The Advantage of Citrus Residues as Feedstock for Biogas Production: A Two-Stage Anaerobic Digestion System
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Citrus Waste
2.2. Characterization of Citrus Residues
2.3. Chemical Analysis
2.4. Hydrolysis Reactor (Packed Bed Reactor)
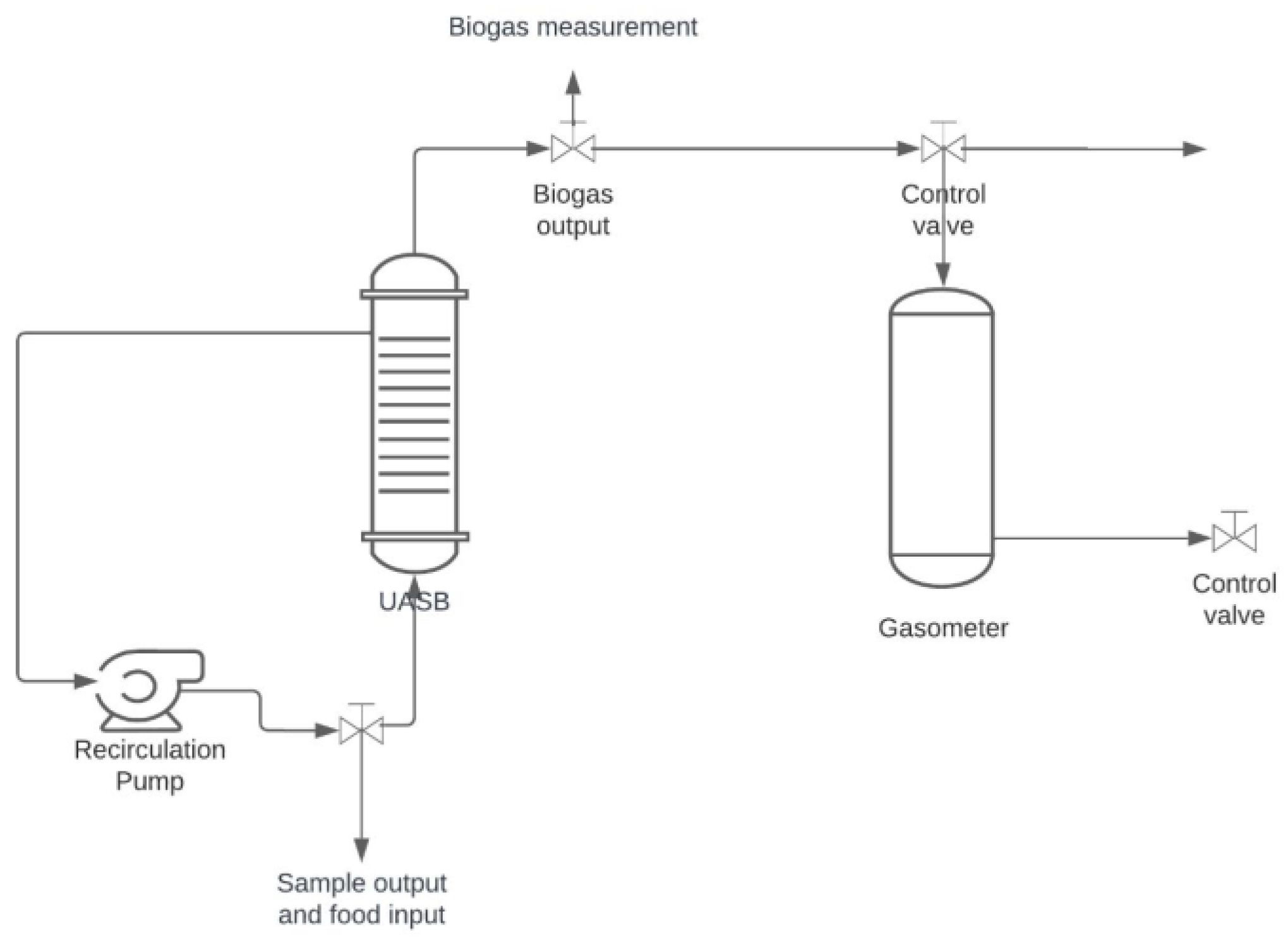
2.5. Methanogenic Reactor (UASB)
3. Results and Discussion
3.1. Characterization of Citrus Residues
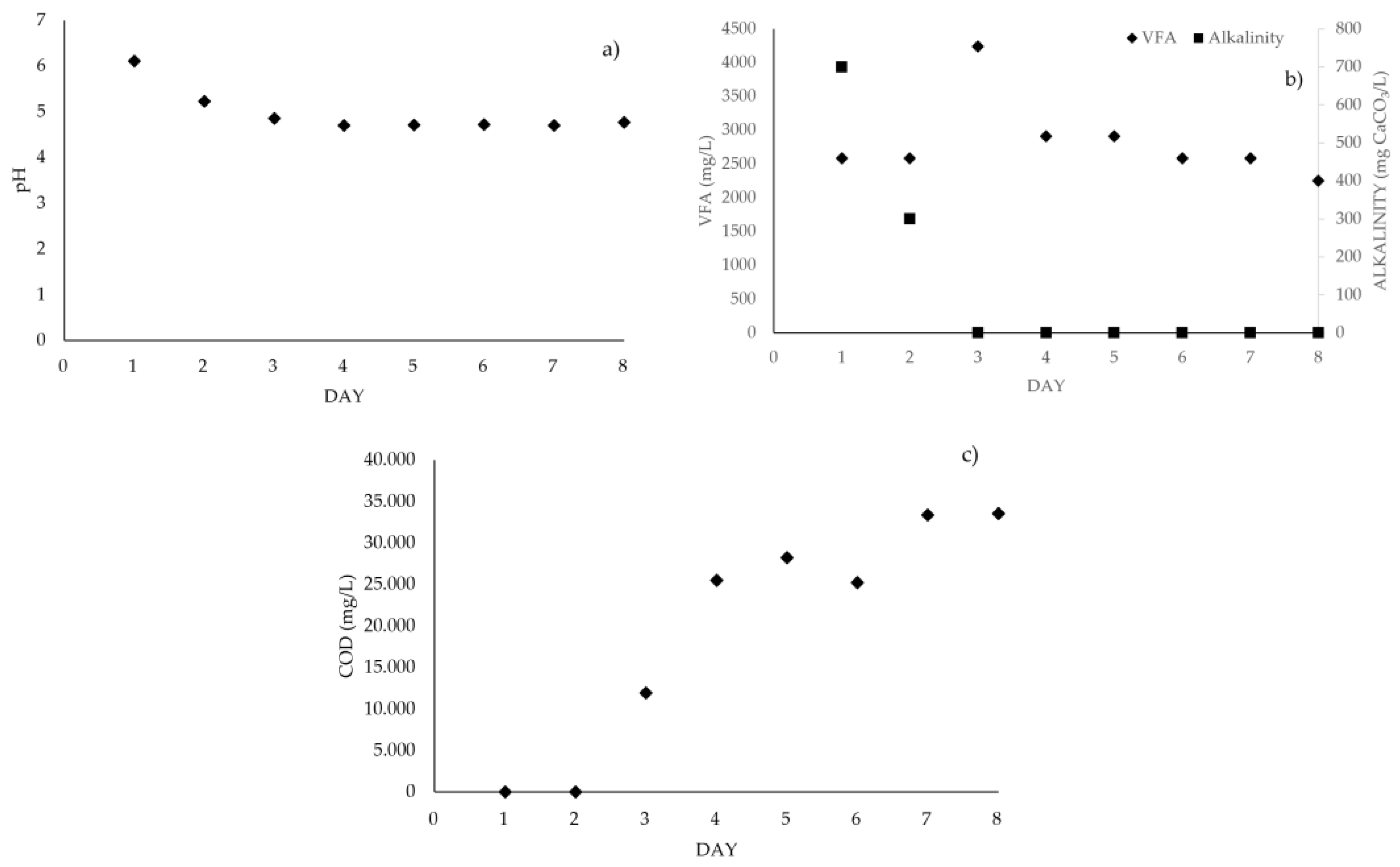
3.2. Stabilization of Methanogenic Microorganisms (UASB Reactor)
3.3. Stabilization of Hydrolytic and Acetogenic Reactor (Packed Bed Reactor)
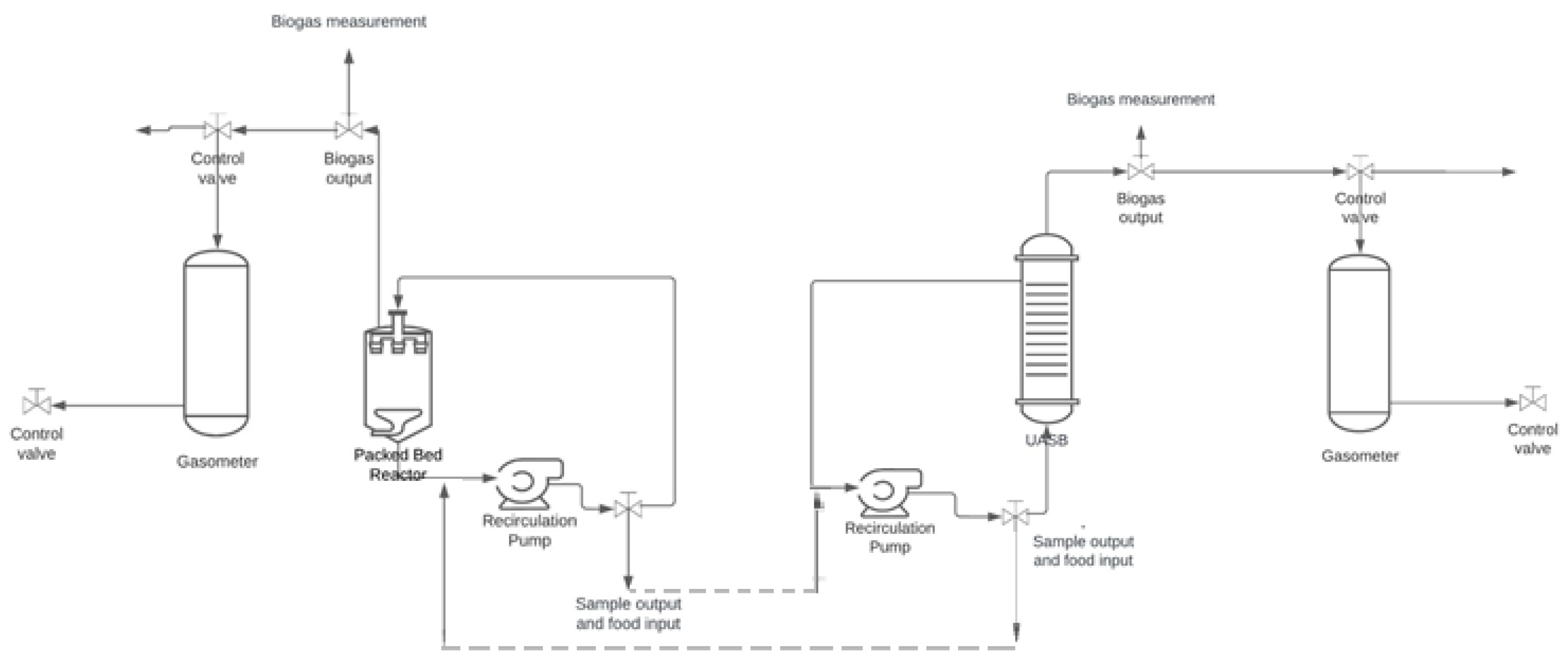
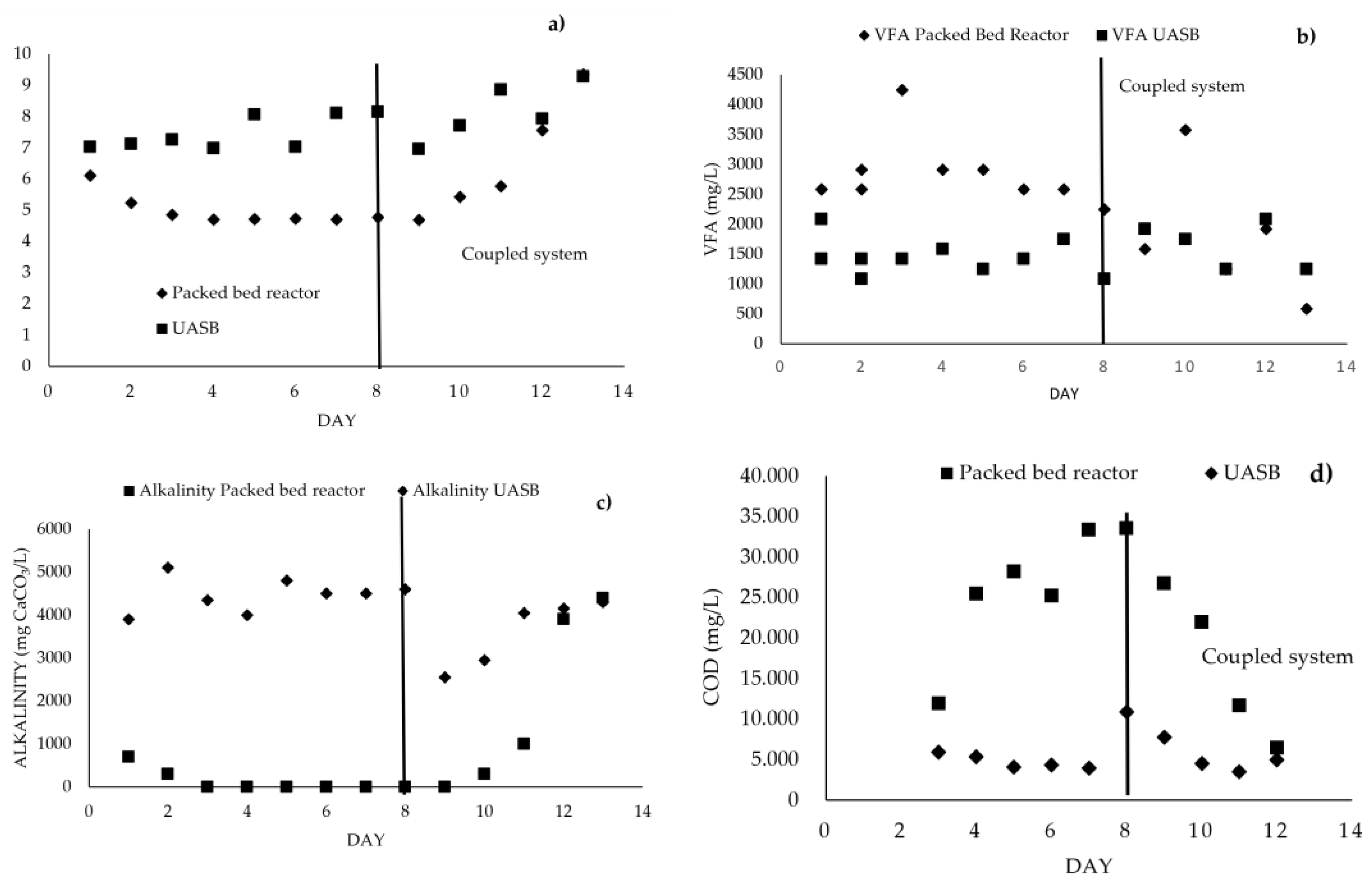
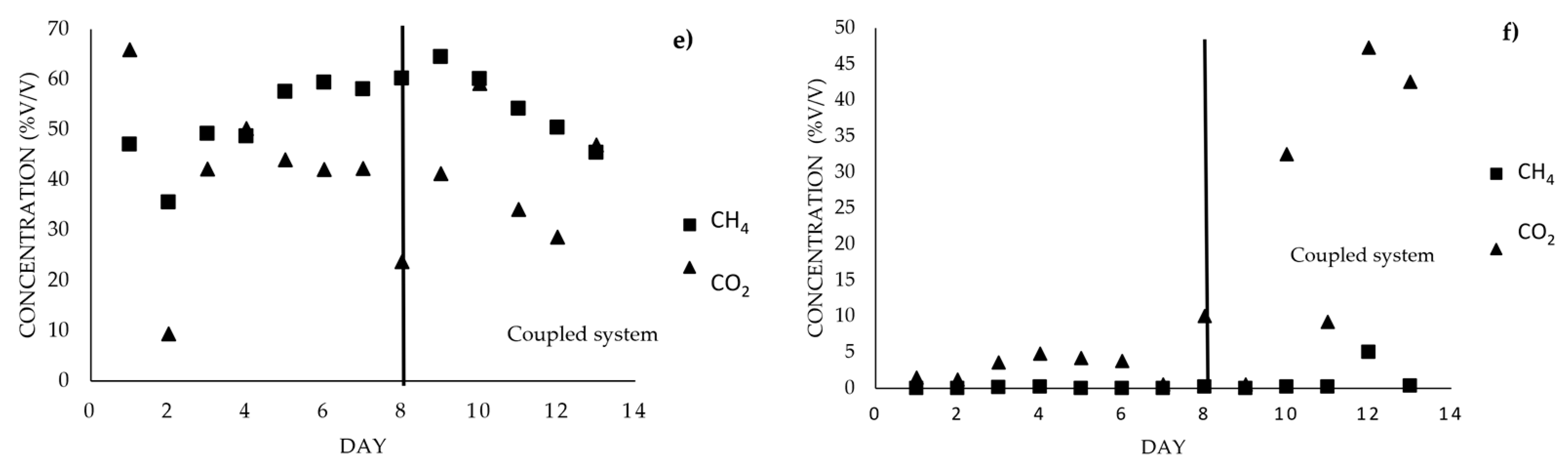
3.4. Coupled System
| Sample | Reference | |
|---|---|---|
| Citrus waste | 0.51 | This study |
| Onion residues | 0.43 | [26] |
| Coffee by product | 0.48 | [47] |
| Dairy cattle waste | 0.22 | [48] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bogota_Environmental_Observatory. Zero Waste Program (Programa Basura Cero). 2013. Available online: https://www.uaesp.gov.co/uaesp_jo/images/BasuraCero/DocumentoBasuraCero.pdf (accessed on 12 November 2023).
- Ministry_of_Agriculture_and_Rural_Development. Citrus Chain, Indicators and Instruments Second Quarter 2021 (Cadena de Citricos, Indicadores e Instrumentos Segundo Trimestre 2021). 2021. Available online: https://sioc.minagricultura.gov.co/Citricos/Documentos/2021-03-31%20Cifras%20Sectoriales.pdf (accessed on 20 October 2023).
- Guerrero-Martin, C.A.; Fernández-Ramírez, J.S.; Arturo-Calvache, J.E.; Milquez-Sanabria, H.A.; Fernandes, F.A.d.S.; Gomes, V.J.C.; e Silva, W.L.; Duarte, E.D.V.; Guerrero-Martin, L.E.; Lucas, E.F. Exergy Load Distribution Analysis Applied to the Dehydration of Ethanol by Extractive Distillation. Energies 2023, 16, 3502. [Google Scholar] [CrossRef]
- Cano, N.A.; Céspedes-Zuluaga, S.; Guerrero-Martin, C.; Gallego, D. Exergy and emergy: Complementary tools for assessing the environmental sustainability use of biosolids generated in wastewater-treatment plant for energy-production. Química Nova 2022, 45, 4–15. [Google Scholar] [CrossRef]
- Ibañez-Gómez, L.F.; Albarracín-Quintero, S.; Céspedes-Zuluaga, S.; Montes-Páez, E.; Junior, O.H.A.; Carmo, J.P.; Ribeiro, J.E.; Moreira, M.M.A.; Siqueira, A.A.G.; Guerrero-Martin, C.A. Process Optimization of the Flaring Gas for Field Applications. Energies 2022, 15, 7655. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Carhuancho, F.; Ramirez, J.; Guerrero, J.J.G. View of Environmental Management of Poultry Waste through Anaerobic Digestion for the Production of Liquid Organic Fertilizers; Universidad Nacional Agraria La Molina: Lima, Peru, 2015. [Google Scholar]
- Zhang, X.; Chen, J.; Li, J. The removal of microplastics in the wastewater treatment process and their potential impact on anaerobic digestion due to pollutants association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal, B.H.; Vanegas, E.; Mariscal, J.P.; Camargo, M.A. Digestión anaerobia de residuos de poda como alternativa para disminuir emisiones de gases de efecto invernadero en rellenos sanitarios. Energética 2015, 46, 29–36. [Google Scholar]
- González-Sánchez, M.E.; Pérez-Fabiel, S.; Wong-Villarreal, A.; Bello-Mendoza, R.; Yañez-Ocampo, G. Residuos agroindustriales con potencial para la producción de metano mediante la digestión anaerobia. Rev. Argent. Microbiol. 2015, 47, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Rustum, R.; Adeloye, A.J. Review of anaerobic digestion modeling and optimization using na-ture-inspired techniques. Processes 2019, 7, 953. [Google Scholar] [CrossRef]
- Adekunle, K.F.; Okolie, J.A. A Review of Biochemical Process of Anaerobic Digestion. Adv. Biosci. Biotechnol. 2015, 6, 205–212. [Google Scholar] [CrossRef]
- Parra Huertas, R.A. Anaerobic digestión: Biotechnological mechanisms in waste water treatments and their application in food industry. Prod. + Limpia 2015, 10, 142–159. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Luo, Z.; Zeng, W.; Huang, H. The role of reflux time in a leach bed reactor coupled with a meth-anogenic reactor for anaerobic digestion of pig manure: Reactor performance and microbial community. J. Clean. Prod. 2020, 242, 118367. [Google Scholar] [CrossRef]
- Sridevi, V.D.; Rema, T.; Srinivasan, S.V. Studies on biogas production from vegetable market wastes in a two-phase anaerobic reactor. Clean Technol. Environ. Policy 2015, 17, 1689–1697. [Google Scholar] [CrossRef]
- Chakraborty, D.; Karthikeyan, O.P.; Selvam, A.; Palani, S.G.; Ghangrekar, M.M.; Wong, J.W. Two-phase anaerobic digestion of food waste: Effect of semi-continuous feeding on acidogenesis and methane production. Bioresour. Technol. 2022, 346, 126396. [Google Scholar] [CrossRef] [PubMed]
- García-Elías, O.; Marín-Peña, O.; Alvarado-Lassman, A.; Vallejo-Cantú, N.A.; Rosas-Mendoza, E.S. Efecto de la hidrólisis en la digestión anaerobia en dos etapas de los residuos de frutas y verduras. Rinderesu 2021, 5, 463–471. [Google Scholar]
- ASTM D2216-19; Standard test methods for laboratory determination of water (moisture) content of soil and rock by mass. ASTM International: West Conshohocken, PA, USA, 2019.
- EPA. Method 160.4: Residue, Volatile (Gravimetric, Ignition at 550 °C) by Muffle Furnace. 2017. Available online: https://19january2017snapshot.epa.gov/sites/production/files/2015-08/documents/method_160-4_1971.pdf (accessed on 10 October 2023).
- EPA. Methods for the Chemical Analysis of Water and Wastes (MCAWW) (EPA/600/4-79/020). 1997. Available online: https://www.nemi.gov/methods/method_summary/5213/ (accessed on 2 October 2023).
- EPA. Method 351.2, Revision 2.0: Determination of Total Kjeldahl Nitrogen by Semi-Automated Colorimetry. 1993. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_351-2_1993.pdf (accessed on 2 October 2023).
- Purser, B.J.; Thai, S.-M.; Fritz, T.; Esteves, S.; Dinsdale, R.; Guwy, A. An improved titration model reducing over estimation of total volatile fatty acids in anaerobic digestion of energy crop, animal slurry and food waste. Water Res. 2014, 61, 162–170. [Google Scholar] [CrossRef]
- Alzate-Gaviria, L.M.; Pérez-Hernández, A.; Nevárez-Moorillón, V.G.; Rinderknecht-Seijas, N.; Poggi-Varaldo, H.M. Comparison of two anaerobic coupled systems for biomethanization of the organic fraction of municipal solid wastes. Interciencia 2003, 28, 436–490. [Google Scholar]
- Lenntech Water Treatment & Purification, Lenntech. Water Treatment Solutions. 2023. Available online: https://www.lenntech.es/productos/IWAKI/MD-10/MD-10-Magnetic-Drive-Pump/index.html (accessed on 15 October 2023).
- Zuo, Z.; Wu, S.; Zhang, W.; Dong, R. Performance of two-stage vegetable waste anaerobic digestion depending on varying recirculation rates. Bioresour. Technol. 2014, 162, 266–272. [Google Scholar] [CrossRef]
- Milquez-Sanabria, H.; Blanco-Cocom, L.; Alzate-Gaviria, L. A fast linear predictive adaptive model of packed bed coupled with UASB reactor treating onion waste to produce biofuel. Microb. Cell Factories 2016, 15, 167. [Google Scholar] [CrossRef][Green Version]
- Pererva, Y.; Miller, C.D.; Sims, R.C. Approaches in Design of Laboratory-Scale UASB Reactors. Processes 2020, 8, 734. [Google Scholar] [CrossRef]
- Cerón-Salazar, I.; Cardona-Alzate, C. Integral evaluation process for obtaining pectin and essential oil from orange peel. Ing. Cienc. 2011, 7, 65–86. [Google Scholar]
- Alvarado-Dávila, T.L.; Hernández-Sierra, A.T. Review of sustainable alternatives for the use of orange pomace (Re-visión de alternativas sostenibles para el aprovechamiento del orujo de naranja). Rev. Colomb. Investig. Agroindustriales 2018, 5, 9–32. [Google Scholar] [CrossRef]
- Lalremruati, M.; Devi, A.S. Changes in physico-chemical properties during composting of three common household organic solid wastes amended with garden soil. Bioresour. Technol. Rep. 2021, 15, 100727. [Google Scholar] [CrossRef]
- De Luna-Vega, A.; Garcia-Sahagun, M.L.; Rodriguez-Guzman, E.; Pimienta-Barrios, E. Agronomic quality of compost with citrus waste (Calidad agronómica de composta con residuos de cítricos). ECORFAN® 2015, 2, 354–361. [Google Scholar]
- Debernardi-Vázquez, T.D.J.; Aguilar-Rivera, N.; Núñez-Pastrana, R. Composting of byproducts from the orange (Citrus sinensis (L.) Osbeck) and sugarcane (Saccharum spp. hybrids) agroindustries. Ing. E Investig. 2020, 40, 8. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Khan, A.A.; Lew, B.; Diamantis, V.; Kazmi, A.A. Performance of Full-Scale UASB Reactors Treating Low or Medium Strength Mu-nicipal Wastewater. Environ. Process. 2017, 4, 137–146. [Google Scholar] [CrossRef]
- Papurello, D.; Silvestri, S.; Lanzini, A. Biogas cleaning: Trace compounds removal with model validation. Sep. Purif. Technol. 2019, 210, 80–92. [Google Scholar] [CrossRef]
- Bacab, F.C.; Gamboa, E.E.; Espinoza, J.E.R.; Leal-Bautista, R.M.; Tussell, R.T.; Maldonado, J.D.; Canché, B.C.; Alzate-Gaviria, L. Two Phase Anaerobic Digestion System of Municipal Solid Waste by Utilizing Microaeration and Granular Activated Carbon. Energies 2020, 13, 933. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Wang, X.; Zhou, X.; Zhu, J. Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour. Technol. 2020, 316, 123851. [Google Scholar] [CrossRef]
- Mohan, S.M.; Swathi, T. Enhanced Degradation of the Substrate Using Modified Upflow Anaerobic Sludge Blanket Reactor–Static Granular Bed Reactor Series with Varying Hydraulic Retention Time at Lab Scale. J. Environ. Eng. 2023, 149, 04022088. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Romano, R.T.; Zhang, R. Co-digestion of onion juice and wastewater sludge using an anaerobic mixed biofilm reactor. Bioresour. Technol. 2008, 99, 631–637. [Google Scholar] [CrossRef]
- Dillon-Viveros, A. Anaerobic Digestion in Bacterial Beds for Nutrient Removal and Methane Gas Generation from Residual Water from a Crude Palm Oil Extractor [USFQ]. Digital Repository. 2016. Available online: http://repositorio.usfq.edu.ec/handle/23000/5703 (accessed on 10 October 2023).
- Eryildiz, B.; Lukitawesa; Taherzadeh, M.J. Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour. Technol. 2020, 302, 122800. [Google Scholar] [CrossRef]
- Cazier, E.A.; Trably, E.; Steyer, J.-P.; Escudie, R. Reversibility of hydrolysis inhibition at high hydrogen partial pressure in dry anaerobic digestion processes fed with wheat straw and inoculated with anaerobic granular sludge. Waste Manag. 2019, 85, 498–505. [Google Scholar] [CrossRef] [PubMed]
- González Paz, J.R.; Vallejo Cantú, N.A.; Alvarado Lassman, A. Tratamiento Anaerobio del Efluente Proveniente del Proceso de una Industria Citrícola en un Reactor Híbrido. Ph.D. Thesis, TecNM Campus Orizaba, Orizaba, Mexico, 2015. [Google Scholar]
- Aziz, T.; Tajuddin, R.M.; Kamarun, D.; Kordi, N.E.; Malini, R. Citrus fruit waste leachate treatment by using newly developed flat sheet membrane. In Proceedings of the Advances in Civil Engineering and Science Technology, Penang, Malaysia, 5–6 September 2018. [Google Scholar]
- Bi, S.; Qiao, W.; Xiong, L.; Ricci, M.; Adani, F.; Dong, R. Effects of organic loading rate on anaerobic digestion of chicken manure under mesophilic and thermophilic conditions. Renew. Energy 2019, 139, 242–250. [Google Scholar] [CrossRef]
- Sani, K.; Kongjan, P.; Pakhathirathien, C.; Cheirsilp, B.; O-Thong, S.; Raketh, M.; Kana, R.; Jariyaboon, R. Effectiveness of using two-stage anaerobic digestion to recover bio-energy from high strength palm oil mill effluents with simultaneous treatment. J. Water Process. Eng. 2021, 39, 101661. [Google Scholar] [CrossRef]
- Acarley, F.; Quipuzco, L. Producción de metano mediante digestión anaerobia de aguamiel, subproducto del beneficio húmedo del café. Agroindustrial Sci. 2020, 10, 7–16. [Google Scholar] [CrossRef]
- Sung, S.; Santha, H. Performance of temperature-phased anaerobic digestion (TPAD) system treating dairy cattle wastes. Water Res. 2003, 37, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Lotito, A.M.; De Sanctis, M.; Pastore, C.; Di Iaconi, C. Biomethanization of citrus waste: Effect of waste characteristics and of storage on treatability and evaluation of limonene degradation. J. Environ. Manag. 2018, 215, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Ariza Calvo, D.; Rincón Ravelo, M.; Paz Cadavid, C.A.; Gutiérrez-Montero, D.J. Evaluación de producción de biogás y reducción de carga orgánica de vinazas mediante digestión anaerobia. Rev. Colomb. Biotecnol. 2019, 21, 118–130. [Google Scholar] [CrossRef]
- Córdoba, V.; Fernández, M.; Santalla, E. The effect of different inoculums on anaerobic digestion of swine wastewater. J. Environ. Chem. Eng. 2016, 4, 115–122. [Google Scholar] [CrossRef]
- Smith, L.G.; Jones, P.J.; Kirk, G.J.; Pearce, B.D.; Williams, A.G. Modelling the production impacts of a widespread conversion to organic agriculture in England and Wales. Land Use Policy 2018, 76, 391–404. [Google Scholar] [CrossRef]


| Sample | TS | VS | %H | %Cz | %SV | Reference |
|---|---|---|---|---|---|---|
| Orange | 5.02 ± 0.04 | 4.99 ± 0.03 | 81.51 ± 0.75 | 0.59 ± 0.01 | 17.89 ± 0.77 | This studio |
| Lemon | 4.99 ± 0.05 | 4.96 ± 0.05 | 85.15 ± 1.06 | 0.62 ± 0.04 | 14.22 ± 1.07 | |
| Citrus waste | 5.01 ± 0.05 | 4.98 ± 0.04 | 83.33 ± 0.91 | 0.60 ± 0.03 | 16.05 ± 0.92 | |
| Citrus waste | 7.10 ± 1.20 | 6.87 ± 1.20 | 85.90 ± 1.60 | 3.29 ± 0.19 | 10.86 ± 0.19 | [28] |
| Orange waste | 20.17 ± 0.08 | 19.31 ± 0.11 | 79.83 ± 0.08 | 4.27 ± 0.11 | 15.90 ± 0.11 | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Martin, C.A.; Rojas-Sanchez, A.N.; Cruz-Pinzón, D.F.; Milquez-Sanabria, H.A.; Sotelo-Tobon, D.L.; da Cunha, A.L.R.; Salinas-Silva, R.; Camacho-Galindo, S.; Costa Gomes, V.J.; Cunha Malagueta, D. The Advantage of Citrus Residues as Feedstock for Biogas Production: A Two-Stage Anaerobic Digestion System. Energies 2024, 17, 1315. https://doi.org/10.3390/en17061315
Guerrero-Martin CA, Rojas-Sanchez AN, Cruz-Pinzón DF, Milquez-Sanabria HA, Sotelo-Tobon DL, da Cunha ALR, Salinas-Silva R, Camacho-Galindo S, Costa Gomes VJ, Cunha Malagueta D. The Advantage of Citrus Residues as Feedstock for Biogas Production: A Two-Stage Anaerobic Digestion System. Energies. 2024; 17(6):1315. https://doi.org/10.3390/en17061315
Chicago/Turabian StyleGuerrero-Martin, Camilo Andrés, Angie Natalia Rojas-Sanchez, David Fernando Cruz-Pinzón, Harvey Andres Milquez-Sanabria, David Leonardo Sotelo-Tobon, Ana Laura Ribeiro da Cunha, Raúl Salinas-Silva, Stefanny Camacho-Galindo, Vando José Costa Gomes, and Diego Cunha Malagueta. 2024. "The Advantage of Citrus Residues as Feedstock for Biogas Production: A Two-Stage Anaerobic Digestion System" Energies 17, no. 6: 1315. https://doi.org/10.3390/en17061315
APA StyleGuerrero-Martin, C. A., Rojas-Sanchez, A. N., Cruz-Pinzón, D. F., Milquez-Sanabria, H. A., Sotelo-Tobon, D. L., da Cunha, A. L. R., Salinas-Silva, R., Camacho-Galindo, S., Costa Gomes, V. J., & Cunha Malagueta, D. (2024). The Advantage of Citrus Residues as Feedstock for Biogas Production: A Two-Stage Anaerobic Digestion System. Energies, 17(6), 1315. https://doi.org/10.3390/en17061315







