Comparative Analysis on Carbon Mitigation by High-Temperature Lithium Adsorption Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Adsorbents Synthesis
2.2. Adsorption and Desorption Test
2.3. Characterization of Adsorbents
3. Results and Discussion
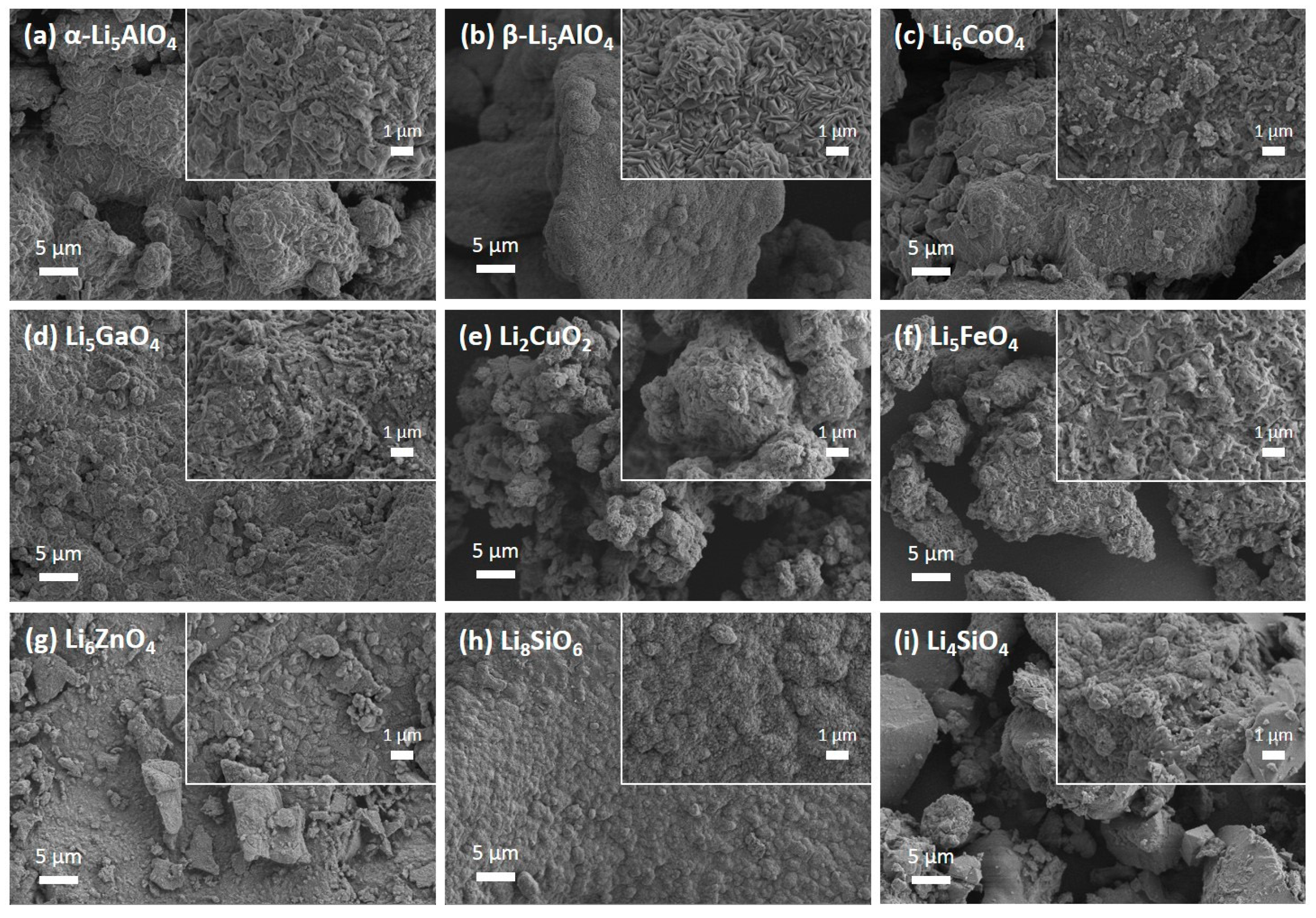
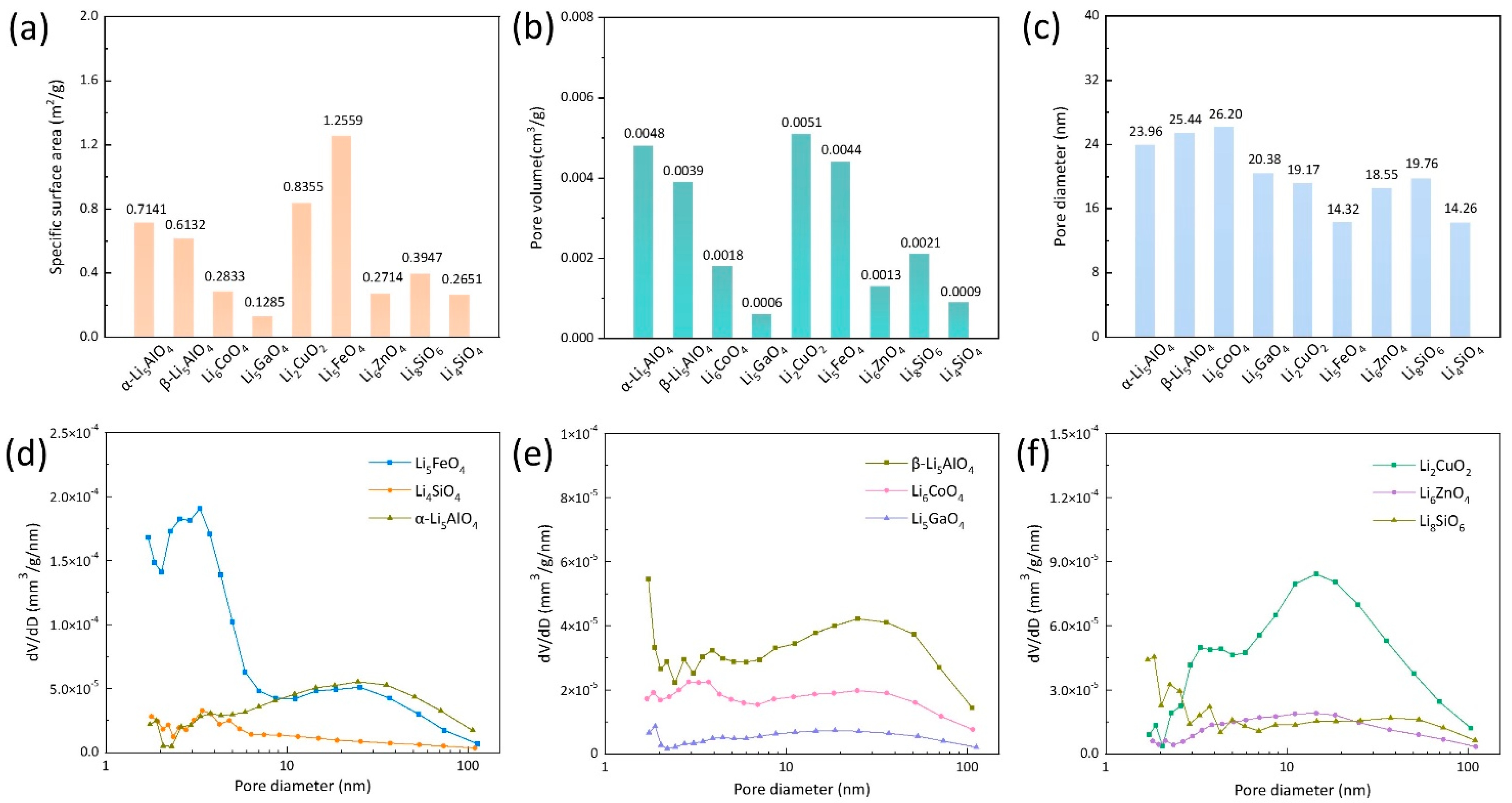
3.1. Microstructural Properties of Fresh Adsorbents
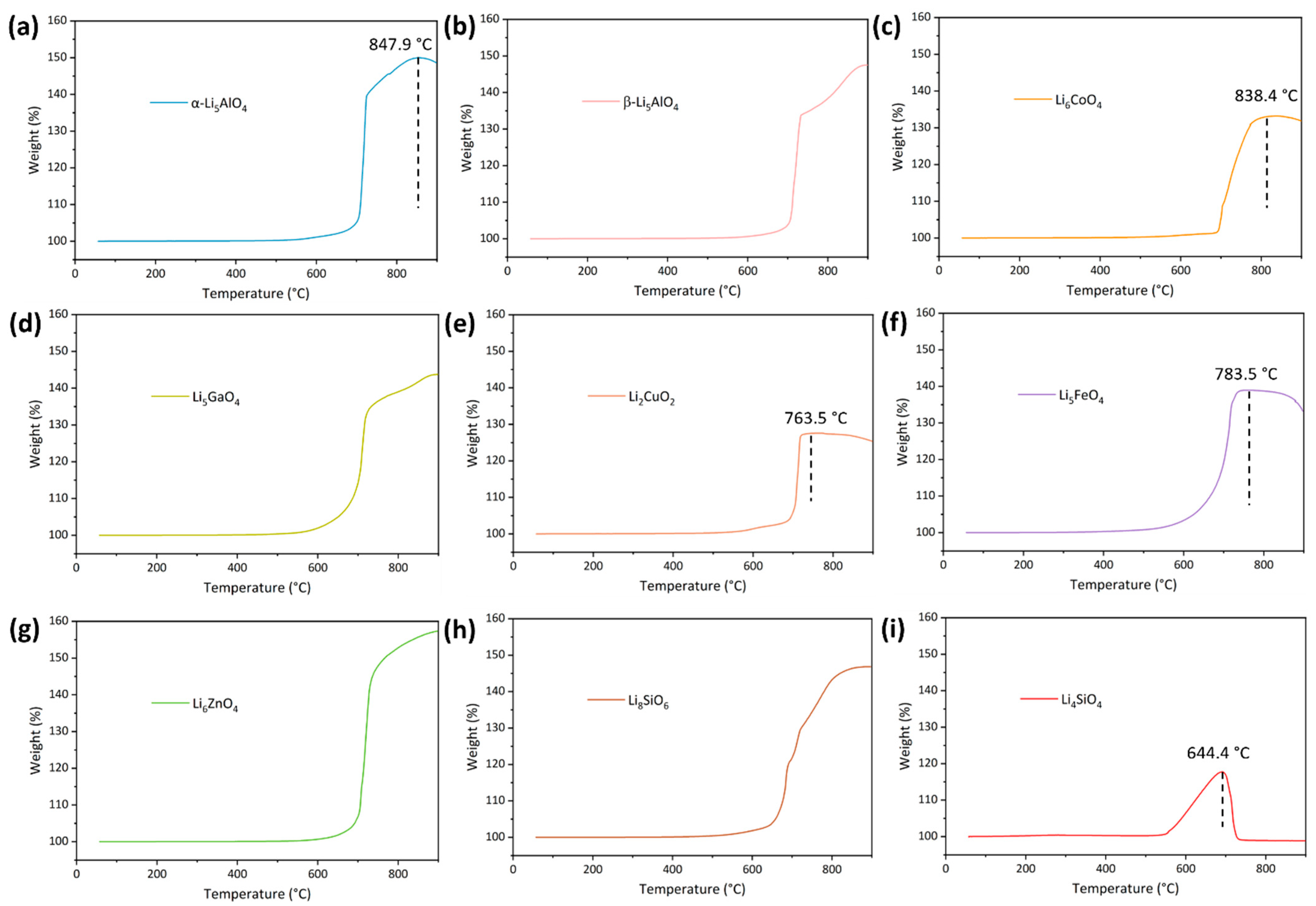
3.2. CO2 Adsorption Characteristics of Nine Adsorbents
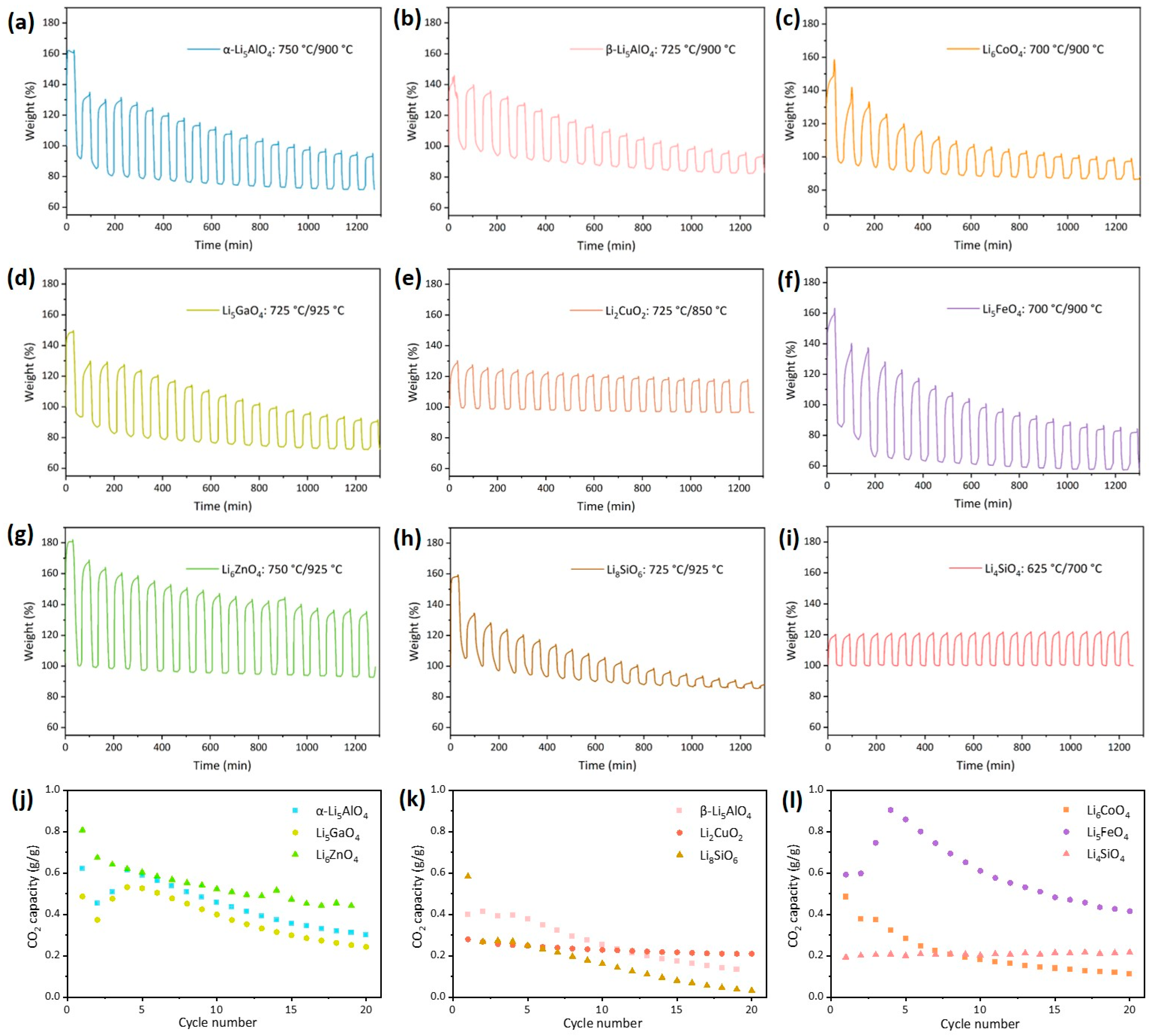
3.3. Cyclic Performance and Evolution of Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Huang, Y.; Liu, W.; Yong, J.Y.; Zhang, X.J.; Wu, C.; Jiang, L. Environmental tradeoff on integrated carbon capture and in-situ methanation technology. Renew. Sustain. Energy Rev. 2025, 208, 115029. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Z. Carbon Capture, Utilization and Storage (CCUS). Appl. Energy 2019, 235, 1289–1299. [Google Scholar] [CrossRef]
- Dunstan, M.T.; Jain, A.; Liu, W.; Ong, S.P.; Liu, T.; Lee, J.; Persson, K.A.; Scott, S.A.; Dennis, J.S.; Grey, C.P. Large scale computational screening and experimental discovery of novel materials for high temperature CO2 capture. Energy Environ. Sci. 2016, 9, 1346–1360. [Google Scholar] [CrossRef]
- Yan, Y.; Borhani, T.N.; Subraveti, S.G.; Pai, K.N.; Prasad, V.; Rajendran, A.; Nkulikiyinka, P.; Asibor, J.O.; Zhang, Z.; Shao, D.; et al. Harnessing the power of machine learning for carbon capture, utilisation, and storage (CCUS)—A state-of-the-art review. Energy Environ. Sci. 2021, 14, 6122–6157. [Google Scholar] [CrossRef]
- Ma, X.; Luo, S.; Hua, Y.; Seetharaman, S.; Zhu, X.; Hou, J.; Zhang, L.; Wang, W.; Sun, Y. An alumina phase induced composite transition shuttle to stabilize carbon capture cycles. Nat. Commun. 2024, 15, 7556. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Zhou, S.; Li, Z.; Sun, J.; Hu, Y.; Yang, Y. A review on granulation of CaO-based sorbent for carbon dioxide capture. Chem. Eng. J. 2022, 446, 136880. [Google Scholar] [CrossRef]
- Yasipourtehrani, S.; Tian, S.; Strezov, V.; Kan, T.; Evans, T. Development of robust CaO-based sorbents from blast furnace slag for calcium looping CO2 capture. Chem. Eng. J. 2020, 387, 124140. [Google Scholar] [CrossRef]
- Deng, T.; Chen, S.; Lv, Z.; Zheng, Y.; Xu, S.; Qin, C. Ionic Diffusion in CO2 Adsorption by Li4SiO4: Inert-Marker Experiment and DFT Calculations. Phys. Rev. Lett. 2024, 133, 198001. [Google Scholar] [CrossRef]
- Hu, Y.; Fu, R.; Liu, W.; Yao, D.; Yan, S. Lithium-based ceramics in nonsilicates for CO2 capture: Current status and new trends. J. Mater. Chem. A 2022, 10, 1706–1725. [Google Scholar] [CrossRef]
- Kierzkowska, A.M.; Pacciani, R.; Müller, C.R. CaO-Based CO2 Sorbents: From Fundamentals to the Development of New, Highly Effective Materials. ChemSusChem 2013, 6, 1130–1148. [Google Scholar] [CrossRef]
- Sun, J.; Liang, C.; Wang, W.; Liu, W. Screening of Naturally Al/Si-Based Mineral Binders to Modify CaO-Based Pellets for CO2 Capture. Energy Fuels 2017, 31, 14070–14078. [Google Scholar] [CrossRef]
- Ma, L.; Qin, C.; Pi, S.; Cui, H. Fabrication of efficient and stable Li4SiO4-based sorbent pellets via extrusion-spheronization for cyclic CO2 capture. Chem. Eng. J. 2020, 379, 122385. [Google Scholar] [CrossRef]
- Stefanelli, E.; Francalanci, F.; Vitolo, S.; Puccini, M. Insights into adsorption mechanism and kinetic modeling of K2CO3-doped Li4SiO4 pellets for CO2 capture at high temperature and low concentration. Fuel 2025, 380, 133161. [Google Scholar] [CrossRef]
- Usas, S.A.; Ricardez-Sandoval, L. Biomass fly-ash derived Li4SiO4 solid for pilot-scale CO2 capture, Part I: Modelling for a waste to capture CO2 process. Chem. Eng. Res. Des. 2025, 214, 219–233. [Google Scholar] [CrossRef]
- Stefanelli, E.; Puccini, M.; Vitolo, S.; Seggiani, M. CO2 sorption kinetic study and modeling on doped-Li4SiO4 under different temperatures and CO2 partial pressures. Chem. Eng. J. 2020, 379, 122307. [Google Scholar] [CrossRef]
- Wang, X.; Wei, J.; Jia, Y.; Geng, L.; Wang, D. Evaluation of Na2CO3-doped MCM-48-Li4SiO4 adsorbent for CO2 capture: Performance and DFT mechanism. Sep. Purif. Technol. 2025, 354, 129417. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Hu, Y. Fabrication of yttrium-doped Li4SiO4 sorbents for CO2 capture and solar energy storage. Fuel 2024, 373, 132255. [Google Scholar] [CrossRef]
- Liao, T.; Qian, Y.; Yu, M.; Tang, A.; Yang, H. CO2 capture utilizing Li4SiO4 from spent lithium-ion batteries and iron tailings offers eco-friendly benefits. Chem. Eng. J. 2024, 493, 152756. [Google Scholar] [CrossRef]
- Guo, A.; Cao, C.; Sun, Y.; Yang, Z.; Wang, Q.; Li, X.; Li, Z.; Huang, L.; Yu, F. Synthesis of highly stable Li4SiO4-based CO2 sorbents using polysilicon by-product SiCl4 as raw material. Chem. Eng. J. 2024, 500, 157055. [Google Scholar] [CrossRef]
- Subha, P.V.; Nair, B.N.; Visakh, V.; Achu, R.; Shikhila, S.; Peer Mohamed, A.; Yamaguchi, T.; Hareesh, U.S. Wet chemically derived Li4SiO4 nanowires as efficient CO2 sorbents at intermediate temperatures. Chem. Eng. J. 2021, 406, 126731. [Google Scholar] [CrossRef]
- Belgamwar, R.; Maity, A.; Das, T.; Chakraborty, S.; Vinod, C.P.; Polshettiwar, V. Lithium silicate nanosheets with excellent capture capacity and kinetics with unprecedented stability for high-temperature CO2 capture. Chem. Sci. 2021, 12, 4825–4835. [Google Scholar] [CrossRef] [PubMed]
- Durán-Muñoz, F.; Romero-Ibarra, I.C.; Pfeiffer, H. Analysis of the CO2 chemisorption reaction mechanism in lithium oxosilicate (Li8SiO6): A new option for high-temperature CO2 capture. J. Mater. Chem. A 2013, 1, 3919–3925. [Google Scholar] [CrossRef]
- Ávalos-Rendón, T.; Pfeiffer, H. Evidence of CO2 Chemisorption at High Temperature in Lithium Gallate (Li5GaO4). Chem. Lett. 2011, 40, 504–505. [Google Scholar] [CrossRef]
- Ávalos-Rendón, T.; Casa-Madrid, J.; Pfeiffer, H. Thermochemical Capture of Carbon Dioxide on Lithium Aluminates (LiAlO2 and Li5AlO4): A New Option for the CO2 Absorption. J. Phys. Chem. A 2009, 113, 6919–6923. [Google Scholar] [CrossRef]
- Togashi, N.; Okumura, T.; Oh-Ishi, K. Synthesis and CO2 Absorption Property of Li4TiO4 as a Novel CO2 Absorbent. J. Ceram. Soc. Jpn. 2007, 115, 324–328. [Google Scholar] [CrossRef]
- Zhou, X.; Plascencia-Hernández, F.; Yu, F.; Pfeiffer, H. Understanding the CO2 chemical reaction path on Li6ZnO4, a new possible high temperature CO2 captor. Chem. Eng. J. 2021, 417, 129205. [Google Scholar] [CrossRef]
- Bernabé-Pablo, E.; Plascencia-Hernández, F.; Yañez-Aulestia, A.; Pfeiffer, H. High and efficient carbon dioxide chemisorption on a new high lithium-content ceramic; hexalithium cobaltate (Li6CoO4). Chem. Eng. J. 2020, 384, 123291. [Google Scholar] [CrossRef]
- Lara-García, H.A.; Sanchez-Camacho, P.; Duan, Y.; Ortiz-Landeros, J.; Pfeiffer, H. Analysis of the CO2 Chemisorption in Li5FeO4, a New High Temperature CO2 Captor Material. Effect of the CO2 and O2 Partial Pressures. J. Phys. Chem. C 2017, 121, 3455–3462. [Google Scholar] [CrossRef]
- Gaultois, M.W.; Dunstan, M.T.; Bateson, A.W.; Chan, M.S.C.; Grey, C.P. Screening and Characterization of Ternary Oxides for High-Temperature Carbon Capture. Chem. Mater. 2018, 30, 2535–2543. [Google Scholar] [CrossRef]
- Palacios-Romero, L.M.; Pfeiffer, H. Lithium Cuprate (Li2CuO2): A New Possible Ceramic Material for CO2 Chemisorption. Chem. Lett. 2008, 37, 862–863. [Google Scholar] [CrossRef]




| Adsorbent | Precursors | Temperature | Atmosphere | Time |
| α-Li5AlO4 | Li2O, γ-Al2O3 | 500 °C | Air | 24 h |
| β-Li5AlO4 | Li2O, γ-Al2O3 | 900 °C | Air | 24 h |
| Li6CoO4 | Li2O, CoO | 800 °C | N2 | 12 h |
| Li5GaO4 | Li2O, Ga2O3 | 500 °C | Air | 24 h |
| Li2CuO2 | Li2O, CuO | 685 °C | Air | 24 h |
| Li5FeO4 | Li2O, Fe2O3 | 850 °C | Air | 20 h |
| Li6ZnO4 | Li2O, ZnO | 800 °C | N2 | 14 h |
| Li8SiO6 | Li2O, SiO2 | 800 °C | Air | 8 h |
| Li4SiO4 | Li2CO3, SiO2 | 900 °C | Air | 24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Ruan, J.; Li, Y.; Qin, C. Comparative Analysis on Carbon Mitigation by High-Temperature Lithium Adsorption Systems. Energies 2025, 18, 2817. https://doi.org/10.3390/en18112817
Du H, Ruan J, Li Y, Qin C. Comparative Analysis on Carbon Mitigation by High-Temperature Lithium Adsorption Systems. Energies. 2025; 18(11):2817. https://doi.org/10.3390/en18112817
Chicago/Turabian StyleDu, Hong, Jiaqi Ruan, Yunlin Li, and Changlei Qin. 2025. "Comparative Analysis on Carbon Mitigation by High-Temperature Lithium Adsorption Systems" Energies 18, no. 11: 2817. https://doi.org/10.3390/en18112817
APA StyleDu, H., Ruan, J., Li, Y., & Qin, C. (2025). Comparative Analysis on Carbon Mitigation by High-Temperature Lithium Adsorption Systems. Energies, 18(11), 2817. https://doi.org/10.3390/en18112817






