Combustion Behaviors, Kinetics, and Thermodynamics of Naturally Decomposed and Torrefied Northern Red Oak (Quercus rubra) Forest Logging Residue
Abstract
1. Introduction
2. Materials and Methods
2.1. Proximate and Ultimate Analyses
2.2. FTIR and Energy Properties
2.3. Combustion Characteristics
2.4. Kinetic Parameters
2.4.1. Model-Free Method
2.4.2. Determination of Reaction Model
2.5. Thermodynamic Analysis
3. Results and Discussion
3.1. Fundamental Characteristics
3.2. Atomic H/C and O/C Ratio, FTIR, and Energy Properties
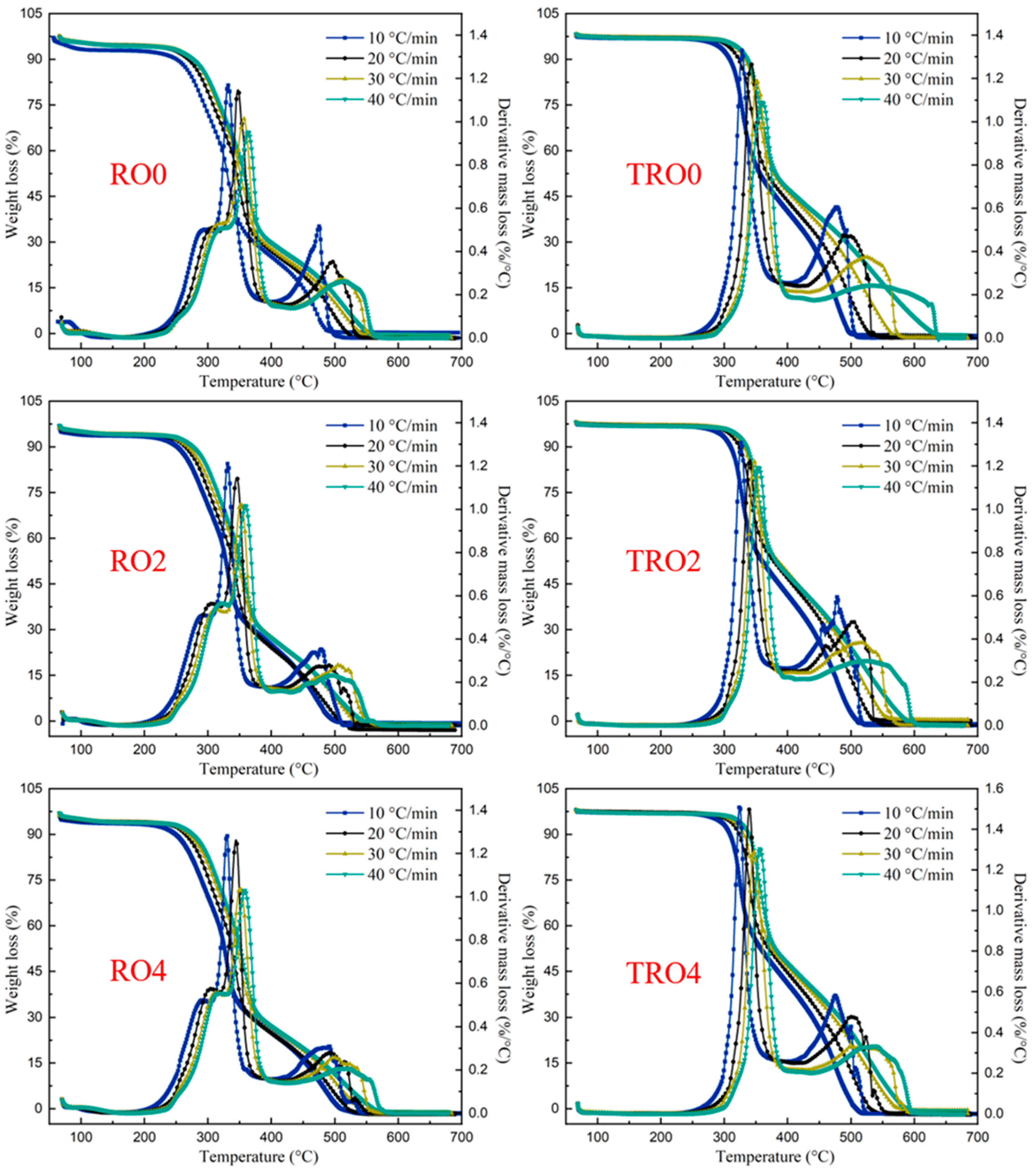
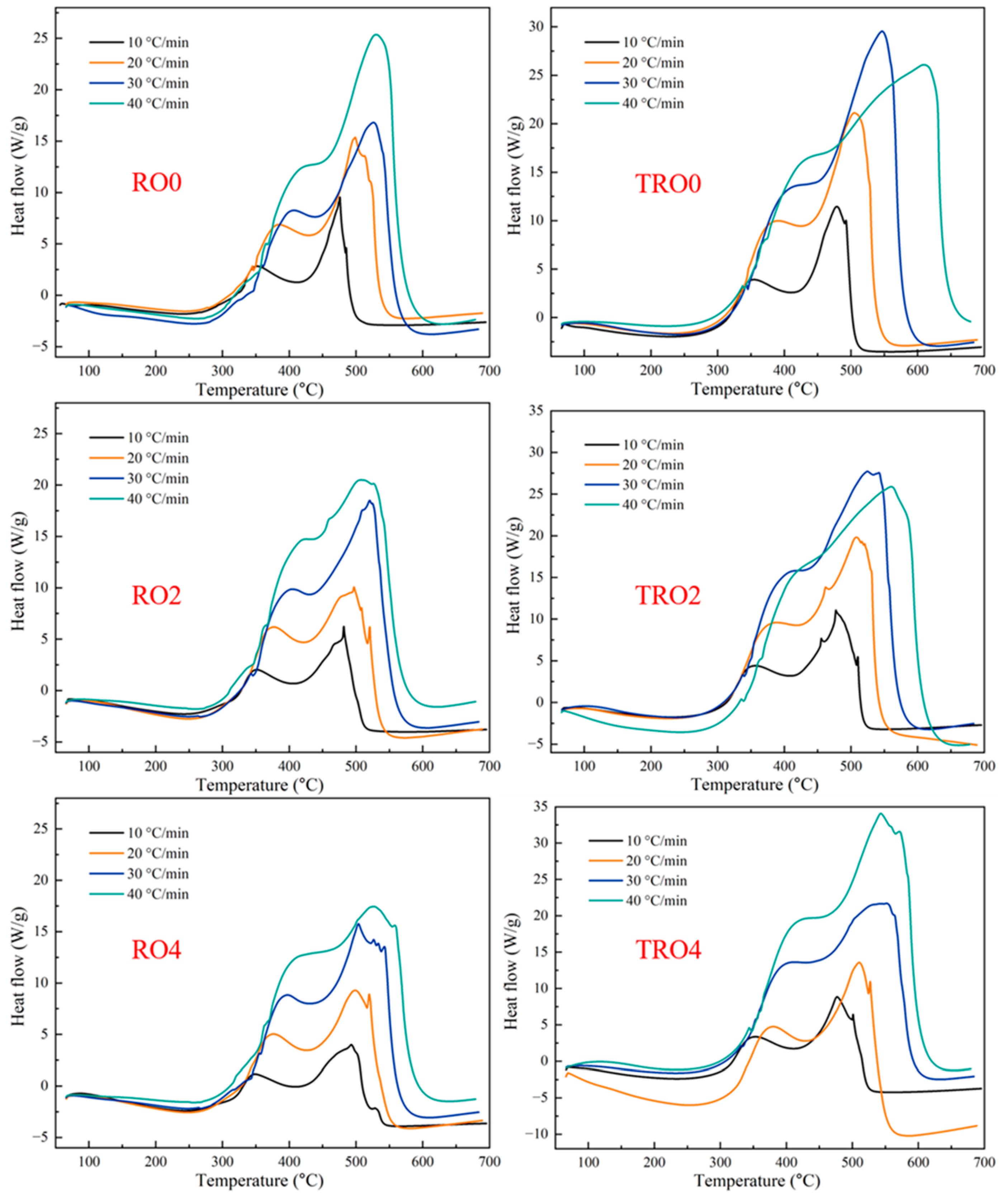
3.3. Combustion Characteristics
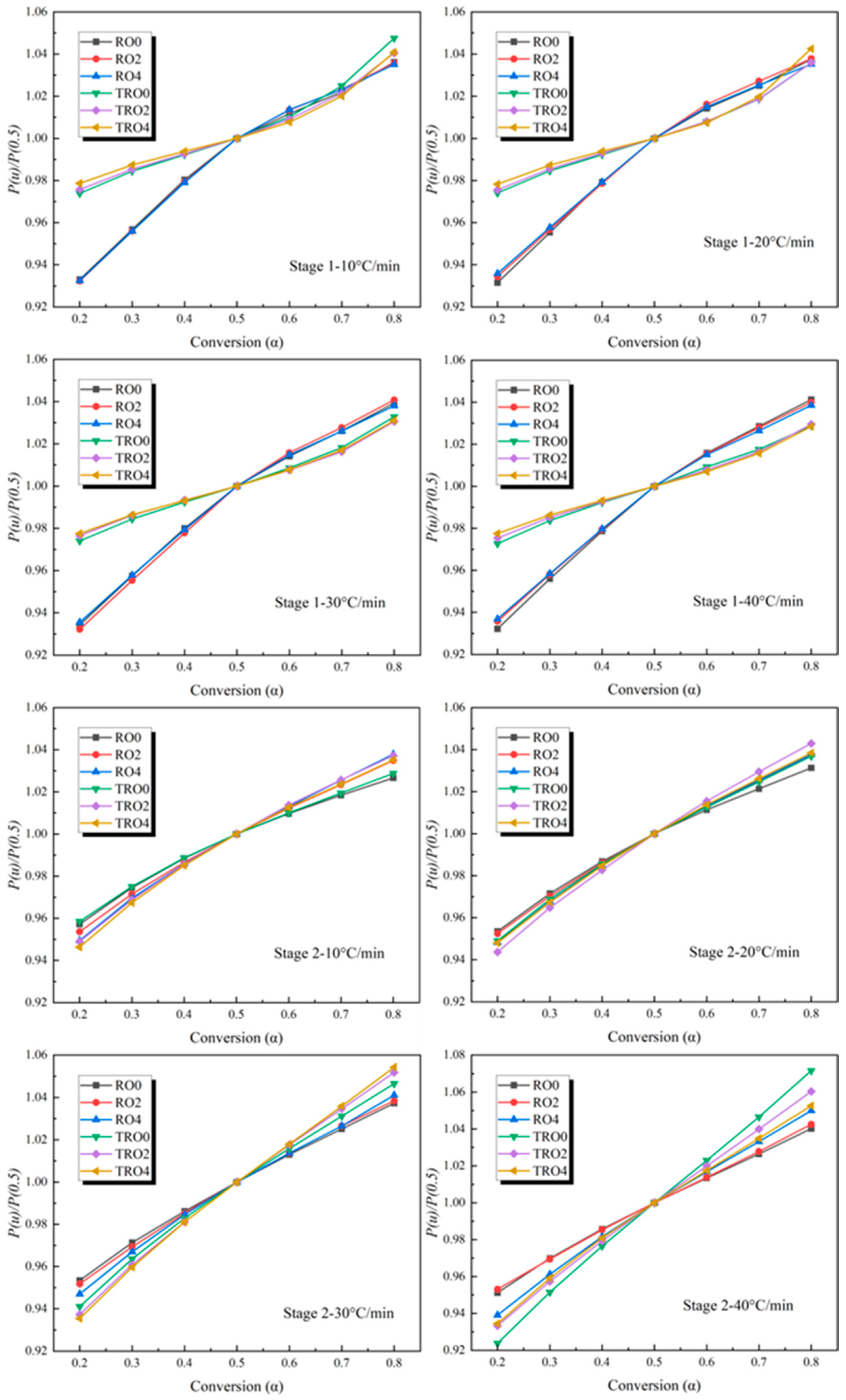
3.4. Kinetics Analysis
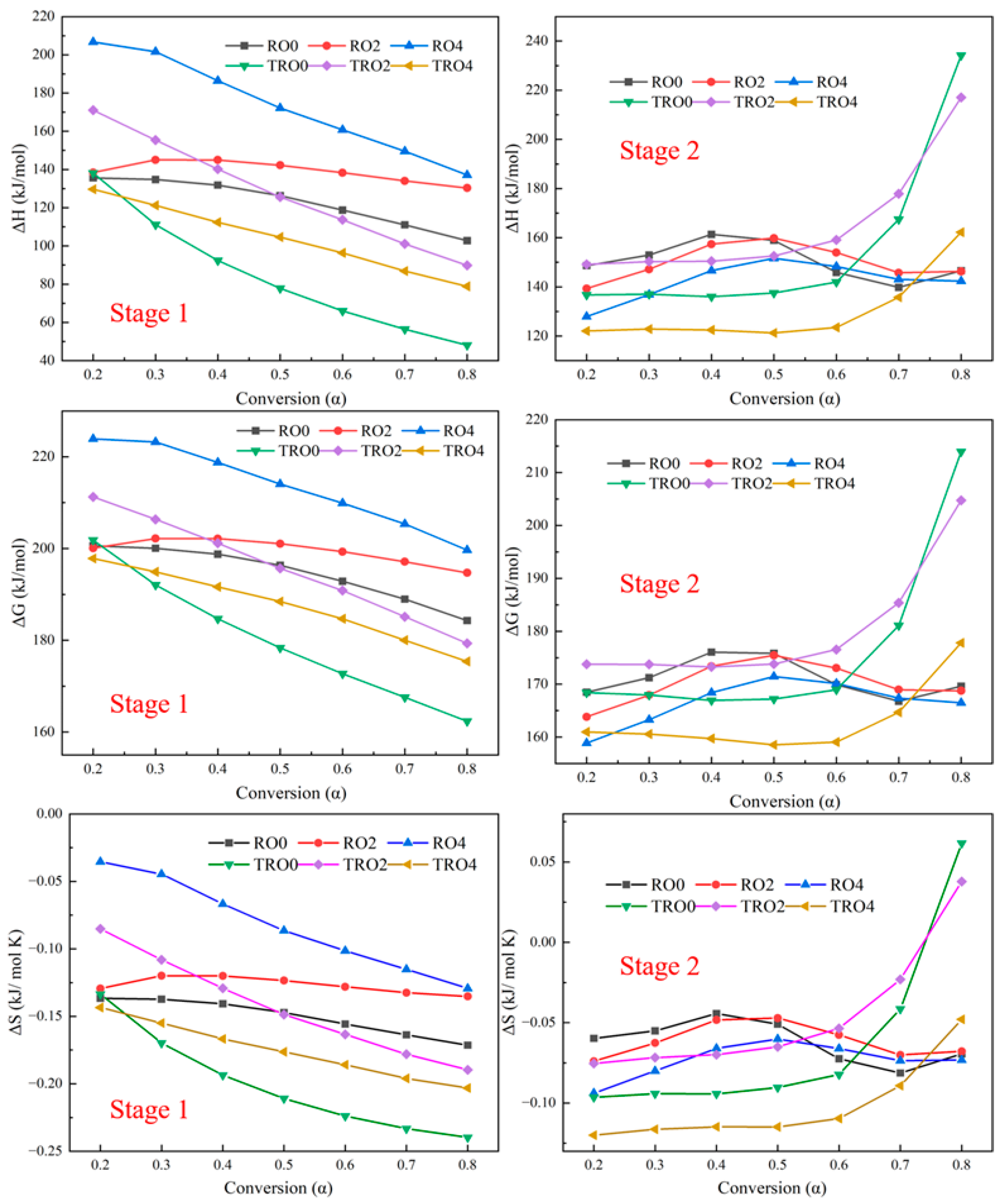
3.5. Thermodynamic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- IEA. Net Zero by 2050; IEA: Paris, France, 2021; Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 23 March 2023).
- Langholtz, M.H.; Stokes, B.J.; Eaton, L.M. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstock; Managed by UT-Battelle, LLC for the US Department of Energy; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016; pp. 1–411.
- Jin, E.; Sutherland, J.W. An integrated sustainability model for a bioenergy system: Forest residues for electricity generation. Biomass Bioenergy 2018, 119, 10–21. [Google Scholar] [CrossRef]
- Hu, W.; Wang, J.; Hu, J.; Schuler, J.; Grushecky, S.; Nan, N.; Smith, W.; Jiang, C. Thermodegradation of naturally decomposed forest logging residues: Characteristics, kinetics, and thermodynamics. Bioresour. Technol. 2023, 376, 128821. [Google Scholar] [CrossRef]
- Seibold, S.; Rammer, W.; Hothorn, T.; Seidl, R.; Ulyshen, M.D.; Lorz, J.; Cadotte, M.W.; Lindenmayer, D.B.; Adhikari, Y.P.; Aragón, R.; et al. The contribution of insects to global forest deadwood decomposition. Nature 2021, 597, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.M.; Ma, X.Q.; Lai, Z.Y. Isoconversional kinetic analysis of co-combustion of sewage sludge with straw and coal. Appl. Energy 2009, 86, 1741–1745. [Google Scholar] [CrossRef]
- Koppejan, J.; Van Loo, S. (Eds.) The Handbook of Biomass Combustion and Co-Firing; Earth Scan; Routledge: London, UK, 2008. [Google Scholar]
- Loeffler, D.; Anderson, N. Emissions tradeoffs associated with cofiring forest biomass with coal: A case study in Colorado, USA. Appl. Energy 2014, 113, 67–77. [Google Scholar] [CrossRef]
- Kukrety, S.; Wilson, D.C.; D’Amato, A.W.; Becker, D.R. Assessing sustainable forest biomass potential and bioenergy implications for the northern Lake States region, USA. Biomass Bioenergy 2015, 81, 167–176. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Hess, J.R.; Boardman, R.D.; Wright, C.T.; Westover, T.L. Formulation, pretreatment, and densification options to improve biomass specifications for co-firing high percentages with coal. Ind. Biotechnol. 2012, 8, 113–132. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Almeida, G.; Brito, J.O.; Perré, P. Alterations in energy properties of eucalyptus wood and bark subjected to torrefaction: The potential of mass loss as a synthetic indicator. Bioresour. Technol. 2010, 101, 9778–9784. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, S.H.; Juang, T.T.; Tsai, C.M.; Zhuang, Y.Q. Characterization of solid and liquid products from bamboo torrefaction. Appl. Energy 2015, 160, 829–835. [Google Scholar] [CrossRef]
- Mi, B.; Liu, Z.; Hu, W.; Wei, P.; Jiang, Z.; Fei, B. Investigating pyrolysis and combustion characteristics of torrefied bamboo, torrefied wood and their blends. Bioresour. Technol. 2016, 209, 50–55. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Doddapaneni, T.R.K.C.; Praveenkumar, R.; Tolvanen, H.; Palmroth, M.R.; Konttinen, J.; Rintala, J. Anaerobic batch conversion of pine wood torrefaction condensate. Bioresour. Technol. 2017, 225, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Xie, L.; Yu, X.; Zhang, X.; Yang, Y.; Rahman, M.M.; Blanco, P.H.; Liu, R.; Bridgwater, A.V.; Cai, J. Poplar wood torrefaction: Kinetics, thermochemistry and implications. Renew. Sustain. Energy Rev. 2021, 143, 110962. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, Z.; Wang, Z.; Ma, C.; Chen, S. A novel on-site wheat straw pretreatment method: Enclosed torrefaction. Bioresour. Technol. 2019, 281, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kai, X.; Meng, Y.; Yang, T.; Li, B.; Xing, W. Effect of torrefaction on rice straw physicochemical characteristics and particulate matter emission behavior during combustion. Bioresour. Technol. 2019, 278, 1–8. [Google Scholar] [CrossRef]
- Gil, M.V.; Casal, D.; Pevida, C.; Pis, J.J.; Rubiera, F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresour. Technol. 2010, 101, 5601–5608. [Google Scholar] [CrossRef]
- Álvarez, A.; Pizarro, C.; García, R.; Bueno, J.L.; Lavín, A.G. Determination of kinetic parameters for biomass combustion. Bioresour. Technol. 2016, 216, 36–43. [Google Scholar] [CrossRef]
- Ma, Q.; Han, L.; Huang, G. Evaluation of different water-washing treatments effects on wheat straw combustion properties. Bioresour. Technol. 2017, 245, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, T.; Chen, K.; Cheng, R.; Chen, S.; Liu, J.; Mei, M.; Li, J.; Xue, Y. Co-combustion behavior of dyeing sludge and rice husk by using TG-MS: Thermal conversion, gas evolution, and kinetic analyses. Bioresour. Technol. 2020, 311, 123527. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Wang, R.; Jiang, C.; Yang, X.; Zhang, T.; Hu, W.; Mi, B.; Liu, Z. Investigating co-combustion characteristics of bamboo and wood. Bioresour. Technol. 2017, 243, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Jenkins, B.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Cordero, T.; Marquez, F.; Rodriguez-Mirasol, J.; Rodriguez, J.J. Predicting heating values of lignocellulosics and carbonaceous materials from proximate analysis. Fuel 2001, 80, 1567–1571. [Google Scholar] [CrossRef]
- Munir, S.; Daood, S.S.; Nimmo, W.; Cunliffe, A.M.; Gibbs, B.M. Thermal analysis and devolatilization kinetics of cotton stalk, sugar cane bagasse and shea meal under nitrogen and air atmospheres. Bioresour. Technol. 2009, 100, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, P.R. Slow pyrolysis of nutshells: Characterization of derived chars and of process kinetics. Energy Sources 2003, 25, 767–778. [Google Scholar] [CrossRef]
- Masiá, A.T.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterising ash of biomass and waste. Fuel Process. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Hu, W.; Feng, Z.; Yang, J.; Gao, Q.; Ni, L.; Hou, Y.; He, Y.; Liu, Z. Combustion behaviors of molded bamboo charcoal: Influence of pyrolysis temperatures. Energy 2021, 226, 120253. [Google Scholar] [CrossRef]
- ASTM D7582-15; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D3176-15; Standard Practice for Ultimate Analysis of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM E711-87; Standard Test Method for Gross Calorific Value of Refuse-Derived Fuel by the Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2004; pp. 1–8.
- Cai, H.; Liu, J.; Xie, W.; Kuo, J.; Buyukada, M.; Evrendilek, F. Pyrolytic kinetics, reaction mechanisms and products of waste tea via TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2019, 184, 436–447. [Google Scholar] [CrossRef]
- Zou, H.; Li, W.; Liu, J.; Buyukada, M.; Evrendilek, F. Catalytic combustion performances, kinetics, reaction mechanisms and gas emissions of Lentinus edodes. Bioresour. Technol. 2020, 300, 1226. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating isothermal life from thermogravimetric data. J. Appl. Polym. Sci. 1962, 6, 639–642. [Google Scholar] [CrossRef]
- Dhyani, V.; Kumar, J.; Bhaskar, T. Thermal decomposition kinetics of sorghum straw via thermogravimetric analysis. Bioresour. Technol. 2017, 245, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Pandey, D.; Patil, T.; Sawarkar, A.N. Pyrolysis of banana leaves biomass: Physico-chemical characterization, thermal decomposition behavior, kinetic and thermodynamic analyses. Bioresour. Technol. 2020, 310, 123464. [Google Scholar] [CrossRef] [PubMed]
- Goodell, B.; Qian, Y.; Jellison, J. Fungal Decay of Wood: Soft Rot-Brown Rot-White Rot. In Development of Commercial Wood Preservatives; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008; Chapter 2; pp. 9–31. [Google Scholar] [CrossRef]
- ISO 17225-2: 2021; Solid Biofuels—Fuel Specifications and Classes—Part 2: Graded Wood Pellets. International Organization for Standardization: Geneva, Switzerland, 2021.
- ISO 17225-2; Solid Biofuels—Fuel Specifications and Classes—Part 2: Graded Wood Pellets. The British Standards Institution: London, UK, 2021. Available online: https://www.iso.org/standard/76088.html (accessed on 23 May 2022).
- Demirbaş, A. Relationships between lignin contents and heating values of biomass. Energy Convers. Manag. 2001, 42, 183–188. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Kim, S.; Kramer, R.W.; Hatcher, P.G. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef]
- El-Hendawy, A.N.A. Variation in the FTIR spectra of a biomass under impregnation, carbonization and oxidation conditions. J. Anal. Appl. Pyrolysis 2006, 75, 159–166. [Google Scholar] [CrossRef]
- Lu, X.; Pellechia, P.J.; Flora, J.R.; Berge, N.D. Influence of reaction time and temperature on product formation and characteristics associated with the hydrothermal carbonization of cellulose. Bioresour. Technol. 2013, 138, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Foong, S.Y.; Ma, N.L.; Liew, R.K.; Mahari, W.A.W.; Xia, C.; Yek, P.N.Y.; Peng, W.; Nam, W.L.; Lim, X.Y.; et al. Vacuum pyrolysis incorporating microwave heating and base mixture modification: An integrated approach to transform biowaste into eco-friendly bioenergy products. Renew. Sustain. Energy Rev. 2020, 127, 109871. [Google Scholar] [CrossRef]
- Liang, F.; Wang, R.; Xiang, H.; Yang, X.; Zhang, T.; Hu, W.; Mi, B.; Liu, Z. Investigating pyrolysis characteristics of moso bamboo through TG-FTIR and Py-GC/MS. Bioresour. Technol. 2018, 256, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Aslan, D.I.; Parthasarathy, P.; Goldfarb, J.L.; Ceylan, S. Pyrolysis reaction models of waste tires: Application of Master-Plots method for energy conversion via devolatilization. Waste Manag. 2017, 68, 405–411. [Google Scholar] [CrossRef] [PubMed]


| Samples | N | C | H | S | O * | Volatile | Ash | FC * | HHV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|
| (wt.%, Dry Basis) | |||||||||
| RO0 | 0.84 | 48.54 | 6.34 | - | 44.29 | 78.90 | 0.29 | 19.93 | 20.14 |
| RO2 | 0.72 | 48.39 | 6.33 | - | 44.56 | 78.20 | 0.31 | 20.59 | 19.74 |
| RO4 | 0.77 | 48.97 | 6.36 | - | 43.91 | 78.40 | 0.16 | 20.48 | 18.85 |
| TRO0 | 0.46 | 53.80 | 5.76 | - | 39.97 | 68.10 | 0.51 | 29.18 | 22.93 |
| TRO2 | 0.68 | 55.14 | 5.46 | - | 38.72 | 66.10 | 0.56 | 30.91 | 22.80 |
| TRO4 | 0.43 | 55.63 | 5.42 | - | 38.53 | 66.30 | 0.22 | 30.50 | 22.99 |
| Samples | Heating Rate (°C/min) | Stage 1 | Stage 2 | ||||
|---|---|---|---|---|---|---|---|
| Temp. Range (°C) | Peak Temp. (°C) | Weight Loss (wt.%) | Temp. Range (°C) | Peak Temp. (°C) | Weight Loss (wt.%) | ||
| RO0 | 10 | 176.4–396.4 | 331.8 | 67.0 | 396.4–513.8 | 475.8 | 25.4 |
| 20 | 184.6–414.2 | 347.5 | 69.8 | 414.2–545.9 | 496.1 | 24.8 | |
| 30 | 197.7–425.4 | 356.9 | 70.5 | 425.4–570.8 | 510.8 | 24.1 | |
| 40 | 202.0–427.7 | 363.0 | 68.8 | 427.7–577.7 | 514.7 | 25.7 | |
| RO2 | 10 | 182.8–391.7 | 331.4 | 67.1 | 391.7–515.4 | 478.5 | 26.4 |
| 20 | 190.6–401.4 | 345.5 | 70.2 | 401.4–530.9 | 489.1 | 23.9 | |
| 30 | 192.7–418.4 | 351.9 | 69.7 | 418.4–566.9 | 507.0 | 24.5 | |
| 40 | 202.6–423.4 | 358.6 | 70.7 | 423.4–582.0 | 496.8 | 23.4 | |
| RO4 | 10 | 181.7–393.8 | 330.0 | 67.3 | 393.8–542.6 | 491.7 | 26.1 |
| 20 | 184.7–405.4 | 344.3 | 68.2 | 405.4–555.5 | 494.5 | 23.9 | |
| 30 | 194.6–414.8 | 351.3 | 69.6 | 414.8–571.5 | 497.8 | 24.4 | |
| 40 | 204.0–414.5 | 358.0 | 69.1 | 414.5–588.4 | 516.8 | 24.9 | |
| TRO0 | 10 | 229.5–402.6 | 328.3 | 57.0 | 402.6–512.7 | 477.3 | 39.7 |
| 20 | 245.2–407.8 | 342.6 | 55.5 | 407.8–548.1 | 490.8 | 41.6 | |
| 30 | 252.3–417.3 | 351.1 | 54.4 | 417.3–583.4 | 526.1 | 42.7 | |
| 40 | 254.7–418.6 | 360.2 | 53.1 | 418.6–639.4 | 533.9 | 43.9 | |
| TRO2 | 10 | 230.9–391.2 | 326.6 | 52.9 | 391.2–523.3 | 478.7 | 43.8 |
| 20 | 240.8–401.7 | 340.6 | 50.7 | 401.7–554.4 | 504.3 | 46.4 | |
| 30 | 246.1–404.2 | 347.6 | 49.3 | 404.2–580.1 | 516.8 | 47.8 | |
| 40 | 252.3–413.9 | 354.4 | 52.1 | 413.9–609.7 | 525.7 | 44.8 | |
| TRO4 | 10 | 226.4–387.8 | 324.5 | 52.6 | 387.8–526.7 | 475.7 | 44.2 |
| 20 | 251.7–409.1 | 340.0 | 54.8 | 409.1–568.9 | 501.8 | 44.3 | |
| 30 | 253.9–410.3 | 347.2 | 52.6 | 410.3–597.4 | 516.9 | 44.2 | |
| 40 | 255.7–418.7 | 356.4 | 53.3 | 418.7–603.4 | 535.8 | 43.6 | |
| Sample | Stage 1 | Stage 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| KAS | FWO | KAS | FWO | |||||
| Ea (KJ/mol) | SE | Ea (KJ/mol) | SE | Ea (KJ/mol) | SE | Ea (KJ/mol) | SE | |
| RO0 | 157.77 | 10.71 | 159.30 | 10.21 | 131.32 | 7.38 | 136.39 | 7.06 |
| RO2 | 157.12 | 7.88 | 158.63 | 7.45 | 147.31 | 29.80 | 151.50 | 28.30 |
| RO4 | 149.52 | 3.03 | 151.39 | 2.89 | 181.83 | 21.08 | 184.42 | 20.00 |
| TRO0 | 163.18 | 10.66 | 164.71 | 10.13 | 92.63 | 18.29 | 99.88 | 17.28 |
| TRO2 | 172.52 | 7.95 | 173.54 | 7.54 | 136.47 | 15.00 | 141.37 | 14.18 |
| TRO4 | 137.30 | 8.87 | 140.05 | 8.42 | 112.63 | 8.37 | 118.73 | 7.90 |
| Sample | Stage 1 | Stage 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| KAS | FWO | KAS | FWO | |||||
| A (min−1) | SE | A (min−1) | SE | A (min−1) | SE | A (min−1) | SE | |
| RO0 | 1.25 × 1011 | 1512.77 | 3.27 × 1017 | 107.81 | 2.45 × 1006 | 808.08 | 9.38 × 1012 | 53.37 |
| RO2 | 1.14 × 1011 | 1392.55 | 3.03 × 1017 | 68.57 | 2.30 × 1007 | 8843.92 | 8.95 × 1013 | 1747.48 |
| RO4 | 2.43 × 1010 | 1206.81 | 6.49 × 1016 | 41.85 | 2.06 × 1011 | 2907.76 | 7.76 × 1017 | 588.13 |
| TRO0 | 2.56 × 1016 | 2355.99 | 7.85 × 1022 | 676.88 | 1.18 × 1006 | 2017.24 | 4.43 × 1012 | 429.93 |
| TRO2 | 1.45 × 1015 | 1515.20 | 4.37 × 1021 | 257.17 | 4.17 × 1008 | 1527.58 | 1.54 × 1015 | 171.97 |
| TRO4 | 4.81 × 1010 | 3890.80 | 1.42 × 1017 | 2263.28 | 4.72 × 1005 | 827.18 | 1.74 × 1012 | 119.442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Wang, J.; Hu, J.; Schuler, J.; Grushecky, S.; Jiang, C.; Smith, W.; Nan, N.; Sabolsky, E.M. Combustion Behaviors, Kinetics, and Thermodynamics of Naturally Decomposed and Torrefied Northern Red Oak (Quercus rubra) Forest Logging Residue. Energies 2024, 17, 1607. https://doi.org/10.3390/en17071607
Hu W, Wang J, Hu J, Schuler J, Grushecky S, Jiang C, Smith W, Nan N, Sabolsky EM. Combustion Behaviors, Kinetics, and Thermodynamics of Naturally Decomposed and Torrefied Northern Red Oak (Quercus rubra) Forest Logging Residue. Energies. 2024; 17(7):1607. https://doi.org/10.3390/en17071607
Chicago/Turabian StyleHu, Wanhe, Jingxin Wang, Jianli Hu, Jamie Schuler, Shawn Grushecky, Changle Jiang, William Smith, Nan Nan, and Edward M. Sabolsky. 2024. "Combustion Behaviors, Kinetics, and Thermodynamics of Naturally Decomposed and Torrefied Northern Red Oak (Quercus rubra) Forest Logging Residue" Energies 17, no. 7: 1607. https://doi.org/10.3390/en17071607
APA StyleHu, W., Wang, J., Hu, J., Schuler, J., Grushecky, S., Jiang, C., Smith, W., Nan, N., & Sabolsky, E. M. (2024). Combustion Behaviors, Kinetics, and Thermodynamics of Naturally Decomposed and Torrefied Northern Red Oak (Quercus rubra) Forest Logging Residue. Energies, 17(7), 1607. https://doi.org/10.3390/en17071607






