A Comprehensive Review of Bimetallic Nanoparticle–Graphene Oxide and Bimetallic Nanoparticle–Metal–Organic Framework Nanocomposites as Photo-, Electro-, and Photoelectrocatalysts for Hydrogen Evolution Reaction
Abstract
1. Introduction
1.1. Production of Hydrogen
1.1.1. Fossil Fuels
- (1)
- Steam Methane Reforming (SMR)
- (2)
- Partial Oxidation
1.1.2. Biomass
1.1.3. Thermochemical Water-Splitting Cycle
1.1.4. Photolysis
1.1.5. Electrolysis
Photovoltaic Electrolysis
Hydrogen Evolution Reaction (HER)
Electrocatalytic HERs
Photocatalytic HERs
Photoelectrocatalytic HERs
2. Graphene Oxide (GO)
2.1. GO Structure
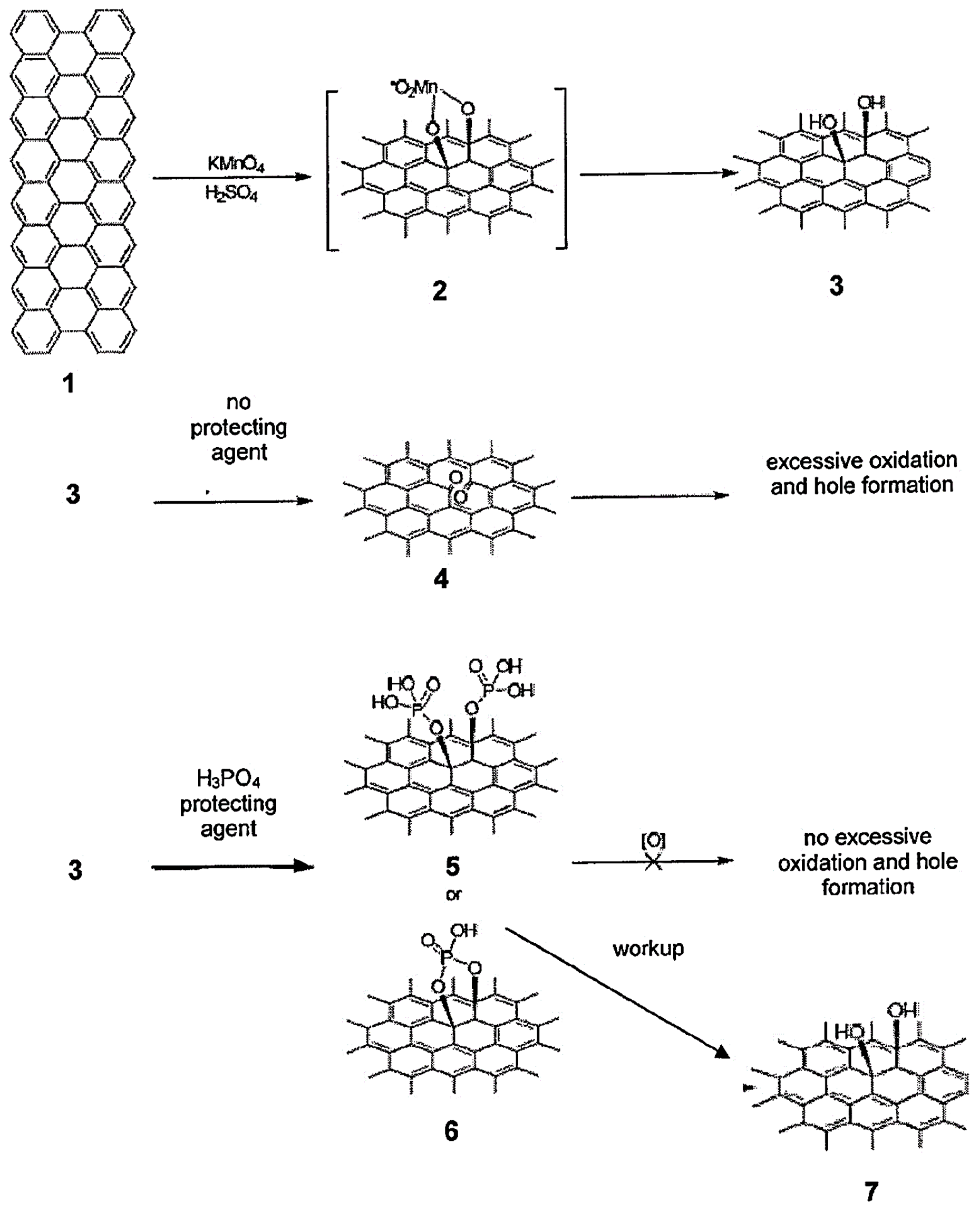
2.2. Synthesis of GO
2.3. GO-Based Materials for HERs
| Material | H2 Source in Electrolyte | Tafel Slope mV.dec−1 | Current Density (io) mA.cm−2 | Ref. |
|---|---|---|---|---|
| GGNR@MoS2 | 0.5 M H2SO4 | 49 | 10.0 | [51] |
| NFO–RGO | 0.5 M H2SO4 | 58 | 25.2 × 10−2 | [88] |
| CFG | 0.5 M H2SO4 | 116.6 | 47.9 | [89] |
| NFG | 0.5 M H2SO4 | 121.4 | 41.2 | [89] |
| rGO–Au48Pd52 | 0.5 M H2SO4 | 149 | - | [90] |
| Pd NPs–GO | 0.5 M H2SO4 | - | 5.2 | [95] |
2.4. Bimetallic Nanoparticle–GO-Based Materials for HERs
3. Metal–Organic Frameworks (MOFs)
3.1. Structure of MOFs
3.1.1. Organic Ligands
3.1.2. Metal Sites
3.1.3. Secondary Building Units (SBUs)
3.1.4. Pores in MOFs
3.1.5. Intrinsic Properties/Features of MOFs
- i.
- Light-harvesting capability
- ii.
- Large surface area and active sites
- iii.
- Tunable band structure
- iv.
- Efficient charge separation and transport
- v.
- Chemical stability under illumination
- vi.
- Synergy between metal ions and organic ligands
- vii.
- Tailorable porosity and surface chemistry
3.2. Synthesis of MOFs
3.2.1. Microwave-Assisted Synthesis
3.2.2. Mechanochemical Synthesis
3.2.3. Electrochemical Synthesis
3.2.4. Solvo/Hydrothermal Synthesis
3.2.5. Epitaxial Growth Method
- i.
- Choice of Substrate
- ii.
- Preparation of Substrate
- iii.
- Introduction of Precursor Solution
- iv.
- Adsorption and Nucleation
- v.
- Epitaxial Growth
- vi.
- Controlled Growth Conditions
- vii.
- Post-Synthesis Treatment
- viii.
- Characterization and Optimization
3.2.6. In Situ Growth Method
- i.
- Substrate Preparation
- ii.
- Deposition of Reactants
- iii.
- MOF Formation and Growth
- iv.
- Film Post-Treatment
3.3. MOF-Based Materials as HER Electrocatalysts
3.4. Bimetallic Nanoparticle–MOF-Based Materials for HERs
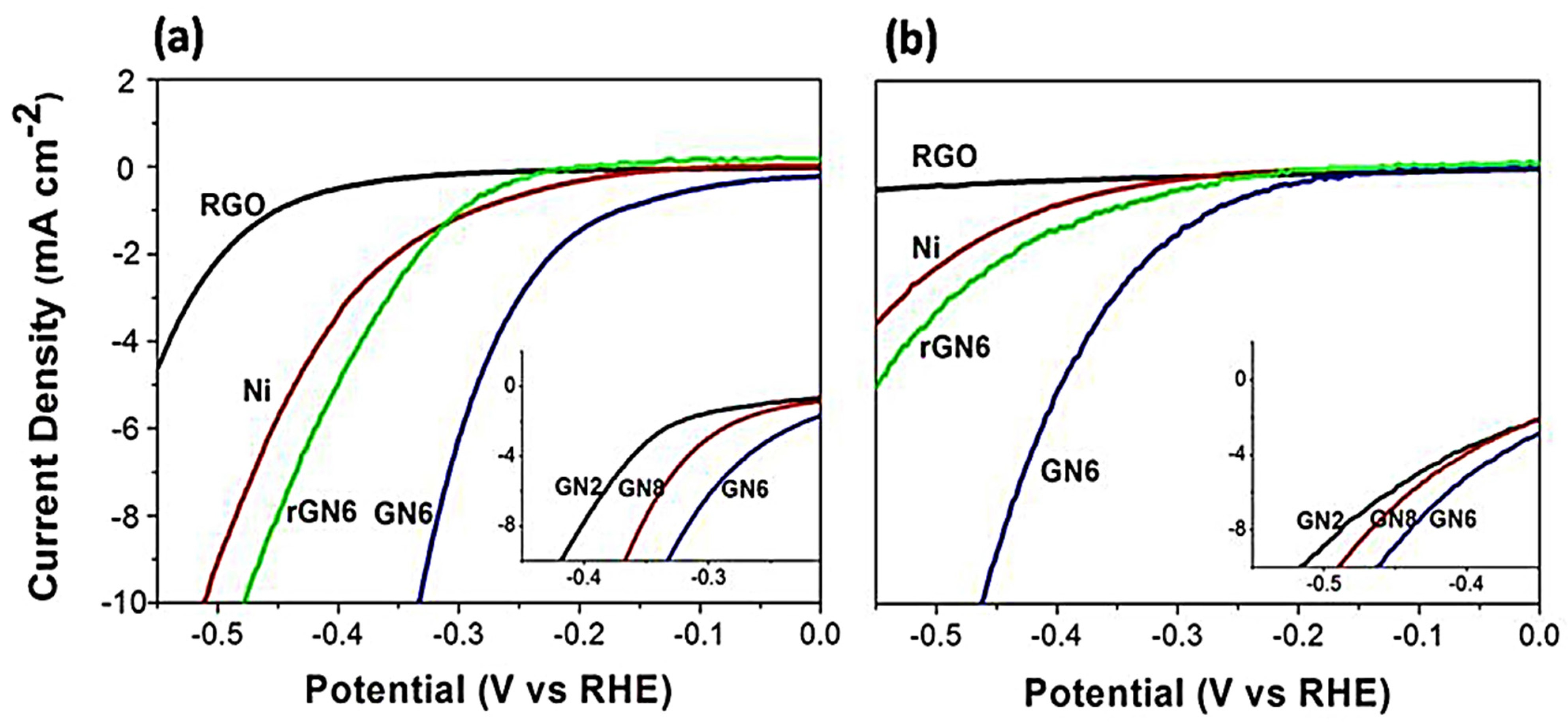
3.5. Influence of Bimetallic Particle Size on Catalytic Performance
4. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Laha, P.; Chakraborty, B. Energy model—A tool for preventing energy dysfunction. Renew. Sustain. Energy Rev. 2017, 73, 95–114. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- AbouSeada, N.; Hatem, T.M. Climate action: Prospects of green hydrogen in Africa. Energy Rep. 2022, 8, 3873–3890. [Google Scholar] [CrossRef]
- Lebrouhi, B.E.; Djoupo, J.J.; Lamrani, B.; Benabdelaziz, K.; Kousksou, T. Global hydrogen development—A technological and geopolitical overview. Int. J. Hydrogen Energy 2022, 47, 7016–7048. [Google Scholar] [CrossRef]
- Fayaz, H.; Saidur, R.; Razali, N.; Anuar, F.S.; Saleman, A.; Islam, M. An overview of hydrogen as a vehicle fuel. Renew. Sustain. Energy Rev. 2012, 16, 5511–5528. [Google Scholar] [CrossRef]
- O’Malley, K.; Ordaz, G.; Adams, J.; Randolph, K.; Ahn, C.C.; Stetson, N.T. Applied hydrogen storage research and development: A perspective from the US Department of Energy. J. Alloys Compd. 2015, 645, S419–S422. [Google Scholar] [CrossRef]
- Schoots, K.; Ferioli, F.; Kramer, G.; Van der Zwaan, B. Learning curves for hydrogen production technology: An assessment of observed cost reductions. Int. J. Hydrogen Energy 2008, 33, 2630–2645. [Google Scholar] [CrossRef]
- Kler, A.M.; Tyurina, E.A.; Potanina, Y.M.; Mednikov, A.S. Estimation of efficiency of using hydrogen and aluminum as environmentally-friendly energy carriers. Int. J. Hydrogen Energy 2015, 40, 14775–14783. [Google Scholar] [CrossRef]
- Sharma, A.; Arya, S.K. Hydrogen from algal biomass: A review of production process. Biotechnol. Rep. 2017, 15, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Burnette, D.D.; Kremer, G.G.; Bayless, D.J. The use of hydrogen-depleted coal syngas in solid oxide fuel cells. J. Power Sources 2008, 182, 329–333. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Lund, H. Renewable energy strategies for sustainable development. Energy 2007, 32, 912–919. [Google Scholar] [CrossRef]
- Lund, H.; Mathiesen, B.V. Energy system analysis of 100% renewable energy systems—The case of Denmark in years 2030 and 2050. Energy 2009, 34, 524–531. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef]
- Asif, M.; Muneer, T. Energy supply, its demand and security issues for developed and emerging economies. Renew. Sustain. Energy Rev. 2007, 11, 1388–1413. [Google Scholar] [CrossRef]
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroglu, T. Current status of hydrogen energy. Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- Dinçer, İ.; Ishaq, H. Renewable Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Safari, F.; Dincer, I. A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Habibi, R. A review on hydrogen production thermochemical water-splitting cycles. J. Clean. Prod. 2020, 275, 123836. [Google Scholar] [CrossRef]
- Khan, J.; Sun, Y.; Han, L. A Comprehensive Review on Graphitic Carbon Nitride for Carbon Dioxide Photoreduction. Small Methods 2022, 6, e2201013. [Google Scholar] [CrossRef] [PubMed]
- Lianos, P. Production of electricity and hydrogen by photocatalytic degradation of organic wastes in a photoelectrochemical cell: The concept of the Photofuelcell: A review of a re-emerging research field. J. Hazard. Mater. 2011, 185, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Llorca, J.; Corberán, V.C.; Divins, N.J.; Fraile, R.O.; Taboada, E. Hydrogen from Bioethanol. In Renewable Hydrogen Technologies; Elsevier: Amsterdam, The Netherlands, 2013; pp. 135–169. [Google Scholar]
- Roeb, M.; Neises, M.; Monnerie, N.; Call, F.; Simon, H.; Sattler, C.; Schmücker, M.; Pitz-Paal, R. Materials-Related Aspects of Thermochemical Water and Carbon Dioxide Splitting: A Review. Materials 2012, 5, 2015–2054. [Google Scholar] [CrossRef]
- Oudejans, D.; Offidani, M.; Constantinou, A.; Albonetti, S.; Dimitratos, N.; Bansode, A. A Comprehensive Review on Two-Step Thermochemical Water Splitting for Hydrogen Production in a Redox Cycle. Energies 2022, 15, 3044. [Google Scholar] [CrossRef]
- Boretti, A.; Castelletto, S. The Perspective of Thermochemical Cycles for Concentrated Solar Energy. ACS Appl. Energy Mater. 2023, 6, 11420–11428. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Patel, C.; Tripathi, P.; Vishwakarma, A.K.; Talat, M.; Soni, P.K.; Yadav, T.; Srivastava, O. Enhanced hydrogen generation by water electrolysis employing carbon nano-structure composites. Int. J. Hydrogen Energy 2018, 43, 3180–3189. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Gopal, P.; Ramesh Kumar, C.; Edwin Geo, V. Effect of solar photovoltaic and various photovoltaic air thermal systems on hydrogen generation by water electrolysis. Int. J. Hydrogen Energy 2022, 47, 3211–3223. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mehrpooya, M. A comprehensive review on coupling different types of electrolyzer to renewable energy sources. Energy 2018, 158, 632–655. [Google Scholar] [CrossRef]
- Rathore, N.; Panwar, N.L.; Yettou, F.; Gama, A. A comprehensive review of different types of solar photovoltaic cells and their applications. Int. J. Ambient Energy 2021, 42, 1200–1217. [Google Scholar] [CrossRef]
- Hussain, A.; Arif, S.M.; Aslam, M. Emerging renewable and sustainable energy technologies: State of the art. Renew. Sustain. Energy Rev. 2017, 71, 12–28. [Google Scholar] [CrossRef]
- Victoria, M.; Haegel, N.; Peters, I.M.; Sinton, R.; Jäger-Waldau, A.; del Cañizo, C.; Breyer, C.; Stocks, M.; Blakers, A.; Kaizuka, I.; et al. Solar photovoltaics is ready to power a sustainable future. Joule 2021, 5, 1041–1056. [Google Scholar] [CrossRef]
- Jesionowski, T. Influence of aminosilane surface modification and dyes adsorption on zeta potential of spherical silica particles formed in emulsion system. Colloids Surf. A Physicochem. Eng. Asp. 2003, 222, 87–94. [Google Scholar] [CrossRef]
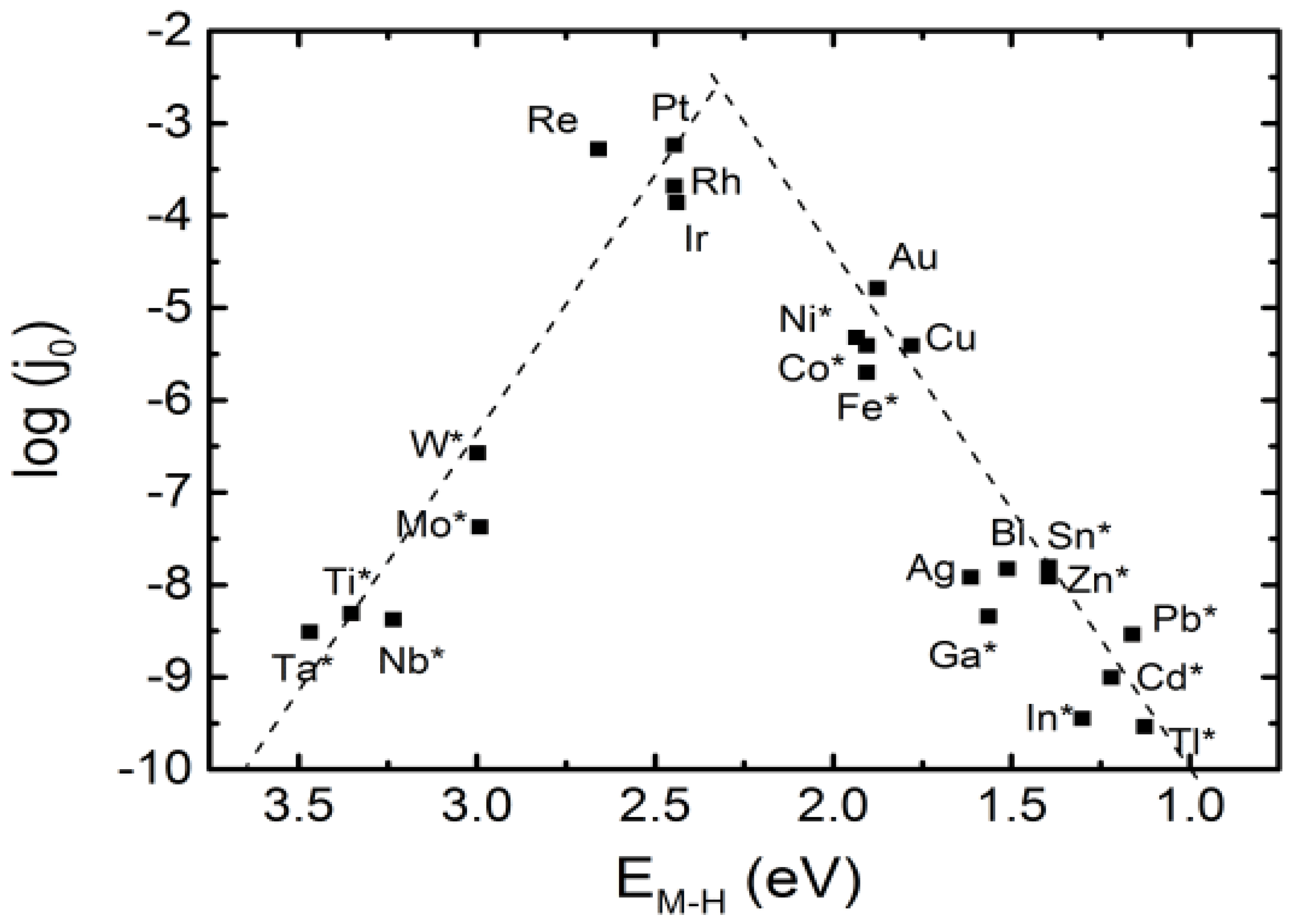
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef]
- Azizi, O.; Jafarian, M.; Gobal, F.; Heli, H.; Mahjani, M. The investigation of the kinetics and mechanism of hydrogen evolution reaction on tin. Int. J. Hydrogen Energy 2007, 32, 1755–1761. [Google Scholar] [CrossRef]
- Rosalbino, F.; Delsante, S.; Borzone, G.; Angelini, E. Electrocatalytic behaviour of Co–Ni–R (R = Rare earth metal) crystalline alloys as electrode materials for hydrogen evolution reaction in alkaline medium. Int. J. Hydrogen Energy 2008, 33, 6696–6703. [Google Scholar] [CrossRef]
- Bocutti, R.; Saeki, M.; Florentino, A.; Oliveira, C.; Angelo, A. The hydrogen evolution reaction on codeposited Ni–hydrogen storage intermetallic particles in alkaline medium. Int. J. Hydrogen Energy 2000, 25, 1051–1058. [Google Scholar] [CrossRef]
- Li, R.; Li, C. Photocatalytic water splitting on semiconductor-based photocatalysts. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2017; Volume 60, pp. 1–57. [Google Scholar]
- Schalenbach, M.; Speck, F.D.; Ledendecker, M.; Kasian, O.; Goehl, D.; Mingers, A.M.; Breitbach, B.; Springer, H.; Cherevko, S.; Mayrhofer, K.J. Nickel-molybdenum alloy catalysts for the hydrogen evolution reaction: Activity and stability revised. Electrochim. Acta 2018, 259, 1154–1161. [Google Scholar] [CrossRef]
- Seredych, M.; Rodriguez-Castellon, E.; Bandosz, T.J. New Cu x S y/nanoporous carbon composites as efficient oxygen reduction catalysts in alkaline medium. J. Mater. Chem. A 2014, 2, 20164–20176. [Google Scholar] [CrossRef]
- Karthik, P.E.; Raja, K.A.; Kumar, S.S.; Phani, K.L.N.; Liu, Y.; Guo, S.-X.; Zhang, J.; Bond, A.M. Electroless deposition of iridium oxide nanoparticles promoted by condensation of [Ir(OH)6]2− on an anodized Au surface: Application to electrocatalysis of the oxygen evolution reaction. RSC Adv. 2015, 5, 3196–3199. [Google Scholar] [CrossRef]
- Ye, F.; Xu, C.; Liu, G.; Li, J.; Wang, X.; Du, X.; Lee, J.K. A novel PtRuIr nanoclusters synthesized by selectively electrodepositing Ir on PtRu as highly active bifunctional electrocatalysts for oxygen evolution and reduction. Energy Convers. Manag. 2018, 155, 182–187. [Google Scholar] [CrossRef]
- Kempahanumakkagari, S.; Vellingiri, K.; Deep, A.; Kwon, E.E.; Bolan, N.; Kim, K.-H. Metal–organic framework composites as electrocatalysts for electrochemical sensing applications. Coord. Chem. Rev. 2018, 357, 105–129. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Esfeden, S.A.; Nadimi, S.R. Fabrication of bimetallic Cu/Pt nanoparticles modified glassy carbon electrode and its catalytic activity toward hydrogen evolution reaction. Int. J. Hydrogen Energy 2010, 35, 3937–3944. [Google Scholar] [CrossRef]
- Ania, C.O.; Seredych, M.; Rodriguez-Castellon, E.; Bandosz, T.J. New copper/GO based material as an efficient oxygen reduction catalyst in an alkaline medium: The role of unique Cu/rGO architecture. Appl. Catal. B Environ. 2015, 163, 424–435. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, W.; Liu, T. Graphene/graphene nanoribbon aerogels as tunable three-dimensional framework for efficient hydrogen evolution reaction. Electrochim. Acta 2017, 250, 91–98. [Google Scholar] [CrossRef]
- Leung, C.-F.; Chen, Y.-Z.; Yu, H.-Q.; Yiu, S.-M.; Ko, C.-C.; Lau, T.-C. Electro-and photocatalytic hydrogen generation in acetonitrile and aqueous solutions by a cobalt macrocyclic Schiff-base complex. Int. J. Hydrogen Energy 2011, 36, 11640–11645. [Google Scholar] [CrossRef]
- Gao, M.-R.; Liang, J.-X.; Zheng, Y.-R.; Xu, Y.-F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.-H. An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat. Commun. 2015, 6, 5982. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ni, D.; Yang, X.; Liu, C.; Yin, J.; Cai, K. Microwave-assisted synthesis of honeycomblike hierarchical spherical Zn-doped Ni-MOF as a high-performance battery-type supercapacitor electrode material. Electrochim. Acta 2018, 278, 114–123. [Google Scholar] [CrossRef]
- Monama, G.R.; Mdluli, S.B.; Mashao, G.; Makhafola, M.D.; Ramohlola, K.E.; Molapo, K.M.; Hato, M.J.; Makgopa, K.; Iwuoha, E.I.; Modibane, K.D. Palladium deposition on copper (II) phthalocyanine/metal organic framework composite and electrocatalytic activity of the modified electrode towards the hydrogen evolution reaction. Renew. Energy 2018, 119, 62–72. [Google Scholar] [CrossRef]
- Tymoczko, J.; Calle-Vallejo, F.; Schuhmann, W.; Bandarenka, A.S. Making the hydrogen evolution reaction in polymer electrolyte membrane electrolysers even faster. Nat. Commun. 2016, 7, 10990. [Google Scholar] [CrossRef] [PubMed]
- Ross, D. Hydrogen storage: The major technological barrier to the development of hydrogen fuel cell cars. Vacuum 2006, 80, 1084–1089. [Google Scholar] [CrossRef]
- Yilanci, A.; Dincer, I.; Ozturk, H.K. A review on solar-hydrogen/fuel cell hybrid energy systems for stationary applications. Prog. Energy Combust. Sci. 2009, 35, 231–244. [Google Scholar] [CrossRef]
- Özgür, T.; Yakaryılmaz, A.C. A review: Exergy analysis of PEM and PEM fuel cell based CHP systems. Int. J. Hydrogen Energy 2018, 43, 17993–18000. [Google Scholar] [CrossRef]
- Haile, S.M. Fuel cell materials and components. Acta Mater. 2003, 51, 5981–6000. [Google Scholar] [CrossRef]
- Lin, R.; Shen, L.; Ren, Z.; Wu, W.; Tan, Y.; Fu, H.; Zhang, J.; Wu, L. Enhanced photocatalytic hydrogen production activity via dual modification of MOF and reduced graphene oxide on CdS. Chem. Commun. 2014, 50, 8533–8535. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huo, F. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Seredych, M.; Bandosz, T.J. Revisiting the chemistry of graphite oxides and its effect on ammonia adsorption. J. Mater. Chem. 2009, 19, 9176–9185. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Dreyer, D.; Park, S.; Ruoff, R. The chemistry of grapheme oxide. Chem. Soc. Rev 2010, 39, 228–240. [Google Scholar]
- Song, K. Interphase characterization in rubber nanocomposites. In Progress in Rubber Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–152. [Google Scholar]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Wang, X.; Wu, W.; Ju, H.; Zou, T.; Qiao, Z.; Gong, H.; Wang, H. Experimental and theoretical studies of the structure and optical properties of nickel phthalocyanine nanowires. Mater. Res. Express 2016, 3, 125002. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Chang, C.-T. Preparation and characterization of graphene oxide. J. Nanomater. 2014, 2014, 276143. [Google Scholar] [CrossRef]
- Rathnayake, R.; Wijayasinghe, H.; Pitawala, H.; Yoshimura, M.; Huang, H.-H. Synthesis of graphene oxide and reduced graphene oxide by needle platy natural vein graphite. Appl. Surf. Sci. 2017, 393, 309–315. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Mindivan, F. The synthesis and charecterization of graphene oxide (GO) and reduced graphene oxide (rGO). Mach. Technol. Mater. 2016, 10, 32–35. [Google Scholar]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J.-M. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and characterisation of hydrophilic and organophilic graphene nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Higginbotham, A.L.; Kosynkin, D.V.; Sinitskii, A.; Sun, Z.; Tour, J.M. Lower-defect graphene oxide nanoribbons from multiwalled carbon nanotubes. ACS Nano 2010, 4, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ma, Y.; Zhang, L.; Mu, J.; Zhang, Z.; Zhang, X.; Che, H.; Bai, Y.; Hou, J. Facile synthesis of manganese ferrite/graphene oxide nanocomposites for controlled targeted drug delivery. J. Magn. Magn. Mater. 2016, 401, 647–650. [Google Scholar] [CrossRef]
- Balogun, S.A.; Fayemi, O.E. Electrochemical Sensors for Determination of Bromate in Water and Food Samples. Biosensors 2021, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng 2014, 2, 58–63. [Google Scholar]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Maktedar, S.S.; Mehetre, S.S.; Singh, M.; Kale, R. Ultrasound irradiation: A robust approach for direct functionalization of graphene oxide with thermal and antimicrobial aspects. Ultrason. Sonochem. 2014, 21, 1407–1416. [Google Scholar] [CrossRef]
- Kumar, V.; Kim, K.-H.; Park, J.-W.; Hong, J.; Kumar, S. Graphene and its nanocomposites as a platform for environmental applications. Chem. Eng. J. 2017, 315, 210–232. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, D.; Wang, C.; Guan, J.; Bao, X. Reduced graphene oxide as a catalyst for hydrogenation of nitrobenzene at room temperature. Chem. Commun. 2011, 47, 2432–2434. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Saleem, M.; Ullah, S.; Saeed, N.; Afridi, A.; Khan, M.; Arif, M. Modified and improved Hummer’s synthesis of graphene oxide for capacitors applications. Mod. Electron. Mater. 2017, 3, 110–116. [Google Scholar] [CrossRef]
- Liu, J.; Jeong, H.; Liu, J.; Lee, K.; Park, J.-Y.; Ahn, Y.; Lee, S. Reduction of functionalized graphite oxides by trioctylphosphine in non-polar organic solvents. Carbon 2010, 48, 2282–2289. [Google Scholar] [CrossRef]
- Gupta, V.; Sharma, N.; Singh, U.; Arif, M.; Singh, A. Higher oxidation level in graphene oxide. Optik 2017, 143, 115–124. [Google Scholar] [CrossRef]
- Ganesh, B.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Z.-H.; Chen, X. Ultrafine Ni–Pt alloy nanoparticles grown on graphene as highly efficient catalyst for complete hydrogen generation from hydrazine borane. ACS Sustain. Chem. Eng. 2015, 3, 1255–1261. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chakrabarty, S.; Su, W.-N.; Basu, S. Nanostructured nickel ferrite embedded in reduced graphene oxide for electrocatalytic hydrogen evolution reaction. Mater. Today Energy 2018, 8, 118–124. [Google Scholar] [CrossRef]
- Nivetha, R.; Chella, S.; Kollu, P.; Jeong, S.K.; Bhatnagar, A.; Andrews, N.G. Cobalt and nickel ferrites based graphene nanocomposites for electrochemical hydrogen evolution. J. Magn. Magn. Mater. 2018, 448, 165–171. [Google Scholar] [CrossRef]
- Cardoso, J.; Amaral, L.; Metin, Ö.; Cardoso, D.; Sevim, M.; Sener, T.; Sequeira, C.; Santos, D. Reduced graphene oxide assembled Pd-based nanoalloys for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 3916–3925. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Zeng, X.; Peng, S. Synergetic effect of metal nickel and graphene as a cocatalyst for enhanced photocatalytic hydrogen evolution via dye sensitization. Sci. Rep. 2015, 5, 10589. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xing, W.; Zhang, L.; Zhuo, S.; Zhou, J.; Wang, G.; Qiao, S. Nickel nanoparticles prepared by hydrazine hydrate reduction and their application in supercapacitor. Powder Technol. 2012, 224, 162–167. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Alfè, M.; Gargiulo, V.; Lisi, L.; Di Capua, R. Synthesis and characterization of conductive copper-based metal-organic framework/graphene-like composites. Mater. Chem. Phys. 2014, 147, 744–750. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Chen, J.; Chen, X.; Xie, Z.; Wang, X. Synthesis of “clean” and well-dispersive Pd nanoparticles with excellent electrocatalytic property on graphene oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695. [Google Scholar] [CrossRef] [PubMed]
- Darabdhara, G.; Amin, M.A.; Mersal, G.A.; Ahmed, E.M.; Das, M.R.; Zakaria, M.B.; Malgras, V.; Alshehri, S.M.; Yamauchi, Y.; Szunerits, S. Reduced graphene oxide nanosheets decorated with Au, Pd and Au–Pd bimetallic nanoparticles as highly efficient catalysts for electrochemical hydrogen generation. J. Mater. Chem. A 2015, 3, 20254–20266. [Google Scholar] [CrossRef]
- Al-Nayili, A.; Albdiry, M. AuPd bimetallic nanoparticles supported on reduced graphene oxide nanosheets as catalysts for hydrogen generation from formic acid under ambient temperature. New J. Chem. 2021, 45, 10040–10048. [Google Scholar] [CrossRef]
- Rakočević, L.; Srejić, I.; Maksić, A.; Golubović, J.; Štrbac, S. Hydrogen evolution on reduced graphene oxide-supported PdAu nanoparticles. Catalysts 2021, 11, 481. [Google Scholar] [CrossRef]
- Song, F.Z.; Zhu, Q.L.; Yang, X.; Zhan, W.W.; Pachfule, P.; Tsumori, N.; Xu, Q. Hydrogen Generation: Metal–Organic Framework Templated Porous Carbon-Metal Oxide/Reduced Graphene Oxide as Superior Support of Bimetallic Nanoparticles for Efficient Hydrogen Generation from Formic Acid. Adv. Energy Mater. 2018, 8, 1770139. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Nasif, O.; Ali Alharbi, S.; Chinni, S.V.; Reddy, L.V.; Reddy, M.R.V.; Sreeramanan, S. Phytogenic synthesis of Pd-Ag/rGO nanostructures using stevia leaf extract for photocatalytic H2 production and antibacterial studies. Biomolecules 2021, 11, 190. [Google Scholar] [CrossRef]
- Darabdhara, G.; Das, M.R.; Amin, M.A.; Mersal, G.A.; Mostafa, N.Y.; Abd El-Rehim, S.S.; Szunerits, S.; Boukherroub, R. AuNi alloy nanoparticles supported on reduced graphene oxide as highly efficient electrocatalysts for hydrogen evolution and oxygen reduction reactions. Int. J. Hydrogen Energy 2018, 43, 1424–1438. [Google Scholar] [CrossRef]
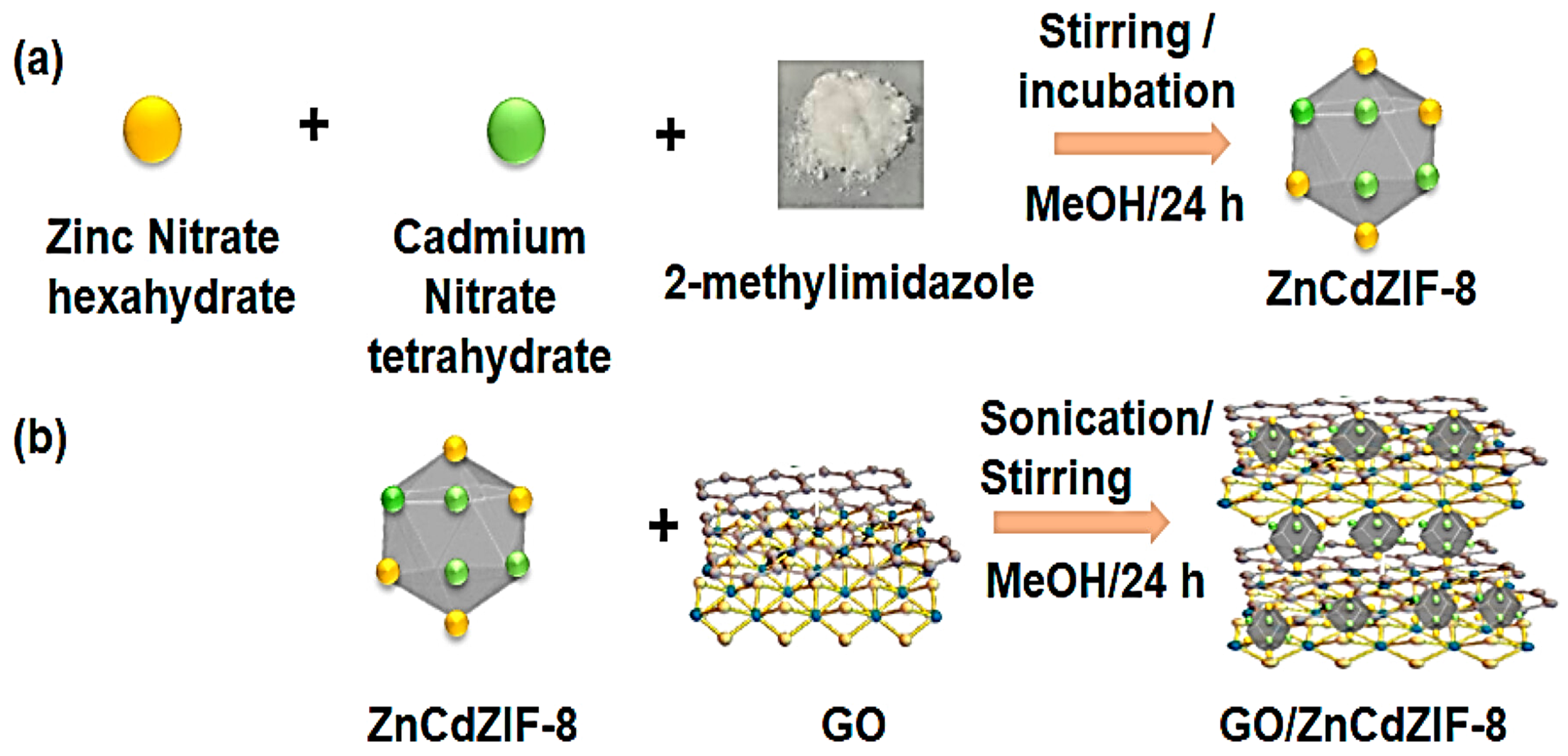
- Gonuguntla, S.; Vennapoosa, C.S.; Abraham, B.M.; Sainath, A.V.S.; Pal, U. Charge Transfer-Regulated Bimetallic ZnCd-ZIF-8/Graphene Oxide Hybrid Nanostructures for Solar Hydrogen Generation. ACS Appl. Nano Mater. 2023. [Google Scholar] [CrossRef]
- Balčiūnaitė, A.; Oliveira, R.C.P.; Yılmaz, M.S.; Metin, Ö.; Sljukic, B.; Santos, D.M. Reduced Graphene Oxide-Supported Bimetallic M-Platinum (M: Co, Ni, Cu) Alloy Nanoparticles for Hydrogen Evolution Reaction. ECS Trans. 2018, 86, 701. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Lin, X.-X.; Zhang, X.-F.; Wang, A.-J.; Zhu, X.-Y.; Feng, J.-J. Bimetallic PtPd alloyed core-shell nanodendrites supported on reduced graphene oxide: One-pot green synthesis and efficient electrocatalytic performances for glycerol oxidation and hydrogen evolution. J. Alloys Compd. 2018, 735, 2123–2132. [Google Scholar] [CrossRef]
- Rakočević, L.; Simatović, I.S.; Maksić, A.; Rajić, V.; Štrbac, S.; Srejić, I. PtAu nanoparticles supported by reduced graphene oxide as a highly active catalyst for hydrogen evolution. Catalysts 2021, 12, 43. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, H.; Li, X. Plasmon-driven engineering in bimetallic CuCo combined with reduced graphene oxide for photocatalytic overall water splitting. Appl. Surf. Sci. 2021, 559, 149865. [Google Scholar] [CrossRef]
- Khalid, M.; Zarate, X.; Saavedra-Torres, M.; Schott, E.; Honorato, A.M.B.; Hatshan, M.R.; Varela, H. Electro-reduced graphene oxide nanosheets coupled with RuAu bimetallic nanoparticles for efficient hydrogen evolution electrocatalysis. Chem. Eng. J. 2021, 421, 129987. [Google Scholar] [CrossRef]
- Du, Z.; Wang, Y.; Li, J.; Liu, J. Facile fabrication of Pt–Ni alloy nanoparticles supported on reduced graphene oxide as excellent electrocatalysts for hydrogen evolution reaction in alkaline environment. J. Nanoparticle Res. 2019, 21, 1–15. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Wang, X.; Su, Y. Engineering bimetallic PtX (X = Sn, Cu) immobilized on graphene as efficient catalysts: Boosted charge migration and hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 17662–17668. [Google Scholar] [CrossRef]
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape engineering of metal–organic frameworks. Polyhedron 2018, 145, 1–15. [Google Scholar] [CrossRef]
- Qin, N.; Pan, A.; Yuan, J.; Ke, F.; Wu, X.; Zhu, J.; Liu, J.; Zhu, J. One-step construction of a hollow Au@ Bimetal–Organic framework core–shell catalytic nanoreactor for selective alcohol oxidation reaction. ACS Appl. Mater. Interfaces 2021, 13, 12463–12471. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-Q.; Shen, J.-Q.; Zhang, J.-P. Metal–organic frameworks for electrocatalysis. Coord. Chem. Rev. 2018, 373, 22–48. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, S.-Y.; Jochems, A.P. Zirconium-based metal organic frameworks: Highly selective adsorbents for removal of phosphate from water and urine. Mater. Chem. Phys. 2015, 160, 168–176. [Google Scholar] [CrossRef]
- Langmi, H.W.; Ren, J.; North, B.; Mathe, M.; Bessarabov, D. Hydrogen storage in metal-organic frameworks: A review. Electrochim. Acta 2014, 128, 368–392. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. New sorbents for hydrogen storage by hydrogen spillover—A review. Energy Environ. Sci. 2008, 1, 268–279. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Bandosz, T.J. Engineering the surface of a new class of adsorbents: Metal–organic framework/graphite oxide composites. J. Colloid Interface Sci. 2015, 447, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, J.; Zhang, J.; Yan, X.-F.; Shen, X.-P.; Yuan, A.-H. Spillover enhanced hydrogen storage in Pt-doped MOF/graphene oxide composite produced via an impregnation method. Inorg. Chem. Commun. 2015, 54, 54–56. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhang, R.; Jiao, L.; Jiang, H.-L. Metal–organic framework-derived porous materials for catalysis. Coord. Chem. Rev. 2018, 362, 1–23. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Houk, R.J.; Jacobs, B.W.; Gabaly, F.E.; Chang, N.N.; Talin, A.A.; Graham, D.D.; House, S.D.; Robertson, I.M.; Allendorf, M.D. Silver cluster formation, dynamics, and chemistry in metal− organic frameworks. Nano Lett. 2009, 9, 3413–3418. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Yuan, Q. Nanoporous metal organic framework materials for hydrogen storage. Particuology 2009, 7, 129–140. [Google Scholar] [CrossRef]
- Wong-Foy, A.G.; Matzger, A.J.; Yaghi, O.M. Exceptional H2 saturation uptake in microporous metal−organic frameworks. J. Am. Chem. Soc. 2006, 128, 3494–3495. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Qiu, L.-G.; Yuan, Y.-P.; Peng, F.-M.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal−organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Azad, F.N.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Pezeshkpour, V. Ultrasonically assisted hydrothermal synthesis of activated carbon–HKUST-1-MOF hybrid for efficient simultaneous ultrasound-assisted removal of ternary organic dyes and antibacterial investigation: Taguchi optimization. Ultrason. Sonochem. 2016, 31, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Abdi, J.; Sisi, A.J.; Hadipoor, M.; Khataee, A. State of the art on the ultrasonic-assisted removal of environmental pollutants using metal-organic frameworks. J. Hazard. Mater. 2022, 424, 127558. [Google Scholar] [CrossRef] [PubMed]
- So, M.C.; Wiederrecht, G.P.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. Metal–organic framework materials for light-harvesting and energy transfer. Chem. Commun. 2015, 51, 3501–3510. [Google Scholar] [CrossRef]
- Jiang, Z.W.; Zhao, T.T.; Zhen, S.J.; Li, C.M.; Li, Y.F.; Huang, C.Z. A 2D MOF-based artificial light-harvesting system with chloroplast bionic structure for photochemical catalysis. J. Mater. Chem. A 2021, 9, 9301–9306. [Google Scholar] [CrossRef]
- Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’keeffe, M.; Yaghi, O.M.; Design, M.; Group, D. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Feng, Y.; Guo, N.; Sun, Y.; Zhang, T.; Ma, L.; Wang, L. Series d–f heteronuclear metal–organic frameworks: Color tunability and luminescent probe with switchable properties. Inorg. Chem. 2017, 56, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, D.; Lin, W. Metal–organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc. 2013, 135, 13222–13234. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, X.; Liu, J.; Feng, C.; Li, Z.; Li, C.; Gong, Y.; Pan, L.; Xu, S.; Sun, C.Q. Efficient charge separation between UiO-66 and ZnIn2S4 flowerlike 3D microspheres for photoelectronchemical properties. Appl. Catal. B Environ. 2018, 226, 234–241. [Google Scholar] [CrossRef]
- Burtch, N.C.; Walton, K.S. Modulating adsorption and stability properties in pillared metal–organic frameworks: A model system for understanding ligand effects. Acc. Chem. Res. 2015, 48, 2850–2857. [Google Scholar] [CrossRef]
- Bosch, M.; Zhang, M.; Zhou, H.-C. Increasing the stability of metal-organic frameworks. Adv. Chem. 2014, 2014, 1155. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Derakhshandeh, P.G.; Depauw, H.; Coudert, F.-X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Cui, X.; Zhang, Z. Metal–Organic Framework Materials for CO2 Photo-/Electro-Conversion. CO2 Convers. Util. Photocatal. Electrochem. Methods Appl. 2023, 6, 111–135. [Google Scholar]
- Sonowal, K.; Saikia, L. Metal–organic frameworks and their composites for fuel and chemical production via CO2 conversion and water splitting. RSC Adv. 2022, 12, 11686–11707. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, M.; Padella, F.; Ennas, G.; Lai, S.; Bellusci, M.; Rombi, E.; Sini, F.; Pentimalli, M.; Delitala, C.; Scano, A. Liquid-assisted mechanochemical synthesis of an iron carboxylate Metal Organic Framework and its evaluation in diesel fuel desulfurization. Microporous Mesoporous Mater. 2015, 213, 14–21. [Google Scholar] [CrossRef]
- Amo-Ochoa, P.; Givaja, G.; Miguel, P.J.S.; Castillo, O.; Zamora, F. Microwave assisted hydrothermal synthesis of a novel CuI-sulfate-pyrazine MOF. Inorg. Chem. Commun. 2007, 10, 921–924. [Google Scholar] [CrossRef]
- Wu, X.; Bao, Z.; Yuan, B.; Wang, J.; Sun, Y.; Luo, H.; Deng, S. Microwave synthesis and characterization of MOF-74 (M = Ni, Mg) for gas separation. Microporous Mesoporous Mater. 2013, 180, 114–122. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Kim, J.; Jhung, S.-H.; Kim, H.-K.; Chang, J.-S.; Chae, H.K. Microwave synthesis of a porous metal-organic framework, zinc terephthalate MOF-5. Bull. Korean Chem. Soc. 2006, 27, 1523–1524. [Google Scholar]
- Lu, C.-M.; Liu, J.; Xiao, K.; Harris, A.T. Microwave enhanced synthesis of MOF-5 and its CO2 capture ability at moderate temperatures across multiple capture and release cycles. Chem. Eng. J. 2010, 156, 465–470. [Google Scholar] [CrossRef]
- Klinowski, J.; Paz, F.A.A.; Silva, P.; Rocha, J. Microwave-assisted synthesis of metal–organic frameworks. Dalton Trans. 2011, 40, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Hillman, I.; Laybourn, A.; Dodds, C.; Kingman, S.W. Realising the environmental benefits of metal–organic frameworks: Recent advances in microwave synthesis. J. Mater. Chem. A 2018, 6, 11564–11581. [Google Scholar] [CrossRef]
- Rahaman, S.K.; Chatterjee, T.; Alam, S.M. Microwave-assisted synthesis of metal–organic frameworks. In Synthesis of Metal-Organic Frameworks via Water-based Routes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 51–72. [Google Scholar]
- Sharma, N.; Sharma, U.K.; Van der Eycken, E.V. Microwave-Assisted Organic Synthesis: Overview of Recent Applications. Green Tech. Org. Synth. Med. Chem. 2018, 17, 441–468. [Google Scholar]
- Mendes, R.F.; Rocha, J.; Paz, F.A.A. Microwave synthesis of metal-organic frameworks. In Metal-Organic Frameworks for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 159–176. [Google Scholar]
- Vakili, R.; Xu, S.; Al-Janabi, N.; Gorgojo, P.; Holmes, S.M.; Fan, X. Microwave-assisted synthesis of zirconium-based metal organic frameworks (MOFs): Optimization and gas adsorption. Microporous Mesoporous Mater. 2018, 260, 45–53. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, S.; Pecharsky, V.; Balema, V. Solvent-free mechanochemical synthesis and magnetic properties of rare-earth based metal-organic frameworks. J. Alloys Compd. 2017, 696, 118–122. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Tanaka, S. Mechanochemical synthesis of MOFs. Met.-Org. Framew. Biomed. Appl. 2020, 2020, 197–222. [Google Scholar]
- Gao, T.; Tang, H.-J.; Zhang, S.-Y.; Cao, J.-W.; Wu, Y.-N.; Chen, J.; Wang, Y.; Chen, K.-J. Mechanochemical synthesis of three-component metal-organic frameworks for large scale production. J. Solid State Chem. 2021, 303, 122547. [Google Scholar] [CrossRef]
- Nath, B. Mechanochemical synthesis of metal–organic frameworks. In Synthesis of Metal-Organic Frameworks via Water-Based Routes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 93–120. [Google Scholar]
- Pagola, S. Outstanding advantages, current drawbacks, and significant recent developments in mechanochemistry: A perspective view. Crystals 2023, 13, 124. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [PubMed]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical synthesis of catalytic materials. Chem. A Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Ng, M.; Milner, P.J. Rapid mechanochemical synthesis of metal–organic frameworks using exogenous organic base. Dalton Trans. 2020, 49, 16238–16244. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Chen, Y.; Li, Y.; Shi, R.; Wu, H.; Sun, X.; Xiao, J.; Xi, H.; Xia, Q.; Li, Z. Efficient mechanochemical synthesis of MOF-5 for linear alkanes adsorption. J. Chem. Eng. Data 2017, 62, 2030–2036. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J. 3D-Superstructured Networks Comprising Fe-MIL-88A Metal-Organic Frameworks Under Mechanochemical Conditions. Eur. J. Inorg. Chem. 2019, 2019, 4597–4600. [Google Scholar] [CrossRef]
- Klimakow, M.; Klobes, P.; Thunemann, A.F.; Rademann, K.; Emmerling, F. Mechanochemical synthesis of metal−organic frameworks: A fast and facile approach toward quantitative yields and high specific surface areas. Chem. Mater. 2010, 22, 5216–5221. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Xu, X.; Xia, F.; Na, H.; Liu, Y.; Qiu, H.; Wang, W.; Gao, J. Magnetic bimetallic nanoparticles supported reduced graphene oxide nanocomposite: Fabrication, characterization and catalytic capability. J. Alloys Compd. 2015, 628, 364–371. [Google Scholar] [CrossRef]
- Van Assche, T.R.; Campagnol, N.; Muselle, T.; Terryn, H.; Fransaer, J.; Denayer, J.F. On controlling the anodic electrochemical film deposition of HKUST-1 metal–organic frameworks. Microporous Mesoporous Mater. 2016, 224, 302–310. [Google Scholar] [CrossRef]
- Varsha, M.; Nageswaran, G. Direct electrochemical synthesis of metal organic frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar]
- Liu, Y.; Wei, Y.; Liu, M.; Bai, Y.; Wang, X.; Shang, S.; Chen, J.; Liu, Y. Electrochemical Synthesis of Large Area Two-Dimensional Metal–Organic Framework Films on Copper Anodes. Angew. Chem. Int. Ed. 2021, 60, 2887–2891. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-M.; Xian, L.; Song, X.-L.; Yang, T.-L.; Liang, Z.-H.; Fan, C.-M. In situ electrochemical synthesis of MOF-5 and its application in improving photocatalytic activity of BiOBr. Trans. Nonferrous Met. Soc. China 2015, 25, 3987–3994. [Google Scholar] [CrossRef]
- Campagnol, N.; Van Assche, T.; Boudewijns, T.; Denayer, J.; Binnemans, K.; De Vos, D.; Fransaer, J. High pressure, high temperature electrochemical synthesis of metal–organic frameworks: Films of MIL-100 (Fe) and HKUST-1 in different morphologies. J. Mater. Chem. A 2013, 1, 5827–5830. [Google Scholar] [CrossRef]
- Cao, W.; Liu, Y.; Xu, F.; Li, J.; Li, D.; Du, G.; Chen, N. In situ electrochemical synthesis of rod-like Ni-MOFs as battery-type electrode for high performance hybrid supercapacitor. J. Electrochem. Soc. 2019, 167, 050503. [Google Scholar] [CrossRef]
- Gascon, J.; Aguado, S.; Kapteijn, F. Manufacture of dense coatings of Cu3 (BTC) 2 (HKUST-1) on α-alumina. Microporous Mesoporous Mater. 2008, 113, 132–138. [Google Scholar] [CrossRef]
- Ding, M.; Liu, W.; Gref, R. Nanoscale MOFs: From synthesis to drug delivery and theranostics applications. Adv. Drug Deliv. Rev. 2022, 190, 114496. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhan, F.; Wang, H.; Xu, W.; Wang, H.; Chen, L. Recent progress of industrial preparation of metal–organic frameworks: Synthesis strategies and outlook. Mater. Today Sustain. 2022, 17, 100104. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.; Yan, X.; Lv, Y. Recent advances in metal-organic frameworks: Synthesis, application and toxicity. Sci. Total Environ. 2023, 902, 165944. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, Q.; Xue, H.; Pang, H.; Xu, Q. A highly alkaline-stable metal oxide@ metal–organic framework composite for high-performance electrochemical energy storage. Natl. Sci. Rev. 2020, 7, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Naeem, A.; Din, I.U.; Farooq, M.; Khan, I.W.; Hamayun, M.; Malik, T. Synthesis of chitosan composite of metal-organic framework for the adsorption of dyes; kinetic and thermodynamic approach. J. Hazard. Mater. 2022, 427, 127902. [Google Scholar] [CrossRef] [PubMed]
- Cravillon, J.; Schröder, C.A.; Bux, H.; Rothkirch, A.; Caro, J.; Wiebcke, M. Formate modulated solvothermal synthesis of ZIF-8 investigated using time-resolved in situ X-ray diffraction and scanning electron microscopy. CrystEngComm 2012, 14, 492–498. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wan, J.; Yu, C. MOF-on-MOF hybrids: Synthesis and applications. Coord. Chem. Rev. 2021, 432, 213743. [Google Scholar] [CrossRef]
- Gu, Z.-G.; Zhang, J. Epitaxial growth and applications of oriented metal–organic framework thin films. Coord. Chem. Rev. 2019, 378, 513–532. [Google Scholar] [CrossRef]
- Gliemann, H.; Wöll, C. Epitaxially grown metal-organic frameworks. Mater. Today 2012, 15, 110–116. [Google Scholar] [CrossRef]
- Chernikova, V.; Shekhah, O.; Eddaoudi, M. Advanced fabrication method for the preparation of MOF thin films: Liquid-phase epitaxy approach meets spin coating method. ACS Appl. Mater. Interfaces 2016, 8, 20459–20464. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Caro, J.; Guo, X.; Song, C.; Liu, Y. In-Plane Epitaxial Growth of Highly c-Oriented NH2-MIL-125 (Ti) Membranes with Superior H2/CO2 Selectivity. Angew. Chem. 2018, 130, 16320–16325. [Google Scholar] [CrossRef]
- Wu, F.; Wan, L.; Li, Q.; Zhang, Q.; Zhang, B. Ternary assembled MOF-derived composite: Anisotropic epitaxial growth and microwave absorption. Compos. Part B: Eng. 2022, 236, 109839. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Sun, Y.; Xu, G.; Ji, T.; Zhang, X.; Wang, F. Fabrication of MIL-96 nanosheets and relevant c-oriented ultrathin membrane through solvent optimization. J. Membr. Sci. 2022, 643, 120064. [Google Scholar] [CrossRef]
- Ruiz-Zambrana, C.L.; Malankowska, M.; Coronas, J. Metal organic framework top-down and bottom-up patterning techniques. Dalton Trans. 2020, 49, 15139–15148. [Google Scholar] [CrossRef]
- Ellis, J.E.; Crawford, S.E.; Kim, K.-J. Metal–organic framework thin films as versatile chemical sensing materials. Mater. Adv. 2021, 2, 6169–6196. [Google Scholar] [CrossRef]
- Bon, V.; Brunner, E.; Pöppl, A.; Kaskel, S. Unraveling structure and dynamics in porous frameworks via advanced in situ characterization techniques. Adv. Funct. Mater. 2020, 30, 1907847. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B. In-situ growth of Ti3C2@ MIL-NH2 composite for highly enhanced photocatalytic H2 evolution. Chem. Eng. J. 2021, 411, 128446. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wang, C.-C.; Chu, H.-Y.; Wang, P.; Zhao, C.; Fu, H. In situ growth of MIL-101 (Fe) on waste PET plastic slices for effective arsenic removal. Sep. Purif. Technol. 2024, 331, 125589. [Google Scholar] [CrossRef]
- Huang, C.; Cai, B.; Zhang, L.; Zhang, C.; Pan, H. Preparation of iron-based metal-organic framework@ cellulose aerogel by in situ growth method and its application to dye adsorption. J. Solid State Chem. 2021, 297, 122030. [Google Scholar] [CrossRef]
- Sun, D.; Li, J.; Shen, T.; An, S.; Qi, B.; Song, Y.-F. In situ construction of MIL-100@ NiMn-LDH hierarchical architectures for highly selective photoreduction of CO2 to CH4. ACS Appl. Mater. Interfaces 2022, 14, 16369–16378. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ding, J.; Zhong, Q. In situ fabrication of amorphous TiO2/NH2-MIL-125 (Ti) for enhanced photocatalytic CO2 into CH4 with H2O under visible-light irradiation. J. Colloid Interface Sci. 2020, 560, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, L.; Wang, Y.; Xiang, W.; Lyu, P.; Tang, B.; Tan, X. Effect of hydrogen content on hydrogen desorption kinetics of titanium hydride. J. Alloys Compd. 2017, 709, 445–452. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, J.; Wen, F.; Zhou, Z.; Zhu, M.; Yin, S.; Wang, H. Platinum nanoparticles deposited nitrogen-doped carbon nanofiber derived from bacterial cellulose for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 6167–6176. [Google Scholar] [CrossRef]
- McKone, J.R.; Marinescu, S.C.; Brunschwig, B.S.; Winkler, J.R.; Gray, H.B. Earth-abundant hydrogen evolution electrocatalysts. Chem. Sci. 2014, 5, 865–878. [Google Scholar] [CrossRef]
- Petit, C.; Burress, J.; Bandosz, T.J. The synthesis and characterization of copper-based metal–organic framework/graphite oxide composites. Carbon 2011, 49, 563–572. [Google Scholar] [CrossRef]
- Loera-Serna, S.; Oliver-Tolentino, M.A.; de Lourdes López-Núñez, M.; Santana-Cruz, A.; Guzmán-Vargas, A.; Cabrera-Sierra, R.; Beltrán, H.I.; Flores, J. Electrochemical behavior of [Cu3(BTC)2] metal–organic framework: The effect of the method of synthesis. J. Alloys Compd. 2012, 540, 113–120. [Google Scholar] [CrossRef]
- Lin, J.; He, J.; Qi, F.; Zheng, B.; Wang, X.; Yu, B.; Zhou, K.; Zhang, W.; Li, Y.; Chen, Y. In-situ selenization of Co-based metal-organic frameworks as a highly efficient electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2017, 247, 258–264. [Google Scholar] [CrossRef]
- Ramohlola, K.E.; Monana, G.R.; Hato, M.J.; Modibane, K.D.; Molapo, K.M.; Masikini, M.; Mduli, S.B.; Iwuoha, E.I. Polyaniline-metal organic framework nanocomposite as an efficient electrocatalyst for hydrogen evolution reaction. Compos. Part B Eng. 2018, 137, 129–139. [Google Scholar] [CrossRef]
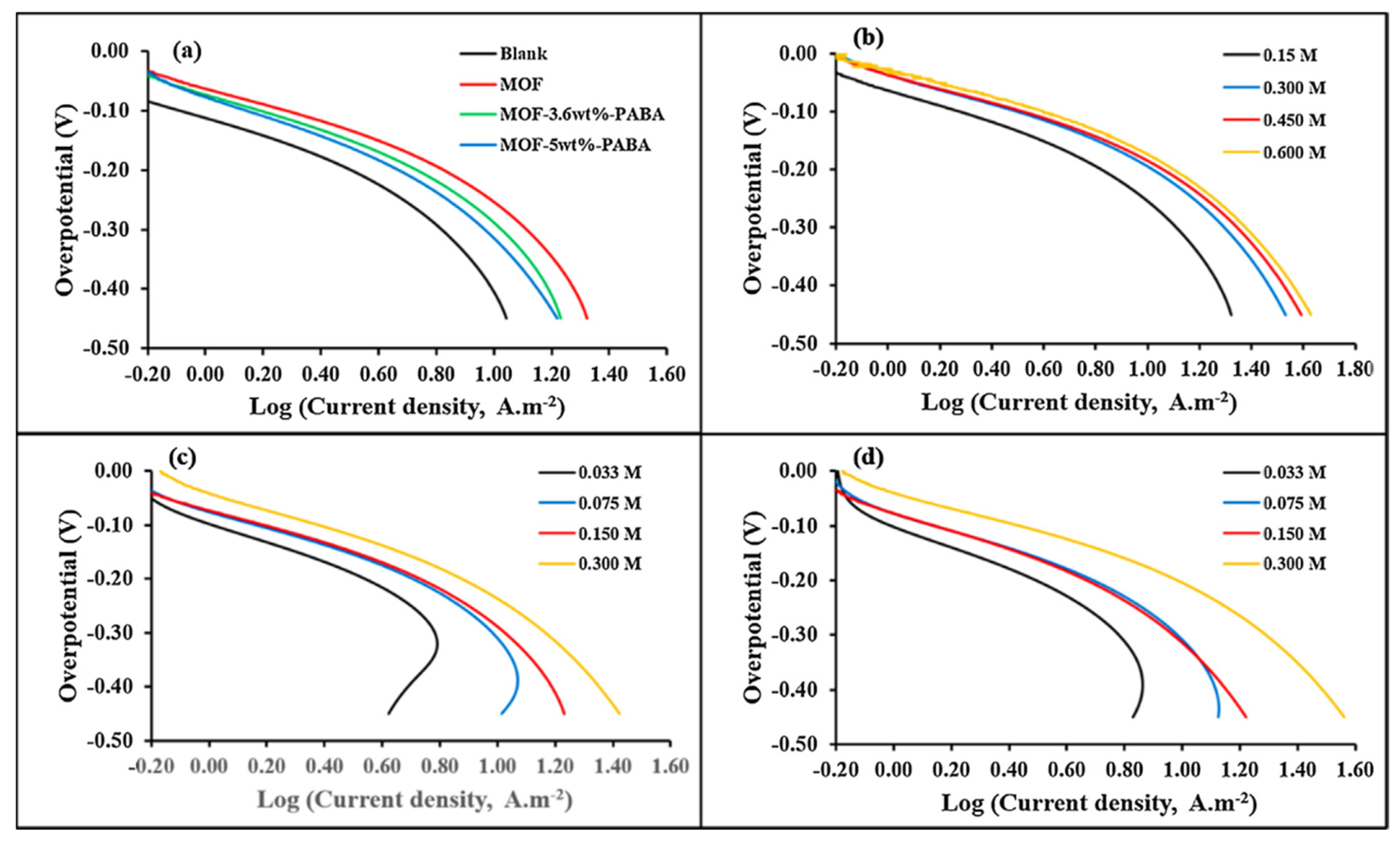
- Ramohlola, K.E.; Masikini, M.; Mdluli, S.B.; Monama, G.R.; Hato, M.J.; Molapo, K.M.; Iwuoha, E.I.; Modibane, K.D. Electrocatalytic hydrogen evolution reaction of metal organic frameworks decorated with poly (3-aminobenzoic acid). Electrochim. Acta 2017, 246, 1174–1182. [Google Scholar] [CrossRef]
- Ramohlola, K.E.; Masikini, M.; Mdluli, S.B.; Monama, G.R.; Hato, M.J.; Molapo, K.M.; Iwuoha, E.I.; Modibane, K.D. Electrocatalytic hydrogen production properties of poly (3-aminobenzoic acid) doped with metal organic frameworks. Int. J. Electrochem. Sci. 2017, 12, 4392–4405. [Google Scholar] [CrossRef]
- Jahan, M.; Liu, Z.; Loh, K.P. A Graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.; Hao, Z.; Li, G.; Liu, T. Immobilization of palladium silver nanoparticles on NH2-functional metal-organic framework for fast dehydrogenation of formic acid. J. Colloid Interface Sci. 2021, 587, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Mandegarzad, S.; Raoof, J.B.; Hosseini, S.R.; Ojani, R. MOF-derived Cu-Pd/nanoporous carbon composite as an efficient catalyst for hydrogen evolution reaction: A comparison between hydrothermal and electrochemical synthesis. Appl. Surf. Sci. 2018, 436, 451–459. [Google Scholar] [CrossRef]
- Zhen, W.; Gao, H.; Tian, B.; Ma, J.; Lu, G. Fabrication of low adsorption energy Ni–Mo cluster cocatalyst in metal–organic frameworks for visible photocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2016, 8, 10808–10819. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Li, Y.; Jia, X.; Shen, Q.; Li, Q.; Liu, X.; Xue, J. Construction of an amino-rich Ni/Ti bimetallic MOF composite with expanded light absorption and enhanced carrier separation for efficient photocatalytic H2 evolution. Mater. Sci. Semicond. Process. 2022, 150, 106914. [Google Scholar] [CrossRef]
- Chen, D.; Han, C.; Sun, Q.; Ding, J.; Huang, Q.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. Bimetallic AgNi nanoparticles anchored onto MOF-derived nitrogen-doped carbon nanostrips for efficient hydrogen evolution. Green Energy Environ. 2021, 8, 258–266. [Google Scholar] [CrossRef]
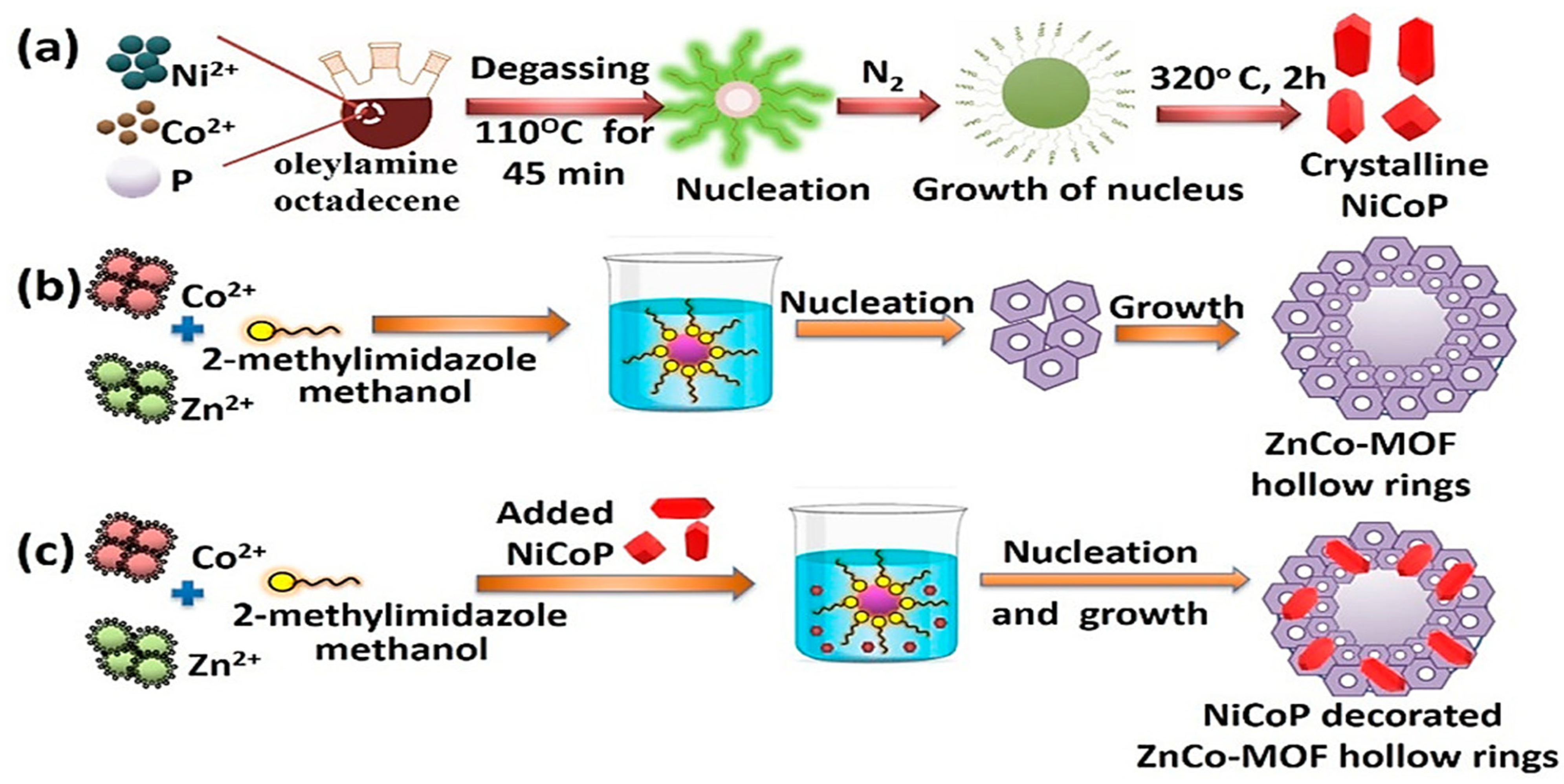
- Antil, B.; Kumar, L.; Das, M.R.; Deka, S. Incorporating NiCoP Cocatalyst into Hollow Rings of ZnCo-Metal–Organic frameworks to deliver Pt cocatalyst like visible light driven hydrogen evolution activity. ACS Appl. Energy Mater. 2022, 5, 11113–11121. [Google Scholar] [CrossRef]
- Yang, M.; Jiao, L.; Dong, H.; Zhou, L.; Teng, C.; Yan, D.; Ye, T.-N.; Chen, X.; Liu, Y.; Jiang, H.-L. Conversion of bimetallic MOF to Ru-doped Cu electrocatalysts for efficient hydrogen evolution in alkaline media. Sci. Bull. 2021, 66, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wang, Z.; Wang, C.; Wang, L.; Chi, Y.; Wang, M.; Zhang, J.; Wu, C.; Gu, Y.; Wang, H. CrPd nanoparticles on NH2-functionalized metal-organic framework as a synergistic catalyst for efficient hydrogen evolution from formic acid. Chem. Eng. J. 2019, 361, 953–959. [Google Scholar] [CrossRef]
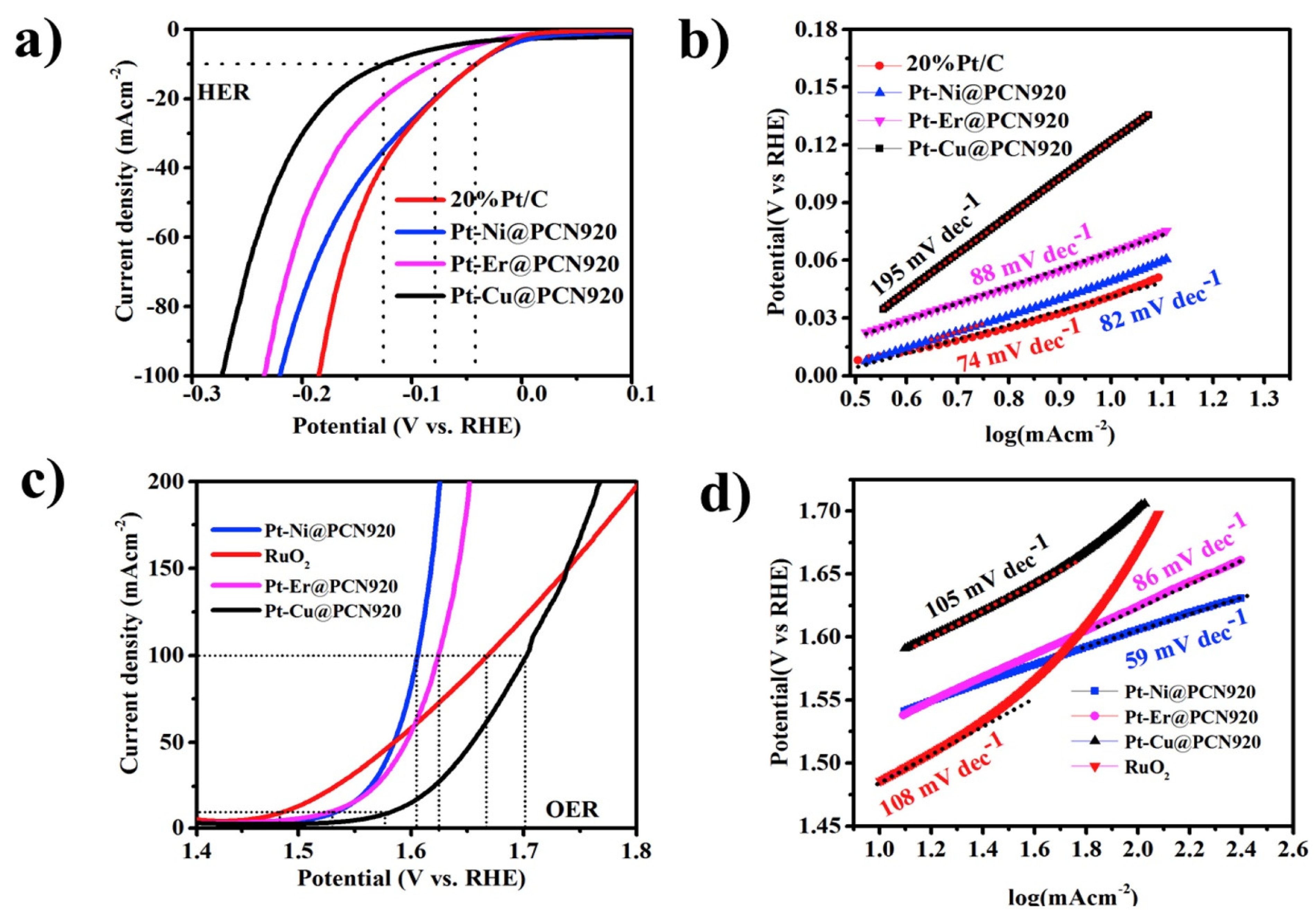
- Nadeem, M.; Yasin, G.; Bhatti, M.H.; Mehmood, M.; Arif, M.; Dai, L. Pt-M bimetallic nanoparticles (M = Ni, Cu, Er) supported on metal organic framework-derived N-doped nanostructured carbon for hydrogen evolution and oxygen evolution reaction. J. Power Sources 2018, 402, 34–42. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.-H.; Debnath, T.; Wang, Y.; Pohl, D.; Besteiro, L.V.; Meira, D.M.; Huang, S.; Yang, F.; Rellinghaus, B.; et al. Silver nanoparticle enhanced metal-organic matrix with interface-engineering for efficient photocatalytic hydrogen evolution. Nat. Commun. 2023, 14, 541. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lian, C.; Hu, S.; Deng, Z.; Gong, J.; Li, M.; Liu, H.; Xing, M.; Zhang, J. Size-dependent activity and selectivity of carbon dioxide photocatalytic reduction over platinum nanoparticles. Nat. Commun. 2018, 9, 1252. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; Alothman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ.-Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Hayden, B.E. Particle size and support effects in electrocatalysis. Acc. Chem. Res. 2013, 46, 1858–1866. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef]
- Xie, J.; Yang, X.; Xie, Y. Defect engineering in two-dimensional electrocatalysts for hydrogen evolution. Nanoscale 2020, 12, 4283–4294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hwang, S.; Wang, M.; Feng, Z.; Karakalos, S.; Luo, L.; Qiao, Z.; Xie, X.; Wang, C.; Su, D. Single atomic iron catalysts for oxygen reduction in acidic media: Particle size control and thermal activation. J. Am. Chem. Soc. 2017, 139, 14143–14149. [Google Scholar] [CrossRef]
- An, K.; Somorjai, G.A. Size and shape control of metal nanoparticles for reaction selectivity in catalysis. ChemCatChem 2012, 4, 1512–1524. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, X.; Wang, M.; Hwang, S.; Karakalos, S.; Chen, M.; Qiao, Z.; Wang, L.; Liu, B.; Ma, Q. Single-iron site catalysts with self-assembled dual-size architecture and hierarchical porosity for proton-exchange membrane fuel cells. Appl. Catal. B Environ. 2020, 279, 119400. [Google Scholar] [CrossRef]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-based bimetallic catalysis: From model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef] [PubMed]
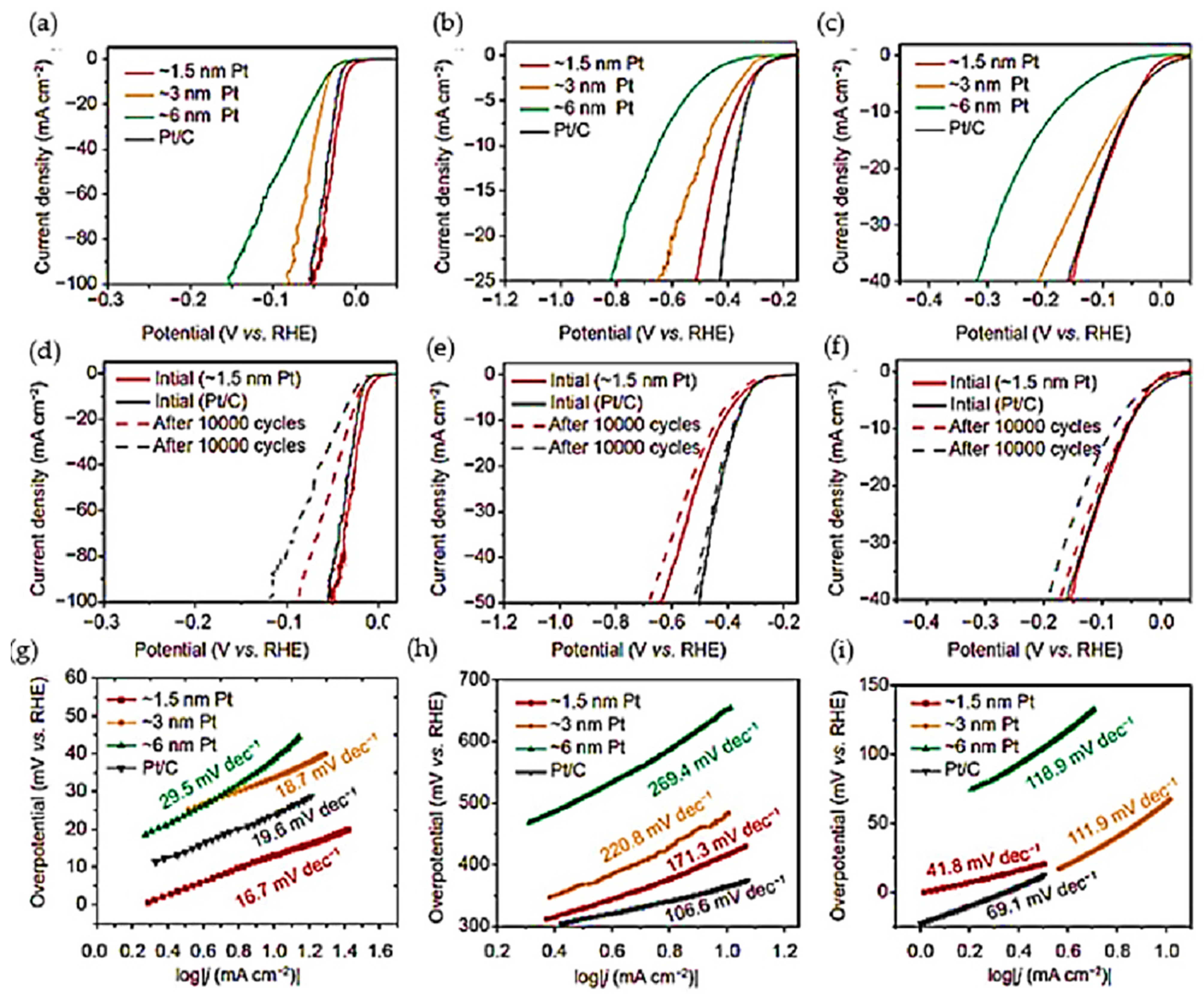
- Ma, Z.; Tian, H.; Meng, G.; Peng, L.; Chen, Y.; Chen, C.; Chang, Z.; Cui, X.; Wang, L.; Jiang, W. Size effects of platinum particles@ CNT on HER and ORR performance. Sci. China Mater. 2020, 63, 2517–2529. [Google Scholar] [CrossRef]
- Yan, Q.-Q.; Wu, D.-X.; Chu, S.-Q.; Chen, Z.-Q.; Lin, Y.; Chen, M.-X.; Zhang, J.; Wu, X.-J.; Liang, H.-W. Reversing the charge transfer between platinum and sulfur-doped carbon support for electrocatalytic hydrogen evolution. Nat. Commun. 2019, 10, 4977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pham, T.H.M.; Ko, Y.; Li, M.; Yang, S.; Koolen, C.D.; Zhong, L.; Luo, W.; Züttel, A. Tandem effect of Ag@ C@ Cu catalysts enhances ethanol selectivity for electrochemical CO2 reduction in flow reactors. Cell Rep. Phys. Sci. 2022, 3, 100949. [Google Scholar] [CrossRef]
- Koolen, C.D.; Luo, W.; Zuttel, A. From single crystal to single atom catalysts: Structural factors influencing the performance of metal catalysts for CO2 electroreduction. ACS Catal. 2022, 13, 948–973. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Yen, H.; Seo, Y.; Kaliaguine, S.; Kleitz, F. Role of metal–support interactions, particle size, and metal–metal synergy in CuNi nanocatalysts for H2 generation. Acs Catal. 2015, 5, 5505–5511. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, P.; Huang, X. Opportunities and challenges of interface engineering in bimetallic nanostructure for enhanced electrocatalysis. Adv. Funct. Mater. 2019, 29, 1806419. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Kim, D.; Park, J.Y. Operando surface studies on metal-oxide interfaces of bimetal and mixed catalysts. ACS Catal. 2021, 11, 8645–8677. [Google Scholar] [CrossRef]
- Kuna, E.; Mrdenovic, D.; Jönsson-Niedziółka, M.; Pieta, P.; Pieta, I.S. Bimetallic nanocatalysts supported on graphitic carbon nitride for sustainable energy development: The shape-structure–activity relation. Nanoscale Adv. 2021, 3, 1342–1351. [Google Scholar] [CrossRef] [PubMed]




| Catalyst | H2 Source in Electrolyte | Tafel Slope mV.dec−1 | Current Density mA.cm−2 | Ref. |
|---|---|---|---|---|
| AuPd–rGO | 0.5 M H2SO4 | 29.0 | 0.47 | [96] |
| PdAu–rGO | 0.5 M H2SO4 | 46 | 22.3 | [98] |
| AuNi–rGO | 0.5 M H2SO4 | 33 | 10 | [101] |
| CoPt–rGO | 8 M KOH | 109 | 0.96 | [103] |
| NiPt–rGO | 8 M KOH | 100 | 0.35 | [103] |
| CuPt–rGO | 8 M KOH | 107 | 0.48 | [103] |
| PtPd–rGO | 0.5 M KOH + 0.5 M glycerol | 36 | 10 | [104] |
| PtAu–rGO | 0.5 M H2SO4 | 38 | 10 | [105] |
| RuAu–rGO | 1 M KOH | 113 | 10 | [107] |
| PtNi–rGO | 1 M KOH | 56 | 10 | [108] |
| Material | H2 Source in Electrolyte | Tafel Slope (mV.dec−1) | Current Density (mA.m−2) | Ref. |
|---|---|---|---|---|
| MOF–CoSe2 | 0.5 M H2SO4 | 42 | 0.080 | [55] |
| Pd@CuPc–MOF | 0.3 M H2SO4 | 176.9 | 8.900 | [55] |
| MOF–PANI | 0.3 M H2SO4 | 199.3 | 7.943 | [199] |
| MOF–5wt.% PABA | 0.3 M H2SO4 | 153.5 | 50.12 | [200] |
| MOF–3wt.% PABA | 0.3 M H2SO4 | 166.7 | 31.62 | [200] |
| PABA–MOF | 0.3 M H2SO4 | 130.5 | 35.48 | [201] |
| Cu–MOF–8 wt.% GO | 0.5 M H2SO4 | - | −300 | [202] |
| Material | Electrolyte | Tafel Slope mV.dec−1 | Current Density mA.cm−2 | H2 Yield/Production Rate | TOF h−1 | Ref. |
|---|---|---|---|---|---|---|
| PdAg–NH2–MIL-101(Cr) | Formic acid | - | - | 144 mL/4.87 min | 1475 | [203] |
| CuPd–NPCC–EC | 0.5 M H2SO4 | 28.2 | 0.03 | - | - | [204] |
| NiMo@MIL-101 | - | 76 | - | 740.2 μmol.h−1 | - | [205] |
| NiTi–NH2–MIL-125 | - | - | - | 699 μmol.g−1.h−1 | - | [206] |
| AgNi–NC | 1 M KOH | 126.2 | 10 | - | - | [207] |
| NiCoP@ZnCo–MOF | - | - | - | 8583.4 μmol.g−1.h−1 | - | [208] |
| RuCu@C | 1 M KOH | 37 | 10 | - | - | [209] |
| CrPd/MIL-101–NH2 | Formic acid | - | - | 225 mL/7.5 min | 2009 | [210] |
| PtNi@PCN920 | 1 M KOH | 82 | 10 | - | - | [211] |
| PtEr@PCN920 | 1 M KOH | 88 | 10 | - | - | [211] |
| PtCu@PCN920 | 1 M KOH | 195 | 10 | - | - | [211] |
| Ag–AgMOF | - | - | - | 1025 μmol.h−1.g−1 | - | [212] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhafola, M.D.; Balogun, S.A.; Modibane, K.D. A Comprehensive Review of Bimetallic Nanoparticle–Graphene Oxide and Bimetallic Nanoparticle–Metal–Organic Framework Nanocomposites as Photo-, Electro-, and Photoelectrocatalysts for Hydrogen Evolution Reaction. Energies 2024, 17, 1646. https://doi.org/10.3390/en17071646
Makhafola MD, Balogun SA, Modibane KD. A Comprehensive Review of Bimetallic Nanoparticle–Graphene Oxide and Bimetallic Nanoparticle–Metal–Organic Framework Nanocomposites as Photo-, Electro-, and Photoelectrocatalysts for Hydrogen Evolution Reaction. Energies. 2024; 17(7):1646. https://doi.org/10.3390/en17071646
Chicago/Turabian StyleMakhafola, Mogwasha Dapheny, Sheriff Aweda Balogun, and Kwena Desmond Modibane. 2024. "A Comprehensive Review of Bimetallic Nanoparticle–Graphene Oxide and Bimetallic Nanoparticle–Metal–Organic Framework Nanocomposites as Photo-, Electro-, and Photoelectrocatalysts for Hydrogen Evolution Reaction" Energies 17, no. 7: 1646. https://doi.org/10.3390/en17071646
APA StyleMakhafola, M. D., Balogun, S. A., & Modibane, K. D. (2024). A Comprehensive Review of Bimetallic Nanoparticle–Graphene Oxide and Bimetallic Nanoparticle–Metal–Organic Framework Nanocomposites as Photo-, Electro-, and Photoelectrocatalysts for Hydrogen Evolution Reaction. Energies, 17(7), 1646. https://doi.org/10.3390/en17071646






