Hydrogen Production from Winery Wastewater Through a Dual-Chamber Microbial Electrolysis Cell
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Consortia
2.2. MEC Experiments
2.3. Analytical Determinations
2.4. Electrochemical Impedance Spectroscopy Acquisition and Modeling
3. Results and Discussion
3.1. MEC Development and Operation
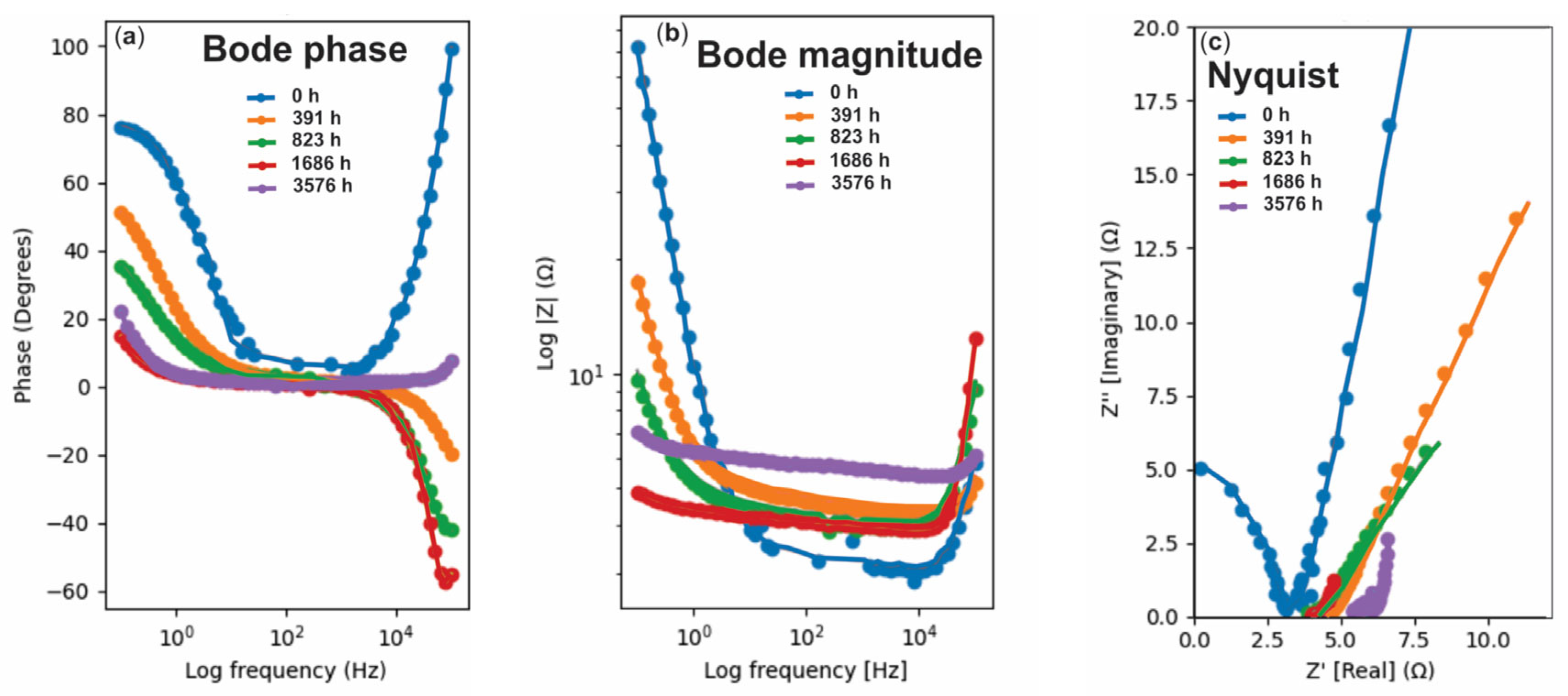
3.2. EIS Analysis
- (1)
- When several spectra separated by thousands of hours are superimposed, Nyquist loci of successive measurements overlap strongly in the high-frequency quarter circle, masking subtle shifts in the solution resistance and high-frequency constant phase element (CPE) exponents. Plotting |Z| and °/Z against log f allows each spectrum to be displayed without overlapping.
- (2)
- At the Bode representation, absolute values and phase angles are separated vertically, so small but systematic drifts remain visible. Also, many kinetic/diffusive phenomena manifest as frequency windows rather than individual semicircles (e.g., Gerischer behavior, finite length diffusion, adsorption inductance).
- (3)
- In a Bode plot, the frequency at which the phase minimum occurs, or the plateau in |Z|, can be read directly and compared with calculated time constants (τ = ½ π fmin).
- (4)
- As the experiments cover 105 Hz decades, at the extreme ends, the Nyquist real part converges towards the solution resistance or 0, making the tails nearly indistinguishable. On the Bode scale, the high-frequency inductive rise and the ultra-low-frequency diffusion plateau separate clearly, even when they change by <1 Ω.
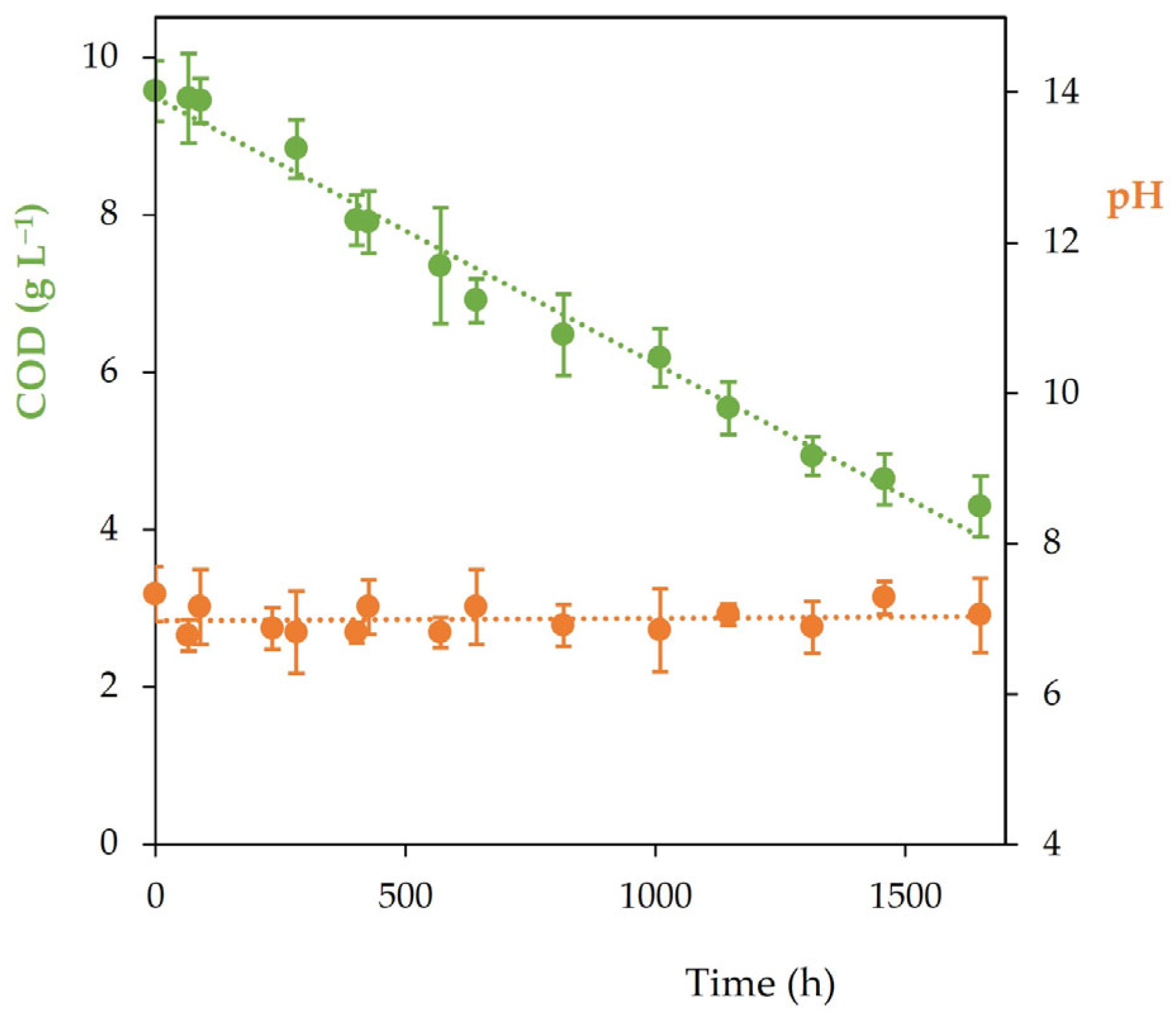
3.3. Winery Wastewater Degradation and Hydrogen Production
| Inoculum | Anode | Cathode | Membrane | HPR (L H2 L−1 d−1) | References |
|---|---|---|---|---|---|
| Mixed microbial culture | Carbon-felt | Stainless-steel | CXM-200 | 0.7 | This study |
| Activated sludge | Graphite granules | Inert polyethylene | Nafion-117 | 0.55−0.66 | [38] |
| Activated sludge | Graphite felts | Pt/C ink | Neosepta AMX | 5.2 | [12] |
| Wastewater sludge | Carbon felt | Ni/Co/Carbon cloth | BPM | 21.0 | [13] |
| Mixed microbial culture | Granular graphite | Stainless-steel | CMI | 1.28 | [39] |
| Food waste | Carbon-felt | Pt-coated Ti | AMI-7001 | 95.6 | [40] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Acdeg | Acetate degradation |
| CEM | Cation exchange membrane |
| COD | Chemical oxygen demand |
| CPE | Constant phase element |
| EIS | Electrochemical impedance spectroscopy |
| EPS | Extracellular polymeric substances |
| Esupp | Electric potential supplemented to the cathode |
| GC | Gas chromatograph |
| HER | Hydrogen evolution reaction |
| HPLC | High-performance liquid chromatography |
| HPR | Hydrogen-production rate |
| MEC | Microbial electrolysis cell |
| MSS | Municipal sewage sludge |
| MWW | Mining wastewater |
| NBAF | Nutrient broth acetate fumarate |
| OCP | Open-circuit potential |
| OD600 | Optical density at 600 nm |
| RC | Resistor-capacitor |
| SHE | Standard hydrogen electrode |
| SLL | Sanitary landfill leachate |
| T | Warburg time constant |
| ΔE | Anode potential variation |
References
- Padhiary, M.; Kumar, R. Assessing the environmental impacts of agriculture, industrial operations, and mining on agro-ecosystems. In Smart Internet of Things for Environment and Healthcare; Mallick, P.K., Tuba, M., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 107–126. [Google Scholar] [CrossRef]
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Wang, D.; Chen, S.; Lai, S.; Dai, W.; Yang, L.; Deng, L.; Luo, S.L. Advanced municipal wastewater treatment and simultaneous energy/resource recovery via photo [electro] catalysis. Chin. Chem. Lett. 2023, 34, 107861. [Google Scholar] [CrossRef]
- El Mekawy, A.; Hegab, H.M.; Alsafar, H.; Yousef, A.F.; Banat, F.; Hasan, S.W. Bacterial nanotechnology: The intersection impact of bacteriology and nanotechnology on the wastewater treatment sector. J. Environ. Chem. Eng. 2023, 11, 109212. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Devi, M.K.; Kumar, P.S.; Rangasamy, G.; Rajendran, S.; Xiao, L. A review on pretreatment methods, photobioreactor design and metabolic engineering approaches of algal biomass for enhanced biohydrogen production. Int. J. Hydrogen Energy 2023, 48, 21110–21127. [Google Scholar] [CrossRef]
- Uddin, M.M.; Wright, M.M. Anaerobic digestion fundamentals, challenges, and technological advances. Phys. Sci. Rev. 2023, 8, 2819–2837. [Google Scholar] [CrossRef]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.A.; Esmaeilion, F.; Silveira, J.L. A conceptual review of sustainable electrical power generation from biogas. Energy Sci. Eng. 2022, 10, 630–655. [Google Scholar] [CrossRef]
- Yerassyl, D.; Jin, Y.; Zhanar, S.; Aigul, K.; Saltanat, Y. The current status and lost biogas production potential of Kazakhstan from anaerobic digestion of livestock and poultry manure. Energies 2022, 15, 3270. [Google Scholar] [CrossRef]
- Guerrero-Sodric, O.; Baeza, J.A.; Guisasola, A. Enhancing bioelectrochemical hydrogen production from industrial wastewater using Ni-foam cathodes in a microbial electrolysis cell pilot plant. Water Res. 2024, 256, 121616. [Google Scholar] [CrossRef]
- Marandola, C.; Cristiani, L.; Zeppilli, M.; Villano, M.; Majone, M.; Fantini, E.; Marone, A. Harvesting Biofuels with Microbial Electrochemical Technologies (METs): State of the Art and Future Challenges. In Wastewater Exploitation: From Microbial Activity to Energy; Springer: Cham, Switzerland, 2024; pp. 305–348. [Google Scholar]
- Timmerman, L.R.; Raghavan, S.; Borole, A.P. Advancing Microbial Electrolysis Technology via Impedance Spectroscopy and Multi-Variate Analysis. Front. Energy Res. 2022, 10, 756900. [Google Scholar] [CrossRef]
- Kim, H.-J.; Nam, J.-Y.; Kim, H.-W.; Jwa, E. Evaluation of a mixture of livestock wastewater and food waste as a substrate in a continuous-flow microbial electrolysis cell. Sci. Total Environ. 2024, 955, 176884. [Google Scholar] [CrossRef]
- Jwa, E.; Kim, S.; Jeong, N.; Han, S.; Song, M.J.; Kang, S.; Nam, J.-Y. Enhanced hydrogen production in zero-gap bipolar membrane microbial electrolysis with binderless cathodes in real wastewater. Chem. Eng. J. 2025, 509, 161416. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Narayanan, A.; Mohamed, S.N. Influence of zinc ferrite nanocomposites for enhancing biohydrogen production during distillery wastewater treatment in microbial electrolysis cell. Fuel 2025, 392, 134872. [Google Scholar] [CrossRef]
- Amanze, C.; Zheng, X.; Anaman, R.; Wu, X.; Fosua, B.A.; Xiao, S.; Xia, M.; Ai, C.; Yu, R.; Wu, X.; et al. Effect of nickel (II) on the performance of anodic electroactive biofilms in bioelectrochemical systems. Water Res. 2022, 222, 118889. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Duan, Z.; Wang, J.; Zhou, W.; Jiang, M.; Li, T.; Ma, H.; Zhu, X. Simultaneous removal of multiple heavy metals using single-chamber microbial electrolysis cells with biocathode in a micro-aerobic environment. Chemosphere 2023, 318, 137982. [Google Scholar] [CrossRef]
- Kanellos, G.; Kyriakopoulos, D.; Lyberatos, G.; Tremouli, A. Electrochemical characterization of a microbial electrolysis cell during the bio-electrochemical conversion of CO2 to CH4. Biochem. Eng. J. 2022, 182, 108431. [Google Scholar] [CrossRef]
- Dubrovin, I.A.; Hirsch, L.O.; Chiliveru, A.; Jukanti, A.; Rozenfeld, S.; Schechter, A.; Cahan, R. Microbial electrolysis cells based on a bacterial anode encapsulated with a dialysis bag including graphite particles. Microorganisms 2024, 12, 1345. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; You, Z.; Bao, H.; Zhang, L.; Wang, J. Electrochemical impedance spectroscopy (EIS) reveals the role of microbial fuel cell-ceramic membrane bioreactor (MFC-CMBR): Electricity utilization and membrane fouling. Water Res. 2022, 222, 117037. [Google Scholar] [CrossRef]
- Fathima, A.; Ilankoon, I.M.S.K.; Zhang, Y.; Chong, M.N. Scaling up of dual-chamber microbial electrochemical systems—An appraisal using systems design approach. Sci. Total Environ. 2024, 912, 169186. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Sodric, O.; Baeza, J.A.; Guisasola, A. Exploring key operational factors for improving hydrogen production in a pilot-scale microbial electrolysis cell treating urban wastewater. Chem. Eng. J. 2023, 469, 144001. [Google Scholar] [CrossRef]
- Yavarinasab, A.; He, J.; Mookherjee, A.; Krishnan, N.; Pestana, L.R.; Fusco, D.; Bizzotto, D.; Tropini, C. Electrogenic dynamics of biofilm formation: Correlation between genetic expression and electrochemical activity in Bacillus subtilis. Biosens. Bioelectron. 2025, 276, 117218. [Google Scholar] [CrossRef]
- Mais, L.; Rodríguez, J.; Campana, R.; Melis, N.; Vacca, A.; Mascia, M. Modelling and EIS characterization of a tubular microbial fuel cell with slip-cast ceramic membrane. J. Power Sources 2025, 630, 236143. [Google Scholar] [CrossRef]
- Huerta-Miranda, G.A.; Arroyo-Escoto, A.I.; Burgos, X.; Juárez, K.; Miranda-Hernández, M. Influence of the major pilA transcriptional regulator in electrochemical responses of Geobacter sulfureducens PilR-deficient mutant biofilm formed on FTO electrodes. Bioelectrochemistry 2019, 127, 145–153. [Google Scholar] [CrossRef]
- O’Brien, J.P.; Malvankar, N.S. A Simple and Low-Cost Procedure for Growing Geobacter sulfurreducens Cell Cultures and Biofilms in Bioelectrochemical Systems. Curr. Protoc. Microbiol. 2016, 43, A-4K. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; APHA Press: Washington, DC, USA, 2023; ISBN 978-0875532998. [Google Scholar]
- Ochiai, I.; Harada, T.; Jomori, S.; Kouzuma, A.; Watanabe, K. Bioaugmentation of microbial electrolysis cells with Geobacter sulfurreducens YM18 for enhanced hydrogen production from starch. Bioresour. Technol. 2023, 386, 129508. [Google Scholar] [CrossRef]
- Murbach, M.D.; Gerwe, B.; Dawson-Elli, N.; Tsui, L.K. impedance.py: A Python package for electrochemical impedance analysis. J. Open Source Softw. 2020, 5, 2349. [Google Scholar] [CrossRef]
- Ibrahim, K.; Sabathiel, S.; Farooq, F.; Hofer, G.; Bergmann, A.; Heer, R. An empirical model for state of charge estimation based on electrochemical impedance spectroscopy. In Proceedings of the 2024 IEEE International Conference on Electrical Systems for Aircraft, Railway, Ship Propulsion and Road Vehicles & International Transportation Electrification Conference (ESARS-ITEC), Naples, Italy, 26–29 November 2024; pp. 1–6. [Google Scholar]
- Leicester, D.D.; Settle, S.; McCann, C.M.; Heidrich, E.S. Investigating variability in microbial fuel cells. Appl. Environ. Microbiol. 2023, 89, e02181-22. [Google Scholar] [CrossRef]
- Santoro, C.; Babanova, S.; Cristiani, P.; Artyushkova, K.; Atanassov, P.; Bergel, A.; Bretschger, O.; Brown, R.K.; Carpenter, K.; Colombo, A.; et al. How comparable are microbial electrochemical systems around the globe? An electrochemical and microbiological cross-laboratory study. ChemSusChem 2021, 14, 2313. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Hoang, N.B. Impact factors and novel strategies for improving biohydrogen production in microbial electrolysis cells. Bioresour. Technol. 2022, 346, 126588. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Melkania, U. Effect of phenolic compounds on hydrogen production from municipal solid waste. Waste Manag. 2018, 78, 115–123. [Google Scholar] [CrossRef]
- Kanellos, G.; Monokrousou, E.; Tremouli, A.; Lyberatos, G. Treatment of two-phase olive mill waste and degradation of phenolic compounds with simultaneous bio-electrochemical conversion of CO2 to CH4 using a microbial electrolysis cell. Sustain. Chem. Pharm. 2024, 39, 101589. [Google Scholar] [CrossRef]
- Jensen, L.S.; Kaul, C.; Juncker, N.B.; Thomsen, M.H.; Chaturvedi, T. Biohydrogen production in microbial electrolysis cells utilizing organic residue feedstock: A review. Energies 2022, 15, 8396. [Google Scholar] [CrossRef]
- Tang, J.; Bian, Y.; Jin, S.; Sun, D.; Ren, Z.J. Cathode material development in the past decade for H2 production from microbial electrolysis cells. ACS Environ. Au 2022, 2, 20–29. [Google Scholar] [CrossRef]
- Cristiani, L.; Zeppilli, M.; Feriaud, D.; Marandola, C.; Papini, M.P.; Da Silva, S.; Erable, B.; Villano, M. Enhancing energy efficiency and H2 production in lab-scale dual chamber microbial electrolysis cells: A focus on catholyte composition and voltage losses. J. Environ. Chem. Eng. 2024, 12, 111782. [Google Scholar] [CrossRef]
- Marchetti, A.; Moreno, M.C.; Lauri, R.; Zeppilli, M. Hydrogen production through efficient organic matter oxidation performed by microbial electrolysis cells. Processes 2025, 13, 1231. [Google Scholar] [CrossRef]
- Kim, D.; Choi, Y.; Cha, J.; Park, H.; Baek, G.; Lee, C. Pre-fermentation and filtration pretreatments enhance hydrogen production from food waste through microbial electrolysis cell. Bioresour. Technol. 2025, 424, 132267. [Google Scholar] [CrossRef]




| Microbial Consortia | ||
|---|---|---|
| Source | Collection Site | Dosage Ratio |
| Sanitary landfill leachate | Intermunicipal sanitary landfill of Associação de Municípios do Alentejo Central, Vila Ruiva, Portugal | 1/3 |
| Mining wastewater | Mina de São Domingos, Mértola, Portugal | 1/3 |
| Municipal sewage sludge | Municipal wastewater treatment plant of Beja, Portugal | 1/3 |
| MEC Inoculum | ||
| Composition | Source | Dosage Ratio |
| Microbial consortia | Developed in this study | 2/3 |
| Geobacter sulfurreducens PCA | Ambifirst, Moita, Portugal | 1/3 |
| Model Code | Topology | Applied Time (h) | Justification |
|---|---|---|---|
| 1 | R0–[R1‖CPE1]–Wo | 0 | Single interfacial process + incipient diffusion |
| 2 | R0–[R1‖CPE1]–[R2‖CPE2]–Wo | 391 | The second time constant appears as the biofilm forms |
| 3 | R0–[R1‖CPE1]–[R2‖CPE2]–G | 823 | Warburg tail becomes Gerischer due to coupled reaction-diffusion |
| 4 | R0–[R1‖CPE1]–[R2‖CPE2]–Wo | 1320–3576 | Thick, but still semi-infinite, diffusion in mature film |
| 5 | R0–[R1‖CPE1]–[R2‖CPE2]–G–Ws | 5102 | Reaction-diffusion + very slow residual semi-infinite transport |
| t (h) | R0 (Ω) | R1 (Ω) | Q1 (S sn) | n1 | R2 (Ω) | Q2 (S sn) | n2 | Diffusive Element | MSE (Ω2) | MSE_w (Ω2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5.0 × 10−1 | 2.4 | 1.6 × 10−6 | 1.0 | — | — | — | Wo (T = 10 Ω s−0.5, p = 0.40) | 5.5 × 10−1 | 1.3 × 10−2 |
| 391 | 3.2 | 1.0 | 1.0 × 10−6 | 6.0 × 10−1 | 1.0 × 10−1 | 1.0 × 10−7 | 5.0 × 10−1 | Wo (T = 9.30 Ω s−0.5) | 7.5 × 10−1 | 4.0 × 10−1 |
| 823 | 3.9 | 1.0 × 10−4 | 1.0 × 10−6 | 6.0 × 10−1 | 1.0 × 10−4 | 1.0 × 10−7 | 5.0 × 10−1 | G (T = 4.66 Ω s−0.5) | 2.8 × 10−1 | 5.6 × 10−2 |
| 1320 | 2.4 | 3.5 × 10−1 | 1.0 × 10−1 | 9.4 × 10−1 | 5.0 | 5.4 × 10−8 | 1.0 | Wo (T = 1.53 Ω s−0.5) | 1.8 × 10−1 | 2.2 × 10−3 |
| 1686 | 3.8 | 3.2 × 10−1 | 2.5 × 10−2 | 7.0 × 10−1 | 1.6 × 10−3 | 1.0 × 10−8 | 5.0 × 10−1 | Wo (T = 0.40 Ω s−0.5) | 6.4 × 10−1 | 6.6 × 10−3 |
| 2520 | 6.0 | 6.1 × 10−1 | 1.0 × 10−2 | 8.5 × 10−1 | 1.0 × 10−2 | 6.1 × 10−5 | 9.9 × 10−1 | Wo (T = 1.79 Ω s−0.5) | 1.7 × 10−1 | 3.1 × 10−3 |
| 3576 | 5.3 × 10−1 | 3.2 × 10−1 | 3.8 × 10−3 | 8.4 × 10−1 | 5.0 | 4.1 × 10−8 | 1.0 | Wo (T = 1.66 Ω s−0.5, p = 0.97) | 1.4 × 10−2 | 3.8 × 10−3 |
| 5102 | 1.5 × 10−1 | 1.9 × 10 | 7.6 × 10−4 | 4.5 × 10−1 | 5.7 × 10 | 1.1 × 10−8 | 1.0 | G (T = 11.7 Ω s−0.5, n = 0.32) + Ws (Z0 = 30.6 Ω, τ = 66.7 s) | 9.5 × 10−2 | 3.6 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baía, A.; Arroyo-Escoto, A.I.; Ramos, N.; Abdelkarim, B.; Pereira, M.; Fernandes, M.C.; Zhang, Y.; Fernandes, A. Hydrogen Production from Winery Wastewater Through a Dual-Chamber Microbial Electrolysis Cell. Energies 2025, 18, 3043. https://doi.org/10.3390/en18123043
Baía A, Arroyo-Escoto AI, Ramos N, Abdelkarim B, Pereira M, Fernandes MC, Zhang Y, Fernandes A. Hydrogen Production from Winery Wastewater Through a Dual-Chamber Microbial Electrolysis Cell. Energies. 2025; 18(12):3043. https://doi.org/10.3390/en18123043
Chicago/Turabian StyleBaía, Ana, Alonso I. Arroyo-Escoto, Nuno Ramos, Bilel Abdelkarim, Marta Pereira, Maria C. Fernandes, Yifeng Zhang, and Annabel Fernandes. 2025. "Hydrogen Production from Winery Wastewater Through a Dual-Chamber Microbial Electrolysis Cell" Energies 18, no. 12: 3043. https://doi.org/10.3390/en18123043
APA StyleBaía, A., Arroyo-Escoto, A. I., Ramos, N., Abdelkarim, B., Pereira, M., Fernandes, M. C., Zhang, Y., & Fernandes, A. (2025). Hydrogen Production from Winery Wastewater Through a Dual-Chamber Microbial Electrolysis Cell. Energies, 18(12), 3043. https://doi.org/10.3390/en18123043








