Experimental Study on Peak Shaving with Self-Preheating Combustion Equipped with a Novel Compact Fluidized Modification Device
Abstract
:1. Introduction
2. Experimental Section
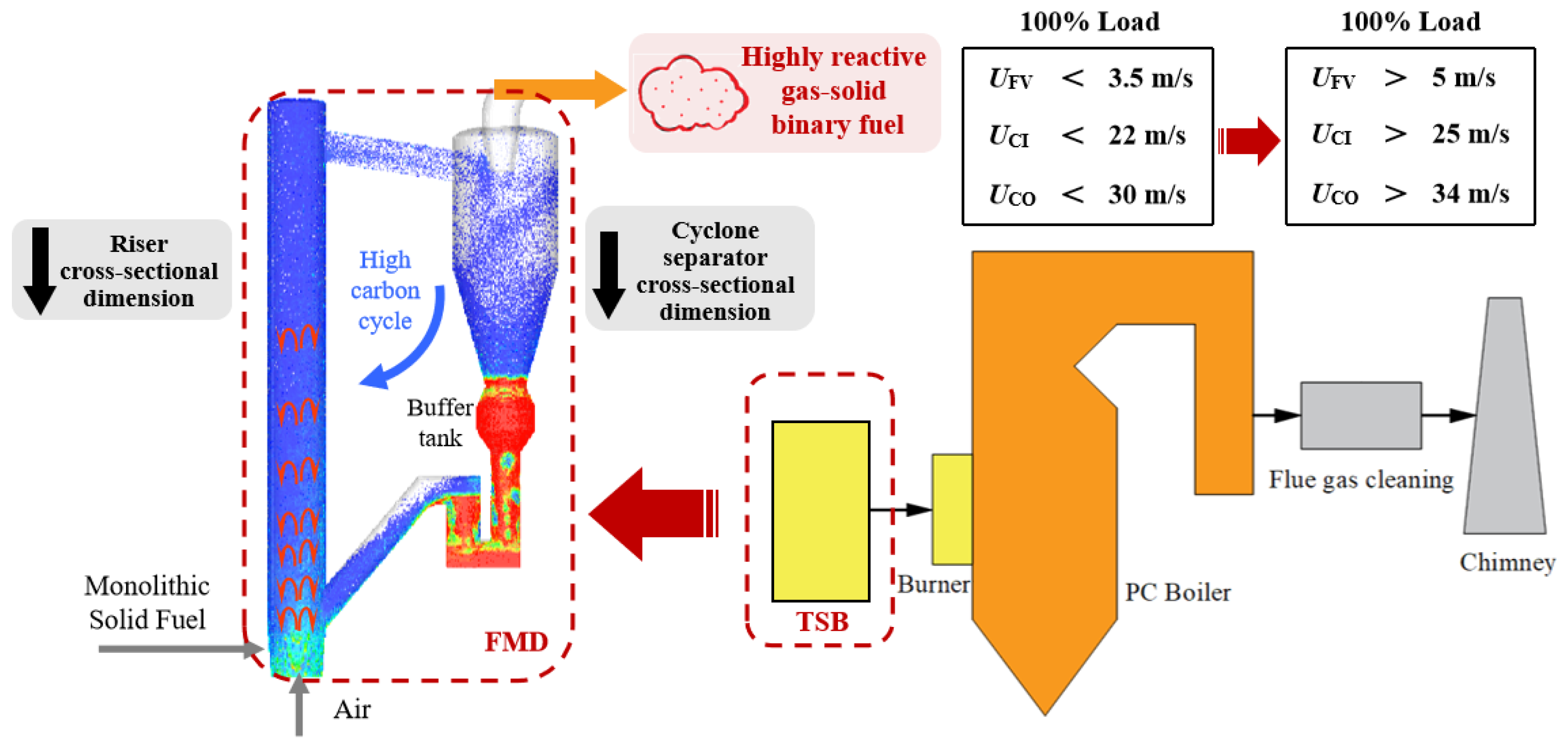
2.1. Experimental Setup
2.2. Experimental Methods and Sampling Ports
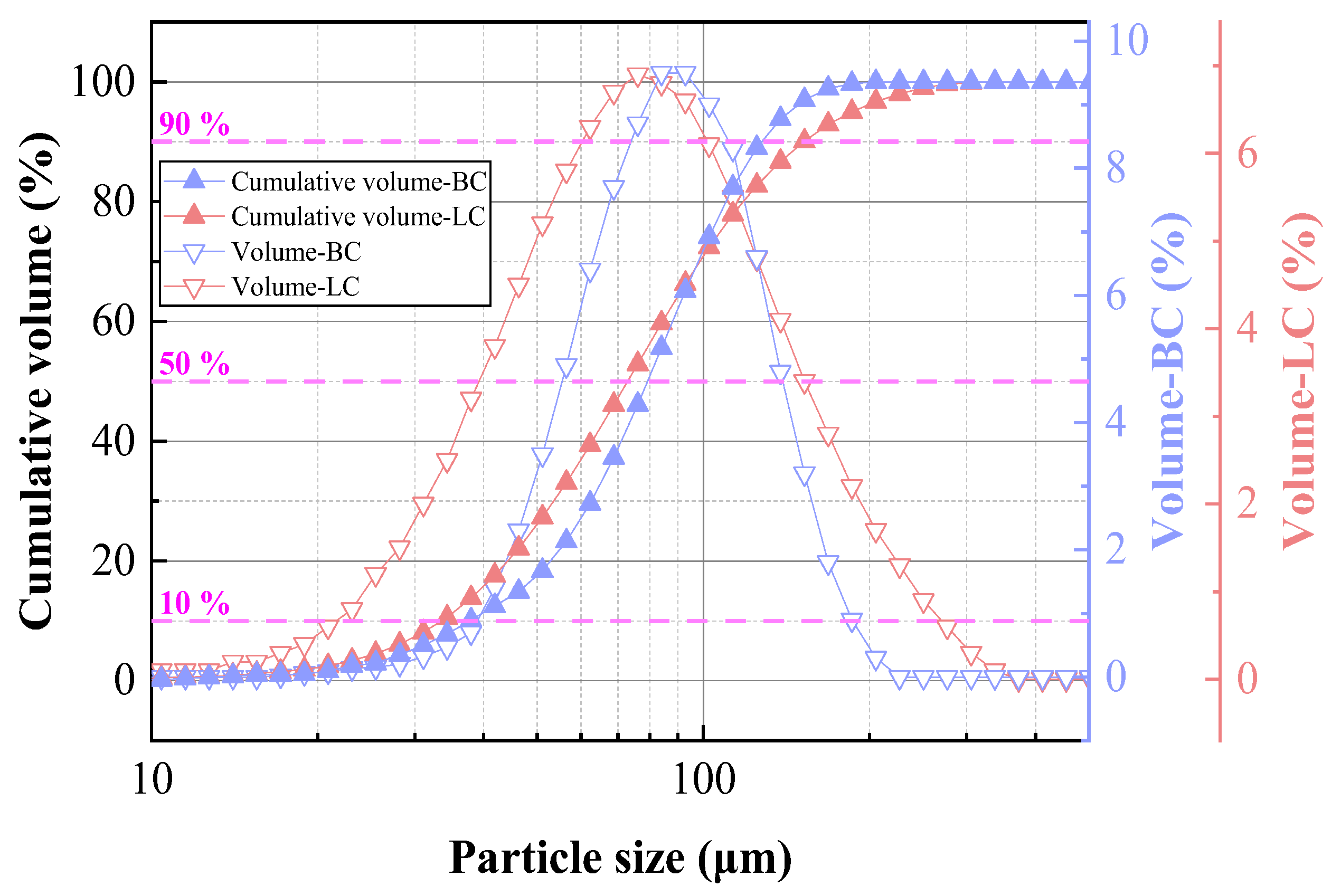
2.3. Fuel Characteristics
2.4. Experimental Conditions
3. Results and Discussion
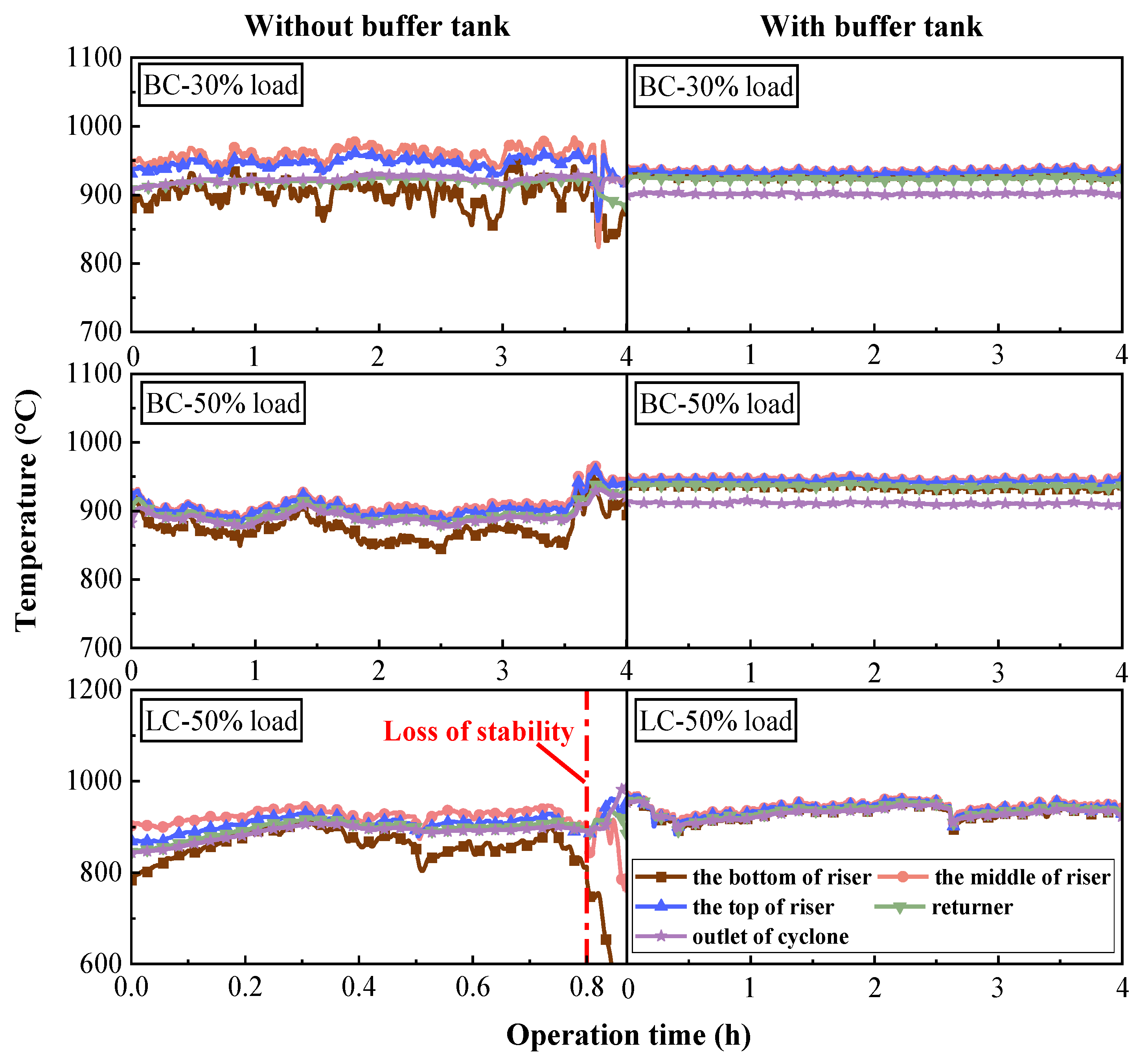
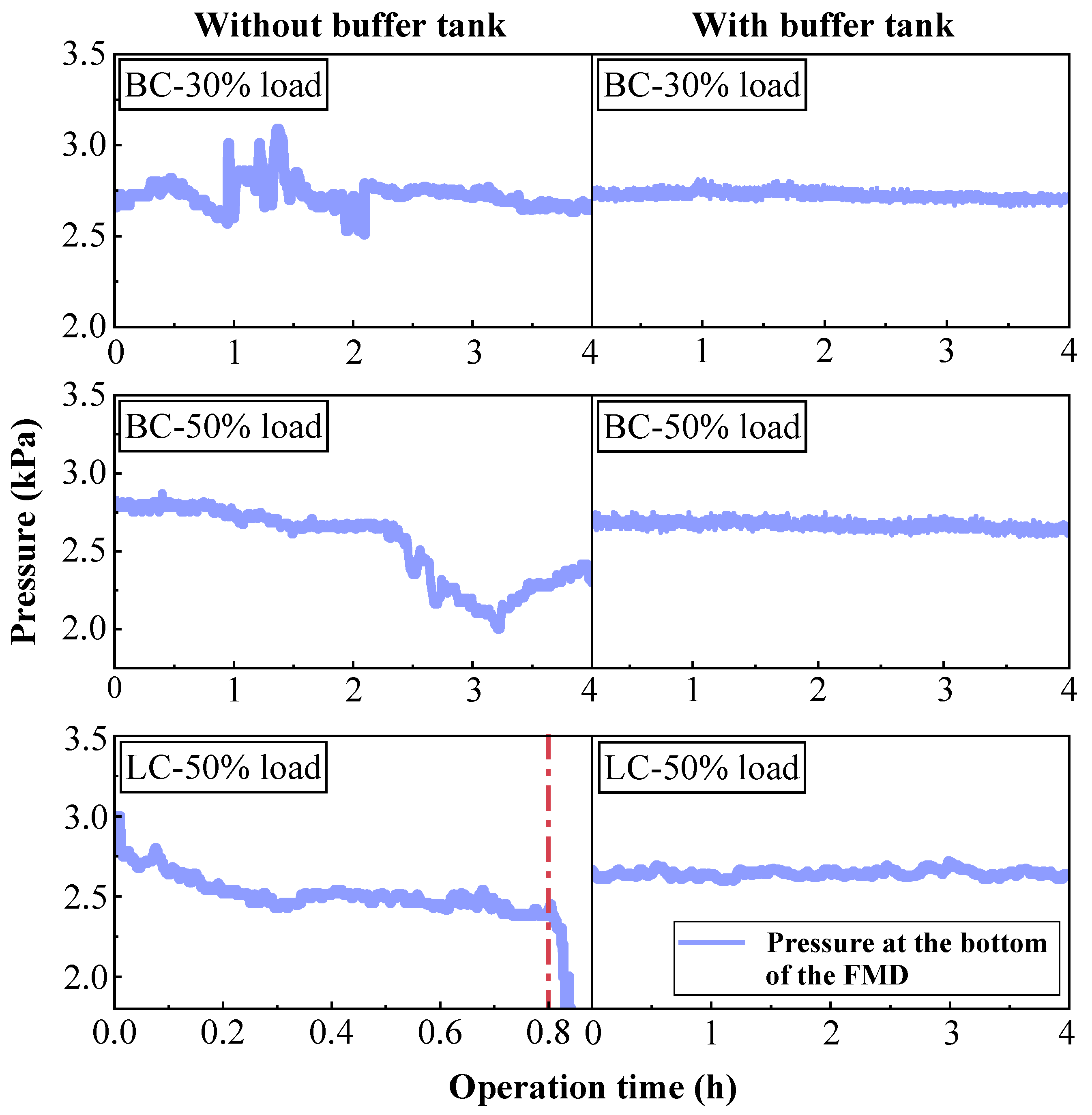
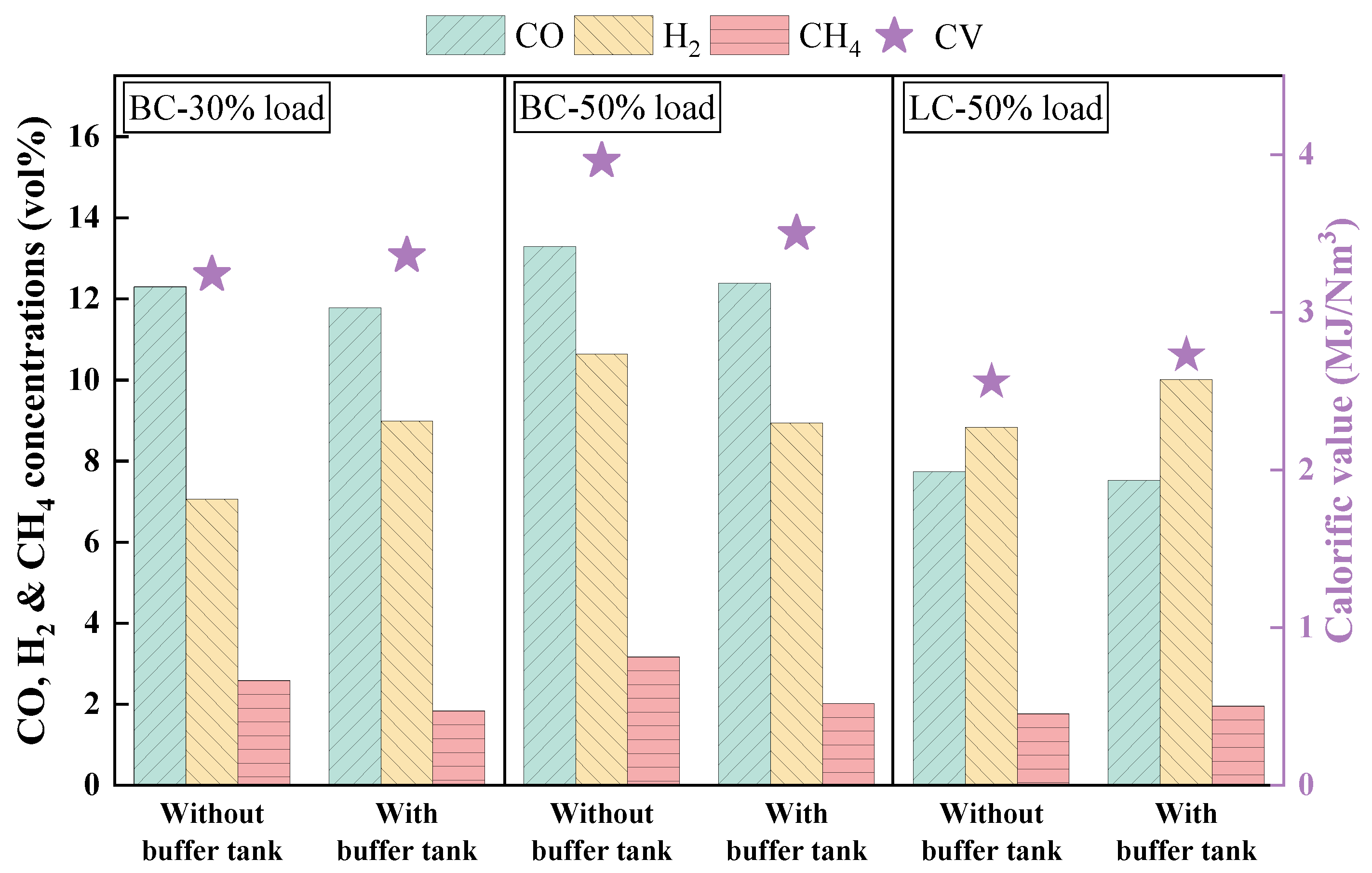
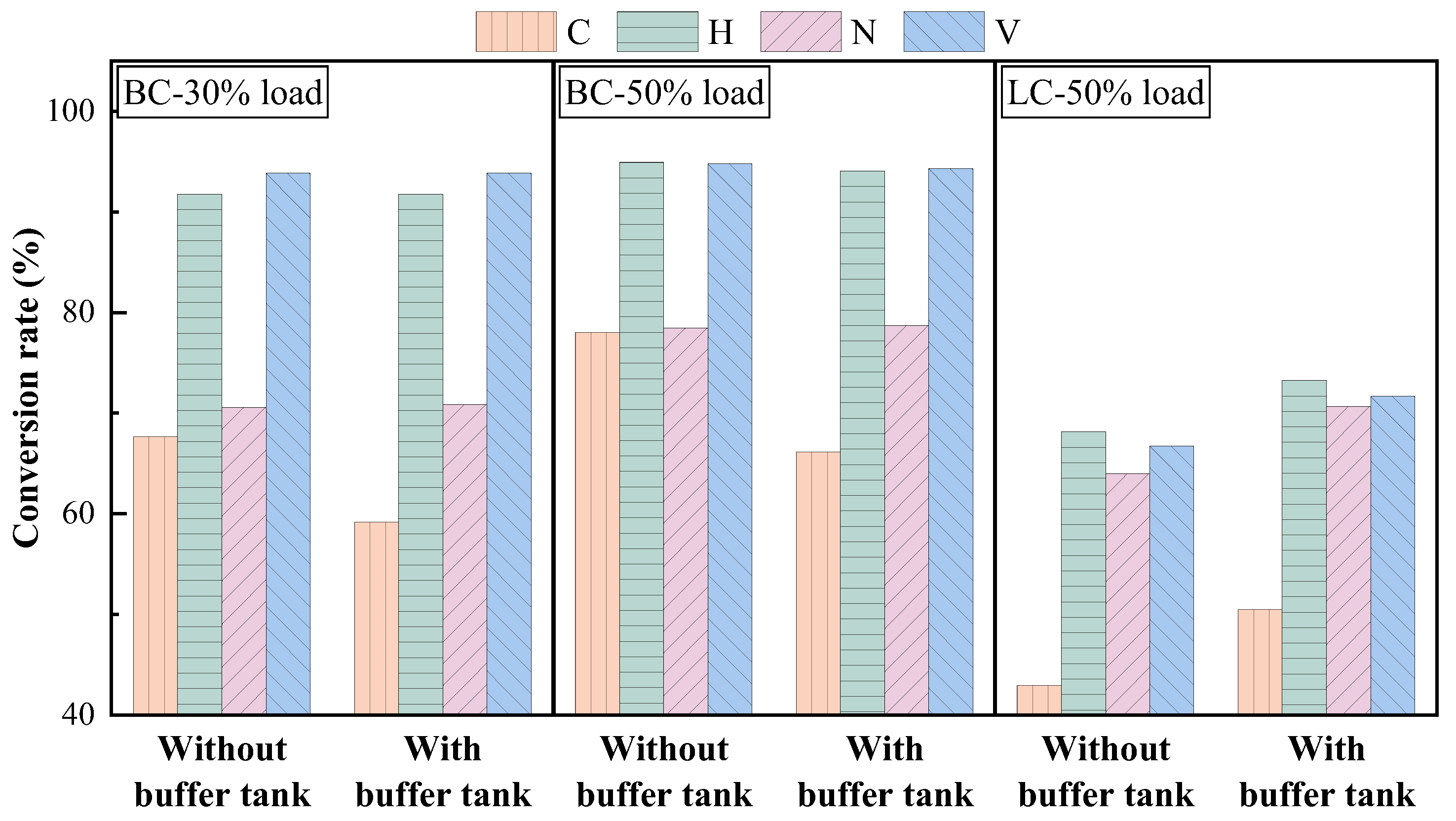
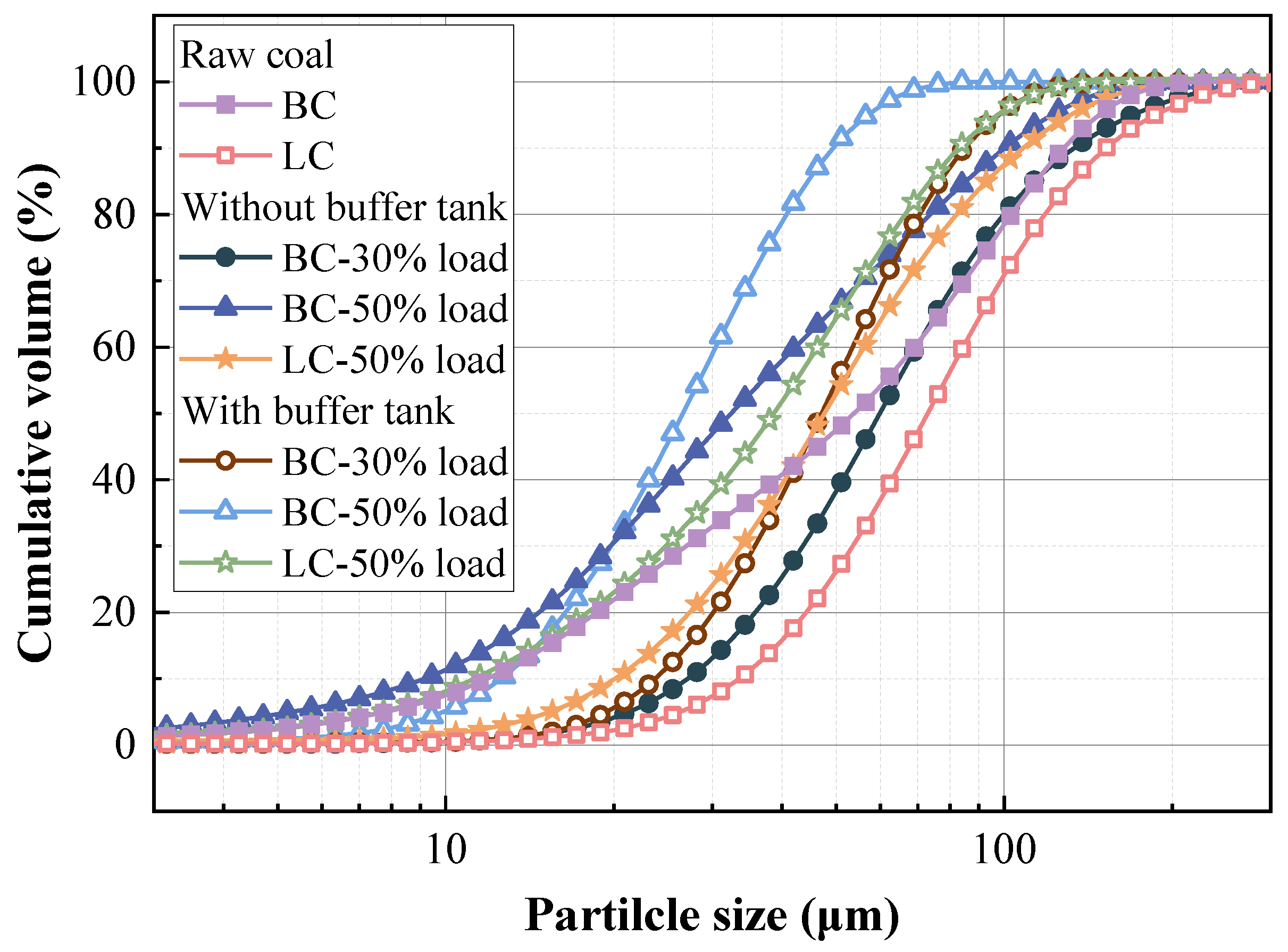
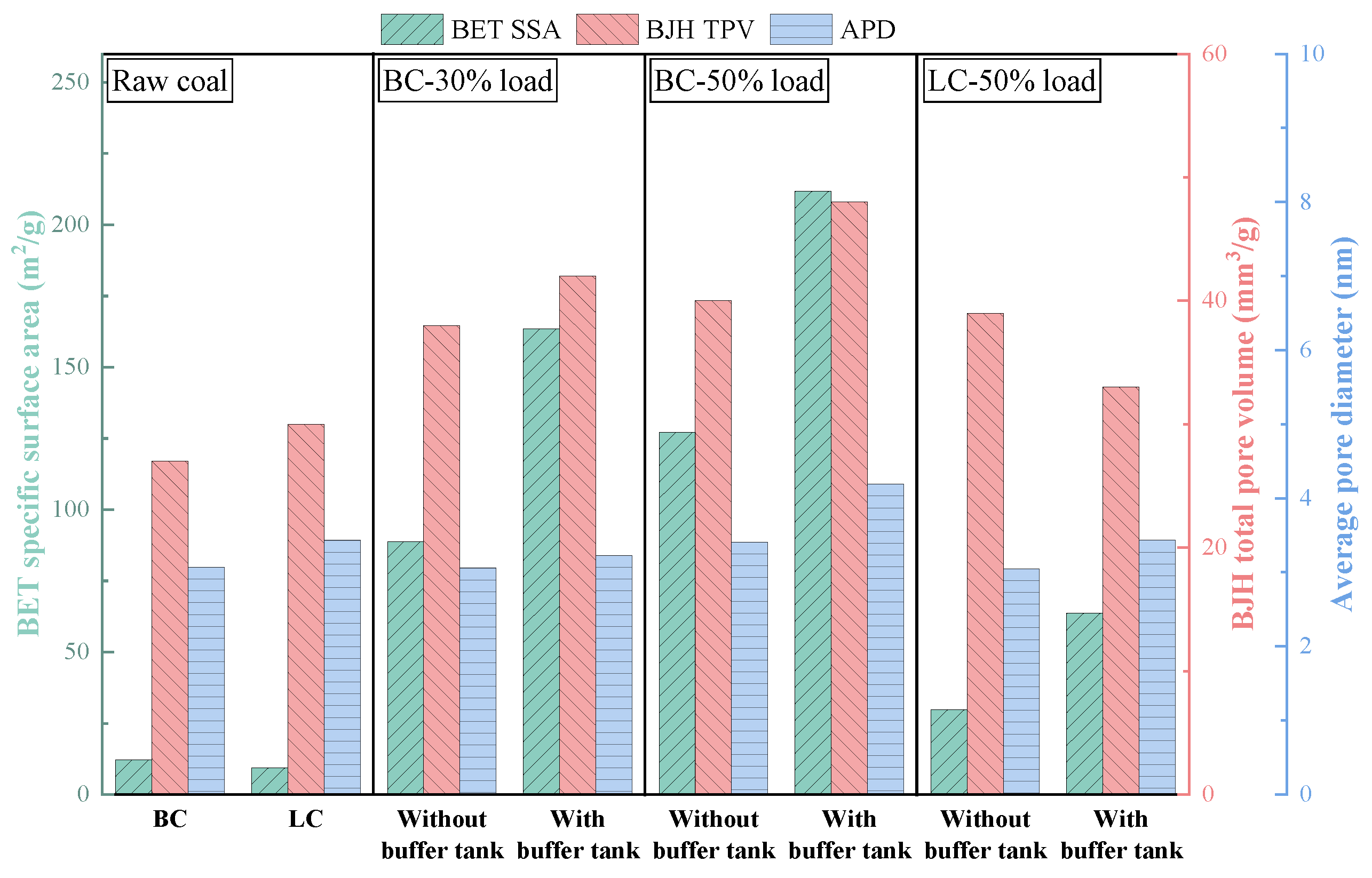
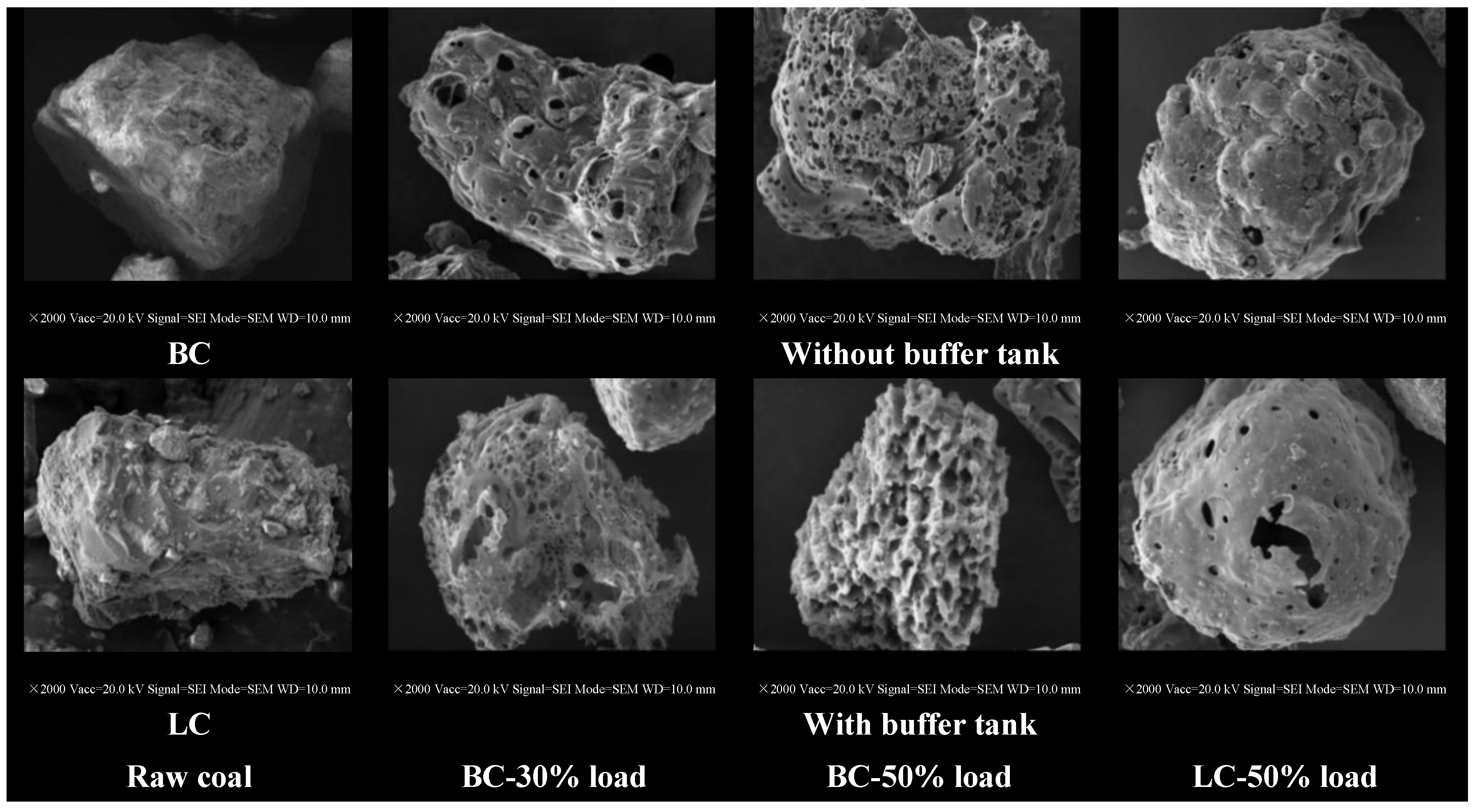
3.1. Effects of Buffer Tank on FMD Operation and Fuel Modification
3.1.1. Operation Characteristics of FMD
3.1.2. Analysis of Gas Composition During Fuel Modification
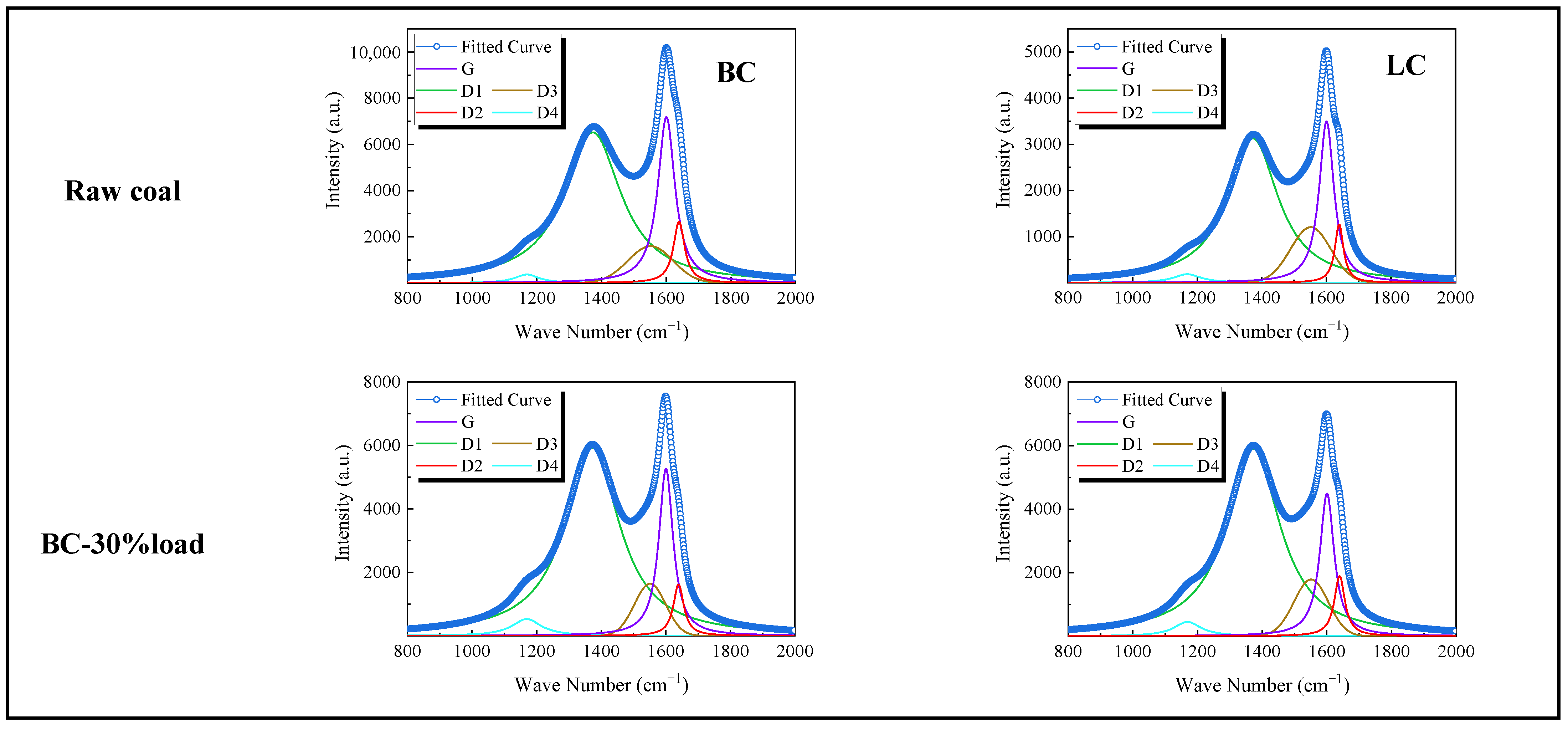
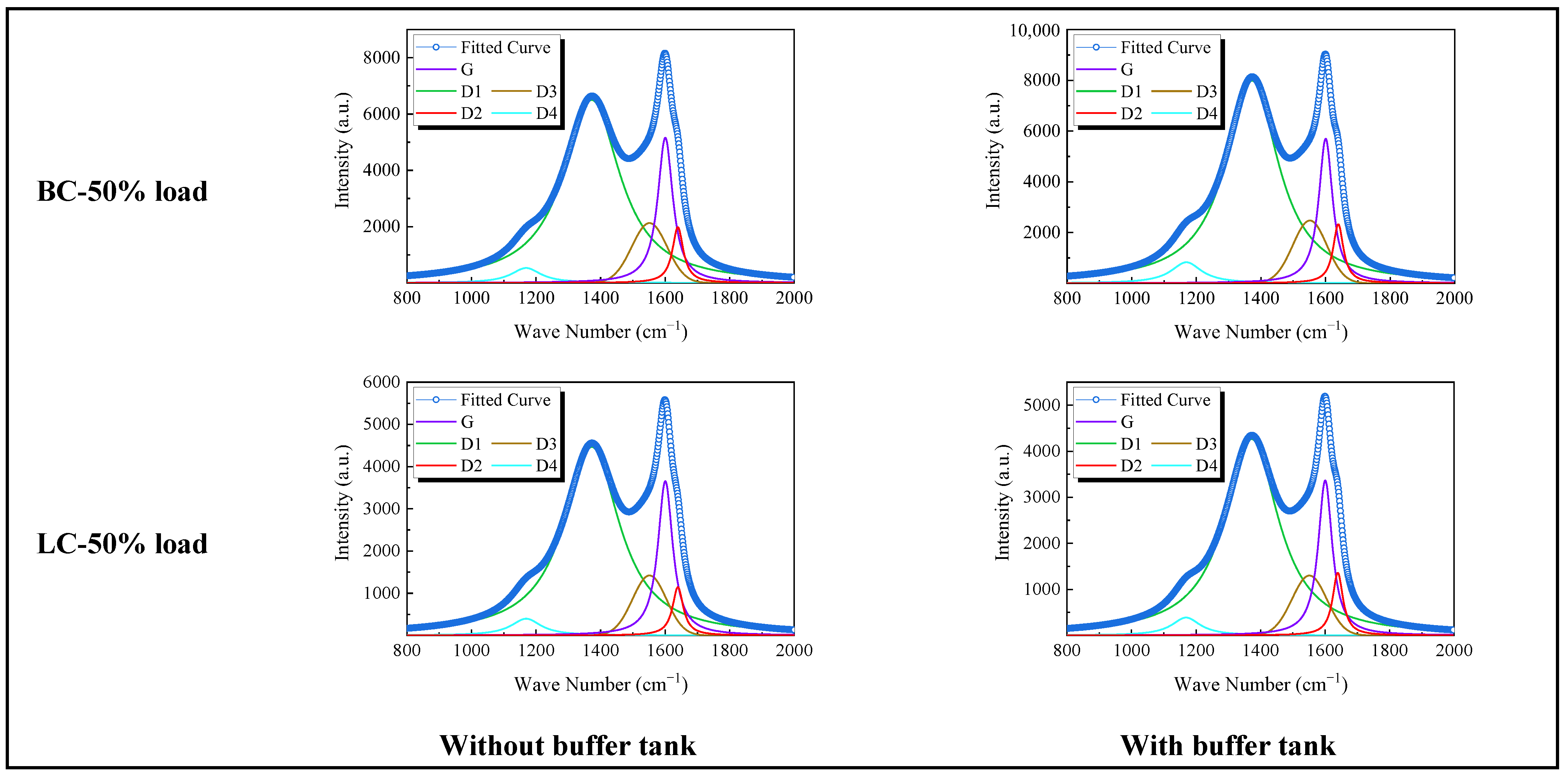
3.1.3. Physicochemical Properties of Preheated Char
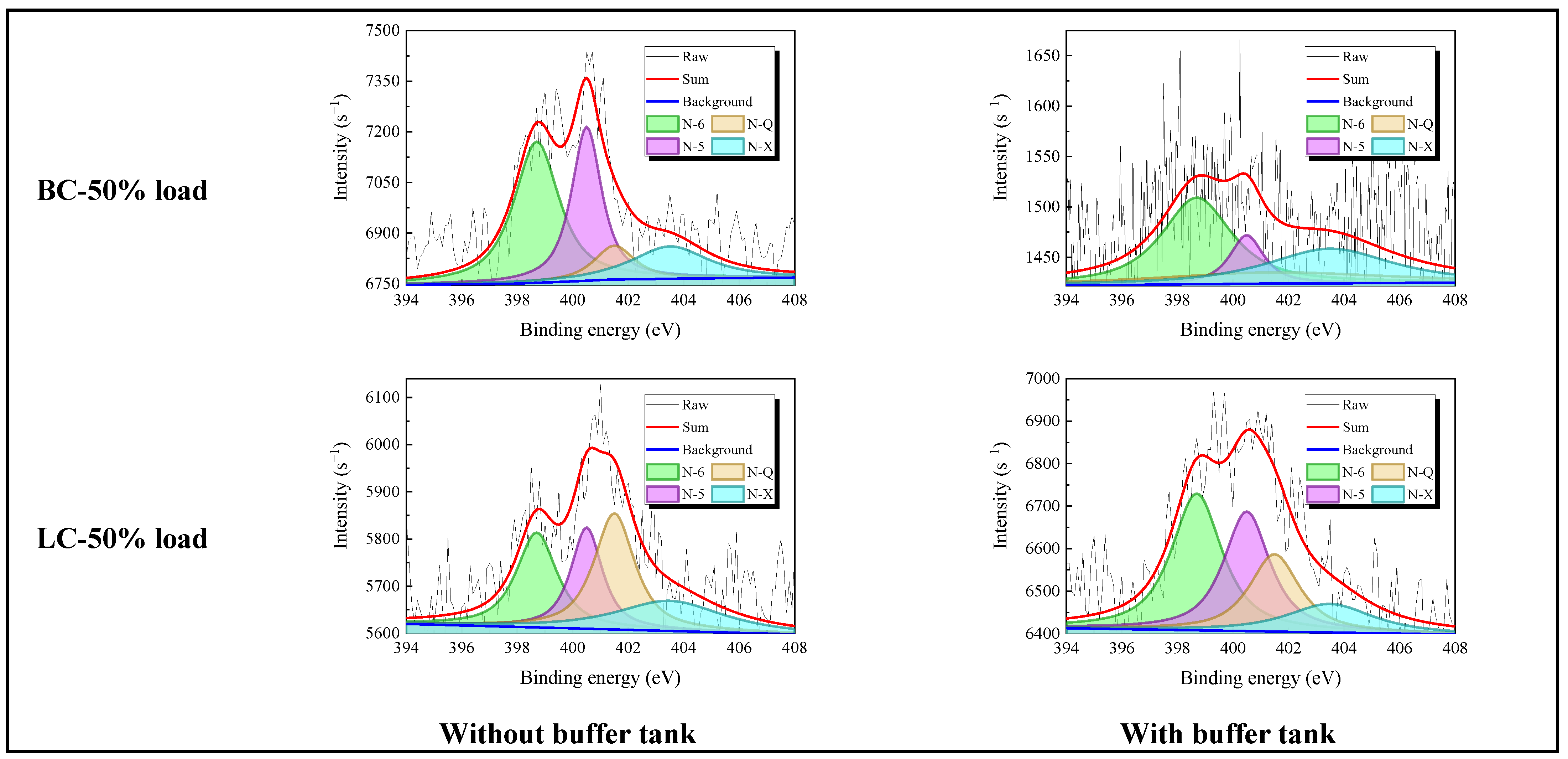
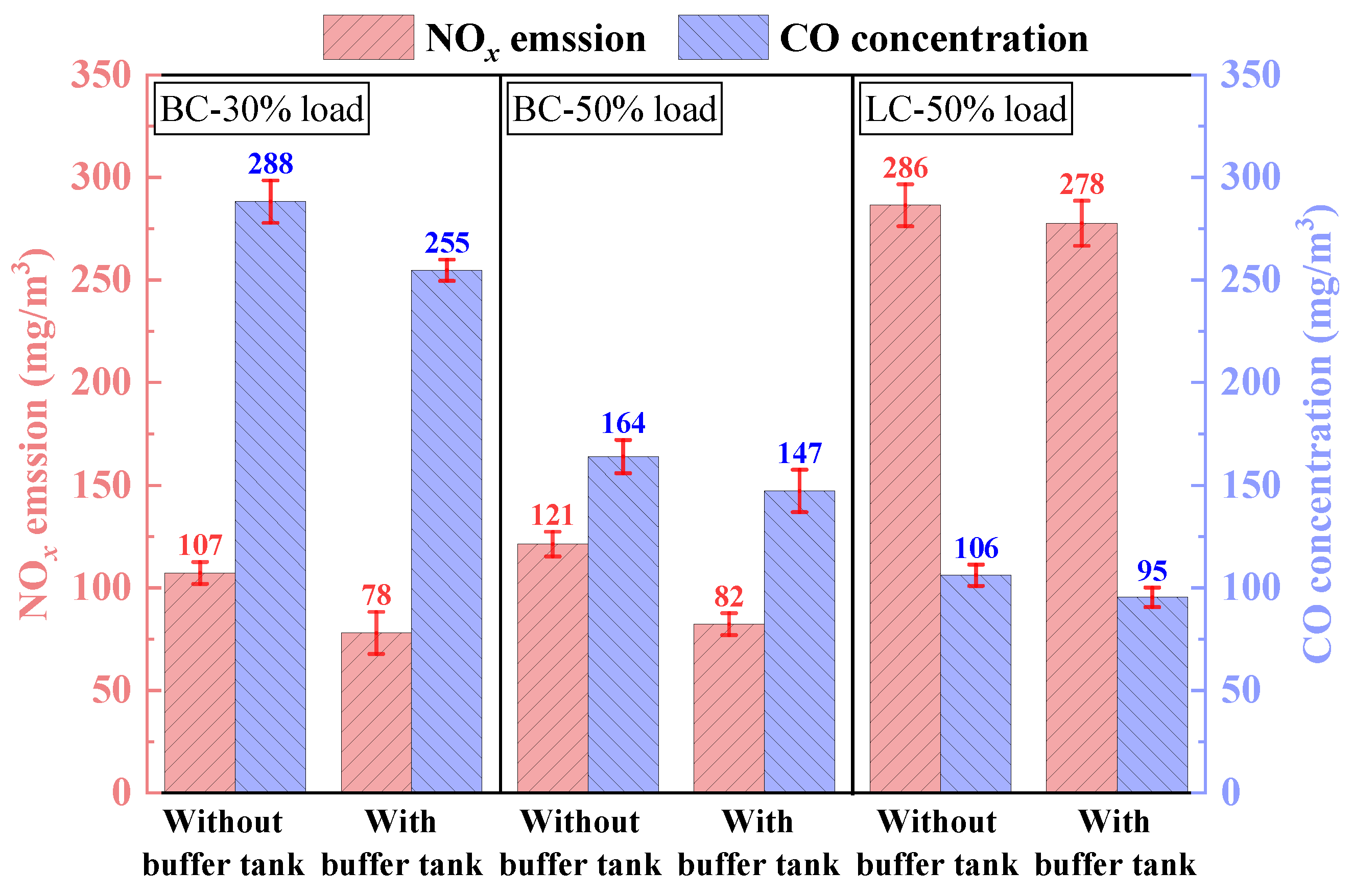
3.1.4. NOx and CO Emission
3.2. Research on Peak Shaving in Self-Preheating Combustion Under Varied FMD Loads
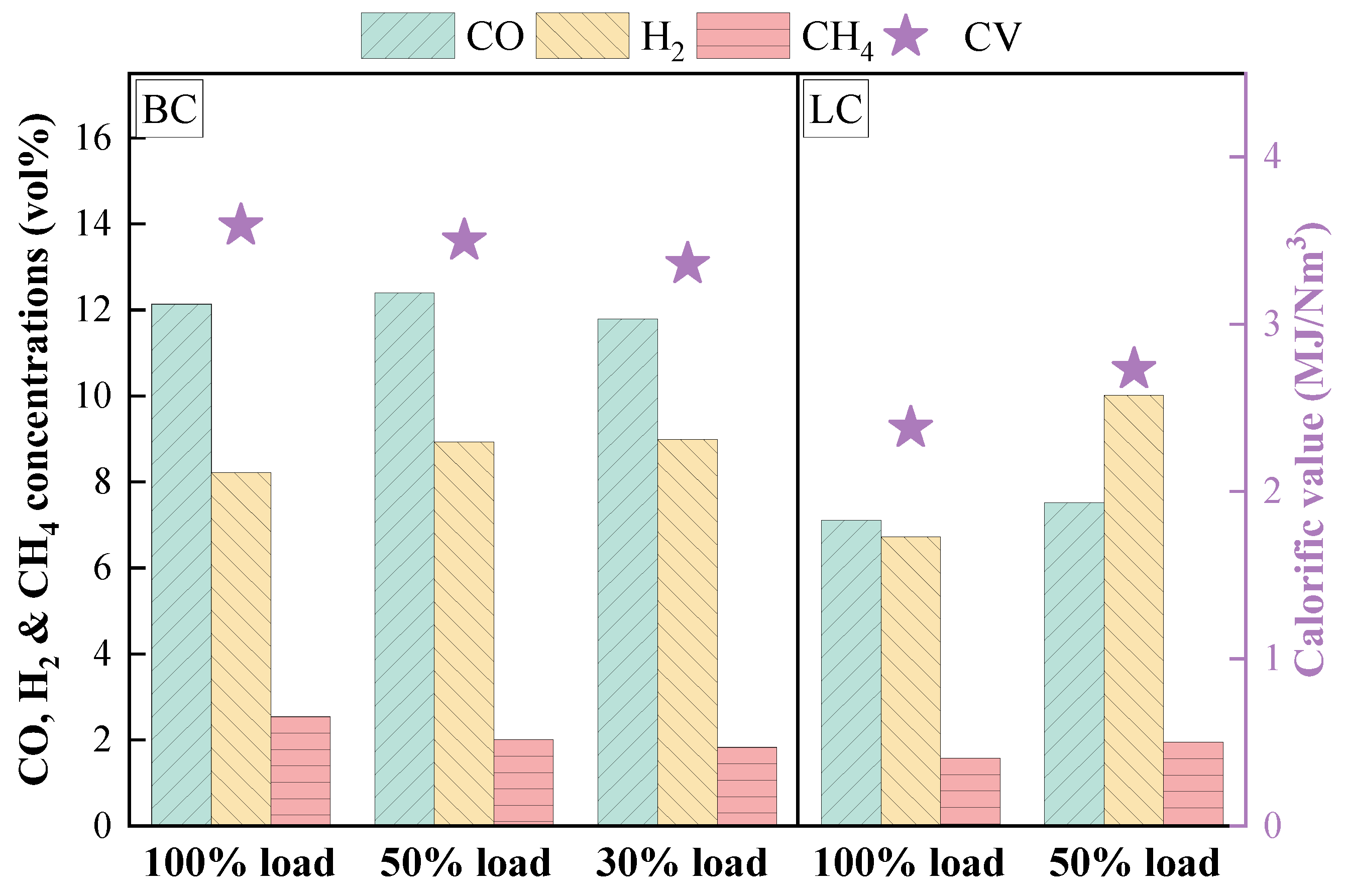
3.2.1. Analysis of Gas Composition During Modification
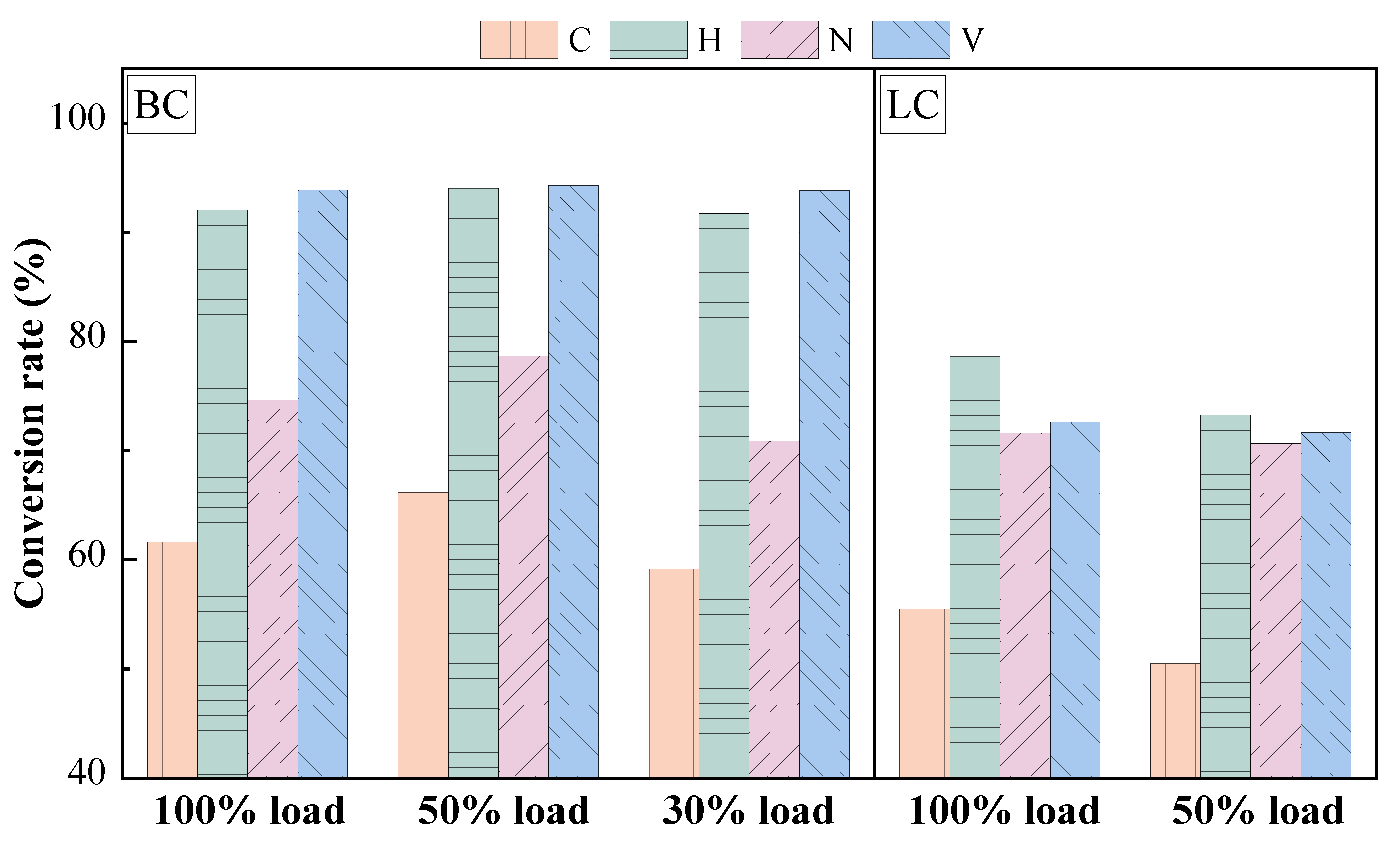
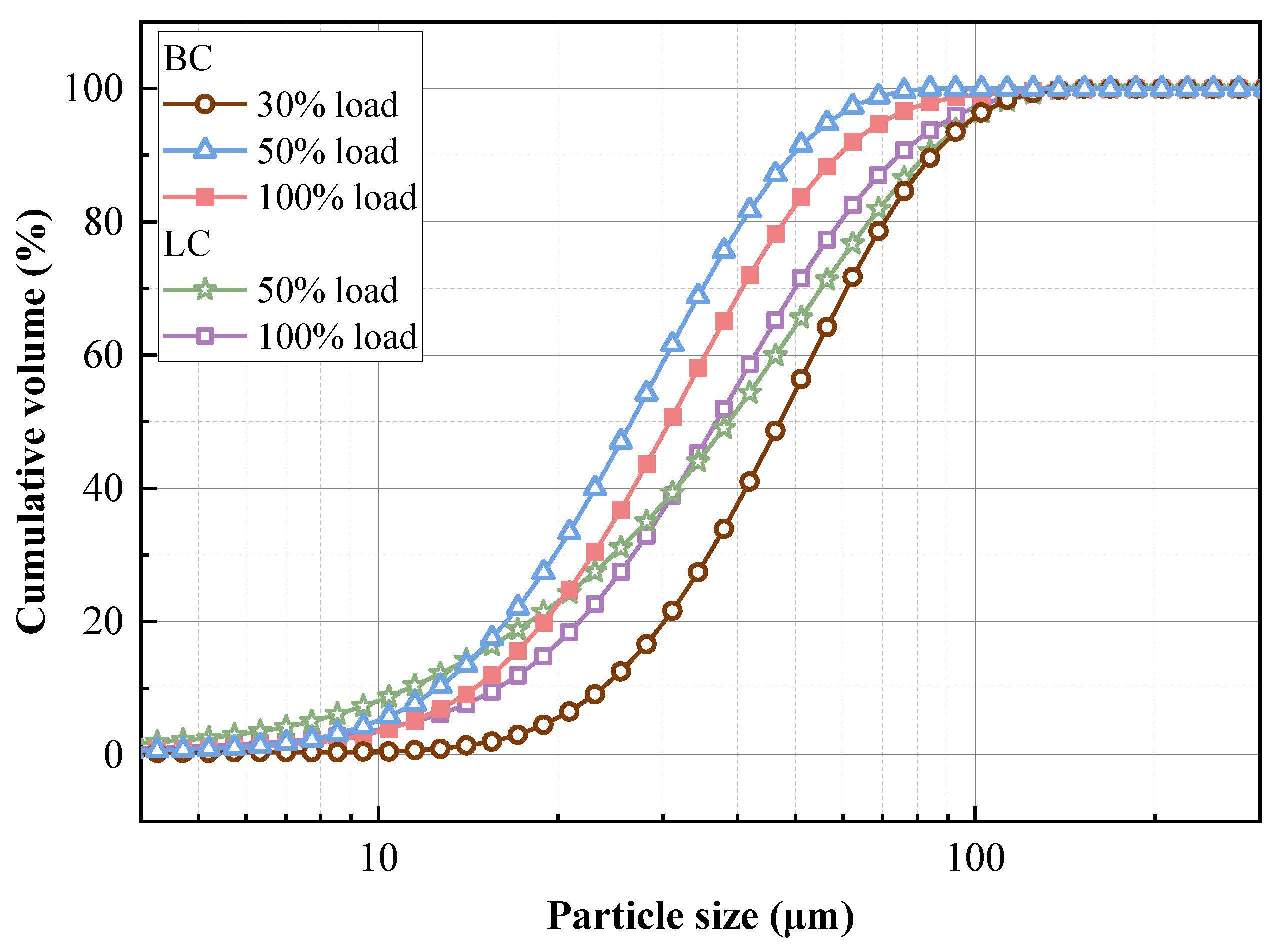
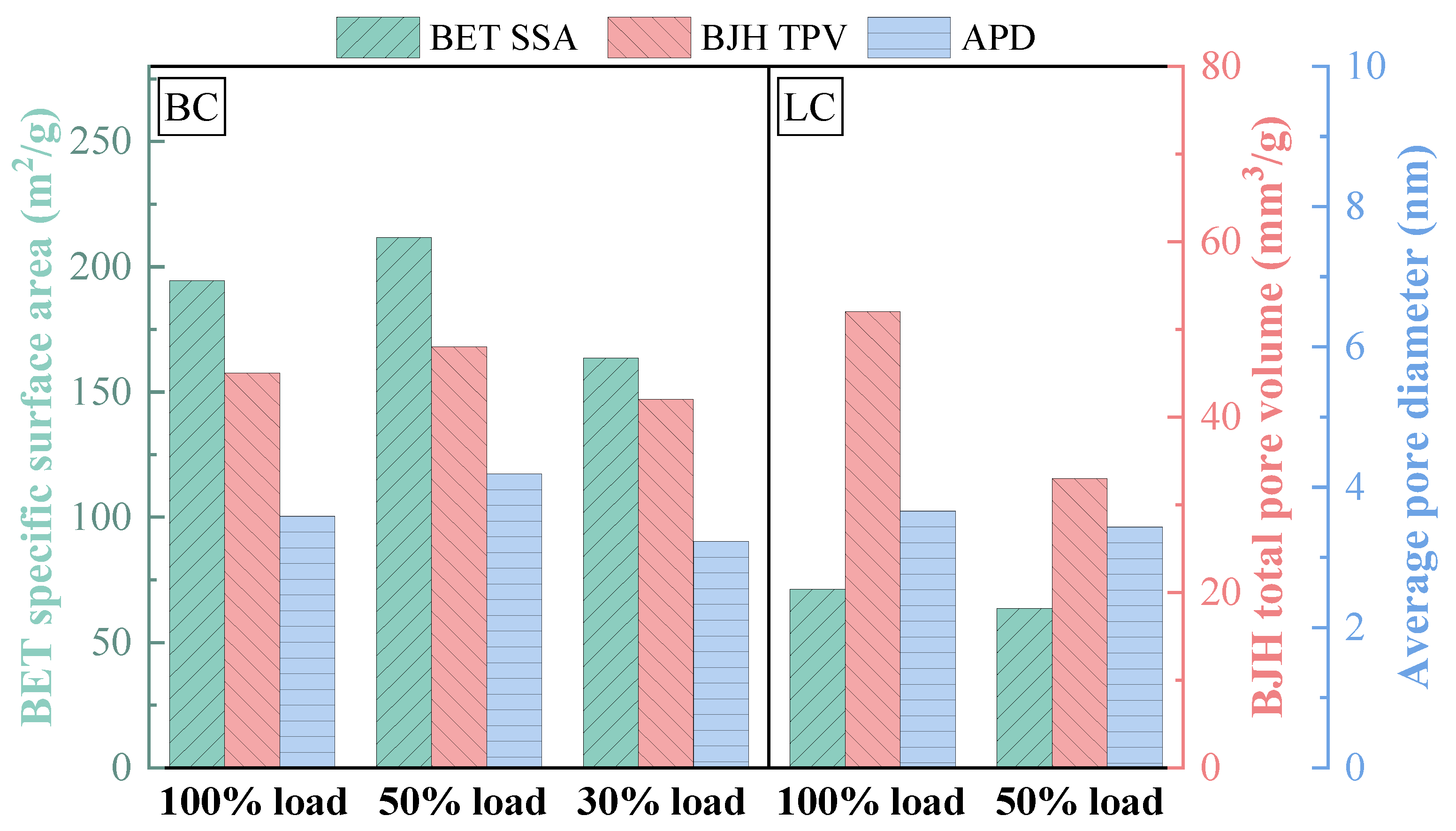
3.2.2. Physicochemical Properties of Preheated Char
3.2.3. NOx and CO Emission
3.3. Comparative Analysis of the FMD and TSB Performance
3.3.1. Operational Characteristics of FMD and TSB
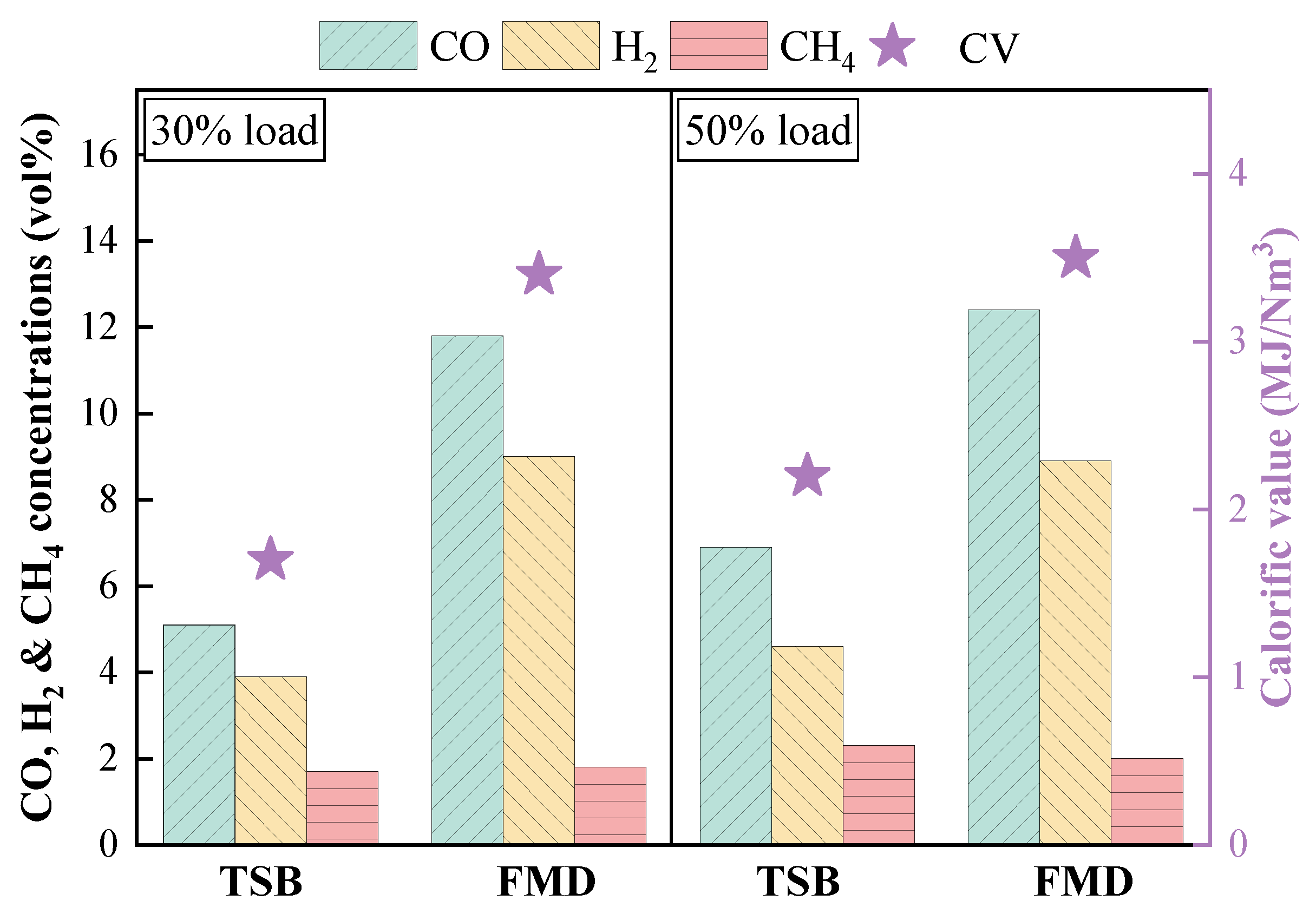
3.3.2. Analysis of Gas Composition During Fuel Modification
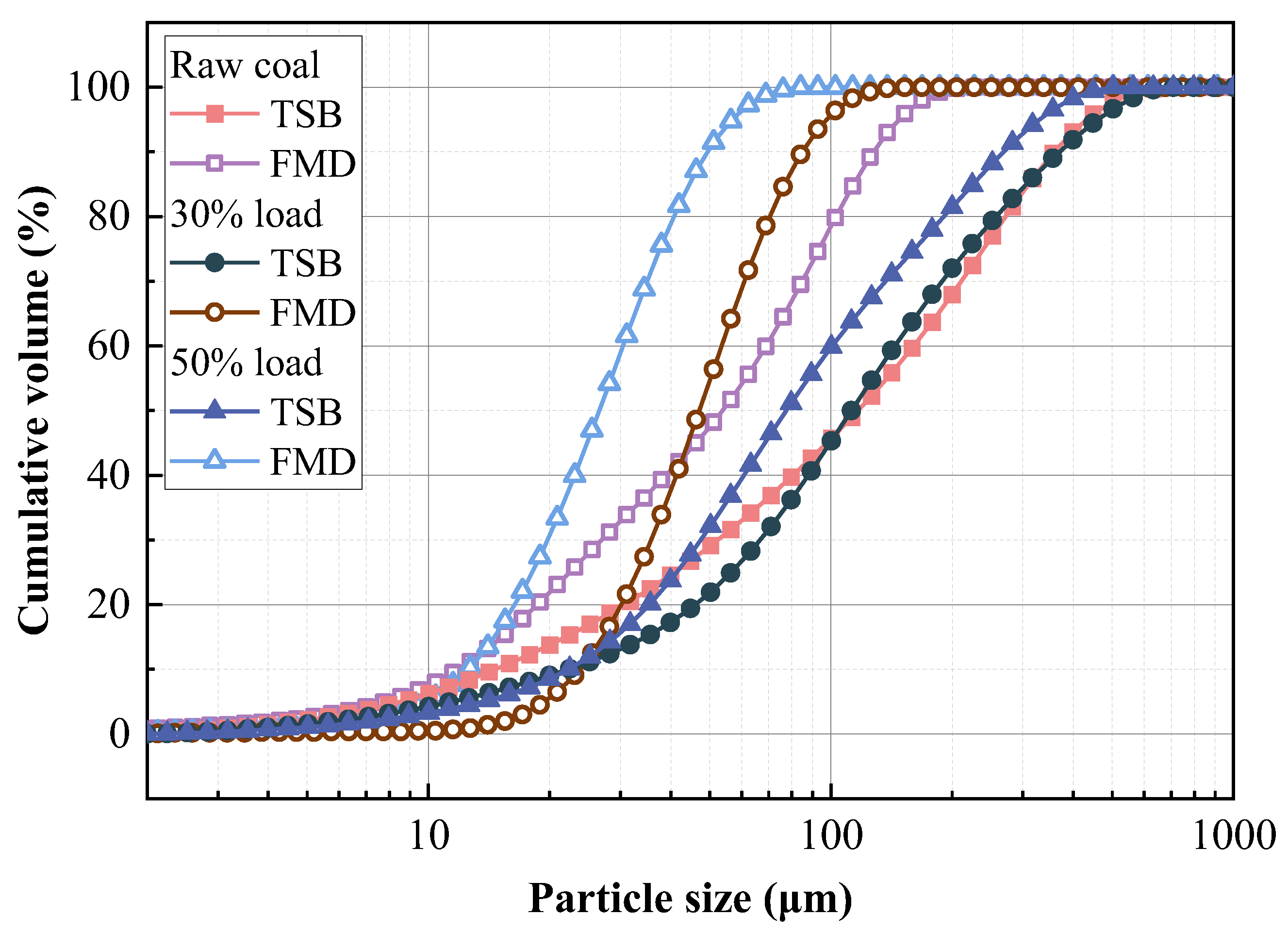
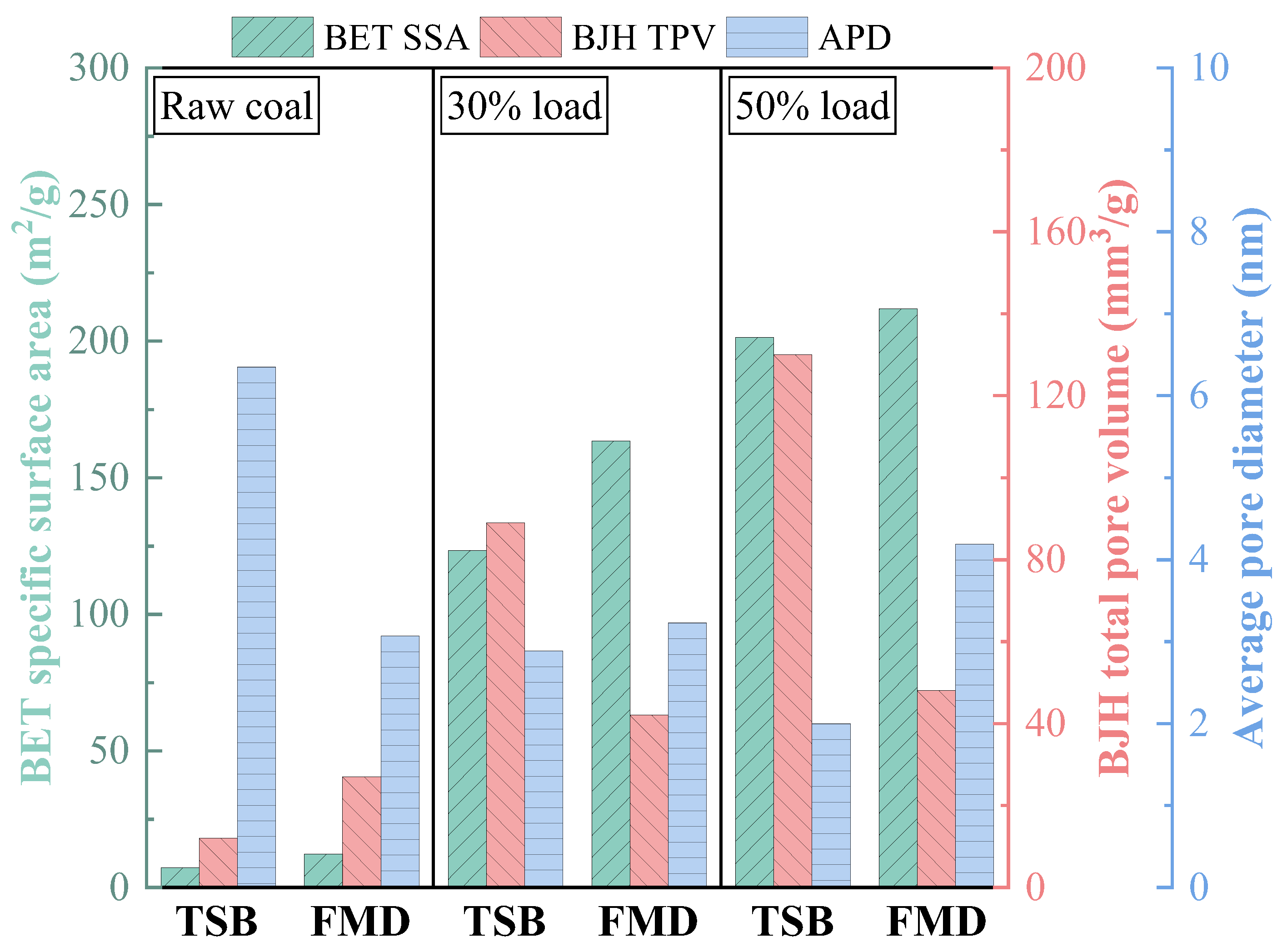
3.3.3. Physicochemical Properties of Preheated Char
3.3.4. NOx Emission and Combustion Efficiency
4. Conclusions
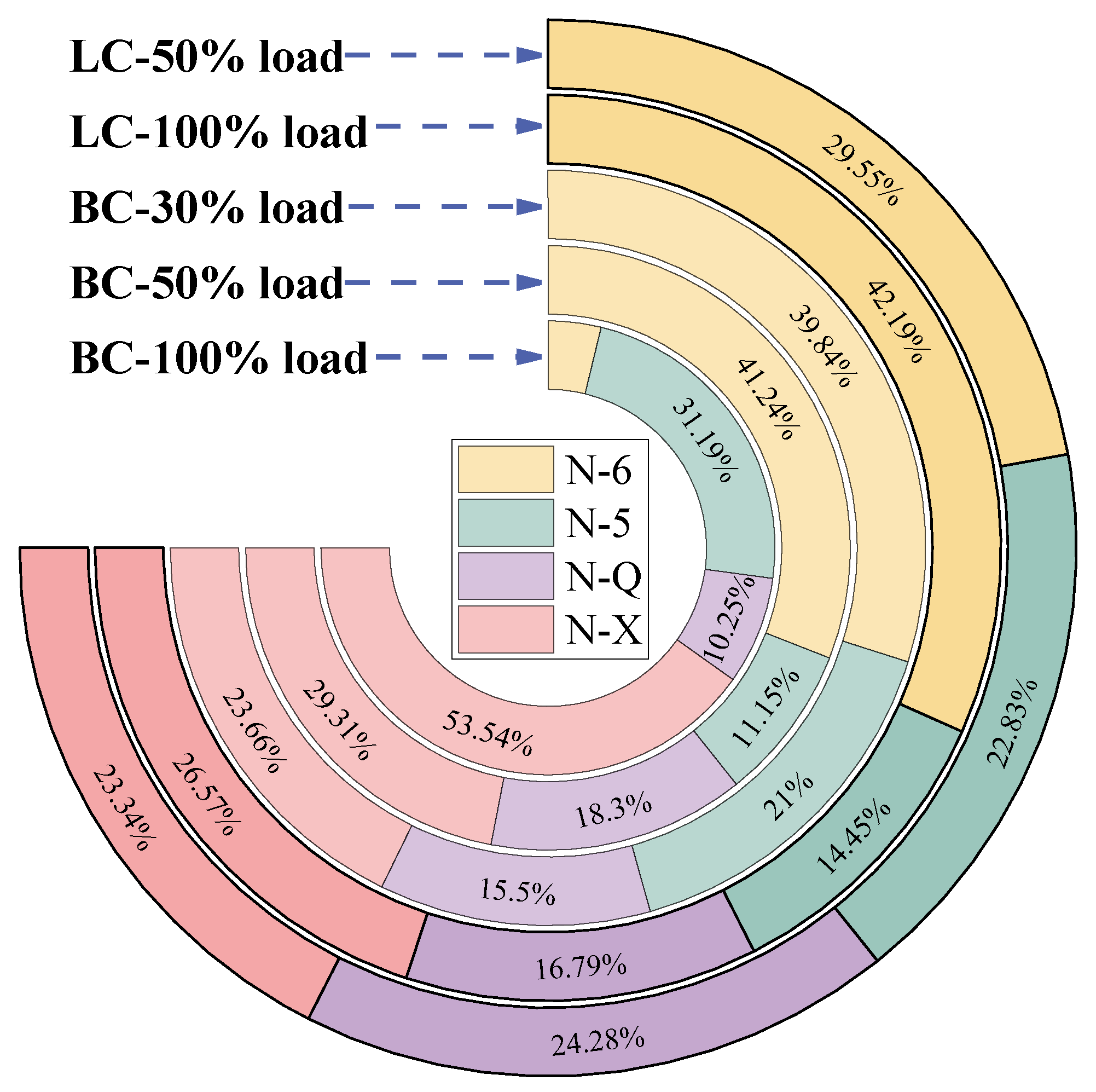
- Adding the buffer tank enhanced operation stability of FMD, improved its modification conditions, and reduced NOx emissions. Under such circumstance, the carbon microcrystalline structure of preheated coal char was improved, and its specific surface area, pore volume, pore diameter, and fuel conversion rate increased. Notably, this condition promoted large migration of stable nitrogen functional groups into nitrogen-containing gases (mainly N2). The optimization of physiochemical properties of preheated coal char and the massive solid-phase nitrogen reduction facilitated NOx emission reduction.
- The influence of load on fuel modification, combustion and NOx emissions was regulated by volatile content. The optimal modification efficiency was achieved at 50% and 100% loads for high-volatile and low-volatile coals, respectively. Moreover, η increased for high-volatile coal as load increased, but with NOx emissions increasing. By contrast, this condition reduced NOx emissions with high η for low-volatile coal.
- In comparison with TSB, FMD (equipped with a buffer tank) illustrated more conspicuous advantages in stable operation, fuel modification and NOx emission control. FMD suggested more remarkable advantages with regard to the enhancement of burnable gas yields in preheated coal gas and the betterment of physicochemical properties of preheated coal char. Moreover, clean and efficient combustion was more easily realized with the FMD design.
- The coal self-preheating combustion technology demonstrated exceptional advantages in stable combustion and NOx emission control at low loads of coal-fired boilers. In industrial applications, the appropriate FMD or TSB should be chosen rooted in diverse application requirements. By optimizing burner structure and operational parameters, original NOx emissions could decrease to a minimum of 77.93 mg/m3 with high η of 98.59% at low load of 30%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbols | |
| Cf | Combustible Percentage of Fly Ash, % |
| MP | Primary Air Flow Rate, Nm3/h |
| MS | Secondary Air Flow Rate, Nm3/h |
| MT | Tertiary Air Flow Rate, Nm3/h |
| M | Total Air Flow Rate, Nm3/h |
| N-5 | Pyrrole and Pyrrolidine Nitrogen |
| N-6 | Pyridine Nitrogen |
| N-X | Pyridine Nitrogen-oxide |
| N-Q | Protonated Pyridine Nitrogen |
| Qnet,ar | Low Heating Value, MJ/kg |
| UFV | Fluidized Air Velocity, m/s |
| UCI | Cyclone Separator Inlet Velocity, m/s |
| UCO | Cyclone Separator Outlet Velocity, m/s |
| Greek letters | |
| λ | Total Air Ratio, % |
| λp | Primary Air Ratio, % |
| λS | Secondary Air Ratio, % |
| λT | Tertiary Air Ratio, % |
| η | Combustion Efficiency, % |
| Abbreviations | |
| APD | Average Pore Diameter, nm |
| BC | Shenmu Bituminous Coal |
| BET | Brunauer Emmett Teller |
| BJH | Barrett Joyner Halenda |
| CFB | Circulating Fluidized Bed |
| CV | Calorific Value, MJ/Nm3 |
| FMD | Novel Compact Fluidized Modification Device |
| LC | Shanxi Lean Coal |
| PLC | Programmable Logic Controller |
| SEM | Scanning Electron Microscopy |
| SCR | Selective Catalytic Reduction |
| SSA | Specific Surface Area, m2/g |
| TPV | Total Pore Volume, cm3/g |
| TSB | Traditional Self-preheating Burners |
| UFC | Up-fired Combustion Chamber |
References
- Chen, Z.; Tan, Y.; Xu, J. Economic and environmental impacts of the coal-to-gas policy on households: Evidence from China. J. Clean. Prod. 2022, 341, 130608. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Yan, P.; Zhou, G.; Liu, J.; Yu, D. Thermodynamics, flexibility and technoeconomics assessment of a novel integration of coal-fired combined heating and power generation unit and compressed air energy storage. Appl. Energy 2023, 339, 120924. [Google Scholar] [CrossRef]
- Qian, Q.; Tian, Y.; Lin, L.; Feng, L.; Shao, Z.; Xie, K. Modelling the socio-economic impacts of coal production reduction: A macro provincial analysis of China. Energy Rep. 2024, 12, 4306–4319. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Li, R.; Jiang, X. Exploring the role of nuclear energy in the energy transition: A comparative perspective of the effects of coal, oil, natural gas, renewable energy, and nuclear power on economic growth and carbon emissions. Environ. Res. 2023, 221, 115290. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Shi, C.; Jin, X.; Xu, Z. A novel data-driven approach for coal-fired boiler under deep peak shaving to predict and optimize NOx emission and heat exchange performance. Energy 2024, 304, 132106. [Google Scholar] [CrossRef]
- Wang, H.; Zou, C.; Hu, H.; Gu, G.; Dong, L.; Huang, Y.; Deng, S.; Li, S. Migration and emission characteristics of trace elements in coal-fired power plant under deep peak load regulation. Sci. Total Environ. 2023, 868, 161626. [Google Scholar] [CrossRef]
- Jin, W.; Si, F.; Kheirkhah, S.; Yu, C.; Li, H.; Wang, Y. Numerical study on the effects of primary air ratio on ultra-low-load combustion characteristics of a 1050 MW coal-fired boiler considering high-temperature corrosion. Appl. Therm. Eng. 2023, 221, 119811. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, S.; He, X.; Zhang, J.; Ding, X. Combustion stability and NOx emission characteristics of a 300 MWe tangentially fired boiler under ultra-low loads with deep-air staging. Energy 2023, 269, 126795. [Google Scholar] [CrossRef]
- Zeng, G.; Zhou, A.; Fu, J.; Ji, Y. Experimental and numerical investigations on NOx formation and reduction mechanisms of pulverized-coal stereo-staged combustion. Energy 2022, 261, 125358. [Google Scholar] [CrossRef]
- Chen, Z.; Qiao, Y.; Guan, S.; Wang, Z.; Zheng, Y.; Zeng, L.; Li, Z. Effect of inner and outer secondary air ratios on ignition, C and N conversion process of pulverized coal in swirl burner under sub-stoichiometric ratio. Energy 2022, 239, 122423. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Chen, W. Provincial pathways to carbon-neutral energy systems in China considering interprovincial electricity transmission development. Appl. Energy 2024, 375, 123953. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Zhou, X.; Wang, X.; Zheng, H.; Liu, Y.; Tan, H. CFD modeling and industry application of a self-preheating pulverized coal burner of high coal concentration and enhanced combustion stability under ultra-low load. Appl. Therm. Eng. 2024, 253, 123831. [Google Scholar] [CrossRef]
- Shen, T.; Song, M.; Huang, Y.; Zhu, R.; Li, Z.; Yu, Q.; Lu, P.; Wang, M. The effectiveness of a novel coal-igniting-fuel technology and application in a direct current burner. Fuel 2021, 306, 121503. [Google Scholar] [CrossRef]
- Li, S.; Chen, Z.; He, E.; Jiang, B.; Li, Z.; Wang, Q. Combustion characteristics and NOx formation of a retrofitted low-volatile coal-fired 330 MW utility boiler under various loads with deep-air-staging. Appl. Therm. Eng. 2017, 110, 223–233. [Google Scholar] [CrossRef]
- Zhu, G.; Gong, Y.; Niu, Y.; Wang, S.; Lei Yu Hui, S. Study on NOx emissions during the coupling process of preheating combustion of pulverized coal with multi-air staging. J. Clean. Prod. 2021, 292, 126012. [Google Scholar] [CrossRef]
- Su, X.; Fang, Q.; Ma, L.; Yao, B.; Li, Y.; Zhao, X.; Mao, R.; Yin, C. Improving combustion and lowering NOx emissions of an industrial coal swirl burner by optimizing its nozzle structure. Appl. Therm. Eng. 2023, 218, 119340. [Google Scholar] [CrossRef]
- Tang, H.; Xu, Z.; Han, X.; Sun, L.; Liu, Y.; Shen, X.; Liu, Z. Design method and experimental study on a novel self-sustaining internal combustion burner. Energy 2024, 308, 133039. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, S.; Zhu, J.; Liu, Y.; Zhang, J.; Hui, J.; Ding, H.; Cao, X.; Lyu, Q. Preheating and combustion characteristics of anthracite under O2/N2, O2/CO2 and O2/CO2/H2O atmospheres. Energy 2023, 274, 127419. [Google Scholar] [CrossRef]
- Su, K.; Ouyang, Z.; Wang, H.; Zhang, J.; Ding, H.; Wang, W. Research on purification, combustion and NO emission characteristics of pulverized coal preheated by a novel self-sustained purifying burner. Fuel 2024, 366, 131436. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Lyu, Q.; Zhu, J.; Pan, F. Microstructure analysis of fluidized preheating pulverized coal under O2/CO2 atmospheres. Fuel 2021, 292, 120386. [Google Scholar] [CrossRef]
- Zhu, S.; Hui, J.; Lyu, Q.; Ouyang, Z.; Zeng, X.; Zhu, J.; Liu, J.; Cao, X.; Zhang, X.; Ding, H.; et al. Experimental study on pulverized coal swirl-opposed combustion preheated by a circulating fluidized bed. Part A. Wide-load operation and low-NOx emission characteristics. Energy 2023, 284, 128573. [Google Scholar] [CrossRef]
- Hui, J.; Zhu, S.; Zhang, X.; Liu, Y.; Lin, J.; Ding, H.; Su, K.; Cao, X.; Lyu, Q. Experimental study of deep and flexible load adjustment on pulverized coal combustion preheated by a circulating fluidized bed. J. Clean. Prod. 2023, 418, 138040. [Google Scholar] [CrossRef]
- Wang, H.; Ouyang, Z.; Ding, H.; Su, K.; Zhang, J.; Hu, Y. Experimental study on the flexible peak shaving with pulverized coal self-preheating technology under load variability. Energy 2024, 289, 129830. [Google Scholar] [CrossRef]
- Wu, H.; Cai, J.; Ren, Q.; Xu, J.; Chu, F.; Lyu, Q. An efficient and economic denitration technology based on fuel pretreatment for cement cleaner production. J. Clean. Prod. 2020, 272, 122669. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Webb, P.; Orr, C.; Camp, R.; Olivier, J.; Yunes, Y. Analytical Methods in Fine Particle Technology; Micromeritics Instrument Corp: Norcross, GA, USA, 1997. [Google Scholar]
- Shao, Z.; Wang, S.; Zhao, Y.; Ren, T.; Chu, Z.; Xiang, P.; Liu, X. Staged Combustion Characteristics of Natural Gas Low-Emission Tower Coaxial Fuel. J. Combust. Sci. Technol. 2023, 29, 606616. [Google Scholar]
- Tong, B.; Li, J.; Cheng, T.; Zhao, Z.; Cheng, L.; Lyu, J. Investigation on the Criterion of the Air Distribution Uniformity in a Circulating Fluidized Bed Boiler and the Improved Test Method. J. Chin. Soc. Power Eng. 2023, 43, 823–828. [Google Scholar] [CrossRef]
- Sekret, R. An analysis of temperature distribution and heat transfer in a large-scale CFB boilers. Rynek Energii 2011, 6, 145–151. [Google Scholar]
- Ding, H.; Ouyang, Z.; Zhang, X.; Zhu, S. The effects of particle size on flameless combustion characteristics and NOx emissions of semi-coke with coal preheating technology. Fuel 2021, 297, 120758. [Google Scholar] [CrossRef]
- Meng, H.; Yang, H.; Wu, Z.; Li, D.; Wang, Z.; Wang, D.; Wang, H.; Li, H.; Li, J. Co-pyrolysis of mushroom residue blended with pine sawdust/wheat straw for sustainable utilization of biomass wastes: Thermal characteristics, kinetic/thermodynamic analysis, and structure evolution of co-pyrolytic char. Sustainability 2024, 16, 6677. [Google Scholar] [CrossRef]
- Ryoichi, K.; Hiromitsu, M.; Hisao, M.; Akira, S. Characteristics of particulate matter generated in pressurized coal combustion for high-efficiency power generation system. Adv. Powder Technol. 2003, 14, 673–694. [Google Scholar] [CrossRef]
- Xi, J.; Liang, J.; Sheng, X.; Shi, L.; Li, S. Characteristics of lump lignite pyrolysis and the influence of temperature on lignite swelling in underground coal gasification. J. Anal. Appl. Pyrolysis 2016, 117, 228–235. [Google Scholar] [CrossRef]
- Rong, L.; Xiao, J.; Wang, X.; Sun, J.; Jia, F.; Chu, M. Low-rank coal drying behaviors under negative pressure: Thermal fragmentation, volume shrinkage and changes in pore structure. J. Clean. Prod. 2020, 272, 122572. [Google Scholar] [CrossRef]
- Pels, J.; Kapteijn, F.; Moulijn, J.; Zhu, Q.; Thomas, K. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Phiri, Z.; Everson, R.; Neomagus, H.; Wood, B. Transformation of nitrogen functional forms and the accompanying chemical-structural properties emanating from pyrolysis of bituminous coals. Appl. Energy 2018, 216, 414–427. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Y.; Liu, Z.; Ding, H.; Huang, X.; Zheng, C. Study on the evolution of the char structure during hydrogasification process using Raman spectroscopy. Fuel 2015, 157, 97–106. [Google Scholar] [CrossRef]
- Guo, S.; Yang, W.; Yuan, S.; Yan, Z.; Geng, W. Experimental investigation of erosion effect on microstructure and oxidation characteristics of long-flame coal. Energy 2022, 259, 124959. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Bula, A. Gasification of biomass wastes in an entrained flow gasifier: Effect of the particle size and the residence time. Fuel Process. Technol. 2010, 91, 681–692. [Google Scholar] [CrossRef]
- Schmiers, H.; Friebel, J.; Streubel, P.; Hesse, R.; Kopsel, R. Change of chemical bonding of nitrogen of polymeric N-heterocyclic compounds during pyrolysis. Carbon 1999, 37, 1965–1978. [Google Scholar] [CrossRef]
- Zhu, S.; Hui, J.; Lyu, Q.; Ouyang, Z.; Liu, J.; Zhu, J.; Zeng, X.; Zhang, X.; Ding, H.; Liu, Y.; et al. Experimental study on pulverized coal combustion preheated by a circulating fluidized bed: Preheating characteristics for peak shaving. Fuel 2022, 324, 124684. [Google Scholar] [CrossRef]



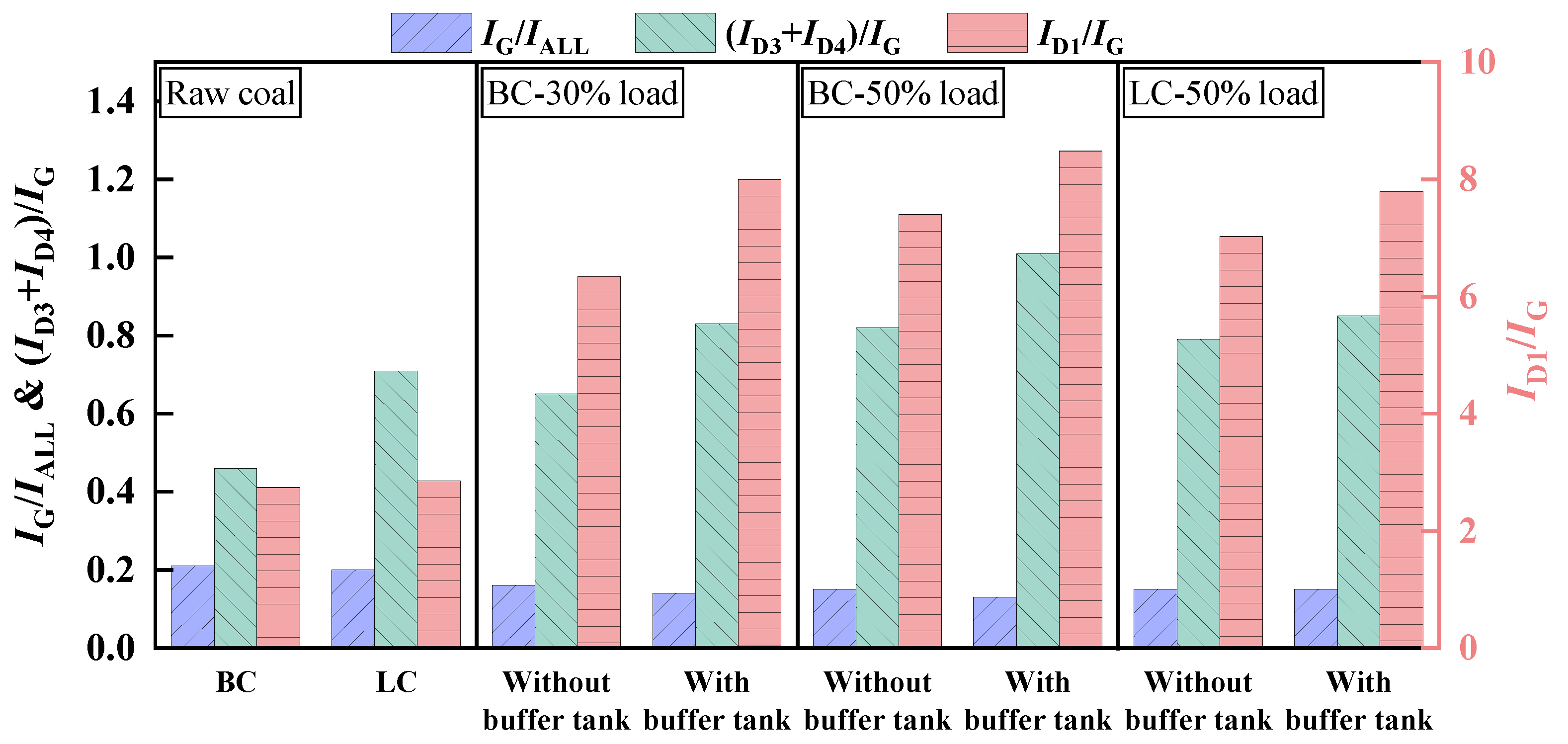







| Items | BC | LC |
|---|---|---|
| Ultimate analysis (wt. %, as-received) | ||
| Carbon (Car) | 73.60 | 54.69 |
| Hydrogen (Har) | 4.30 | 2.82 |
| Oxygen (Oar) | 11.43 | 4.86 |
| Nitrogen (Nar) | 0.94 | 0.85 |
| Sulfur (Sar) | 0.32 | 1.66 |
| Proximate analysis (wt. %, as-received) | ||
| Moisture (Mar) | 4.93 | 0.72 |
| Volatile matter (Var) | 32.59 | 13.36 |
| Fixed carbon (FCar) | 58.00 | 51.52 |
| Ash (Aar) | 4.48 | 34.40 |
| Low heating value (Qnet, ar, MJ/kg) | 28.49 | 21.03 |
| Items | Unit | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 |
|---|---|---|---|---|---|---|---|---|---|
| Fuel | - | BC | LC | ||||||
| Load | % | 30 | 30 | 50 | 50 | 100 | 50 | 50 | 100 |
| Fuel feed rate | kg/h | 39 | 38 | 64 | 65 | 129 | 87 | 85 | 172 |
| Thermal power | MW | 0.30 | 0.30 | 0.50 | 0.50 | 1.00 | 0.50 | 0.50 | 1.00 |
| Buffer tank | - | Without | With | Without | With | With | Without | With | With |
| MP | Nm3/h | 50 | 51 | 82 | 87 | 170 | 85 | 83 | 178 |
| λP | - | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.19 |
| UFV | m/s | 1.53 | 1.56 | 2.50 | 2.66 | 5.20 | 2.57 | 2.51 | 5.39 |
| UCI | m/s | 7.42 | 7.57 | 12.17 | 12.92 | 25.24 | 12.50 | 12.21 | 26.18 |
| UCO | m/s | 10.05 | 10.25 | 16.48 | 17.49 | 34.18 | 16.93 | 16.53 | 35.45 |
| MS | Nm3/h | 145 | 136 | 234 | 238 | 490 | 244 | 243 | 492 |
| λS | - | 0.51 | 0.49 | 0.50 | 0.50 | 0.52 | 0.51 | 0.52 | 0.52 |
| MB | Nm3/h | 142 | 150 | 243 | 238 | 490 | 254 | 239 | 496 |
| λB | - | 0.50 | 0.54 | 0.52 | 0.50 | 0.52 | 0.53 | 0.51 | 0.52 |
| M | Nm3/h | 337 | 337 | 559 | 563 | 1150 | 583 | 565 | 1166 |
| λ | - | 1.19 | 1.21 | 1.20 | 1.18 | 1.22 | 1.22 | 1.21 | 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Li, S.; Ouyang, Z.; Zhu, S.; Zeng, X.; Zhou, H.; Su, K.; Wang, H.; Hui, J. Experimental Study on Peak Shaving with Self-Preheating Combustion Equipped with a Novel Compact Fluidized Modification Device. Energies 2025, 18, 2555. https://doi.org/10.3390/en18102555
Ding H, Li S, Ouyang Z, Zhu S, Zeng X, Zhou H, Su K, Wang H, Hui J. Experimental Study on Peak Shaving with Self-Preheating Combustion Equipped with a Novel Compact Fluidized Modification Device. Energies. 2025; 18(10):2555. https://doi.org/10.3390/en18102555
Chicago/Turabian StyleDing, Hongliang, Shuyun Li, Ziqu Ouyang, Shujun Zhu, Xiongwei Zeng, Haoyang Zhou, Kun Su, Hongshuai Wang, and Jicheng Hui. 2025. "Experimental Study on Peak Shaving with Self-Preheating Combustion Equipped with a Novel Compact Fluidized Modification Device" Energies 18, no. 10: 2555. https://doi.org/10.3390/en18102555
APA StyleDing, H., Li, S., Ouyang, Z., Zhu, S., Zeng, X., Zhou, H., Su, K., Wang, H., & Hui, J. (2025). Experimental Study on Peak Shaving with Self-Preheating Combustion Equipped with a Novel Compact Fluidized Modification Device. Energies, 18(10), 2555. https://doi.org/10.3390/en18102555






