1. Introduction
Economic growth, a population that has doubled since 1970, and accelerated material extraction, coupled with an increase in waste, have led to substantial transformations in land use and forest cover. These factors have also induced land degradation, climate change, biodiversity decrease, eutrophication, and pollution of waterways and soils [
1]. Cities, which are responsible for generating 80% of the global domestic product [
2], are currently undergoing rapid economic growth, resulting in increased rural-to-urban migration [
3]. Presently, 55% of the world’s population resides in urban areas [
4], while in Europe, nearly 75% of the population lives in cities [
5]. Many cities grapple with problems such as inadequate infrastructure, traffic congestion [
3], energy-inefficient building stock, and air pollution. Nonetheless, in the forthcoming decades, urban areas are expected to be the most profoundly affected by climate change [
5]. Conversely, cities are significant contributors to climate change, emitting 71–76% of global anthropogenic carbon dioxide (CO
2). The realisation of the 11th Sustainable Development Goal (Sustainable Cities and Communities) from Resolution 70/1, adopted by the UN General Assembly on 25 September 2015, titled “Transforming our world: the 2030 Agenda for Sustainable Development”, necessitates intelligent urban planning aimed at creating resilient cities [
6]. In the European Union (EU), the European Green Deal set a target to transform Europe into a climate-neutral continent by 2050 through a reduction in greenhouse gas (GHG) emissions of at least 55%, compared to 1990 levels. Achieving this goal requires, among other measures, an increase in the share of energy from renewable sources in the EU’s energy mix to 40% [
7]. The European urban landscape is characterised by small and medium-sized cities, which are expected to play a pivotal role in the development of a sustainable and climate-neutral Europe [
5].
Urban green areas, as defined by the Nature Conservation Act of April 2004 [
8], encompass areas with technical infrastructure and buildings functionally linked to them, covered with vegetation and serving public functions, particularly including parks; promenades; boulevards; botanical, zoological, and historical gardens; and cemeteries. Urban green areas also include greenery along roads in built-up areas, squares, historic fortifications, buildings, landfills, airports, railway stations, and industrial facilities. Urban green areas are situated within the administrative borders of cities, providing aesthetic, recreational, and health functions [
9]. Green areas in urban landscapes enhance air quality, mitigate extreme weather events, and regulate the hydrological cycle [
10,
11]. Additionally, green spaces in cities effectively alleviate the urban heat island effect [
12,
13] and play a crucial role in city resilience [
14]. Consequently, urban systems with extensive green infrastructure exhibit greater resilience to crises and are more human-friendly [
15]. The maintenance of urban green areas is indispensable for their diverse roles in cities, encompassing aesthetic aspects, environmental benefits, stormwater management, urban heat island mitigation, and community cohesion. Maintenance tasks in urban green spaces include trimming, irrigation, fertilisation, and pesticide application [
16]. Mowing grass on road verges is conducted to maintain visibility and safety. The cut grass is either left to decay or collected and utilised. However, the maintenance of green areas involves significant labour and machine input, consuming energy resources and resulting in waste generation. Trimming, fertilising, and waste transport consume fossil fuels and emit GHGs into the atmosphere [
17]. Despite recent reductions in maintenance workload through improved working plans, decreased trimming frequency, the introduction of wildflowers and meadows, and the self-maintenance of green spaces, large areas, such as sports fields and road verges, are still frequently trimmed. In addition, the policy of urban greenery extension results in increase in both the workload associated with its maintenance and with the amount of biomass produced, which needs to be utilised in a sustainable way [
17].
The biobased and circular economy, considered a viable approach for sustainable development, directs societies toward the sequential utilisation of resources, with an emphasis on biomass and bio-waste. The diminishing availability of resources, coupled with an escalating demand for energy and food, underscores the need to optimize the efficient use of biomass and bio-waste. Grass-trimming biomass is commonly subjected to composting, an aerobic process that transforms lignocellulosic waste into a value-added product, namely compost. However, this process is associated with substantial GHG emissions. Furthermore, the utilisation of immature compost may result in water pollution, odour emissions, and adverse effects on plant germination and development [
18]. As an alternative, other methods of bio-waste utilisation, such as biogas production, are being explored. The utilisation of green waste for energy generation has the potential to mitigate the elevated fuel consumption and GHG emissions associated with maintaining expanded green spaces within urban areas.
The anaerobic digestion (AD) of grass offers benefits, including waste reduction, decreased GHG emissions, and the generation of renewable energy and valuable fertilizer. However, the biogas potential of grass is relatively low, particularly when compared to biogas production from maize [
19]. The specific methane yield (SMY) has been extensively studied across various wild and cultivated grass species [
20,
21,
22,
23,
24,
25,
26]. Additionally, co-digestion of grass and other substrates as a strategy to enhance biogas production has been investigated [
27,
28]. The harvesting date is a critical factor affecting grass SMY, as the lignification process intensifies with advancing maturity, limiting material digestibility [
29]. Dragoni et al. [
24] noted higher SMY from AD of leaves compared to stems due to their elevated protein content [
20].
Another possibility for utilising waste generated during road verges maintenance involves the production of biodiesel. Biodiesel, primarily comprising Fatty Acid Methyl Esters (FAMEs), can be derived from various waste biomasses, including olive pomace oil [
30], cooking palm oil [
31], beef tallow [
32], fish fat [
33], chicken fat [
34], citrus wax [
35], and sewage sludge [
36]. Additionally, biodiesel production from spent coffee grounds [
37], cherry stone waste [
38], and herbal waste [
39] represents another feasible approach to resource utilisation. This not only aids in reducing crude oil consumption but also contributes to mitigating GHG emissions and air pollution.
The aim of this study was to determine the potential to obtain material from grass from road verges for the production of liquid biofuels (biodiesel) and to determine the specific biogas yield (SBY) from anaerobic mono-digestion of the studied grass in relation to the time of cutting and the preservation method of the studied material. Since a continuous supply of feedstock is essential for biofuel production, the study was conducted on both fresh and ensiled grass.
4. Discussion
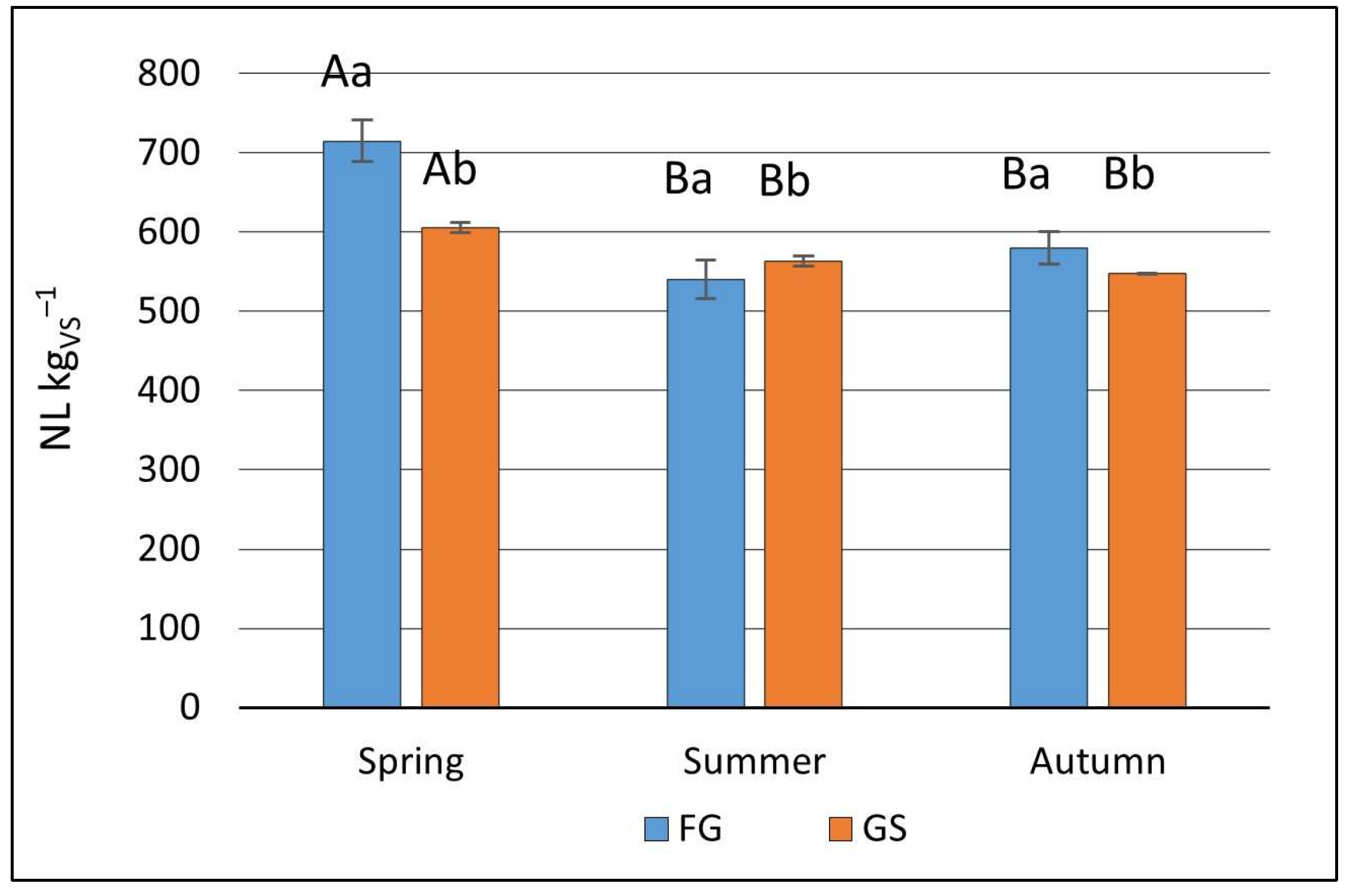
In this study, the biogas production from both grass and grass silage ranged from 540.19 ± 24.32 NL kg
VS−1 to 715.05 ± 26.43 NL kg
VS−1, exhibiting similarity to biogas yields from typical feedstock like farm manures or maize silage [
46]. These values slightly surpassed the range provided by Rajendran et al. [
46], who reported SBY for grass between 280 NL kg
VS−1 and 550 NL kg
VS−1. However, Żurek and Martyniak [
47] documented a biogas yield from silage of three species of perennial grasses within the range of 485–612 NL kg
VS−1.
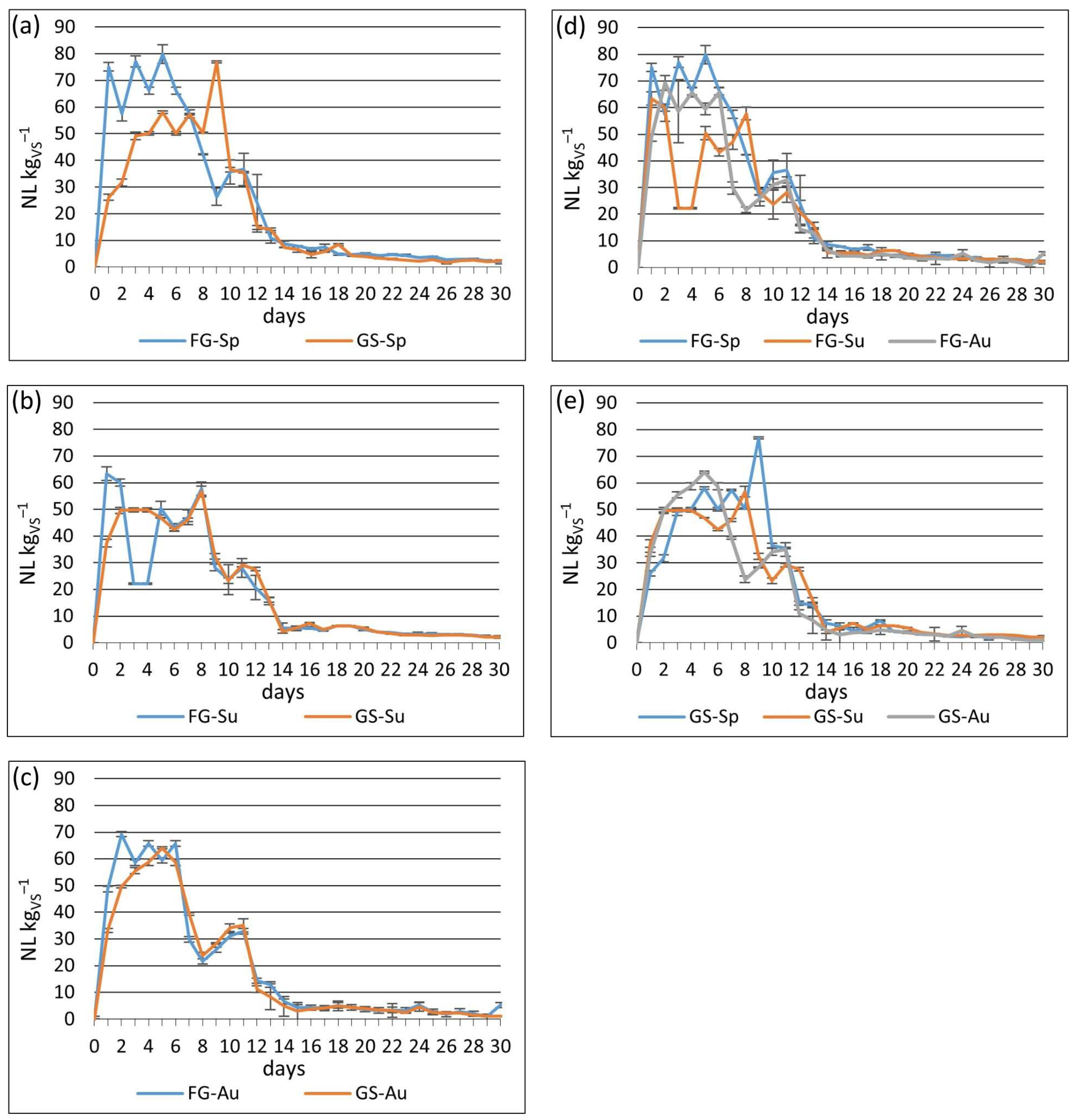
In the present study, both biogas yield and CH
4 concentration were influenced by the cutting time. Despite being statistically significant, the differences were relatively minor. Several authors [
20,
21,
24,
26,
48] have reported lower SMY from grass harvested later in the vegetation season. According to Korres et al. [
49], cutting time significantly impacts biogas production due to alterations in the proportion of cell wall components, namely cellulose, hemicellulose, and lignin, with increasing lignin content. Lignin, being the most recalcitrant, limits the biodegradability of grass and grass silage during the AD process [
29,
48]. Moreover, the CH
4 content in biogas from late-season-mown grass decreases due to reductions in crude protein and crude fat contents [
50] and an increase in the stem-to-leaves ratio [
49], given that stems produce lesser CH
4 amounts [
24]. The lignin content in the studied grass increased in summer and remained similar in autumn, influencing the biogas yield and CH
4 content [
51]. The marginal differences in biogas yield and CH
4 content may be attributed to frequent mowing conducted multiple times a year, shortening the physiological vegetation age of grasses and reducing lignification, as suggested by Triolo et al. [
48] and Piepenschneider et al. [
23].
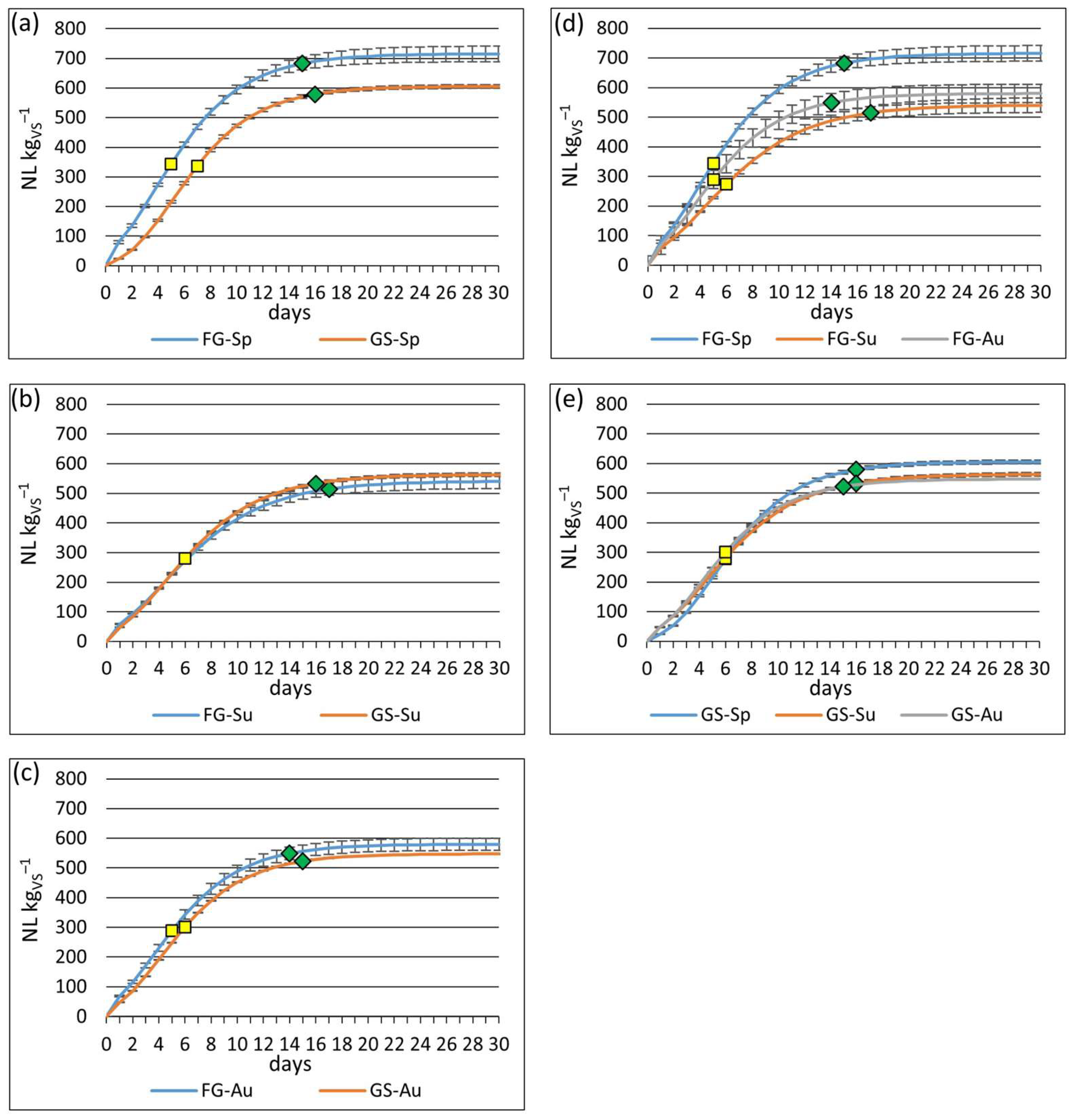
Continuous feedstock supply is imperative for sustained biogas production, while the biomass can be harvested only during the growing season. Consequently, ensiling becomes essential to avoid feedstock shortages throughout the year [
52]. In this study, ensiling had minimal adverse effects on biogas yield and no impact on the CH
4 concentration in biogas. Results on the impact of ensiling on CH
4 potential present contradictory findings. Cui et al. [
53] reported a higher SMY from ensiled wilted maize stover, while Feng et al. [
54] found that ensiling, although an appropriate storage method for
Festuca arundinacea, had no positive effect on CH
4 yield. Similar conclusions were drawn by Hillion et al. [
55], who reported that co-ensiling was effective for storing highly fermentable fresh waste, but CH
4 potential remained unaffected during storage. Menardo et al. [
56] demonstrated that although ensiling improved the CH
4 production rate initially, it did not affect the cumulative CH
4 production of corn stalks. Conversely, Liu et al. [
57] observed a higher CH
4 yield from ensiled giant reed compared to fresh material. Sun et al. [
58] reported that ensiling material with relatively high biodigestibility did not significantly increase CH
4 yield, while in the case of raw materials with relatively low biodigestibility values, it could enhance CH
4 production. In practical terms, the total CH
4 yield is crucial for the economic efficiency of biogas plants, and thus, studies on the effects of ensiling on biogas production should consider the trade-off between storage loss and CH
4 enhancement [
58]. Hermann et al. [
59] reported that ensiling showed little effect on CH
4 yield considering the increase in CH
4 concentration, with a mutual decrease in dry matter content during the storage. Teixeira Franco et al. [
60] suggested that ensiling may increase CH
4 potential only under specific conditions, accounting for storage losses.
In addition to CH
4 and CO
2, biogas encompasses nitrogen (N
2), hydrogen (H
2), carbon monoxide (CO), oxygen (O
2), H
2S, and NH
3. The latter two compounds, released during the digestion of feedstock, may exhibit inhibitory effects on biogas production. The H
2S content in biogas is contingent upon the feedstock and AD technology, fluctuating between 2 and 12,000 ppm [
61,
62,
63]. Elevated concentrations of H
2S result from the decomposition of sulphur-containing compounds, such as amino acids, sulphoxides, sulphonic acids, and the biological reduction of sulphates in the feedstock [
64].
The presence of H
2S not only hampers the AD process by denaturing proteins in microorganisms responsible for feedstock digestion, but also leads to the formation of a corrosive condensate with water in biogas, causing damage to combined heat and power (CHP) units and installations [
65,
66]. The toxicity of sulphur oxides (SOx) released into the atmosphere [
65,
67] and the corrosion of installations or engine damage in biogas plants compel operators to eliminate H
2S from biogas. However, the threshold value is contingent not only on the safety of the biogas installation but also on its subsequent applications. In biogas used in microturbines, the threshold value is high (70,000 ppm); in CHP units, the acceptable range is between 100 and 500 ppm [
67,
68], and biogas upgraded to biomethane should contain 4–10 ppm [
69].
In this study, the initial H
2S concentrations for summer- and autumn-cut grass exceeded the threshold values for CHP units, whereas the H
2S concentration in biogas from spring-cut grass remained very low, even at the beginning of the experiment. Similar disparities in H
2S concentration, influenced by cutting time, were reported by Chumenti et al. [
25], who observed a significantly higher H
2S content in biogas produced from summer-cut grass compared to biogas from spring-cut grass. Chumenti et al. [
70] also reported significant differences in H
2S concentrations in biogas produced from fresh and ensiled grass, contradicting the results of this study. Studies by Żurek and Martyniak [
47] indicated relatively low H
2S concentrations in biogas from perennial grasses, ranging from 272 to 298 ppm.
Another studied inhibitor is produced during the AD process. NH
4+ is released through the degradation of nitrogen-rich compounds, primarily proteins, urea, and nucleic acids [
64,
71,
72,
73]. This compound is not degraded under anaerobic conditions and is in equilibrium with NH
3, whose concentration is influenced by pH and temperature. A decrease in pH may lead to an increase in NH
3 concentration, adversely affecting the community structure of archaea, which is responsible for CH
4 production, and consequently reducing CH
4 yield [
74]. Inhibition of archaea leads to an increase in Volatile Fatty Acids (VFA) and a reduction in pH value [
75]. Threshold values for NH
3 concentration range from 80 to 400 ppm [
76]; however, the toxicity limits in the literature vary significantly, ranging from 60 to 14,000 ppm [
64,
77]. In this study, even the highest NH
3 value was lower than the threshold value and decreased significantly by the end of the experiment.
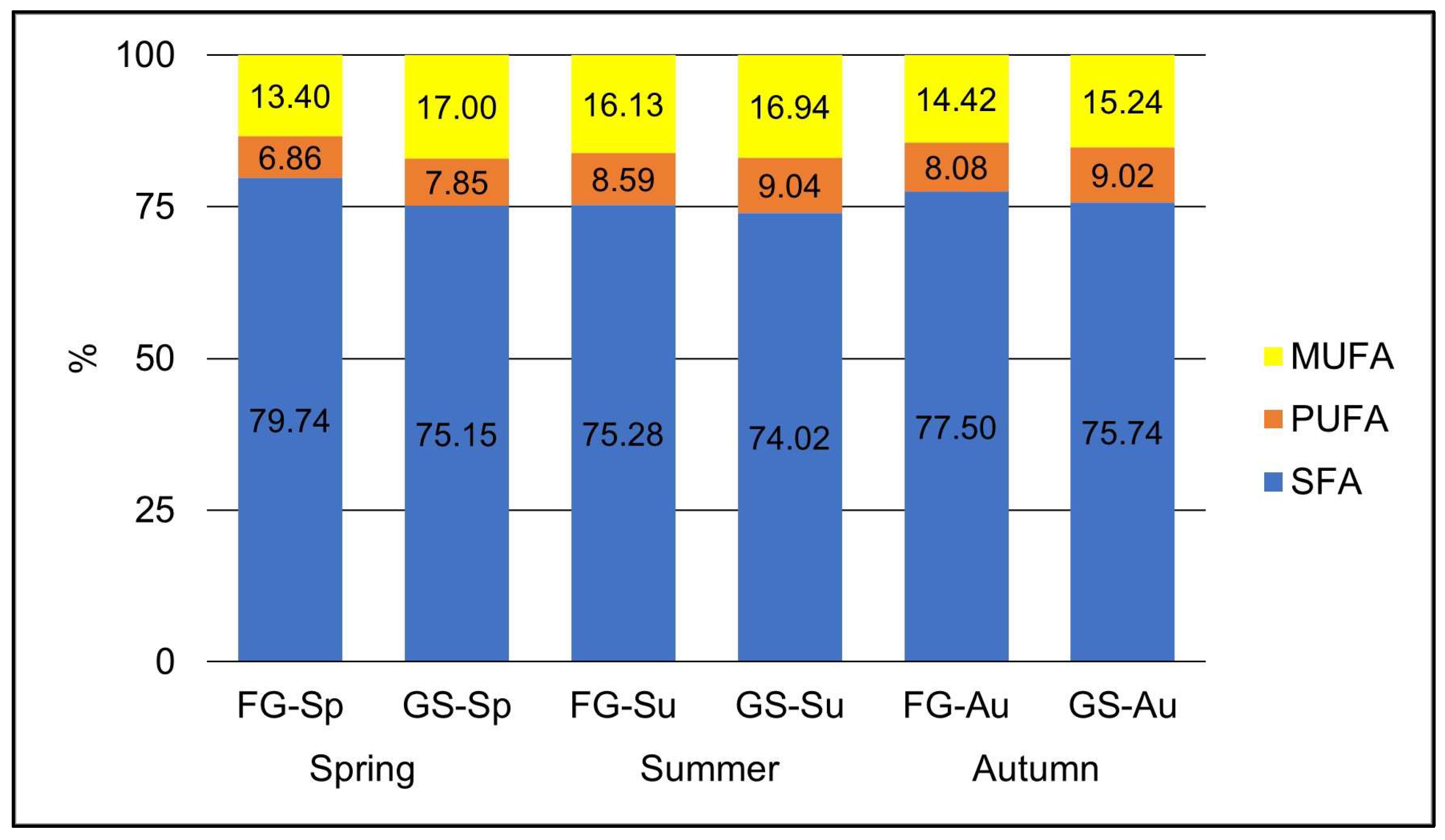
The compositional analysis of grass identified predominant FAMEs such as C16:0, C18:0, C18:1n9t/c, C18:2n6c, and C18:3n6, which are considered suitable for fuel production. Synthesised biodiesel, as reported in the literature, demonstrated the highest yield when derived from waste cooking oil (80.6%), followed by a mix of waste cooking oil and animal fats (79.3%). Characterisation of the produced biodiesel revealed the presence of various FAMEs components, with oleic acid (C18:1n9c), palmitic acid (C16:0), and linoleic acid (C18:2n6c) identified as major constituents [
78]. Spectroscopic studies assessing the quality of FAMEs obtained from waste cooking oil confirmed their compliance with the European Standard EN 14214:2006 requirements [
79]. FAMEs extracted from spent coffee grounds exhibited a composition composed of C16:0 (41.7%) and C18:0 (48.2%), rendering these extracts suitable for conversion into biodiesel. Furthermore, the residual solid fraction resulting from lignin and FAME extraction underwent AD under mesophilic conditions, yielding CH
4 at a rate of 360 NL kg
VS−1 [
37]. The FAME composition derived from cherry stone waste indicated a notable unsaturated to saturated fatty acid ratio [
38]. Similarly, herbal waste exhibited higher amounts of unsaturated FAMEs compared to saturated ones, with linoleic acid identified as the major polyunsaturated FAME and palmitic acid as the major saturated FAME [
39].
The comparative analysis of the compositional profiles between the examined grass samples and other waste materials, such as municipal sewage sludge [
80], revealed analogous distributions of FAMEs. Palmitic acid (C16:0) emerged as the predominant saturated fatty acid, constituting 37.5%, followed by stearic acid (C18:0) at 12.0%. Oleic acid (C18:1n9c) dominated the unsaturated fatty acids, accounting for 29.0%, while linoleic acid (C18:2n6c) represented 6.2% of the total FAMEs. Similarly, in lipids extracted from primary sludge, Villalobos-Delgado et al. [
81] identified palmitic acid (C16:0) as the major saturated fatty acid (42–58%), trailed by stearic acid (C18:0) at 18.3–24.5%, and oleic (C18:1n9c) and linoleic (C18:2n6c) acids at 9.5–22.3%.
The concentration of total FAMEs in the grass samples varied from 98.08 mg g
DM−1 in FG-Au to 56.37 mg g
DM−1 in FG-Sp. Notably, the C16:0 fatty acid ranged from 37.82 to 66.87 mg g
DM−1, and C18:0 from 4.50 to 6.83 mg g
DM−1, constituting the most abundant components. These specific FAMEs are considered ideal for biofuel production. In comparison, integrated processes for food waste yielded 248.21 g of FAMEs per 1 kg [
82]. Similarly, high lipid concentrations (248 mg g
DM−1) were observed in
Chlorella vulgaris [
83], highlighting the advantage of microalgae biomass production from waste in a more spatially efficient manner than other crop types. In herbal waste, the highest total FAMEs concentration was observed in rye bran (35.79 mg g
DM−1), herbal tea (11.69 mg g
DM−1), and chicory (8.78 mg g
DM−1), with the majority of herbal waste (62.5%) falling within the total FAMEs content range of 1.42 to 5.02 mg g
DM−1 [
39].
The lipid content in temperate grasses is relatively low and tends to decrease as the plant matures [
84]. The highest concentration of total FAMEs was observed in fresh grass samples collected in autumn and summer, with values of 98.08 mg g
DM−1 and 90.03 mg g
DM−1, respectively. Whetsell and Rayburn [
85] highlighted that vegetative growth and leafiness significantly influence the Fatty Acid (FA) content in grasses, emphasising the negative impact of summer months, specifically May, June, and July, on total FA content. Furthermore, the total FA content exhibited a stronger correlation with linolenic acid (C18:3) than with linoleic acid (C18:2), with lower correlations observed between linoleic acid (C18:2) and linolenic acid (C18:3) content. Concentrations of linoleic acid (C18:2), linolenic acid (C18:3), and total FAs were higher during the summer compared to spring growth [
86]. Consistent with these findings, the present study reported the lowest concentration of total FAMEs in FS-Sp (56.37 mg g
DM−1) and GS-Sp (58.42 mg g
DM−1). Intriguingly, GS-Sp exhibited a higher content of these compounds than FG-Sp.
The ensiling process significantly influenced the content of total FAMEs in spring grass, resulting in a reduction in total saturated fatty acids and an increase in total unsaturated fatty acids. These results are in good agreement with the findings of Khan et al. [
87], who attributed variations in plant maturity at harvest as the primary explanation for the variability in FA content, highlighting higher contents of C18:3n3 in silages from young grass. Notably, the FAMEs content in grass silage from summer and autumn was lower than in fresh grass from these cutting times. The highest concentration of total FAMEs in ensiled grass (75.81 mg g
DM−1) was detected in samples from summer, closely related to the dry matter content in the analysed samples.
Biodiesel production from waste oils presents challenges, including elevated Free Fatty Acids (FFA) during transesterification. The presence of FFA and water leads to the formation of glycerol (propane-1,2,3-triol) as a by-product and reduces methyl ester levels [
88]. The amount of glycerol is contingent on the conversion methods, as well as the type of alcohol and catalyst employed [
89]. A substantial portion (70–95%) of the total biodiesel production cost is associated with raw materials [
90]. Utilising waste materials, such as waste cooking oil, can significantly reduce production costs, with the cost of obtaining waste cooking oil being 2.5 to 3.5 times lower than that of edible vegetable oils [
91]. Osman et al. [
92] have explored computational and machine learning techniques, biodiesel characteristics, transesterification processes, waste materials, and policies encouraging biodiesel production from waste. Consequently, the studied grasses represent a potential source for biodiesel production. However, further investigations into their properties are needed.
Although the studied grass exhibits potential for application in both biodiesel and biogas production, its limited availability results in a low energy yield per hectare [
51]. Hence, grass waste from the maintenance of road verges should not be viewed as the primary substrate for the production of liquid or gaseous biofuels. Instead, it should be considered a supplementary feedstock, to be used alongside other resources that are available in quantities sufficient for the operations of biofuel plants. The findings underscore the potential for alternative utilisation of biowaste, thereby prompting consideration for future policy adjustments in urban waste management strategies, integrating energy generation in alignment with the principles of circular economy.