Enhancing the Viability of a Promising E-Fuel: Oxymethylene Ether–Decanol Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Fuels
2.2. FKM Compatibility Test
2.3. Engine Dyno Measurement Setup
2.4. Engine Dyno Test Methods and Calculations
3. Results and Discussion
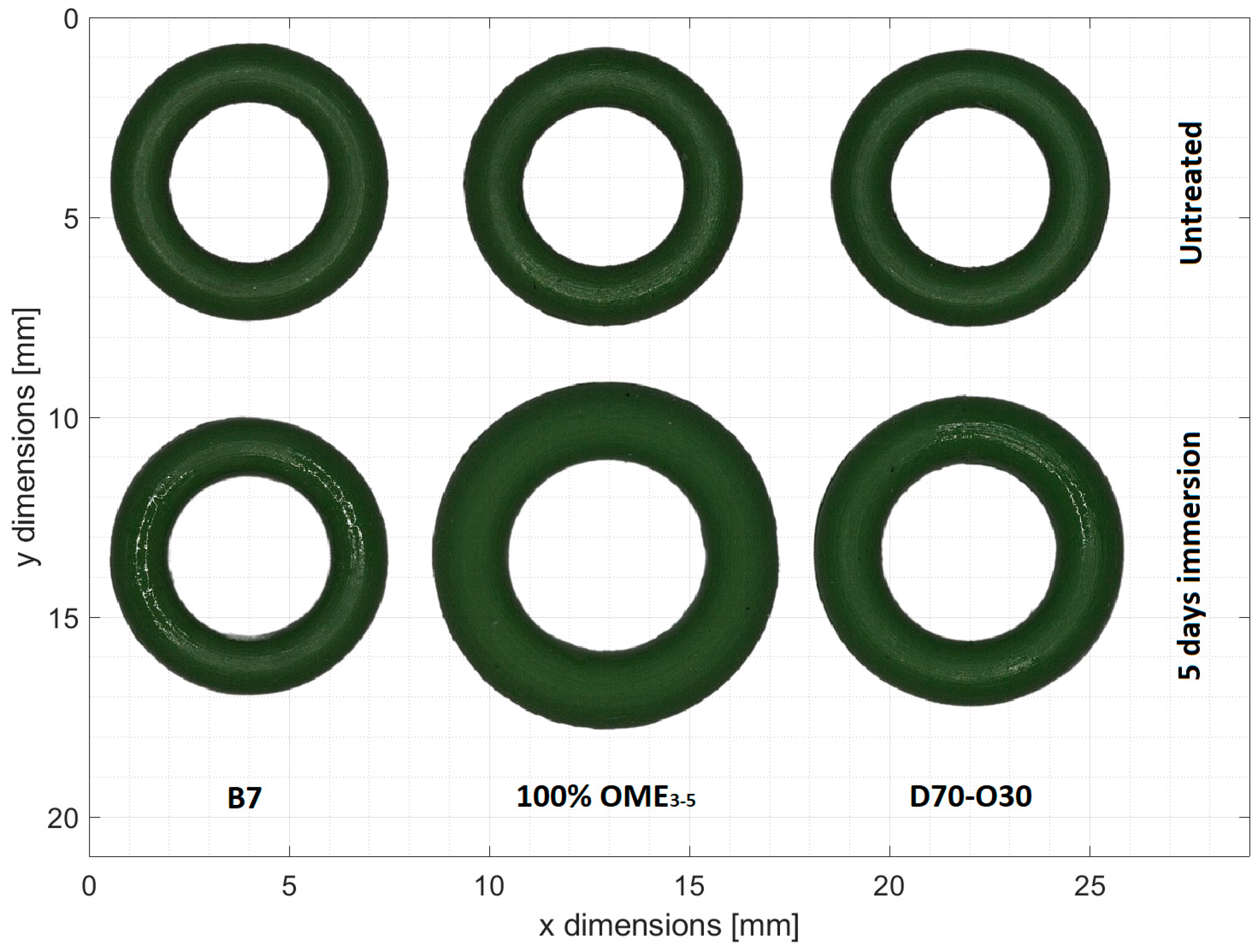
3.1. Compatibility with FKM Sealing Materials
3.2. Emissions
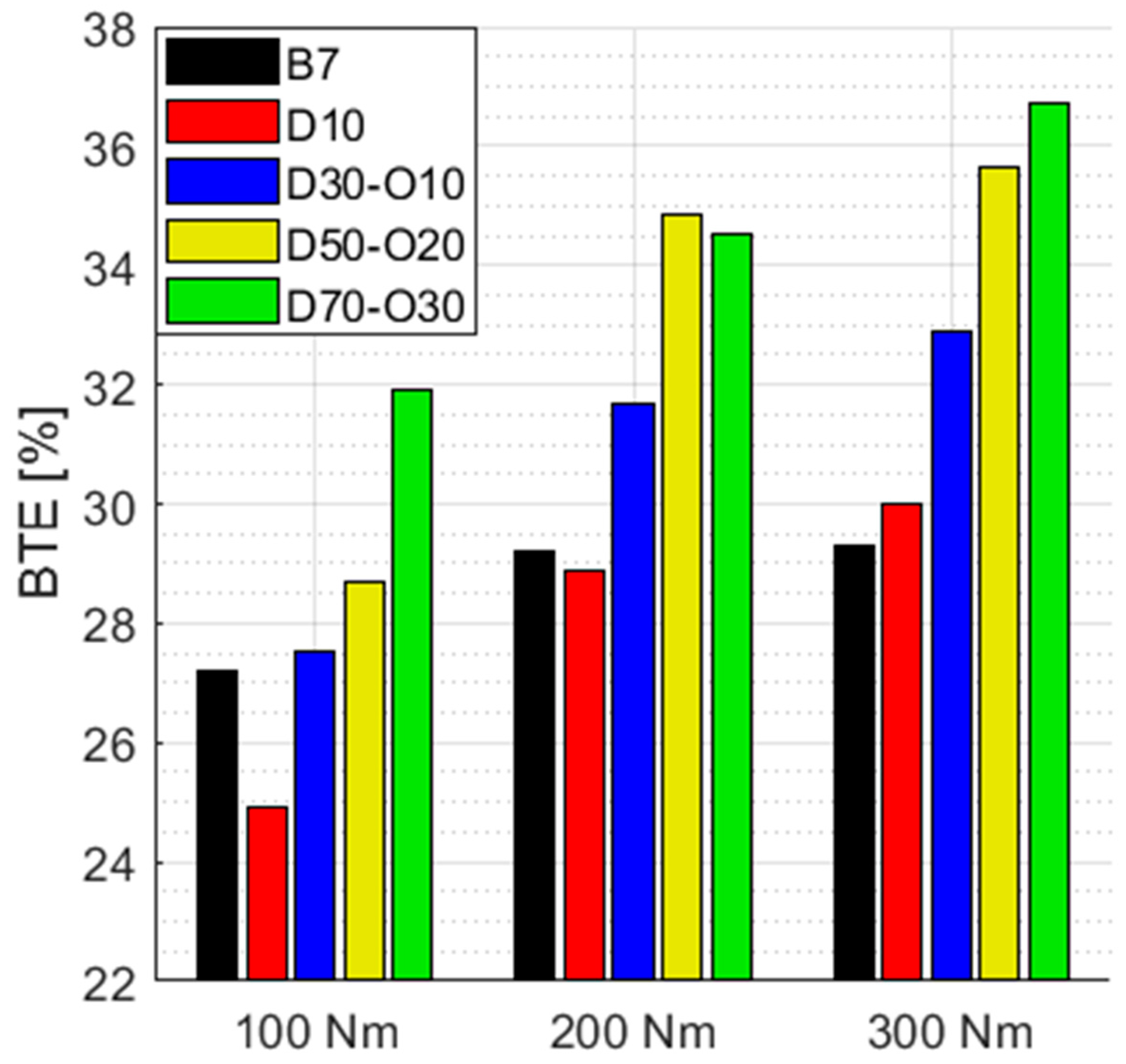
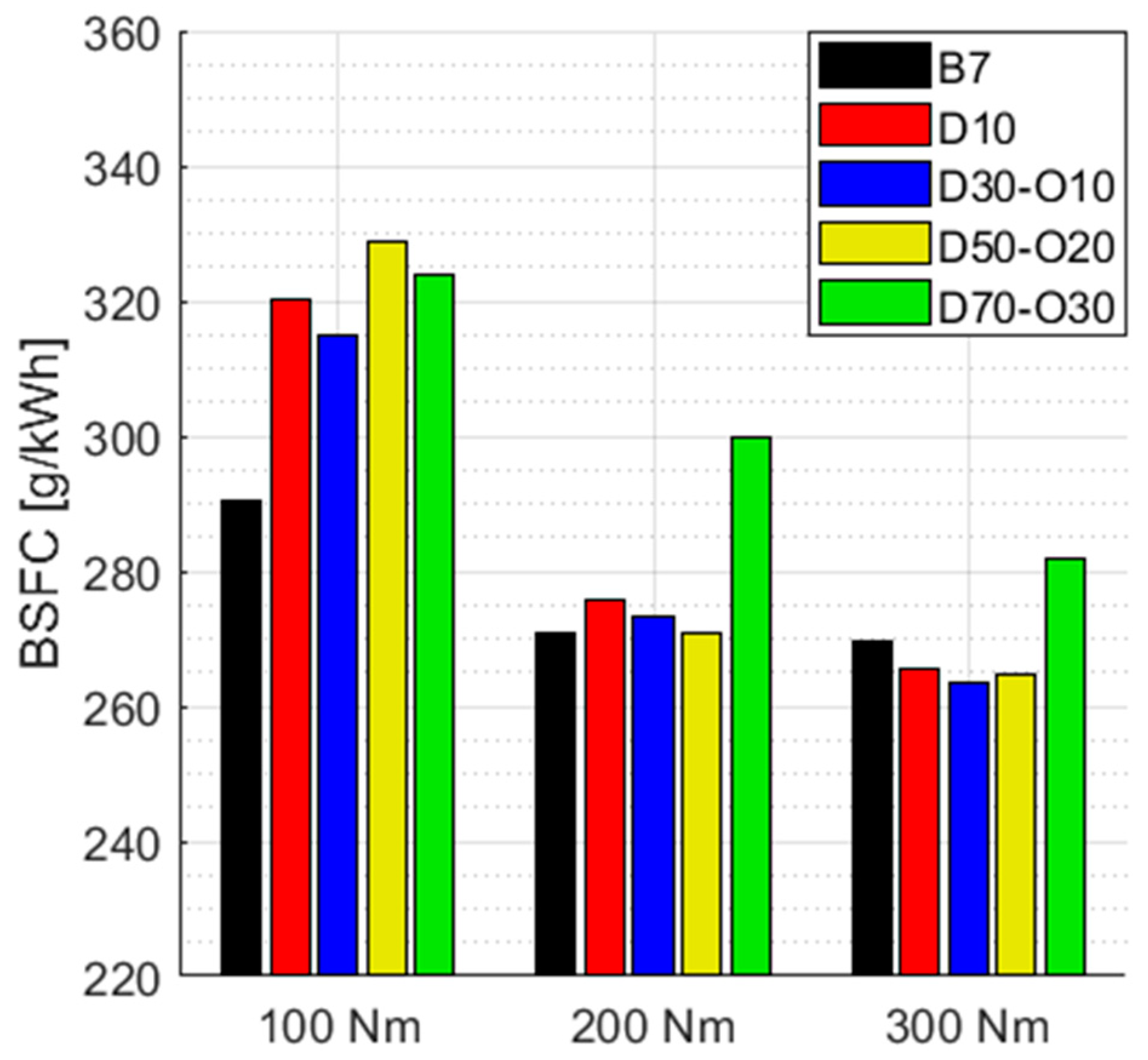
3.3. Economy
3.4. Combustion
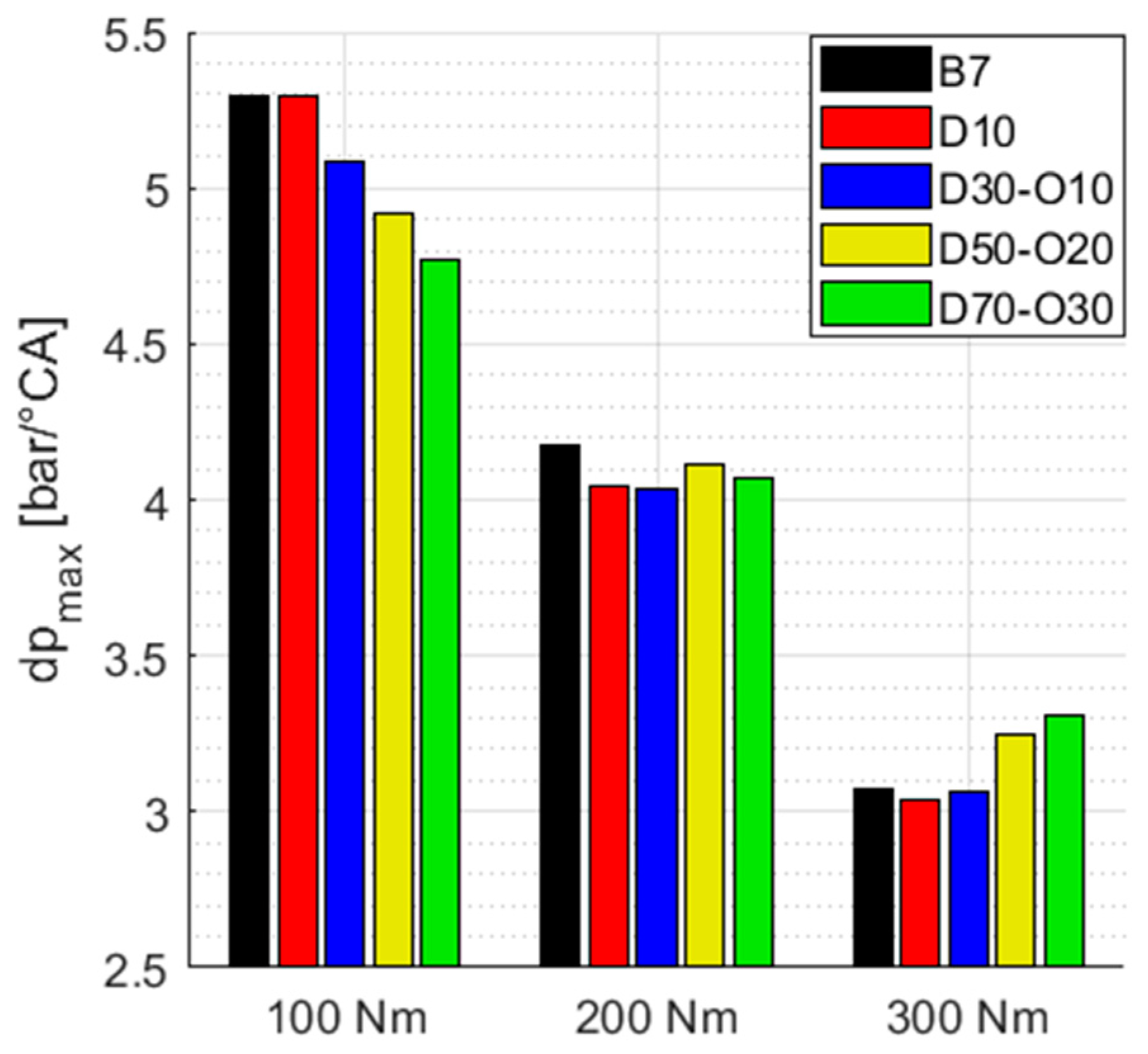
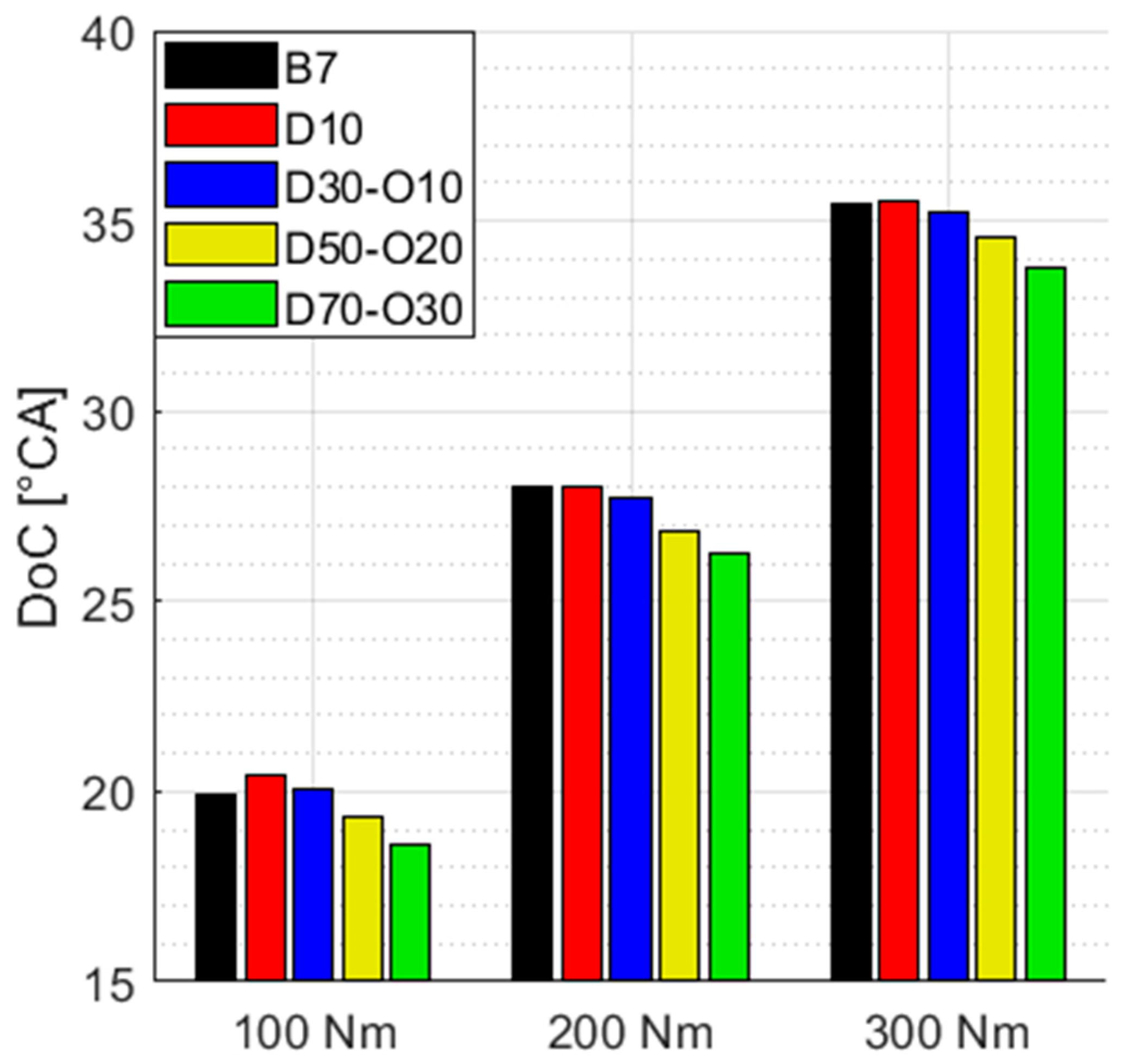
3.4.1. Mean Combustion Properties
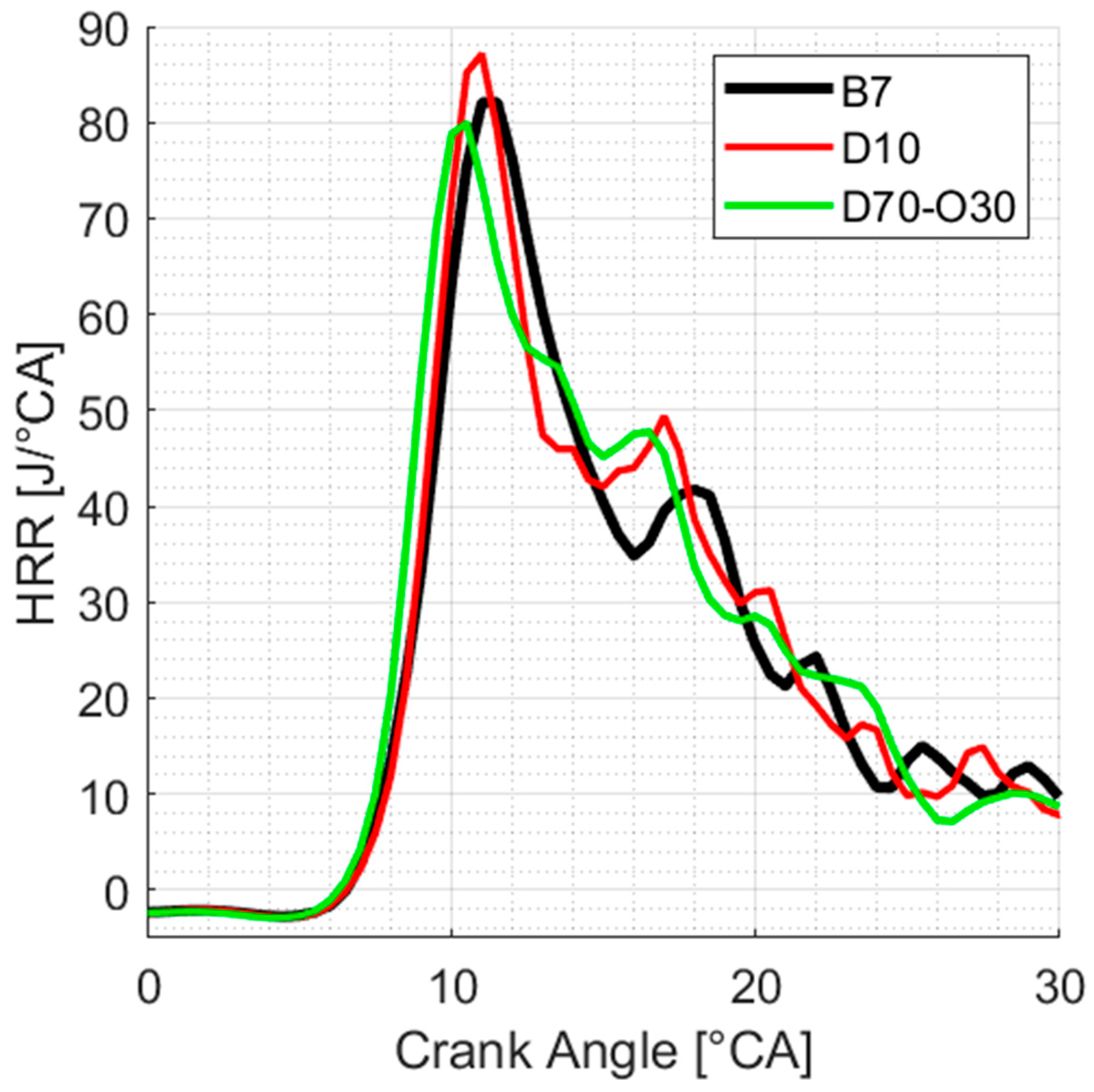
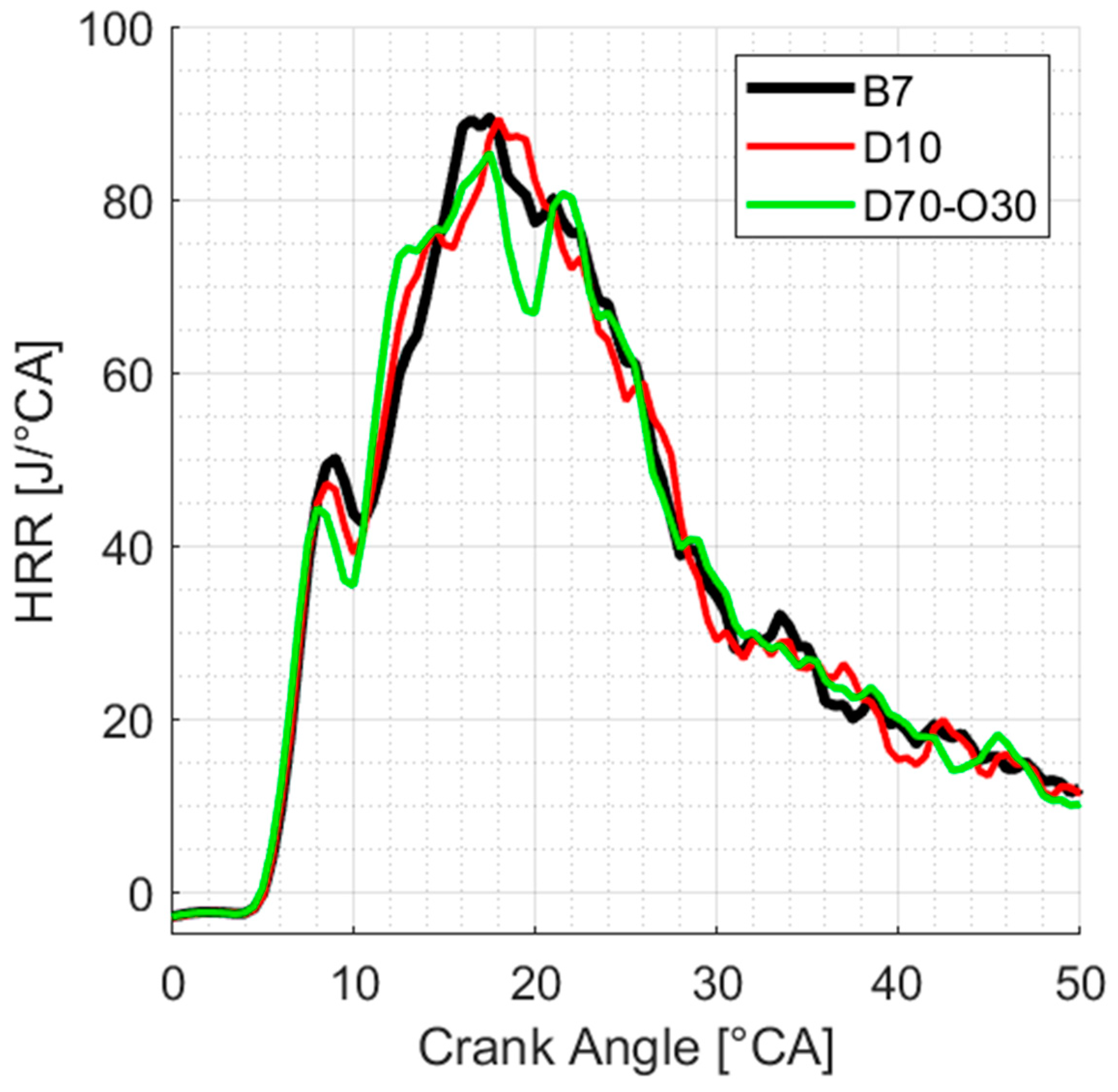
3.4.2. Heat Release Rates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Delbeke, J.; Runge-Metzger, A.; Slingenberg, V.; Werksman, J. The Paris Agreement in Towards a Climate-Neutral Europe, 1st ed.; Routledge: London, UK, 2019. [Google Scholar] [CrossRef]
- Ovaere, M.; Proost, S. Cost-effective reduction of fossil energy use in the European transport sector: An assessment of the Fit for 55 Package. Energy Policy 2022, 168, 113085. [Google Scholar] [CrossRef]
- eFuel Alliance: Press Release. 2022. Available online: https://www.efuel-alliance.eu/fileadmin/Downloads/E_PM_eFA_EU-Parlament_vor_Richtungsentscheidung_02062022.pdf (accessed on 10 December 2023).
- Wu, F.; Fang, S.; Kuenzel, M.; Mullaliu, A.; Kim, J.K.; Gao, X.; Diemant, T.; Kim, G.-T.; Passerini, S. Dual-anion ionic liquid electrolyte enables stable Ni-rich cathodes in lithium-metal batteries. Joule 2021, 5, 2177–2194. [Google Scholar] [CrossRef]
- Blakey, S.; Rauch, B.; Oldani, A.; Lee, T. Advanced Fuel Property Data Platform: Overview and Potential Applications. Front. Energy Res. 2022, 10, 771325. [Google Scholar] [CrossRef]
- Fu, J.; Turn, S.Q. Characteristics and stability of biofuels used as a drop-in replacement for NATO marine diesel. Fuel 2019, 236, 516–524. [Google Scholar] [CrossRef]
- Dhinesh, B.; Lalvani, J.I.J.; Parthasarathy, M.; Annamalai, K. An assessment on performance, emission and combustion characteristics of single cylinder diesel engine powered by Cymbopogon flexuosus biofuel. Energy Convers. Manag. 2016, 117, 466–474. [Google Scholar] [CrossRef]
- Schmitz, R.; Russo, C.; Ferraro, F.; Apicella, B.; Hasse, C.; Sirignano, M. Effect of oxymethylene ether-2-3-4 (OME2-4) on soot particle formation and chemical features. Fuel 2022, 324, 124617. [Google Scholar] [CrossRef]
- Prentice, I.C.; Farquhar, G.D.; Fasham, M.J.R.; Goulden, M.L.; Heimann, M.; Jaramillo, V.J.; Kheshgi, H.S.; Le Quéré, C.; Scholes, R.J.; Wallace, D.W.; et al. The carbon cycle and atmospheric carbon dioxide. In Climate Change 2001: The Scientific Basis, Intergovernmental Panel on Climate Change. 2001, pp. 183–238. Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/TAR-03.pdf (accessed on 12 December 2023).
- Wicke, B.; Verweij, P.; Van Meijl, H.; Van Vuuren, D.P.; Faaij, A.P. Indirect land use change: Review of existing models and strategies for mitigation. Biofuels 2012, 3, 87–100. [Google Scholar] [CrossRef]
- Bongartz, D.; Burre, J.; Mitsos, A. Production of Oxymethylene Dimethyl Ethers from Hydrogen and Carbon Dioxide Part I: Modeling and Analysis for OME1. Ind. Eng. Chem. Res. 2019, 58, 4881–4889. [Google Scholar] [CrossRef]
- Cipriano, E.; da Silva Major, T.C.F.; Pessela, B.; Barros, A.A.C. Production of Anhydrous Ethyl Alcohol from the Hydrolysis and Alcoholic Fermentation of Corn Starch. Cogn. Sustain. 2022, 1. [Google Scholar] [CrossRef]
- Alahmer, A.; Rezk, H.; Aladayleh, W.; Mostafa, A.O.; Abu-Zaid, M.; Alahmer, H.; Gomaa, M.R.; Alhussan, A.A.; Ghoniem, R.M. Modeling and Optimization of a Compression Ignition Engine Fueled with Biodiesel Blends for Performance Improvement. Mathematics 2022, 10, 420. [Google Scholar] [CrossRef]
- Pali, H.S.; Sharma, A.; Kumar, M.; Annakodi, V.A.; Singh, N.K.; Singh, Y.; Balasubramanian, D.; Deepanraj, B.; Truong, T.H.; Nguyen, P.Q.P. Enhancement of combustion characteristics of waste cooking oil biodiesel using TiO2 nanofluid blends through RSM. Fuel 2023, 331, 125681. [Google Scholar] [CrossRef]
- Kowthaman, C.N.; Rahman, S.M.A.; Fattah, I.M.R. Exploring the Potential of Lignocellulosic Biomass-Derived Polyoxymethylene Dimethyl Ether as a Sustainable Fuel for Internal Combustion Engines. Energies 2023, 16, 4679. [Google Scholar] [CrossRef]
- Härtl, M.; Seidenspinner, P.; Jacob, E.; Wachtmeister, G. Wachtmeister: Oxygenate screening on a heavy-duty diesel engine and emission characteristics of highly oxygenated oxymethylene ether fuel OME1. Fuel 2015, 153, 328–335. [Google Scholar] [CrossRef]
- BS EN 590:2022; Automotive Fuels. Diesel. Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2023.
- Agarwal, A.K.; Chandra, K. Di-ethyl ether-diesel blends fuelled off-road tractor engine: Part-I: Technical feasibility. Fuel 2022, 308, 121972. [Google Scholar] [CrossRef]
- Virt, M.; Arnold, U. Effects of Oxymethylene Ether in a Commercial Diesel Engine. Cogn. Sustain. 2022, 1. [Google Scholar] [CrossRef]
- Zhang, X.; Oyedun, A.O.; Kumar, A.; Oestreich, D.; Arnold, U.; Sauer, J. An optimized process design for oxymethylene ether production from woody-biomass-derived syngas. Biomass Bioenergy 2016, 90, 7–14. [Google Scholar] [CrossRef]
- Debergh, P.; Gutiérrez-Sánchez, O.; Khan, M.N.; Birdja, Y.Y.; Pant, D.; Bulut, M. The Economics of Electrochemical Syngas Production via Direct Air Capture. ACS Energy Lett. 2023, 8, 3398–3403. [Google Scholar] [CrossRef]
- Omari, A.; Heuser, B.; Pischinger, S.; Rüdinger, C. Potential of long-chain oxymethylene ether and oxymethylene ether-diesel blends for ultra-low emission engines. Appl. Energy 2019, 239, 1242–1249. [Google Scholar] [CrossRef]
- Pélerin, D.; Gaukela, K.; Härtl, M.; Jacob, E.; Wachtmeister, G. Potentials to simplify the engine system using the alternative diesel fuels oxymethylene ether OME1 and OME3−6 on a heavy-duty engine. Fuel 2020, 259, 116231. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, P.; Sun, P.; Liu, H.; Meng, Z.; Zhang, L.; Ma, H. An overview of polyoxymethylene dimethyl ethers as alternative fuel for compression ignition engines. Fuel 2022, 318, 123582. [Google Scholar] [CrossRef]
- Parravicini, M.; Barro, C.; Boulouchos, K. Experimental characterization of GTL, HVO, and OME based alternative fuels for diesel engines. Fuel 2021, 292, 120177. [Google Scholar] [CrossRef]
- El-Seesy, A.I.; Xuan, T.; He, Z.; Hassan, H. Enhancement the combustion aspects of a CI engine working with Jatropha biodiesel/decanol/propanol ternary combinations. Energy Convers. Manag. 2020, 226, 113524. [Google Scholar] [CrossRef]
- Nanthagopal, K.; Ashok, B.; Saravanan, B.; Pathy, M.R.; Sahil, G.; Ramesh, A.; Nabi, M.N.; Rasul, M.G. Study on decanol and Calophyllum Inophyllum biodiesel as ternary blends in CI engine. Fuel 2019, 239, 862–873. [Google Scholar] [CrossRef]
- Preuß, J.; Munch, K.; Denbratt, I. Performance and emissions of long-chain alcohols as drop-in fuels for heavy duty compression ignition engines. Fuel 2018, 216, 890–897. [Google Scholar] [CrossRef]
- Ashok, B.; Nanthagopal, K.; Darla, S.; Chyuan, O.H.; Ramesh, A.; Jacob, A.; Sahil, G.; Thiyagarajan, S.; Geo, V.E. Comparative assessment of hexanol and decanol as oxygenated additives with calophyllum inophyllum biodiesel. Energy 2019, 173, 494–510. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Damodharan, D.; Gopal, K.; Kumar, B.R.; De Poures, M.V. Collective influence of 1-decanol addition, injection pressure and EGR on diesel engine characteristics fueled with diesel/LDPE oil blends. Fuel 2020, 277, 118166. [Google Scholar] [CrossRef]
- Hamilton-Kemp, T.; Newman, M.; Collins, R.; Elgaali, H.; Yu, K.; Archbold, D. Production of the Long-Chain Alcohols Octanol, Decanol, and Dodecanol by Escherichia coli. Curr. Microbiol. 2005, 51, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Rutter, C.D.; Rao, C.V. Production of 1-decanol by metabolically engineered Yarrowia lipolytica. Metab. Eng. 2016, 38, 139–147. [Google Scholar] [CrossRef]
- Haltenort, P.; Hackbarth, K.; Oestreich, D.; Lautenschütz, L.; Arnold, U.; Sauer, J. Heterogeneously catalyzed synthesis of oxymethylene dimethyl ethers (OME) from dimethyl ether and trioxane. Catal. Commun. 2018, 109, 80–84. [Google Scholar] [CrossRef]
- Csemány, D.; DarAli, O.; Rizvi, S.A.H.; Józsa, V. Comparison of volatility characteristics and temperature-dependent density, surface tension, and kinematic viscosity of n-butanol-diesel and ABE-diesel fuel blends. Fuel 2021, 312, 122909. [Google Scholar] [CrossRef]
- DIN EN 17155; Liquid Petroleum Products—Determination of Indicated Cetane Number (ICN) of Middle Distillate Fuels—Primary Reference Fuels Calibration Method Using a Constant Volume Combustion Chamber. German Version; Deutsches Institut für Normung [DIN]: Berlin, Germany, 2018.
- ISO 12156 1; Diesel Fuel Assessment of Lubricity Using the High-Frequency Reciprocating Rig (HFRR). International Organization for Standardization: Geneva, Switzerland, 2018.
- ASTM D 7094; Standard Test Method for Flash Point by Modified Continuously Closed Cup (MCCCFP) Tester. ASTM International: West Conshohocken, PA, USA, 2013.
- Virt, M.; Horváth, L.; Zöldy, M. Density and viscosity measurements for diesel-decanol-oxymethylene ether blends. Angolan Miner. Oil Gas J. 2023, 4, 1–5. [Google Scholar]
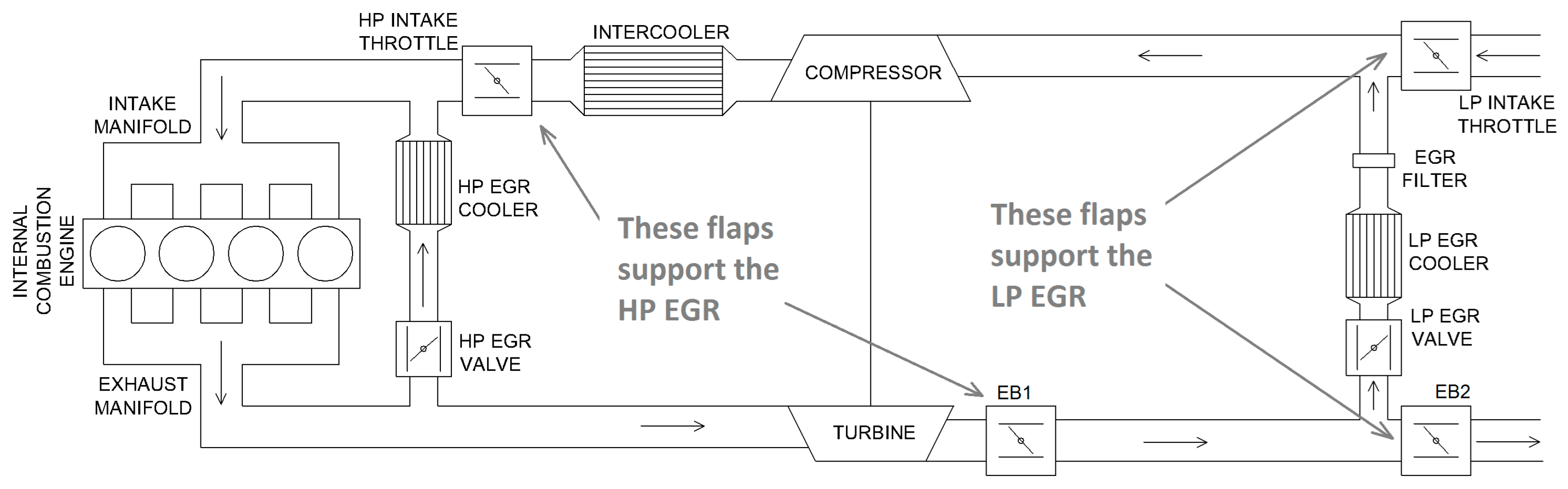
- Nyerges, Á.; Zöldy, M. Verification and Comparison of Nine Exhaust Gas Recirculation Mass Flow Rate Estimation Methods. Sensors 2020, 20, 7291. [Google Scholar] [CrossRef]
- Virt, M.; Granovitter, G.; Zöldy, M.; Bárdos, Á.; Nyerges, Á. Multipulse Ballistic Injection: A Novel Method for Improving Low Temperature Combustion with Early Injection Timings. Energies 2021, 14, 3727. [Google Scholar] [CrossRef]
- Nyerges, Á.; Zöldy, M. Ranking of four dual loop EGR modes. Cogn. Sustain. 2023, 2. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Lakshminarayanan, P.A.; Aswin, S. Estimation of Particulate Matter from Smoke, Oil Consumption and Fuel Sulphur; SAE Technical Paper: Warrendale, PA, USA, 2016; p. 22016-32-0066. [Google Scholar]
- Zeldovich, Y.B. The oxidation of nitrogen in combustion and explosions. Acta Physicochem. 1946, 21, 577–628. [Google Scholar]
- Virt, M.; Zöldy, M. The Effects of Oxygen from the Fuel and the Intake Air; OGÉT; Hungarian Technical Scientific Society of Transylvania: Cluj, Romania, 2023. [Google Scholar]
- Liu, J.; Sun, P.; Huang, H.; Meng, J.; Yao, X. Experimental investigation on performance, combustion and emission characteristics of a common-rail diesel engine fueled with polyoxymethylene dimethyl ethers-diesel blends. Appl. Energy 2017, 202, 527–536. [Google Scholar] [CrossRef]
- Yin, X.; Li, Z.; Yang, B.; Sun, T.; Wang, Y.; Zeng, K. Experimental study of the combustion characteristics prediction model for a sensor-less closed-loop control in a heavy-duty NG engine. Fuel 2021, 300, 120945. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Zhang, J.; Wang, J.; Shuai, S. Study on combustion and emission characteristics of Polyoxymethylene Dimethyl Ethers/diesel blends in light-duty and heavy-duty diesel engines. Appl. Energy 2015, 185, 1393–1402. [Google Scholar] [CrossRef]


| Property | EN 590 | OME1 | OME2 | OME3 | OME4 | OME5 | OME6 |
|---|---|---|---|---|---|---|---|
| Lower heating value [MJ/kg] | - | 23.3 | 21 | 19.6 | 19 | 18.5 | 17.7 |
| Cetane number [-] | >51 | 28 | 68 | 72 | 84 | 93 | 104 |
| Density at 15 °C [kg/m3] | 820–845 | 860 | 980 | 1030 | 1070 | 1110 | 1140 |
| Kinematic viscosity at 40 °C [mm2/s] | 2–4.5 | 0.37 | 0.559 | 0.866 | 1.33 | 1.96 | n.i |
| Boiling point [°C] | - | 42 | 105 | 156 | 202 | 242 | 273 |
| Flashpoint [°C] | >55 | −32 | 12 | 54 | 88 | 115 | 169 |
| Oxygen content [wt%] | - | 42.1 | 45.2 | 47 | 48.1 | 48.9 | 49.5 |
| Fuel | B7 | OME3-5 | n-Decanol | EN590 |
|---|---|---|---|---|
| Density at 15 °C [kg/m3] | 844.2 | 1063.2 | 832.6 | 820–845 |
| Kinematic viscosity at 40 °C [mm2/s] | 2.780 | 1.163 | 6.910 | 2–4.5 |
| Oxygen concentration [wt%] | ~1.00 | 47.73 | 10.11 | - |
| Indicated cetane number [-] | 52.7 | 76.9 | 46.7 | >51 |
| WSD at 60 °C [μm] | 190 | 440 | 390 | <460 |
| Lower heating value [MJ/kg] | 45.55 | 21.93 | 41.85 | - |
| Flash point [°C] | 67 | 50 | 112 | >55 |
| Fuel | D10 | D30-O10 | D50-O20 | D70-O30 | EN590 |
|---|---|---|---|---|---|
| B7 content [vol%] | 90 | 60 | 30 | 0 | - |
| 1-decanol content [vol%] | 10 | 30 | 50 | 70 | - |
| OME3-5 content [vol%] | 0 | 10 | 20 | 30 | - |
| Density at 15 °C [kg/m3] | 842.3 | 851.4 | 863.2 | 898.0 | 820–845 |
| Kinematic viscosity at 40 °C [mm2/s] | 3.23 | 2.90 | 2.93 | 2.99 | 2–4.5 |
| Oxygen concentration [wt%] | 1.09 | 8.87 | 16.31 | 23.42 | - |
| Indicated cetane number [-] | 53.2 | 54.1 | 54.4 | 51.1 | >51 |
| WSD at 60 °C [μm] | 430 | 170 | 160 | 160 | <460 |
| Lower heating value [MJ/kg] | 45.18 | 41.53 | 38.14 | 34.82 | - |
| Flash point [°C] | 69 | 64 | 64 | 68 | >55 |
| Parameter | Specification |
|---|---|
| Engine displacement | 3922 cm3 |
| Bore | 102 mm |
| Stroke | 120 mm |
| Compression ratio | 17.3 |
| Rated effective power | 125 kW |
| Fuel | ∆m [mg] | ∆m [%] | ∆V [mm3] | ∆V [%] |
|---|---|---|---|---|
| B7 | 0.3 | 0.48 | −0.88 | −2.75 |
| OME3-5 | 36 | 56.69 | 33.76 | 106.38 |
| n-decanol | 0.5 | 0.78 | −0.64 | −2.01 |
| D10 | 0.7 | 1.11 | −0.54 | −1.68 |
| D30-O10 | 5.2 | 8.10 | 4.83 | 14.75 |
| D50-O20 | 9.3 | 15.20 | 8.42 | 27.41 |
| D70-O30 | 14.1 | 22.00 | 13.42 | 41.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virt, M.; Zöldy, M. Enhancing the Viability of a Promising E-Fuel: Oxymethylene Ether–Decanol Mixtures. Energies 2024, 17, 1348. https://doi.org/10.3390/en17061348
Virt M, Zöldy M. Enhancing the Viability of a Promising E-Fuel: Oxymethylene Ether–Decanol Mixtures. Energies. 2024; 17(6):1348. https://doi.org/10.3390/en17061348
Chicago/Turabian StyleVirt, Márton, and Máté Zöldy. 2024. "Enhancing the Viability of a Promising E-Fuel: Oxymethylene Ether–Decanol Mixtures" Energies 17, no. 6: 1348. https://doi.org/10.3390/en17061348
APA StyleVirt, M., & Zöldy, M. (2024). Enhancing the Viability of a Promising E-Fuel: Oxymethylene Ether–Decanol Mixtures. Energies, 17(6), 1348. https://doi.org/10.3390/en17061348







