Reduction of Iron Oxides for CO2 Capture Materials
Abstract
1. Introduction
2. Iron Oxides in the Context of Energy Transition and CCS
2.1. Motivation for the Use of Iron Oxides in CCS
2.2. Hematite
2.3. Magnetite
2.4. Wüstite
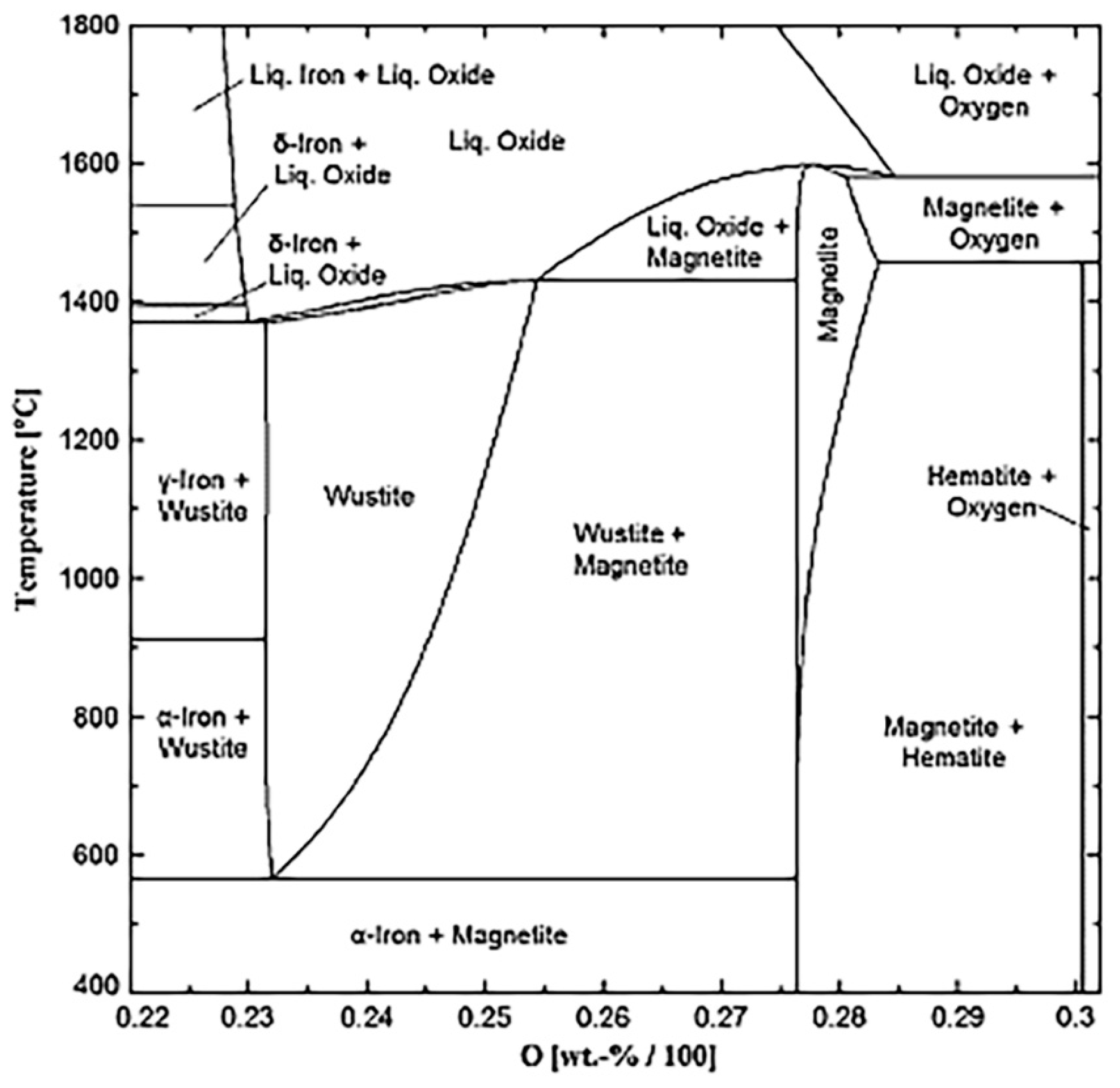
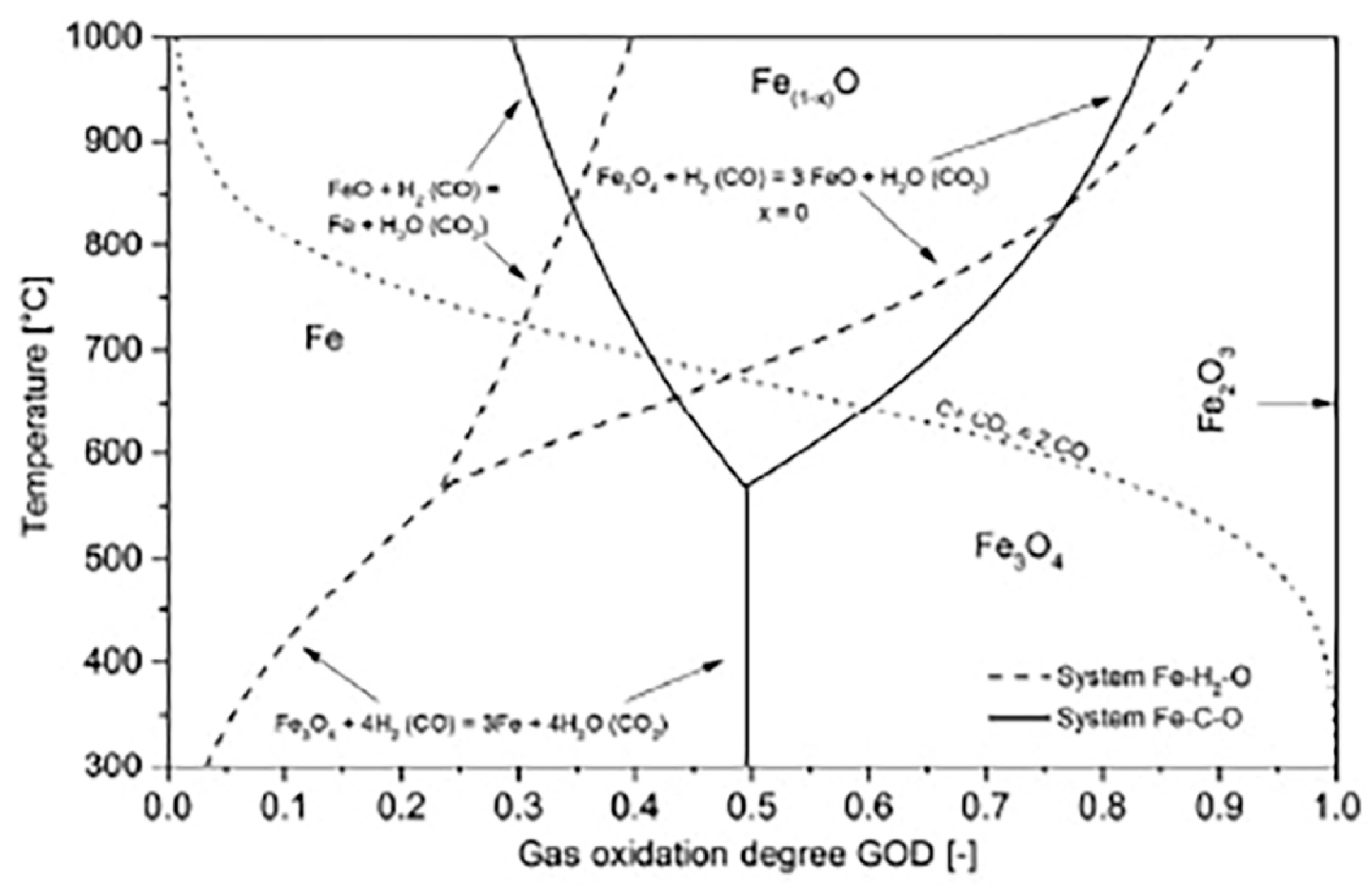
3. Thermodynamics of Carbon Capture with Iron Oxides
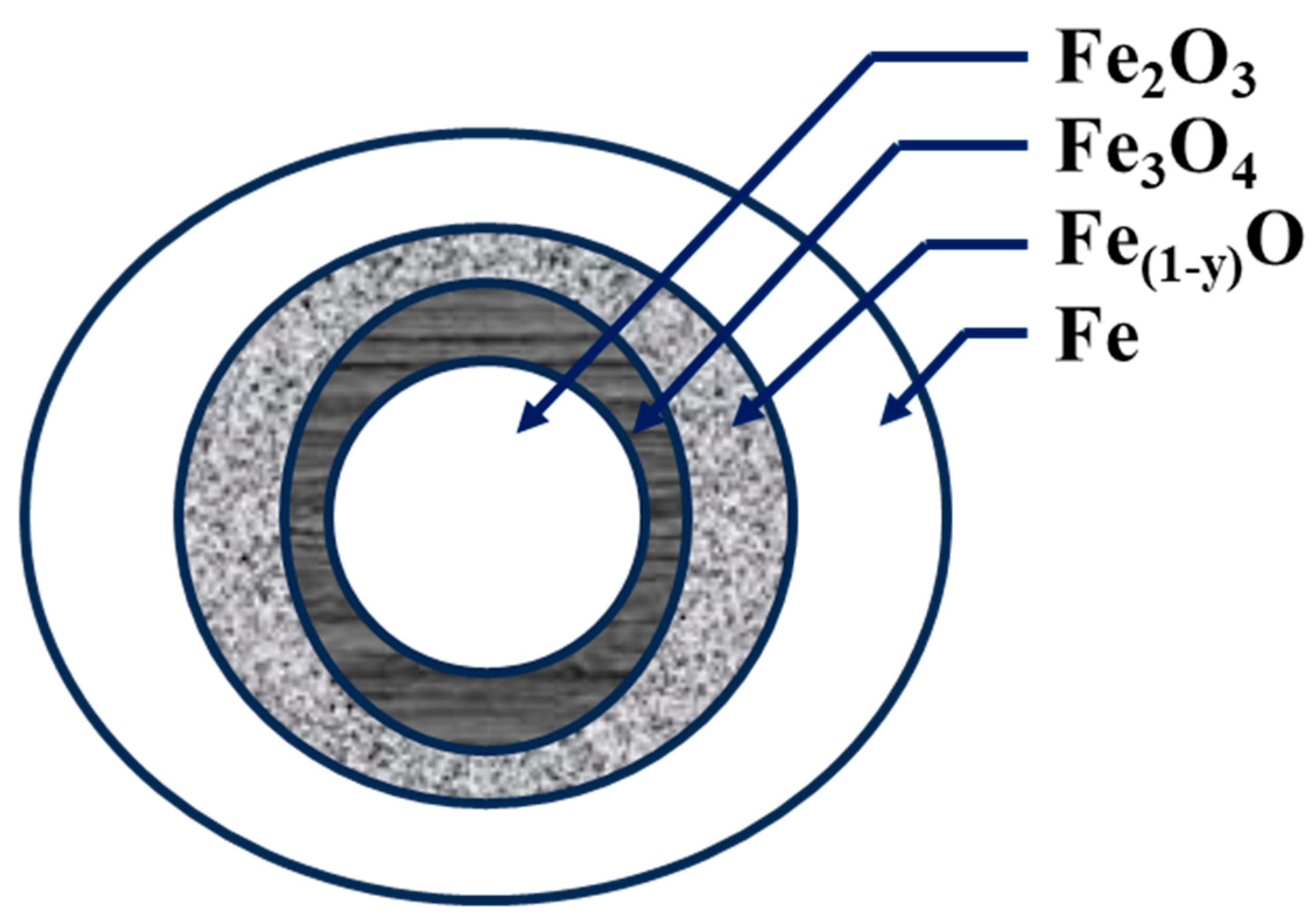
4. Morphology and Microstructural Properties of Iron Oxides
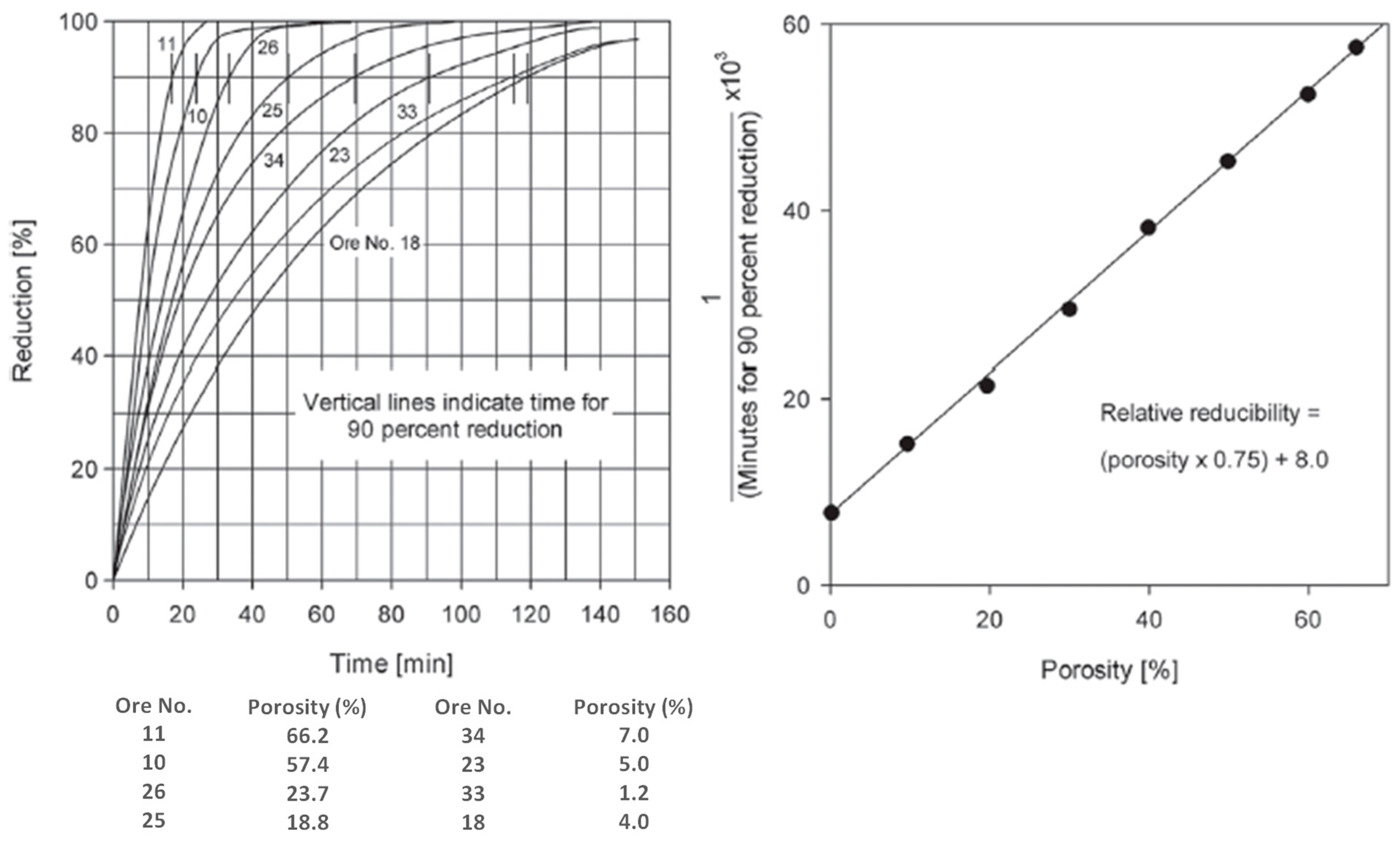
4.1. Porosity Changes in Reduction of Iron Ores
4.2. Effect of Gangue on Reduction Kinetics and Porosity
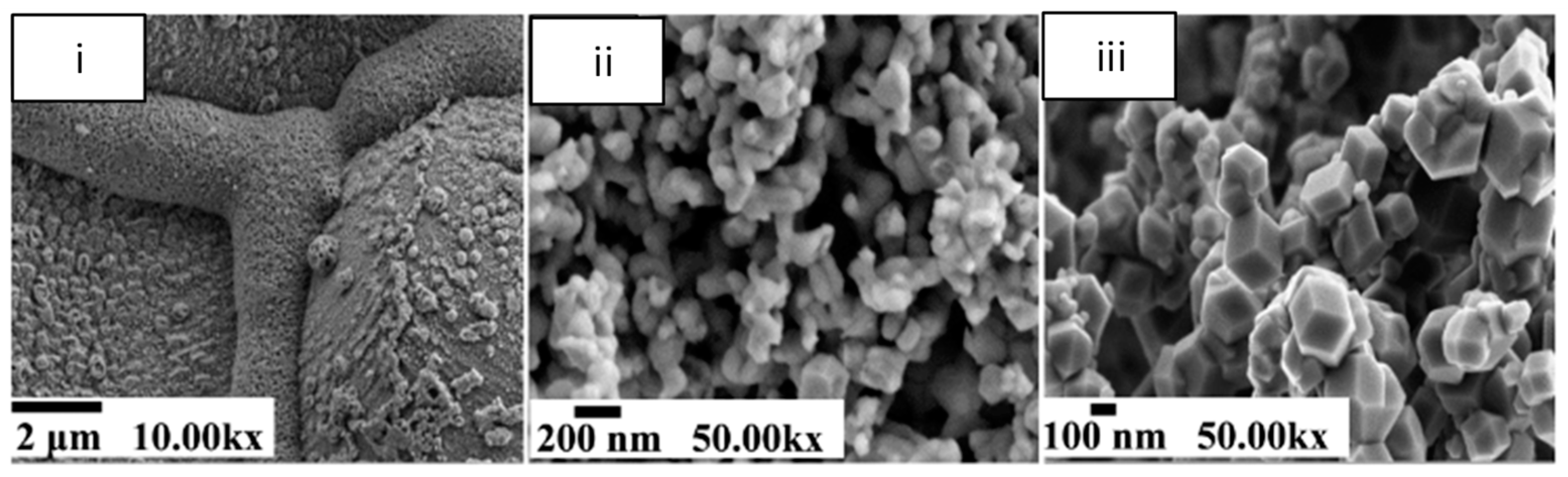
4.3. Microstructural Changes in CO2 Capture by Iron Oxides
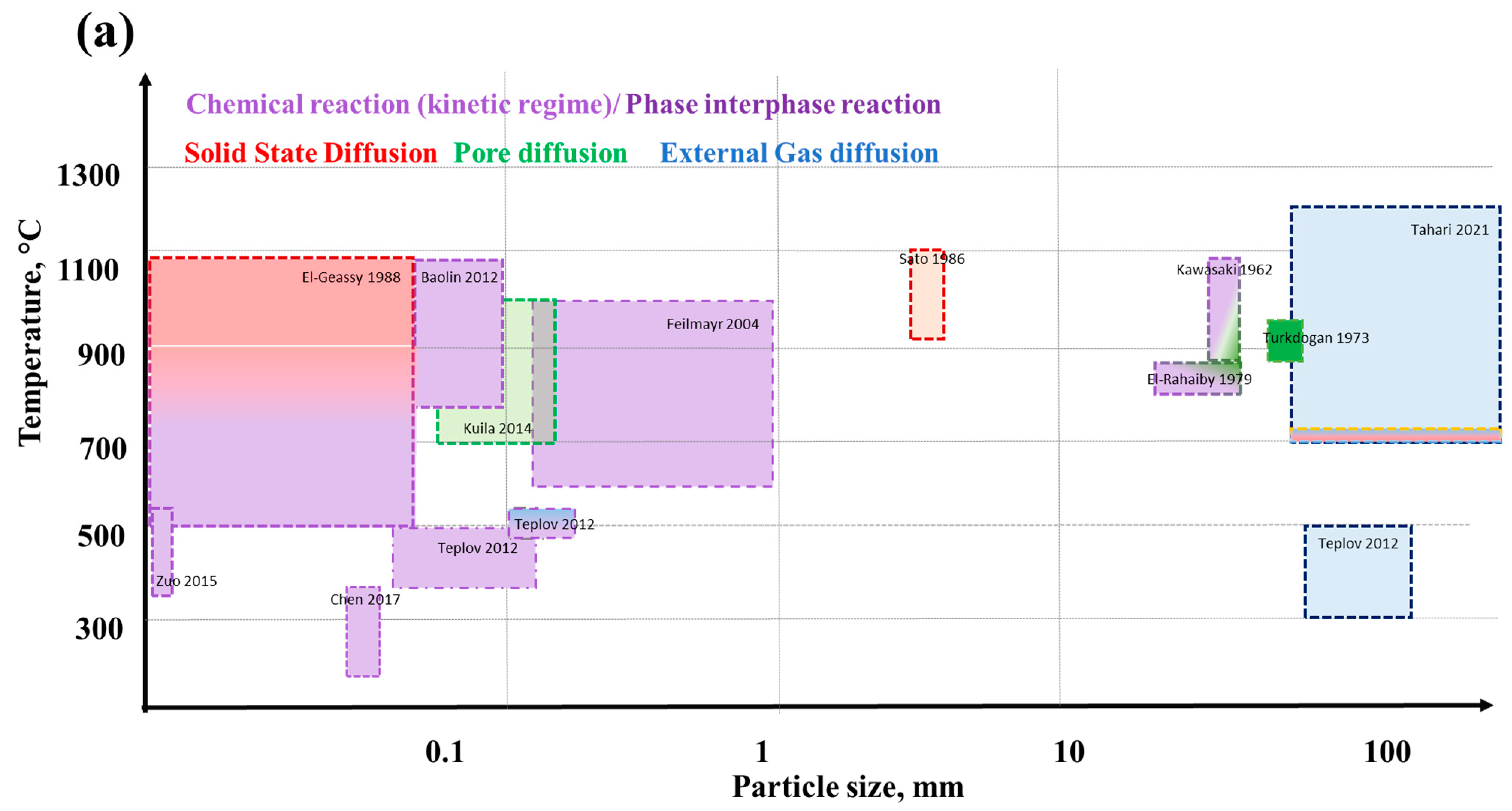
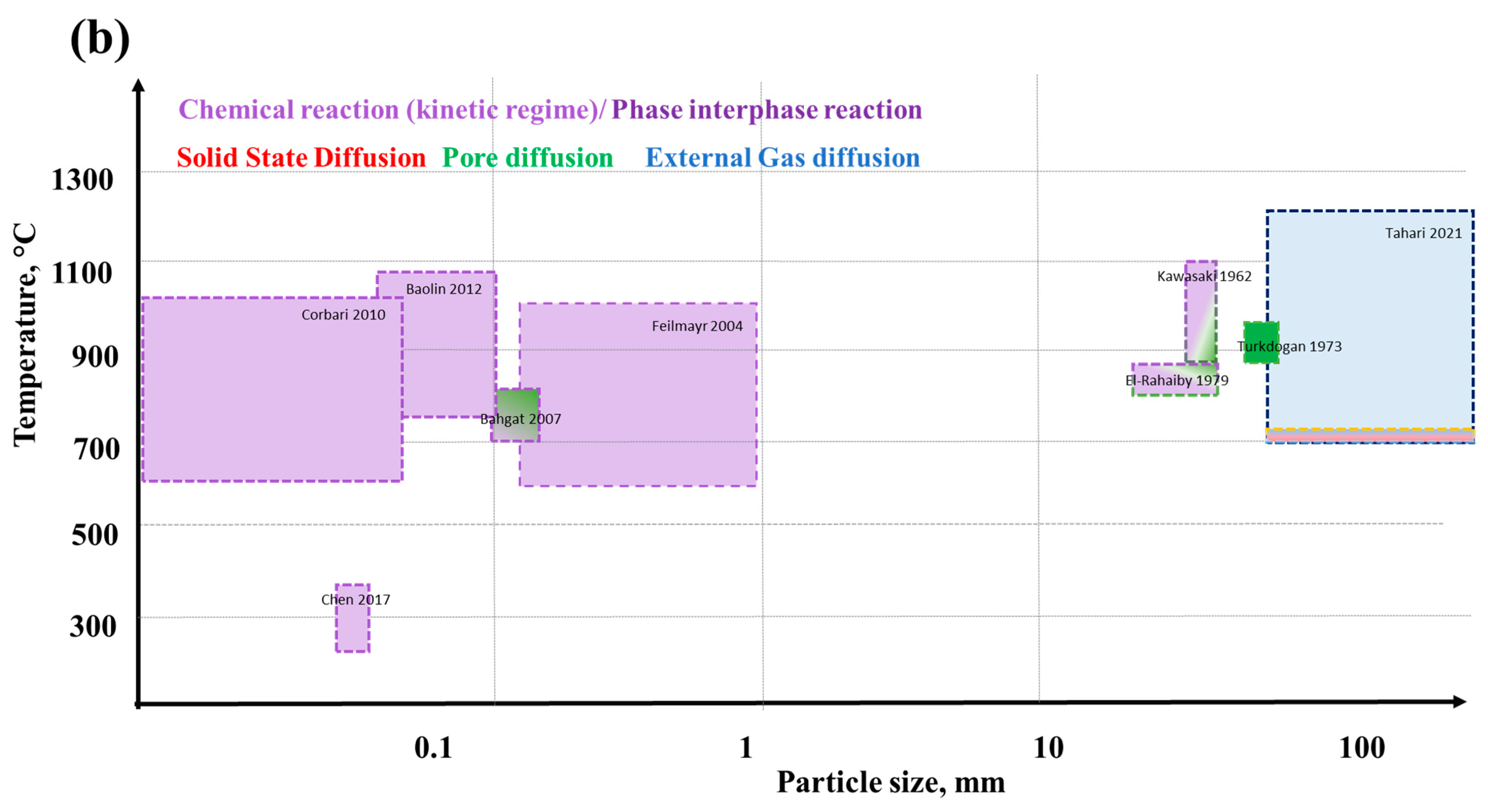
5. Kinetics of Iron Ore Reduction
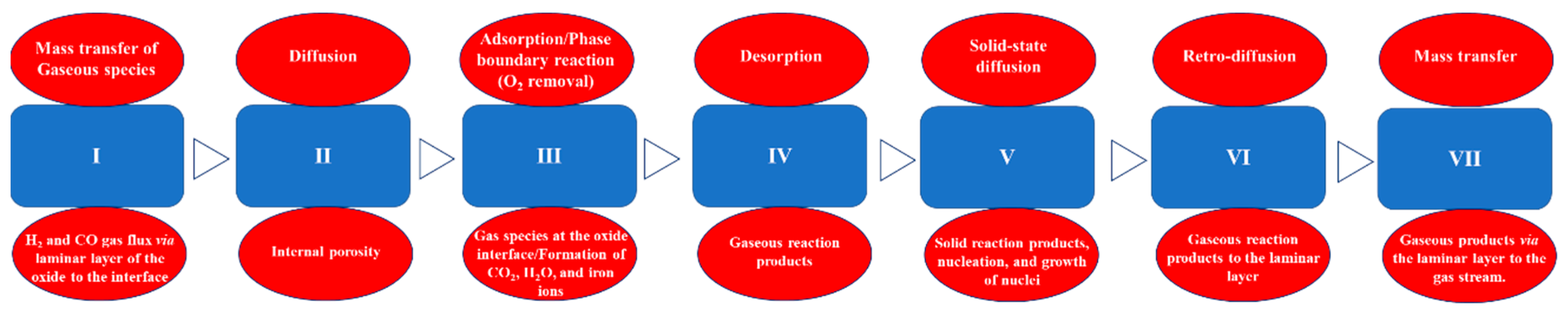
6. Effect of Physical and Chemical Phenomena on the Reaction Rate
| Reducing Agent | Particle Size | Porosity (ε) | T (°C) | P (bar) | Ea (kJ/mol) | Limitation Steps (2nd Reaction Step) | Reference |
|---|---|---|---|---|---|---|---|
| H2 | 4 mm × 4 mm × 8 mm | - | 900–1100 | - | 99.2 | Solid-state diffusion | [138] |
| H2, CO | 125 to 500 μm | 0.15 | 350–600 | 10 | 91 | Phase boundary reaction | [125] |
| CO | 100–150 μm | - | 700–850 | - | 80 | Nucleation of FeO and then internal diffusion | [136] |
| H2 | <74 µm | 0.54 | 500–1100 | 68 | 51 for T < 650 °C; 84 for 650–900 °C; 176 for T > 900 °C | T < 650 °C chemical reaction, 650–900 °C chemical reaction + solid state diffusion, T > 900 °C solid state diffusion | [105] |
| CO-CO2 H2 | 50.8 µm | 0.0115 | 240–417 | - | 71 | Phase boundary reaction | [134] |
| H2/CO/mixtures | 1.07–1.24 cm | 0.06 | 850 | - | - | Mixed (chemical reaction + pore diffusion) at the beginning, pore diffusion at the end | [143] |
| H2/CO | - | - | 800–1000 | - | 48.64 with CO 63.19 with H2 | First phase boundary reaction, then pore diffusion and mixed regime (at 800 °C and 900 °C). | [144] |
| H2 | 75–180 µm | 0.27 | 700–1000 | 0.25–1 | 33 | Pore diffusion | [123] |
| H2/CO | - | - | 150–900 | - | - | Solid-state diffusion | [135] |
| H2/CO | 1.5–4.4 cm | 0.31 | 700–1200 | 1–2 | - | Gas diffusion At 700 °C in the late stages solid state diffusion | [133] |
| H2 CO/CO2 | 14 mm | - | 900 | - | - | Pore diffusion | [101] |
| H2/CO | 12 mm | - | 800–1000 | - | - | Chemical reaction at the beginning, pore diffusion at the end | [137] |
| H2 | 50–160 µm | 0.5 | 400 | 1 | - | Chemical reaction | [106] |
| H2 | 100–160 µm | 0.5 | 500 | 1 | - | External gas transport + chemical reaction | [106] |
| H2 | pellets | 0.5 | 300–500 | 1 | - | Transport in the external H2O layer | [106] |
| CO-CO2 | ~150 µm | 0.69 | 590–1000 | 1 | - | T < 700 °C, mixed control in the pores T > 700 °C external gas transport | [130] |
| CO-CO2 | 150–500 µm | 0.42 | 590–1000 | 1 | - | Chemical reaction | [130] |
| CO-CO2 | <75 µm | 0.23 | 590–1000 | 1 | - | Chemical reaction | [130] |
| H2 | 0.249 µm | - | 400–570 | 131.5 Fe3O4 → FeO 76.0 FeO → Fe | Chemical reaction | [131] | |
| H2 CO | <100 µm | - | 800–1100 | - | 41.15 with H2 54.19 with CO | Phase boundary reaction | [145] |
7. Conclusions, Challenges and Future Perspectives
- H2, for thermodynamic and kinetic reasons, is a better reduction agent than CO thanks to better diffusion behavior. Indeed, the viscosity and the molecule size of H2 are lower than CO, affecting the diffusion behavior.
- The first step of reduction, leading from Fe2O3 to Fe3O4, below 570 °C, is relatively fast compared to further reduction from Fe3O4 to Fe(1−y)O or Fe; however, it is important because it opens up porosity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Name |
| CO2 | Carbon dioxide |
| Fe2O3 | Hematite |
| Fe3O4 | Magnetite |
| Fe(1−y)O | Wüstite |
| IEA | International Energy Agency |
| H2 | Hydrogen |
| CO | Carbon oxide |
| CCS | Carbon Capture and Sequestration |
| EOR | Enhance Oil Recovery |
| CaO | Calcium oxides |
| TRL | Technology Readiness Level |
| N2 | Nitrogen |
| MgO | Magnesium oxides |
| CLC | Chemical Looping Combustion |
| FeCO3 | Siderite |
| MOFs | Metal organic frameworks |
| O2 | Oxygen |
| Al2O3 | Alumina |
| SiO2 | Silica |
| Al | Aluminium |
| Fe | Iron |
| ATS | (3-Aminopropyl) triethoxysilane |
| TeOS | Tetraethyl orthosilicate |
| A | L (+)-ascorbic acid |
| C | Carbon |
| Mn | Manganese |
| Mn3O4 | Hausmannite |
| CH4 | Methane |
| GOD | Gas Oxidation Degree |
| SEM | Scanning electron microscopy |
| dpore | Pore diameter |
| Vpore | Pore volume |
| Ti2O3 | Tistarite |
| V2O3 | Karelianite |
| H2O | Water |
References
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar]
- Jeffry, L.; Ong, M.Y.; Nomanbhay, S.; Mofijur, M.; Mubashir, M.; Show, P.L. Greenhouse gases utilization: A review. Fuel 2021, 301, 121017. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, H. Carbon sequestration potential of disturbed and non-disturbed forest ecosystem: A tool for mitigating climate change. Afr. J. Environ. Sci. Technol. 2020, 14, 385–393. [Google Scholar]
- Nica, A.; Popescu, A.; Ibanescu, D.-C. Human influence on the climate system. Curr. Trends Nat. Sci. 2019, 8, 209–215. [Google Scholar]
- Saleh, T.A. Nanomaterials and hybrid nanocomposites for CO2 capture and utilization: Environmental and energy sustainability. RSC Adv. 2022, 12, 23869–23888. [Google Scholar] [CrossRef] [PubMed]
- Wani, O.A.; Kumar, S.S.; Hussain, N.; Wani, A.I.A.; Subhash, B.; Parvej, A.; Rashid, M.; Popescu, S.M.; Mansoor, S. Multi-scale processes influencing global carbon storage and land-carbon-climate nexus: A critical review. Pedosphere 2023, 33, 250–267. [Google Scholar] [CrossRef]
- Gajdzik, B.; Sroka, W.; Vveinhardt, J. Energy Intensity of Steel Manufactured Utilising EAF Technology as a Function of Investments Made: The Case of the Steel Industry in Poland. Energies 2021, 14, 5152. [Google Scholar] [CrossRef]
- Kim, J.; Sovacool, B.K.; Bazilian, M.; Griffiths, S.; Lee, J.; Yang, M.; Lee, J. Decarbonizing the iron and steel industry: A systematic review of sociotechnical systems, technological innovations, and policy options. Energy Res. Soc. Sci. 2022, 89, 102565. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Farooqi, Z.U.R.; Lee, C. Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Berdysheva, S.; Ikonnikova, S. The energy transition and shifts in fossil fuel use: The study of international energy trade and energy security dynamics. Energies 2021, 14, 5396. [Google Scholar] [CrossRef]
- Kober, T.; Schiffer, H.-W.; Densing, M.; Panos, E. Global energy perspectives to 2060–WEC’s World Energy Scenarios 2019. Energy Strategy Rev. 2020, 31, 100523. [Google Scholar] [CrossRef]
- Griffin, P.W.; Hammond, G.P. Industrial energy use and carbon emissions reduction in the iron and steel sector: A UK perspective. Appl. Energy 2019, 249, 109–125. [Google Scholar] [CrossRef]
- Holappa, L. A general vision for reduction of energy consumption and CO2 emissions from the steel industry. Metals 2020, 10, 1117. [Google Scholar] [CrossRef]
- Budinis, S.; Krevor, S.; Mac Dowell, N.; Brandon, N.; Hawkes, A. An assessment of CCS costs, barriers and potential. Energy Strategy Rev. 2018, 22, 61–81. [Google Scholar] [CrossRef]
- Van der Spek, M.; Roussanaly, S.; Rubin, E.S. Best practices and recent advances in CCS cost engineering and economic analysis. Int. J. Greenh. Gas Control. 2019, 83, 91–104. [Google Scholar] [CrossRef]
- Jiang, K.; Ashworth, P. The development of Carbon Capture Utilization and Storage (CCUS) research in China: A bibliometric perspective. Renew. Sustain. Energy Rev. 2021, 138, 110521. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K. Recent trends in the development of adsorption technologies for carbon dioxide capture: A brief literature and patent reviews (2014–2018). J. Clean. Prod. 2020, 253, 119707. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.Ó.; Sigfússon, B.; Marieni, C.; Goldberg, D.; Gislason, S.R.; Oelkers, E.H. Carbon dioxide storage through mineral carbonation. Nat. Rev. Earth Environ. 2020, 1, 90–102. [Google Scholar] [CrossRef]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An overview of the status and challenges of CO2 storage in minerals and geological formations. Front. Clim. 2019, 1, 9. [Google Scholar] [CrossRef]
- Ajayi, T.; Gomes, J.S.; Bera, A. A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches. Pet. Sci. 2019, 16, 1028–1063. [Google Scholar] [CrossRef]
- Herzog, H.J. Scaling up carbon dioxide capture and storage: From megatons to gigatons. Energy Econ. 2011, 33, 597–604. [Google Scholar] [CrossRef]
- Fabozzi, A.; Della Sala, F.; di Gennaro, M.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. Polymer based nanoparticles for biomedical applications by microfluidic techniques: From design to biological evaluation. Polym. Chem. 2021, 12, 6667–6687. [Google Scholar] [CrossRef]
- Fabozzi, A.; Vitiello, R.; Krauss, I.R.; Iuliano, M.; De Tommaso, G.; Amoresano, A.; Pinto, G.; Paduano, L.; Jones, C.; Di Serio, M.; et al. Synthesis, Surface Properties, and Self-Aggregation Behavior of a Branched N,N-Dimethylalkylamine Oxide Surfactant. J. Surfactants Deterg. 2019, 22, 115–124. [Google Scholar] [CrossRef]
- Fabozzi, A.; Krauss, I.R.; Vitiello, R.; Fornasier, M.; Sicignano, L.; King, S.; Guido, S.; Jones, C.; Paduano, L.; Murgia, S.; et al. Branched alkyldimethylamine oxide surfactants: An effective strategy for the design of high concentration/low viscosity surfactant formulations. J. Colloid Interface Sci. 2019, 552, 448–463. [Google Scholar] [CrossRef]
- Scermino, L.; Fabozzi, A.; De Tommaso, G.; Valente, A.J.M.; Iuliano, M.; Paduano, L.; D’Errico, G. pH-responsive micellization of an amine oxide surfactant with branched hydrophobic tail. J. Mol. Liq. 2020, 316, 113799. [Google Scholar] [CrossRef]
- Savignano, L.; Fabozzi, A.; Vitiello, R.; Fornasier, M.; Murgia, S.; Guido, S.; Guida, V.; Paduano, L.; D’Errico, G. Effect of tail branching on the phase behavior and the rheological properties of amine oxide/ethoxysulfate surfactant mixtures. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 613, 126091. [Google Scholar]
- Massarweh, O.; Abushaikha, A.S. A review of recent developments in CO2 mobility control in enhanced oil recovery. Petroleum 2022, 8, 291–317. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Yu, X.; Wang, C.; Yao, B.; Wang, S.; Winterfeld, P.H.; Wang, X.; Yang, Z.; Wang, Y. Advances in improved/enhanced oil recovery technologies for tight and shale reservoirs. Fuel 2017, 210, 425–445. [Google Scholar] [CrossRef]
- Vega, F.; Baena-Moreno, F.; Fernández, L.M.G.; Portillo, E.; Navarrete, B.; Zhang, Z. Current status of CO2 chemical absorption research applied to CCS: Towards full deployment at industrial scale. Appl. Energy 2020, 260, 114313. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. [Google Scholar] [CrossRef]
- Odunlami, O.; Vershima, D.; Oladimeji, T.; Nkongho, S.; Ogunlade, S.; Fakinle, B. Advanced techniques for the capturing and separation of CO2—A review. Results Eng. 2022, 15, 100512. [Google Scholar] [CrossRef]
- Salvi, B.L.; Jindal, S. Recent developments and challenges ahead in carbon capture and sequestration technologies. SN Appl. Sci. 2019, 1, 885. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Mendoza, E.Y.M.; Santos, A.S.; López, E.V.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Iron oxides as efficient sorbents for CO2 capture. J. Mater. Res. Technol. 2019, 8, 2944–2956. [Google Scholar] [CrossRef]
- Ramirez-Vidal, P.; Sdanghi, G.; Celzard, A.; Fierro, V. High hydrogen release by cryo-adsorption and compression on porous materials. Int. J. Hydrogen Energy 2022, 47, 8892–8915. [Google Scholar] [CrossRef]
- Nicotera, I.; Policicchio, A.; Conte, G.; Agostino, R.G.; Rehman, M.H.U.; Lufrano, E.; Simari, C. Quaternized polyepichlorohydrin-based membrane as high-selective CO2 sorbent for cost-effective carbon capture. J. CO2 Util. 2022, 63, 102135. [Google Scholar] [CrossRef]
- Tao, H.; Qian, X.; Zhou, Y.; Cheng, H. Research progress of clay minerals in carbon dioxide capture. Renew. Sustain. Energy Rev. 2022, 164, 112536. [Google Scholar] [CrossRef]
- Powell, C.E.; Qiao, G.G. Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J. Membr. Sci. 2006, 279, 1–49. [Google Scholar] [CrossRef]
- Barbera, E.; Mio, A.; Pavan, A.M.; Bertucco, A.; Fermeglia, M. Fuelling power plants by natural gas: An analysis of energy efficiency, economical aspects and environmental footprint based on detailed process simulation of the whole carbon capture and storage system. Energy Convers. Manag. 2022, 252, 115072. [Google Scholar] [CrossRef]
- Sanchez Moore, C.C.; Kulay, L. Effect of the implementation of carbon capture systems on the environmental, energy and economic performance of the Brazilian electricity matrix. Energies 2019, 12, 331. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-based CO2 capture technology development from the beginning of 2013 A Review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Yamada, H. Amine-based capture of CO2 for utilization and storage. Polym. J. 2021, 53, 93–102. [Google Scholar] [CrossRef]
- Haaf, M.; Anantharaman, R.; Roussanaly, S.; Ströhle, J.; Epple, B. CO2 capture from waste-to-energy plants: Techno-economic assessment of novel integration concepts of calcium looping technology. Resour. Conserv. Recycl. 2020, 162, 104973. [Google Scholar] [CrossRef]
- Aghel, B.; Janati, S.; Wongwises, S.; Shadloo, M.S. Review on CO2 capture by blended amine solutions. Int. J. Greenh. Gas Control 2022, 119, 103715. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon dioxide capture using liquid absorption methods: A review. Environ. Chem. Lett. 2021, 19, 77–109. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon dioxide emissions, capture, storage and utilization: Review of materials, processes and technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Siegelman, R.L.; Milner, P.J.; Kim, E.J.; Weston, S.C.; Long, J.R. Challenges and opportunities for adsorption-based CO2 capture from natural gas combined cycle emissions. Energy Environ. Sci. 2019, 12, 2161–2173. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of carbon dioxide for post-combustion capture: A review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Sifat, N.S.; Haseli, Y. A critical review of CO2 capture technologies and prospects for clean power generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef]
- Ramli, N.A.; Hashim, N.A.; Aroua, M.K. Prediction of CO2/O2 absorption selectivity using supported ionic liquid membranes (SILMs) for gas–liquid membrane contactor. Chem. Eng. Commun. 2018, 205, 295–310. [Google Scholar] [CrossRef]
- da Silva Freitas, W.; Mecheri, B.; Vecchio, C.L.; Gatto, I.; Baglio, V.; Ficca, V.C.; Patra, A.; Placidi, E.; D’Epifanio, A. Metal-organic-framework-derived electrocatalysts for alkaline polymer electrolyte fuel cells. J. Power Sources 2022, 550, 232135. [Google Scholar] [CrossRef]
- Ruhaimi, A.; Aziz, M.; Jalil, A. Magnesium oxide-based adsorbents for carbon dioxide capture: Current progress and future opportunities. J. CO2 Util. 2021, 43, 101357. [Google Scholar] [CrossRef]
- Dunstan, M.T.; Donat, F.; Bork, A.H.; Grey, C.P.; Müller, C.R. CO2 capture at medium to high temperature using solid oxide-based sorbents: Fundamental aspects, mechanistic insights, and recent advances. Chem. Rev. 2021, 121, 12681–12745. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, C.; Shen, B.; Zhang, X.; Zhang, Y.; Huang, J. Progress in the development and application of CaO-based adsorbents for CO2 capture—A review. Mater. Today Sustain. 2018, 1, 1–27. [Google Scholar]
- Abd, A.A.; Othman, M.R.; Kim, J. A review on application of activated carbons for carbon dioxide capture: Present performance, preparation, and surface modification for further improvement. Environ. Sci. Pollut. Res. 2021, 28, 43329–43364. [Google Scholar] [CrossRef] [PubMed]
- André, L.; Abanades, S. Recent advances in thermochemical energy storage via solid–gas reversible reactions at high temperature. Energies 2020, 13, 5859. [Google Scholar] [CrossRef]
- Jin, B.; Wei, K.; Ouyang, T.; Fan, Y.; Zhao, H.; Zhang, H.; Liang, Z. Chemical looping CO2 capture and in-situ conversion: Fundamentals, process configurations, bifunctional materials, and reaction mechanisms. Appl. Energy Combust. Sci. 2023, 16, 100218. [Google Scholar] [CrossRef]
- Goyal, P.; Tiwary, C.S.; Misra, S.K. Ion exchange based approach for rapid and selective Pb (II) removal using iron oxide decorated metal organic framework hybrid. J. Environ. Manag. 2021, 277, 111469. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, K.; Zhang, J.; Guo, Z. A review on low carbon emissions projects of steel industry in the World. J. Clean. Prod. 2021, 306, 127259. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Taghizadeh, S.-M.; Berenjian, A.; Zare, M.; Ebrahiminezhad, A. New perspectives on iron-based nanostructures. Processes 2020, 8, 1128. [Google Scholar] [CrossRef]
- Hamza, A.; Hussein, I.A.; Jalab, R.; Saad, M.; Mahmoud, M. Review of iron sulfide scale removal and inhibition in oil and gas wells: Current status and perspectives. Energy Fuels 2021, 35, 14401–14421. [Google Scholar] [CrossRef]
- Garibello, C.F.; Eldridge, D.S.; Malherbe, F.; Hocking, R.K. Abiotic transformations of nitrogen mediated by iron sulfides and related species from early Earth to catalyst design. Inorg. Chem. Front. 2023, 10, 6792–6811. [Google Scholar] [CrossRef]
- Ubando, A.T.; Chen, W.H.; Show, P.L.; Ong, H.C. Kinetic and thermodynamic analysis of iron oxide reduction by graphite for CO2 mitigation in chemical-looping combustion. Int. J. Energy Res. 2020, 44, 3865–3882. [Google Scholar] [CrossRef]
- Abuelgasim, S.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2021, 764, 142892. [Google Scholar] [CrossRef] [PubMed]
- Raganati, F.; Alfe, M.; Gargiulo, V.; Chirone, R.; Ammendola, P. Kinetic study and breakthrough analysis of the hybrid physical/chemical CO2 adsorption/desorption behavior of a magnetite-based sorbent. Chem. Eng. J. 2019, 372, 526–535. [Google Scholar] [CrossRef]
- Manchisi, J.; Matinde, E.; Rowson, N.A.; Simmons, M.J.; Simate, G.S.; Ndlovu, S.; Mwewa, B. Ironmaking and steelmaking slags as sustainable adsorbents for industrial effluents and wastewater treatment: A critical review of properties, performance, challenges and opportunities. Sustainability 2020, 12, 2118. [Google Scholar] [CrossRef]
- Gaikwad, S.; Kim, Y.; Gaikwad, R.; Han, S. Enhanced CO2 capture capacity of amine-functionalized MOF-177 metal organic framework. J. Environ. Chem. Eng. 2021, 9, 105523. [Google Scholar] [CrossRef]
- Hussein, A.; Burra, K.; Bassioni, G.; Hammouda, R.; Gupta, A. Production of CO from CO2 over mixed-metal oxides derived from layered-double-hydroxides. Appl. Energy 2019, 235, 1183–1191. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Bowen, C.; Zhang, P.; Li, Z.; Yuan, Q.; Ren, X.; Deng, L. Spinel photocatalysts for environmental remediation, hydrogen generation, CO2 reduction and photoelectrochemical water splitting. J. Mater. Chem. A 2018, 6, 11078–11104. [Google Scholar] [CrossRef]
- Nikolaeva, N.V.; Aleksandrova, T.N.; Chanturiya, E.L.; Afanasova, A. Mineral and technological features of magnetite–hematite ores and their influence on the choice of processing technology. ACS Omega 2021, 6, 9077–9085. [Google Scholar] [CrossRef] [PubMed]
- Cabello, A.; Abad, A.; García-Labiano, F.; Gayán, P.; De Diego, L.; Adánez, J. Kinetic determination of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for use in gas-fueled Chemical Looping Combustion. Chem. Eng. J. 2014, 258, 265–280. [Google Scholar] [CrossRef]
- Enoch, K.; Sundaram, A.; Ponraj, S.S.; Sathya, P.; George, S.D.B.; Kumar, M.R. Enhancement of MXene optical properties towards medical applications via metal oxide incorporation. Nanoscale 2023, 15, 16874–16889. [Google Scholar] [CrossRef]
- Zhang, S.; Saha, C.; Yang, Y.; Bhattacharya, S.; Xiao, R. Use of Fe2O3-containing industrial wastes as the oxygen carrier for chemical-looping combustion of coal: Effects of pressure and cycles. Energy Fuels 2011, 25, 4357–4366. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Xiao, J.; Zhang, S.; Song, T. Chemical looping combustion of biomass/coal with natural iron ore as oxygen carrier in a continuous reactor. Energy Fuels 2011, 25, 446–455. [Google Scholar] [CrossRef]
- Song, T.; Shen, T.; Shen, L.; Xiao, J.; Gu, H.; Zhang, S. Evaluation of hematite oxygen carrier in chemical-looping combustion of coal. Fuel 2013, 104, 244–252. [Google Scholar] [CrossRef]
- Pawar, A.A.; Bandal, H.A.; Kim, H. Spinel type Fe3O4 polyhedron supported on nickel foam as an electrocatalyst for water oxidation reaction. J. Alloys Compd. 2021, 863, 158742. [Google Scholar] [CrossRef]
- Santos-Carballal, D.; Roldan, A.; Dzade, N.Y.; De Leeuw, N.H. Reactivity of CO2 on the surfaces of magnetite (Fe3O4), greigite (Fe3S4) and mackinawite (FeS). Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170065. [Google Scholar] [CrossRef]
- Zhu, H.; Ren, X.; Yang, X.; Liang, X.; Liu, A.; Wu, G. Fe-based catalysts for nitrogen reduction toward ammonia electrosynthesis under ambient conditions. SusMat 2022, 2, 214–242. [Google Scholar] [CrossRef]
- Raza, S.; Orooji, Y.; Ghasali, E.; Hayat, A.; Karimi-Maleh, H.; Lin, H. Engineering approaches for CO2 converting to biomass coupled with nanobiomaterials as biomediated towards circular bioeconomy. J. CO2 Util. 2023, 67, 102295. [Google Scholar] [CrossRef]
- Shan, J.; Wang, L.; Yu, H.; Ji, J.; Amer, W.; Chen, Y.; Jing, G.; Khalid, H.; Akram, M.; Abbasi, N. Recent progress in Fe3O4 based magnetic nanoparticles: From synthesis to application. Mater. Sci. Technol. 2016, 32, 602–614. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.W.; Ahn, H.; Kang, Y.T. Development of novel nanoabsorbents by amine functionalization of Fe3O4 with intermediate ascorbic acid coating for CO2 capture enhancement. J. CO2 Util. 2022, 65, 102228. [Google Scholar] [CrossRef]
- Yu, W.; Wang, T.; Park, A.-H.A.; Fang, M. Review of liquid nano-absorbents for enhanced CO2 capture. Nanoscale 2019, 11, 17137–17156. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.; Van Wachem, B.; Kuster, B.; Schouten, J. Mass transfer in sparged and stirred reactors: Influence of carbon particles and electrolyte. Chem. Eng. Sci. 2003, 58, 4719–4728. [Google Scholar] [CrossRef]
- Li, L.; Kang, Y.T. Enhancement mechanisms of mass transfer performance by nanoabsorbents during CO2 absorption process. Int. J. Heat Mass Transf. 2021, 164, 120444. [Google Scholar] [CrossRef]
- Kars, R.; Best, R.; Drinkenburg, A. The sorption of propane in slurries of active carbon in water. Chem. Eng. J. 1979, 17, 201–210. [Google Scholar] [CrossRef]
- Kim, W.-g.; Kang, H.U.; Jung, K.-m.; Kim, S.H. Synthesis of silica nanofluid and application to CO2 absorption. Sep. Sci. Technol. 2008, 43, 3036–3055. [Google Scholar] [CrossRef]
- Li, L.; Kang, Y.T. Effects of bubble coalescence and breakup on CO2 absorption performance in nanoabsorbents. J. CO2 Util. 2020, 39, 101170. [Google Scholar] [CrossRef]
- Abad, A.; de Las Obras-Loscertales, M.; García-Labiano, F.; de Diego, L.; Gayán, P.; Adánez, J. In situ gasification Chemical-Looping Combustion of coal using limestone as oxygen carrier precursor and sulphur sorbent. Chem. Eng. J. 2017, 310, 226–239. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Abad, A.; Gayán, P.; de Diego, L.; García-Labiano, F.; Adánez, J. Development of (Mn0.77Fe0.23)2O3 particles as an oxygen carrier for coal combustion with CO2 capture via in-situ gasification chemical looping combustion (iG-CLC) aided by oxygen uncoupling (CLOU). Fuel Process. Technol. 2017, 164, 69–79. [Google Scholar] [CrossRef]
- Chen, J.L.; Dong, X.Y.M.; Shi, C.L.; Li, S.H.; Wang, Y.; Zhu, J.H. Fabrication of strong solid base FeO–MgO for warm CO2 capture. CLEAN–Soil Air Water 2019, 47, 1800447. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Reduction of iron oxides with hydrogen—A review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Kim, S.-H.; Zhang, X.; Ma, Y.; Souza Filho, I.R.; Schweinar, K.; Angenendt, K.; Vogel, D.; Stephenson, L.T.; El-Zoka, A.A.; Mianroodi, J.R. Influence of microstructure and atomic-scale chemistry on the direct reduction of iron ore with hydrogen at 700 C. Acta Mater. 2021, 212, 116933. [Google Scholar] [CrossRef]
- Cavaliere, P. Hydrogen Ironmaking. In Hydrogen Assisted Direct Reduction of Iron Oxides; Springer: Berlin/Heidelberg, Germany, 2022; pp. 131–183. [Google Scholar]
- Akiyama, T.; Ohta, H.; Takahashi, R.; Waseda, Y.; Yagi, J.-I. Measurement and modeling of thermal conductivity for dense iron oxide and porous iron ore agglomerates in stepwise reduction. ISIJ Int. 1992, 32, 829–837. [Google Scholar] [CrossRef]
- Hakim, A.; Marliza, T.S.; Abu Tahari, N.M.; Wan Isahak, R.W.; Yusop, R.M.; Mohamed Hisham, W.M.; Yarmo, A.M. Studies on CO2 adsorption and desorption properties from various types of iron oxides (FeO, Fe2O3, and Fe3O4). Ind. Eng. Chem. Res. 2016, 55, 7888–7897. [Google Scholar] [CrossRef]
- Sarkar, A.; Chavan, V.; Pai, N.; Prakash, A.; Hazra, B.; Raut, P.; Sunilkumar, D.; Sivananda, C.; Kundu, S.; Nag, S. Reduction of Iron Ore Pellets: A Microstructural Perspective? Metall. Mater. Trans. A 2023, 55, 537–549. [Google Scholar] [CrossRef]
- Forsmo, S.; Forsmo, S.-E.; Samskog, P.-O.; Björkman, B. Mechanisms in oxidation and sintering of magnetite iron ore green pellets. Powder Technol. 2008, 183, 247–259. [Google Scholar] [CrossRef]
- Zare Ghadi, A.; Valipour, M.S.; Vahedi, S.M.; Sohn, H.Y. A review on the modeling of gaseous reduction of iron oxide pellets. Steel Res. Int. 2020, 91, 1900270. [Google Scholar] [CrossRef]
- Turkdogan, E.; Vinters, J. Reducibility of iron ore pellets and effect of additions. Can. Metall. Q. 1973, 12, 9–21. [Google Scholar] [CrossRef]
- Hayes, P. The kinetics of formation of H2O and CO2 during iron oxide reduction. Metall. Trans. B 1979, 10, 211–217. [Google Scholar] [CrossRef]
- Joseph, T. Porosity, reducibility and size preparation of iron ores. Trans. AIME 1936, 120, 72–89. [Google Scholar]
- El-Geassy, A.; Nasr, M. Influence of the original structure on the kinetics of hydrogen reduction of hematite compacts. Trans. Iron Steel Inst. Jpn. 1988, 28, 650–658. [Google Scholar] [CrossRef]
- Teplov, O. Kinetics of the low-temperature hydrogen reduction of magnetite concentrates. Russ. Metall. (Met.) 2012, 2012, 8–21. [Google Scholar] [CrossRef]
- Kapelyushin, Y.; Xing, X.; Zhang, J.; Jeong, S.; Sasaki, Y.; Ostrovski, O. Effect of alumina on the gaseous reduction of magnetite in CO/CO2 gas mixtures. Metall. Mater. Trans. B 2015, 46, 1175–1185. [Google Scholar] [CrossRef]
- Kapelyushin, Y.; Sasaki, Y.; Zhang, J.; Jeong, S.; Ostrovski, O. Formation of a network structure in the gaseous reduction of magnetite doped with alumina. Metall. Mater. Trans. B 2017, 48, 889–899. [Google Scholar] [CrossRef]
- Paananen, T.; Heinänen, K.; Härkki, J. Degradation of iron oxide caused by alumina during reduction from magnetite. ISIJ Int. 2003, 43, 597–605. [Google Scholar] [CrossRef]
- Pal, J. Innovative development on agglomeration of iron ore fines and iron oxide wastes. Miner. Process. Extr. Metall. Rev. 2018, 40, 248–264. [Google Scholar] [CrossRef]
- Cores, A.; Babich, A.; Muñiz, M.; Ferreira, S.; Mochon, J. The influence of different iron ores mixtures composition on the quality of sinter. ISIJ Int. 2010, 50, 1089–1098. [Google Scholar] [CrossRef]
- Hsieh, L.-H.; JA, W. Effect of oxygen potential on mineral formation in lime-fluxed iron ore sinter. ISIJ Int. 1989, 29, 625–634. [Google Scholar] [CrossRef]
- Mazanek, E.; Wyderko, M. Zur Optimierung der Eigenschaften von Eisenerzsintern. Arch. Für Das Eisenhüttenwesen 1976, 47, 457–463. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Gao, Z.; Fu, J.; Ao, W.; Dai, J. CO2 capture with chemical looping combustion of gaseous fuels: An overview. Energy Fuels 2017, 31, 3475–3524. [Google Scholar] [CrossRef]
- Munteanu, G.; Ilieva, L.; Andreeva, D. TPR data regarding the effect of sulfur on the reducibility of α-Fe2O3. Thermochim. Acta 1999, 329, 157–162. [Google Scholar] [CrossRef]
- Munteanu, G.; Ilieva, L.; Andreeva, D. Kinetic parameters obtained from TPR data for α-Fe2O3 and Auα-Fe2O3 systems. Thermochim. Acta 1997, 291, 171–177. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chen, Y.-W.; Li, C. The mechanism of reduction of iron oxide by hydrogen. Thermochim. Acta 2003, 400, 61–67. [Google Scholar] [CrossRef]
- Jozwiak, W.; Kaczmarek, E.; Maniecki, T.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Sastri, M.; Viswanath, R.; Viswanathan, B. Studies on the reduction of iron oxide with hydrogen. Int. J. Hydrogen Energy 1982, 7, 951–955. [Google Scholar] [CrossRef]
- Abd Elhamid, M.; Khader, M.; Mahgoub, A.; El Anadouli, B.; Ateya, B. Autocatalytic reduction of hematite with hydrogen under conditions of surface control: A vacancy-based mechanism. J. Solid State Chem. 1996, 123, 249–254. [Google Scholar] [CrossRef]
- Barde, A.A.; Klausner, J.F.; Mei, R. Solid state reaction kinetics of iron oxide reduction using hydrogen as a reducing agent. Int. J. Hydrogen Energy 2016, 41, 10103–10119. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Choi, J.-P.; Song, J.-I.; Jung, S.-S.; Lee, J.-S. The kinetics of isothermal hydrogen reduction of nanocrystalline Fe2O3 powder. Mater. Trans. 2014, 55, 1611–1617. [Google Scholar] [CrossRef]
- Kuila, S.K.; Chaudhuri, S.; Chatterjee, R.; Ghosh, D. In Reduction of magnetite ore fines with hydrogen. In Proceedings of the 4th International Conference on Chemical Engineering, Dhaka, Bangladesh, 29–30 December 2014; Chemical Engineering Department, BUET: Dhaka, Bangladesh, 2014; p. 81. [Google Scholar]
- Kuila, S.K.; Chatterjee, R.; Ghosh, D. Kinetics of hydrogen reduction of magnetite ore fines. Int. J. Hydrogen Energy 2016, 41, 9256–9266. [Google Scholar] [CrossRef]
- Feilmayr, C.; Thurnhofer, A.; Winter, F.; Mali, H.; Schenk, J. Reduction behavior of hematite to magnetite under fluidized bed conditions. ISIJ Int. 2004, 44, 1125–1133. [Google Scholar] [CrossRef]
- Swimm, K.; Reichenauer, G.; Vidi, S.; Ebert, H.-P. Gas pressure dependence of the heat transport in porous solids with pores smaller than 10 μm. Int. J. Thermophys. 2009, 30, 1329–1342. [Google Scholar] [CrossRef]
- Du, Z.; Liu, J.; Liu, F.; Pan, F. Relationship of particle size, reaction and sticking behavior of iron ore fines toward efficient fluidized bed reduction. Chem. Eng. J. 2022, 447, 137588. [Google Scholar] [CrossRef]
- Komatina, M.; Gudenau, H.W. The sticking problem during direct reduction of fine iron ore in the fluidized bed. Metall. Mater. Eng. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chung, U.-C.; Lee, I.-O.; Kim, H.-G.; Sahajwalla, V.; Chung, W.-B. Degradation characteristics of iron ore fines of a wide size distribution in fluidized-bed reduction. ISIJ Int. 1998, 38, 943–952. [Google Scholar] [CrossRef]
- Corbari, R.; Fruehan, R. Reduction of iron oxide fines to wustite with CO/CO2 gas of low reducing potential. Metall. Mater. Trans. B 2010, 41, 318–329. [Google Scholar] [CrossRef]
- Gudenau, H. Materialsammlung Zum Praktikum Metallurgie; Trans-Aix-Press: Aachen, Germany, 2002. [Google Scholar]
- Qu, Y.; Xing, L.; Shao, L.; Luo, Y.; Zou, Z. Microstructural characterization and gas-solid reduction kinetics of iron ore fines at high temperature. Powder Technol. 2019, 355, 26–36. [Google Scholar] [CrossRef]
- Wolfinger, T.; Spreitzer, D.; Zheng, H.; Schenk, J. Influence of a prior oxidation on the reduction behavior of magnetite iron ore ultra-fines using hydrogen. Metall. Mater. Trans. B 2022, 53, 14–28. [Google Scholar] [CrossRef]
- Sato, K.; Ueda, Y.; Nishikawa, Y.; Goto, T. Effect of Pressure on Reduction Rate of Iron Ore with High Pressure Fluidized Bed. Trans. Iron Steel Inst. Jpn. 1986, 26, 697–703. [Google Scholar] [CrossRef]
- Bahgat, M.; Khedr, M. Reduction kinetics, magnetic behavior and morphological changes during reduction of magnetite single crystal. Mater. Sci. Eng. B 2007, 138, 251–258. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, Z.; Chen, Z.; Yu, W.; Yue, J. Multistep reduction kinetics of fine iron ore with carbon monoxide in a micro fluidized bed reaction analyzer. Metall. Mater. Trans. B 2017, 48, 841–852. [Google Scholar] [CrossRef]
- El-Rahaiby, S.; Rao, Y. The kinetics of reduction of iron oxides at moderate temperatures. Metall. Trans. B 1979, 10, 257–269. [Google Scholar] [CrossRef]
- Bonalde, A.; Henriquez, A.; Manrique, M. Kinetic analysis of the iron oxide reduction using hydrogen-carbon monoxide mixtures as reducing agent. ISIJ Int. 2005, 45, 1255–1260. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Zhang, J.; Liu, Z.; Jiao, K.; Liu, X.; Wang, R. Study on the controlling steps and reduction kinetics of iron oxide briquettes with CO-H2 mixtures. Metall. Res. Technol. 2017, 114, 611. [Google Scholar] [CrossRef]
- Tahari, M.N.A.; Salleh, F.; Saharuddin, T.S.T.; Samsuri, A.; Samidin, S.; Yarmo, M.A. Influence of hydrogen and carbon monoxide on reduction behavior of iron oxide at high temperature: Effect on reduction gas concentrations. Int. J. Hydrogen Energy 2021, 46, 24791–24805. [Google Scholar] [CrossRef]
- Kawasaki, E.; Sanscrainte, J.; Walsh, T.J. Kinetics of reduction of iron oxide with carbon monoxide and hydrogen. AIChE J. 1962, 8, 48–52. [Google Scholar] [CrossRef]
- Zuo, H.-B.; Wang, C.; Dong, J.-J.; Jiao, K.-X.; Xu, R.-S. Reduction kinetics of iron oxide pellets with H2 and CO mixtures. Int. J. Miner. Metall. Mater. 2015, 22, 688–696. [Google Scholar] [CrossRef]
- Baolin, H.; Zhang, H.; Hongzhong, L.; Qingshan, Z. Study on kinetics of iron oxide reduction by hydrogen. Chin. J. Chem. Eng. 2012, 20, 10–17. [Google Scholar]
- Hammam, A.; Li, Y.; Nie, H.; Zan, L.; Ding, W.; Ge, Y.; Li, M.; Omran, M.; Yu, Y. Isothermal and non-isothermal reduction behaviors of iron ore compacts in pure hydrogen atmosphere and kinetic analysis. Min. Metall. Explor. 2021, 38, 81–93. [Google Scholar] [CrossRef]
- Moradmand, S.; Allen, J. Magnetic carbon formation via in-situ CO2 capture and electrolysis in a molten carbonate system. Mater. Today Sustain. 2024, 25, 100645. [Google Scholar] [CrossRef]
- Ouyang, T.; Jin, B.; Mao, Y.; Wei, D.; Liang, Z. Control of strong electronic oxide-support interaction in iron-based redox catalysts for highly efficient chemical looping CO2 conversion. Appl. Catal. B Environ. 2024, 343, 123531. [Google Scholar] [CrossRef]
- Rao, Q.; Zhang, J.; Yang, T.; Li, Y.; Gai, Z.; Li, P.; Wang, X.; Pan, Y.; Jin, H. A nickel-modified perovskite-supported iron oxide oxygen carrier for chemical looping dry reforming of methane for syngas production. Chem. Eng. J. 2024, 485, 150033. [Google Scholar] [CrossRef]
- Saqline, S.; Wang, H.; Fan, Q.; Donat, F.; Müller, C.; Liu, W. Investigation of barium iron oxides for CO2 capture and chemical looping oxygen uncoupling. Appl. Energy Combust. Sci. 2024, 17, 100238. [Google Scholar] [CrossRef]
- Guo, D.; Hu, M.; Pu, C.; Xiao, B.; Hu, Z.; Liu, S.; Wang, X.; Zhu, X. Kinetics and mechanisms of direct reduction of iron ore-biomass composite pellets with hydrogen gas. Int. J. Hydrogen Energy 2015, 40, 4733–4740. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Rajakumar, V. Gaseous reduction of wustite with H2, CO and H2-CO mixtures. Trans. Iron Steel Inst. Jpn. 1985, 25, 449–458. [Google Scholar] [CrossRef]
- El-Geassy, A.-H.A. In Rate Controlling Step in the Reduction of Iron Oxides; Kinetics and Mechanism of Wüstite-Iron Step in H2, CO and H2/CO Gas Mixtures; IOP Conference Series: Materials Science and Engineering, 2017; IOP Publishing: Bristol, UK, 2017; p. 012002. [Google Scholar]

| Operative Conditions | Reduction Reaction | Ea (kJ/mol) | Reference |
|---|---|---|---|
| Non-Isothermal with H2 | Fe2O3 → Fe3O4 | 246 | [115] |
| Fe3O4 → Fe | 93.2 | ||
| Fe2O3 → Fe3O4 | 162.1 | ||
| Fe3O4 → Fe | 103.6 | ||
| Fe2O3 → Fe3O4 | 139.2 | [116] | |
| Fe3O4 → FeO | 77.3 | ||
| FeO → Fe | 85.7 | ||
| Fe2O3 → Fe3O4 | 89.1 | [117] | |
| Fe3O4 → Fe | 70.4 | ||
| FeO → Fe | 104.0 | [118] | |
| Isothermal with H2 | Fe2O3 → Fe | 57.1 | [119] |
| Fe2O3 → Fe | 72.7 | ||
| Fe2O3 → Fe | 89.9 | ||
| Fe2O3 → Fe3O4 | 30.1 | [120] | |
| Fe2O3 → FeO | 47.0 | [121] | |
| FeO → Fe | 30.0 | ||
| Fe2O3 → Fe | 47.2 | [122] | |
| Fe2O3 → Fe | 51.5 | ||
| Fe2O3 → FeO | 42.0 | [123] | |
| FeO → Fe | 55.0 | ||
| Fe2O3 → FeO | 33.0 | [124] | |
| FeO → Fe | 11.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabozzi, A.; Cerciello, F.; Senneca, O. Reduction of Iron Oxides for CO2 Capture Materials. Energies 2024, 17, 1673. https://doi.org/10.3390/en17071673
Fabozzi A, Cerciello F, Senneca O. Reduction of Iron Oxides for CO2 Capture Materials. Energies. 2024; 17(7):1673. https://doi.org/10.3390/en17071673
Chicago/Turabian StyleFabozzi, Antonio, Francesca Cerciello, and Osvalda Senneca. 2024. "Reduction of Iron Oxides for CO2 Capture Materials" Energies 17, no. 7: 1673. https://doi.org/10.3390/en17071673
APA StyleFabozzi, A., Cerciello, F., & Senneca, O. (2024). Reduction of Iron Oxides for CO2 Capture Materials. Energies, 17(7), 1673. https://doi.org/10.3390/en17071673








