Cold Energy Storage via Hydrates Production with Pure CO2 and CO2/N2 (70/30 and 50/50 vol%) Mixtures: Quantification and Comparison between Energy Stored and Energy Spent
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus and Materials
2.2. Methodology
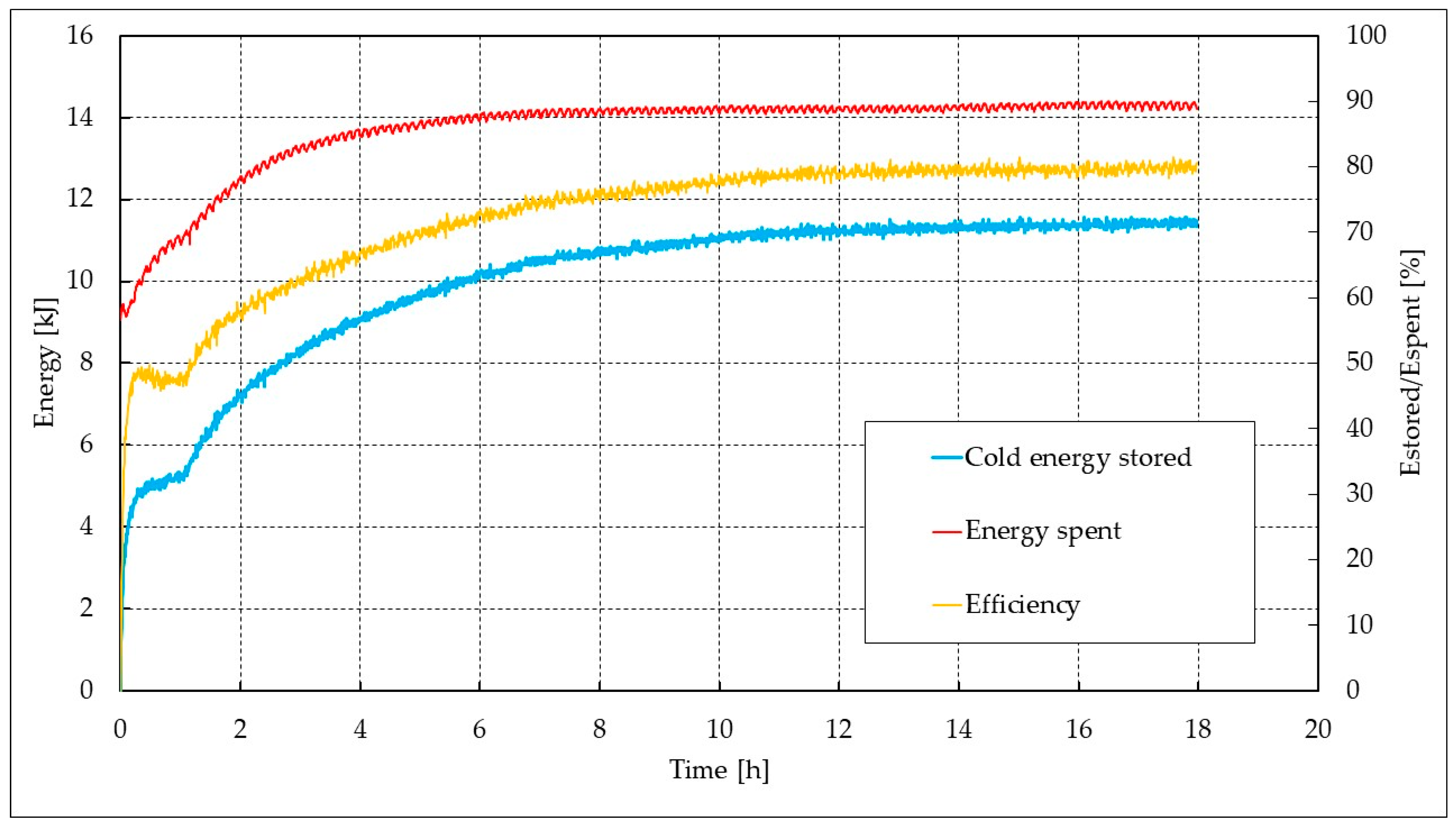
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate hydrates of natural gases, 3rd ed.CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Gambelli, A.M.; Li, Y.; Rossi, F. Influence of different proportion of CO2/N2 binary gas mixture on methane recovery through replacement processes in natural gas hydrates. Chem. Eng. Process. 2022, 175, 108932. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yin, S.; He, G.; Li, J.; Wu, Q. Research progress of the kinetics on natural gas hydrate replacement by CO2-containing mixed gas: A review. J. Nat. Gas Sci. Eng. 2022, 108, 104837. [Google Scholar] [CrossRef]
- Demirbas, A. Methane from gas hydrates in the black sea. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 32, 165–171. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Chen, J.; Yin, Z.; Rossi, F.; Tronconi, E.; Mei, S. Experimental study on the competition between carbon dioxide hydrate and ice below the freezing point. Chem. Eng. Sci. 2023, 268, 118426. [Google Scholar] [CrossRef]
- Gambelli, A.M. Variations in terms of CO2 capture and CH4 recovery during replacement processes in gas hydrate reservoirs, associated to the “memory effect”. J. Clean. Prod. 2022, 360, 132154. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, C.G.; Zhang, Y.; Ruan, X.K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrates: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef]
- Nair, V.C.; Prasad, S.K.; Kumar, R.; Sangway, J.S. Energy recovery from simulated clayey gas hydrate reservoir using depressurization by constant rate gas release, thermal stimulation and their combination. Appl. Energy 2018, 225, 755–768. [Google Scholar] [CrossRef]
- Xuan, K.; Yi, W.; Li, X.S.; Zhang, Y.; Chen, Z.Y. Influence of heat conduction and heat convection on gas hydrate dissociation by depressurization in a pilot-scale hydrate simulator. Appl. Energy 2019, 251, 113045. [Google Scholar]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y. Experimental investigation of optimization of well spacing for gas recovery from methane hydrate reservoir in sandy sediment by heat stimulation. Appl. Energy 2017, 207, 562–572. [Google Scholar] [CrossRef]
- Go, W.; Yun, S.; Lee, D.; Seo, Y. Experimental and computational investigation of hydrophilic monomeric substances as novel CO2 hydrate inhibitors and potential synergists. Energy 2022, 244, 123136. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, C.; Wang, H.; Yang, L.; Zhang, L.; Zhao, J.; Song, Y. Atomistic insight into the performance of thermodynamic inhibitors in the nucleation of methane hydrate. Chem. Eng. J. 2022, 431, 133479. [Google Scholar] [CrossRef]
- Li, Y.; Wu, N.; He, C.; Sun, Z.; Zhang, Z.; Hao, X.; Chen, Q.; Bu, Q.; Liu, C.; Sun, J. Nucleation probability and memory effect of methane-propane mixed gas hydrate. Fuel 2021, 291, 120103. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.; Rochelle, C.; Manovìc, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Cao, X.; Wang, H.; Yang, K.; Wu, S.; Chen, Q.; Bian, J. Hydrate-based CO2 sequestration technology: Feasibilities, mechanisms, influencing factors, and applications. J. Petrol. Sci. Eng. 2022, 219, 111121. [Google Scholar] [CrossRef]
- Teng, Y.H.; Zhang, D.X. Long-term viability of carbon sequestration in deep-sea sediments. Sci. Adv. 2018, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, C.A.; Camps, A.P.; Long, D.; Milodowski, A.; Bateman, K. Can CO2 hydrate assist in the underground storage of carbon dioxide? Geol. Soc. Lond. Spec. Publ. 2015, 319, 171–183. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F. Permanent carbon dioxide sequestration in deep-sea sediments. Proc. Natl. Acad. Sci. USA 2006, 103, 12291. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.Q.; Ahluwalia, R.K.; Peng, J.K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical assessment compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Energy 2011, 36, 3037–3049. [Google Scholar] [CrossRef]
- Yanxing, Z.; Maoqiong, G.; Yuan, Z.; Xueqiang, D.; Jun, S. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int. J. Hydrogen Energy 2019, 22, 16833–16840. [Google Scholar] [CrossRef]
- Sethia, G.; Sayari, A. Activated carbon with optimum pore size distribution for hydrogen storage. Carbon 2016, 99, 289–294. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational design, synthesis, purification, and activation of metal-organic framework materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, G.; Liu, G.Q.; Luo, S.J.; Guo, R.B. Effects of surfactants micelles and surfactant-coated nanospheres on methane hydrate growth pattern. Chem. Eng. Sci. 2016, 144, 108–115. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Re-definition of the region suitable for CO2/CH4 replacement into hydrates as a function of the thermodynamic difference between CO2 hydrate formation and dissociation. Process Saf. Environ. Prot. 2023, 169, 132–141. [Google Scholar] [CrossRef]
- Dyadin, Y.A.; Larionov, E.G.; Manakov, A.Y.; Zhurko, F.V.; Aladko, E.Y.; Mikina, T.V.; Komarov, V.Y. Clathrate hydrates of hydrogen and neon. Mendeleev Commun. 1999, 9, 209–210. [Google Scholar] [CrossRef]
- Smirnov, G.S.; Stegailov, V.V. Toward determination of new hydrogen hydrate clathrate structures. J. Phys. Chem. Lett. 2013, 4, 3560–3564. [Google Scholar] [CrossRef]
- Du, J.; Wang, L.; Liang, D.; Li, D. Phase equilibria and dissociation enthalpies of hydrogen semi-clathrate hydrate with tetrabutyl ammonium nitrate. J. Chem. Eng. Data 2021, 57, 603–609. [Google Scholar] [CrossRef]
- Hashimoto, S.; Sugahara, T.; Moritoki, M.; Sato, H.; Ohgaki, K. Thermodynamic stability of hydrogen + tetra-n-butyl ammonium bromide mixed gas hydrate in nonstoichiometric aqueous solutions. Chem. Eng. Sci. 2008, 63, 1092–1097. [Google Scholar] [CrossRef]
- Sinehbaghizadeh, S.; Saptoro, A.; Mohammadi, A.H. CO2 hydrate properties and applications: A state of the art. Prog. Energy Conbustion Sci. 2022, 93, 101026. [Google Scholar] [CrossRef]
- Tromp, R.H.; Neilson, G.W.; Soper, A.K. Water structure in concentrated lithium chloride solutions. J. Chem. Phys. 1992, 96, 8460–8469. [Google Scholar] [CrossRef]
- Karamoddin, M.; Varaminian, F. Water desalination using R141b gas hydrate formation. Desalination Water Treat. 2014, 52, 2450–2456. [Google Scholar] [CrossRef]
- Nam Park, K.; Hong, S.Y.; Lee, J.W.; Kang, K.C.; Lee, Y.C.; Ha, M.G.; Lee, J.D. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B3+). Desalination 2011, 274, 91–96. [Google Scholar] [CrossRef]
- Gaikward, N.; Nakka, R.; Khavala, V.; Bhadani, A.; Mamane, H.; Kumar, R. Gas hydrate-based process for desalination of heavy metal ions from an aqueous solution: Kinetics and rate of recovery. ACS EsT Water 2021, 1, 134–144. [Google Scholar] [CrossRef]
- Claben, T.; Seidl, P.; Loekman, S.; Gatternig, B.; Rauch, C.; Delgado, A. Review on the food technological potentials for gas hydrate technology. Curr. Opin. Food Sci. 2019, 29, 48–55. [Google Scholar]
- Srivastava, S.; Hitzmann, B.; Zettel, V. A future road map for carbon dioxide (CO2) gas hydrate as an emerging technology in food research. Food Bioprocess Technol. 2021, 14, 1758–1762. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Z.; Zhang, P. State-of-the-art of cold energy storage, release and transport using CO2 double hydrate slurry. Appl. Energy 2024, 358, 122531. [Google Scholar] [CrossRef]
- Kauffeld, M.; Wang, M.J.; Goldstein, V. Ice slurry applications. Int. J. Refrigerat. 2010, 33, 1491–1505. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, J.; Kim, H. Hydrates for cold energy storage and transport: A review. Adv. Appl. Energy 2021, 2, 100022. [Google Scholar] [CrossRef]
- Hassan, M.H.A.; Sher, F.; Zarren, G. Kinetic and thermodynamic evaluation of effective combined promoters for CO2 hydrate formation. J. Nat. gas Sci. Eng. 2020, 78, 103313. [Google Scholar] [CrossRef]
- Fournaison, L.; Anthony, D.; Chatti, I. CO2 hydrates in refrigeration processes. Indust. Eng. Chem. Res. 2004, 43, 6521–6526. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Rossi, F. Experimental study on the effect of SDS and micron copper particles mixture on carbon dioxide hydrates formation. Energies 2022, 15, 6540. [Google Scholar] [CrossRef]
- Song, C.; Pan, W.; Srimat, T.S.; Zheng, J.; Li, Y.; Wang, Y.H.; Xu, B.Q.; Zhu, Q.M. Trireforming of methane over Ni catalysts for CO2 conversion to syngas with desired H2/CO ratios using flue gas of power plants without CO2 separation. Stud. Surf. Sci. Catal. 2004, 153, 315–322. [Google Scholar]
- Kacem, M.; Pellerano, M.; Delebarre, A. Pressure swing adsorption for CO2/N2 and CO2/CH4 separation: Comparison between activated carbons and zeolites performances. Fuel Process. Technol. 2015, 138, 271–283. [Google Scholar] [CrossRef]
- Zueco, J.; Lopez-Asensio, D.; Férnandez, F.J.; Lopez-Gonzalez, L.M. Exergy analysis of a steam-turbine power plant using thermocombustion. Appl. Therm. Eng. 2020, 180, 115812. [Google Scholar] [CrossRef]
- International Panel on Climate Control (IPCC), Carbon Dioxide Capture and Storage, Special Report. Cambridge University Press: New York, NY, USA, 2005.
- Belandria, V.; Mohammadi, A.H.; Eslamimanesh, A.; Richon, D.; Sanchez-Mora, M.F.; Galicia-Luna, L.A. Phase equilibrium measurements for semi-clathrate hydrates of the (CO2+N2+tetra-n-butylammonium bromide) aqueous solution systems: Part 2. Fluid Phase Equilibr. 2012, 322-323, 101–112. [Google Scholar] [CrossRef]
- Rossi, F.; Li, Y.; Gambelli, A.M. Thermodynamic and kinetic description of the main effects related to the memory effect during carbon dioxide hydrates formation in a confined environment. Sustainability 2021, 13, 13797. [Google Scholar] [CrossRef]
- Giovannetti, R.; Gambelli, A.M.; Castellani, B.; Rossi, A.; Minicucci, M.; Zannotti, M.; Li, Y.; Rossi, F. May sediments affect the inhibiting properties of NaCl on CH4 and CO2 hydrates formation? An experimental report. J. Mol. Liq. 2022, 359, 119300. [Google Scholar] [CrossRef]
- Tupsakhare, S.S.; Castaldi, M.J. Efficiency enhancements in methane recovery from natural gas hydrates using injection of CO2/N2 gas mixture simulating in-situ combustion. Appl. Energy 2019, 236, 825–836. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Presciutti, A.; Rossi, F. Kinetic considerations and formation rate for carbon dioxide hydrate, formed in presence of a natural silica-based porous medium: How initial thermodynamic conditions may modify the process kinetics. Thermochim. Acta 2021, 705, 179039. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Lee, J.; Seo, Y. Structure identification and dissociation enthalpy measurements of the CO2+N2 hydrates for their application to CO2 capture and storage. Chem. Eng. J. 2014, 246, 20–26. [Google Scholar] [CrossRef]
- Herri, J.M.; Bouchemoua, A.; Kwatersky, M.; Fezoua, A.; Ouabbas, Y.; Cameirao, A. Gas hydrate equilibria for CO2-N2 and CO2-CH4 gas mixtures-Experimental studies and thermodynamic modelling. Fluid Phase Equilibr. 2011, 301, 171–190. [Google Scholar] [CrossRef]
- Jarrahian, A.; Nakhaee, A. Hydrate-liquid-vapor equilibrium condition for N2+CO2+H2O system: Measurement and modelling. Fuel 2019, 237, 769–774. [Google Scholar] [CrossRef]
- Yasuda, K.; Oto, Y.; Shen, R.; Uchida, T.; Ohmura, R. Phase equilibrium condition measurement in nitrogen and air clathrate hydrate forming systems at temperatures below freezing point of water. J. Chem. Thermodyn. 2013, 67, 143–147. [Google Scholar] [CrossRef]
- Roozeboom, H.W.B. Sur l’hydrate de l’acide sulfureux. Recl. Trav. Chim. Pays-Bas 1884, 3, 29–58. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Experimental characterization of the difference in induction period between CH4 and CO2 hydrates: Motivations and possible consequences on the replacement process. J. Nat. Gas Sci. Eng. 2022, 108, 104848. [Google Scholar] [CrossRef]
- Wilson, P.W.; Haymet, A.D.J. Hydrate formation and re-formation in nucleating THF/water mixtures show no evidence to support a “memory effect”. Chem. Eng. J. 2010, 161, 146–150. [Google Scholar] [CrossRef]

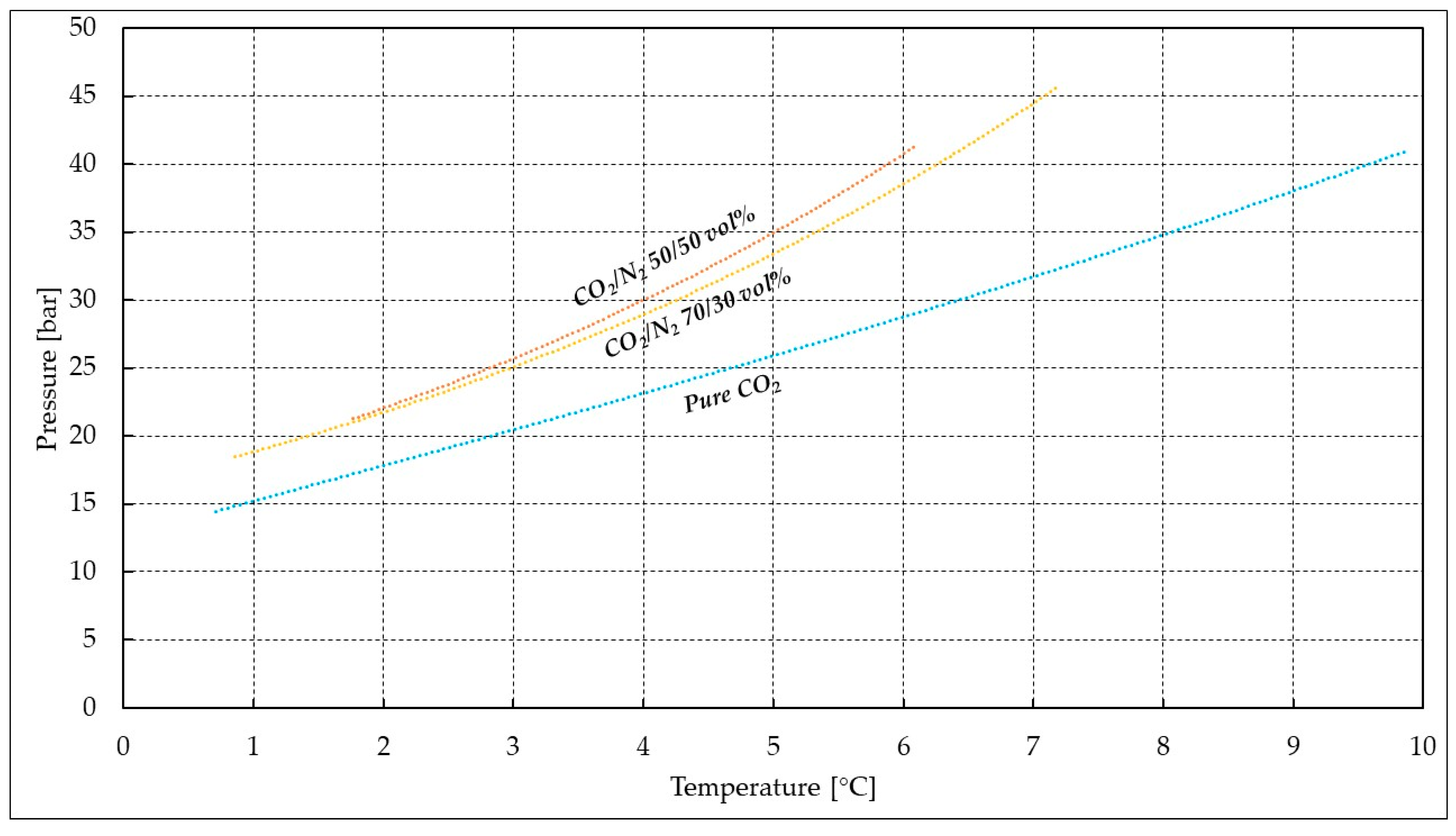
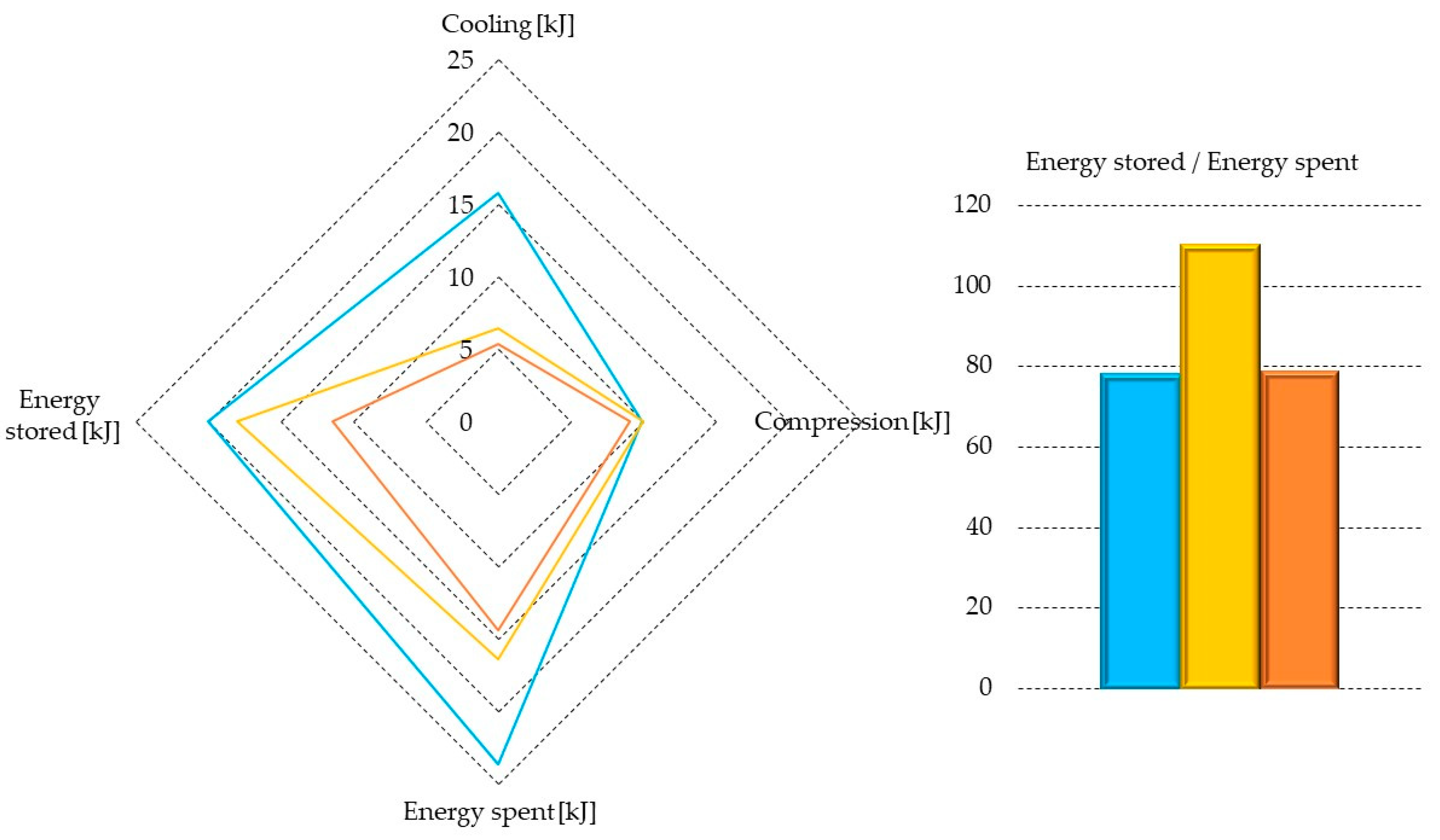
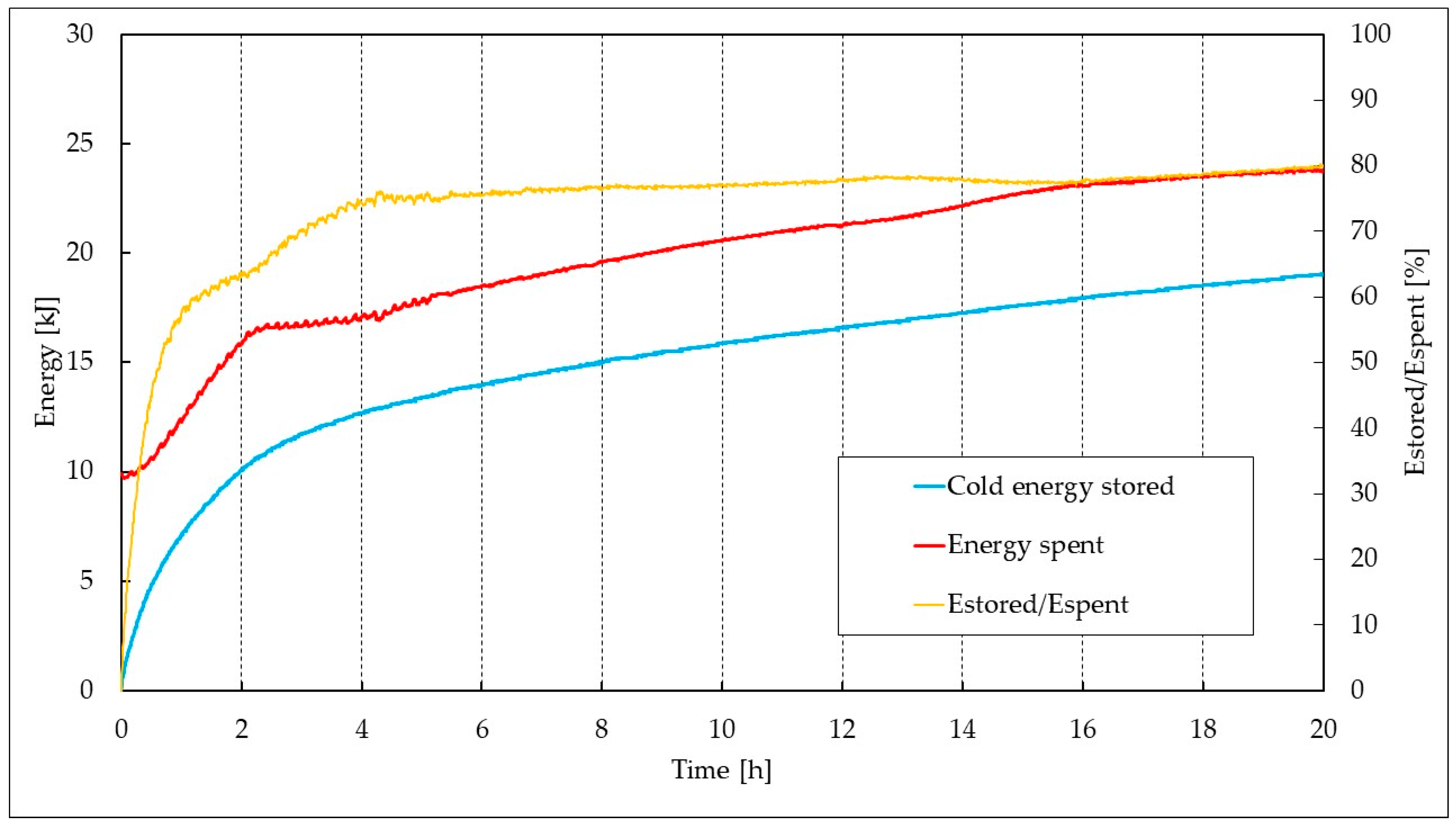
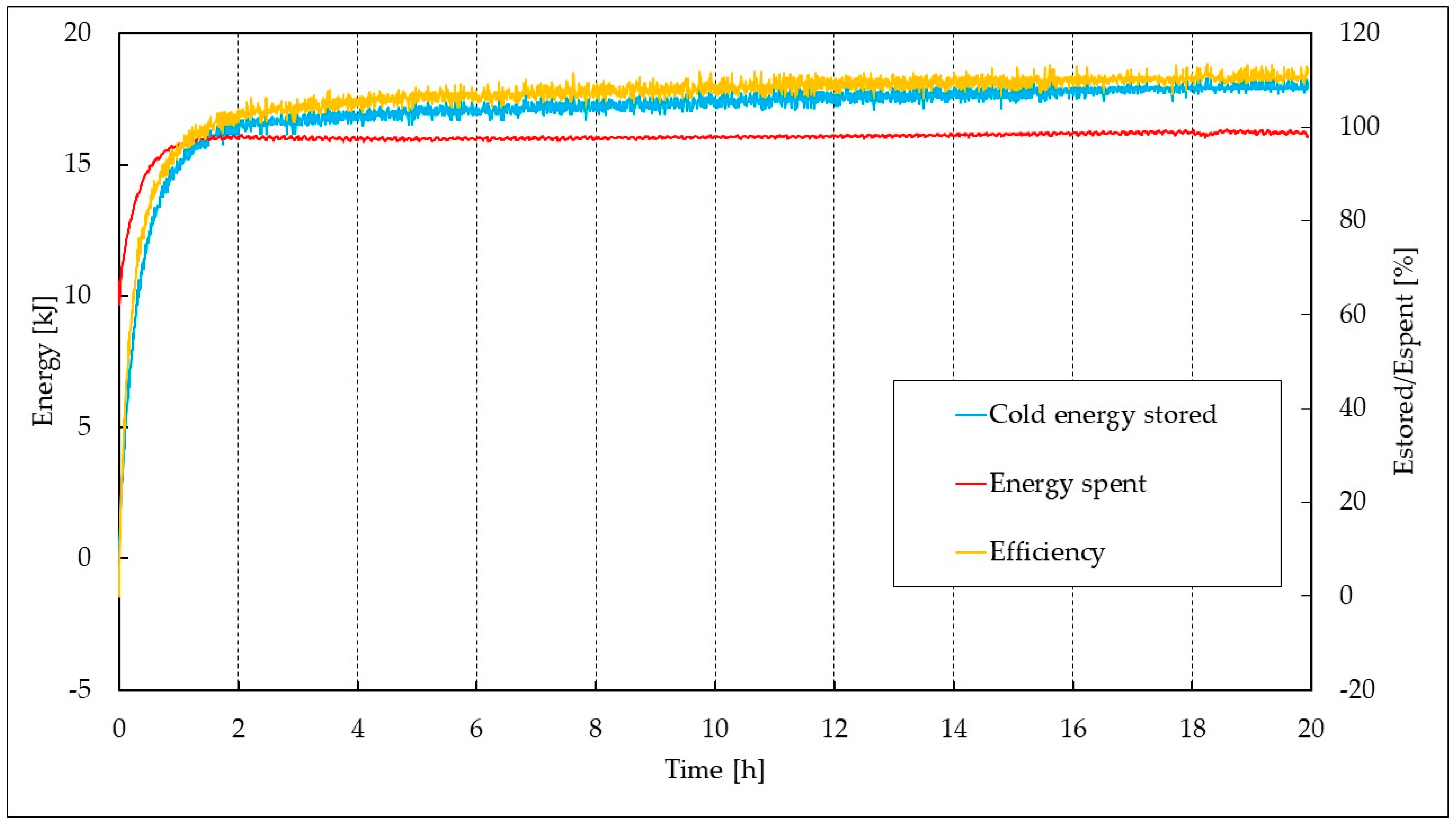
| Parameter | Pure CO2 | CO2/N2 (70/30 vol%) | CO2/N2 (50/50 vol%) |
|---|---|---|---|
| Pi [bar] | 44.23 | 45.20 | 41.02 |
| Ti [°C] | 9.76 | 5.03 | 5.45 |
| Pf [bar] | 14.91 | 19.74 | 22.84 |
| Tf [°C] | 0.79 | 1.35 | 2.37 |
| molINJ [mol] | 0.622 | 0.527 | 0.479 |
| molHYD [mol] | 0.345 | 0.349 | 0.215 |
| Cooling [kJ] | Compression [kJ] | Energy Spent [kJ] | Energy Stored [kJ] | Estored/Espent [%] | |
|---|---|---|---|---|---|
| Pure CO2 | 15.72 | 9.91 | 23.63 | 20.00 | 78.06 |
| CO2/N2 (70/30 vol%) | 6.39 | 10.01 | 16.41 | 18.04 | 109.94 |
| CO2/N2 (50/50 vol%) | 5.38 | 9.06 | 14.44 | 11.40 | 78.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambelli, A.M.; Rossi, F.; Gigliotti, G. Cold Energy Storage via Hydrates Production with Pure CO2 and CO2/N2 (70/30 and 50/50 vol%) Mixtures: Quantification and Comparison between Energy Stored and Energy Spent. Energies 2024, 17, 2211. https://doi.org/10.3390/en17092211
Gambelli AM, Rossi F, Gigliotti G. Cold Energy Storage via Hydrates Production with Pure CO2 and CO2/N2 (70/30 and 50/50 vol%) Mixtures: Quantification and Comparison between Energy Stored and Energy Spent. Energies. 2024; 17(9):2211. https://doi.org/10.3390/en17092211
Chicago/Turabian StyleGambelli, Alberto Maria, Federico Rossi, and Giovanni Gigliotti. 2024. "Cold Energy Storage via Hydrates Production with Pure CO2 and CO2/N2 (70/30 and 50/50 vol%) Mixtures: Quantification and Comparison between Energy Stored and Energy Spent" Energies 17, no. 9: 2211. https://doi.org/10.3390/en17092211
APA StyleGambelli, A. M., Rossi, F., & Gigliotti, G. (2024). Cold Energy Storage via Hydrates Production with Pure CO2 and CO2/N2 (70/30 and 50/50 vol%) Mixtures: Quantification and Comparison between Energy Stored and Energy Spent. Energies, 17(9), 2211. https://doi.org/10.3390/en17092211






