1. Introduction
Perovskite solar cells (PSCs) have achieved unprecedented power conversion efficiencies (PCEs) that rival or even surpass those of traditional solar cells mainly composed of silicon [
1,
2]. This exceptional performance results from the distinct photophysical attributes of perovskite materials, such as their strong light-harvesting capabilities, extended carrier diffusion ranges, and easy bandgap tunability [
3,
4,
5]. Additionally, the low-temperature, solution-processable fabrication methods associated with PSCs make them particularly attractive for scalable and cost-effective renewable energy applications. As PSCs approach commercialization, challenges related to device stability, reproducibility, and large-scale manufacturing must be addressed [
6,
7,
8]. A critical aspect of overcoming these challenges lies in the optimization of perovskite precursor formulations, where solvents and additives play pivotal roles in determining the quality of perovskite films and the overall efficiency of devices [
9].
Historically, dimethyl sulfoxide (DMSO) and N,N-dimethylformamide (DMF) have been the most widely used solvents for perovskite precursor solutions [
10]. These solvents are highly effective in dissolving essential components, such as lead halides and organic cations, while facilitating controlled crystallization during film formation [
11]. The 1:4 volume ratio of DMSO to DMF has been widely recognized as the standard for achieving structurally coherent perovskite layers with reliable control of morphology and desirable optoelectronic properties [
12]. Despite their widespread use, DMSO-based systems are not without limitations. The high volatility of DMSO can disrupt the evaporation dynamics during deposition, leading to non-uniform crystallization and suboptimal film quality [
13,
14,
15,
16,
17]. Furthermore, the rapid crystallization kinetics associated with DMSO often result in smaller grain sizes and higher defect densities, which negatively impact both efficiency and stability [
18,
19]. These limitations have prompted researchers to explore alternative solvent systems capable of addressing these challenges while maintaining or enhancing device performance.
Among alternative solvents, N-methyl-2-pyrrolidone (NMP) and N,N′-dimethylpropyleneurea (DMPU) have drawn growing interest recently for their suitability in perovskite precursor solution design [
20,
21]. These solvents exhibit distinct physicochemical properties, including lower volatility, higher boiling points, and stronger coordination capabilities with precursor components, which allow for more controlled film formation [
22]. This enhanced control over the evaporation and crystallization processes supports the formation of perovskite films with greater uniformity, larger grain sizes, and reduced defect densities. Additionally, the compatibility of these solvents with scalable deposition methods, for instance, blade coating and slot-die coating, makes them particularly attractive for industrial-scale manufacturing [
23]. Among these, blade coating has attracted increasing attention as a promising technique for roll-to-roll processing and could be well-suited to the controlled crystallization behavior observed in NMP-based systems [
24]. In particular, NMP, when combined with DMF at a 1:9 volume ratio, has shown particular promise, producing films with enhanced crystallinity and optoelectronic properties [
25,
26]. These advancements suggest that NMP could address many of the challenges posed by DMSO while providing a pathway to scalable production. However, while extensive efforts have been made to optimize solvent volume ratios, comparatively little attention has been given to the role of additives in alternative solvent systems.
Additives, such as methylammonium chloride (MACl), are instrumental in tailoring the structural and photovoltaic characteristics of perovskite films [
27,
28,
29]. MACl is widely recognized for its ability to enhance crystallinity, promote larger grain sizes, and reduce defect densities, which collectively contribute to higher PCEs and improved device stability. In DMSO-based systems, an optimal MACl concentration of approximately 50 mol% has been established across numerous studies [
30,
31]. This concentration facilitates uniform crystallization and provides sufficient chloride ions to passivate defects at grain boundaries and charge-trap sites [
32]. These effects result in films with superior optoelectronic properties and greater stability under operational conditions. However, the transferability of this optimal concentration to alternative solvent systems, such as NMP/DMF, remains largely unexplored. Given the distinct physicochemical properties of NMP, including its lower volatility and unique coordination interactions, it is reasonable to hypothesize that the optimal concentration of MACl in NMP-based systems would differ significantly from that in DMSO-based systems.
The interplay between solvents and additives is a critical but often overlooked factor in perovskite precursor engineering. Solvents influence the solubility, distribution, and reactivity of additives, as well as the kinetics of crystallization and defect passivation [
33,
34]. For example, NMP’s lower evaporation rate and stronger coordination with precursor components may alter the behavior of MACl during film formation, potentially reducing the required MACl concentration while maintaining or even enhancing film quality. Additionally, the unique chemical interactions between NMP and precursor components may lead to differences in the crystallization dynamics, resulting in improved grain growth and defect reduction. Despite these possibilities, few studies have systematically investigated the combined effects of solvents and additives in NMP-based systems. This gap in understanding limits the ability to fully exploit the advantages offered by alternative solvent systems, particularly in reducing material consumption while achieving high efficiency.
To tackle those issues, we examined the relationship between solvent systems and additive concentrations, with a specific focus on the NMP/DMF (1:9) solvent system. We systematically evaluated how the optimal concentration of MACl in NMP-based systems compared to that in traditional DMSO-based systems. Our findings revealed that the optimal MACl concentration in NMP-based systems could be substantially reduced to 20–30 mol%, representing a notable decrease from the 50 mol% commonly used in DMSO-based systems. Remarkably, this reduction was achieved without compromising film quality or device performance. Films prepared with NMP and 20–30 mol% MACl exhibited superior crystallinity, as evidenced by narrower FWHM values in XRD measurements, along with reduced defect densities. In addition to XRD, surface and morphological analyses using scanning electron microscopy (SEM) and atomic force microscopy (AFM) further confirmed that these films possessed uniform, pinhole-free surfaces and larger grain sizes compared with other conditions. These improvements translated into enhanced optoelectronic properties, with devices achieving PCEs exceeding 23%, compared with ~20% for DMSO-based systems [
35]. Furthermore, devices fabricated under these conditions demonstrated improved long-term stability under continuous illumination, highlighting the potential of NMP-based systems for scalable PSC manufacturing.
2. Materials and Methods
2.1. Materials
All chemicals were stored in a nitrogen (N2)-filled glovebox and used without further purification. The following materials were used in the experiments: formamidine hydroiodide (FAI; 99.99%, TCI), lithium bis(trifluoromethanesulfonyl)imide (Li-TFSI; 98%, TCI), lead(II) iodide (PbI2; 99.99%, TCI), anhydrous N,N-dimethylformamide (DMF; 99.8%, Sigma-Aldrich, St. Louis, MO, USA), anhydrous N-methyl-2-pyrrolidone (NMP; 99.5%, Sigma-Aldrich), 4-tert-butylpyridine (tBP; 98%, Sigma-Aldrich), isopropanol (IPA; 99.5%, Sigma-Aldrich), lead(II) bromide (PbBr2; 99.999%, Sigma-Aldrich), anhydrous acetonitrile (ACN; 99.8%, Sigma-Aldrich), methylammonium chloride (MACl; 99.5%, Lumtec, New Taipei City, Taiwan), 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenyl-amine)9,9′-spirobifluorene (Spiro-OMeTAD; 99.5%, Lumtec, New Taipei City, Taiwan), deionized water (H2O; 100%, DAEJUNG), propanone (acetone; 99.7%, SAMCHUN), cyclohexylmethylammonium iodide (CHMAI; GreatCellSolar, Queanbeyan, Australia), tin(IV) oxide (SnO2; 15% H2O colloidal dispersion, Alfa Aesar, Ward Hill, MA, USA), and chlorobenzene (CB; 99.5%, Alfa Aesar).
The perovskite precursor solution was prepared to a total molar concentration of 1.35 M by dissolving FAI (1.35 M), PbI
2 (1.32975 M), and PbBr
2 (0.02025 M) in a mixed solvent of DMF and NMP with a 9:1 volume ratio. MACl was added as an additive at various molar concentrations (0%, 10%, 20%, 30%, 40%, and 50%) relative to the total precursor concentration, corresponding to 0 M, 0.135 M, 0.27 M, 0.405 M, 0.54 M, and 0.675 M, respectively. The solution was agitated at room temperature until fully dissolved and used without filtration. The perovskite composition used in this study was FAPb(I
0.99Br
0.01)
3, which was selected based on preliminary results demonstrating superior photovoltaic performance compared with other compositions. Supporting data for this composition’s performance can be found in
Figure S1.
2.2. Device Fabrication
The indium tin oxide (ITO) substrates were sequentially ultrasonically cleaned in DI water, acetone, and IPA solutions, each for 15 min. The cleaned substrates were further treated with UV-ozone for 15 min to enhance surface wettability.
The electron transport layer (ETL) was deposited using a spin-coating method. A SnO2 solution was formulated by mixing commercial SnO2 dispersion with DI water, maintaining a 1:3 volume ratio. After UV-ozone treatment (15 min), 100 µL of the diluted SnO2 solution was statically dropped onto each substrate and spin-coated at 5000 rpm for 30 s. The resulting films were then annealed on a hot plate at 155 °C for at least 15 min to complete the ETL formation.
After ETL deposition, an additional UV-ozone treatment (10 min) was applied before depositing the perovskite layer. All processes up to this step were performed under ambient conditions, while the perovskite, interlayer, and hole transport layer (HTL) deposition steps were conducted in a dry-air environment. For perovskite layer deposition, 50 μL of precursor solution was statically dropped onto the ETL-coated substrate, followed by spin-coating at 3000 rpm for 30 s. CB (30 μL) was dripped at 15 s into the spin-coating process as an anti-solvent. The perovskite films were then annealed at 155 °C for exactly 15 min. An interlayer was applied between the perovskite and HTL by spin-coating a CHMAI solution (0.025 M in IPA). A volume of 40 μL was dynamically deposited onto each substrate and spin-coated at 5000 rpm for 30 s without additional annealing.
For the HTL deposition, a Spiro-OMeTAD solution was obtained by dissolving Spiro-OMeTAD in CB at a concentration of 0.059 M, followed by doping with t-BP and Li-TFSI (1.18 M). The prepared solution was dynamically spin-coated onto the HTL at 2500 rpm for 30 s, with 50 μL of solution dispensed per substrate to ensure uniform coating. No additional annealing step was performed after the coating. Before depositing the metal electrode, the ITO surface at both edges of the substrate was carefully scraped with a blade to ensure direct electrical contact between the electrode and the underlying layers. Finally, gold (Au) electrodes (54.9 nm) were thermally evaporated using a metal mask to complete the device fabrication.
2.3. Measurement Condition
The J-V characteristics were measured under 1 sun equivalent illumination using an Ossila Solar Cell I-V Test System equipped with a LumiSun-50TM LED Solar Simulator (Innovations in Optics, Woburn, MA, USA). The LumiSun-50 TM provides Class A+A+A+ illumination, closely replicating the AM1.5G solar spectrum with exceptional spectral match and uniformity, ensuring highly accurate photovoltaic performance measurements under standard testing conditions. Incident photon-to-current efficiency (IPCE) measurements were performed using a quantX-300 system (Newport, RI, USA).
AFM data were obtained using an MFP-3D Origin+ system (Oxford Instruments, Oxfordshire, UK). SEM images were captured in planar view using an Hitachi SU8600. XRD analysis was conducted with a Rigaku SmartLab instrument (Rigaku, Tokyo, Japan), employing Cu Kα radiation and recording data at 0.02° intervals. UV-vis absorption was measured using a UV-3600i Plus spectrophotometer (Shimadzu, Kyoto, Japan). Fourier-transform infrared (FT/IR) spectroscopy was performed using an FT/IR-6X spectrometer (Jasco, Tokyo, Japan).
AFM, SEM, and XRD analyses were conducted at the Central Laboratory of Hankyong National University using samples prepared with a structure consisting of ITO substrates coated with SnO2 and perovskite layers only. In contrast, J-V measurements were performed on fully fabricated devices, including the HTL and metal electrode.
3. Results and Discussion
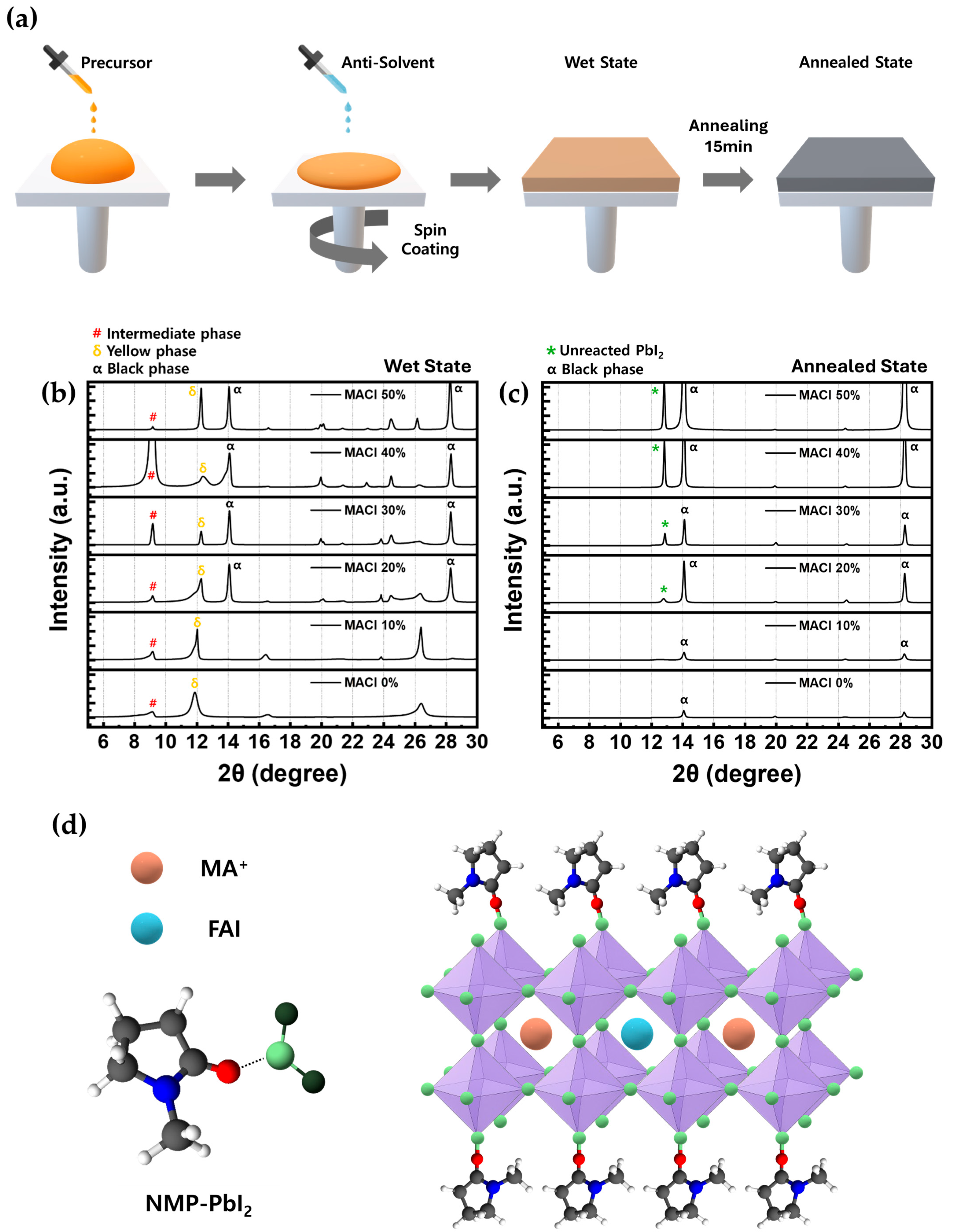
In order to evaluate the impact of MACl molar concentration on the crystal structure of perovskite films, XRD analysis was performed. As illustrated in
Figure 1a, the perovskite precursor solution was prepared using an NMP-based solvent system and deposited via the anti-solvent method [
36]. Measurements were conducted under two different conditions: one in the wet state, referring to the moment immediately after spin-coating the precursor solution, before any thermal treatment; and the other in the annealed state, following thermal annealing. The perovskite composition was fixed as FAPb(I
0.99Br
0.01)
3, and MACl was added at six different molar concentrations: 0%, 10%, 20%, 30%, 40%, and 50%. All samples were analyzed in the 2θ range of 5° to 30°.
Since the wet state reflects the initial stage of crystallization before thermal annealing, it has a substantial impact on the resulting perovskite film quality. As such, analyzing the phase composition in this early stage can provide key insights into film formation mechanisms [
37]. In the XRD results obtained from the wet state (
Figure 1b), three major peaks were identified: the intermediate phase around 9°, the
δ-phase (yellow phase) around 12°, and the
α-phase (black phase) around 14°. The
α-phase peak was not observed in the 0% or 10% MACl samples, but became evident at 20% and above. The
δ-phase appeared from 0% and gradually decreased in intensity from 20% as the MACl concentration increased, although an unusually high intensity was observed at 50%. This unexpectedly high
δ-phase intensity at 50% indicates that this condition may not be favorable for high-quality perovskite formation, given the non-photoactive nature of the
δ-phase. The intermediate phase was present across all concentrations, showing the highest intensity at 40%. This intermediate phase, commonly observed in NMP-based solvent systems, originated from the coordination between PbI
2 and NMP, as illustrated in
Figure 1d [
38].
A clearly observed
α-phase peak in the wet state indicated the presence of a favorable
α-phase seed that facilitated the formation of a strong
α-phase peak in the annealed state, as shown in
Figure 1c [
39]. Therefore, the 20%, 30%, and 40% MACl conditions (
Figure 1b), which exhibited a well-defined
α-phase peak without excessively high
δ-phase intensity, were selected for comparison. The
δ-phase, known as a non-photoactive phase, showed a decrease in intensity as the MACl concentration increased. In contrast, the intermediate phase, which required an appropriate amount to facilitate proper crystallization, exhibited an increasing trend. To more accurately assess the crystal quality, the FWHM of the
α-phase peak observed in the wet state was calculated using Voigt function-based peak fitting.
The Voigt function, a convolution of a Gaussian and a Lorentzian function, is expressed as follows:
In this equation,
I(
x) denotes the XRD intensity,
x0 represents the central peak position,
σ corresponds to the Gaussian width,
γ to the Lorentzian width, and
η is the weighting factor between the two functions. The FWHM values calculated using this model for the 20%, 30%, and 40% MACl concentrations are summarized in
Table 1. Among these, the 40% condition exhibited the highest FWHM values across all three fitting functions: 0.388 (Gaussian), 0.284 (Lorentzian), and 0.129 (pseudo-Voigt). The relatively broad
α-phase peak and the pronounced intensity of the intermediate phase under the 40% condition suggest inferior crystallinity and less uniform crystal growth compared with the other samples [
40,
41,
42].
In the XRD patterns measured in the annealed state (
Figure 1c), the intermediate-phase peaks completely disappeared across all conditions, indicating full solvent evaporation and stabilization of the crystal structure [
43,
44]. MACl, while essential for guiding initial crystallization and stabilizing the structure, must also be completely removed during annealing, along with the solvent. As this process enhances the crystallinity of the films, leading to more pronounced
α-phase peaks, the involvement of MA
+ ions in the perovskite structure during crystallization is schematically depicted in
Figure 1d. The
α-phase peak near 14° showed very low intensity under the 0% and 10% MACl conditions, making it difficult to confirm the presence of a well-formed crystal structure. In contrast, samples containing 20% or more MACl exhibited significantly stronger
α-phase peaks, indicating that a crystalline perovskite phase was formed. This suggests that the crystallization behavior observed in the annealed state was strongly influenced by the initial phase formation in the wet state, where favorable
α-phase seeds could serve as the foundation for high-quality crystal growth during subsequent annealing. The promotion of
α-phase formation with increasing MACl content could also be visually confirmed when the substrates in the wet state were partially dried through solvent evaporation. Supporting visual evidence for this observation is provided in
Figure S2.
Additionally, a new diffraction peak appeared near 13°, corresponding to residual PbI2. This peak was not observed in the 0% and 10% conditions, was faintly detected at 20% and 30%, and became markedly more intense at 40% and 50%. These findings indicate that while MACl promotes the α-phase formation, it also leads to an increase in residual PbI2. Among the samples with well-formed α-phase (i.e., 20% and above), only the 20% and 30% conditions showed relatively low PbI2 peak intensities. These results collectively suggest that MACl concentrations of 20% and 30% are optimal for producing perovskite films with superior crystallinity, as consistently supported by both pre- and post-annealing XRD analysis.
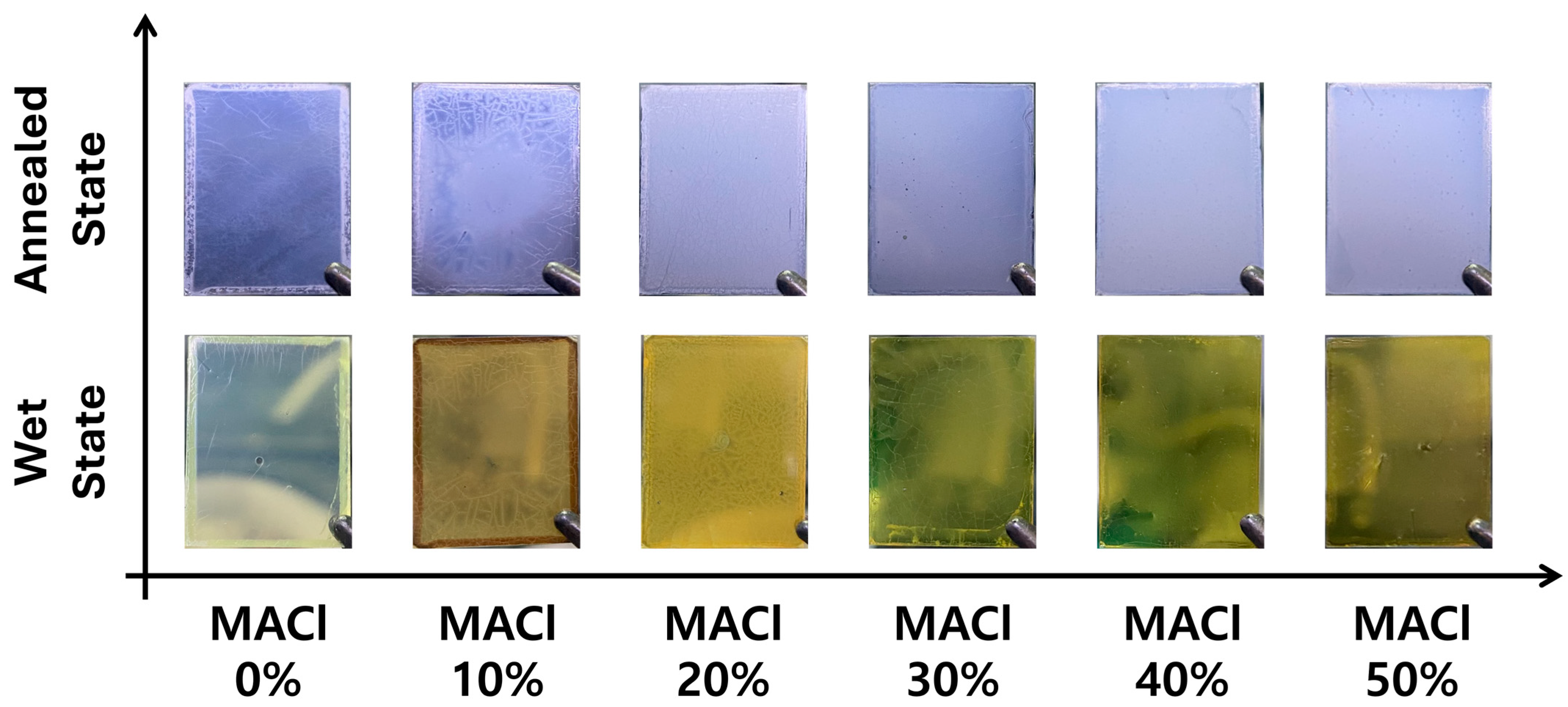
To complement the XRD results,
Figure 2 presents optical images of the perovskite films, visually illustrating the film appearance and surface evolution for each MACl concentration (0%, 10%, 20%, 30%, 40%, and 50%) at two different stages: the wet state (immediately after spin-coating) and the annealed state (after complete thermal processing). In the wet state, the film with 0% MACl appeared very light and transparent, with negligible crystallization. The 10% sample displayed an unusual brownish hue and poor crystallinity, especially at the center of the film. In contrast, samples with MACl concentrations of 20% or higher exhibited deeper coloration and more uniformly formed crystals, with the crystal size increasing with MACl content. However, in the 40% and 50% conditions, the crystal grains became excessively large, resulting in the formation of pinholes and appearance of abnormal particulate features.
In the annealed state, the films maintained a trend consistent with the wet-state observations, but showed significantly improved grain growth and surface uniformity. The visual results presented in
Figure 2 are in strong agreement with the crystallization behavior observed in the XRD analysis and clearly suggest a threshold MACl concentration necessary for initiating effective perovskite crystallization.
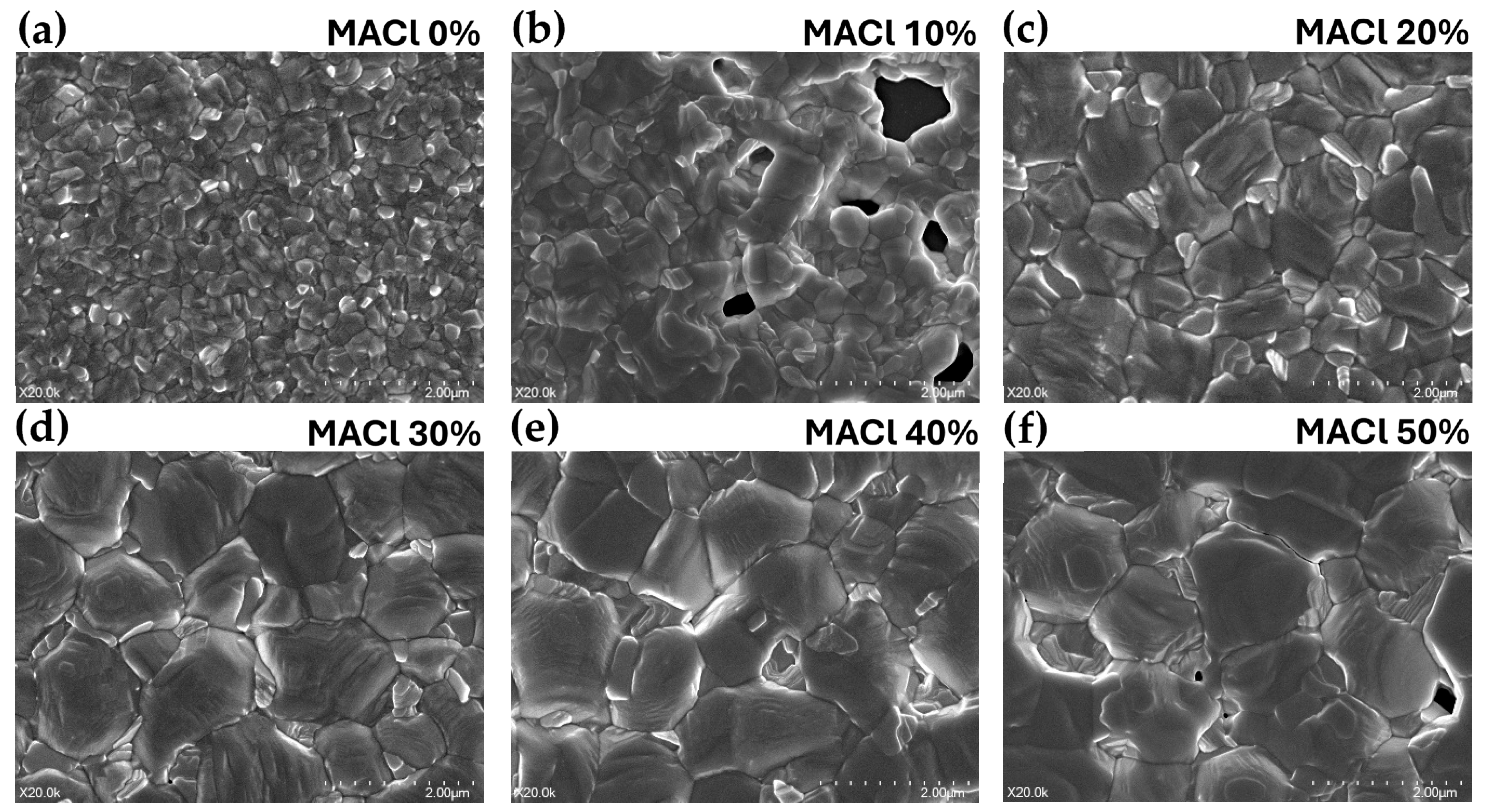
Based on the previous XRD analysis, the crystallization behavior of perovskite films was investigated as a function of MACl molar concentration in an NMP-based solvent system. To further examine the morphological characteristics of the films in greater detail, surface structures of the perovskite films in the annealed state were observed using SEM, as presented in
Figure 3. All samples were imaged at a magnification of 20,000×, and the MACl concentrations were varied from 0% to 50%. The perovskite composition and other fabrication conditions were kept consistent with those used in prior measurements (
Figure 3a–f). Under the 0% MACl condition (
Figure 3a), significantly smaller grain sizes were observed compared with the other samples. Although the surface was relatively uniform and no prominent defects or pinholes were visible, the small grain size indicated poor overall crystallinity. In the case of 10% MACl (
Figure 3b), the film exhibited the poorest crystallinity among all samples, with non-uniform particle distribution and numerous pinholes across a large area, suggesting incomplete crystal growth and low film density.
At 20% MACl (
Figure 3c), the grains were densely packed and uniform in size, with well-defined grain boundaries and a smooth surface. No observable defects or pinholes were present, indicating the formation of a compact, high-quality crystal structure. The 30% MACl sample (
Figure 3d) displayed a similarly dense and uniform morphology, though with slightly larger grains, while still maintaining excellent film uniformity and coverage. For the 40% MACl condition (
Figure 3e), further grain growth was observed, accompanied by increased height variation between grains, resulting in a less uniform surface. Grain boundaries appeared more disconnected, and slight height differences were noticeable between adjacent grains. At 50% MACl (
Figure 3f), the grains became even larger and more irregular in shape, leading to a notable decrease in uniformity and an increase in surface roughness. Pinholes were observed both between and within the grains, likely due to excessive grain growth.
These SEM observations highlight the evolution of perovskite film microstructure and surface morphology depending on MACl concentration. However, since SEM is limited in its ability to quantitatively assess surface topography and roughness, AFM was subsequently employed to more precisely evaluate height distribution and film compactness.
To supplement the limitations of SEM in providing detailed surface information, AFM was employed to more precisely assess the surface height distribution, roughness, and compactness of the perovskite films.
Figure 4 presents 3D surface morphology images of perovskite films fabricated with six different MACl concentrations, ranging from 0% to 50%, using an NMP-based solvent system (
Figure 4a–f). The perovskite composition and other fabrication conditions were kept consistent throughout all measurements. In the 0% MACl condition (
Figure 4a), relatively small yet uniform crystal grains were observed, and the surface morphology appeared overall flat. The RMS (root-mean-square) roughness was measured to be 28.43 nm, which was consistent with the trend of small and dense crystal formation observed in SEM analysis. In contrast, the 10% condition (
Figure 4b) exhibited highly non-uniform crystal formation, with localized depressions present on the surface, resulting in an increased RMS roughness of 35.57 nm.
The 20% condition (
Figure 4c) exhibited the lowest RMS roughness of 25.42 nm and showed a dense and uniform surface morphology, representing an ideal crystal structure. This result closely aligns with the SEM observations of optimal crystal formation. The 30% condition (
Figure 4d) also displayed similar uniformity and morphology; however, as the grain size increased slightly, the RMS roughness also increased to 42.39 nm. At 40% MACl (
Figure 4e), excessive grain growth led to noticeable height differences between adjacent grains, increasing the RMS roughness to 53.65 nm. Both grain size irregularity and surface roughness were clearly observed. Finally, the 50% condition (
Figure 4f) showed further grain growth and pronounced height variation between grains, resulting in the highest RMS roughness of 59.59 nm. The surface appeared rough and uneven, and the non-uniform grain size suggested the possibility of local defect formation.
Through AFM analysis, it was confirmed that MACl concentrations in the range of 20–30% led to the most compact and smooth surface morphologies, which closely matched the trends observed in XRD, SEM, and visual inspection. This comprehensive analysis of crystallographic structure, surface morphology, and roughness as a function of MACl concentration provided a systematic understanding of the physical property variations in perovskite films. Based on these findings, we proceeded to investigate how these differences in film properties affected the photovoltaic performance of actual solar cell devices. To this end, current-voltage (J-V) measurements and other electrical performance evaluations were conducted on devices fabricated under the same conditions.
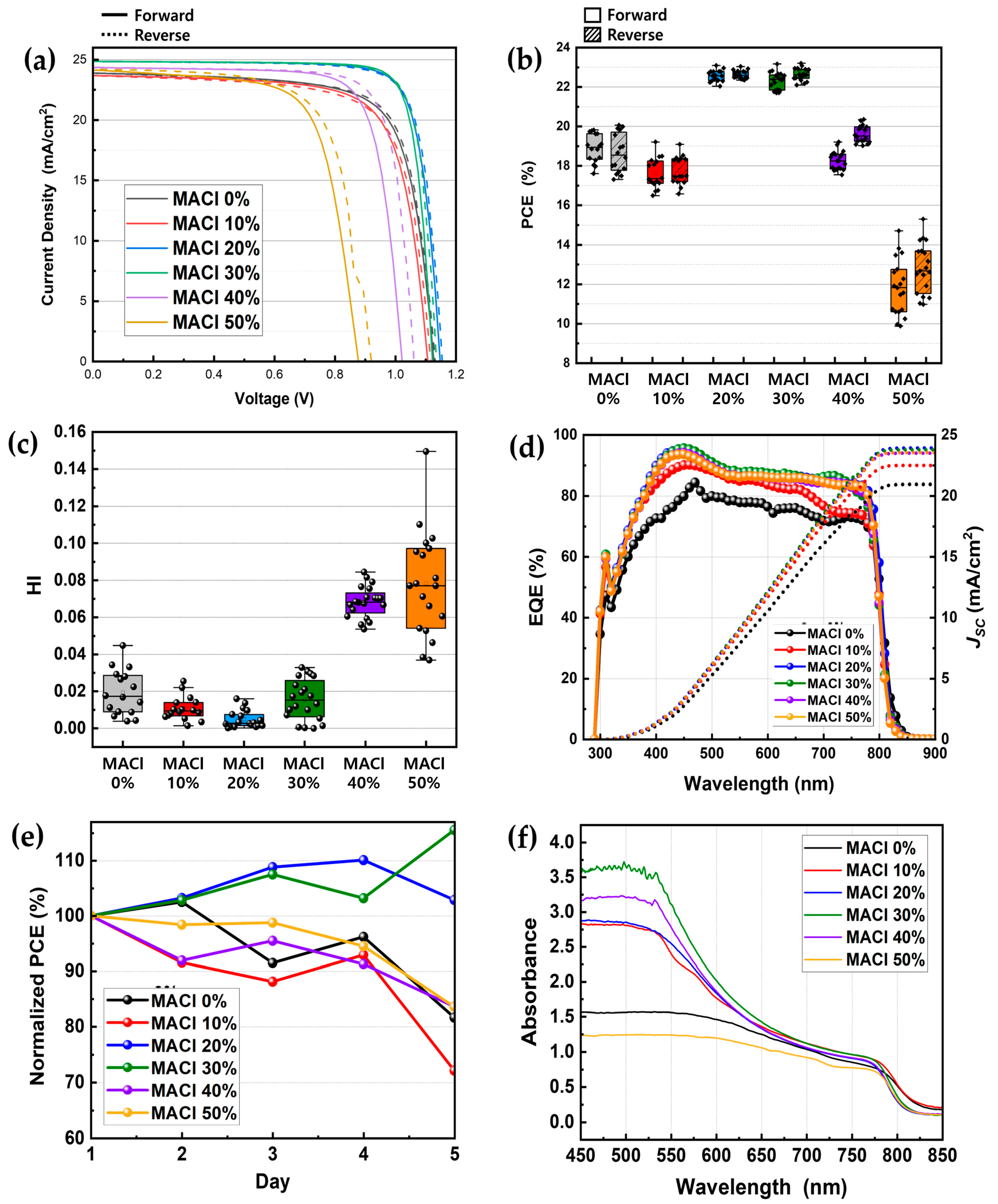
To assess the actual performance of the devices, PSCs were fabricated using an NMP-based solvent system, as described in
Section 2 (Materials and Methods), with only the molar concentration of MACl in the perovskite precursor solution varied among the samples. The resulting perovskite solar cells were characterized through a comprehensive set of analyses, including J-V measurements, PCE distribution, EQE analysis, HI evaluation, long-term stability testing, and UV-vis absorption spectroscopy, as shown in
Figure 5. In addition, more detailed distributions of parameters for each MACl concentration are presented in the form of box plots in
Figure S3.
Figure 5a presents representative J-V curves measured under different MACl concentrations. The 20% and 30% MACl conditions exhibited the highest performance with elevated
JSC,
VOC, and FF values. In particular, the 30% sample achieved the highest PCE of 23.2%. This aligned with earlier results in which these conditions yielded the most compact and uniform crystalline films. In contrast, devices fabricated with 0% and 10% MACl showed relatively low
JSC and FF values, resulting in poor performance, consistent with prior characterization. Notably, the 10% condition yielded even lower efficiency than the 0% case, suggesting that the addition of an insufficient amount of additive may be more detrimental than excluding it entirely. Devices with 40% and 50% MACl showed a performance decline with increasing concentration, as evidenced by reductions in
JSC,
VOC, and FF values, which supported the hypothesis that excessive grain growth can lead to surface non-uniformity and pinhole formation. A comprehensive comparison of PCE,
JSC,
VOC, and FF values, including their standard deviations, is provided in
Table 2. For reference, control experiments with a DMSO/DMF (1:4) solvent system (
Figure S5) showed optimal performance at 40–50% MACl, highlighting the reduced additive requirement specific to NMP-based formulations.
Figure 5b visualizes the distribution of PCE values for multiple devices in the form of a box plot. The 20% and 30% samples exhibited the highest average PCEs with the smallest deviations, indicating both superior performance and excellent reproducibility. Additionally,
Figure 5c shows a box plot comparison of HI values, revealing the lowest hysteresis in the 20% and 30% conditions, and significantly greater hysteresis in the 40% and 50% samples.
Figure 5d presents the EQE spectra of selected devices, measured over a wavelength range of 290–900 nm. The
JSC values calculated from EQE integration closely matched those obtained from the J-V measurements.
Figure 5e shows the long-term stability of the devices over 5 days. Samples were stored in a nitrogen-filled glovebox and measured periodically under a dry-air atmosphere. While the 20% and 30% MACl samples exhibited a slight increase in performance, all other samples showed a gradual decline, with a more rapid drop observed after day 5.
Figure 5f displays the UV-vis absorption of perovskite films coated on glasses. The 30%, 40%, and 20% MACl samples exhibited the highest absorption, in that order, which was consistent with the other characterization results.
These final results, which reflect the true photovoltaic performance of the devices, show excellent agreement with earlier material and morphological characterizations such as XRD, SEM, and AFM. Collectively, they allow for the reliable identification of the optimal MACl additive concentration for perovskite solar cell fabrication.