Study on Characteristics of Ash Accumulation During Co-Combustion of Salix Biomass and Coal
Abstract
1. Introduction
2. Materials and Methods
2.1. Mechanism of Ash Deposition and Slagging
2.2. Raw Material
2.3. Experiment
2.4. Analytical Instruments
3. Results and Discussion
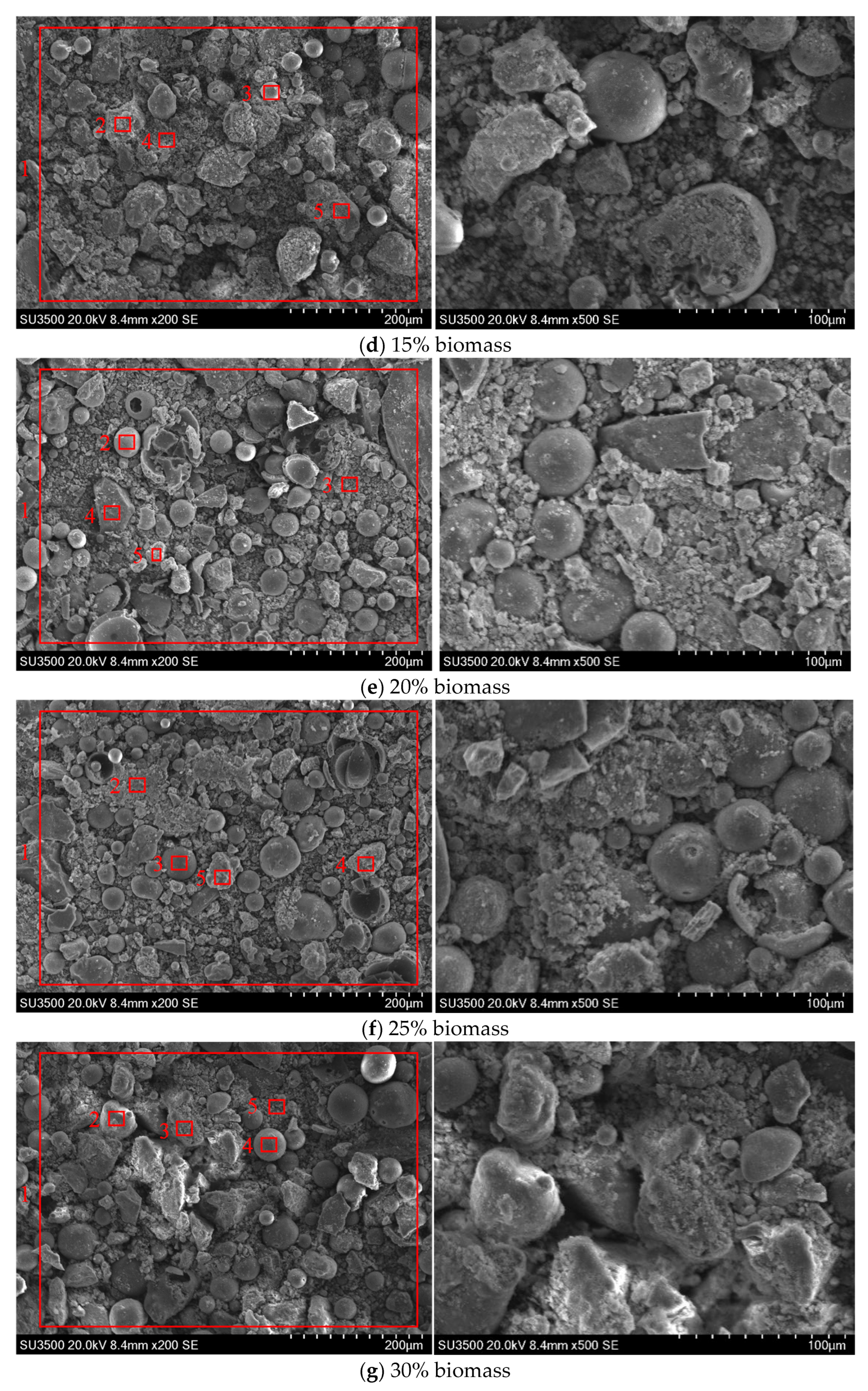
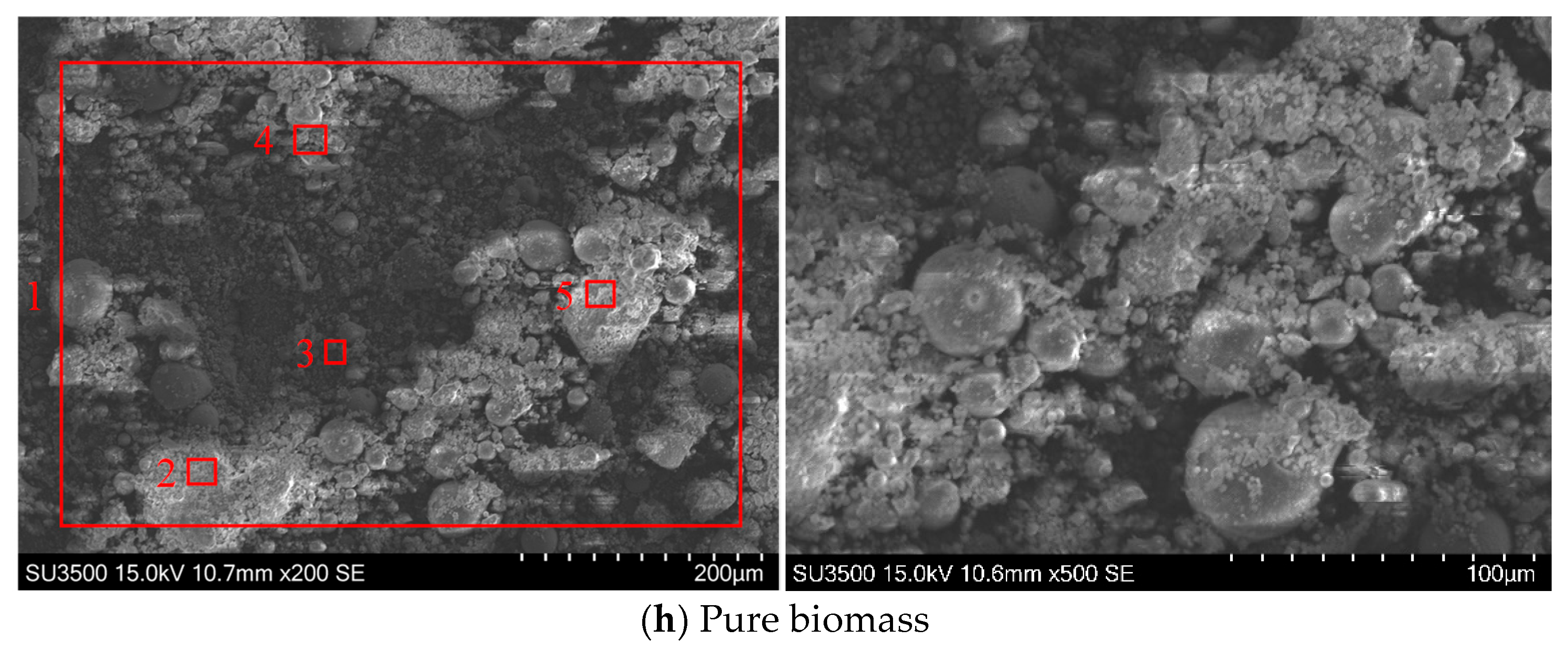
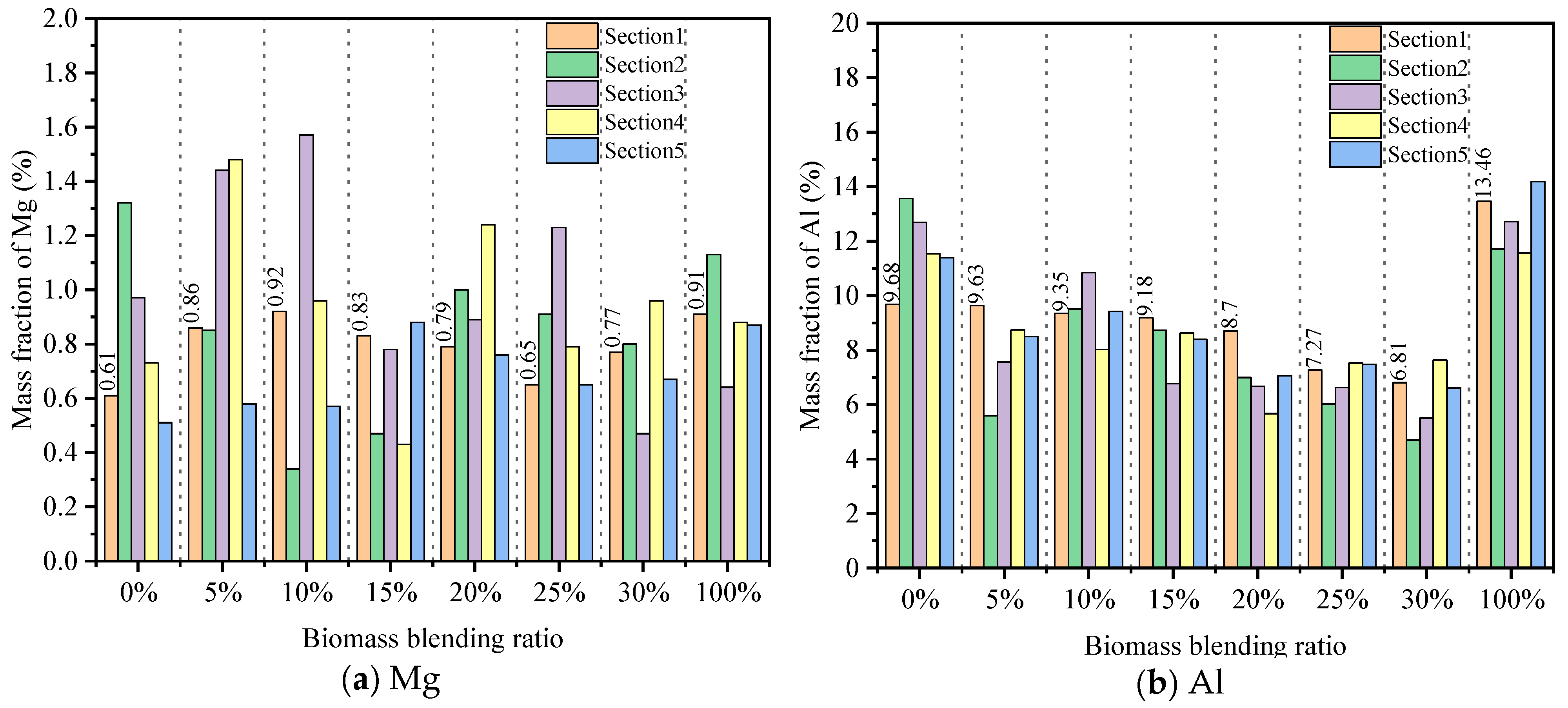
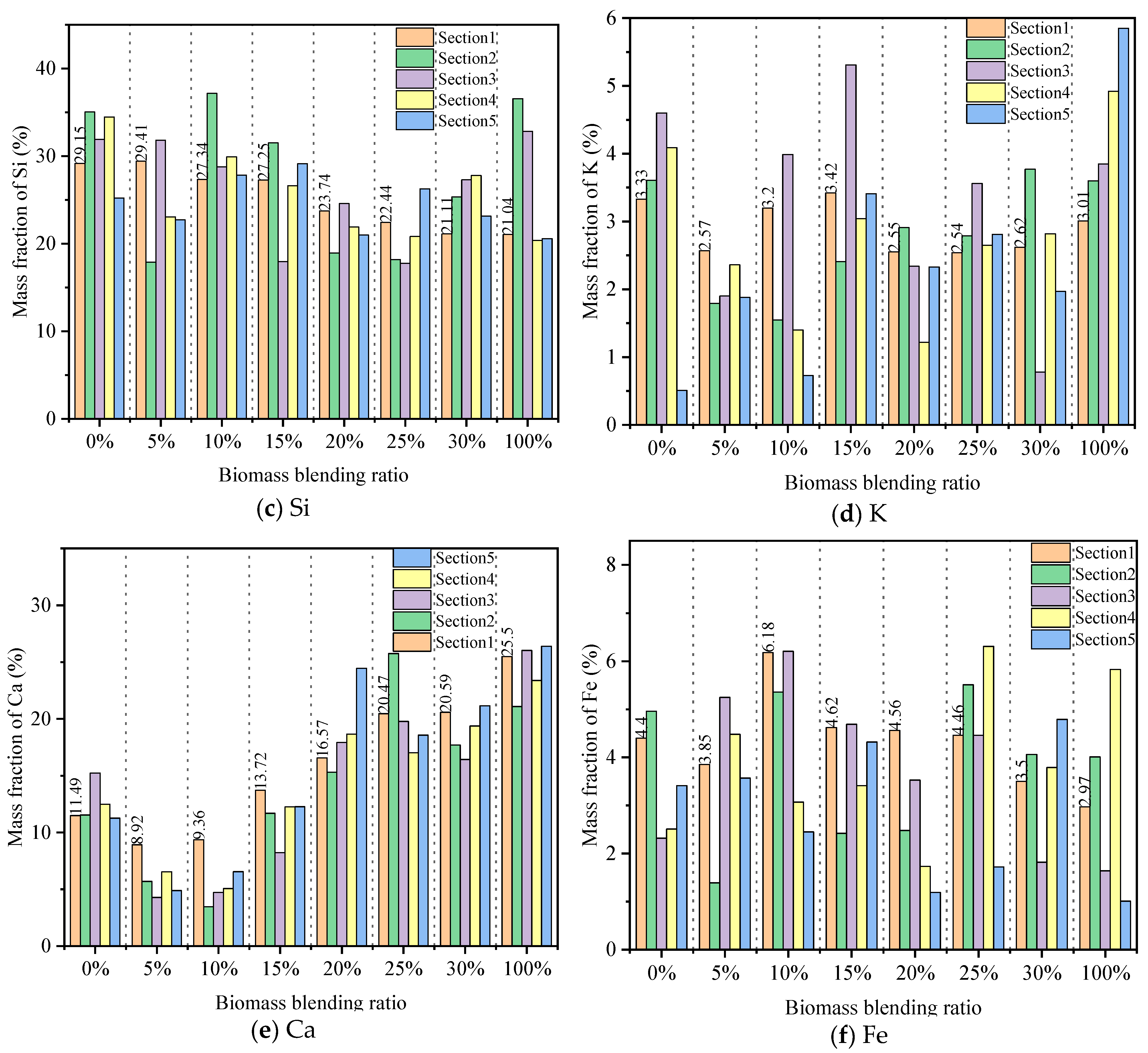
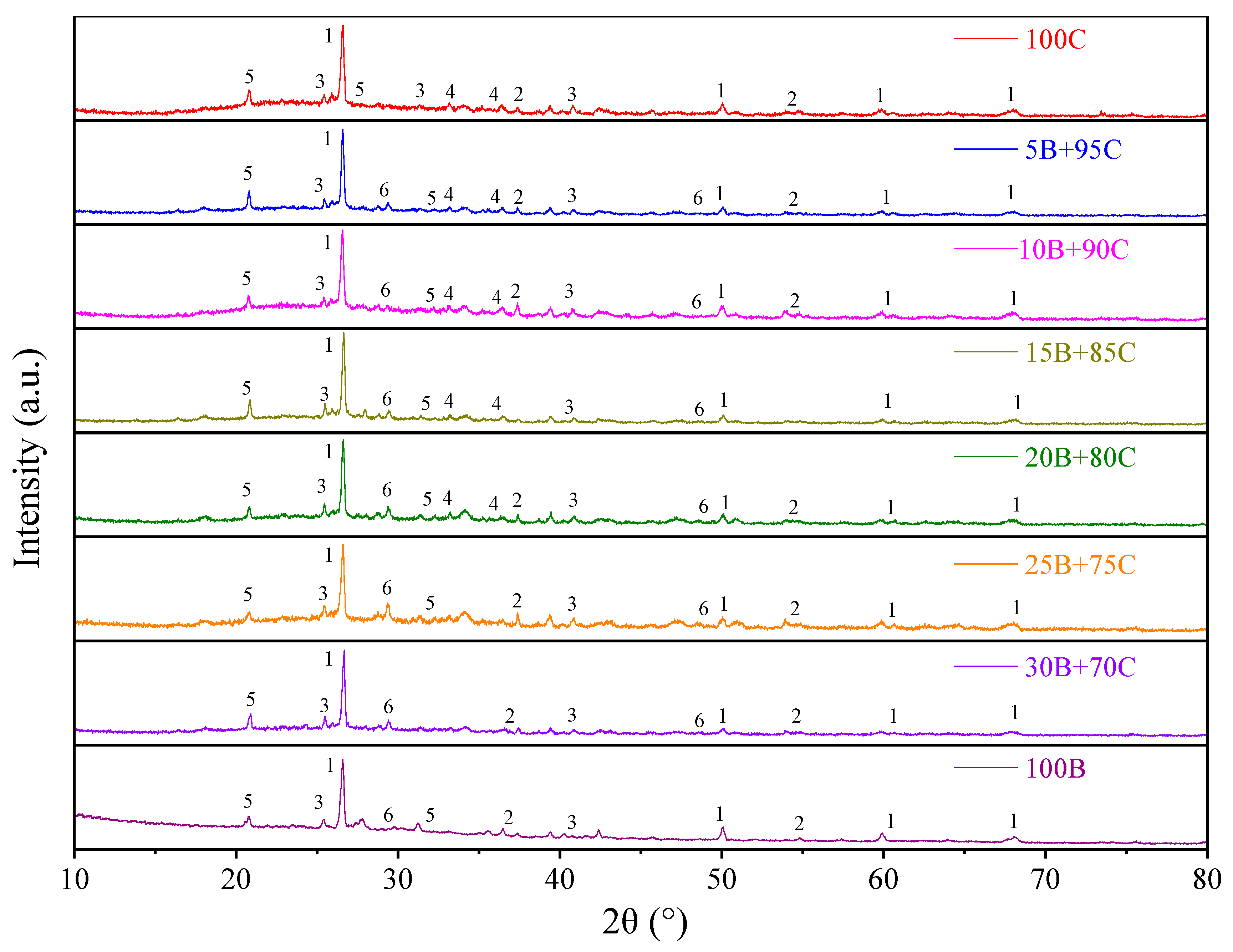
3.1. Effect of Biomass Blending Ratio on Ash Deposition
3.1.1. SEM-EDS Analysis
3.1.2. XRD Analysis
3.2. Effect of Flue Gas Temperature on Ash Deposition Characteristics
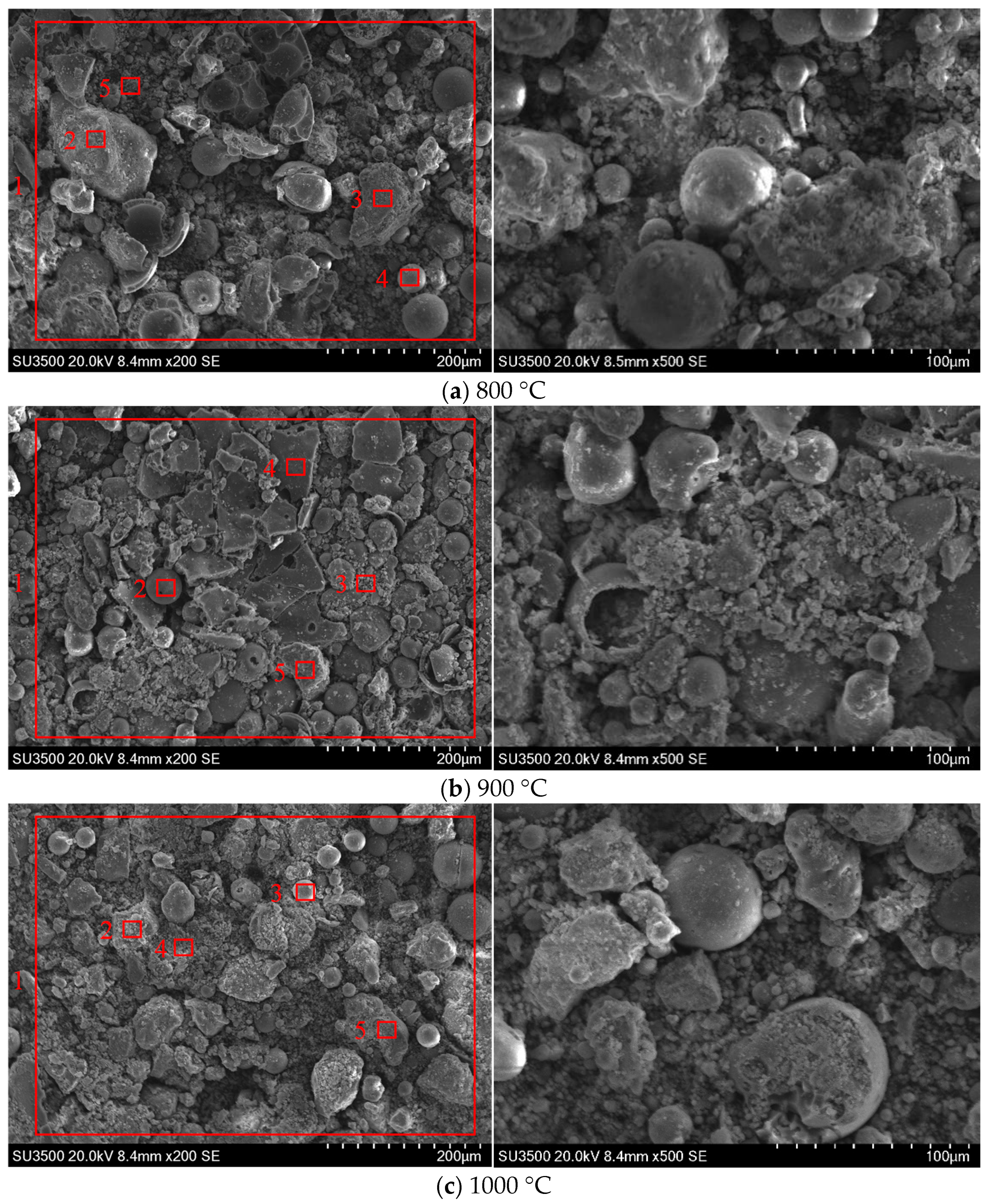
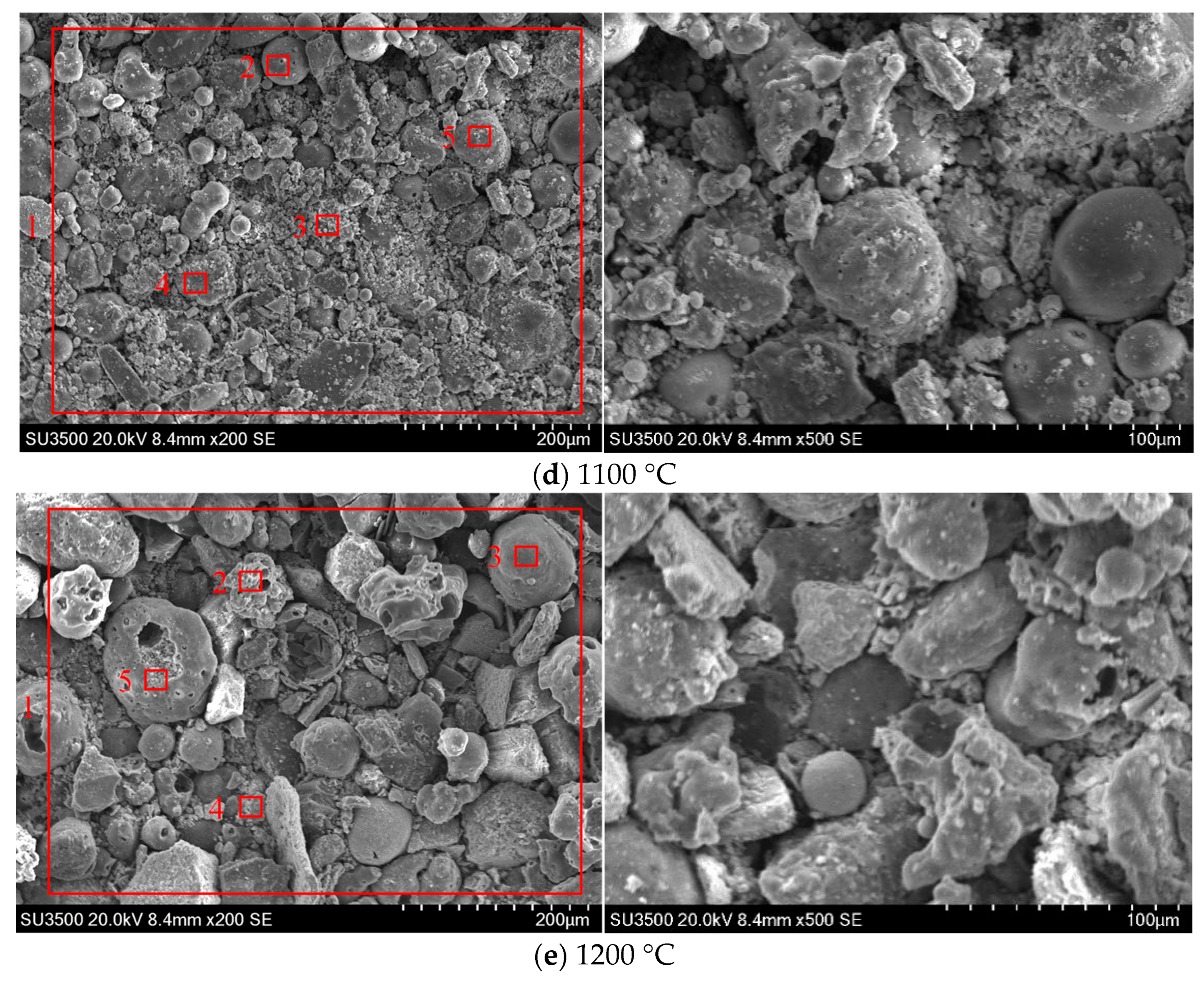
3.2.1. SEM-EDS Analysis
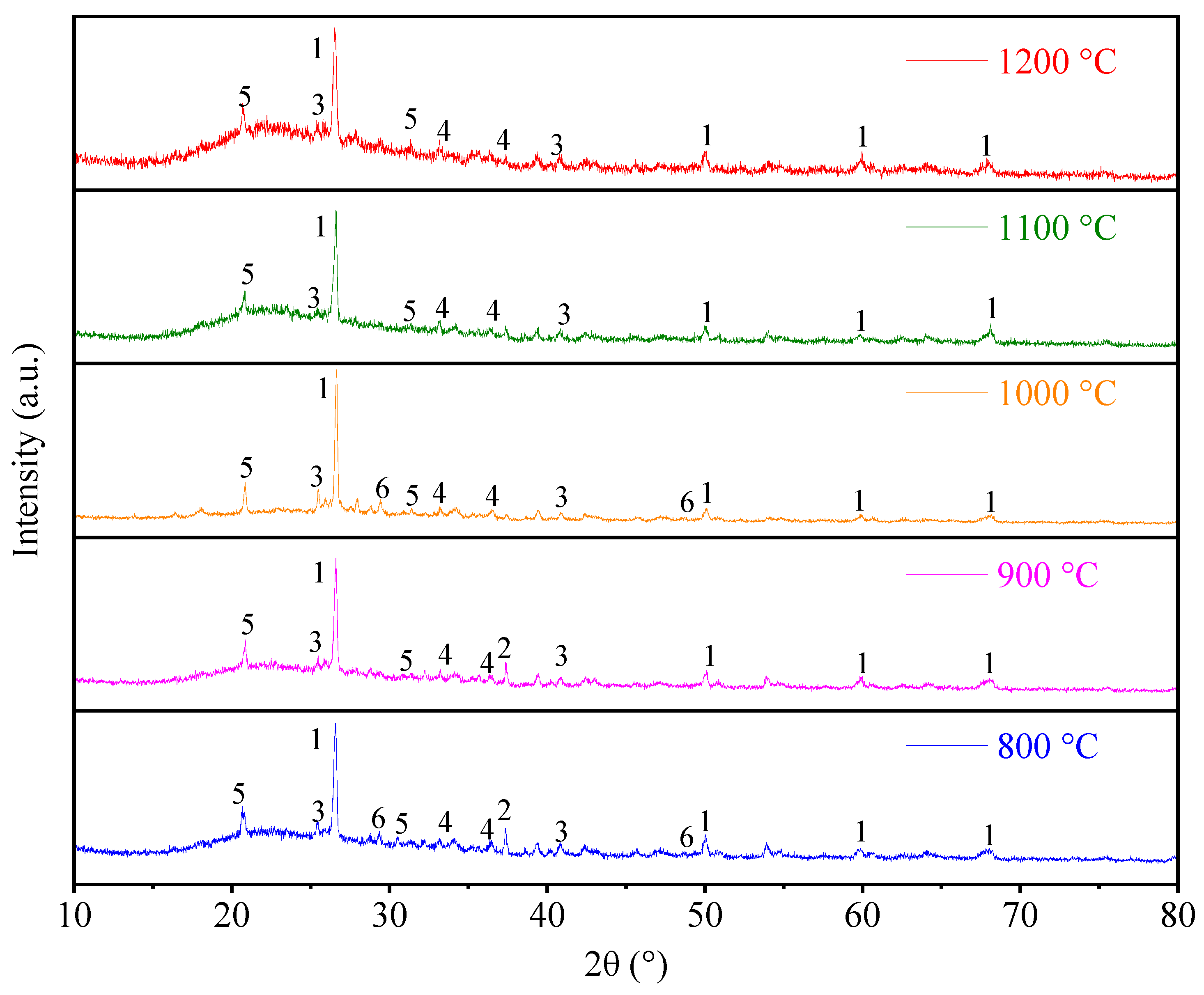
3.2.2. XRD Analysis
3.3. Effect of Wall Temperature on Ash Deposition Characteristics
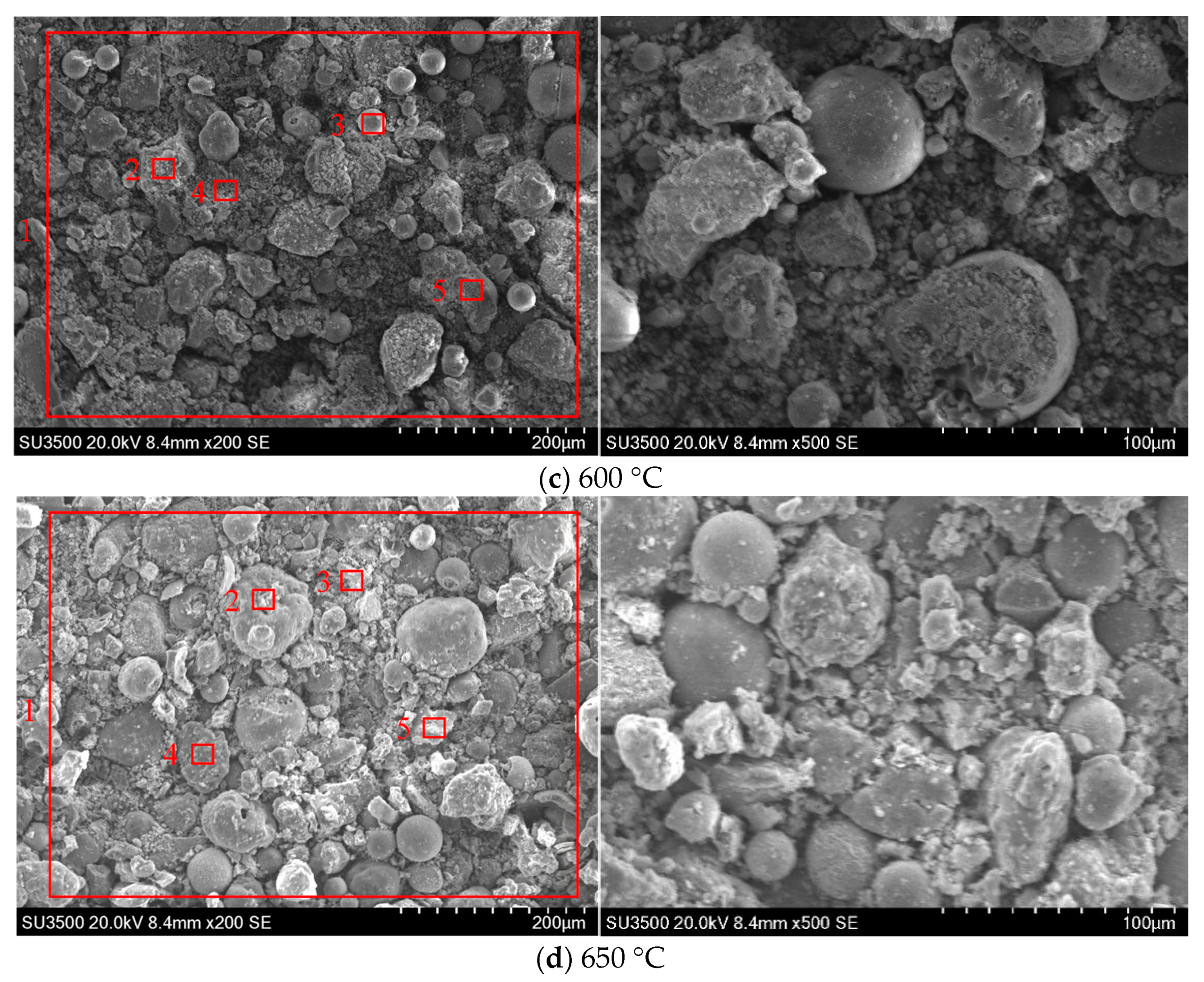
3.3.1. SEM-EDS Analysis
3.3.2. XRD Analysis
4. Conclusions
- (1)
- Salix, a key afforestation species in China’s arid regions, has fostered a significant planting industry in Inner Mongolia. The abundance of pruned salix branches exceeds the fuel demands of local power plants, suggesting a wide variety of potential applications. Salix biomass exhibits significant industrial value as a renewable resource. Its high calorific value (comparable to coal) makes it a key feedstock for bioenergy production, including power generation and biofuel synthesis. The high CaO content in salix ash (31.94%) and its inherently low sulfur composition synergistically contribute to the reduction in sulfur oxide emissions during combustion.
- (2)
- The addition of biomass will exacerbate the fuel ash deposition, which is related to the high alkali metal content of biomass fuel. As the biomass blending ratio increases from 5% to 30%, the content of Ca in ash increases from 8.92% to 20.59%, forming a lower melting point, which results in an obvious melt flow. The surface viscosity of the ash deposition probe increases, enhancing the ability to capture large particle sizes. In particular, when the biomass blending ratio exceeds 20%, serious adhesive ash accumulation will be caused. Therefore, it is recommended to control the biomass blending ratio to not exceed 20%.
- (3)
- As flue gas temperature increases, a distinct melting and flow phenomenon becomes apparent between ash particles. The shape of ash particles gradually becomes irregular and they lose their individual integrity under high-temperature thermal treatment. Ash particles begin to adhere to each other, during which a pronounced molten flow phenomenon becomes observable. When the flue gas temperature reaches 1200 °C, there is a serious ash particle melting flow under different biomass combustion ratio conditions, and the low-melting-point material containing calcium covers the surface of the ash particles, making the ash particles adhere to each other. Practical boiler systems must integrate real-time temperature monitoring and adaptive combustion controls. Therefore, it is recommended that the flue gas temperature entering the convective heating surface be controlled below 1200 °C to avoid the growth of cohesive ash deposition on the convective heating surface.
- (4)
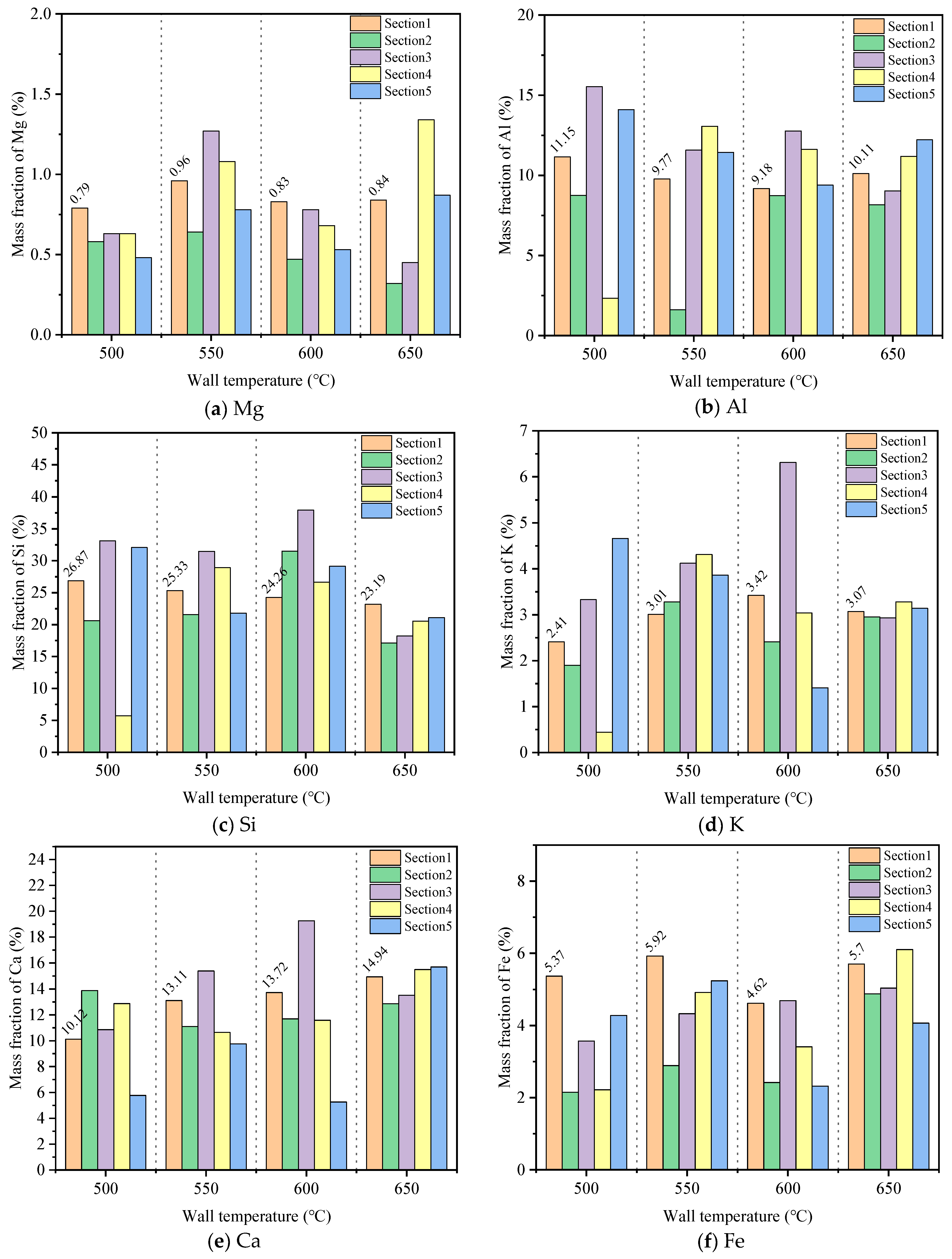
- As the wall temperature increases, the adhesion between the ash particles increases significantly, resulting in a denser ash deposit structure. The experimental results show that this is related to the increase in alkali metals and the decrease in Si content. When the wall temperature is increased from 500 °C to 600 °C, the Ca and K contents increase by 35.6% and 41.9%, respectively, while the Si content decreases by 9.7%. The increase in K and Ca content leads to the thickening of the initial layer of the ash deposit, which facilitates the formation of the sintered layer of the deposited ash. The reduction in Si content is not conducive to maintaining the shape and independence of the particles, which makes the particles fuse and adhere, resulting in difficulty in removing the ash accumulation. When the wall temperature reaches 600 °C, the degree of ash slagging increases markedly, especially in the section of high alkali metal content, and the bonding strength between particles increases, forming ash that is more difficult to remove. Therefore, it is recommended that the wall temperature should not exceed 600 °C.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, W.; Jia, X.; Wu, L.; Guo, Y.; Yang, T.; Wang, E.; Xiao, P. Research on regional differences in the impact of clean energy development on carbon dioxide emissions and economic growth. Humanit. Soc. Sci. Commun. 2022, 9, 25. [Google Scholar] [CrossRef]
- Gu, C.; Ye, X.; Cao, Q.; Guan, W.; Peng, C.; Wu, Y.; Zhai, W. System dynamics modelling of urbanization under energy constraints in China. Sci. Rep. 2020, 10, 9956. [Google Scholar] [CrossRef] [PubMed]
- Mydlarz, K.; Wieruszewski, M. The energy potential of firewood and by-products of round wood processing—Economic and technical aspects. Energies 2024, 17, 4797. [Google Scholar] [CrossRef]
- Eka, P.M.Z.; Prida, P.H.; Nesha, A.; Meida, L.I.; Ifanda, I.; Adi, P.; Arif, D.; Juli, H.; Solistia, W.S.; Muhammad, A.; et al. Co-combustion performance of oil palm biomass with coal: Thermodynamics and kinetics analyses. J. Therm. Anal. Calorim. 2024, 149, 2873–2891. [Google Scholar]
- Owusu, P.A.; Asumadu-Sarkodie, S.; Dubey, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Ni, Z.; Zhang, Y.; Liu, X.; Shi, H.; Yao, Y.; Tian, J.; Hu, P.; He, L.; Lin, Q.; Liu, L. Co-combustion of sewage sludge with corn stalk based on TG-MS and TG-DSC: Gas products, interaction mechanisms, and kinetic behavior. Energy 2024, 308, 132747. [Google Scholar] [CrossRef]
- Lourinho, G.; Alves, O.; Garcia, B.; Rijo, B.; Brito, P.; Nobre, C. Costs of gasification technologies for energy and fuel production: Overview, analysis, and numerical estimation. Recycling 2023, 8, 49. [Google Scholar] [CrossRef]
- Rivera Sasso, O.; Carreño Gallardo, C.; Soto Castillo, D.M.; Ojeda Farias, O.F.; Bojorquez Carrillo, M.; Prieto Gomez, C.; Herrera Ramirez, J.M. Valorization of biomass and industrial wastes as alternative fuels for sustainable cement production. Clean. Technol. 2024, 6, 814–825. [Google Scholar] [CrossRef]
- Nunes, L.J.R. Torrefied biomass as an alternative in coal-fueled power plants: A case study on grindability of agroforestry waste forms. Clean. Technol. 2020, 2, 270–289. [Google Scholar] [CrossRef]
- Noh, Y.-H.; Lee, D.-G.; Park, J.-H.; Song, G.-S.; Seung Kim, J.; Park, S.-J.; Won Choi, J.; Ho Song, K.; Choi, Y.-C.; Lee, Y.-J. Ashless herbaceous biomass for slagging and fouling reduction in solid-fuel boiler: Combustion and ash fusion characterizations. Fuel 2024, 379, 132957. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, S.; Chen, T.; Cao, Y.; Ma, X. The alkali metal characteristic during biomass combustion with additives. Energy Procedia 2015, 75, 124–129. [Google Scholar] [CrossRef]
- Demirbas, A. Potential applications of renewable energy sources, biomass combustion problems in boiler power systems, and combustion related environmental issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Toft, A.J.; Brammer, J.G. A techno-economic comparison of power production by biomass fast pyrolysis with gasification and combustion. Renew. Sustain. Energy Rev. 2002, 6, 181–246. [Google Scholar] [CrossRef]
- Jingkun, H.; Dunxi, Y.; Jianqun, W.; Xin, Y.; Fangqi, L.; Minghou, X. Effects of torrefaction on ash-related issues during biomass combustion and co-combustion with coal. Part 3: Ash slagging behavior. Fuel 2023, 339, 126925. [Google Scholar]
- Jiang, J.; Tie, Y.; Deng, L.; Che, D. Influence of water-washing pretreatment on ash fusibility of biomass. Renew. Energy 2022, 200, 125–135. [Google Scholar] [CrossRef]
- Long, J.; Deng, L.; Che, D. Analysis on organic compounds in water leachate from biomass. Renew. Energy 2020, 155, 1070–1078. [Google Scholar] [CrossRef]
- Lv, Y.; Lei, Y.; Hui, S.E.; Li, Y.; Niu, Y. Co-firing biomass with coal on ash deposition behavior at various temperatures in a down-fired furnace. Fuel 2024, 364, 131049. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, S.; Wang, X.; Shao, J.; Zhang, X.; Wang, X.; Yang, H.; Chen, H. Co-combustion of wheat straw and camphor wood with coal slime: Thermal behaviour, kinetics, and gaseous pollutant emission characteristics. Energy 2021, 234, 121292. [Google Scholar]
- Kupka, T.; Mancini, M.; Irmer, M.; Weber, R. Investigation of ash deposit formation during co-firing of coal with sewage sludge, saw-dust and refuse derived fuel. Fuel 2008, 87, 2824–2837. [Google Scholar] [CrossRef]
- Wang, G.; Pinto, T.; Costa, M. Investigation on ash deposit formation during the co-firing of coal with agricultural residues in a large-scale laboratory furnace. Fuel 2014, 117, 269–277. [Google Scholar] [CrossRef]
- Weichen, M.; Hao, Z.; Jiakai, Z.; Kun, Z.; Dan, L.; Chenying, Z.; Kefa, C. Behavior of slagging deposits during coal and biomass co-combustion in a 300 kW down-fired furnace. Energy Fuels 2018, 32, 4399–4409. [Google Scholar]
- Yuan, L.; Liang, X.; Yanqing, N.; Guangyao, W.; Yu, L.; Haiyu, H.; Shi, H. Investigation on ash deposition formation during co-firing of coal with wheat straw. J. Energy Inst. 2022, 100, 148–159. [Google Scholar]
- Zhou, W.; He, X.; Wei, Z.; Chen, D.; Shi, H.; Ma, D. Effects of combustion temperature on the ash melting properties of Zhundong coal. J. Chin. Soc. Power Eng. 2016, 36, 945–950. [Google Scholar]
- Llorente, M.J.F.; García, J.E.C. Comparing methods for predicting the sintering of biomass ash in combustion. Fuel 2005, 84, 1893–1900. [Google Scholar] [CrossRef]
- Jing, N.; Wang, Q.; Luo, Z.; Cen, K. Effect of different reaction atmospheres on the sintering temperature of Jincheng coal ash under pressurized conditions. Fuel 2011, 90, 2645–2651. [Google Scholar] [CrossRef]
- Al-Otoom, A.Y.; Elliott, L.K.; Wall, T.F.; Moghtaderi, B. Measurement of the sintering kinetics of coal ash. Energy Fuels 2000, 14, 994–1001. [Google Scholar] [CrossRef]
- Fenghai, L.; Jiejie, H.; Yitian, F.; Yang, W. Research on the effect of the fusion characteristics of Xiaolongtan lignite ashes. Clean. Coal Technol. 2010, 16, 49–53. [Google Scholar]
- Jia, M.; Zhang, Q. Key factors affecting fusion temperature of coal ash. Coal Chem. Ind. 2007, 3, 1–5. [Google Scholar]
- Li, H.; Jiao, F.; Li, H. Research on flux effected to ash fusion. Coal Sci. And. Technol. 2007, 35, 81–84. [Google Scholar]
- Jin, X.; Ye, J.; Li, Y.; Deng, L.; Che, D. Experimental research on condensation characteristics of alkali salts. J. Eng. Thermophys. 2017, 38, 894–899. [Google Scholar]
- Aleksandar, M.; Srdjan, B.; Nenad, C.; Ivan, T.; Andrijana, S.; Dragan, T.; Deng, L.; Defu, C. Numerical study of co-firing lignite and agricultural biomass in utility boiler under variable operation conditions. Int. J. Heat. Mass. Transf. 2021, 181, 121728. [Google Scholar]
- Ahmed, I.I.; Gupta, A.K. Sugarcane bagasse gasification: Global reaction mechanism of syngas evolution. Appl. Energy 2012, 91, 75–81. [Google Scholar] [CrossRef]
- Putra, H.P.; Hilmawan, E.; Darmawan, A.; Mochida, K.; Aziz, M. Theoretical and experimental investigation of ash-related problems during coal co-firing with different types of biomass in a pulverized coal-fired boiler. Energy 2023, 269, 126784. [Google Scholar]
- Chen, G.; Zhao, W.; He, S.; Fu, X. Biomass allocation and allometric relationship in aboveground components of salix psammophila branches. J. Desert Res. 2016, 36, 357–363. [Google Scholar]
- Hai, L.; Wang, X.; Zhang, W.; Zhang, L.; Hong, G.; Li, Z. Stumping Rejuvenation Technology of Salix psammophila Artificial Shrubbery in the Mu Us Sandy Land. J. Desert Res. 2016, 36, 131–136. [Google Scholar]
- Pu, Y.; Wang, H.; Wang, X.; Lim, M.; Yao, B.; Yang, H.; Lou, C. Experimental study of the influence of synergistic effects on the co-firing characteristics of biomass and coal. J. Energy Inst. 2024, 115, 101687. [Google Scholar] [CrossRef]
- Yanquan, L.; Wenyi, T.; Shaohua, L.; Xiaojun, P. Study on the co-combustion behavior of semi-coke and typical biomass: Combustion, NO emission and ash characteristics analysis. Fuel 2024, 358, 130068. [Google Scholar]
- Zhenrong, L.; Yuwei, H.; Junhua, W.; Junquan, M.; Yancheng, Z.; Rong, C. Study on the combustion characteristics and kinetics of water hyacinth co-combustion with anthracite. Chem. Eng. Res. Des. 2023, 200, 637–645. [Google Scholar]
- Yajuan, Z.; Xuebin, Z.; Yanshu, L.; Yun, L.; Wenxiu, F. Water use process of salix psammophila and salix cheilophila in Gonghe basin, Qinghai province. J. Desert Res. 2017, 37, 281–287. [Google Scholar]
- Yan, W.; Fu, J.; Li, Z.; Yan, L. Research on macro and meso simulation in compression process of salix psammophila granules. Acta Energiae Solaris Sin. 2023, 44, 449–454. [Google Scholar]
- Mendes, L.J.; Bazzo, E. Characterization and growth modeling of ash deposits in coal fired boilers. Powder Technol. 2012, 217, 61–68. [Google Scholar] [CrossRef]
- Niu, Y.Q.; Tan, H.Z.; Wang, X.B.; Liu, Z.N.; Liu, Y.; Xu, T.M. Study on Deposits on the Surface, Upstream, and Downstream of Bag Filters in a 12 MW Biomass-Fired Boiler. Energy Fuels 2010, 24, 2127–2132. [Google Scholar] [CrossRef]
- Niu, Y.Q.; Zhu, Y.M.; Tan, H.Z.; Hui, S.; Jing, Z.; Xu, W.G. Investigations on biomass slagging in utility boiler: Criterion numbers and slagging growth mechanisms. Fuel Process. Technol. 2014, 128, 499–508. [Google Scholar] [CrossRef]
- Zhou, H.S.; Jensen, P.A.; Frandsen, F.J. Dynamic mechanistic model of superheater deposit growth and shedding in a biomass fired grate boiler. Fuel 2007, 86, 1519–1533. [Google Scholar] [CrossRef]
- Wall, T.F.; Bhattacharya, S.P.; Zhang, D.K.; Gupta, R.P.; He, X. The properties and thermal effects of ash deposits in coal-fired furnaces. Prog. Energy Combust. Sci. 1993, 19, 487–504. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, B.; Zhang, H.L.; Li, L.T. Behavior of Fouling Deposits Formed on a Probe with Different Surface Temperatures. Energy Fuels 2014, 28, 7701–7711. [Google Scholar] [CrossRef]
- Wang, X.H.; You, C.F.; Liu, R.L.; Yang, R.C. Particle deposition on the wall driven by turbulence, thermophoresis and particle agglomeration in channel flow. Proc. Combust. Inst. 2011, 33, 2821–2828. [Google Scholar] [CrossRef]
- Shimogori, M.; Mine, T.; Ohyatsu, N.; Takarayama, N.; Matsumura, Y. Effects of fine ash particles and alkali metals on ash deposition characteristics at the initial stage of ash deposition determined in 1.5 MWth pilot plant tests. Fuel 2012, 97, 233–240. [Google Scholar] [CrossRef]
- Paz, C.; Suárez, E.; Eirís, A.; Porteiro, J. Experimental evaluation of the critical local wall shear stress around cylindrical probes fouled by diesel exhaust gases. Exp. Therm. Fluid Sci. 2012, 38, 85–93. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Y.J.; Cheng, W.; Sang, H.Y.; Yang, H.P.; Chen, H.P. Characteristics of Particulate Matter Emitted from Agricultural Biomass Combustion. Energy Fuels 2017, 31, 7493–7501. [Google Scholar] [CrossRef]
- Regueiro, A.; Patiño, D.; Granada, E.; Porteiro, J. Experimental study on the fouling behaviour of an underfeed fixed-bed biomass combustor. Appl. Therm. Eng. 2017, 112, 523–533. [Google Scholar] [CrossRef]
- Wang, Y.B.; Tan, H.Z.; Wang, X.B.; Cao, R.J.; Wei, B. The condensation and thermodynamic characteristics of alkali compound vapors on wall during wheat straw combustion. Fuel 2017, 187, 33–42. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Z.W.; Cheng, H.R.; Liang, S.W.; Hu, Y.Z.; Dong, X.Y.; Wu, J.W. Characteristics of water-soluble ions in condensable particulate matter emitted from stationary sources in Wuhan. Fuel 2021, 295, 120626. [Google Scholar] [CrossRef]
- ASTM E830-87(2004); Standard Test Method for Ash in the Analysis Sample of Refuse-Derived Fuel (Withdrawn 2004). ASTM International: West Conshohocken, PA, USA, 2004.
- Maria, Z.; Patrik, Y.; Johan, S.B.; Mikko, H. Characterization of ash-forming matter in various solid fuels by selective leaching and its implications for fluidized-bed combustion. Energy Fuels 2012, 26, 6366–6386. [Google Scholar]
- Niu, Y.; Tan, H.; Hui, S. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Guo, F.; He, Y.; Hassanpour, A.; Gardy, J.; Zhong, Z. Thermogravimetric analysis on the co-combustion of biomass pellets with lignite and bituminous coal. Energy 2020, 197, 117147. [Google Scholar] [CrossRef]





| Sample | Proximate Analysis w/% | Net Calorific Value (MJ·kg−1) | Ultimate Analysis w/% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mar | Aar | Var | FCar * | Car | Har | Nar | Oar * | St,ar | ||
| Biomass | 8.20 | 2.75 | 75.49 | 13.56 | 17.14 | 45.00 | 5.82 | 0.43 | 37.72 | 0.08 |
| Raw coal | 4.50 | 18.03 | 29.66 | 47.81 | 23.52 | 61.17 | 3.93 | 0.79 | 10.95 | 0.63 |
| Sample | Ash Composition/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | Al2O3 | CaO | MgO | SiO2 | TiO2 | SO3 | K2O | Na2O | MnO2 | |
| Pure coal | 4.96 | 14.04 | 9.16 | 1.10 | 54.51 | 0.85 | 5.39 | 2.68 | 1.32 | 0.127 |
| 5% biomass | 5.52 | 16.60 | 8.71 | 1.12 | 53.52 | 0.80 | 5.45 | 2.64 | 1.18 | 0.132 |
| 10% biomass | 5.44 | 16.64 | 8.82 | 1.12 | 53.20 | 0.85 | 4.94 | 2.69 | 1.28 | 0.133 |
| 15% biomass | 5.60 | 16.46 | 9.61 | 1.14 | 53.65 | 0.81 | 5.04 | 2.84 | 1.26 | 0.13 |
| 20% biomass | 5.36 | 16.01 | 9.83 | 1.18 | 54.53 | 0.85 | 5.50 | 2.89 | 1.29 | 0.12 |
| 30% biomass | 5.60 | 14.51 | 10.84 | 1.21 | 50.58 | 0.77 | 4.75 | 3.17 | 1.34 | 0.14 |
| Pure biomass | 3.27 | 5.62 | 31.94 | 3.06 | 13.22 | 0.14 | 1.85 | 7.02 | 0.52 | 0.225 |
| Sample | Deformation Temperature (DT)/°C | Softening Temperature (ST)/°C | Hemisphere Temperature (HT)/°C | Flow Temperature (FT)/°C |
|---|---|---|---|---|
| Pure coal | 1130 | 1170 | 1200 | 1260 |
| 5% biomass | 1120 | 1180 | 1210 | 1280 |
| 10% biomass | 1140 | 1210 | 1220 | 1290 |
| 15% biomass | 1170 | 1200 | 1210 | 1300 |
| 20% biomass | 1170 | 1230 | 1250 | 1290 |
| 30% biomass | 1210 | 1250 | 1280 | 1350 |
| Pure biomass | 1460 | >1500 | >1500 | >1500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shen, C.; Wang, D.; Zhang, J.; Yang, K.; Yang, H.; Liu, H.; Wen, X.; Zhang, Y.; Shao, Y.; et al. Study on Characteristics of Ash Accumulation During Co-Combustion of Salix Biomass and Coal. Energies 2025, 18, 2713. https://doi.org/10.3390/en18112713
Zhang Y, Shen C, Wang D, Zhang J, Yang K, Yang H, Liu H, Wen X, Zhang Y, Shao Y, et al. Study on Characteristics of Ash Accumulation During Co-Combustion of Salix Biomass and Coal. Energies. 2025; 18(11):2713. https://doi.org/10.3390/en18112713
Chicago/Turabian StyleZhang, Yan, Chengzhe Shen, Dongxv Wang, Jinbao Zhang, Kai Yang, Haisong Yang, Hailong Liu, Xintong Wen, Yong Zhang, Yunhao Shao, and et al. 2025. "Study on Characteristics of Ash Accumulation During Co-Combustion of Salix Biomass and Coal" Energies 18, no. 11: 2713. https://doi.org/10.3390/en18112713
APA StyleZhang, Y., Shen, C., Wang, D., Zhang, J., Yang, K., Yang, H., Liu, H., Wen, X., Zhang, Y., Shao, Y., Yan, R., Ye, N., & Deng, L. (2025). Study on Characteristics of Ash Accumulation During Co-Combustion of Salix Biomass and Coal. Energies, 18(11), 2713. https://doi.org/10.3390/en18112713







