The Influence of Ventilation Conditions on LPG Leak Dispersion in a Commercial Kitchen
Abstract
1. Introduction
2. Numerical Simulation
2.1. Model Development
2.1.1. Physical Model
2.1.2. Mathematical Model
2.2. Model Verification
2.2.1. Mathematical Model Verification
2.2.2. Grid Independence Verification
2.3. Numerical Method
2.3.1. Boundary Conditions
2.3.2. Computational Parameters
3. Results and Discussion
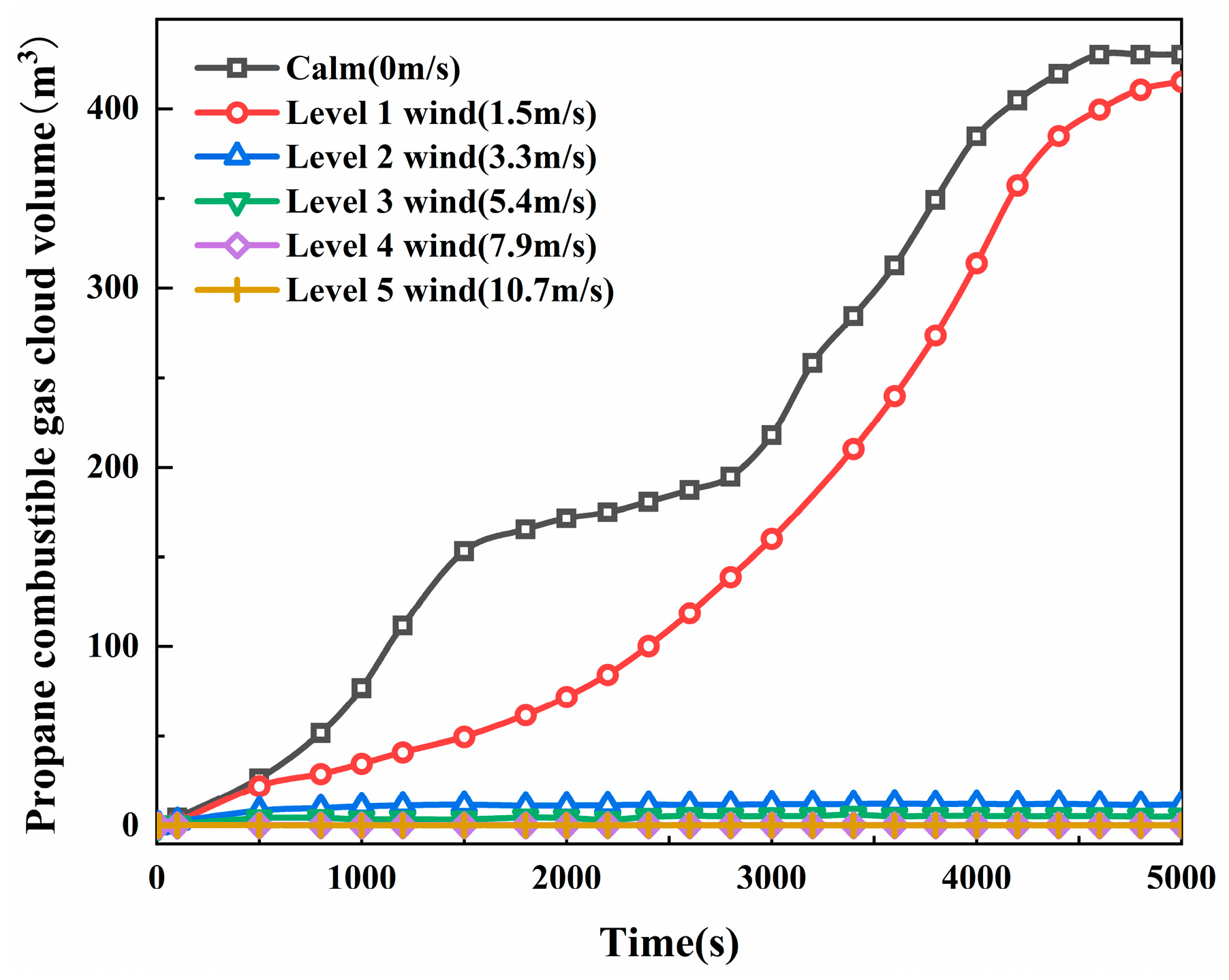
3.1. The Impact of Natural Ventilation on LPG Leakage Dispersion Patterns
3.2. The Impact of Forced Ventilation on LPG Leakage Dispersion Patterns
3.2.1. Operating Conditions Setup
3.2.2. The Impact of Air Exchange Rate on Propane Leakage
3.2.3. The Impact of Fan and Window Positions on Propane Leakage
4. Conclusions
- (1)
- Under natural ventilation conditions, when the window ventilation wind speed is more than 7.9 m/s, the propane concentration at the indoor alarm monitoring point remains consistently below the lower explosive limit (2.1%). There is a logarithmic relationship between wind speed and propane combustible gas cloud volume. Within the wind speed ranges of 1.5–3.3 m/s and 7.9–10.7 m/s, a small increase in wind speed can significantly reduce the volume of the combustible gas cloud (97.2% and 95.05%, respectively), effectively reducing the explosion risk of leaking propane;
- (2)
- Under forced ventilation conditions, at lower air exchange rates (5.98 to 10.6 times/h), the turbulence kinetic energy of the airflow is weak, and the dispersion effect on the combustible gas cloud is poor. In contrast, higher air exchange rates (22.1 times/h, 24.3 times/h) effectively increase the airflow speed, enhance the turbulence kinetic energy, and reduce the explosion risk. Once the air exchange rate reaches 22.1 times/h, further increases in exchange rate no longer effectively reduce the volume of the combustible gas cloud. Taking all factors into account, it is recommended to choose an air exchange rate of 22.1 times/h for houses with a similar confined space size.
- (3)
- When the window opening is at position 1, the exhaust airflow path of the fan is the shortest and the turbulence kinetic energy is the highest, which makes the dispersion effect of the fan the most obvious. The distance of the fan leakage source is exponentially increased with the volume of propane combustible gas cloud. To ensure that the volume of the combustible gas cloud does not exceed 0.5 m3, and to effectively control the diffusion and explosion risk of the propane gas cloud, the fan should be installed within 2.786 m from the leak hole. In practical applications, it is suggested to place the fan close to the potential leak source to maximize the reduction in the volume of the combustible gas cloud.
5. Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ditta, A.; Figueroa, O.; Galindo, G.; Yie-Pinedo, R. A review on research in transportation of hazardous materials. Socio-Econ. Plan. Sci. 2019, 68, 100665. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, Z.; Cui, X.; Zhu, J. Characteristics of malignant urban gas accidents in China from 2013 to 2022. Heliyon 2024, 10, e34568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, F.; Xu, S.; Xie, Y.; Shu, C. Building urban gas process safety management (UG-PSM) system: Based on root cause analysis with 160 urban gas accidents in China. J. Loss Prev. Proc. 2023, 84, 105101. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, L.; Li, X.; Shi, S.; Lu, Y. Trends of hazardous material accidents (HMAs) during highway transportation from 2013 to 2018 in China. J. Loss Prev. Proc. 2020, 66, 104150. [Google Scholar] [CrossRef]
- Yang, D.; Zheng, Y.; Peng, K.; Pan, L.; Zheng, J.; Xie, B.; Wang, B. Characteristics and Statistical Analysis of Large and above Hazardous Chemical Accidents in China from 2000 to 2020. Int. J. Environ. Res. Public Health 2022, 19, 15603. [Google Scholar] [CrossRef]
- Liu, J.; Ye, Q.; Jia, Z.; Yang, Y.; Xu, T. Analysis of Factors Affecting Emergency Response Linkage in Coal Mine Gas Explosion Accidents. Sustainability 2024, 16, 6325. [Google Scholar] [CrossRef]
- Luketa-Hanlin, A. A review of large-scale LNG spills: Experiments and modeling. J. Hazard. Mater. 2006, 132, 119–140. [Google Scholar] [CrossRef]
- Mokhtarzadeh-Dehghan, M.R.; Akcayoglu, A.; Robins, A.G. Numerical study and comparison with experiment of dispersion of a heavier-than-air gas in a simulated neutral atmospheric boundary layer. J. Wind. Eng. Ind. Aerod. 2012, 110, 10–24. [Google Scholar] [CrossRef]
- Goldwire, H.C.; Rodean, H.C.; Cederwall, R.T.; Kansa, E.J.; Kiefer, R.D. Coyote Series Data Report LLNL/NWC 1981 LNG Spill Tests Dispersion, Vapor Burn, and Rapid-Phase-Transition; Appendices; Technical Report; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1983; Volume 2.
- Brown, T.C.; Cederwall, R.T.; Chan, S.T.; Ermak, D.L.; Koopman, R.P. Falcon Series Data Report: 1987 LNG Vapor Barrier Verification Field Trials; Liquefied Natural Gas; Technical Report; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2019.
- Britter, R.E.; Mcquaid, J. Workbook on the Dispersion of Dense Gases; Health & Safety Executive: Bootle, UK, 1988. [Google Scholar]
- Hall, D.J.; Walker, S. Scaling rules for reduced-scale field releases of hydrogen fluoride. J. Hazard. Mater. 1997, 54, 89–111. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Zhao, W.W.; Zhang, Y.Z. Study on the Diffusion Law of Heavy Gas Leakage in Complex Scenarios Based on Scaled-Down Experiments. Acs Omega 2024, 9, 31533–31545. [Google Scholar] [CrossRef]
- Havens, J.; Walker, H.; Spicer, T.O. Wind tunnel study of air entrainment into two-dimensional dense gas plumes at the Chemical Hazards Research Center. Atmos. Env. 2001, 35, 2305–2317. [Google Scholar] [CrossRef]
- Guo, X.; Yan, X.; Zheng, Y.; Yu, J.; Zhang, Y.; Chen, S.; Chen, L.; Mahgerefteh, H.; Martynov, S.; Collard, A. Under-expanded jets and dispersion in high pressure CO2 releases from an industrial scale pipeline. Energy 2017, 119, 53–66. [Google Scholar] [CrossRef]
- Rottman, J.W. Workbook on the dispersion of dense gases: By R.E. Britter and J. McQuaid. J. Fluid Mech. 1988, 211, 656–657. [Google Scholar] [CrossRef]
- Berenblut, B. Guidelines for the Use of Vapour Cloud Dispersion Models. Phys. Bull. 1987, 38, 466. [Google Scholar] [CrossRef]
- Hankin, R.K.S.; Britter, R.E. Twodee: The Health and Safety Laboratory’s shallow layer model for heavy gas dispersion Part 1. Mathematical basis and physical assumptions. J. Hazard. Mater. 1999, 66, 211–226. [Google Scholar] [CrossRef]
- Uchida, T.; Ohya, Y. Large-eddy simulation of turbulent airflow over complex terrain. J. Wind. Eng. Ind. Aerod 2003, 91, 219–229. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Liu, Y.; Li, X. Simulation of the Gas Distribution Law and Operational Risk Analysis of a Vertical Gas Tank in a Ventilation State. Energies 2023, 16, 6855. [Google Scholar] [CrossRef]
- Ma, Q.; Zhong, M.; Guo, Y.; You, J.; He, Y.; Chen, J.; Zhang, Z. Study on the characteristic of boiling expansion process of superheated LPG and its vapor cloud explosion. J. Loss Prev. Proc. 2022, 78, 104831. [Google Scholar] [CrossRef]
- Sklavounos, S.; Rigas, F. Validation of turbulence models in heavy gas dispersion over obstacles. J. Hazard. Mater. 2004, 108, 9–20. [Google Scholar] [CrossRef]
- Liu, A.; Huang, J.; Li, Z.; Chen, J.; Huang, X.; Chen, K.; Xu, W.B. Numerical simulation and experiment on the law of urban natural gas leakage and diffusion for different building layouts. J. Nat. Gas. Sci. Eng. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Shi, B.J.; Wang, H.; Nie, S.M.; Cheng, C.; Li, T.J. Experimental and numerical research on the characteristics of heavy gas leakage and diffusion. Process Saf. Prog. 2022, 41, 567–580. [Google Scholar] [CrossRef]
- Poulsen, T.G.; Furman, A.; Liberzon, D. Effects of wind speed and wind gustiness on subsurface gas transport. Vadose Zone J. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Han, O.; Zhang, Y.; Li, A.; Li, J.; Li, Y.; Liu, H. Experimental and numerical study on heavy gas contaminant dispersion and ventilation design for industrial buildings. Sustain. Cities Soc. 2020, 55, 102016. [Google Scholar] [CrossRef]
- Hou, X.; Lan, H.; Zhao, Z.; Li, J.; Hu, C.; Li, Y. Effect of obstacle location on hydrogen dispersion in a hydrogen fuel cell bus with natural and mechanical ventilation. Process Saf. Env. 2023, 171, 995–1008. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.; Seo, J.; Choi, S. Numerical study on the internal ventilation of the engine room of a ship under construction. Alex. Eng. J. 2024, 100, 232–245. [Google Scholar] [CrossRef]
- Hao, H. Research on Building Ventilation Condition and Numerical Simulation Based on Fluid Mechanics Model. J. Phys. Conf. Ser. 2022, 2280, 012021. [Google Scholar] [CrossRef]
- Kit, L.W.; Mohamed, H.; Luon, N.Y.; Chan, L. Numerical Simulation of Ventilation in a Confined Space. J. Adv. Res. Fluid. Mech. Therm. Sci. 2023, 107, 1–18. [Google Scholar] [CrossRef]
- ANSYS Fluent 19.0 User’s Guide; ANSYS Inc.: Canonsburg, PA, USA, 2012.
- Wang, Z.; Li, Y.; Tong, X.; Gong, J. Risk probability evaluation for the effect of obstacle on CO2 leakage and dispersion indoors based on uncertainty theory. J. Loss Prev. Proc. 2022, 74, 104652. [Google Scholar] [CrossRef]
- GB 35844-2018; Pressure Regulators for Liquefied Petroleum Gas Cylinders. Ministry of Housing and Urban—Rural Development of the People’s Republic of China: Beijing, China, 2018.
- GB50180-93; Urban Residential Area Planning and Design Code (2002 Edition). China Architecture & Building Press: Beijing, China, 2002.
- GB50016-2014; Code for Fire Protection Design of Buildings. China Architecture & Building Press: Beijing, China, 2014.
- GBTSRCA000003; Code for Design of Commercial Kitchens. China Architecture & Building Press: Beijing, China, 2019.
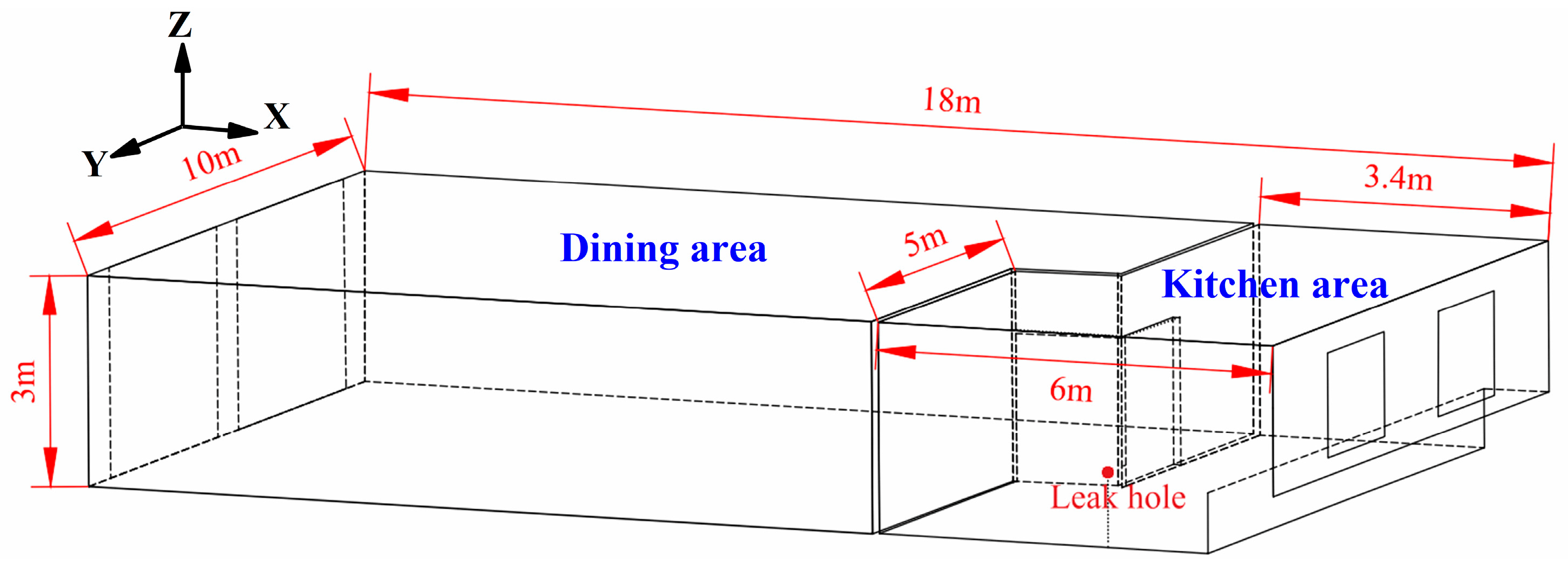


















| Number of Grids | Grid Size of Encrypted Area (mm) | Grid Size in Normal Areas (mm) |
|---|---|---|
| 804,094 | 10 | 110 |
| 954,943 | 7 | 105 |
| 1,090,132 | 6 | 100 |
| 1,281,170 | 5 | 95 |
| 1,466,607 | 4 | 90 |
| Boundary | Boundary Type | Parameter Settings |
|---|---|---|
| Leak Hole | Mass flow–inlet | Mass flow rate, 0.0962 kg/s; initial gauge pressure, 5000 Pa; Substance, pure propane |
| Window | Velocity–inlet /Pressure–outlet | initial gauge pressure, 0 Pa; substance, air |
| Door | Wall | Default wall roughness settings |
| Wall | Wall | Default wall roughness settings |
| Exhaust fan | Wall/Exhaust fan | Piecewise–linear |
| Mass Flow Rate (kg/s) | Leak Hole Size (mm) | Room Dimensions (m) | Natural Ventilation Wind Speed (m/s) | Forced Ventilation Air Exchange Rate (times/h) |
|---|---|---|---|---|
| 0.009225 | 10 | 18 × 10 × 3 | 0, 1.5, 3.3, 5.4, 7.9, 10.7 | 5.98, 7.86, 10.6, 12.5, 16.4, 19.6, 22.1, 24.3 |
| Fan Model | BT35-11-5 | BT35-11-5.6 | ||||||
|---|---|---|---|---|---|---|---|---|
| Air volume (m3/h) | 3142 | 4129 | 5566 | 6595 | 8667 | 10,379 | 11,682 | 12,812 |
| Static pressure (Pa) | 53 | 59 | 65 | 151 | 169 | 174 | 186 | 232 |
| Wind speed (m/s) | 4.45 | 5.86 | 7.88 | 7.44 | 9.78 | 11.7 | 13.18 | 14.45 |
| Air exchange rate (times/h) | 5.98 | 7.86 | 10.6 | 12.5 | 16.4 | 19.6 | 22.1 | 24.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Li, X.; Zhang, Y.; Zhou, N.; Chen, B.; Liang, Y.; Yang, C.; Huang, W.; Sun, C. The Influence of Ventilation Conditions on LPG Leak Dispersion in a Commercial Kitchen. Energies 2025, 18, 2678. https://doi.org/10.3390/en18112678
Yuan X, Li X, Zhang Y, Zhou N, Chen B, Liang Y, Yang C, Huang W, Sun C. The Influence of Ventilation Conditions on LPG Leak Dispersion in a Commercial Kitchen. Energies. 2025; 18(11):2678. https://doi.org/10.3390/en18112678
Chicago/Turabian StyleYuan, Xiongjun, Xue Li, Yanxia Zhang, Ning Zhou, Bing Chen, Yiting Liang, Chunhai Yang, Weiqiu Huang, and Chengye Sun. 2025. "The Influence of Ventilation Conditions on LPG Leak Dispersion in a Commercial Kitchen" Energies 18, no. 11: 2678. https://doi.org/10.3390/en18112678
APA StyleYuan, X., Li, X., Zhang, Y., Zhou, N., Chen, B., Liang, Y., Yang, C., Huang, W., & Sun, C. (2025). The Influence of Ventilation Conditions on LPG Leak Dispersion in a Commercial Kitchen. Energies, 18(11), 2678. https://doi.org/10.3390/en18112678





