Abstract
Converting carbon dioxide (CO2) into valuable chemicals such as fossil resources via photocatalysis requires the development of advanced materials. Herein, we coupled zirconium-based metal–organic frameworks (MOFs) containing porphyrin and Cu-porphyrin with anatase TiO2. The effect of the porphyrin metalation proportion was also investigated. Notably, while the use of free-base porphyrin as the organic linker resulted in the development of PCN-224, the presence of Cu-porphyrin provided mixed-phase MOF structures containing both PCN-224 and PCN-222. MOF/TiO2 composites bearing partial (50%) metalated porphyrin were proven more active and selective towards the production of CH4, at ambient conditions, in the gas phase and using water vapors without the use of hole scavengers. The optimized composite bearing 15 wt.% of the partial metalated MOF was three times more active than pure TiO2 towards CH4 production. This study provides insights on the effect of precise materials engineering at a molecular level on the development of advanced MOF-based photocatalysts for CO2 reduction.
1. Introduction
Currently, there is a great need for alternatives to deal with the increasing global energy requirements, the fossil resource depletion, but mostly the environmental crisis originating from the rapid CO2 accumulation in the atmosphere due to fossil fuel usage. To this end, photocatalysis has the potential to contribute both to clean energy production and to the utilization of CO2 as a cheap and easily available carbon-source, converting it into valuable feedstocks [1]. This process greatly depends on the properties of the photocatalysts used. Therefore, many different types of materials have been developed and applied in CO2 photocatalytic reduction. As in other photocatalytic reactions, TiO2 has been intensively studied in CO2 reduction [2,3,4]. However, inherent drawbacks related to fast charge carriers’ recombination and UV-light response prevent further application of TiO2. Different strategies have been applied to develop improved TiO2-based photocatalysts for CO2 reduction including modification via ion doping [5], surface engineering [6], photosensitization [7] and formation of composites and heterojunctions [3,8]. The latter approach synergizes materials, offering the possibility to develop advanced systems and improve the properties of traditional catalysts such as TiO2 [9].
Over the last decade, the interest in metal–organic frameworks (MOFs) as heterogeneous photocatalysts has grown significantly, mostly due to their tunable properties and high specific surface area that facilitates adsorption [10,11,12]. MOFs have been applied as photocatalysts either individually [10,12,13,14] or as part of a composite material [9,10,11,15] for CO2 reduction. TiO2-based hybrid photocatalysts bearing MOF structures have been developed and tested in CO2 reduction as a way to integrate increased light harvesting, active catalytic sites and high surface areas [3,9,16,17,18], presenting superior activity in CO2 reduction but also in selectivity towards C-products. Improvements in several important parameters have been reported that enhanced CO2 conversion in such composite materials [9,16,18]. Among the different MOFs studied, of particular importance are stable structures that contain porphyrins as organic linkers. These structures offer the advantage of visible light absorption capacity [19,20], the ability for efficient and fast exciton migration and transportation from the photoexcited porphyrin moiety to suitable catalysts [21,22] and the presence of a metalation center [23] that may affect the electronic properties and control catalytic activity.
Porphyrinic zirconium MOFs have been studied in CO2 photocatalytic reduction [14,23,24,25]. The Zr ions in the metal clusters may have multiple redox states (i.e., 4+ or 3+) [23,25] and act as semiconductors while the photoexcited porphyrin linker can activate these clusters [25,26,27]. Heterojunctions have been developed via the coupling of such MOF structures with other photocatalysts [18,27], improving charge recombination phenomena. In addition, metalation of the porphyrin linker as well as the degree of metalation (partial metalated porphyrins) may also affect the overall photocatalytic activity [19,23,28,29,30].
In view of the recent contributions and driven by the different possibilities to tune the activity of porphyrin MOF/TiO2 photoactive materials, a series of porphyrinic-MOF/TiO2 composite materials were prepared by adjusting the degree of metalation using free-base or copper-metalated porphyrin as the linker. It is shown that both photocatalytic activity and selectivity towards H2, CO and CH4 may be controlled by varying the metalation degree of the porphyrin linker.
2. Materials and Methods
Zirconium chloride, benzoic acid and N,N-Dimethylformamide (anhydrous, 99.8%), Cu(NO3)2•3H2O and tetra(4-carboxylphenyl)porphyrin (H2TCPP) were supplied by Sigma Aldrich, Burlington, MA, USA. TiO2 (Hombikat UV100) was purchased from Sachtleben Chemie GmbH (Duisburg, Germany). The chemical reagents were used without further purification.
2.1. H2TCPP Metalation
Metalation of the H2TCPP was performed using a reported protocol with small modifications [31]. Specifically, 0.3047 g of Cu(NO3)2•3H2O and 0.1970 g H2TCPP were dissolved in 15 mL of DMF. The mixture was heated at 150 °C under reflux for 5 h. Then, the solution was cooled down naturally, and the precipitate was collected by filtration and was rinsed with DMF and EtOH. The complex formed was labeled CuTCPP.
2.2. MOF Synthesis
MOF structures were synthesized using a typical protocol already reported in the literature for the development of PCN-224 [32]. Specifically, a mixture of 0.173 g H2TCPP, 0.288 g ZrCl4 and 9.73 g benzoic acid was dissolved in 29 mL DMF and put in sonication till all the precursors were dissolved. Then, the solution was transferred to a 90 mL teflon-lined stainless steel autoclave and heated at 120 °C for 48 h. After cooling down, the resultant black powder was collected with filtration and washed several times with fresh DMF and, finally, with acetone. Then, the powder was vacuum dried at 120 °C overnight to remove any remaining solvent. For the development of the MOF structure containing the metalated porphyrin, the same procedure was followed, but CuTCPP was used. Two different approaches were used, where the organic ligand concentration was identical to the synthesis using the free-base H2TCPP. In the first, a molar ratio of H2TCPP:CuTCPP equal to 1:1 was used, while in the second, only CuTCPP was used. The resulted MOFs were denoted as MOF-a, MOF-b and MOF-c using only H2TCPP, H2TCPP:CuTCPP at 1:1 molar ratio and only CuTCPP, respectively.
2.3. Synthesis of MOF/TiO2 Composite Materials
The development of the composites was performed by in situ synthesis of the MOF structure in the presence of TiO2, using the same protocol as the one used for the pure MOF synthesis. The precursors of the MOF structure were mixed with 1.663 g of TiO2 powder and sonicated for 30 min. Three composite materials were developed by adding either H2TCPP, a mixture of H2TCPP:CuTCPP at 1:1 molar ratio or only CuTCPP. The composites were denoted as MOF-a/TiO2, MOF-b/TiO2 and MOF-c/TiO2 using H2TCPP, a mixture of H2TCPP:CuTCPP or only CuTCPP as precursors, respectively. An illustration depicting the steps used in the synthesis protocol is given in Scheme 1.
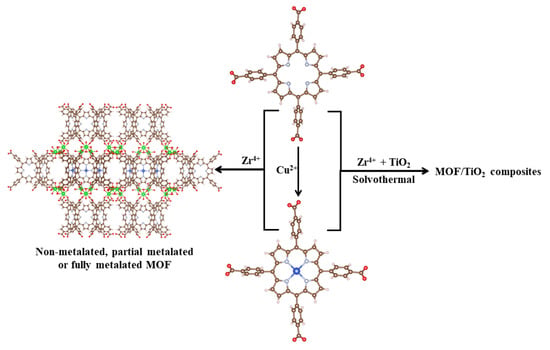
Scheme 1.
Schematic illustration of the synthesis protocol used to develop the MOFs and composite materials.
2.4. Pt Deposition
The introduction of Pt (1 wt.%) was realized by a chemical reduction deposition method, using H2PtCl6 and NaBH4 as the reducing agent following a process already reported [4].
2.5. Materials Characterization
X-ray diffraction (XRD) patterns were recorded using a Bruker D8-Advance (Karlsruhe, Germany) diffractometer equipped with a scintillation counter detector, with a Cu-Kα radiation. Attenuated Total Reflectance IR spectra were measured with a Thermo Fisher Nicolet iS10 spectrometer (Waltham, MA, USA). Nitrogen adsorption–desorption isotherms were obtained using a Micromeritics Asap 2420 porosimeter (Norcross, GA, USA) at 77 K. Prior to the analysis, the samples were degassed at 150 °C under vacuum for 5 h. Thermogravimetric analyses (TGA) were performed using a TA Instrument Q5000IR (New Castle, DE, USA) under the air flow (flow rate of 25 mL·min−1) with the temperature ranging from 50 to 600 °C, with a dynamic ramping rate depending on the weight loss. Diffuse Reflectance UV-vis absorption spectra were recorded in the range of 200–800 nm using a PerkinElmer Lambda 950 Scan spectrophotometer (Waltham, MA, USA) equipped with integrating sphere using BaSO4 as a reference; the energy bandgap was calculated by using the Tauc plot. A Zeiss Gemini SEM 500 (Munich, Germany) equipped with FEG Schottky was used for Scanning Electron Microscopy. X-ray photoelectron spectroscopy (XPS) measurements were performed on an ultrahigh vacuum (UHV) Thermo-VG scientific Spectrometer source, and the spectra were calibrated by the Adventitious Carbon peak at 284.6 eV. A Zeiss Gemini SEM 500 equipped with FEG Schottky was used for Scanning Electron Microscopy.
2.6. Photocatalytic Reactions
The photocatalytic evaluation was performed under continuous flow conditions. Fifty mg of the catalyst suspension was drop-casted on a glass disc and dried under vacuum for 2 h at 100 °C. The disc was then placed in a stainless-steel reactor (6 mL) that was irradiated from the top through a quartz glass window. A defined flow (0.3 mL·min−1) of research-grade CO2 was passed through a thermo-controlled water saturator, feeding the reactor. Prior to the test, the reactor was purged with CO2/H2O till all impurities were eliminated. During the test, a Hg lamp (150 W, Ceramic-Metal-Halide, C-Topspot, StrassElec SARL, Strasbourg, France) simulating solar light was used as an irradiation source (irradiances in the 300–800 nm region: UV = 46 W·m−2, Visible = 4271 W·m−2, the emission spectrum is given in Figure S1). The evolved gases were analyzed every 15 min by a micro-GC (Agilent 3000 A SRA Instrument, Santa Clara, CA, USA).
3. Results and Discussion
Figure S2a presents the UV-Vis spectra of the H2TCPP before (free-base) and after the metalation (CuTCPP). The H2TCPP presented an intense Soret band centered at ca. 415 nm and four Q bands (516, 555, 580 and 635 nm), typical of the free-base H2TCPP. After metalation, a blue shift of the Soret band and the presence of only two Q-bands were evidenced for the CuTCPP complex. This observation suggests the successful incorporation of copper in the porphyrin core [33]. To further evidence the TCPP metalation, infrared spectra were also recorded (Figure S2b). The peak centered at 966 cm−1 assigned to the N–H in-plane bending vibration of pyrrolic nitrogen became undetectable after the treatment with Cu2+, suggesting an interaction between the –NH groups inside the porphyrin ring and copper ions [34,35,36]. In addition, a peak at ca. 1000 cm−1 was detected at the CuTCPP complex that is assigned to the bond stretching/bending vibration between Cu2+ and TCPP ligand [37]. The shift of the peak at 1685 cm−1 to 1654 cm−1 and the presence of a strong peak at 1400 cm−1 suggest alteration of the –COOH groups of the porphyrin ring, probably via the interaction with Cu2+ ions. As will be shown later, this did not affect the development of the MOF structure. Therefore, both UV-Vis and ATR spectroscopies verified the successful coordination of Cu2+ within the porphyrin core.
XRD analysis was then performed in order to get information on the structure of both TiO2 and the MOF synthesized. The diffraction patterns are given in Figure 1. A typical diffraction pattern of PCN-224 was acquired for the material synthesized using only the free-base H2TCPP, i.e., MOF-a [38]. In the MOF-b and MOF-c, an increase in the diffraction peak width was evidenced with increasing the CuTCPP content. In addition to this, the intensity of the peak centered at ca 7.2° increased going from MOF-b to MOF-c, and a peak centered at ca. 9.9° was clearly observed. These new peaks are fingerprints of the PCN-222 structure [39] and suggest the presence of more than one porphyrinic MOFs when CuTCPP is used. More particular, in addition to the PCN-224, PCN-222 was also formed using the CuTCPP complex in the synthesis process [39,40]. As expected, the TiO2 was of pure anatase phase. Close inspection of the anatase (101) main diffraction peak centered at ca. 25.4° revealed that the synthesis employed for the development of the MOF did not alter the characteristics of the TiO2 phase in terms of phase composition and crystallinity. This is probably related to the mild conditions applied for the development of the MOF. The XRD patterns of all nanocomposite materials contained the diffraction peaks of the two individual counterparts, as displayed in Figure 1b. The low intensity of the diffraction peaks corresponding to the MOF structure probably originates from the low MOF amount in the final composite material.
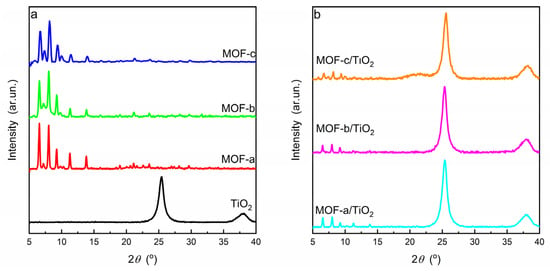
Figure 1.
XRD patterns of (a) pure TiO2 and MOFs and (b) nanocomposite materials.
Further evidence for the presence of both phases in the composites was obtained from ATR spectroscopy (Figure S3). Typical spectra of the pure TiO2 and porphyrin MOFs were acquired. Interestingly, the peak centered at ca. 1683 cm−1 that corresponds to the C=O stretching vibration from the carboxyl functional groups greatly weakened in the MOF spectra when compared with the H2TCPP, suggesting the successful coordination of the porphyrin’s –COOH groups with Zr to form the MOF crystal [41,42]. Most importantly, the peak centered at ca. 1000 cm−1, assigned to the successful coordination of Cu2+ in the porphyrin core was also detected in the MOFs prepared by using in part or only CuTCPP as the organic linker [43]. This was accompanied by the disappearance of the peak assigned to the N–H in-plane bending vibration of pyrrolic nitrogen (centered at ca. 963 cm−1) [34,35,36] that was only observed in the MOF-a material developed using the free-base H2TCPP. These data suggest that Cu2+ remained in the porphyrin core even after the development of the MOF structure. TiO2 gave rise to a featureless broad peak at low wavenumbers (400–920 cm−1) that was also evidenced in the composite materials [44]. As in the case of XRD, the ATR spectra of the composite materials contained features from the two individual counterparts.
Light absorption properties were studied by means of UV–vis diffuse reflectance spectroscopy (DRS). The corresponding spectra are given in Figure S4. The light absorption onset of the pure TiO2 was below 400 nm corresponding to a band gap energy (Eg) of ca. 3.20 eV, typical for pure anatase materials. On the contrary, light absorption extended to the whole visible region for the pure MOF materials. This is due to the Soret and the Q bands of the porphyrin linker that absorb light from 350 up to 800 nm [45]. Similar light absorption properties were also evidenced for all composites. These findings clearly suggest that the extension of light absorption to the visible region detected for the composite materials can be solely attributed to the presence of the MOF structure.
Furthermore, differences were also detected in the absorption in the visible light region when comparing the MOF structures developed using the free-base H2TCPP and the CuTCPP complex. Specifically, the four Q bands detected on the MOF structure where the free-base TCPP ligand was used were converted into two, both in the pure MOFs and the composite materials. This is in line with results in the literature where metalated porphyrins were used to develop porphyrinic MOF structures [43,45]. These findings further suggest that the porphyrin core was metalated in the MOFs where CuTCPP was used as the organic linker, in agreement with the ATR results.
Quantification of the two parts in the final composites was performed by thermogravimetric analysis (TGA). Representative TGA profiles are given in Figure S5. The small mass drop up to 200 °C can be attributed to impurities on the surface of TiO2 and guest species in the MOF structures. The temperature onset of the organic decomposition in the pure MOFs is observed at ca. 425 °C. This is slightly shifted to lower values in the composites, probably due to the presence of the oxide. The remaining weight at 600 °C was used to calculate the content of the MOF in the hybrid materials. This was practically constant within the three composite samples ranging from (13 to 15 wt.%).
The composition of the prepared materials was further investigated using XPS. The survey spectra revealed the presence of Ti, O, C, N, Zr and Cu in the corresponding samples (Figure S6). Cu was only detected in the MOF structures where CuTCPP was used as the organic ligand. High resolution XPS spectra are shown in Figure 2. The peak centered at 184.7 and 182.3 eV are ascribed to Zr 3d3/2 and Zr 3d5/2. The Zr peaks were shifted to lower binding energies in the case of the composite materials compared to the signal recorded for the bare MOFs. Similarly, the Ti 2p peak in the composite materials was shifted to higher binding energies (ca. 0.2 eV), compared to the Ti 2p3/2 of the pure TiO2 (Figure 2b). This observation indicates a strong interaction between the MOF and TiO2 and suggests the presence of a pathway for charge transfer between the two parts of the composite [46]. High resolution XPS spectra in the 402–397 eV region revealed the presence of N-species from the organic linker (Figure 2c). Two peaks were observed for MOF-a. More precisely, the peak at ca. 397.8 eV originates from the iminic nitrogen (–C=N–), and the peak at 400 eV is ascribed to pyridinic nitrogen (–NH–) of the free-base porphyrin [47]. Τhe N 1s spectrum of the MOF-c, developed using the metalated CuTCPP as the organic linker, presented only one peak, attributed to N interacting with Cu [48], centered between the two peaks of free-base porphyrin at 398.4 eV [49]. This further verifies that the porphyrin remained metalated even after the synthesis of the MOF, in agreement with ATR and DRS-UV-Vis. Finally, based on XPS surface composition analysis, the Cu content was estimated to be 0.47% in the pure MOF-c and 0.06% in the corresponding MOF-c/TiO2 composite. This is expected, taking into account the ca. 15% of the MOF-c in the MOF-c/TiO2 composite, as extracted from TGA.
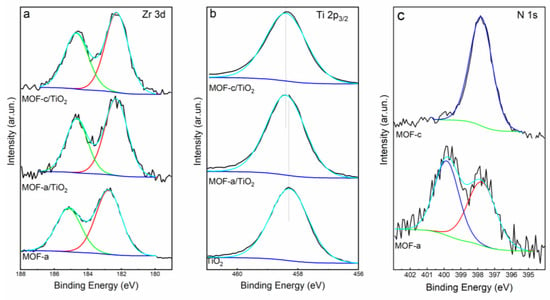
Figure 2.
High resolution XPS spectra of Zr 3d (a), Ti 2p3/2 (b) and N 1s (c) of the prepared pure and composite materials and the corresponding simulated spectra.
N2 sorption–desorption isotherms were contacted to evaluate the textural properties (Figure S7). The MOF-a material developed using only H2TCPP as the organic linker presented a type I isotherm indicating microporosity [35] and a Brunauer–Emmett–Teller (BET) surface area of 1617 m2 g−1. On the contrary, MOF-c presented a type IV isotherm, with two plateaus at ca. P/P0 = 0.3 and a slightly increased BET surface area (1739 m2 g−1). The presence of mesoporosity is characteristic of the PCN-222 structure [39] and is in line with the XRD results. Surface area significantly decreased in all composite materials, with the isotherm resembling that of the pure TiO2, probably due to the low MOF content.
To further establish the formation of composites and to elucidate morphological characteristics that may have given rise to the different textural properties and crystal structures, a microscopy analysis of the prepared materials was undertaken. Figure 3 presents SEM images of the pure MOF structures. A cubic morphology in the range of micrometers was evidenced for the MOF-a material developed using H2TCPP, typical of the PCN-224 structure (Figure 3a). In addition to the cubes that were clearly formed when CuTCPP was used in the synthesis process, needle-shaped crystals were also observed (MOF-b and MOF-c, Figure 3b–d). Their length varied from a few up to tenths of micrometers and their width from hundreds of nanometers up to a few micrometers. Particles of this morphology are ascribed to the formation of PCN-222 [39], revealing that MOF-b and MOF-c are mixed phase materials containing both PCN-224 and PCN-222. These two MOFs have identical building units but differ in topology [14]. The PCN-222 content as well as the size of the needle-shaped particles increased by increasing the CuTCPP content during the synthesis. In addition, more uniform-sized PCN-222 particles and less resolved cubic particles were detected in the MOF-c material when compared with the MOF-b. This clearly suggests that the use of CuTCPP as the organic linker precursor favors the formation of PCN-222 under the conditions of our experiment. Formation of PCN-222 also explains the two plateaus in the N2 isotherms and the mesoporosity observed for the MOF-b and MOF-c materials.
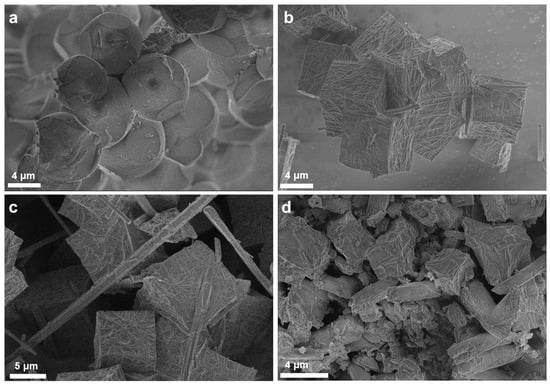
Figure 3.
SEM images of the MOF-a (a), MOF-b (b,c) and the MOF-c (d) materials.
These morphological characteristics were maintained in the composite materials (Figure 4). In addition to the MOF particles, large TiO2 agglomerates of few micrometers were observed in all three composites. Well dispersed TiO2 particles on the surface of the MOFs were also resolved, forming agglomerates of significantly smaller size. For example, the size of TiO2 agglomerates in the pure TiO2 ranged between few hundreds of nanometers to micrometers, while agglomerates in the nanometer scale were detected on the surface of the MOF particles in the composites (Figure 4f). This may affect catalytic activity due to the increase in the exposed active sites due to the smaller TiO2 agglomerates in the composites [50].
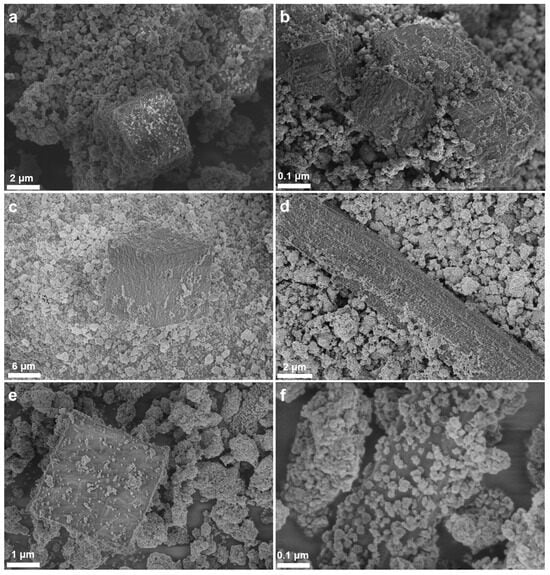
Figure 4.
SEM images of the MOF-a/TiO2 (a,b), MOF-b/TiO2 (c,d) and the MOF-c/TiO2 (e,f) composite materials.
Following the characterization, all materials were tested as photocatalysts for the reduction of CO2 in gas phase using water vapor as the electron donor. Under these conditions, H2, CO and CH4 were only detected in the gas phase. Figure 5 presents the average production rates of the catalytic reactions performed over 10 h using the pure TiO2 and the three composite materials. Under the same catalytic conditions, no products were detected using the pure MOF structures. Only H2 and minimal amount of CH4 were detected using the MOF-a/TiO2 and the pure TiO2. Photocatalytic activity improved significantly when CuTCPP was used in the synthesis process. Specifically, H2 and CH4 production increased five and three times in the MOF-b/TiO2 compared with the TiO2 and MOF-a/TiO2 composite. In addition, CO was also detected in the gas phase. Photocatalytic activity for H2 and CH4 production decreased in the MOF-c/TiO2 compared with the MOF-b/TiO2; however, it was higher compared with the pure TiO2 and the MOF-a/TiO2 composite. The smaller PCN-222 crystals observed in MOF-b compared with MOF-c (Figure 3) could play a role in the improved catalytic activity. Besides activity, the selectivity among the C-products was also improved towards CH4 production using the MOF-b/TiO2 compared with the MOF-c/TiO2. The increased catalytic activity of the MOF-b/TiO2 and MOF-c/TiO2 is also evidenced in Figure S8, which presents the total e− utilized by the catalysts taking into account all reaction products (H2, CH4 and CO). The use of the metalated CuTCPP complex resulted in an impressive enhancement in activity. The MOF-b/TiO2 composite presented the highest activity, an approximately 4-fold increase compared to the reference pure TiO2 catalyst. These results are in accordance with recent contributions to the literature. PCN-222 has been previously reported more active in CO2 photocatalytic reduction compared with PCN-224 using a light sensitizer [14]. In addition, partial metalated porphyrins have been also proven more active in photocatalytic CO2 reduction using a hydrogen-bonded organic framework [29].
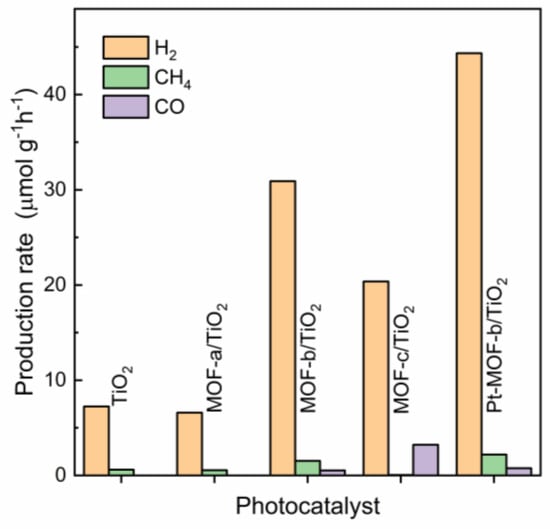
Figure 5.
Photocatalytic activity of the prepared materials.
Of importance is to mention that no carbon-products were detected when CO2 was replaced with argon. This clearly evidences that the C-products detected in the gas phase using the composite materials as catalysts originate from CO2 and not from any carbons on the surface of TiO2 or the MOF structure. Furthermore, to exclude the effect of the porphyrin ligand acting simply as an antenna, a TiO2 material loaded with porphyrin was prepared and tested, resulting in no production of any gaseous products. This reference reaction provides clear evidence for the beneficial effect of the MOF in the observed increased activity of the composite materials. Finally, since Pt nanoparticles have been widely applied as co-catalyst in similar reactions, the best performing composite was loaded with 1 wt.% Pt. It is generally accepted that Pt improves charge separation efficiency acting as an e− sink. The presence of Pt increased H2 and CH4 production by ca. 50%, which could be assigned to the improved charge availability.
Several parameters could contribute to improved catalytic efficiency. The integrated MOF structure in the composite provided extension of the light harvesting to the visible region [18]. Formation of a heterojunction in such a type of composites may improve charge handling properties [9,32,50,51]. The conduction band (CB) and valence band of both PCN-224 and PCN 222 have been reported to be less positive than that of anatase [18,23,32]. Therefore, taking into account that both TiO2 and MOF are excited under artificial solar light irradiation and the tight interaction of the two parts in the composite as revealed by XPS, efficient charge separation may take place due to their matched band structure [18,23,32]. In addition, the presence of CuTCPP complex as an organic ligand in the MOF structure could also function as a catalytic site, improving CO2 reduction and H2 production [52]. Regarding the effect of the relative CuTCPP portion, it has been recently demonstrated in similar structures that the CuTCPP/H2TCPP loading ratio affects greatly the charge carrier separation efficiency where charge is transferred from the free-base H2TCPP to the CuTCPP [29]. This could also improve charge formation and separation MOFs containing both H2TCPP and CuTCPP. All these parameters could contribute in the observed improved catalytic activity.
4. Conclusions
For the first time, porphyrinic zirconium MOF/TiO2 composites controlling the free-base porphyrin/metalloporphyrin (CuTCPP) loading ratio were developed. Detailed characterization of the prepared materials revealed that the use of CuTCPP as the organic linker resulted in the development of mixed phase MOFs, allowing the development of both PCN-224 and PCN-222. Photocatalytic activity evaluation revealed that the use of metalated porphyrin as well as regulating the proportion significantly affects CO2 reduction efficiency and selectivity, presenting a 5- and 3-fold increased H2 and CH4 production, respectively, compared with the pure TiO2 or composites with MOF structures bearing only the free-base porphyrin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en17061483/s1, Figure S1: Irradiance spectra; Figure S2: UV-Vis (a) and ATR (b) spectra of the of the H2TCPP and CuTCPP complex; Figure S3: ATR spectra of TiO2, pure MOFs and composite nanomaterials; Figure S4: DR-UV-Vis spectra of the pure MOFs and TiO2 and composite materials; Figure S5: TGA profile of TiO2, synthesized MOFs and composite materials; Figure S6: Survey spectra of the prepared materials; Figure S7: Nitrogen sorption/desorption isotherms; Figure S8: electrons used in the reactions.
Author Contributions
Conceptualization, K.C.C.; methodology, K.C.C.; software, V.K.; validation, M.A.; formal analysis, M.A., V.K. and K.C.C.; investigation, M.A.; resources, V.K. and K.C.C.; data curation, M.A.; writing—original draft preparation, M.A.; writing—review and editing, V.K. and K.C.C.; visualization, M.A. and K.C.C.; supervision, V.K. and K.C.C.; project administration, K.C.C.; funding acquisition, K.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research benefited from state aid managed by the French National Research Agency (ANR) under the Investments for the Future (program “Make Our Planet Great Again”) with reference ANR-18-MOPGA-0014. This research has received funding from the European Union under grant agreement No. 101099717—ECOLEFINS project.
Data Availability Statement
The data presented in this study are available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sayre, H.J.; Tian, L.; Son, M.; Hart, S.M.; Liu, X.; Arias-Rotondo, D.M.; Rand, B.P.; Schlau-Cohen, G.S.; Scholes, G.D. Solar Fuels and Feedstocks: The Quest for Renewable Black Gold. Energy Environ. Sci. 2021, 14, 1402–1419. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Duflot, M.; Marchal, C.; Caps, V.; Artero, V.; Christoforidis, K.; Keller, V. Optimization of NH2-UiO-66/TiO2/Au Composites for Enhanced Gas-Phase CO2 Photocatalytic Reduction into CH4. Catal. Today 2023, 413–415, 114018. [Google Scholar] [CrossRef]
- Ioannidou, T.; Anagnostopoulou, M.; Vasiliadou, I.A.; Marchal, C.; Alexandridou, E.-O.; Keller, V.; Christoforidis, K.C. Mixed Phase Anatase Nanosheets/Brookite Nanorods TiO2 Photocatalysts for Enhanced Gas Phase CO2 Photoreduction and H2 Production. J. Environ. Chem. Eng. 2024, 12, 111644. [Google Scholar] [CrossRef]
- Xin, X.; Xu, T.; Wang, L.; Wang, C. Ti3+-Self Doped Brookite TiO2 Single-Crystalline Nanosheets with High Solar Absorption and Excellent Photocatalytic CO2 Reduction. Sci. Rep. 2016, 6, 23684. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Cheng, B.; Yu, J. Surface Modification and Enhanced Photocatalytic CO2 Reduction Performance of TiO2: A Review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of Material Design and Reactor Engineering on TiO2 Photocatalysis for CO2 Reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Wei, L.; Yu, C.; Zhang, Q.; Liu, H.; Wang, Y. TiO2-Based Heterojunction Photocatalysts for Photocatalytic Reduction of CO2 into Solar Fuels. J. Mater. Chem. A Mater. 2018, 6, 22411–22436. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Kafizas, A.; Zafeiratos, S.; Petit, C. CO2 Capture and Photocatalytic Reduction Using Bifunctional TiO2MOF Nanocomposites under UV–Vis Irradiation. Appl. Catal. B 2017, 210, 131–140. [Google Scholar] [CrossRef]
- Swetha, S.; Janani, B.; Khan, S.S. A Critical Review on the Development of Metal-Organic Frameworks for Boosting Photocatalysis in the Fields of Energy and Environment. J. Clean. Prod. 2022, 333, 130164. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Pang, H. A Review of MOFs and Their Composites-Based Photocatalysts: Synthesis and Applications. Adv. Funct. Mater. 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; Ferrer, B.; García, H. Metal–Organic Frameworks as Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Rev. 2023, 123, 445–490. [Google Scholar] [CrossRef]
- Jin, J. Porphyrin-Based Metal–Organic Framework Catalysts for Photoreduction of CO2: Understanding the Effect of Node Connectivity and Linker Metalation on Activity. New J. Chem. 2020, 44, 15362–15368. [Google Scholar] [CrossRef]
- Stanley, P.M.; Hemmer, K.; Hegelmann, M.; Schulz, A.; Park, M.; Elsner, M.; Cokoja, M.; Warnan, J. Topology- and Wavelength-Governed CO2 Reduction Photocatalysis in Molecular Catalyst-Metal–Organic Framework Assemblies. Chem. Sci. 2022, 13, 12164–12174. [Google Scholar] [CrossRef]
- Majewski, M.B.; Peters, A.W.; Wasielewski, M.R.; Hupp, J.T.; Farha, O.K. Metal–Organic Frameworks as Platform Materials for Solar Fuels Catalysis. ACS Energy Lett. 2018, 3, 598–611. [Google Scholar] [CrossRef]
- Li, R.; Hu, J.; Deng, M.; Wang, H.; Wang, X.; Hu, Y.; Jiang, H.; Jiang, J.; Zhang, Q.; Xie, Y.; et al. Integration of an Inorganic Semiconductor with a Metal–Organic Framework: A Platform for Enhanced Gaseous Photocatalytic Reactions. Adv. Mater. 2014, 26, 4783–4788. [Google Scholar] [CrossRef]
- Wang, M.; Wang, D.; Li, Z. Self-Assembly of CPO-27-Mg/TiO2 Nanocomposite with Enhanced Performance for Photocatalytic CO2 Reduction. Appl. Catal. B 2016, 183, 47–52. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Huang, J.; She, H.; Wang, Q. Integration of Copper(II)-Porphyrin Zirconium Metal–Organic Framework and Titanium Dioxide to Construct Z-Scheme System for Highly Improved Photocatalytic CO2 Reduction. ACS Sustain. Chem. Eng. 2019, 7, 15660–15670. [Google Scholar] [CrossRef]
- Lin, C.; Han, C.; Zhang, H.; Gong, L.; Gao, Y.; Wang, H.; Bian, Y.; Li, R.; Jiang, J. Porphyrin-Based Metal–Organic Frameworks for Efficient Photocatalytic H2 Production under Visible-Light Irradiation. Inorg. Chem. 2021, 60, 3988–3995. [Google Scholar] [CrossRef] [PubMed]
- Rajasree, S.S.; Li, X.; Deria, P. Physical Properties of Porphyrin-Based Crystalline Metal–organic Frameworks. Commun. Chem. 2021, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Jung, W.-J.; Park, K.; Kim, S.-Y.; Baeg, J.-O.; Kim, C.H.; Son, H.-J.; Pac, C.; Kang, S.O. Rapid Exciton Migration and Amplified Funneling Effects of Multi-Porphyrin Arrays in a Re(I)/Porphyrinic MOF Hybrid for Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2021, 13, 2710–2722. [Google Scholar] [CrossRef]
- Son, H.-J.; Jin, S.; Patwardhan, S.; Wezenberg, S.J.; Jeong, N.C.; So, M.; Wilmer, C.E.; Sarjeant, A.A.; Schatz, G.C.; Snurr, R.Q.; et al. Light-Harvesting and Ultrafast Energy Migration in Porphyrin-Based Metal–Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, K.; Wang, H.; Zhou, M.; Zhou, B.; Li, Y.; Li, Q.; Wang, Q.; Shen, H.-M.; She, Y. Metalloporphyrin-Based Metal–Organic Frameworks for Photocatalytic Carbon Dioxide Reduction: The Influence of Metal Centers. Processes 2023, 11, 1042. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; He, J.; Zeng, X.; Hou, X.; Long, Z. Enhancement of Photoredox Catalytic Properties of Porphyrinic Metal–Organic Frameworks Based on Titanium Incorporation via Post-Synthetic Modification. Chem. Commun. 2018, 54, 8610–8613. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Song, S.; Gu, H.; Li, Y.; Sun, Q.; Ding, N.; Tang, H.; Zheng, L.; Liu, S.; Li, Z.; et al. Enhancing the Photocatalytic Activity of Zirconium-Based Metal–Organic Frameworks Through the Formation of Mixed-Valence Centers. Adv. Sci. 2023, 10, 2303206. [Google Scholar] [CrossRef]
- Xu, C.; Liu, H.; Li, D.; Su, J.-H.; Jiang, H.-L. Direct Evidence of Charge Separation in a Metal–Organic Framework: Efficient and Selective Photocatalytic Oxidative Coupling of Amines via Charge and Energy Transfer. Chem. Sci. 2018, 9, 3152–3158. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, Z.; Li, Y.; Sun, Q.; Ding, N.; Zheng, L.; Liu, S.; Chen, W.; Li, S.; Pang, S. Modulating the Conduction Band of MOFs by Introducing Tiny TiO2 Nanoparticles for Enhanced Photocatalytic Performance: Importance of the Loading Position. Inorg. Chem. 2023, 62, 10572–10581. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.-B.; Liu, T.-T.; Liu, J.; Jin, S.; Wu, X.-P.; Gong, X.-Q.; Wang, K.; Yin, Q.; Liu, T.-F.; Cao, R.; et al. Boosting Interfacial Charge-Transfer Kinetics for Efficient Overall CO2 Photoreduction via Rational Design of Coordination Spheres on Metal–Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 12515–12523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Si, D.; Huang, H.; Xie, L.; Fang, Z.; Liu, T.; Cao, R. Partial Metalation of Porphyrin Moieties in Hydrogen-Bonded Organic Frameworks Provides Enhanced CO2 Photoreduction Activity. Angew. Chem. Int. Ed. 2022, 61, e202203955. [Google Scholar] [CrossRef]
- Yu, C.-J.; Krzyaniak, M.D.; Fataftah, M.S.; Wasielewski, M.R.; Freedman, D.E. A Concentrated Array of Copper Porphyrin Candidate Qubits. Chem. Sci. 2019, 10, 1702–1708. [Google Scholar] [CrossRef]
- Wang, L.; Duan, S.; Jin, P.; She, H.; Huang, J.; Lei, Z.; Zhang, T.; Wang, Q. Anchored Cu(II) Tetra(4-Carboxylphenyl)Porphyrin to P25 (TiO2) for Efficient Photocatalytic Ability in CO2 Reduction. Appl. Catal. B 2018, 239, 599–608. [Google Scholar] [CrossRef]
- Jin, P.; Wang, L.; Ma, X.; Lian, R.; Huang, J.; She, H.; Zhang, M.; Wang, Q. Construction of Hierarchical ZnIn2S4@PCN-224 Heterojunction for Boosting Photocatalytic Performance in Hydrogen Production and Degradation of Tetracycline Hydrochloride. Appl. Catal. B 2021, 284, 119762. [Google Scholar] [CrossRef]
- Hartle, M.D.; Prell, J.S.; Pluth, M.D. Spectroscopic Investigations into the Binding of Hydrogen Sulfide to Synthetic Picket-Fence Porphyrins. Dalton Trans. 2016, 45, 4843–4853. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, P.; Duan, S.; Huang, J.; She, H.; Wang, Q.; An, T. Accelerated Fenton-like Kinetics by Visible-Light-Driven Catalysis over Ironiii Porphyrin Functionalized Zirconium MOF: Effective Promotion on the Degradation of Organic Contaminants. Environ. Sci. Nano 2019, 6, 2652–2661. [Google Scholar] [CrossRef]
- Ma, C.; Wolterbeek, H.T.; Denkova, A.G.; Serra Crespo, P. Porphyrinic Metal–Organic Frameworks as Molybdenum Adsorbents for the 99Mo/99mTc Generator. Inorg. Chem. Front. 2023, 10, 2239–2249. [Google Scholar] [CrossRef]
- Wang, N.; Liu, S.; Sun, Z.; Han, Y.; Xu, J.; Xu, Y.; Wu, J.; Meng, H.; Zhang, B.; Zhang, X. Synergistic Adsorption and Photocatalytic Degradation of Persist Synthetic Dyes by Capsule-like Porphyrin-Based MOFs. Nanotechnology 2021, 32, 465705. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Peng, C.; He, Y.; Zhang, W.; Zhang, Q.; Zhang, T. Conjugated Microspheres FeTCPP–TDI–TiO2 with Enhanced Photocatalytic Performance for Antibiotics Degradation Under Visible Light Irradiation. Catal. Lett. 2016, 146, 2543–2554. [Google Scholar] [CrossRef]
- Liu, X.; Qi, W.; Wang, Y.; Lin, D.; Yang, X.; Su, R.; He, Z. Rational Design of Mimic Multienzyme Systems in Hierarchically Porous Biomimetic Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2018, 10, 33407–33415. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Martin, C.B.; Arumuganainar, S.; Gilman, A.; Koel, B.E.; Sarazen, M.L. Mechanistic Elucidations of Highly Dispersed Metalloporphyrin Metal-Organic Framework Catalysts for CO2 Electroreduction. Angew. Chem. Int. Ed. 2023, 62, e202218208. [Google Scholar] [CrossRef]
- Yu, K.; Lee, Y.-R.; Seo, J.Y.; Baek, K.-Y.; Chung, Y.-M.; Ahn, W.-S. Sonochemical Synthesis of Zr-Based Porphyrinic MOF-525 and MOF-545: Enhancement in Catalytic and Adsorption Properties. Microporous Mesoporous Mater. 2021, 316, 110985. [Google Scholar] [CrossRef]
- Zhao, Y.; Kuang, Y.; Liu, M.; Wang, J.; Pei, R. Synthesis of Metal–Organic Framework Nanosheets with High Relaxation Rate and Singlet Oxygen Yield. Chem. Mater. 2018, 30, 7511–7520. [Google Scholar] [CrossRef]
- Cao, X.; Tong, R.; Tang, S.; Jang, B.W.-L.; Mirjalili, A.; Li, J.; Guo, X.; Zhang, J.; Hu, J.; Meng, X. Design of Pd–Zn Bimetal MOF Nanosheets and MOF-Derived Pd3.9Zn6.1/CNS Catalyst for Selective Hydrogenation of Acetylene under Simulated Front-End Conditions. Molecules 2022, 27, 5736. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Liu, Y.; Lei, L.; Wang, J.; Wang, Z.; Zheng, Z.; Wang, P.; Cheng, H.; Dai, Y.; Huang, B. Light-Promoted CO2 Conversion from Epoxides to Cyclic Carbonates at Ambient Conditions over a Bi-Based Metal–Organic Framework. ACS Catal. 2021, 11, 1988–1994. [Google Scholar] [CrossRef]
- Madhusudan Reddy, K.; Gopal Reddy, C.V.; Manorama, S.V. Preparation, Characterization, and Spectral Studies on Nanocrystalline Anatase TiO2. J. Solid. State Chem. 2001, 158, 180–186. [Google Scholar] [CrossRef]
- Sheng, W.; Wang, X.; Wang, Y.; Chen, S.; Lang, X. Integrating TEMPO into a Metal–Organic Framework for Cooperative Photocatalysis: Selective Aerobic Oxidation of Sulfides. ACS Catal. 2022, 12, 11078–11088. [Google Scholar] [CrossRef]
- da Trindade, L.G.; Borba, K.M.N.; Trench, A.B.; Zanchet, L.; Teodoro, V.; Pontes, F.M.L.; Longo, E.; Mazzo, T.M. Effective Strategy to Coupling Zr-MOF/ZnO: Synthesis, Morphology and Photoelectrochemical Properties Evaluation. J. Solid. State Chem. 2021, 293, 121794. [Google Scholar] [CrossRef]
- Mette, G.; Sutter, D.; Gurdal, Y.; Schnidrig, S.; Probst, B.; Iannuzzi, M.; Hutter, J.; Alberto, R.; Osterwalder, J. From Porphyrins to Pyrphyrins: Adsorption Study and Metalation of a Molecular Catalyst on Au(111). Nanoscale 2016, 8, 7958–7968. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.M.; Flechtner, K.; Kretschmann, A.; Lukasczyk, T.; Steinrück, H.-P. Direct Synthesis of a Metalloporphyrin Complex on a Surface. J. Am. Chem. Soc. 2006, 128, 5644–5645. [Google Scholar] [CrossRef]
- Diller, K.; Papageorgiou, A.C.; Klappenberger, F.; Allegretti, F.; Barth, J.V.; Auwärter, W. In Vacuo Interfacial Tetrapyrrole Metallation. Chem. Soc. Rev. 2016, 45, 1629–1656. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Gregg, A.; Moss, B.; Kafizas, A.; Petit, C. The Effect of Materials Architecture in TiO2/MOF Composites on CO2 Photoreduction and Charge Transfer. Small 2019, 15, 1805473. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Gao, J.; Rao, S.; Jin, C.; Jiang, H.; Shen, J.; Yu, X.; Wang, W.; Wang, L.; Yang, J.; et al. A Multifunctional Membrane Based on TiO2/PCN-224 Heterojunction with Synergistic Photocatalytic-Photothermal Activity under Visible-Light Irradiation. Appl. Catal. B 2024, 342, 123374. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Lu, H.; Gu, Z.; Chen, L. Au-Nanorod-Modified PCN-222(Cu) for H2 Evolution from HCOOH Dehydrogenation by Photothermally Enhanced Photocatalysis. Chem. Commun. 2022, 58, 8520–8523. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).