Surfactant-Enhanced Assisted Spontaneous Imbibition for Enhancing Oil Recovery in Tight Oil Reservoirs: Experimental Investigation of Surfactant Types, Concentrations, and Temperature Impact
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Equipment
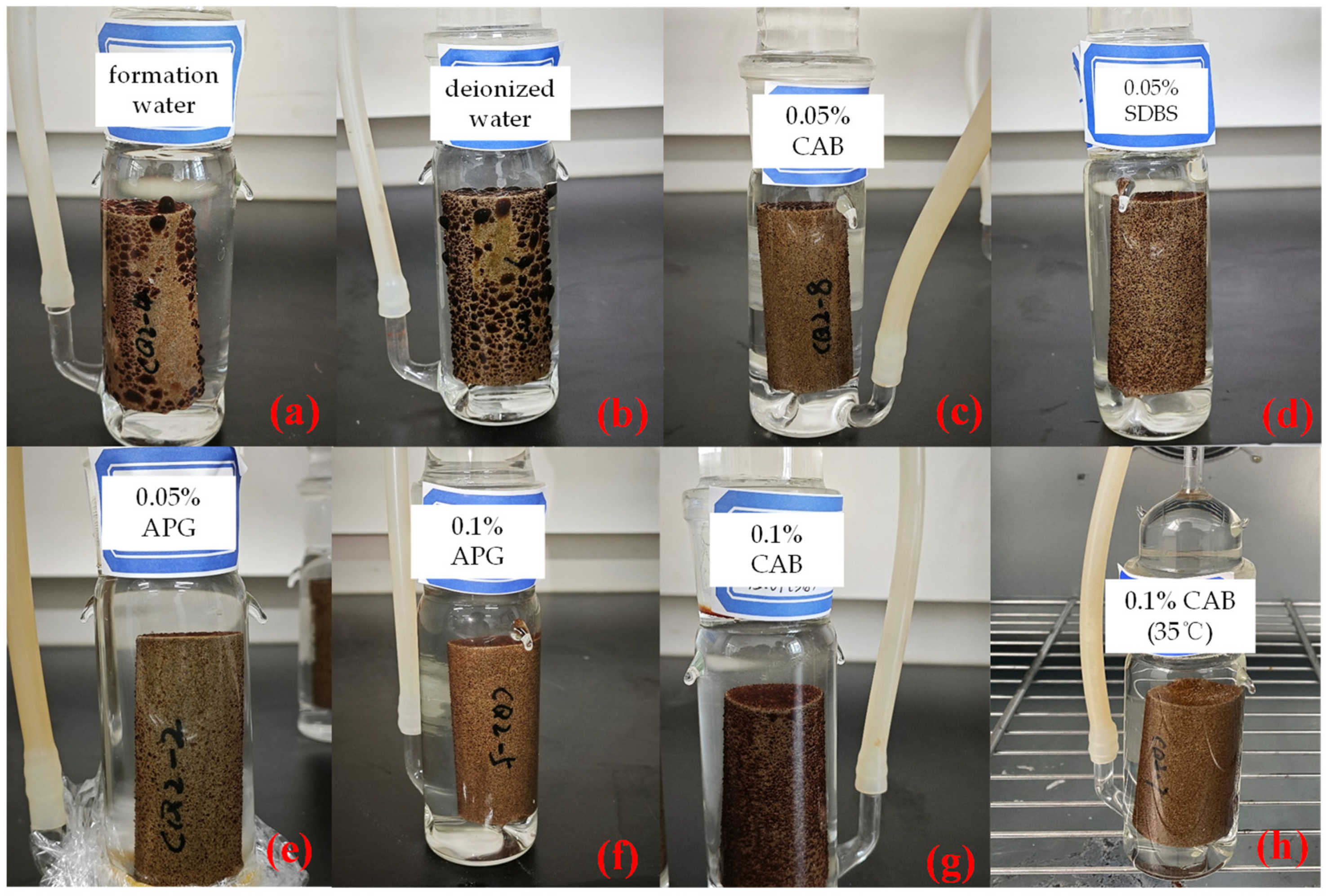
2.3. Experimental Design and Procedure
- The dry and weighed core were placed into the core chamber. The simulated oil was injected into the storage tank, and the storage tank and core chamber were sealed.
- The vacuum pump was turned on in order for 48 h to evacuate the sandstone core and simulated oil, until the pressure within the core stabilized.
- After the core and simulated oil had been evacuated, the vacuum pump was turned off.
- The liquid storage tank valve and the liquid inlet valve were opened to allow the simulated oil in a vacuum state to enter the core chamber. The hand pump was turned clockwise to increase the pressure in the core chamber to 25 MPa and the core was pressurized and saturated for 72 h, until the pressure within the core stabilized.
- The hand pump was turned counterclockwise to relieve the pressure in the core chamber. The core chamber was opened and the core was removed. An electronic balance was used to measure the wet weight of the core.
- The volume of core saturated with simulated oil was calculated.
- Before the experiment, the imbibition cell was rinsed with the prepared imbibition solution, and then the weighed core was put into the imbibition cell.
- The imbibition liquid from the rubber tube was injected into the imbibition cell until the imbibition liquid rose to 1/3~2/3 of the scale tube of the imbibition cell. The openings of the scale tube and rubber tube were sealed with plastic wrap.
- After ensuring that there was no leakage at the interface of the imbibition cell, the imbibition experiment was initiated.
- The initial time of core imbibition was recorded. Then, the amount of oil imbibed at intervals was recorded according to experimental conditions, and photos were taken to observe the adsorption state of crude oil.
3. Results and Discussion
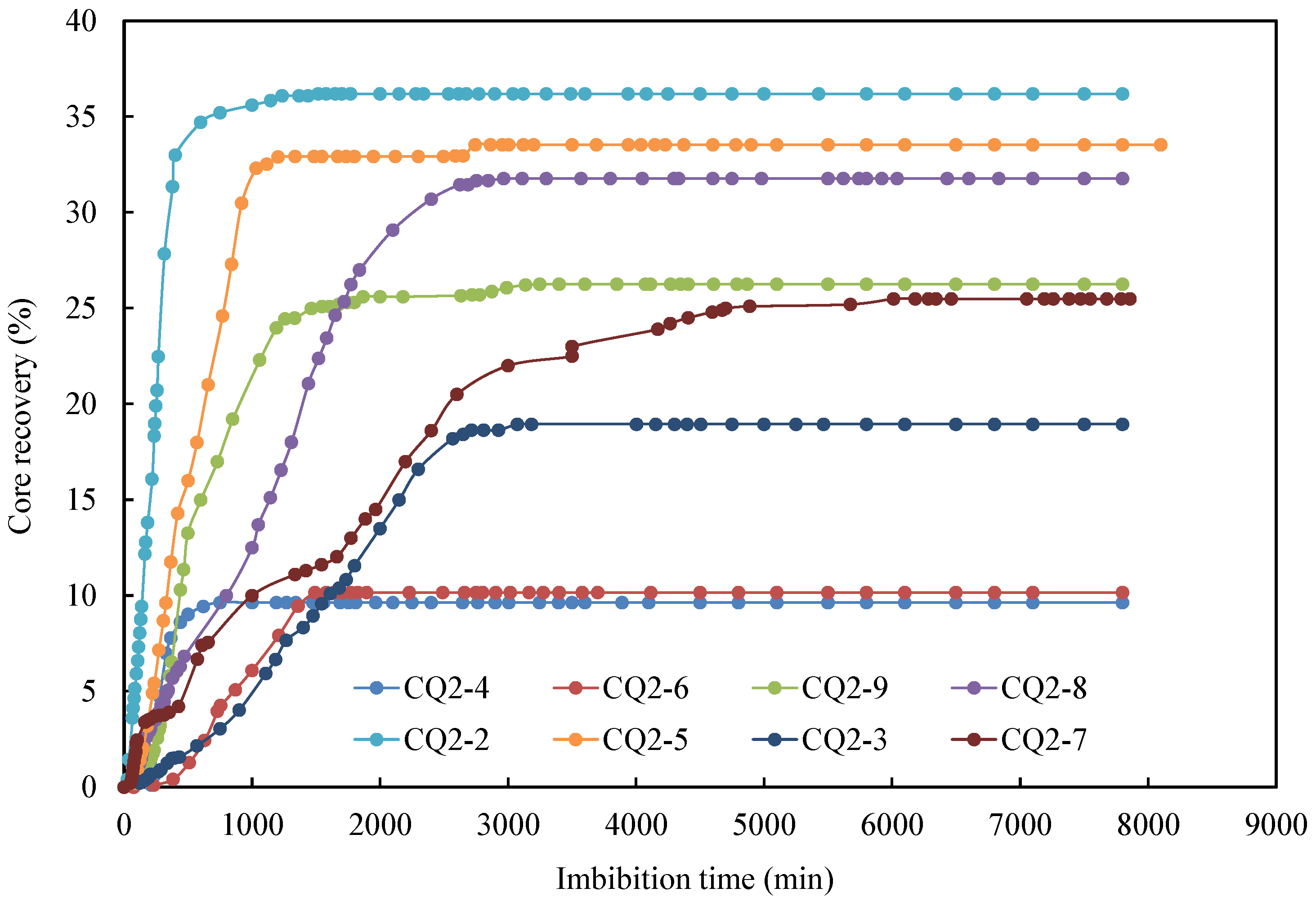
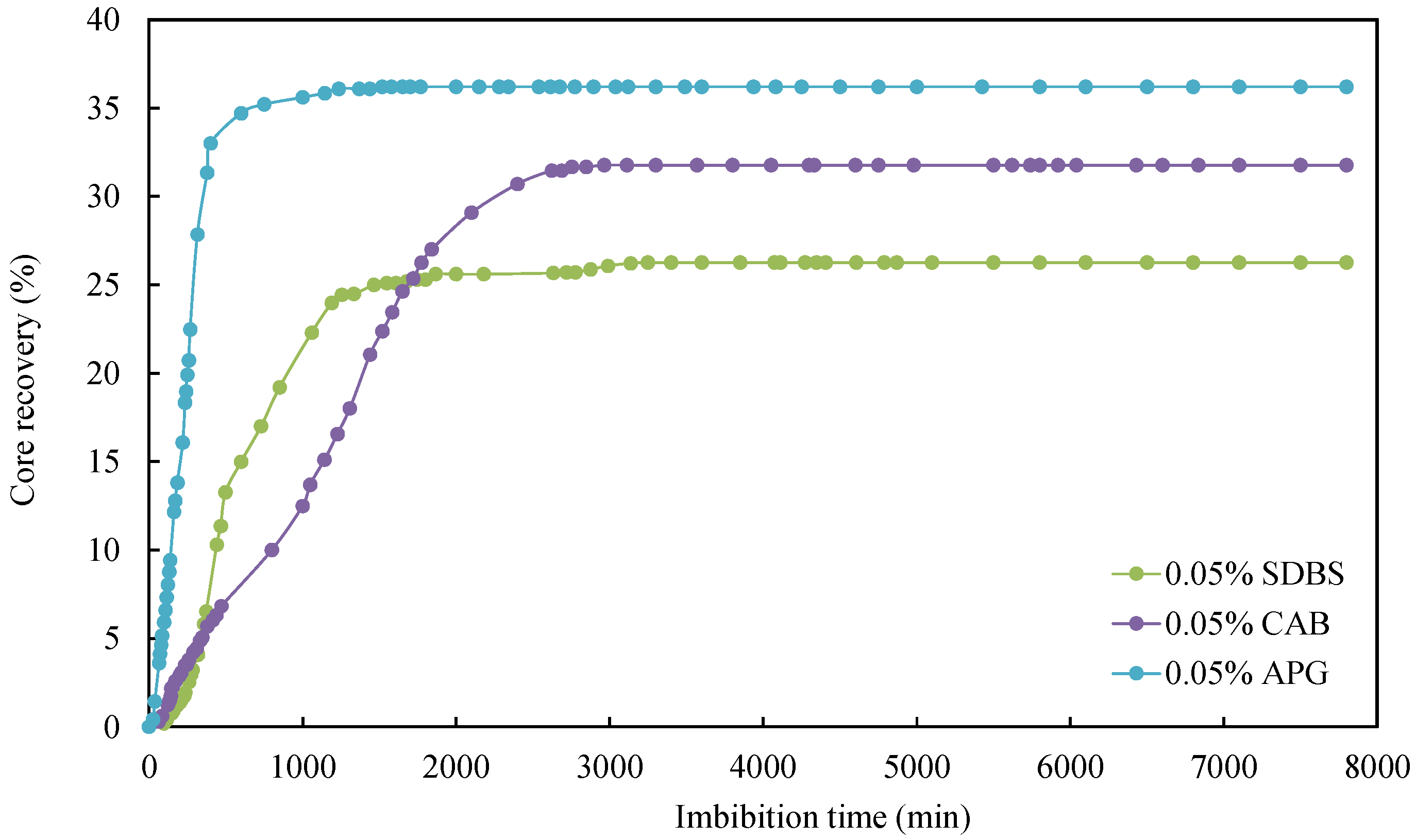
3.1. Effect of Surfactant Type
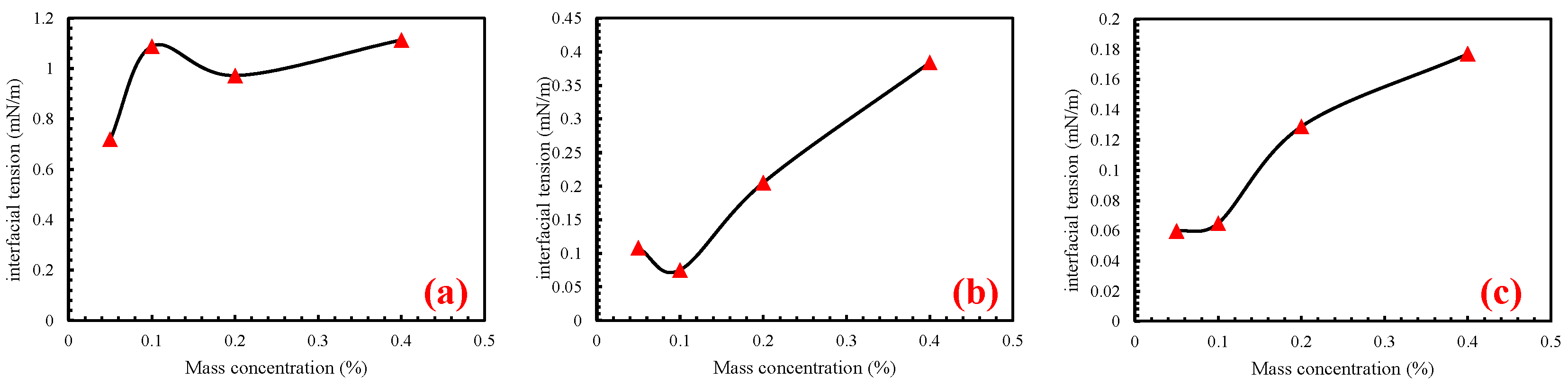
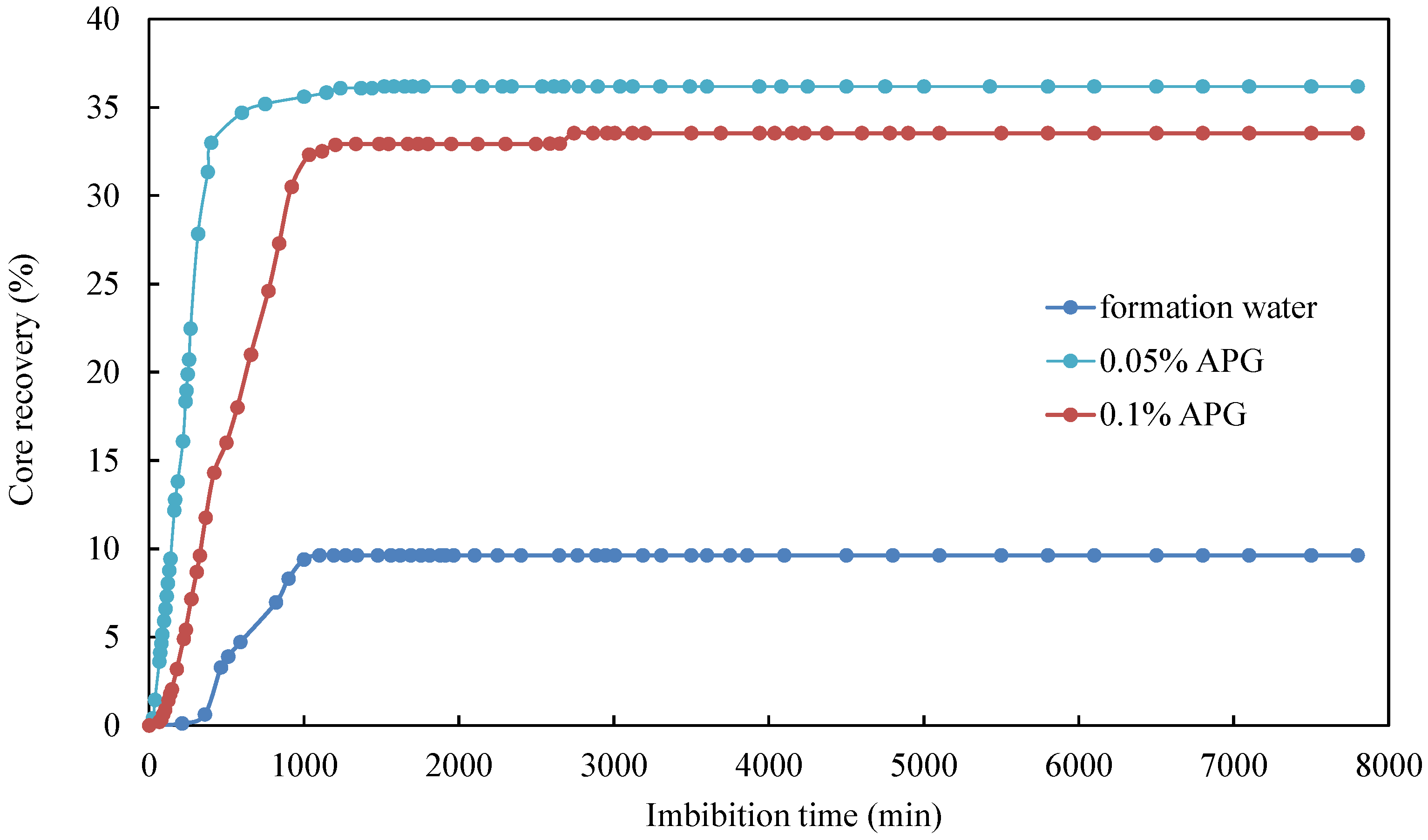
3.2. Effect of Surfactant Concentration
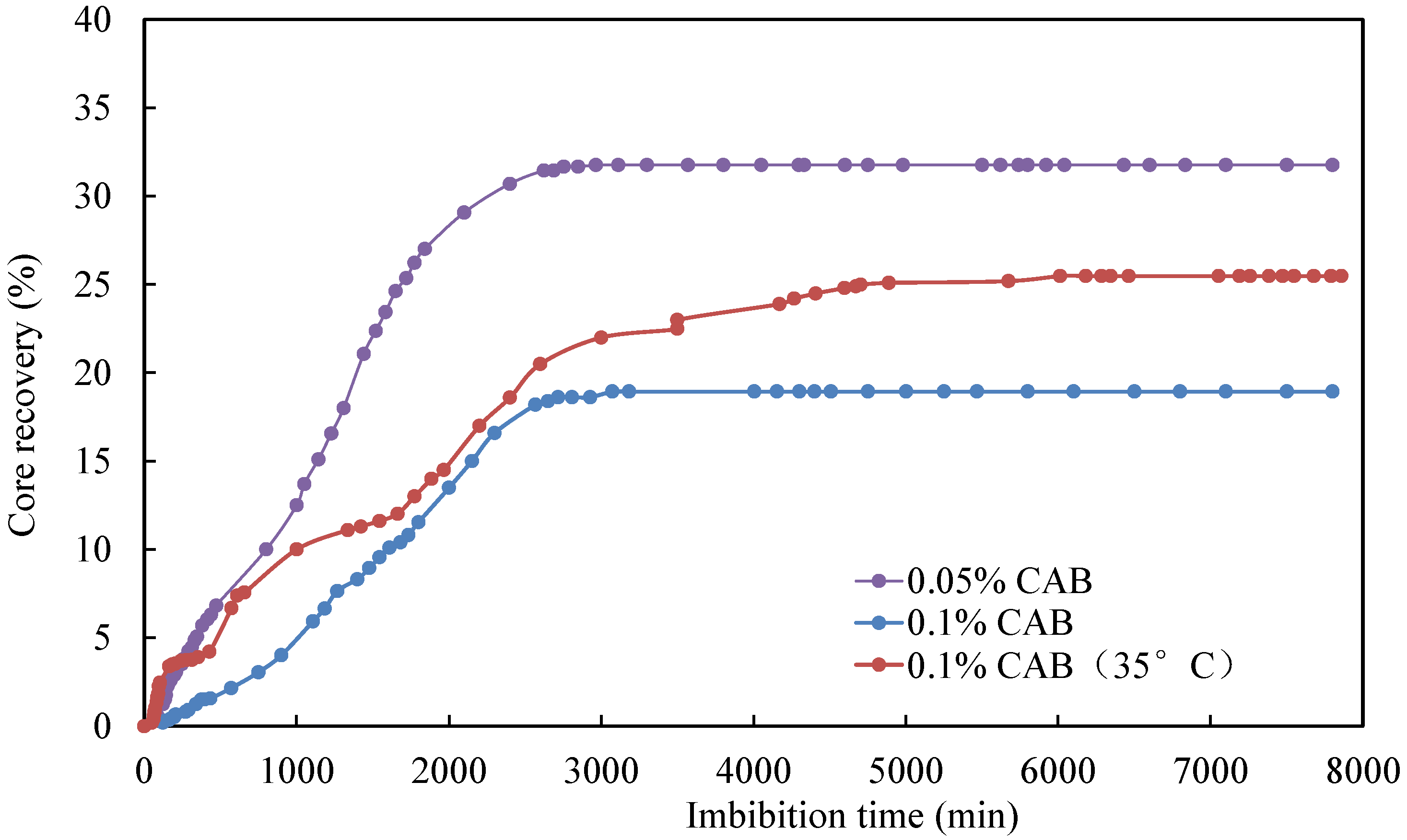
3.3. Effect of Temperature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Yao, Y.; Zhang, C.; Liu, Y. A novel method for evaluation of the spontaneous imbibition process in tight reservoir rocks: Mathematical model and experimental verification. Geoenergy Sci. Eng. 2023, 223, 211554. [Google Scholar] [CrossRef]
- Wang, F.; Fu, J. A capillary bundle model for the forced imbibition in the shale matrix with dual-wettability. Can. J. Chem. Eng. 2023, 101, 2330–2340. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, F.; Wang, L.; Yue, H.; Chang, S. Experimental Study of Surfactant-Aided Dynamic Spontaneous Imbibition in Tight Oil Reservoirs: The Effect of Fluid Flow, Displacement Pressure, Temperature, and Fracture. Energy Fuels 2023, 37, 18632–18641. [Google Scholar] [CrossRef]
- Morrow, N.R.; Mason, G. Recovery of oil by spontaneous imbibition. Curr. Opin. Colloid. 2001, 6, 321–337. [Google Scholar] [CrossRef]
- Chen, Y.; Zhi, D.; Qin, J.; Song, P.; Zhao, H.; Wang, F. Experimental study of spontaneous imbibition and CO2 huff and puff in shale oil reservoirs with NMR. J. Pet. Sci. Eng. 2022, 209, 109883. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J. A mathematical model for co-current spontaneous water imbibition into oil-saturated tight sandstone: Upscaling from pore-scale to core-scale with fractal approach. J. Pet. Sci. Eng. 2019, 178, 376–388. [Google Scholar] [CrossRef]
- Wu, R.; Yang, S.; Xie, J.; Wang, M.; Yan, J. Experiment and mechanism of spontaneous imbibition of matrix core in tight oil-gas reservoirs. Pet. Geol. Recovery Effic. 2017, 24, 98–104. [Google Scholar]
- Cheng, H.; Wang, F. Mathematical model of the spontaneous imbibition of water into oil-saturated fractured porous media with gravity. Chem. Eng. Sci. 2021, 231, 116317. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J. Mathematical model of liquid spontaneous imbibition into gas-saturated porous media with dynamic contact angle and gravity. Chem. Eng. Sci. 2021, 229, 116139. [Google Scholar] [CrossRef]
- Wang, F.; Yue, H. A comprehensive mathematical model for spontaneous imbibition in oil-saturated fractured tight sandstones: Incorporating fracture distribution, displacement pressure, gravity, and buoyancy effects. Phys. Fluids 2023, 35, 062122. [Google Scholar]
- Dou, L.; Xiao, Y.; Gao, H.; Wang, R.; Liu, C.; Sun, H. The study of enhanced displacement efficiency in tight sandstone from the combination of spontaneous and dynamic imbibition. J. Pet. Sci. Eng. 2021, 199, 108327. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; Zou, C.; You, Q.; Zhao, M.; Zhao, G.; Sun, Y. Impact of flow rate on dynamic imbibition in fractured tight sandstone cores. Pet. Sci. 2022, 19, 2895–2904. [Google Scholar] [CrossRef]
- Alvarez, J.O.; Schechter, D.S. Improving oil recovery in the Wolfcamp unconventional liquid reservoir using surfactants in completion fluids. J. Pet. Sci. Eng. 2017, 157, 806–815. [Google Scholar] [CrossRef]
- Chen, W.; Schechter, D.S. Surfactant selection for enhanced oil recovery based on surfactant molecular structure in unconventional liquid reservoirs. J. Pet. Sci. Eng. 2021, 196, 107702. [Google Scholar] [CrossRef]
- Sheng, J.J. What type of surfactants should be used to enhance spontaneous imbibition in shale and tight reservoirs? J. Pet. Sci. Eng. 2017, 159, 635–643. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, J.J.; Wang, X.; Ge, H.; Yao, E. Experimental study of wettability alteration and spontaneous imbibition in Chinese shale oil reservoirs using anionic and nonionic surfactants. J. Pet. Sci. Eng. 2019, 175, 624–633. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Houston, TX, USA, 2010. [Google Scholar]
- Rudin, J.; Bernard, C.; Wasan, D.T. Effect of added surfactant on interfacial tension and spontaneous emulsification in alkali/acidic oil systems. Ind. Eng. Chem. Res. 1994, 33, 1150–1158. [Google Scholar] [CrossRef]
- Rosen, M.J.; Wang, H.; Shen, P.; Zhu, Y. Ultralow interfacial tension for enhanced oil recovery at very low surfactant concentrations. Langmuir 2005, 21, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, G.; Ge, J.; Jiang, P.; Tang, J.; Liu, Y. Research of the heavy oil displacement mechanism by using alkaline/surfactant flooding system. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 63–71. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, G.; Ge, J.; Liao, K.; Pei, H.; Jiang, P.; Li, X. Study on organic alkali-surfactant-polymer flooding for enhanced ordinary heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 230–239. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Liu, X.; Wang, L.; Cui, Z. 1, 3-Dialkyl glyceryl ethers derivatives as surfactants for enhanced oil recovery in high salinity and high temperature reservoir conditions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124425. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.; Fang, Y.; Liu, H.; Xia, Y. Comparative study of conventional/ethoxylated/extended n-alkylsulfate surfactants. Langmuir 2019, 35, 3116–3125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, Y.; Qiao, W.; Li, Z. Interfacial tensions upon the addition of alcohols to phenylalkane sulfonate monoisomer systems. Fuel 2004, 83, 2059–2063. [Google Scholar] [CrossRef]
- Jarrahian, K.; Seiedi, O.; Sheykhan, M.; Sefti, M.V.; Ayatollahi, S. Wettability alteration of carbonate rocks by surfactants: A mechanistic study. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 1–10. [Google Scholar] [CrossRef]
- Li, S.; Yang, K.; Li, Z.; Zhang, K.; Jia, N. Properties of CO2 foam stabilized by hydrophilic nanoparticles and nonionic surfactants. Energy Fuels 2019, 33, 5043–5054. [Google Scholar] [CrossRef]
- Yang, K.; Li, S.; Zhang, K.; Wang, Y. Synergy of hydrophilic nanoparticle and nonionic surfactant on stabilization of carbon dioxide-in-brine foams at elevated temperatures and extreme salinities. Fuel 2021, 288, 119624. [Google Scholar] [CrossRef]
- Iglauer, S.; Wu, Y.; Shuler, P.; Tang, Y.; Goddard III, W.A. Alkyl polyglycoside surfactant–alcohol cosolvent formulations for improved oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 48–59. [Google Scholar] [CrossRef]
- Yan, L.; Ma, J.; Cui, Z.; Jiang, J.; Song, B.; Pei, X. A new series of double-chain single-head sulfobetaine surfactants derived from 1, 3-dialkyl glyceryl ether for reducing crude oil/water interfacial tension. J. Surfactants Deterg. 2019, 22, 47–60. [Google Scholar] [CrossRef]
- Ma, J.; Yan, L.; Cui, Z.; Jiang, J.; Pei, X.; Song, B. Synthesis of a new sulfobetaine surfactant with double long alkyl chains and its performances in surfactant-polymer flooding. J. Dispers. Sci. Technol. 2018, 39, 1185–1191. [Google Scholar] [CrossRef]
- Cui, Z.; Du, X.; Pei, X.; Jiang, J.; Wang, F. Synthesis of didodecylmethylcarboxyl betaine and its application in surfactant–polymer flooding. J. Surfactants Deterg. 2012, 15, 685–694. [Google Scholar] [CrossRef]
- Hu, X.; Qi, D.; Yan, L.; Cui, Z.; Song, B.; Pei, X.; Jiang, J. Inhibiting hydrophobization of sandstones via adsorption of alkyl carboxyl betaines in SP flooding by using gentle alkali. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 75–82. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Cui, Z.; Song, B.; Jiang, J.; Wang, Z. A new type of sulfobetaine surfactant with double alkyl polyoxyethylene ether chains for enhanced oil recovery. J. Surfactants Deterg. 2016, 19, 967–977. [Google Scholar] [CrossRef]
- Yahya, Z.N.M.; Puspaseruni, N.P.; Kurnia, R.; Wahyuningrum, D.; Mulyani, I.; Wijayanto, T.; Kurihara, M.; Waskito, S.S.; Aslam, B.M.; Marhaendrajana, T. The effect of aluminosilicate in anionic–nonionic surfactant mixture on wetness and interfacial tension in its application for enhanced oil recovery. Energy Rep. 2022, 8, 1013–1025. [Google Scholar] [CrossRef]
- Kesarwani, H.; Saxena, A.; Mandal, A.; Sharma, S. Anionic/nonionic surfactant mixture for enhanced oil recovery through the investigation of adsorption, interfacial, rheological, and rock wetting characteristics. Energy Fuels 2021, 35, 3065–3078. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, C.; Ma, Q.; Li, H.; Song, Z. Effect of Hydrophobic Carbon Chain Length of Alkyl Betaine Surfactant on Spontaneous Imbibition of Low-permeability Core. Spec. Oil Gas. Reserv. 2021, 28, 102–107. [Google Scholar]
- Chen, S.; Han, M.; AlSofi, A.; Fuseni, A. Non-Ionic Surfactant Formulation with Ultra-Low Interfacial Tension at High-Temperature and High-Salinity Conditions; SPE Conference at Oman Petroleum & Energy Show: Muscat, Oman, 2022; p. D022S057R001. [Google Scholar]
- Chan, K.S.; Shah, D.O. The molecular mechanism for achieving ultra low interfacial tension minimum in a petroleum sulfonate/oil/brine system. J. Dispers. Sci. Technol. 1980, 1, 55–95. [Google Scholar] [CrossRef]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. 2 Surfactants and their applications. Annu. Rep. Sect. “C” 2003, 99, 3–48. [Google Scholar] [CrossRef]



| Core No. | Dry Weight (g) | Permeability (mD) | Porosity (%) | Particle Density (g/cm3) | Density (g/cm3) |
|---|---|---|---|---|---|
| CQ2–2 | 81.59 | 0.3425 | 15.76 | 2.74 | 2.31 |
| CQ2–3 | 80.87 | 0.3211 | 15.86 | 2.75 | 2.31 |
| CQ2–4 | 80.28 | 0.3343 | 15.86 | 2.74 | 2.31 |
| CQ2–5 | 81.09 | 0.3174 | 16.03 | 2.74 | 2.3 |
| CQ2–6 | 81.24 | 0.3451 | 15.67 | 2.73 | 2.3 |
| CQ2–7 | 80.85 | 0.3245 | 15.92 | 2.74 | 2.3 |
| CQ2–8 | 80.62 | 0.3172 | 15.94 | 2.73 | 2.3 |
| CQ2–9 | 80.87 | 0.3226 | 15.9 | 2.74 | 2.3 |
| Hydronium | K+ + Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | Total Mineralization |
|---|---|---|---|---|---|---|---|
| (mg/L) | 1853.3 | 133.4 | 37.4 | 1936.7 | 560.3 | 1455.7 | 5976.8 |
| Reagent Name | Chemical Formula | Source | Purity | Type |
|---|---|---|---|---|
| SDBS | C18H29SO3Na | MACKLIN, Shanghai, China | >98% | Anionic surfactant |
| CAB | C19H38N2O3 | Linyilvsen, Linyi, China | >98% | Zwitterionic surfactant |
| APG | C18H36O6 | Shanghai Fine Chemical Co., Ltd., Shanghai, China | >98% | Nonionic surfactant |
| Deionized water | H2O | 100% |
| IFT (mN/m) | ||||
|---|---|---|---|---|
| Concentration (%) | 0.4 | 0.2 | 0.1 | 0.05 |
| SDBS | 1.113 | 0.972 | 1.088 | 0.719 |
| CAB | 0.384 | 0.205 | 0.075 | 0.108 |
| APG | 0.177 | 0.129 | 0.065 | 0.060 |
| Imbibed Fluid Type | IFT (mN/m) |
|---|---|
| Formation water | 11.632 |
| Deionized water | 14.759 |
| Formation water + 0.05% SDBS | 0.682 |
| Formation water + 0.05% CAB | 0.108 |
| Formation water + 0.1% CAB | 0.075 |
| Formation water + 0.05% APG | 0.065 |
| Formation water + 0.1% APG | 0.060 |
| Core No | Imbibed Fluid Type |
|---|---|
| CQ2–2 | Formation water + 0.05% APG |
| CQ2–8 | Formation water + 0.05% CAB |
| CQ2–9 | Formation water + 0.05% SDBS |
| CQ2–3 | Formation water + 0.1% CAB |
| CQ2–5 | Formation water + 0.1% APG |
| CQ2–7 | Formation water + 0.1% CAB (35 °C) |
| CQ2–4 | Formation water |
| CQ2–6 | Deionized water |
| Core No | Dry Weight (g) | Wet Weight (g) | Saturated Oil Volume (mL) |
|---|---|---|---|
| CQ2–2 | 81.590 | 85.569 | 4.849 |
| CQ2–8 | 80.620 | 84.585 | 4.832 |
| CQ2–9 | 80.870 | 84.892 | 4.902 |
| CQ2–3 | 80.870 | 84.812 | 4.804 |
| CQ2–5 | 81.090 | 85.102 | 4.890 |
| CQ2–7 | 80.850 | 84.842 | 4.865 |
| CQ2–4 | 80.280 | 84.283 | 4.879 |
| CQ2–6 | 81.240 | 85.279 | 4.923 |
| CoreNo. | Absorption Fluid Type | Saturated Oil Volume (mL) | Produced Oil Volume (mL) | Oil Recovery Factor (%) |
|---|---|---|---|---|
| CQ2–2 | formation water + 0.05% APG | 4.849 | 1.755 | 36.19 |
| CQ2–8 | formation water + 0.05% CAB | 4.832 | 1.535 | 31.76 |
| CQ2–9 | formation water + 0.05% SDBS | 4.902 | 1.287 | 26.26 |
| CQ2–3 | formation water + 0.1% CAB | 4.804 | 0.910 | 18.94 |
| CQ2–5 | formation water + 0.1% APG | 4.89 | 1.640 | 33.54 |
| CQ2–7 | formation water + 0.1% CAB (35 °C) | 4.865 | 1.240 | 25.49 |
| CQ2–4 | formation water | 4.879 | 0.470 | 9.63 |
| CQ2–6 | Deionized water | 4.923 | 0.500 | 10.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Hua, H.; Wang, L. Surfactant-Enhanced Assisted Spontaneous Imbibition for Enhancing Oil Recovery in Tight Oil Reservoirs: Experimental Investigation of Surfactant Types, Concentrations, and Temperature Impact. Energies 2024, 17, 1794. https://doi.org/10.3390/en17081794
Wang F, Hua H, Wang L. Surfactant-Enhanced Assisted Spontaneous Imbibition for Enhancing Oil Recovery in Tight Oil Reservoirs: Experimental Investigation of Surfactant Types, Concentrations, and Temperature Impact. Energies. 2024; 17(8):1794. https://doi.org/10.3390/en17081794
Chicago/Turabian StyleWang, Fuyong, Haojie Hua, and Lu Wang. 2024. "Surfactant-Enhanced Assisted Spontaneous Imbibition for Enhancing Oil Recovery in Tight Oil Reservoirs: Experimental Investigation of Surfactant Types, Concentrations, and Temperature Impact" Energies 17, no. 8: 1794. https://doi.org/10.3390/en17081794
APA StyleWang, F., Hua, H., & Wang, L. (2024). Surfactant-Enhanced Assisted Spontaneous Imbibition for Enhancing Oil Recovery in Tight Oil Reservoirs: Experimental Investigation of Surfactant Types, Concentrations, and Temperature Impact. Energies, 17(8), 1794. https://doi.org/10.3390/en17081794






