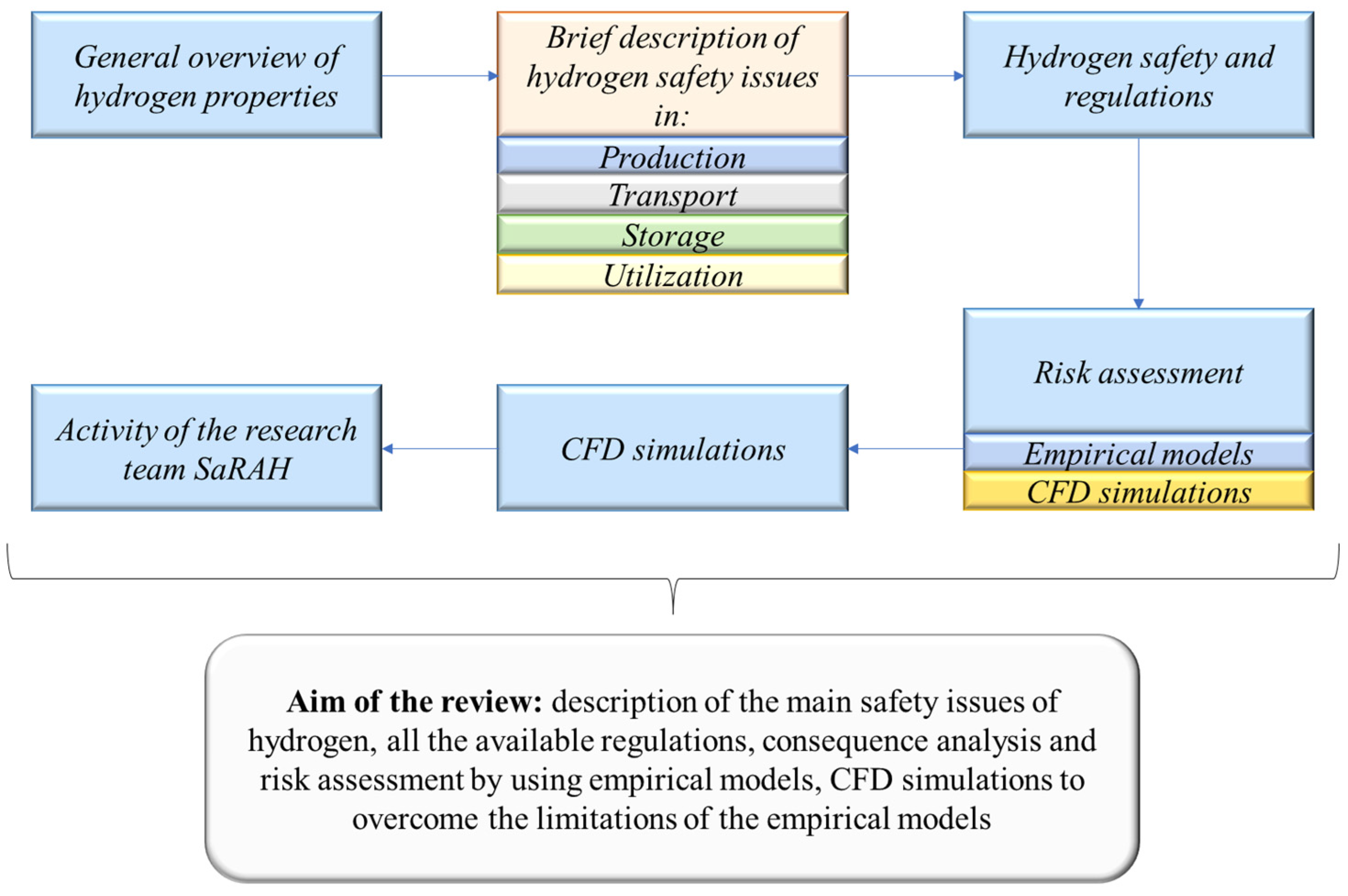
Hydrogen Safety Challenges: A Comprehensive Review on Production, Storage, Transport, Utilization, and CFD-Based Consequence and Risk Assessment
Abstract
1. Introduction
- Growth of the hydrogen economy: Many countries have announced ambitious plans to invest in hydrogen as a clean energy carrier. These include plans for the production, distribution, and utilisation of hydrogen in various sectors such as transport, industry, and power generation.
- Advances in technology: Significant progress has been made in hydrogen production technologies, including electrolysis (both alkaline and PEM), steam methane reforming (SMR) with carbon capture and storage (CCS), thee gasification of biomass, and more recently the production of green hydrogen from renewable energy sources.
- Infrastructure development: One of the biggest challenges facing the hydrogen industry is the development of infrastructure for the production, transport, storage, and distribution of hydrogen. This includes the construction of pipelines, storage facilities, and hydrogen refuelling stations for transport.
- Challenges in the area of safety as follows:
- Handling hydrogen: Hydrogen is highly flammable and can easily ignite. The safe handling and storage of hydrogen requires special equipment and procedures to prevent leaks and minimise risks.
- Hydrogen embrittlement: hydrogen can embrittle metals, which can cause problems with the structural integrity of equipment and infrastructure as well as pose a safety risk.
- Transport safety: transporting hydrogen safely over long distances can be a challenge due to its low energy density and the need for specialised containers or pipelines.
- Public awareness and education: in order to prevent accidents, it is important to ensure that both the public and those working in the hydrogen industry are aware of the safety risks associated with hydrogen and are trained in its proper handling.
- Regulations framework: the development and the implementation of regulation frameworks and standards for hydrogen safety are critical for ensuring that industry practices meet safety requirements and effectively mitigate risks.
- Research and development: continuous research and development efforts are essential to address safety issues and improve technologies for the production, storage, transport, and utilisation of hydrogen.
2. The Hydrogen Pyramid: Safety in Production, Transport, Storage, and Utilisation
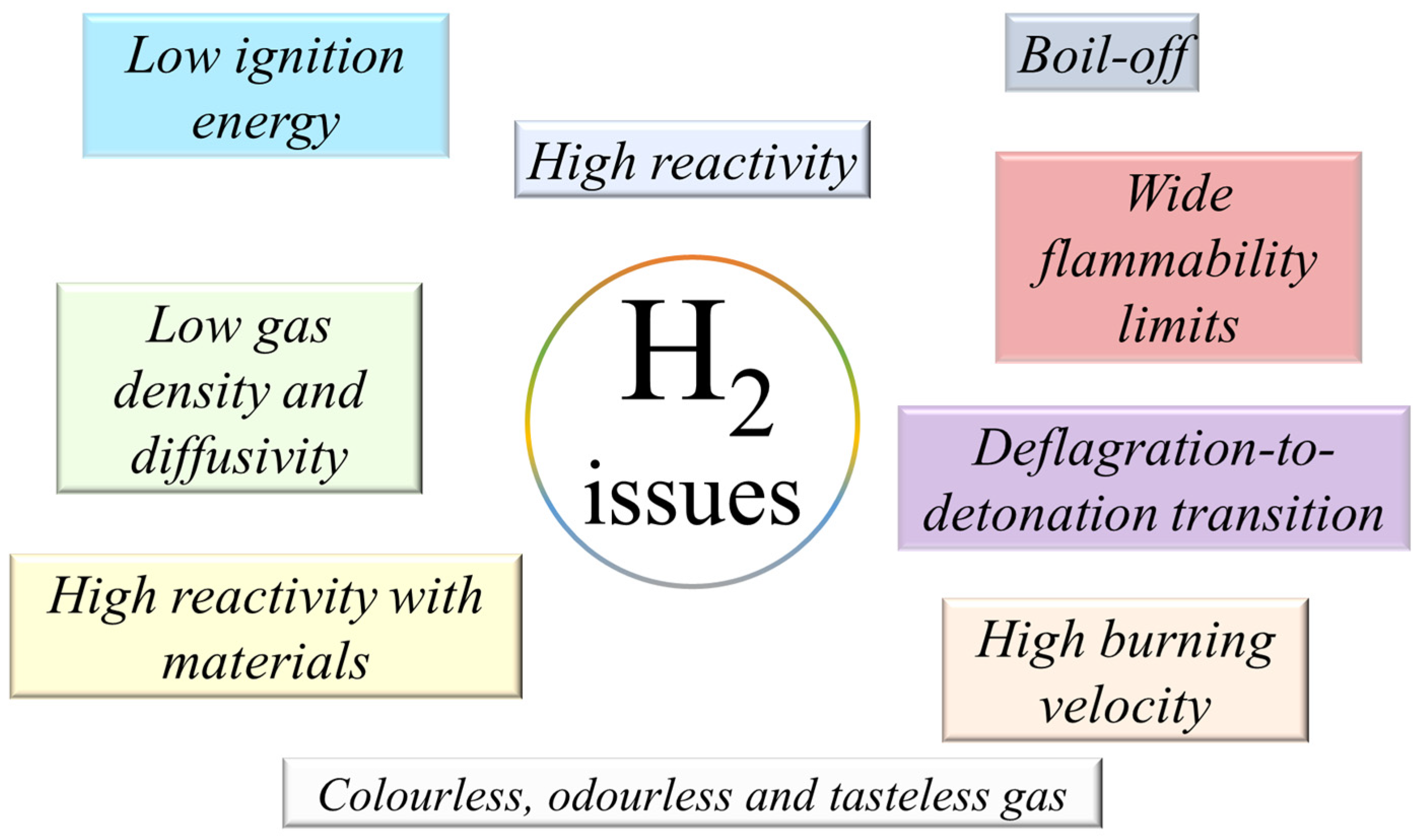
2.1. Hydrogen Properties
- Low ignition energy: one order of magnitude lower than the hydrocarbons;
- High reactivity due to its particular chemical and physical properties;
- Boil-off tendency: this can cause safety issues and economic losses;
- Wide flammability limits: 4–75% in air, being very wide with respect to methane (different ATEX category);
- Deflagration-to-detonation transition: the transition can easily occurs and is often observed in the case of a high-scale system;
- High burning velocity: the laminar burning velocity is significantly higher than that of many other fuels;
- Hydrogen is colourless, odourless, and tasteless: additives cannot be easily added;
- High reactivity with materials (embrittlement): huge investments are needed on material investigation;
- Low gas density and diffusivity: particular behaviour in the case of release, and it can stratified in the upper part of confined spaces.
2.2. Safety Issues in Production
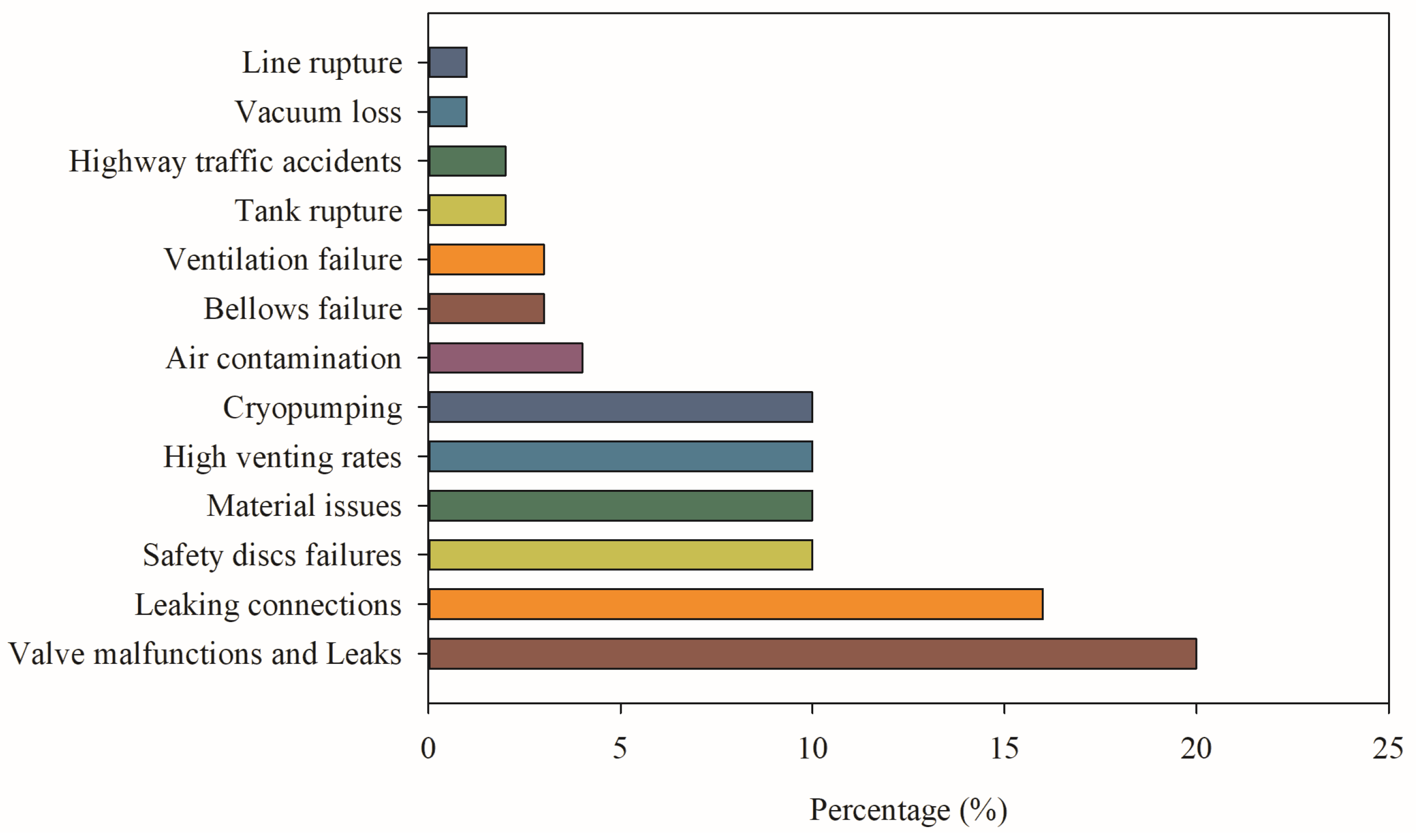
2.3. Safety Issues in Transportation
2.4. Safety Issues in Storage
2.5. Safety Issues in Utilisation
3. Hydrogen Safety and Regulations
- Providing example scenarios to demonstrate the application of performance-based concepts for analysing and designing fire and gas systems (FGS);
- Providing a performance-based methodology for the allocation of fire and gas detectors. The methodology provides considerations for more effective hazard detection and detector placement in cases where fusible plugs (fire) may be required;
- Defining a methodology that addresses the design and effectiveness of FGS mitigation features that are consistent with the underlying principles used to design and evaluate the effectiveness of preventive features.
| Standard | General Scope | Main Sections | Section Indications |
|---|---|---|---|
| NFPA 2 | Fundamental safeguards for the generation, installation, storage, piping, use, and handling of hydrogen in a compressed gas (GH2) form or a cryogenic liquid (LH2) form; fuelling stations. | 6. General H2 requirements | Appropriate design for gas detection, maintenance, and control; inspection; calibration; testing. |
| 7. GH2 supply and storage |
| ||
| 8. LH2 bulk storage |
| ||
| 10. Gaseous hydrogen fuelling systems |
| ||
| NFPA 55 | Protection from over-pressurisation, explosive, and flammability hazards associated with compressed gases and cryogenic fluids. | 10. Gaseous hydrogen systems | Strong references to NFPA 2 [44]. |
| 11. Liquid hydrogen systems | Strong references to NFPA 2 [44]. | ||
| NFPA 853 | Fire prevention and protection requirements for safeguarding life and physical property associated with buildings or facilities that employ stationary fuel cell systems of all sizes. | 6. Fuel supplies and storage arrangements | Storage following NFPA 55 [45]. |
| 8. Fire protection | In the case of hydrogen, the position and detection range are suggested. | ||
| 9. Fuel cell power systems of 50 kW or less | Requirements about indoor and outdoor installation, ventilation, and fire protection. |
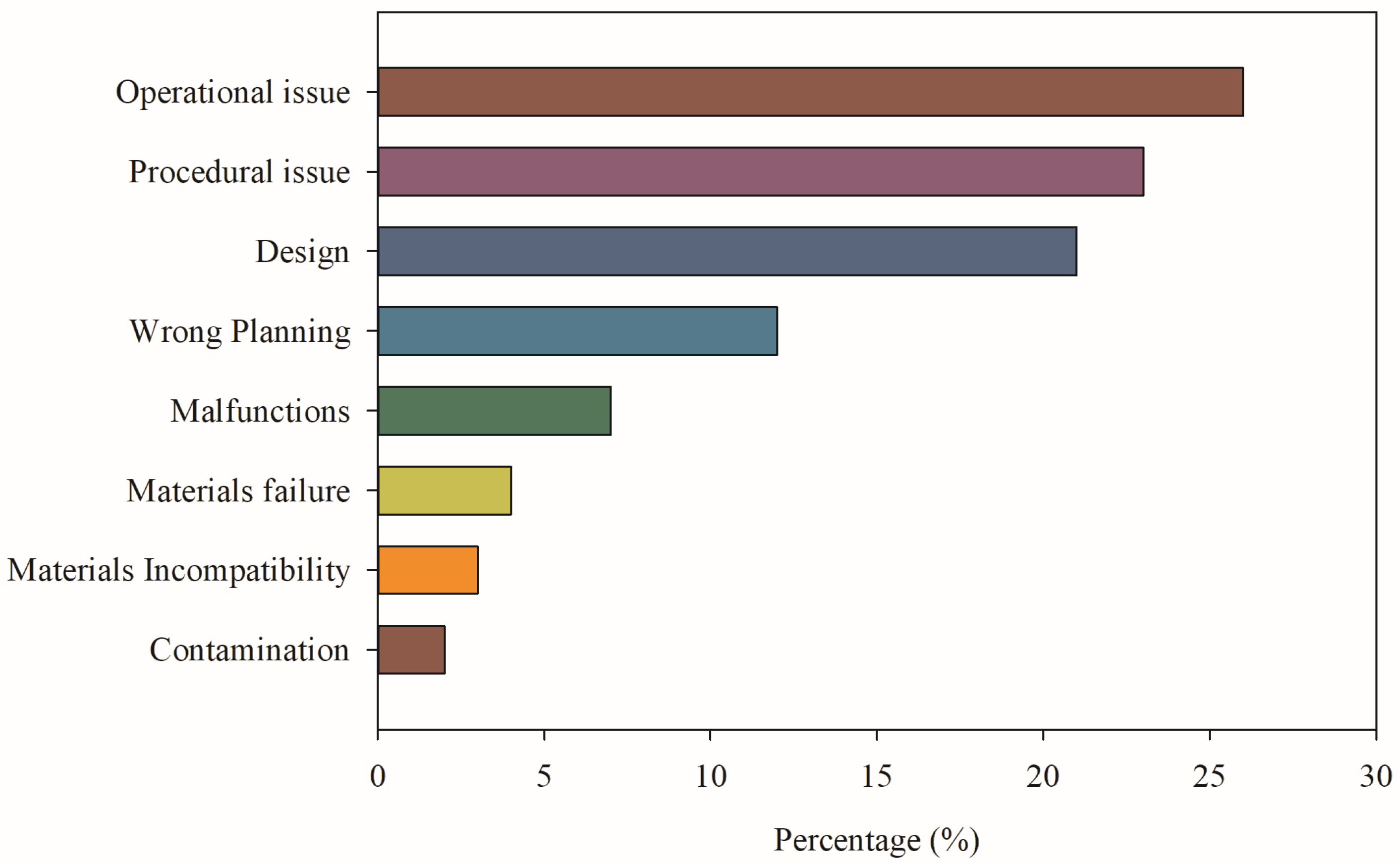
4. Risk Assessment and Consequence Analysis of Hydrogen Systems: Criticism of Empirical Models
5. CFD Simulations of Hydrogen Dispersion and the Consequences and Risk Assessment
- Worst-case assessment, with the stoichiometric cloud covering the entire geometry;
- “Realistic worst-case” assessment, where releases are simulated with ventilation and the worst case of a “realistic” flammable cloud is estimated;
- Probabilistic risk assessment, where a range of release and ventilation conditions is simulated, the cloud size distribution is established, and explosions with various cloud sizes are simulated.
6. Our Activity with the Combination of Risk Assessments and CFDs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brodny, J.; Tutak, M. Challenges of the Polish Coal Mining Industry on Its Way to Innovative and Sustainable Development. J. Clean. Prod. 2022, 375, 134061. [Google Scholar] [CrossRef]
- Baloch, M.A.; Danish. The Nexus between Renewable Energy, Income Inequality, and Consumption-Based CO2 Emissions: An Empirical Investigation. Sustain. Dev. 2022, 30, 1268–1277. [Google Scholar] [CrossRef]
- Khorasani, M.; Sarker, S.; Kabir, G.; Ali, S.M. Evaluating Strategies to Decarbonize Oil and Gas Supply Chain: Implications for Energy Policies in Emerging Economies. Energy 2022, 258, 124805. [Google Scholar] [CrossRef]
- Jing, R.; Hua, W.; Lin, J.; Lin, J.; Zhao, Y.; Zhou, Y.; Wu, J. Cost-Efficient Decarbonization of Local Energy Systems by Whole-System Based Design Optimization. Appl. Energy 2022, 326, 119921. [Google Scholar] [CrossRef]
- Seck, G.S.; Hache, E.; Sabathier, J.; Guedes, F.; Reigstad, G.A.; Straus, J.; Wolfgang, O.; Ouassou, J.A.; Askeland, M.; Hjorth, I.; et al. Hydrogen and the Decarbonization of the Energy System in Europe in 2050: A Detailed Model-Based Analysis. Renew. Sustain. Energy Rev. 2022, 167, 112779. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Markets & Markets Hydrogen Generation Market. Available online: https://www.marketsandmarkets.com/ (accessed on 5 February 2024).
- National Academies of Sciences Engineering and Medicine. The Future of Hydrogen: Seizing Today’s Opportunities; National Academies of Sciences Engineering and Medicine: Washington, DC, USA, 2019. [Google Scholar]
- International Energy Agency Hydrogen. Available online: https://www.iea.org/energy-system/low-emission-fuels/hydrogen (accessed on 12 July 2023).
- European Commission Hydrogen. Available online: https://energy.ec.europa.eu/topics/energy-systems-integration/hydrogen_en (accessed on 12 July 2023).
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Labidine Messaoudani, Z.; Rigas, F.; Binti Hamid, M.D.; Che Hassan, C.R. Hazards, Safety and Knowledge Gaps on Hydrogen Transmission via Natural Gas Grid: A Critical Review. Int. J. Hydrogen Energy 2016, 41, 17511–17525. [Google Scholar] [CrossRef]
- Yang, F.; Wang, T.; Deng, X.; Dang, J.; Huang, Z.; Hu, S.; Li, Y.; Ouyang, M. Review on Hydrogen Safety Issues: Incident Statistics, Hydrogen Diffusion, and Detonation Process. Int. J. Hydrogen Energy 2021, 46, 31467–31488. [Google Scholar] [CrossRef]
- West, M.; Al-Douri, A.; Hartmann, K.; Buttner, W.; Groth, K.M. Critical Review and Analysis of Hydrogen Safety Data Collection Tools. Int. J. Hydrogen Energy 2022, 47, 17845–17858. [Google Scholar] [CrossRef]
- Abohamzeh, E.; Salehi, F.; Sheikholeslami, M.; Abbassi, R.; Khan, F. Review of Hydrogen Safety during Storage, Transmission, and Applications Processes. J. Loss Prev. Process Ind. 2021, 72, 104569. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook, NIST Standard Reference Database Number 69. [CrossRef]
- ISO/TR 15916:2015; Basic Considerations for the Safety of Hydrogen Systems. International Standard Organization: Geneva, Switzerland, 2015.
- Astbury, G.R.; Hawksworth, S.J. Spontaneous Ignition of Hydrogen Leaks: A Review of Postulated Mechanisms. Int. J. Hydrogen Energy 2007, 32, 2178–2185. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Tretsiakova-McNally, S. LECTURE–Hydrogen Properties Relevant to Safety (HyResponse). Available online: https://hyresponder.eu/ (accessed on 5 February 2024).
- Molkov, V. Fundamentals of Hydrogen Safety Engineering; Part I and Part II; Australian Refrigeration Mechanics Association: Loganholme, QLD, Australia, 2012. [Google Scholar]
- Han, W.; Dai, P.; Gou, X.; Chen, Z. A Review of Laminar Flame Speeds of Hydrogen and Syngas Measured from Propagating Spherical Flames. Appl. Energy Combust. Sci. 2020, 1–4, 100008. [Google Scholar] [CrossRef]
- Muscetta, M.; Portarapillo, M.; Di Benedetto, A.; Andreozzi, R. Risk Analysis of the Sodium Hypochlorite Production Process: Focus on the Chlorine Line. Chem. Eng. J. Adv. 2022, 12, 100381. [Google Scholar] [CrossRef]
- Portarapillo, M.; Muscetta, M.; Benedetto, A.D.; Andreozzi, R. Risk Analysis of Sodium Hypochlorite Production Process. Chem. Eng. Trans. 2020, 82, 49–54. [Google Scholar] [CrossRef]
- Wen, J.X.; Marono, M.; Moretto, P.; Reinecke, E.A.; Sathiah, P.; Studer, E.; Vyazmina, E.; Melideo, D. Statistics, Lessons Learned and Recommendations from Analysis of HIAD 2.0 Database. Int. J. Hydrogen Energy 2022, 47, 17082–17096. [Google Scholar] [CrossRef]
- Pacific Northwest National Laboratory. Hydrogen Tools Portal; Pacific Northwest National Laboratory: Washington, DC, USA, 2023. [Google Scholar]
- Di Nardo, A.; Calabrese, M.; Venezia, V.; Portarapillo, M.; Turco, M.; Di Benedetto, A.; Luciani, G. Addressing Environmental Challenges: The Role of Hydrogen Technologies in a Sustainable Future. Energies 2023, 16, 7908. [Google Scholar] [CrossRef]
- Tsai, Y.; Cai, J.; Pan, Y.; Jiang, J. Explosion Risk Assessment of a Liquid Organic Hydrogen Carrier System by Using Toluene–Methylcyclohexane on Varying Hydrogen Storage Scenarios. J. Loss Prev. Process Ind. 2023, 86. [Google Scholar] [CrossRef]
- Guo, L.; Su, J.; Wang, Z.; Shi, J.; Guan, X.; Cao, W.; Ou, Z. Hydrogen Safety: An Obstacle That Must Be Overcome on the Road towards Future Hydrogen Economy. Int. J. Hydrogen Energy 2024, 51, 1055–1078. [Google Scholar] [CrossRef]
- Liu, W.; Christopher, D.M. Dispersion of Hydrogen Leaking from a Hydrogen Fuel Cell Vehicle. Int. J. Hydrogen Energy 2015, 40, 16673–16682. [Google Scholar] [CrossRef]
- Shen, Y.; Lv, H.; Zheng, T.; Liu, Y.; Zhou, W.; Zhang, C. Temporal and Spatial Evolution of Hydrogen Leakage and Diffusion from Tube Fittings on Fuel Cell Vehicles under the Effect of Ambient Wind. Renew. Sustain. Energy Rev. 2023, 185, 113596. [Google Scholar] [CrossRef]
- Salehi, F.; Abbassi, R.; Asadnia, M.; Chan, B.; Chen, L. Overview of Safety Practices in Sustainable Hydrogen Economy–An Australian Perspective. Int. J. Hydrogen Energy 2022, 47, 34689–34703. [Google Scholar] [CrossRef]
- European Gas Association EIGA Database, Brussels, Belgium. 2023. Available online: https://www.eiga.eu/ (accessed on 5 February 2024).
- National Fire Protection Association NFPA Standards, Quincy, Massachusetts, U.S. 2023. Available online: https://www.nfpa.org/ (accessed on 5 February 2024).
- European Committee for Electrotechnical Standardization CEN-CENELEC Standards, Brussels, Belgium. 2023. Available online: https://www.cencenelec.eu/ (accessed on 5 February 2024).
- International Organization for Standardization ISO Standards, Vernier, Geneva, Switzerland. 2023. Available online: https://www.iso.org/home.html (accessed on 5 February 2024).
- UL Association UL Standards, New York, US. 2023. Available online: https://ulse.org/ (accessed on 5 February 2024).
- CSA Group CSA Standards, Toronto, Canada. 2023. Available online: https://www.csagroup.org/ (accessed on 5 February 2024).
- SAE Foundation SAE Standards for Mobility Knowledge and Solutions, New York City, U.S. 2023. Available online: https://www.sae.org/standards (accessed on 5 February 2024).
- American Society of Mechanical Engineers ASME Standards and Certification, New York City, U.S. 2023. Available online: https://www.asme.org/ (accessed on 5 February 2024).
- International Code Council 2021 International Fire Code. 2023. Available online: https://codes.iccsafe.org/content/IFC2021P1/preface (accessed on 5 February 2024).
- ISO/TS 19880-1:2020; International Organization for Standardization-Gaseous Hydrogen, Fuelling Stations, Part 1: General Requirements. ISO: Geneva, Switzerland, 2020.
- National Fire Protection Association NFPA 2—Hydrogen Technologies Code, Quincy, Massachusetts, U.S. 2023. Available online: https://www.nfpa.org/codes-and-standards/nfpa-2-standard-development/2 (accessed on 5 February 2024).
- National Fire Protection Association NFPA 55—Compressed Gases and Cryogenic Fluids Code, Quincy, Massachusetts, U.S. 2023. Available online: https://www.nfpa.org/product/nfpa-55-code/p0055code (accessed on 5 February 2024).
- National Fire Protection Association NFPA 853—Standard for the Installation of Stationary Fuel Cell Power Systems, Quincy, Massachusetts, U.S. 2023. Available online: https://www.nfpa.org/codes-and-standards/8/5/3/nfpa-853 (accessed on 5 February 2024).
- International Society of Automation ISA-TR84.00.07-2018, Guidance on the Evaluation of Fire, Combustible Gas, and Toxic Gas System Effectiveness, Research Triangle Park, North Carolina. 2023. Available online: https://webstore.ansi.org/preview-pages/ISA/preview_ISA+TR84.00.07-2018.pdf (accessed on 5 February 2024).
- Department of Transportation DOT Standards, Washington, DC, US. 2023. Available online: https://www.transportation.gov/ (accessed on 5 February 2024).
- Compressed Gas Association CGA Standards, Virginia, US. 2023. Available online: https://www.cganet.com/standards/ (accessed on 5 February 2024).
- Hydrogen Safety Panel. Hydrogen Equipment Certification Guide. 2017. Available online: https://h2tools.org/hsp/certification-guide (accessed on 5 February 2024).
- Crowl, D.A.; Louvar, J.F. Chemical Process Safety: Fundamentals with Applications; Prentice Hall PTR: Hoboken, NJ, USA, 2002; ISBN 9780130181763. [Google Scholar]
- Center for Chemical Process Safety. Guidelines for Chemical Process Quantitative Risk Analysis, 2nd ed.; Center for Chemical Process Safety: New York, NY, USA, 2010. [Google Scholar]
- Bentaib, A.; Meynet, N.; Bleyer, A. Overview on Hydrogen Risk Research and Development Activities: Methodology and Open Issues. Nucl. Eng. Technol. 2015, 47, 26–32. [Google Scholar] [CrossRef]
- Birch, A.D.; Brown, D.R.; Dodson, M.G.; Swaffield, F. The Structure and Concentration Decay of High Pressure Jets of Natural Gas. Combust. Sci. Technol. 1984, 36, 249–261. [Google Scholar] [CrossRef]
- Birch, A.D.; Hughes, D.J.; Swaffield, F. Velocity Decay of High Pressure Jets. Combust. Sci. Technol. 1987, 52, 161–171. [Google Scholar] [CrossRef]
- Yüceil, K.B.; Ötügen, M.V. Scaling Parameters for Underexpanded Supersonic Jets. Phys. Fluids 2002, 14, 4206–4215. [Google Scholar] [CrossRef]
- Molkov, V.; Makarov, D.; Bragin, M. Physics and Modelling of Underexpanded Jets and Hydrogen Dispersion in Atmosphere. In Physics of Extreme States of Matter-2009; Russian Academy of Sciences: Moscow, Russia, 2009; pp. 146–149. ISBN 978-5-901675-89-2. [Google Scholar]
- Ewan, B.C.R.; Moodie, K. Structure and Velocity Measurements in Underexpanded Jets. Combust. Sci. Technol. 1986, 45, 275–288. [Google Scholar] [CrossRef]
- Houf, W.; Schefer, R. Analytical and Experimental Investigation of Small-Scale Unintended Releases of Hydrogen. Int. J. Hydrogen Energy 2008, 33, 1435–1444. [Google Scholar] [CrossRef]
- Houf, W.G.; Winters, W.S. Simulation of High-Pressure Liquid Hydrogen Releases. Int. J. Hydrogen Energy 2013, 38, 8092–8099. [Google Scholar] [CrossRef]
- Gerrit, A. Horizontal Jets in Stagnant Fluid of Other Density. J. Hydraul. Div. 1965, 91, 139–154. [Google Scholar] [CrossRef]
- Briggs, G.A. Plume Rise and Buoyancy Effects. Atmospheric Science and Power Production; Report No. DOE/TIC-27601; U.S. Dept. of Energy: Washington, DC, USA, 1984. [Google Scholar]
- Houf, W.; Schefer, R. Predicting Radiative Heat Fluxes and Flammability Envelopes from Unintended Releases of Hydrogen. Int. J. Hydrogen Energy 2007, 32, 136–151. [Google Scholar] [CrossRef]
- Ekoto, I.W.; Ruggles, A.J.; Creitz, L.W.; Li, J.X. Updated Jet Flame Radiation Modeling with Buoyancy Corrections. Int. J. Hydrogen Energy 2014, 39, 20570–20577. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Sáez, C.; Linares, J.J. A Comparison of Hydrogen Cloud Explosion Models and the Study of the Vulnerability of the Damage Caused by an Explosion of H2. Int. J. Hydrogen Energy 2006, 31, 1780–1790. [Google Scholar] [CrossRef]
- Li, Y.; Bi, M.; Zhou, Y.; Jiang, H.; Huang, L.; Zhang, K.; Gao, W. Experimental and Theoretical Evaluation of Hydrogen Cloud Explosion with Built-in Obstacles. Int. J. Hydrogen Energy 2020, 45, 28007–28018. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Ma, C.; Wang, X. Influencing Factors of the Chain Effect of Spherical Gas Cloud Explosion. Process Saf. Environ. Prot. 2020, 142, 359–369. [Google Scholar] [CrossRef]
- Casal, J. Evaluation of the Effects and Consequences of Major Accidents in Industrial Plants; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780444638830. [Google Scholar]
- Lachance, J.; Tchouvelev, A.; Engebo, A. Development of Uniform Harm Criteria for Use in Quantitative Risk Analysis of the Hydrogen Infrastructure. Int. J. Hydrogen Energy 2011, 36, 2381–2388. [Google Scholar] [CrossRef]
- Eisenberg, N.A.; Lynch, C.J.; Breeding, R.J. Vulnerability Model. A Simulation System for Assessing Damage Resulting from Marine Spills; Final Report; US Coast Guard, Office of Research and Development: Washington, DC, USA, 1975. [Google Scholar]
- Tsao, C.K.; Perry, W.W. Modifications to the Vulnerability Model: A Simulation System for Assessing Damage Resulting from Marine Spills; Final Report; US Coast Guard, Office of Research and Development: Washington, DC, USA, 1979. [Google Scholar]
- Lees, F.P. The Assessment of Major Hazard: A Model for Fatal Injury from Burns. Process Saf. Environ. Prot. 1994, 72, 127–134. [Google Scholar]
- Health and Safety Executive. Health and Safety Executive (HSE). Methods of Approximation and Determination of Human Vulnerability for Offshore Major Accident Hazard Assessment; UK Health and Safety Executive: Bootle, UK, 2010.
- UK Health and Safety Executive. HSE, Major Hazard Aspects of the Transport of Dangerous Substances; UK Health and Safety Executive: Bootle, UK, 1991.
- The Netherlands Organization of Applied Scientific Research (TNO). Methods for the Determination of Possible Damage, CPR 16E; The Netherlands Organization of Applied Scientific Research (TNO): Voorburg, The Netherlands, 1992. [Google Scholar]
- Liu, Y.; Ozbayoglu, E.M.; Upchurch, E.R.; Baldino, S. Computational Fluid Dynamics Simulations of Taylor Bubbles Rising in Vertical and Inclined Concentric Annuli. Int. J. Multiph. Flow 2023, 159, 10433. [Google Scholar] [CrossRef]
- Ke, W.; Zeng, H.; Wang, Z.; Yu, H.; Liu, Y.; Zheng, D.; Zhu, J.; Zhu, H. A Numerical Study on Labyrinth Screw Pump (LSP) Performance under Viscous Fluid Flow. Energies 2023, 16, 5997. [Google Scholar] [CrossRef]
- Middha, P.; Hansen, O.R.; Storvik, I.E. Validation of CFD-Model for Hydrogen Dispersion. J. Loss Prev. Process Ind. 2009, 22, 1034–1038. [Google Scholar] [CrossRef]
- Wilkening, H.; Baraldi, D. CFD Modelling of Accidental Hydrogen Release from Pipelines. Int. J. Hydrogen Energy 2007, 32, 2206–2215. [Google Scholar] [CrossRef]
- Lakshmipathy, S.; Skjold, T.; Hisken, H.; Atanga, G. Consequence Models for Vented Hydrogen Deflagrations: CFD vs. Engineering Models. Int. J. Hydrogen Energy 2019, 44, 8699–8710. [Google Scholar] [CrossRef]
- Papanikolaou, E.A.; Venetsanos, A.G.; Heitsch, M.; Baraldi, D.; Huser, A.; Pujol, J.; Garcia, J.; Markatos, N. HySafe SBEP-V20: Numerical Studies of Release Experiments inside a Naturally Ventilated Residential Garage. Int. J. Hydrogen Energy 2010, 35, 4747–4757. [Google Scholar] [CrossRef]
- Guan, W.; Chen, J.; Chen, L.; Cao, J.; Fan, H. Safe Design of a Hydrogen-Powered Ship: CFD Simulation on Hydrogen Leakage in the Fuel Cell Room. J. Mar. Sci. Eng. 2023, 11, 651. [Google Scholar] [CrossRef]
- Hussein, H.; Brennan, S.; Molkov, V. Dispersion of Hydrogen Release in a Naturally Ventilated Covered Car Park. Int. J. Hydrogen Energy 2020, 45, 23882–23897. [Google Scholar] [CrossRef]
- Atanga, G.; Lakshmipathy, S.; Skjold, T.; Hisken, H.; Hanssen, A.G. Structural Response for Vented Hydrogen Deflagrations: Coupling CFD and FE Tools. Int. J. Hydrogen Energy 2019, 44, 8893–8903. [Google Scholar] [CrossRef]
- Lucas, M.; Skjold, T.; Hisken, H. Computational Fluid Dynamics Simulations of Hydrogen Releases and Vented Deflagrations in Large Enclosures. J. Loss Prev. Process Ind. 2020, 63, 103999. [Google Scholar] [CrossRef]
- Bauwens, C.R.; Dorofeev, S.B. CFD Modeling and Consequence Analysis of an Accidental Hydrogen Release in a Large Scale Facility. Int. J. Hydrogen Energy 2014, 39, 20447–20454. [Google Scholar] [CrossRef]
- Giannissi, S.G.; Shentsov, V.; Melideo, D.; Cariteau, B.; Baraldi, D.; Venetsanos, A.G.; Molkov, V. CFD Benchmark on Hydrogen Release and Dispersion in Confined, Naturally Ventilated Space with One Vent. Int. J. Hydrogen Energy 2015, 40, 2415–2429. [Google Scholar] [CrossRef]
- Brennan, S.L.; Makarov, D.V.; Molkov, V. LES of High Pressure Hydrogen Jet Fire. J. Loss Prev. Process Ind. 2009, 22, 353–359. [Google Scholar] [CrossRef]
- Palacios, A.; Rengel, B. Computational Analysis of Vertical and Horizontal Jet Fires. J. Loss Prev. Process Ind. 2020, 65, 104096. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, J.; Pan, Y.; Ni, Y.; Ma, C.; Zhou, W.; Wang, Y. Hazard Analysis on Tunnel Hydrogen Jet Fire Based on CFD Simulation of Temperature Field and Concentration Field. Saf. Sci. 2020, 122, 104532. [Google Scholar] [CrossRef]
- Jang, C.B.; Choi, S.W.; Baek, J.B. CFD Modeling and Fire Damage Analysis of Jet Fire on Hydrogen Pipeline in a Pipe Rack Structure. Int. J. Hydrogen Energy 2015, 40, 15760–15772. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, S.; Shen, B. A Numerical Simulation of the Suppression of Hydrogen Jet Fires on Hydrogen Fuel Cell Ships Using a Fine Water Mist. Int. J. Hydrogen Energy 2021, 46, 13353–13364. [Google Scholar] [CrossRef]
- Daniel Crowl, J.L. Chemical Process Safety: Fundamentals with Applications; Pearson Education: London, UK, 2020. [Google Scholar]
- Giannissi, S.G.; Venetsanos, A.G.; Markatos, N.; Bartzis, J.G. CFD Modeling of Hydrogen Dispersion under Cryogenic Release Conditions. Int. J. Hydrogen Energy 2014, 39, 15851–15863. [Google Scholar] [CrossRef]
- Giannissi, S.G.; Venetsanos, A.G. Study of Key Parameters in Modeling Liquid Hydrogen Release and Dispersion in Open Environment. Int. J. Hydrogen Energy 2018, 43, 455–467. [Google Scholar] [CrossRef]
- Jin, T.; Wu, M.; Liu, Y.; Lei, G.; Chen, H.; Lan, Y. CFD Modeling and Analysis of the Influence Factors of Liquid Hydrogen Spills in Open Environment. Int. J. Hydrogen Energy 2017, 42, 732–739. [Google Scholar] [CrossRef]
- Ichard, M.; Hansen, O.R.; Middha, P.; Willoughby, D. CFD Computations of Liquid Hydrogen Releases. Int. J. Hydrogen Energy 2012, 37, 17380–17389. [Google Scholar] [CrossRef]
- Sun, R.; Pu, L.; He, Y.; Wang, T.; Tan, H. Phase Change Modeling of Air at the Liquid Hydrogen Release. Int. J. Hydrogen Energy 2023, 50, 717–731. [Google Scholar] [CrossRef]
- Kangwanpongpan, T.; Makarov, D.; Cirrone, D.; Molkov, V. LES Model of Flash-Boiling and Pressure Recovery Phenomena during Release from Large-Scale Pressurised Liquid Hydrogen Storage Tank. Int. J. Hydrogen Energy 2024, 50, 390–405. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Y.; Zhao, Y.; Wang, C. Flame Characteristics of Under-Expanded, Cryogenic Hydrogen Jet Fire. Combust. Flame 2022, 244, 112294. [Google Scholar] [CrossRef]
- Ba, Q.; Zhao, Z.; Zhang, Y.; Liu, Y.; Christopher, D.M.; Ge, P.; Li, X. Modeling of Cryogenic Compressed Hydrogen Jet Flames. Int. J. Hydrogen Energy 2024, 51, 917–927. [Google Scholar] [CrossRef]
- Lim, S.J.; Woo, D.H.; Lee, Y.H. Numerical Analysis on Extinguishing of Sprinklers in a Hydrogen Pool Fire. Int. J. Hydrogen Energy 2024, 54, 118–126. [Google Scholar] [CrossRef]
- Elena Vyazmina, S.J.L.G. Delayed Explosion of Hydrogen High Pressure Jets: An Inter Comparison Benchmark Study. In Proceedings of the International Conference on Hydrogen Safety, Hamburg, Germany, 11 September 2017. [Google Scholar]
- Jallais, S.; Liquide, A.; Vyazmina, E.; Miller, D. Effects of the Ignition Position on the Overpressure Originated from a Delayed Ignition of High Pressure Releases of Hydrogen. In Proceedings of the 11th International Symposium on Hazards, Prevention, and Mitigation of Industrial Explosion (ISHPMIE), Dalian, China, 24–29 July 2016. [Google Scholar]
- Middha, P.; Hansen, O.R. Using Computational Fluid Dynamics as a Tool for Hydrogen Safety Studies. J. Loss Prev. Process Ind. 2009, 22, 295–302. [Google Scholar] [CrossRef]
- Middha, P.; Hansen, O.R. CFD Simulation Study to Investigate the Risk from Hydrogen Vehicles in Tunnels. Int. J. Hydrogen Energy 2009, 34, 5875–5886. [Google Scholar] [CrossRef]
- Hansen, O.R.; Hinze, P.; Engel, D.; Davis, S. Using Computational Fluid Dynamics (CFD) for Blast Wave Predictions. J. Loss Prev. Process Ind. 2010, 23, 885–906. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Q.; Pang, L.; Huang, Y.; Chen, J. Effects of Hydrogen Addition on the Confined and Vented Explosion Behavior of Methane in Air. J. Loss Prev. Process Ind. 2014, 27, 65–73. [Google Scholar] [CrossRef]
- Witkowski, A.; Rusin, A.; Majkut, M.; Stolecka, K. Analysis of Compression and Transport of the Methane/Hydrogen Mixture in Existing Natural Gas Pipelines. Int. J. Press. Vessel. Pip. 2018, 166, 24–34. [Google Scholar] [CrossRef]
- Pluvinage, G.; Capelle, J.; Meliani, M.H. Pipe Networks Transporting Hydrogen Pure or Blended with Natural Gas, Design and Maintenance. Eng. Fail Anal. 2019, 106, 104164. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, H.; Huang, X.; Peng, S. Numerical Simulation on Methane-Hydrogen Explosion in Gas Compartment in Utility Tunnel. Process Saf. Environ. Prot. 2020, 140, 100–110. [Google Scholar] [CrossRef]
- Middha, P.; Engel, D.; Hansen, O.R. Can the addition of hydrogen to natural gas reduce the explosion risk? Int. J. Hydrogen Energy 2011, 36, 2628–2636. [Google Scholar] [CrossRef]
- Fang, W.; Wu, J.; Bai, Y.; Zhang, L.; Reniers, G. Quantitative Risk Assessment of a Natural Gas Pipeline in an Underground Utility Tunnel. Process Saf. Prog. 2019, 38, e12051. [Google Scholar] [CrossRef]
- He, Q.; Gu, M.; Tang, F.; Sun, X.; Wang, Y. Ceiling Radiation Heat Flux and Downward Received Radiation Heat Flux of Methane Jet Fire with Hydrogen Addition. Int. J. Hydrogen Energy 2024, 51, 741–753. [Google Scholar] [CrossRef]
- Molkov, V.; Dery, W. The Blast Wave Decay Correlation for Hydrogen Tank Rupture in a Tunnel Fire. Int. J. Hydrogen Energy 2020, 45, 31289–31302. [Google Scholar] [CrossRef]
- Kashkarov, S.; Dadashzadeh, M.; Sivaraman, S.; Molkov, V. Quantitative Risk Assessment Methodology for Hydrogen Tank Rupture in a Tunnel Fire. Hydrogen 2022, 3, 512–530. [Google Scholar] [CrossRef]
- Wang, T.; Huang, T.; Hu, S.; Li, Y.; Yang, F.; Ouyang, M. Simulation and Risk Assessment of Hydrogen Leakage in Hydrogen Production Container. Int. J. Hydrogen Energy 2023, 48, 20096–20111. [Google Scholar] [CrossRef]
- Lin, H.; Luan, H.; Yang, L.; Han, C.; Zhang, S.; Zhu, H.; Chen, G. Numerical Simulation and Consequence Analysis of Accidental Hydrogen Fires in a Conceptual Offshore Hydrogen Production Platform. Int. J. Hydrogen Energy 2023, 48, 10250–10263. [Google Scholar] [CrossRef]
- Portarapillo, M.; Di Benedetto, A. Risk Assessment of the Large-Scale Hydrogen Storage in Salt Caverns. Energies 2021, 14, 2856. [Google Scholar] [CrossRef]
| Blue Hydrogen | Green Hydrogen |
|---|---|
| Release of hydrogen: large quantities of hydrogen are handled during production, posing the risk of accidental release that leads to a flammable atmosphere. | Release of hydrogen: similarly to the production of blue hydrogen, the production of green hydrogen also involves the handling of significant quantities of hydrogen, thereby leading to potential release hazards. |
| Carbon capture and storage: the transport and storage of captured carbon dioxide (CO2) raises concerns about leaks and potential environmental impacts. | Electrical hazard: high electrical currents are used during electrolysis, creating a risk of electric shocks, short circuits, and fires. |
| High temperatures and pressures: the reforming processes take place at high temperatures and pressures, thereby requiring robust equipment and safety measures. | Chemical exposure: electrolysis uses electrolytes that can result in chemical exposure. |
| Typical accidents: hydrogen release and ignition, with CO2 release having an impact on the environment | Typical accidents: fire in chlorine electrolyser cells [24,25], hydrogen explosion, hydrogen–oxygen explosion, explosion during the operation of a HP WE, membrane perforation in a PEM-FC cell, destruction of a PEM FC short stack, deflagration of H2/O2 with a short circuit, fire, hydrogen gas holder exploded due to a malfunction in the electrolyser. |
| Storage | Safety Issues |
|---|---|
| Compressed hydrogen | High pressure, strong interaction with materials, high frequency of occurrence of release. |
| Cryogenic hydrogen | Low temperature, strong interaction with materials, difficult thermal management, blow-out, ground release for the high density, freezer burns, complex phenomena close to the release point. |
| Cryo-compressed hydrogen | High pressure, low temperature, strong interaction with materials, difficult thermal management, blow-out, ground release for the high density, freezer burns, complex phenomena close to the release point. |
| LOHC | Formic acid: corrosive chemical that causes severe burns to the skin and eyes. DBT: low flammability, and toxicity is not well defined since it is a mixture of different regioisomers. |
| Chemical storage | Methanol: toxic (ingestion of 56.2 g per person and for inhalation a concentration of 4000–13,000 ppm), low FP, low BP. Ammonia: toxic (the lethal dose after 10 min of exposure is already estimated to be 2700 ppm, with severe irritation already estimated to be 220 ppm. The lethal dose of ammonia after 8 h of exposure can be as low as 390 ppm) and flammable. |
| Hydrogen Value Chain Step | Operation | European Standards | US Standards |
|---|---|---|---|
| Production | Electrolysis | CEN-CENELEC [36] ISO [37] NFPA [35] | UL [38] CSA [39] |
| Traditional steam reforming | |||
| Conditioning | Compression | ISO [37] NFPA [35] | NFPA [35] |
| Liquification | |||
| Storage and transport | Pipeline | ISO [37] EIGA [34] SAE [40] NFPA [35] | ASME [41] CSA [39] NFPA [35] |
| Storage | |||
| Cryogenic tank | |||
| End use | Fuel cell mobility | ISO [37] SAE [40] CEN-CENELEC [36] | UL [38] SAE [40] |
| Refuelling | ISO [37] SAE [40] CEN-CENELEC [36] NFPA [35] | UL [38] SAE [40] NFPA [35] | |
| Process control | Sensor and detectors | IEC [36] SAE [40] CEN-CENELEC [36] ISO [37] NFPA [35] | UL [38] NFPA [35] |
| Model Name | Safety Issues | Limitations | References |
|---|---|---|---|
| Notional nozzle | Mass flow rate in the case of high-pressure hydrogen jets | Highly dependent on jet temperature, expansion type, real gas properties, the conservation of energy, and the position of the notional nozzle as a function of the Mach number | [54,55,56,57,58] |
| Pasquill-Gifford | Dispersion in the atmosphere | Model developed for passive dispersion, with the density of the substance being similar to that of air | [51] |
| Houf and Winters (2013) | Dispersion in the atmosphere | No consideration for positive buoyancy in the case of gas and not applicable in the near-field zone in the case of cryogenic hydrogen | [59,60,61] |
| Briggs (1984) | Dispersion in the atmosphere | Combination of momentum and positive buoyancy | [62] |
| Houf and Schefer | Jet fire | Underestimation of the radiation portion of the flame by 40% or more | [63] |
| Ekoto et al. (2014) | Jet fire with buoyancy effects | Applicable for hydrogen gas stored at 60 bar | [64] |
| TNT model | Overpressure of an unconfined vapour cloud explosion | Overestimation in most cases | [65,66,67,68] |
| TNO model | Overpressure of an unconfined vapour cloud explosion | Underestimation in most cases | [65,66,67,68] |
| Baker–Strehlow–Tang (BST) model | Overpressure of an unconfined vapour cloud explosion | Underestimation in most cases | [65,66,67,68] |
| Dorofeev model | Overpressure of an unconfined vapour cloud explosion | Underestimation in most cases | [65,66,67,68] |
| Eisenberg and the Tsao and Perry probit models | Thermal harm | Generic probit but the most applicable one for hydrogen | [70,71,72] |
| TNO probit models | Overpressure harm | Generic probit but the most applicable one for hydrogen | [52,73,74,75] |
| Model | Aim and Results | References |
|---|---|---|
| Hydrogen dispersion | Validation of the CFD tool FLACS–HYDROGEN (https://www.gexcon.com/software/flacs-cfd/) and next (RANS approach) in the case of GH2 and LH2 dispersion. Reasonable agreement was seen for many different kinds of release conditions. | [78] |
| Hydrogen dispersion | Two-dimensional CFD modelling of accidental hydrogen release from pipelines using CFD-ACE (RANS approach). The hydrogen clouds are further away from the ground or buildings than the methane clouds, so the probability of ignition is reduced and flame acceleration is reduced due to obstacles in the event of ignition. | [79] |
| Hydrogen dispersion | Hydrogen dispersion from tube fittings on fuel cell vehicles under the effect of ambient wind using FLACS (RANS approach). In the case of a crosswind, the flammable region becomes far away from the car in 20 s. | [32] |
| Hydrogen dispersion | Hydrogen dispersion in enclosed spaces like a residential garage, fuel cell room, covered car park, and large enclosures. Simulations were carried out with FLACS-Hydrogen and validated against experimental data. The results are highly dependent on the used geometry. | [80,81,82,83,84,85,86] |
| Jet fires | CFD simulations of a vertical and horizontal high-pressure jet fire, mainly using the LES approach and Ansys Fluent (https://www.ansys.com/products/fluids/ansys-fluent) and next but also Kameleon FireEx (KFX) (https://www.dnv.co.kr/services/cfd-simulation-kameleon-fireex-kfx-110598) with the RANS approach. All the results were validated against experimental data. | [88,89,90,91] |
| Jet fires | Suppression of hydrogen jet fires on hydrogen fuel cell ships using a fine water mist with a Fire Dynamics Simulator (FDS) (LES approach). Water mist with a spray velocity of 30 m/s and average droplet size of 30 μm can effectively reduce the fire field temperature of hydrogen jet fires and prevent the fire from developing further. | [92] |
| Liquid hydrogen release and dispersion | Release and dispersion in an open environment was always simulated with the RANS approach using FLACS, Ansys Fluent, or ADREA-HF (http://www2.ipta.demokritos.gr/pages/ADREA-HF.html), and the effect of the influence of ground temperature, wind speed, wind temperature, and both air liquefaction and solidification was investigated. The results were generally validated against NASA data and/or experimental results. | [94,95,96,97] |
| Liquid hydrogen release and dispersion | Flash-boiling and pressure recovery phenomena during release from high-scale pressurized liquid hydrogen storage tank with the LES approach. | [99] |
| Liquid hydrogen fires | CFD simulations (LES approach) and novel empirical models were proposed to capture the features of the phenomenon. The effect of pressure and temperature was considered, and the results were validated against experimental data. | [100,101] |
| Liquid hydrogen fires | Extinguishing the action of sprinklers in a hydrogen pool fire using the Fire Dynamics Simulator (FDS). A higher spray velocity and a smaller droplet size enhanced the extinguishing efficiency, and the number of sprinklers required to suppress hydrogen pool fire was optimized. | [102] |
| Vapor cloud explosions | Blast wave predictions performed using FLACS-Hydrogen, with a focus on the dynamic effects in the near- and far-field zones, time-dependent pressure loads, reflection, and blast waves. | [103,104,105,106,107] |
| Hythane | Methane–hydrogen explosion in the gas compartment in utility tunnels using FLACS. The results show that higher overpressures were seen with methane compared with hythane. | [111,112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, M.; Portarapillo, M.; Di Nardo, A.; Venezia, V.; Turco, M.; Luciani, G.; Di Benedetto, A. Hydrogen Safety Challenges: A Comprehensive Review on Production, Storage, Transport, Utilization, and CFD-Based Consequence and Risk Assessment. Energies 2024, 17, 1350. https://doi.org/10.3390/en17061350
Calabrese M, Portarapillo M, Di Nardo A, Venezia V, Turco M, Luciani G, Di Benedetto A. Hydrogen Safety Challenges: A Comprehensive Review on Production, Storage, Transport, Utilization, and CFD-Based Consequence and Risk Assessment. Energies. 2024; 17(6):1350. https://doi.org/10.3390/en17061350
Chicago/Turabian StyleCalabrese, Marcella, Maria Portarapillo, Alessandra Di Nardo, Virginia Venezia, Maria Turco, Giuseppina Luciani, and Almerinda Di Benedetto. 2024. "Hydrogen Safety Challenges: A Comprehensive Review on Production, Storage, Transport, Utilization, and CFD-Based Consequence and Risk Assessment" Energies 17, no. 6: 1350. https://doi.org/10.3390/en17061350
APA StyleCalabrese, M., Portarapillo, M., Di Nardo, A., Venezia, V., Turco, M., Luciani, G., & Di Benedetto, A. (2024). Hydrogen Safety Challenges: A Comprehensive Review on Production, Storage, Transport, Utilization, and CFD-Based Consequence and Risk Assessment. Energies, 17(6), 1350. https://doi.org/10.3390/en17061350











