Abstract
This study explores the possibility of utilising electrochemically assisted anaerobic digestion supplemented with carbon-based materials to stimulate methanogenesis. Two different carbonaceous materials—commercial activated carbon (AC), and pyrolysed argan (PA, derived from argan shells)—were employed as supplements, with cheese whey (CW) being used as the substrate. Methane production slightly increased in the electrochemically assisted digesters, potentially translating into a 2–4% increase in the output of industrial digesters. In addition, reactors supplemented with PA also exhibited better production rates (496–508 L·kgVS−1), although there was no observed improvement in the quantity of biogas at the end of the biodegradability experiment. In contrast, when commercial AC was used as the supplement, the start-up phase was accelerated (5 days), although methane productivity decreased (273–352 L·kgVS−1). These observations were supported by microbiological analyses, demonstrating that the reactors with the poorest performance (those supplemented with AC) experienced the most significant decrease in both archaeal and bacterial populations.
1. Introduction
The dairy industry is a major segment of the European agricultural market [1]. Cheese whey (CW), which is a by-product resulting from the transformation of milk into cheese, can cause serious environmental problems if not adequately handled [2]. Still, CW can also represent a huge opportunity for bioenergy and biochemicals production [3]. In this regard, anaerobic digestion (AD), a proven technology typically used for the management of different agro-wastes [4,5], can provide a suitable alternative for CW valorisation [6,7,8,9]. However, this waste typically has a low alkalinity and contains a high concentration of organics, all of which makes CW a challenging waste product for AD, usually causing acidification and inhibition of the methanogenic activity [10,11].
In recent years, several studies have reported on the benefits of integrating microbial electrochemical technologies, such as microbial electrolysis cells (MEC), with anaerobic digestion to alleviate some of its limitations [12,13,14]. MEC are a type of bio-electrochemical system that can degrade organic matter and, depending on the reactor configuration, generate hydrogen (H2) and/or methane (CH4) (with a small input of electrical energy) [14,15]. The main benefit of integrating MEC within AD is that this may help to balance the composition of the microbial community and, consequently, the electron transfer pathways, something that can enhance methane productivity, kinetics and process stability [12,16]. Moreover, some authors have reported that the addition of carbon-based materials (e.g., biochar, carbon nanotubes, granular activated carbon, among others) to the MEC-AD system stimulates direct interspecies electron transfer [17,18,19], which can result in greater methane productivity and improved process stability [20]. For example, Yin et al. [18] found that dosing 1.0 g/g dry matter sludge-based biochar to an MEC-AD reactor helped to increase methane production (24.7% improvement) and the removal of volatile solids (17.9% improvement). Huang et al. [21] reported that the use of granular activated carbon in an MEC-AD enhanced the kinetics of the hydrolysis step and increased by up to 38% the CH4 yield.
Nevertheless, other authors have reported contrary observations. For instance, Jiang et al. [22] showed that although the addition of granular activated carbon accelerated the start-up of a methanogenic reactor, it also reduced CH4 production yields. Moreover, Fujinawa et al. [23] found that Vulcan black carbon (2% w/v) could even inhibit the methanogenic process. Therefore, there seems to be controversy over whether the use of carbon-based materials as supplements can stimulate methanogenesis in AD and in MEC-AD systems. Consequently, and considering these contrasting results, we aim in this paper at clarifying whether the addition of two different carbon-based materials (activated carbon and pyrolysed argan) to an MEC-AD system provides any benefit to the energy valorisation of CW. We pay special attention to the kinetics of methane production and to the structure of the microbial communities that develop on the bulk of the MEC-AD.
2. Materials and Methods
2.1. Inoculum, Substrate and Additives
Anaerobic sludge obtained from a local wastewater treatment plant (WWTP) was used as inoculum. The cheese whey (CW) used as substrate was collected from Industrias Lácteas San Vicente (León, Spain). The detailed characterisations of both inoculum and substrate are given in Table 1. The CW contained a high concentration of organics, evidenced by the total organic carbon (TOC) and the chemical oxygen demand (COD), that were around 21.5 ± 0.6 g·L−1 and 85.5 ± 4.9 g·L−1, respectively. TOC was measured using an analyser (Multi N/C 3100, Analytikjena, Jena, Germany). COD was analysed with Hach Lange tubes LCK 514 (100–2000 mg L−1) (HACH LANGE GmbH, Düsseldorf, Germany) by a high-performance spectrophotometer DR 3900 (Hach Lange, Barcelona, Spain).

Table 1.
Characterisation of inoculum and substrate.
Two different types of additives were used: activated carbon (AC) and pyrolysed argan shell (PA). AC was obtained from EMD Millipore Corporation (Darmstadt, Germany) with a mean diameter with respect to particle number of 2.2 µm, and a microporous surface area of 530.2 m2·g−1. The PA consisted of argan shells ground in a ball mill to a size between 2 and 8 mm and subsequently pyrolysed. The crushed sample was washed with distilled water, dried at 60 °C for 24 h and pyrolysed at 575 °C using helium as a carrier gas at a flow rate of 200 mL·min−1. During the pyrolysis phase, the sample mass used was 100 g, the temperature was increased to 250 °C at a rate of 10 °C·min−1, held for 10 min and increased again to 575 °C, where it was held for 90 min. The final PA sample was 33.5 g and had a mean diameter with respect to particle number of 3.4 µm and a microporous surface area of 249.2 m2·g−1. The mean diameter with respect to particle number graphs for AC and PA are shown in the Supplementary Material Figure S1.
2.2. Reactors Setup
Experiments were carried out in thirteen single-chamber reactors (modified Duran® bottles, Merck, Darmstadt, Germany) with a working volume of 0.4 L each (Figure 1), equipped with a gas bag (Ritter, Bochum, Germany) to collect the gas produced. Six of them also contained an electrolytic module (anode + cathode) and an Ag/AgCl as a reference electrode (Sigma-Aldrich, St. Louis, MO, USA). Each electrolytic module consisted of four graphite rods (diameter: 1 cm; length: 5 cm) in cross-shaped configuration as indicated in Figure 1a. Two sticks were connected as anodes while the other two operated as cathodes. This arrangement is based on the configuration proposed by Alonso et al. [24]. The electrodes were connected to a potentiostat (applied voltage: 1 V) using titanium wires covered with heat-shrink tubing (as insulator), as shown in Figure 1a. The remaining 7 reactors were operated as conventional anaerobic digesters (Figure 1b).
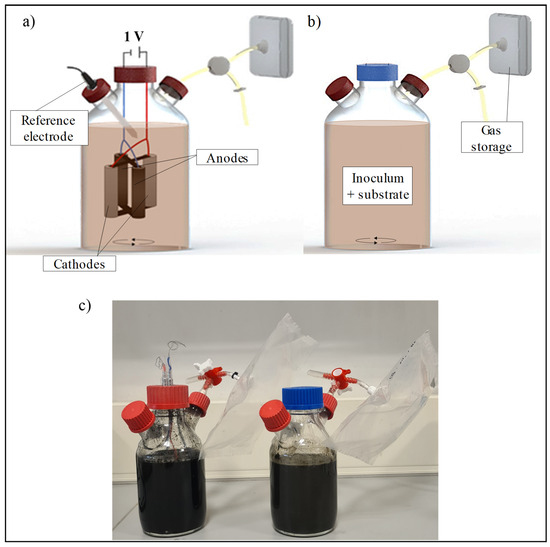
Figure 1.
Schematic representation of the laboratory setup of (a) MEC-AD and (b) AD reactors, and (c) the actual lab reactors (left: MEC-AD, right: AD).
All the reactors were filled with a mixture inoculum and substrate (ratio of 1:1 in terms of volatile solids), resulting in a total volume of 400 mL (215 mL inoculum + 186 mL substrate). They were operated in batch mode for 40 days at a constant temperature of 30 ± 2 °C and a stirring speed of 300 rpm (magnetic stirrer plate IKA-WERKE RO 15, Germany), to allow mixing and to facilitate mass transfer inside the chambers. In addition, and to ensure a neutral pH, 5 g·L−1 of bicarbonate was added to each reactor (≥99%, GPR RECTAPUR®, VWR Chemicals, Leuven, Belgium), and they were bubbled with nitrogen for 15 min to ensure anaerobic conditions.
The thirteen reactors were divided into four different experimental conditions that are summarised in Table 2 (the tests were carried out in duplicate): (i) Four reactors were dosed with 8 g·L−1 of AC (value within literature range [19,22,25,26]), two of which integrated an electrolytic module (named as MECAC) and the other two were operated as conventional AD (named as ADAC), (ii) Four reactors were dosed with 8 g·L−1 of PA, two of which integrated an electrolytic module (named as MECPA) and the other two were operated as conventional AD (named as ADPA), (iii) Four reactors lacking any additives, two of which integrated an electrolytic module (named as MECNA) and the other two were operated as conventional AD (named as ADNA), (iv) A reactor used as a blank (named as Blank) that contained only a mixture of inoculum and distilled water (215 mL inoculum + 186 mL water) to determine the methane production potential of the inoculum itself. At the end of the experiments, the CH4 production in the Blank was subtracted from the production of the other reactors in order to determine the productivity that could be specifically attributed to the substrate.

Table 2.
Summary of the operating conditions used during the experimental phase.
2.3. Calculations
The maximum theoretical volume of methane that can be attributed to charge circulation in the reactors that contain an MEC module (VEC), expressed in LCH4·kgVSˉ¹added, was calculated as:
where is the molar volume (24.88 L·mol−1) at 30 °C and 1 atm, I is the electrical current (A) circulating through the MEC, to is the initial time (s) and tf is the final time (s). F is the Faraday constant (96,485 C·mol−1), n (8) is the number of electrons involved in the production of 1 mol of methane, and VS are the volatile solids added at the beginning of the experiment (3.7 g VSadded).
2.4. Analytical Techniques and Electrochemical Characterisation
Gas composition was measured every two/three days using a gas chromatograph (GC) (Agilent 990 micro-GC) equipped with a thermal conductivity detector. Column 1 (Molesieve 5A, 20m + 3m CP-PoraBOND Q) followed by column 2 (CP-PoraPLOT U, 10m + 1m CP-PoraBOND Q) were used to determine the gas composition in terms of hydrogen (H2), carbon dioxide (CO2), oxygen (O2), nitrogen (N2) and methane (CH4). Column 1 was operated with an initial pressure of 250 kPa and a temperature of 100 °C in the column and injector, and column 2 with an initial pressure of 130 kPa and 90 °C in the column and injector. The carrier gas used in both columns was helium. The sample inlet temperature was 110 °C in continuous flow with a sample time of 1 s.
Conductivity (Hach, HQ40d—two-channel digit multimeter), redox (pH-Meter, pH 91; Wissenschaftlich Technische Werkstätten, WTW, Weilheim, Germany) and pH (pH-Meter BASIC 20+, Crison) measurements were carried out according to standard methodologies and were measured at the beginning and at the end of the experiment.
A Biologic VSP potentiostat (Seyssinet-Pariset, France) equipped with an EC-Lab software v.11.36 was used to run simultaneous multi-technique electrochemical test in the reactors that contained an MEC system. During normal operation, the reactors were operated in a two-electrode configuration with an applied voltage of 1 V (vs. Ag/AgCl—3 M KCl) and the current production was monitored every 600 s. Cyclic voltammetry tests were carried out at the beginning and end of the experiment in a three-electrode configuration using an Ag/AgCl (3M) electrode as a reference (see Figure 1a) with a potential window between 0.3 V and −0.6 V at a scanning potential rate of 5 mV·s−1.
2.5. DNA Extraction and Microbial Community Characterisation
Approximately 50 mL of sample was extracted from the bulk and centrifuged to extract about 300 mg of sediment from all reactors plus the initial inoculum. These samples were used to characterise the microbial communities that had developed in each reactor at the end of the experiment. Microbial communities were analysed and followed along the experimental time by high-throughput sequencing of massive 16S rRNA gene libraries. Total bacteria and archaea were analysed. Genomic DNA was extracted with a DNeasy PowerSoil kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. All polymerase chain reactions (PCR) were carried out in a Mastercycler (Eppendorf, Hamburg, Germany), and PCR samples were checked for size of the product on a 1% agarose gel and quantified by NanoDrop 1000 (Thermo Scientific, Waltham, MA, USA). The entire DNA extract was used for high-throughput sequencing of 16S rRNA gene-based massive libraries with 16S rRNA gene-based primers for Bacteria 515F-806R and for Archaea 349F-806R. The Novogene Company (Cambridge, UK) carried out Illumina sequencing using an HiSeq 2500 PE250 platform.
The obtained DNA reads were compiled in FASTq files for further bioinformatics processing carried out using QIIME software version 1.7.0. [27] Sequence analyses were performed by Uparse software (version 7.0.1001) using all the effective tags. Sequences with ≥97% similarity were assigned to the same operative taxonomic units (OTUs). Representative sequences for each OTU were screened for further annotation. For each representative sequence, Mothur software was performed against the SSUrRNA database of the SILVA Database [28] for species annotation at each taxonomic rank (Threshold: 0.8–1).
The quantitative analysis of all samples was analysed by means of quantitative-PCR reaction (qPCR) using PowerUp SYBR Green Master Mix (Applied Biosystems, Waltham, MA, USA) in a StepOnePlus Real-Time PCR System (Applied Biosystems). The qPCR amplification was performed for the 16S rRNA gene in order to quantify the entire eubacteria community and for the mcrA gene to quantify the total methanogen community. The primer set 314F qPCR (5′-CCTACGGGAGGCAGCAG-3) and 518R qPCR (5′-ATTACCGCGGCTGCTGG-3′) at an annealing temperature of 60 °C for 30 s was used for Bacteria and Archaea 349F (5′-GYGCASCAGKCGMGAAW-3′) and Arc 806R (5′-GGACTACVSGGGTATCTAAT-3′) for Archaea quantification.
3. Results and Discussion
3.1. Biomethanisation Potential
Although the reactors that were supplemented with activated carbon (MECAC and ADAC) started to produce methane 5 days earlier than the rest, their cumulative methane production at the end of the tests was notably lower. Similar results have been reported by other authors: in Jiang et al. [22], for instance, it was observed that granular activated carbon favoured the start-up of an AD process (probably because it provides enhanced direct interspecies electron transfer (DIET)), although it ultimately resulted in a diminishment of the overall CH4 production yields; and Fujinawa et al. [23] found that the use of conductive carbon nanoparticles (2% w/v) inhibited the methanogenic process. However, and despite the negative impact of activated carbon on methane production, it is important to note that it was only in the AC-supplemented reactor that the MEC seemed to provide any substantial contribution to the total methane production, allowing the methane yield to increase by approximately 22%. For all other circumstances, the presence of the MEC did not add any additional benefit in terms of biogas yield, which is coherent with the results reported by Park et al. [29], where the contribution of MEC to the total biogas production in an MEC-AD system was negligible. However, results in Figure 2 seem to indicate that the MEC in the non-supplemented reactors (MECNA) accelerated the kinetics of methane production (in comparison to ADNA) which, for continuous industrial systems (that operate with very tight retention times), can become a practical advantage by accelerating the process in one or two days (this could mean an increase of 2–4% in methane production in real-life continuous AD systems).
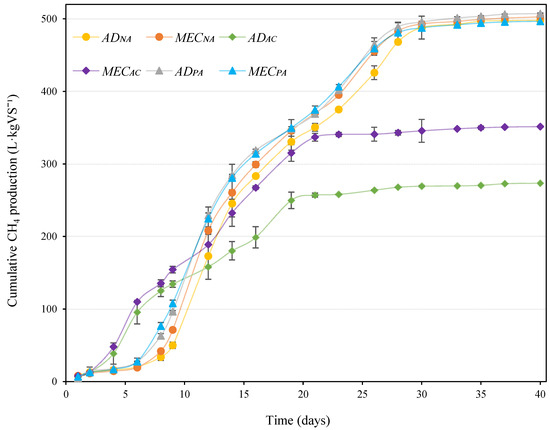
Figure 2.
Cumulative CH4 production for the different experimental conditions described in Table 2.
The methane yield measured for those reactors supplemented with pyrolysed argan (MECPA and ADPA) was similar to that recorded for the non-supplemented reactors. However, pyrolysed argan provided a slight advantage (again compared to the non-supplemented reactors) in terms of production rates, at least until day 25 of the experiment. This improvement in performance can probably be attributed to biomass retention in the pyrolysed argan.
The productivity of the inoculum itself (measured in the blank reactor according to the methods described in Section 2.2) was negligible (1.9 L·kgVS−1) compared to the productivity measured for the different experimental conditions (Figure 2).
Regarding the biogas quality, the results showed a minimal variation in the methane composition across the different reactors. At the end of the experiments, all reactors produced a biogas with an average methane concentration of 80% ± 2%, also containing carbon dioxide (~20%) and traces of hydrogen.
3.2. Current Production
Current profiles in Figure 3 can provide an indication of the activity of electroactive bacteria. The first thing we notice is that the non-supplemented reactor (MECNA) produced both the largest total charge (14,231 ± 33 °C) and the largest peak current density (7–8 A·m−2), clearly outperforming those reactors that incorporated any supplementation. Indeed, although MECAC was the first to show any electrical activity (3 days after inoculation), the cumulative charge production was only 29% of that measured in the MECNA. Interestingly, and as we have seen in the previous section, methane production also started earlier in the AC-supplemented reactors, so it seems that this additive is favouring a fast development of both methanogenic and electroactive bacteria, although in the long run it penalises current and methane productivity. The addition of PA also deteriorated charge production, but to a lesser extent: a 57% reduction compared to MECNA. Still, and in contrast to AC, PA slightly accelerated methane production, which suggests that PA has a differential impact on the methanogenic and electroactive bacteria.
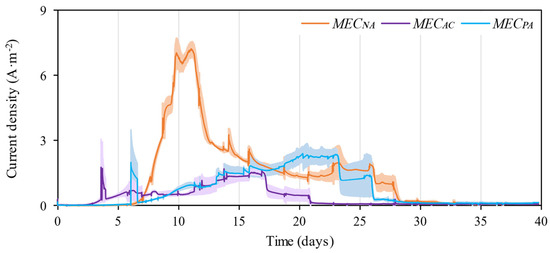
Figure 3.
Averaged current profiles measured in the reactors containing an MEC. The shaded areas mark the interval of variability across replicates.
We have seen in the previous section that when only using AC as supplement, the MEC brought additional benefits compared to the AD operated alone. However, the question remains of the actual contribution of the circulating coulombs to the total methane production in all other cases. It can be assumed that, in our reactors, methane has two possible origins: (i) Conventional methanogenesis, (ii) (Bio)-electromethanogenesis (either through direct interspecies electron transfer (DIET) or direct interspecies electron transfer (IIET)). Although it is not easy to discriminate between their individual contribution (as the AD and MEC processes are occurring simultaneously and within the same reactor), it is still possible to have an estimate of the theoretical maximum contribution of the MEC to the overall methane production, using Equation (1), and assuming that all the circulating current is converted into this gas (the amount of hydrogen in the off-gas was below the detention limit of the chromatograph). The estimates (Table 3) indicate that the MEC might provide a significant contribution to the overall methane production, although we did not see any improvement in the overall methane yield in MECNA or MECPA (Figure 2) in comparison to that measured without the assistance of the MEC (i.e., ADNA and ADPA).

Table 3.
Estimates of the maximum theoretical methane yield (VEC) that can be attributed to the MEC systems, and the fraction it represents of the total methane yield.
Overall, these results seem to indicate that the MEC and the AD are competing for the substrate, and when there are no external limitations on the AD (these being, in our experiments, the presence of activated carbon), the MEC only provides a slight improvement in terms of production rates. However, when the refereed limitations are present, our results suggest that the MEC helps to overcome them, allowing for a notable increase in both methane production rates and methane yield.
Electrochemical and Biological Characterisation
To further understand the impact of the different supplements on the AD and MEC-AD processes, cyclic voltammetry (CV) and microbiology analyses were carried out at the end of the experiments. CV results (Figure 4) suggest the existence of a developed electroactive biofilm for all the biotic electrodes, with a well-defined oxidation peak around −0.2 V vs Ag/AgCl, that can be attributed to acetate oxidation (dashed circle) [14,30]. The largest peak was observed for the MECNA (followed by MECPA and MECAC), which is coherent with the current profiles measured during the experimental phase (Figure 3). The different behaviour between AC and PA could be explained by a larger micropore surface area present in AC (see Section 2.1 for the surface characterisation of the tow additives), which could cause more damage to the cell walls/membranes and therefore contribute to the destruction of the biofilm.
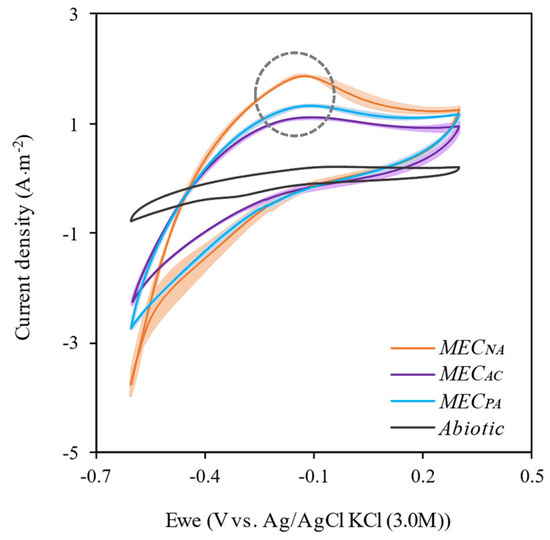
Figure 4.
Cyclic voltammetry for abiotic and MEC reactors. The shaded areas mark the interval of variability across replicates.
Microbiology analyses were carried out to determine the influence of the MEC and the carbonaceous materials on the different microbial communities. Figure 5a shows the quantifications (qPCR) of the archaea populations of the initial inoculum and of the bulk of the different reactors at the end of the experiments. The reactors without additives (ADNA and MECNA) showed the largest presence of archaea, undergoing a significant growth compared to the inoculum. In contrast, this domain experienced a much-limited growth in the reactors supplemented with AC, something that could explain the low productivity of the ADAC and MECAC (Figure 2). These results support the theory previously proposed by Fujinawa et al. [23] that some structures of carbonaceous materials could damage the cell membrane/walls, leading to lysis of microorganisms and therefore a reduction in the microbial population (the biophysical processes that induce the destruction of phospholipid membranes are complex and can be attributed to several factors [31]). Although the PA-supplemented reactors also showed a diminishment in the population of archaea (Figure 5a), this reduction was less dramatic than that observed for the AC tests (the number of gene copies of archaea in PA doubled that of AC). In addition, the relative abundance of Methanosaeta (a robust and efficient methanogenic archaea) is larger in the PA reactors compared to that in the AC reactors (Figure 5b). It seems that the combined effect of a larger archaea population and a larger relative abundance of Methanosaeta might explain, at least in part, the better performance of PA compared to AC.
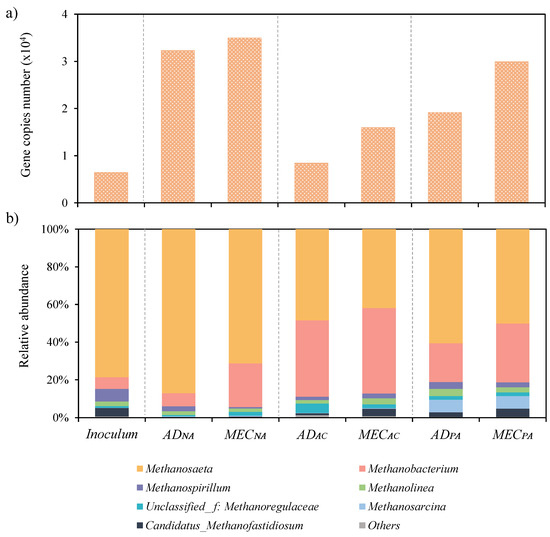
Figure 5.
Archaea classification for the inoculum and reactor bulks in terms of (a) total gene copy number for, and (b) taxonomic classification of 16S rDNA amplicon sequencing gene at genus level.
It is also important to note that all the reactors that contained an MEC unit (MECNA, MECAC and MECPA) showed 8–47% more biomass growth than the rest (ADNA, ADAC and ADPA). It seems then that the applied voltage in the MEC is promoting the electron transfer between species, which further enhances microbial growth [32,33].
Figure 5b shows the taxonomic classification of the archaea community. Relative abundances below 2% were categorised as “others”. The main methanogen species in the reactors were Methanosaeta (42–87%), Methanobacterium (6–45%), Methanospirillum (0.8–6.7%), Methanolinea (1.8–3.8%) and Methanosarcina (0.1–6.7%). The inoculum and the non-additive reactor (ADNA) where mainly dominated by Methanosaeta (80–87%), which is a strictly acetoclastic methanogen capable of producing CH4 from acetate [34], being typically found in anaerobic digestion processes with a stable performance [35,36]. Therefore, it can be hypothesised that the acetolactic pathway is the main methanogenic route in these two systems.
In contrast, all the other reactors (MECNA, ADAC, MECAC, ADPA and MECPA) underwent a slightly different reorganisation of the microbial community, resulting in a co-dominance between Methanosaeta (42–71%) and Methanobactherium (20–45%). The latter is a hydrogenotrophic methanogen able to produce CH4 from CO2 in the presence of H2 [37], which suggests that the presence of the carbonaceous supplements is promoting the hydrogenotrophic pathway [38].
Figure 6a shows the results of the qPCR analyses of bacteria. As with archaea, all reactors experienced a notable growth in bacterial population (in comparison to the inoculum). Again, and similarly to archaea, the reactors that were not supplemented underwent the largest bacterial growth.
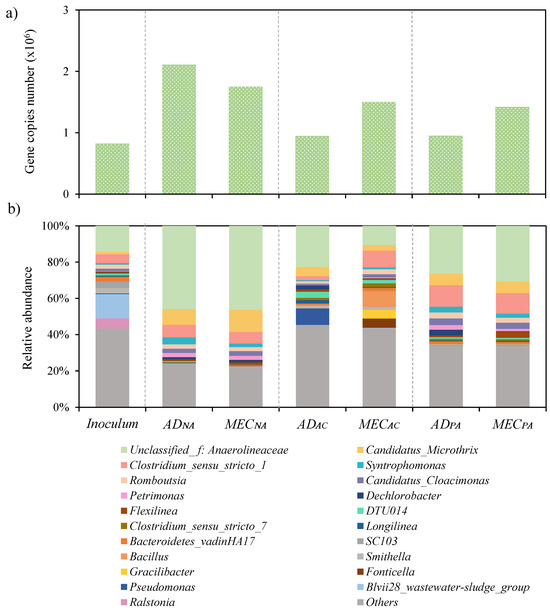
Figure 6.
Bacteria classification for the inoculum and reactor bulks in terms of (a) total gene copy number for, and (b) taxonomic classification of 16S rDNA amplicon sequencing gene at genus level.
The Unclassified_f:Anaerolineaceae (10–46%) is the most abundant bacteria found in all reactors (Figure 6b), followed by Candidatus_Microthrix (3–12%) and Clostridium_sensu_stricto_1 (2–12%). Anaerolineaceae species are known to have a fermentative metabolism using carbohydrates to produce small molecules (e.g., formate, lactate, acetate, hydrogen and carbon dioxide) [39,40], being capable of cooperating syntrophically with certain archaea, as, for example Methanosaeta [40,41]. This means that Anaerolineaceae can supply organic acids to acetoclastic methanogens [40,42], which are the predominant archaea in all the reactors. On the other hand, Candidatus Microthrix and/or Clostridium_sensu_stricto_1 are microorganisms with an important role in the degradation of organic compounds in AD [43,44]. They are acetogens that generally metabolise various types of sugars while producing precursors for the methanogenesis, including acetate, butyrate, CO2 and H2 [45].
Unclassified_f:Anaerolineaceae and Candidatus Microthrix were the two most affected bacteria in all reactors with additives, showing the highest reduction (41–77%) in the ADAC and MECAC reactors compared to the non-additive reactors (MECNA and ADNA). As described above, these microorganisms are involved in the methanogenic process, which would contribute to explaining the lowest methane productivity in those reactors (see Figure 2).
4. Conclusions
This study investigates the use of two carbonaceous materials (PA and AC) as supplements to both traditional AD and MEC-assisted AD. From the results reported above, we can attain the following conclusions:
- The AC amendment has a negative impact on methane productivity (a result that was supported by the microbiological analyses, where it was shown that the reactors that contained AC underwent the largest reduction in the population of both archaea and bacteria). Nevertheless, the MEC attenuated the low productivity caused by the AC.
- The addition of the pyrolysed material (PA) did not result in any increase in biogas production (compared to conventional AD), although it slightly accelerated the methane production rate, something that could be advantageous in real anaerobic digesters that usually operate at very tight hydraulic retention times. A similar improvement was observed in the MEC-assisted AD without supplements.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en17061290/s1, Figure S1: Mean diameter with respect to particle number of activated carbon (top) and pyrolysed argan (bottom).
Author Contributions
D.C.-P.: Conceptualisation, Investigation, Methodology, Writing—original draft. R.M.: Supervision, Validation, Investigation, Methodology. A.M.: Supervision, Methodology, Funding acquisition, Project administration. A.E.: Conceptualisation, Supervision, Methodology, Writing—Reviewing and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the ‘Ministerio de Ciencia e Innovación (Gobierno de España)’ project ref: PID2020-115948RB-I00 funded by MCIN/AEI/10.13039/501100011033.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
There are no conflicts to declare.
References
- Milk and Dairy Products. Available online: https://agriculture.ec.europa.eu/farming/animal-products/milk-and-dairy-products_en (accessed on 18 July 2023).
- Rao, M.; Bast, A.; de Boer, A. Valorized Food Processing By-Products in the EU: Finding the Balance between Safety, Nutrition, and Sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Gómez-Falcon, N.; Brar, S.K.; Ramírez, A.A. Cheese Whey as a Potential Feedstock for Producing Renewable Biofuels: A Review. Energies 2022, 15, 6828. [Google Scholar] [CrossRef]
- Curry, N.; Pillay, P. Biogas Prediction and Design of a Food Waste to Energy System for the Urban Environment. Renew Energy 2012, 41, 200–209. [Google Scholar] [CrossRef]
- Iglesias, R.; Muñoz, R.; Polanco, M.; Díaz, I.; Susmozas, A.; Moreno, A.D.; Guirado, M.; Carreras, N.; Ballesteros, M. Biogas from Anaerobic Digestion as an Energy Vector: Current Upgrading Development. Energies 2021, 14, 2742. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Dessì, P.; Isipato, M.; Lens, P.N.L.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. The Dairy Biorefinery: Integrating Treatment Processes for Cheese Whey Valorisation. J. Environ. Manag. 2020, 276, 111240. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Cuetos, M.J.; Martínez, E.J.; Gómez, X. Thermophilic Anaerobic Digestion of Cheese Whey: Coupling H2 and CH4 Production. Biomass Bioenergy 2015, 81, 55–62. [Google Scholar] [CrossRef]
- Comino, E.; Riggio, V.A.; Rosso, M. Biogas Production by Anaerobic Co-Digestion of Cattle Slurry and Cheese Whey. Bioresour. Technol. 2012, 114, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Escalante, H.; Castro, L.; Amaya, M.P.; Jaimes, L.; Jaimes-Estévez, J. Anaerobic Digestion of Cheese Whey: Energetic and Nutritional Potential for the Dairy Sector in Developing Countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Escalante-Hernández, H.; Castro-Molano, L.D.P.; Besson, V.; Jaimes-Estévez, J. Feasibility of the Anaerobic Digestion of Cheese Whey in a Plug Flow Reactor (PFR) under Local Conditions. Ing. Investig. Y Tecnol. 2017, 18, 264–277. [Google Scholar] [CrossRef][Green Version]
- Jaimes-Estévez, J.; Castro, L.; Escalante, H.; Carrillo, D.; Portillo, S.; Sotres, A.; Morán, A. Cheese Whey Co-Digestion Treatment in a Tubular System: Microbiological Behaviour along the Axial Axis. Biomass Convers. Biorefinery 2020, 12, 5719–5728. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, B.; Yin, C.; Zhang, C.; Dai, X.; Yuan, H.; Zhu, N. Biostimulation by Direct Voltage to Enhance Anaerobic Digestion of Waste Activated Sludge. RSC Adv. 2015, 6, 1581–1588. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Chen, S.; Quan, X. Enhanced Production of Methane from Waste Activated Sludge by the Combination of High-Solid Anaerobic Digestion and Microbial Electrolysis Cell with Iron–Graphite Electrode. Chem. Eng. J. 2015, 259, 787–794. [Google Scholar] [CrossRef]
- Carrillo-Peña, D.; Escapa, A.; Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R.; Mateos, R. Bioelectrochemical Enhancement of Methane Production from Exhausted Vine Shoot Fermentation Broth by Integration of MEC with Anaerobic Digestion. Biomass Convers. Biorefinery 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Escapa, A.; Gil-Carrera, L.; García, V.; Morán, A. Performance of a Continuous Flow Microbial Electrolysis Cell (MEC) Fed with Domestic Wastewater. Bioresour. Technol. 2012, 117, 55–62. [Google Scholar] [CrossRef]
- Park, J.G.; Lee, B.; Park, H.R.; Jun, H.B. Long-Term Evaluation of Methane Production in a Bio-Electrochemical Anaerobic Digestion Reactor according to the Organic Loading Rate. Bioresour. Technol. 2019, 273, 478–486. [Google Scholar] [CrossRef]
- Blasco-Gómez, R.; Batlle-Vilanova, P.; Villano, M.; Balaguer, M.D.; Colprim, J.; Puig, S. On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis. Int. J. Mol. Sci. 2017, 18, 874. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Shen, Y.; Yuan, R.; Zhu, N.; Yuan, H.; Lou, Z. Sludge-Based Biochar-Assisted Thermophilic Anaerobic Digestion of Waste-Activated Sludge in Microbial Electrolysis Cell for Methane Production. Bioresour. Technol. 2019, 284, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Johnravindar, D.; Liang, B.; Fu, R.; Luo, G.; Meruvu, H.; Yang, S.; Yuan, B.; Fei, Q. Supplementing Granular Activated Carbon for Enhanced Methane Production in Anaerobic Co-Digestion of Post-Consumer Substrates. Biomass Bioenergy 2020, 136, 105543. [Google Scholar] [CrossRef]
- Wang, X.T.; Zhang, Y.F.; Wang, B.; Wang, S.; Xing, X.; Xu, X.J.; Liu, W.Z.; Ren, N.Q.; Lee, D.J.; Chen, C. Enhancement of Methane Production from Waste Activated Sludge Using Hybrid Microbial Electrolysis Cells-Anaerobic Digestion (MEC-AD) Process—A Review. Bioresour. Technol. 2022, 346, 126641. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, Y.; Dhar, B.R. Boosting Resilience of Microbial Electrolysis Cell-Assisted Anaerobic Digestion of Blackwater with Granular Activated Carbon Amendment. Bioresour. Technol. 2023, 381, 129136. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, H.; Zhang, Y.; Cui, M.H.; Fu, B.; Liu, H. Insight into Sludge Anaerobic Digestion with Granular Activated Carbon Addition: Methanogenic Acceleration and Methane Reduction Relief. Bioresour. Technol. 2021, 319, 124131. [Google Scholar] [CrossRef]
- Fujinawa, K.; Nagoya, M.; Kouzuma, A.; Watanabe, K. Conductive Carbon Nanoparticles Inhibit Methanogens and Stabilize Hydrogen Production in Microbial Electrolysis Cells. Appl. Microbiol. Biotechnol. 2019, 103, 6385–6392. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.M.; Escapa, A.; Sotres, A.; Morán, A. Integrating Microbial Electrochemical Technologies with Anaerobic Digestion to Accelerate Propionate Degradation. Fuel 2020, 267, 117158. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.; Xie, D.; Guan, W.; Yang, M.; Zhao, P.; Gao, M.; Wang, Q.; Wu, C. Adding Activated Carbon to the System with Added Zero-Valent Iron Further Improves Anaerobic Digestion Performance by Alleviating Ammonia Inhibition and Promoting DIET. J. Environ. Chem. Eng. 2021, 9, 106616. [Google Scholar] [CrossRef]
- Orrantia, M.; Meza-Escalante, E.R.; Burboa-Charis, V.A.; García-Reyes, R.B.; Atilano-Camino, M.M.; Serrano-Palacios, D.; Leyva, L.A.; Del Angel, Y.A.; Alvarez, L.H. Granular Activated Carbon Enhances the Anaerobic Digestion of Solid and Liquid Fractions of Swine Effluent at Different Mesophilic Temperatures. Anaerobe 2023, 83, 102782. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, B.; Tian, D.; Jun, H. Bioelectrochemical Enhancement of Methane Production from Highly Concentrated Food Waste in a Combined Anaerobic Digester and Microbial Electrolysis Cell. Bioresour. Technol. 2018, 247, 226–233. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Lacroix, R.; Trably, E.; Da Silva, S.; Bernet, N. On the Actual Anode Area That Contributes to the Current Density Produced by Electroactive Biofilms. Electrochim. Acta 2018, 259, 395–401. [Google Scholar] [CrossRef]
- Wang, X.F.; Xu, K.; Li, X.R.; Liu, Y.X.; Cheng, J.M. Damage Effect of Amorphous Carbon Black Nanoparticle Aggregates on Model Phospholipid Membranes: Surface Charge, Exposure Concentration and Time Dependence. Int. J. Environ. Res. Public Health 2023, 20, 2999. [Google Scholar] [CrossRef]
- Guo, H.; Hua, J.; Cheng, J.; Yue, L.; Zhou, J. Microbial Electrochemistry Enhanced Electron Transfer in Lactic Acid Anaerobic Digestion for Methane Production. J. Clean. Prod. 2022, 358, 131983. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of Methane Production in Anaerobic Digestion Process: A Review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Chen, S.; He, Q. Persistence of Methanosaeta Populations in Anaerobic Digestion during Process Instability. J. Ind. Microbiol. Biotechnol. 2015, 42, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Conklin, A.; Stensel, H.D.; Ferguson, J. Growth Kinetics and Competition Between Methanosarcina and Methanosaeta in Mesophilic Anaerobic Digestion. Water Environ. Res. 2006, 78, 486–496. [Google Scholar] [CrossRef]
- Jannat, A.H.; Park, S.H.; Chairattanawat, C.; Yulisa, A.; Hwang, S. Effect of Different Microbial Seeds on Batch Anaerobic Digestion of Fish Waste. Bioresour. Technol. 2022, 349, 126834. [Google Scholar] [CrossRef]
- Carrillo-Peña, D.; Mateos, R.; Morán, A.; Escapa, A. Reduced Graphene Oxide Improves the Performance of a Methanogenic Biocathode. Fuel 2022, 321, 123957. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.H.; Lee, S.H.; Jung, S.P.; Kim, S.H. Enhancing Anaerobic Digestion for Rural Wastewater Treatment with Granular Activated Carbon (GAC) Supplementation. Bioresour. Technol. 2020, 315, 123890. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Watson, J.; Sharma, B.K.; Si, B.; Zhang, Y. Adsorption or Direct Interspecies Electron Transfer? A Comprehensive Investigation of the Role of Biochar in Anaerobic Digestion of Hydrothermal Liquefaction Aqueous Phase. Chem. Eng. J. 2022, 435, 135078. [Google Scholar] [CrossRef]
- Liang, B.; Wang, L.Y.; Mbadinga, S.M.; Liu, J.F.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Anaerolineaceae and Methanosaeta Turned to Be the Dominant Microorganisms in Alkanes-Dependent Methanogenic Culture after Long-Term of Incubation. AMB Express 2015, 5, 37. [Google Scholar] [CrossRef]
- Zamorano-López, N.; Borrás, L.; Giménez, J.B.; Seco, A.; Aguado, D. Acclimatised Rumen Culture for Raw Microalgae Conversion into Biogas: Linking Microbial Community Structure and Operational Parameters in Anaerobic Membrane Bioreactors (AnMBR). Bioresour. Technol. 2019, 290, 121787. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic Promotion of Syntrophic Methane Production from Anaerobic Digestion of Complex Organic Wastes by Biochar: Performance and Associated Mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.K.; Hoshiko, Y.; Toya, S.; Maeda, T. Effect of Different Concentrations of Sodium Selenite on Anaerobic Digestion of Waste Sewage Sludge. Environ. Technol. Innov. 2022, 27, 102403. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Wei, B.; Song, C.; Cai, F.; Liu, G.; Chen, C. Effects of Different Microbial Pretreatments on the Anaerobic Digestion of Giant Grass under Anaerobic and Microaerobic Conditions. Bioresour. Technol. 2021, 337, 125456. [Google Scholar] [CrossRef]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane Production through Anaerobic Digestion: Participation and Digestion Characteristics of Cellulose, Hemicellulose and Lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).