Improving Photocatalytic Hydrogen Production with Sol–Gel Prepared NiTiO₃/TiO₂ Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pure NiTiO3 and NiTiO3/TiO2 Composite Synthesis
2.3. Characterization
2.4. Hydrogen Evolution Test
3. Results
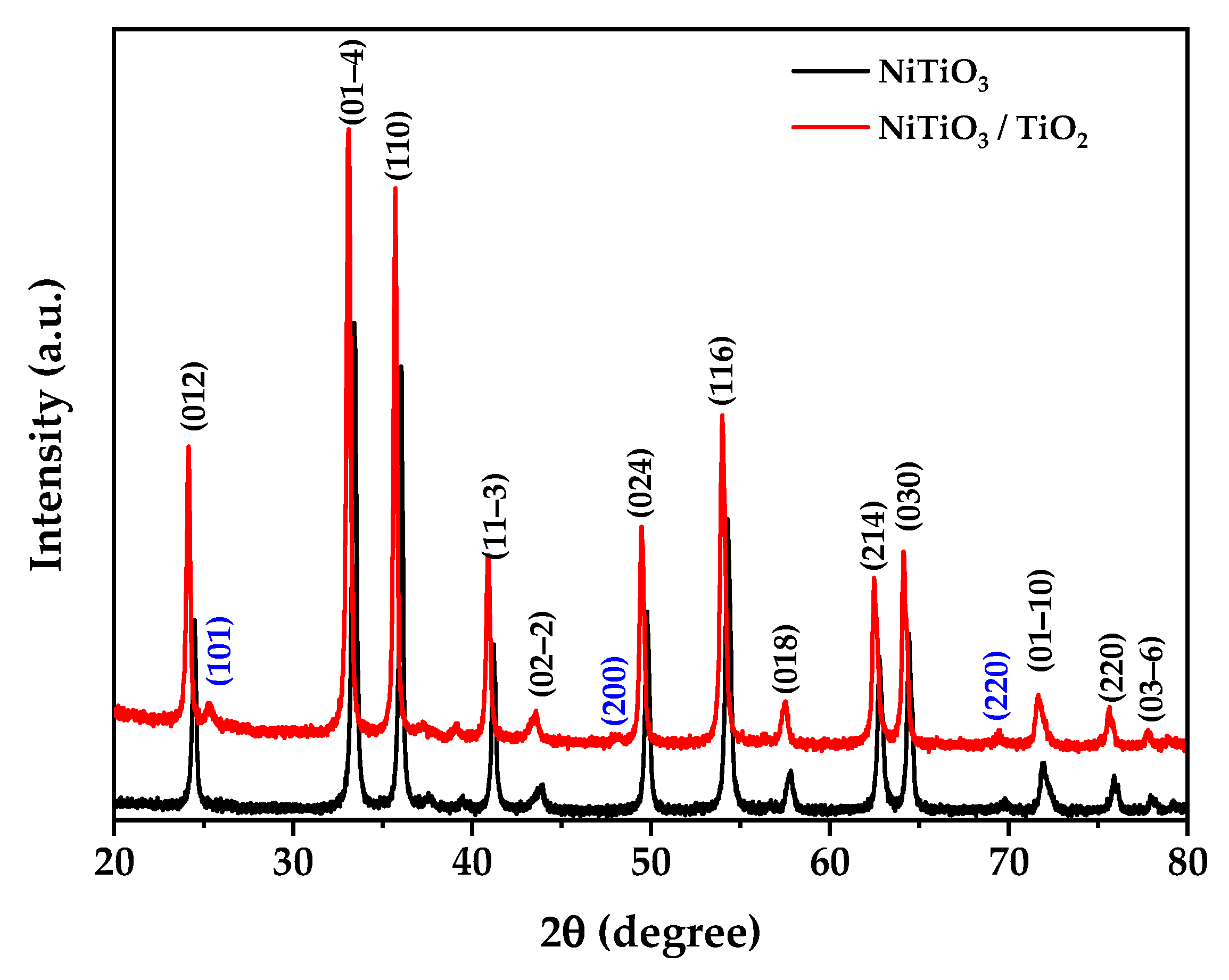
3.1. XRD Results
3.2. Raman Spectroscopy Results
3.3. Fourier-Transform Infrared Spectroscopy Results
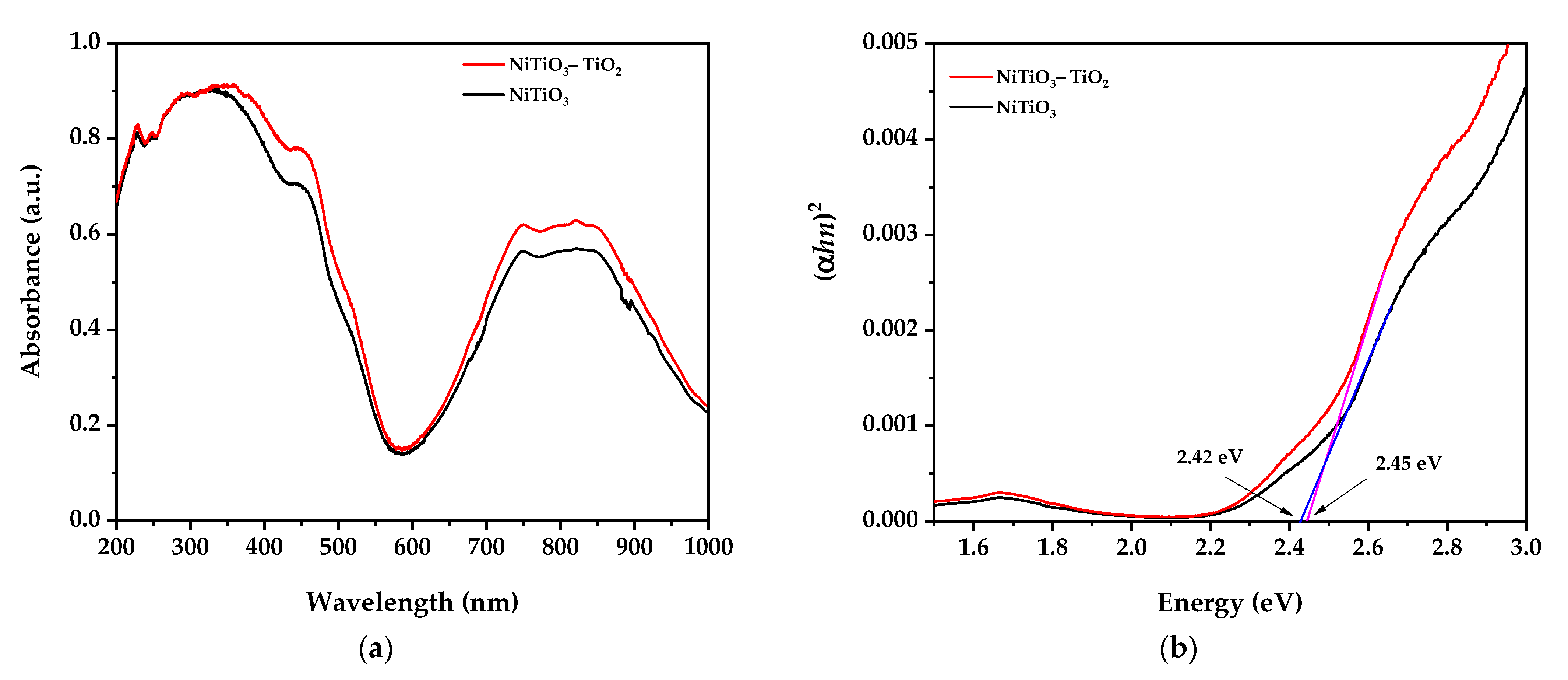
3.4. UV–Visible Spectroscopy Results
3.5. Photoluminiscence Spectroscopy Results
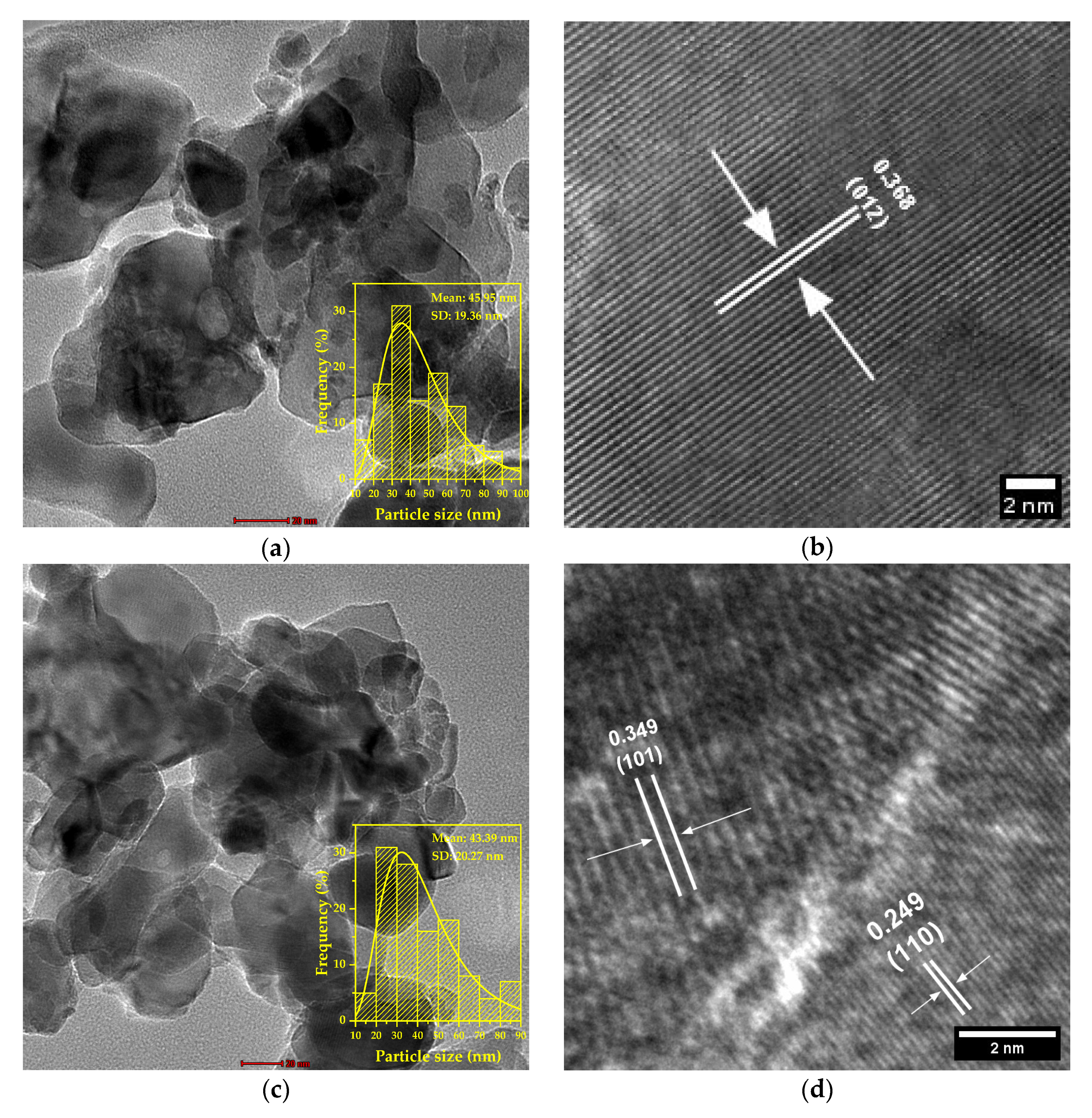
3.6. Transmission Electron Mycroscopy Results
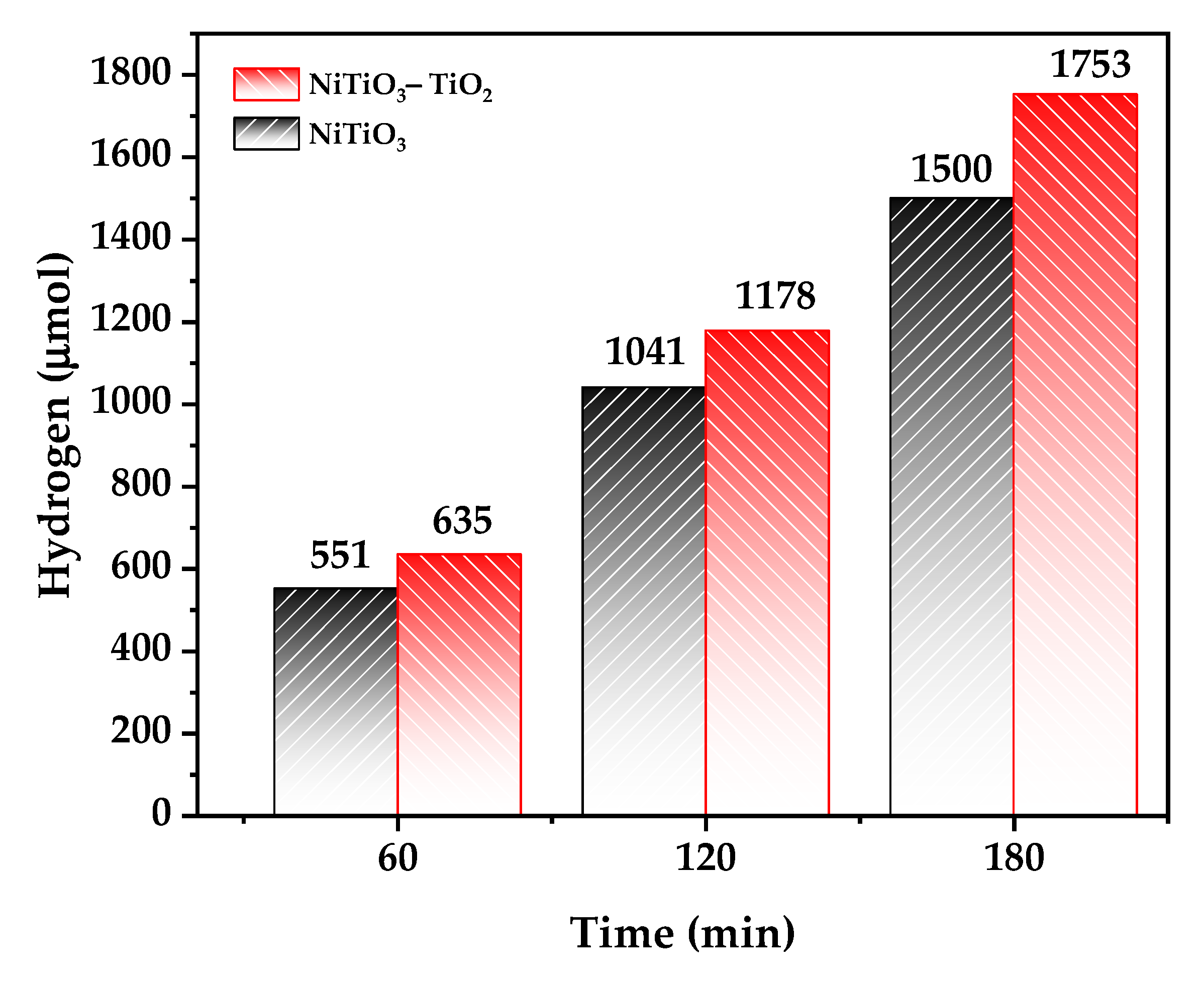
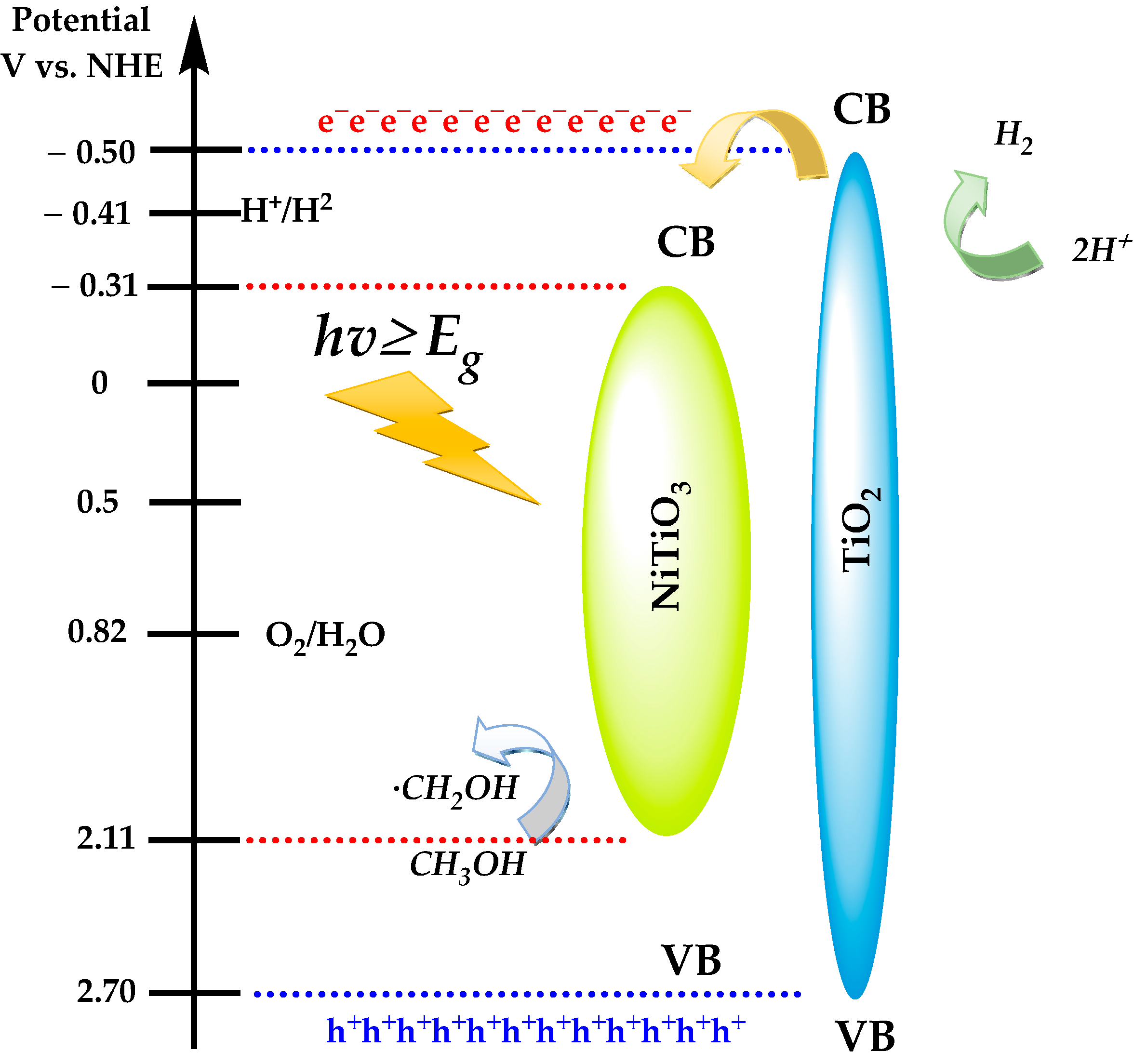
3.7. Hydrogen Generation Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakrabartty, I.; Hakeem, K.R. (Eds.) Green Nanomaterials in Energy Conversion and Storage Applications; Apple Academic Press: New York, NY, USA, 2023; ISBN 9781003398578. [Google Scholar]
- Soeder, D. Energy Futures; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-031-15380-8. [Google Scholar]
- Diab, G.A.A.; Da Silva, M.A.R.; Rocha, G.F.S.R.; Noleto, L.F.G.; Rogolino, A.; de Mesquita, J.P.; Jiménez-Calvo, P.; Teixeira, I.F. A Solar to Chemical Strategy: Green Hydrogen as a Means, Not an End. Glob. Chall. 2024, 8, 2300185. [Google Scholar] [CrossRef]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef]
- Cao, S.; Piao, L.; Chen, X. Emerging Photocatalysts for Hydrogen Evolution. Trends Chem. 2020, 2, 57–70. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Current trends in strategies to improve photocatalytic performance of perovskites materials for solar to hydrogen production. Renew. Sustain. Energy Rev. 2020, 132, 110073. [Google Scholar] [CrossRef]
- Xiao, Q.; An, Z.; Wang, Y.; Zhao, M.; Guo, X.; Jin, Z. Enhanced photocatalytic hydrogen evolution by S-scheme heterojunction and metal phosphide in NiTiO3/Graphdiyne. Int. J. Hydrogen Energy 2024, 89, 1012–1024. [Google Scholar] [CrossRef]
- Hussain, E.; Ishaq, A.; Abid, M.Z.; Waleed, M.Z.; Rauf, A.; Jin, R.; Rafiq, K. Sunlight-Driven Hydrogen Generation: Acceleration of Synergism between Cu–Ag Cocatalysts on a CdS System. ACS Appl. Energy Mater. 2024, 7, 1914–1926. [Google Scholar] [CrossRef]
- Rafique, M.; Hajra, S.; Irshad, M.; Usman, M.; Imran, M.; Assiri, M.A.; Ashraf, W.M. Hydrogen Production Using TiO2-Based Photocatalysts: A Comprehensive Review. ACS Omega 2023, 8, 25640–25648. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Marín, D.; Oza, G.; Real, J.D.; Cervantes-Uribe, A.; Pérez-Vidal, H.; Kesarla, M.K.; Torres, J.T.; Godavarthi, S. Distinguishing between type II and S-scheme heterojunction materials: A comprehensive review. Appl. Surf. Sci. Adv. 2024, 19, 100536. [Google Scholar] [CrossRef]
- Balapure, A.; Ray Dutta, J.; Ganesan, R. Recent advances in semiconductor heterojunctions: A detailed review of the fundamentals of photocatalysis, charge transfer mechanism and materials. RSC Appl. Interfaces 2024, 1, 43–69. [Google Scholar] [CrossRef]
- Tavakoli-Azar, T.; Mahjoub, A.R.; Sadjadi, M.S.; Ghaznavi-Ghoushchi, M.B. Synergistic effect of band gap and surface area on the improvement of NiTiO3 sunlight-driven photocatalysts via NiTiO3@S nanocomposites. Inorg. Chem. Commun. 2023, 152, 110658. [Google Scholar] [CrossRef]
- Subramanian, S.; Ganapathy, S.; Subramanian, S. Superior photocatalytic activities of p-CdTe QDs/n-NiTiO3 NFs system for the treatment of hazardous dye industrial effluents. J. Environ. Chem. Eng. 2022, 10, 106941. [Google Scholar] [CrossRef]
- Rawal, S.B.; Bera, S.; Lee, D.; Jang, D.-J.; Lee, W.I. Design of visible-light photocatalysts by coupling of narrow bandgap semiconductors and TiO2: Effect of their relative energy band positions on the photocatalytic efficiency. Catal. Sci. Technol. 2013, 3, 1822. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; Li, G.; Xue, C.; Guo, W. Hetero-structural NiTiO3/TiO2 nanotubes for efficient photocatalytic hydrogen generation. Renew. Energy 2017, 111, 410–415. [Google Scholar] [CrossRef]
- Moghiminia, S.; Farsi, H.; Raissi, H. Comparative optical and electrochemical studies of nanostructured NiTiO3 and NiTiO3-TiO2 prepared by a low temperature modified Sol-Gel route. Electrochim. Acta 2014, 132, 512–523. [Google Scholar] [CrossRef]
- Xing, C.; Liu, Y.; Zhang, Y.; Liu, J.; Zhang, T.; Tang, P.; Arbiol, J.; Soler, L.; Sivula, K.; Guijarro, N.; et al. Porous NiTiO3/TiO2 nanostructures for photocatatalytic hydrogen evolution. J. Mater. Chem. A 2019, 7, 17053–17059. [Google Scholar] [CrossRef]
- Ruiz-Preciado, M.A.; Kassiba, A.; Gibaud, A.; Morales-Acevedo, A. Comparison of nickel titanate (NiTiO3) powders synthesized by sol–gel and solid state reaction. Mater. Sci. Semicond. Process. 2015, 37, 171–178. [Google Scholar] [CrossRef]
- Althomali, R.H.; Jabbar, H.S.; Kareem, A.T.; Alazbjee, A.A.A.; Abdullaeva, B.; Abdullaev, S.S.; Alsalamy, A.; Hussien, B.M.; Balasim, H.M.; Mohammed, Y. Various methods for the synthesis of NiTiO3 and ZnTiO3 nanomaterials and their optical, sensor and photocatalyst potentials: A review. Inorg. Chem. Commun. 2023, 158, 111493. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Wang, J.-G.; Sun, H.-H.; Wei, B. Heterostructured TiO2/NiTiO3 Nanorod Arrays for Inorganic Sensitized Solar Cells with Significantly Enhanced Photovoltaic Performance and Stability. ACS Appl. Mater. Interfaces 2018, 10, 11580–11586. [Google Scholar] [CrossRef]
- Quispe Cohaila, A.B.; Sacari Sacari, E.J.; Lanchipa Ramos, W.O.; Tamayo Calderón, R.M.; Medina Salas, J.P.; Gamarra Gómez, F.; Viswanathan Mangalaraja, R.; Rajendran, S. Photocatalytic Hydrogen Production Enhancement of NiTiO3 Perovskite through Cobalt Incorporation. Energies 2024, 17, 3704. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction: New International Edition; Pearson Education: Harlow, UK, 2014; ISBN 9781292040547. [Google Scholar]
- Amadine, O.; Essamlali, Y.; Fihri, A.; Larzek, M.; Zahouily, M. Effect of calcination temperature on the structure and catalytic performance of copper–ceria mixed oxide catalysts in phenol hydroxylation. RSC Adv. 2017, 7, 12586–12597. [Google Scholar] [CrossRef]
- Enhessari, M.; Sakhaei, M.; Salehabadi, A.; Etemad, L. CeO2/NiTiO3 nanocomposites; synthesis, photoluminescence and magnetic behavior. Mater. Sci.-Pol. 2017, 35, 275–282. [Google Scholar] [CrossRef]
- Alam, U.; Pandey, A.; Verma, N. An anthraquinone-integrated S-scheme-based NiTiO3-g-C3N4 composite with enhanced hydrogen production activity. Int. J. Hydrogen Energy 2023, 48, 2532–2541. [Google Scholar] [CrossRef]
- Diab, K.R.; El-Maghrabi, H.H.; Nada, A.A.; Youssef, A.M.; Hamdy, A.; Roualdes, S.; Abd El-Wahab, S. Facile fabrication of NiTiO3/graphene nanocomposites for photocatalytic hydrogen generation. J. Photochem. Photobiol. A Chem. 2018, 365, 86–93. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Nguyen, T.; Ha, C.A.; Hoang, T.C.; van Nguyen, T.T.; Nguyen, D.T.; Luu, C.L. Exceptional photodecomposition activity of heterostructure NiTiO3–TiO2 catalyst. J. Sci. Adv. Mater. Devices 2022, 7, 100407. [Google Scholar] [CrossRef]
- He, X.; Wang, F.; Liu, H.; Li, J.; Niu, L. Fabrication of highly dispersed NiTiO3@TiO2 yellow pigments with enhanced NIR reflectance. Mater. Lett. 2017, 208, 82–85. [Google Scholar] [CrossRef]
- Araújo, E.S.; Libardi, J.; Faia, P.M.; de Oliveira, H.P. Hybrid ZnO/TiO2 Loaded in Electrospun Polymeric Fibers as Photocatalyst. J. Chem. 2015, 2015, 476472. [Google Scholar] [CrossRef]
- Pavithra, C.; Madhuri, W. Electrical and magnetic properties of NiTiO3 nanoparticles synthesized by the sol–gel synthesis method and microwave sintering. J. Mater. Res. Technol. 2019, 8, 3097–3101. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Nithya, V.D.; Fathima, K.S.; Sanjeeviraja, C.; Selvan, G.K.; Arumugam, S.; Selvan, R.K. Investigations on the temperature dependent electrical and magnetic properties of NiTiO3 by molten salt synthesis. Mater. Res. Bull. 2013, 48, 1110–1116. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, P.; Subramanian, M.A.; Cao, W. Synthesis, properties and applications of novel inorganic yellow pigments based on Ni-doped Al2TiO5. Solid State Sci. 2023, 135, 107088. [Google Scholar] [CrossRef]
- De Haart, L.; de Vries, A.J.; Blasse, G. Photoelectrochemical properties of MgTiO3 and other titanates with the ilmenite structure. Mater. Res. Bull. 1984, 19, 817–824. [Google Scholar] [CrossRef]
- Kumar, B.S.; Shanmugharaj, A.M.; Kalpathy, S.K.; Anandhan, S. Some new observations on the structural and phase evolution of nickel titanate nanofibers. Ceram. Int. 2017, 43, 6845–6857. [Google Scholar] [CrossRef]
- Agui, A.; Mizumaki, M. Intermetallic charge transfer and band gap of MTiO3 (M = Mn, Fe, Co, and Ni) studied by O 1s-edge X-ray emission spectroscopy. J. Electron Spectrosc. Relat. Phenom. 2011, 184, 463–467. [Google Scholar] [CrossRef]
- Salvador, P.; Gutiérrez, C.; Goodenough, J.B. Photoelectrochemical properties of n-type NiTiO3. J. Appl. Phys. 1982, 53, 7003–7013. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Hafeez, H.Y.; Veluswamy, P.; Ganesh, V.; Khan, A.; Ikeda, H.; Neppolian, B. Enhanced photocatalytic degradation and hydrogen production activity of in situ grown TiO2 coupled NiTiO3 nanocomposites. Appl. Surf. Sci. 2018, 449, 790–798. [Google Scholar] [CrossRef]
- Boudjellal, L.; Belhadi, A.; Brahimi, R.; Boumaza, S.; Trari, M. Physical and photoelectrochemical properties of the ilmenite NiTiO3 prepared by wet chemical method and its application for O2 evolution under visible light. Mater. Sci. Semicond. Process. 2018, 75, 247–252. [Google Scholar] [CrossRef]
- Xing, C.; Liu, Y.; Zhang, Y.; Wang, X.; Guardia, P.; Yao, L.; Han, X.; Zhang, T.; Arbiol, J.; Soler, L.; et al. A Direct Z-Scheme for the Photocatalytic Hydrogen Production from a Water Ethanol Mixture on CoTiO3/TiO2 Heterostructures. ACS Appl. Mater. Interfaces 2021, 13, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Cañas-Martínez, D.M.; Cipagauta-Díaz, S.; Manrique, M.; Gómez, R.; Pedraza-Avella, J.A. Photocatalytic hydrogen production using FeTiO3 concentrates modified by high energy ball milling and the presence of Mg precursors. Top. Catal. 2021, 64, 2–16. [Google Scholar] [CrossRef]
- Saadetnejad, D.; Yıldırım, R. Photocatalytic hydrogen production by water splitting over Au/Al-SrTiO3. Int. J. Hydrogen Energy 2018, 43, 1116–1122. [Google Scholar] [CrossRef]
- Alzahrani, A. Photocatalytic Hydrogen Production Using Metallic Silver Loaded Calcium Titanate Photocatalyst. Ph.D. Thesis, Rutgers University-Camden Graduate School, Camden, NJ, USA, 2016. [Google Scholar]
- Lu, L.; Lv, M.; Liu, G.; Xu, X. Photocatalytic hydrogen production over solid solutions between BiFeO3 and SrTiO3. Appl. Surf. Sci. 2017, 391, 535–541. [Google Scholar] [CrossRef]
- Pantoja-Espinoza, J.C.; Domínguez-Arvizu, J.L.; Jiménez-Miramontes, J.A.; Hernández-Majalca, B.C.; Meléndez-Zaragoza, M.J.; Salinas-Gutiérrez, J.M.; Herrera-Pérez, G.M.; Collins-Martínez, V.H.; López-Ortiz, A. Comparative Study of Zn2Ti3O8 and ZnTiO3 Photocatalytic Properties for Hydrogen Production. Catalysts 2020, 10, 1372. [Google Scholar] [CrossRef]



| Structural | Sample | ||
|---|---|---|---|
| Parameter | NiTiO3 | NiTiO3—TiO2 | |
| Phase Name | NiTiO3 | NiTiO3 | TiO2 (Anatase) |
| Phase (%) | 100% | 99.2 | 0.8 |
| Crystal system | Hexagonal | Hexagonal | Tetragonal |
| Space group | R − 3 | R − 3 | I 41/a m d |
| a (Å) | 5.02588 | 5.2576 | 3.78503 |
| b (Å) | 5.02588 | 5.2576 | 3.78503 |
| c (Å) | 13.80987 | 13.8102 | 9.50425 |
| α (°) | 90 | 90 | 90 |
| β (°) | 90 | 90 | 90 |
| γ (°) | 120 | 120 | 90 |
| ρ (g/cm3) | 5 | 5.03 | 3.59 |
| D (nm) | 53.46 | 46.35 | 68.94 |
| Rexp (%) | 6.322 | 6.13182 | |
| Rp (%) | 4.197 | 3.7737 | |
| Rwp (%) | 6.164 | 5.4464 | |
| GOF | 0.97 | 0.88 | |
| Sample | Peak | Area Intg | FWHM | Max Height | Center Grvty | Area IntgP |
|---|---|---|---|---|---|---|
| NiTiO3 | 1 (green line) | 7,181,083.94 | 56.65583 | 119,077.172 | 404.1403 | 65.44915 |
| 2 (blue line) | 3,790,920.64 | 63.46499 | 56,329.4278 | 361.95239 | 34.55085 | |
| NiTiO3/TiO2 | 1 (green line) | 1,107,529.89 | 59.48056 | 17,493.1386 | 400.0478 | 67.32776 |
| 2 (blue line) | 537,452.619 | 56.70732 | 8947.51769 | 352.23357 | 32.67224 |
| N° | Catalyst | Cocatalyst | Light | Sacrificial Agent | H2 Production Rate | Reference |
|---|---|---|---|---|---|---|
| 1 | SrTiO3 | Au-Al | Solar–Xenon (150 W) | Methanol | ~285 µmol g−1 h−1 | [44] |
| 2 | CaTiO3 | Ag | Mercury lamp (450 W) | Glycerol (10%) | 167 µmol g−1 h−1 | [45] |
| 3 | SrTiO3 | Bi-Fe | λ ≥ 250 nm | Sodium sulfite (0.05 M) | ~180 µmol g−1 h−1 | [46] |
| 4 | FeTiO3 | - | Pen-Ray Ultraviolet lamp | Methanol | 240.5 µmol g−1 h−1 | [43] |
| 5 | NiTiO3 | TiO2 | Xe Lamp 250 W | Glycerol (5%) | 87 µmol g−1 h−1 | [40] |
| 6 | CoTiO3 | - | UV light (365 ± 5 nm and 79.1 ± 0.5 mW cm−2) | Ethanol (10%)–water | 0.3 mmol g−1 h−1 | [42] |
| 7 | ZnTiO3 | - | visible light emitted by a 250 W metal-halide Philips lamp MH/U | Methanol (10%)–water | 31 µmol g−1 h−1 | [47] |
| 8 | NiTiO3 | UV Lamp (300 W) | 500 mL methanol (10%)–water solution | 499.2 µmol g−1 h−1 | Present Work | |
| 9 | NiTiO3 | TiO2 | UV Lamp (300 W) | 500 mL methanol (10%)–water solution | 584.4 µmol g−1 h−1 | Present Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe Cohaila, A.B.; Sacari Sacari, E.J.; Lanchipa Ramos, W.O.; Canahua Loza, H.B.; Tamayo Calderón, R.M.; Medina Salas, J.P.; Gamarra Gómez, F.; Mangalaraja, R.V.; Rajendran, S. Improving Photocatalytic Hydrogen Production with Sol–Gel Prepared NiTiO₃/TiO₂ Composite. Energies 2024, 17, 5830. https://doi.org/10.3390/en17235830
Quispe Cohaila AB, Sacari Sacari EJ, Lanchipa Ramos WO, Canahua Loza HB, Tamayo Calderón RM, Medina Salas JP, Gamarra Gómez F, Mangalaraja RV, Rajendran S. Improving Photocatalytic Hydrogen Production with Sol–Gel Prepared NiTiO₃/TiO₂ Composite. Energies. 2024; 17(23):5830. https://doi.org/10.3390/en17235830
Chicago/Turabian StyleQuispe Cohaila, Alberto Bacilio, Elisban Juani Sacari Sacari, Wilson Orlando Lanchipa Ramos, Hugo Benito Canahua Loza, Rocío María Tamayo Calderón, Jesús Plácido Medina Salas, Francisco Gamarra Gómez, Ramalinga Viswanathan Mangalaraja, and Saravanan Rajendran. 2024. "Improving Photocatalytic Hydrogen Production with Sol–Gel Prepared NiTiO₃/TiO₂ Composite" Energies 17, no. 23: 5830. https://doi.org/10.3390/en17235830
APA StyleQuispe Cohaila, A. B., Sacari Sacari, E. J., Lanchipa Ramos, W. O., Canahua Loza, H. B., Tamayo Calderón, R. M., Medina Salas, J. P., Gamarra Gómez, F., Mangalaraja, R. V., & Rajendran, S. (2024). Improving Photocatalytic Hydrogen Production with Sol–Gel Prepared NiTiO₃/TiO₂ Composite. Energies, 17(23), 5830. https://doi.org/10.3390/en17235830








