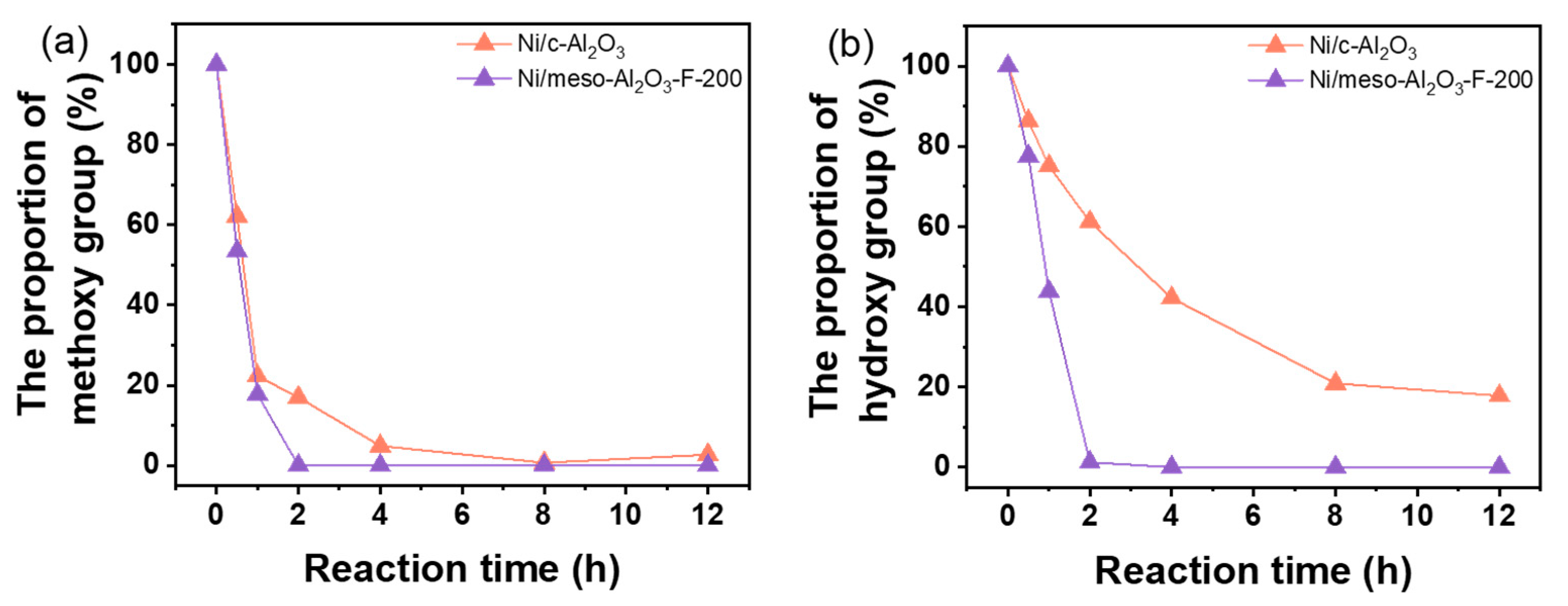
| Catalyst | Surface Area (m2/g) a | Total Pore Volume (cm3/g) b | Mesoporous Volume (cm3/g) c | Mesoporous Size (nm) d |
|---|---|---|---|---|
| c-Al2O3 | 138.8 | 0.197 | 0.184 | 3.83 |
| meso-Al2O3 | 173.2 | 0.275 | 0.268 | 5.54 |
| meso-Al2O3-P-200 | 207.9 | 0.366 | 0.357 | 4.64 |
| meso-Al2O3-F-25 | 324.9 | 0.958 | 0.856 | 4.37 |
| meso-Al2O3-F-50 | 292.2 | 0.419 | 0.348 | 4.59 |
| meso-Al2O3-F-100 | 264.4 | 0.380 | 0.305 | 4.05 |
| meso-Al2O3-F-200 | 240.6 | 0.345 | 0.286 | 4.07 |
| meso-Al2O3-F-400 | 232.4 | 0.338 | 0.273 | 4.08 |
| Substrate | Conversion | Product and Yield |
|---|---|---|
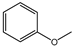 | 100% |  97.1% 97.1% |
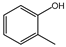 | 100% | 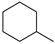 95.7% 95.7% |
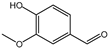 | 100% | 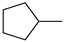 12.1% 12.1% 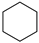 8.8% 8.8% 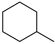 62.2% 62.2% |
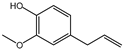 | 100% | 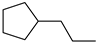 14.1% 14.1% 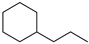 74.2% 74.2% |
| Raw Lignin Oil Component | Content (%) | Upgraded Lignin Oil Component | Product Distribution (%) | |
|---|---|---|---|---|
| Ni/meso-Al2O3-F-200 | Ni/c-Al2O3 | |||
| Hydrocarbons | 22.0 | Hydrocarbons | 65.9 | 40.5 |
| Longifolene | 9.7 | Cyclopentane | 1.3 | 0 |
| Retene | 12.3 | Methylcyclopentane | 3.2 | 1.1 |
| Guaiacols | 70.3 | Cyclohexane | 14.4 | 3.9 |
| o-Cresol | 3.0 | Methylcyclohexane | 8.4 | 6.0 |
| p-Cresol | 4.7 | Ethylcyclohexane | 4.3 | 0 |
| Guaiacol | 10.1 | Propylcyclohexane | 3.5 | 0 |
| 3,5-Dimethylphenol | 3.7 | Longifolene | 0 | 8.0 |
| 2-Methoxy-4-methylphenol | 19.9 | Retene | 2.3 | 7.2 |
| 2-Methoxy-4-ethylphenol | 14.2 | Retene’s derivatives | 28.5 | 14.3 |
| 2-Methoxy-5-propylphenol | 3.1 | Guaiacols | 34.1 | 59.5 |
| 3-Allyl-6-methoxyphenol | 5.2 | Guaiacol | 3.9 | 8.5 |
| trans-Isoeugenol | 6.4 | 2-Methoxy-4-methylphenol | 8.9 | 17.5 |
| Other Oxygenated Compounds | 7.7 | 2-Methoxy-4-ethylphenol | 10.7 | 14.5 |
| Methyl dehydroabietate | 7.7 | 3-Allyl-6-methoxyphenol | 10.6 | 19.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Wen, C.; Su, Y.; Zhang, X.; Ma, L. Effect of Mesopore Structural Parameters in Alumina Supports on Catalytic Hydrodeoxygenation of Guaiacol to Cycloalkanes via Ni-Supported Al2O3 Catalysts. Energies 2025, 18, 3044. https://doi.org/10.3390/en18123044
Huang W, Wen C, Su Y, Zhang X, Ma L. Effect of Mesopore Structural Parameters in Alumina Supports on Catalytic Hydrodeoxygenation of Guaiacol to Cycloalkanes via Ni-Supported Al2O3 Catalysts. Energies. 2025; 18(12):3044. https://doi.org/10.3390/en18123044
Chicago/Turabian StyleHuang, Wen, Chengyan Wen, Yanting Su, Xinghua Zhang, and Longlong Ma. 2025. "Effect of Mesopore Structural Parameters in Alumina Supports on Catalytic Hydrodeoxygenation of Guaiacol to Cycloalkanes via Ni-Supported Al2O3 Catalysts" Energies 18, no. 12: 3044. https://doi.org/10.3390/en18123044
APA StyleHuang, W., Wen, C., Su, Y., Zhang, X., & Ma, L. (2025). Effect of Mesopore Structural Parameters in Alumina Supports on Catalytic Hydrodeoxygenation of Guaiacol to Cycloalkanes via Ni-Supported Al2O3 Catalysts. Energies, 18(12), 3044. https://doi.org/10.3390/en18123044






