Production of CH4/C3H8 (85/15 vol%) Hydrate in a Lab-Scale Unstirred Reactor: Quantification of the Promoting Effect Due to the Addition of Propane to the Gas Mixture
Abstract
1. Introduction
- (i)
- Limitations due to the heat and mass transfer properties of the system;
- (ii)
- The thermodynamic region, existing between the phase equilibrium curves of pure methane and carbon dioxide hydrates, is relatively narrow;
- (iii)
- If the process is carried out with pure carbon dioxide, the maximum ideal efficiency is much lower than one;
- (iv)
- The local concentration of replacing and replaced species plays a crucial role.
2. Role of C3H8 in the Capture of CO2 and CH4 into Hydrate Structures
3. Materials and Methods
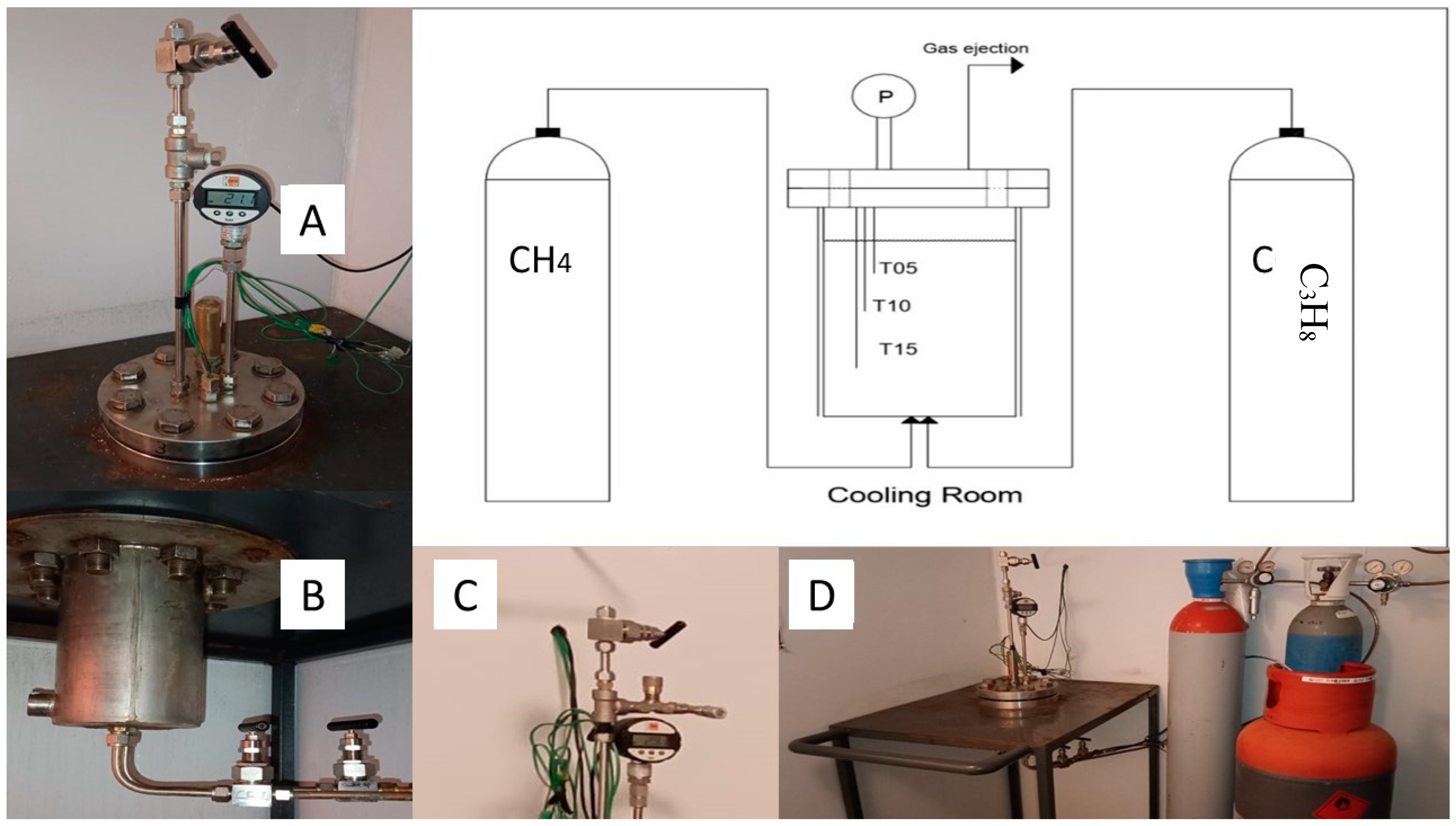
3.1. Experimental Apparatus and Materials
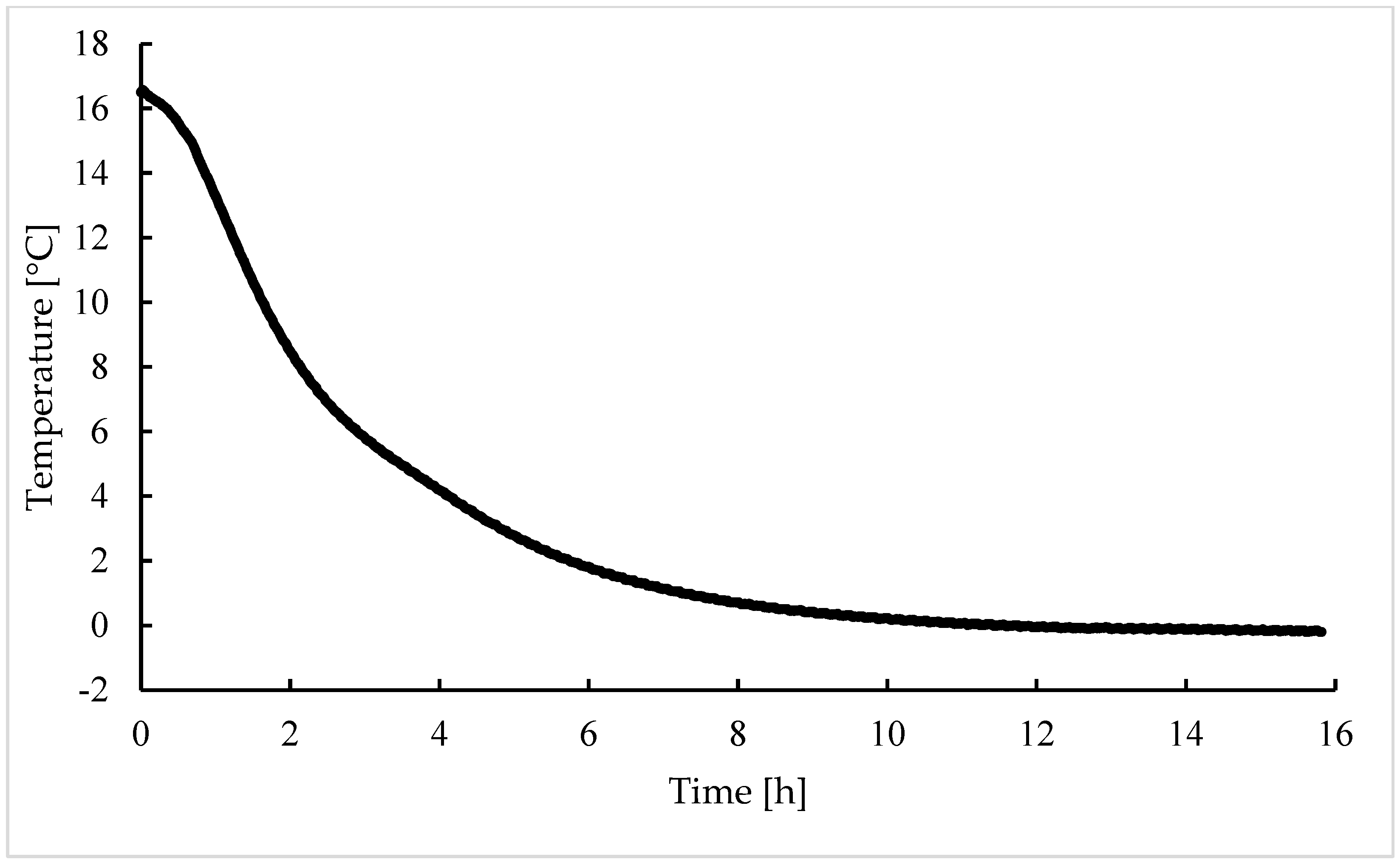
3.2. Procedure
- -
- VPORE: the portion of internal volume available for the production of hydrates;
- -
- Z: compressibility factor (calculated with the Peng–Robinson equation);
- -
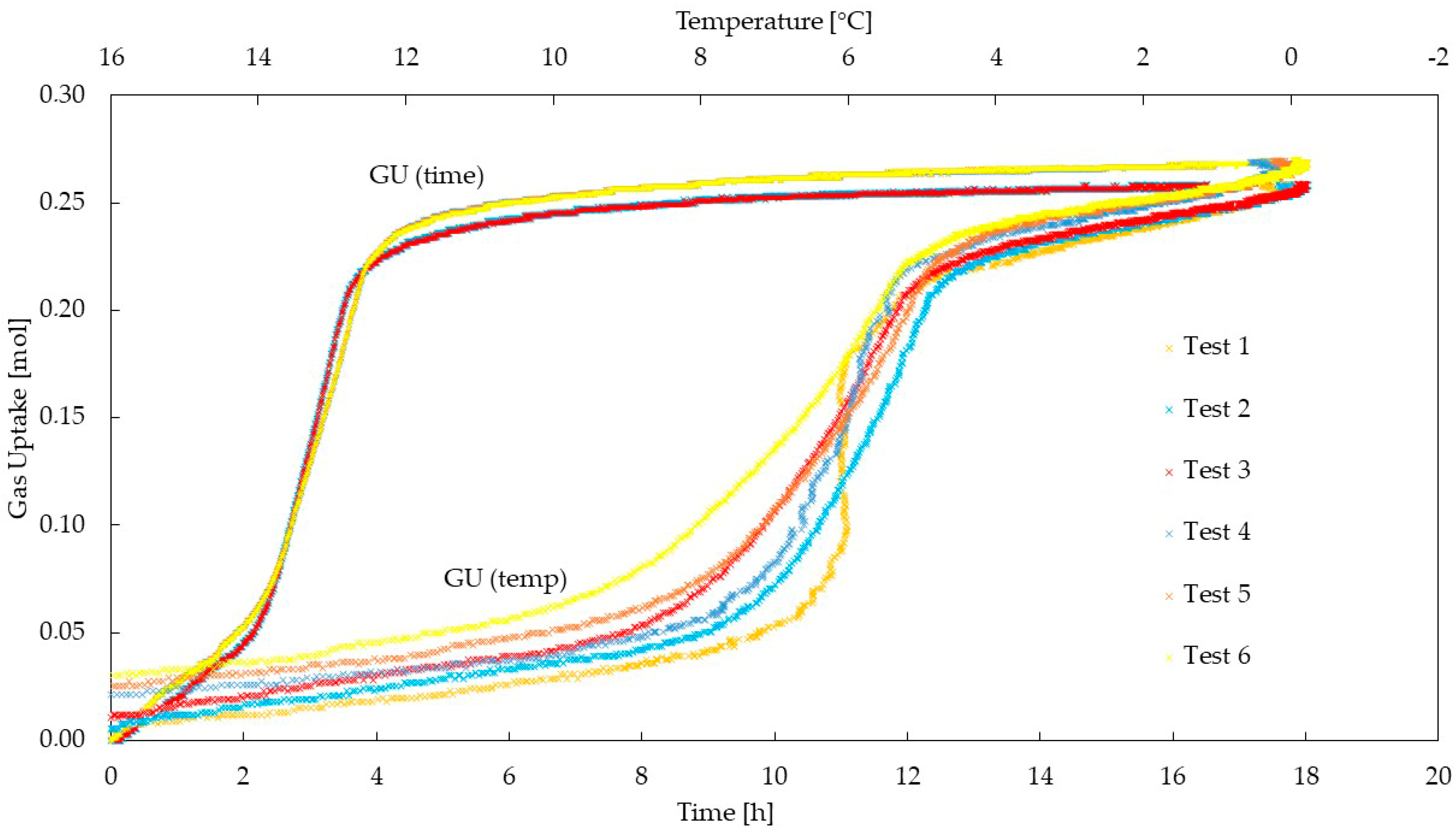
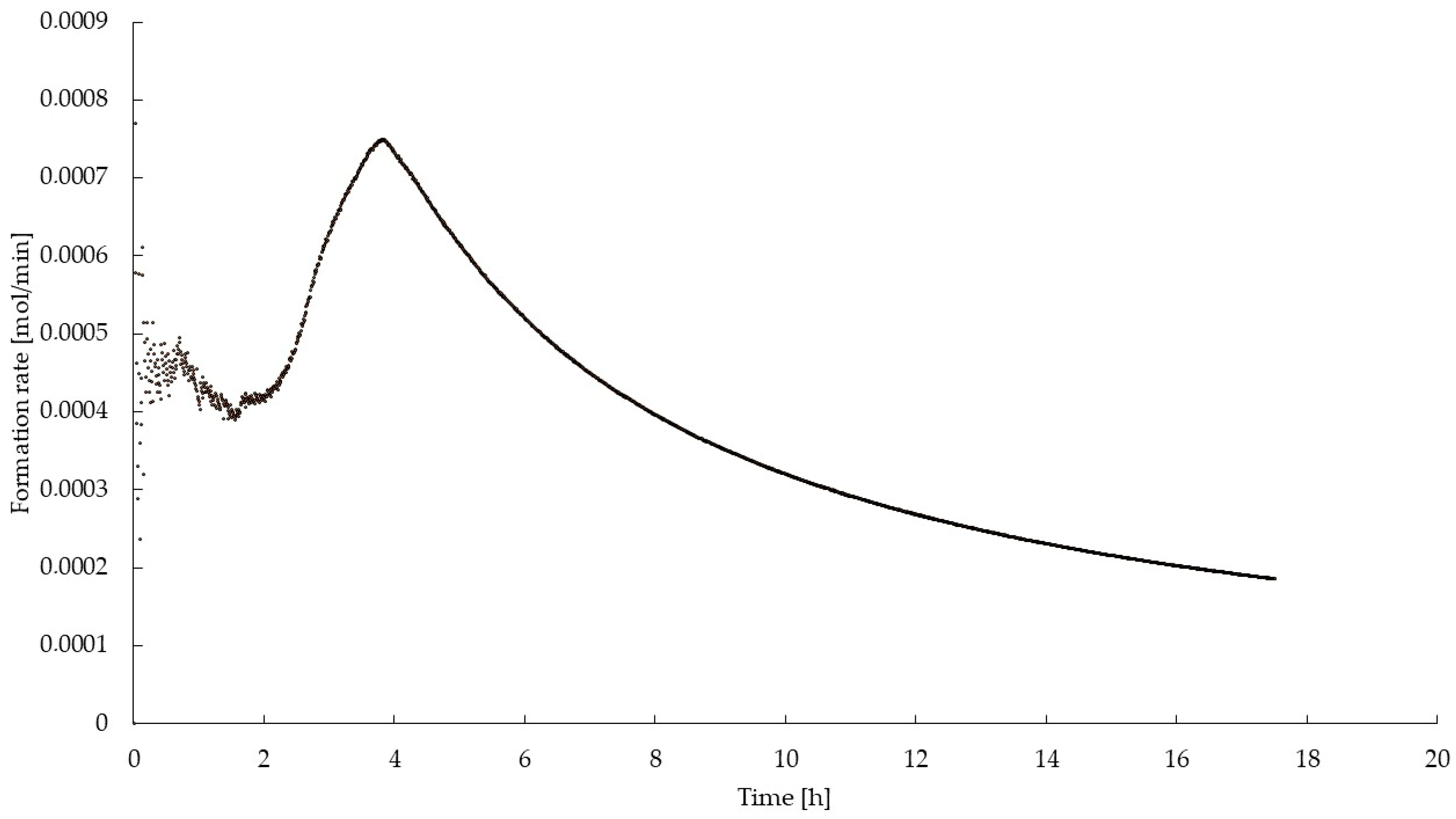
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Argentino, C.; Witting, C.; Peckmann, J.; Panieri, G. Nitrogen uptake by methanotrophic consortia in deep-water gas hydrate-bearing sediments. Chem. Geol. 2023, 636, 121638. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Thermodynamic and kinetic characterization of methane hydrate nucleation, growth and dissociation processes, according to the Labile Cluster Theory. Chem. Eng. J. 2021, 425, 130706. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yin, S.; He, G.; Li, J.; Wu, Q. Research progress of the kinetics on natural gas hydrate replacement by CO2-containing mixed gas: A review. J. Nat. Gas Sci. Eng. 2022, 108, 104837. [Google Scholar] [CrossRef]
- Demirbas, A. Methane from gas hydrates in the Black Sea. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 32, 165–171. [Google Scholar] [CrossRef]
- Shaibu, R.; Sambo, C.; Guo, B.; Dudun, A. An assessment of methane gas production from natural gas hydrates: Challenges, technology and market outlook. Adv. Geo-Energy Res. 2021, 5, 318–332. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Rossi, F.; Li, Y.; Gambelli, A.M. Thermodynamic and kinetic description of the main effects related to the memory effect during carbon dioxide hydrates formation in a confined environment. Sustainability 2021, 13, 13797. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Tse, J.S.; Ratcliffe, C.I.; Powell, B.M. A new clathrate hydrate structure. Nature 1987, 325, 135–136. [Google Scholar] [CrossRef]
- Mahant, B.; Kushwaha, O.S.; Kumar, R. Synthesis of Cocos nucifera derived surfactant and its application in growth kinetics of methane gas hydrates for energy storage and transportation. Energy Conv. Manag. 2022, 269, 116044. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Castellani, B.; Nicolini, A.; Rossi, F. Gas hydrate formation as a strategy for CH4/CO2 separation: Experimental study on gaseous mixtures produced via Sabatier reaction. J. Nat. Gas Sci. Eng. 2019, 71, 102985. [Google Scholar] [CrossRef]
- Lv, Y.; Xia, X.; Wang, F.; Wu, X.; Cheng, C.; Zhang, L.; Yang, L.; Zhao, J.; Song, Y. Clathrate hydrate for phase change cold storage: Simulation advances and potential applications. J. Energy Storage 2022, 55, 105835. [Google Scholar] [CrossRef]
- Chen, L.; Sasaki, H.; Watanabe, T.; Okajima, J.; Komiya, A.; Maruyama, S. Production strategy for oceanic methane hydrate extraction and power generation with Carbon Capture and Storage (CCS). Energy 2017, 126, 256–272. [Google Scholar] [CrossRef]
- Falahieh, M.M.; Bonyadi, M.; Lashanizadegan, A. Seawater desalination by coupling cyclopentane hydrate formation and capacitive deionization processes in the presence of Fe3O4 nanoparticles and magnetic field. Desalination 2022, 543, 116120. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Pezzolla, D.; Rossi, F.; Gigliotti, G. Thermodynamic description of CO2 hydrates production in aqueous systems containing NH4Cl; evaluation of NH4+ removal from water via spectrophotometric analysis. Chem. Eng. Sci. 2023, 281, 119137. [Google Scholar] [CrossRef]
- Sun, L.; Hassanpouryouzband, A.; Sun, H.; Wang, T.; Zhang, L.; Yang, L.; Zhao, J.; Song, Y. Advancement in sewage sludge dewatering with hydrate crystal phase change: Unveiling the micro-moisture migration and dewaterability mechanism. ACS Sustain. Chem. Eng. 2023, 11, 12075–12083. [Google Scholar] [CrossRef]
- Sun, L.; Dong, H.; Lu, Y.; Zhang, L.; Yang, L.; Zhao, J.; Song, Y. A hydrate-based zero liquid discharge method for high-concentration organic wastewater: Resource recovery and water reclamation. Water 2023, 6, 49. [Google Scholar] [CrossRef]
- Wang, F.; Guo, G.; Liu, G.Q.; Luo, S.J.; Guo, R.B. Effects of surfactants micelles and surfactant-coated nanospheres on methane hydrate growth pattern. Chem. Eng. Sci. 2016, 144, 108–115. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, J.; Kim, H.; Seo, Y.; Linga, P. Hydrates for cold energy storage and transport: A review. Adv. Appl. Energy 2021, 2, 100022. [Google Scholar] [CrossRef]
- Zhang, Y.; Bhattacharjee, G.; Kumar, R.; Linga, P. Solidified hydrogen storage (Solid-HyStore) via clathrate hydrates. Chem. Eng. J. 2022, 431, 133702. [Google Scholar] [CrossRef]
- Chang, Y.; Sun, Z. Synthesis and thermal properties of n-tetradecane phase change microcapsules for cold storage technology. Renew. Sust. Energ. Rev. 2020, 117, 104959. [Google Scholar]
- Cheng, C.; Wang, F.; Tian, Y.; Wu, X.; Zheng, J.; Zhang, J.; Li, L.; Yang, P.; Zhao, J. Review and prospects of hydrate cold storage technology. Renew. Sust. Energ. Rev. 2020, 117, 109492. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, M.; Wang, T.; Yang, L.; Zhang, X.; Zhao, J.; Song, Y. An in-situ MRI method for quantifying temperature changes during crystal hydrate growth in porous medium. J. Therm. Sci. 2022, 31, 1542–1550. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Partoon, B.; Lal, B.; Gonfa, G.; Foo Khor, S.; Sharif, A.M. Inhibition effect of amino acids on carbon dioxide hydrates. Chem. Eng. Sci. 2017, 171, 331–339. [Google Scholar] [CrossRef]
- Nam Park, K.; Hong, S.Y.; Lee, J.W.; Kang, K.C.; Lee, Y.C.; Ha, M.G.; Lee, J.D. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B3+). Desalination 2011, 274, 91–96. [Google Scholar] [CrossRef]
- Gaikward, N.; Nakka, R.; Khavala, V.; Bhadani, A.; Mamane, H.; Kumar, R. Gas hydrate-based process for desalination of heavy metal ions from an aqueous solution: Kinetics and rate of recovery. ACS EsT Water 2021, 1, 134–144. [Google Scholar] [CrossRef]
- Karamoddin, M.; Varaminian, F. Water purification by freezing and gas hydrate processes, and removal of dissolved minerals (Na+, K+, Mg2+, Ca2+). J. Mol. Liq. 2016, 223, 1021–1031. [Google Scholar] [CrossRef]
- Montazeri, S.M.; Kolliopoulos, G. Hydrate based desalination for sustainable water treatment: A review. Desalination 2022, 537, 115855. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Chen, Z.; Li, Q.; Zhang, Y.; Wang, Y.; Xia, Z.; You, C. Combined styles of depressurization and electrical heating for methane hydrate production. Appl. Energy 2021, 282, 266–273. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Rossi, F. Experimental study on the effect of SDS and micron copper particles mixture on carbon dioxide hydrates formation. Energies 2022, 15, 6540. [Google Scholar] [CrossRef]
- Xu, C.G.; Zhang, W.; Yan, K.F.; Cai, J.; Chen, Z.Y.; Li, X.S. Research on micro mechanism and influence of hydrate-based methane-carbon dioxide replacement for realizing simultaneous clean energy exploitation and carbon emission reduction. Chem. Eng. Sci. 2022, 248, 117266. [Google Scholar] [CrossRef]
- Wang, P.; Teng, Y.; Zhu, J.; Bao, W.; Han, S.; Li, Y.; Zhao, Y.; Xie, H. Review on the synergistic effect between metal-organic frameworks and gas hydrates for CH4 storage and CO2 separation applications. Renew. Sust. Energ. Rev. 2022, 167, 112807. [Google Scholar] [CrossRef]
- Xuan, K.; Yi, W.; Li, X.S.; Zhang, Y.; Chen, Y.Z. Influence of heat conduction and heat convection on hydrate dissociation by depressurization in a pilot-scale hydrate simulator. Appl. Energy 2019, 251, 113405. [Google Scholar]
- Nair, V.C.; Prasad, S.K.; Kumar, R.; Sangway, J.S. Energy recovery from simulated clayey gas hydrate reservoir using depressurization by constant rate gas release, thermal stimulation and their combination. Appl. Energy 2018, 225, 755–768. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y. Experimental investigation of optimization of well spacing for gas recovery from methane hydrate reservoir in sandy sediment by heat stimulation. Appl. Energy 2017, 207, 562–572. [Google Scholar] [CrossRef]
- Lei, G.; Tang, J.; Zhang, L.; Wu, Q.; Li, J. Effective thermal conductivity for hydrate-bearing sediments under stress and local thermal stimulation conditions: A novel analytical model. Energy 2024, 288, 129704. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Castellani, B.; Nicolini, A.; Rossi, F. Water salinity as potential aid for improving the carbon dioxide replacement process effectiveness in natural gas hydrate reservoirs. Processes 2020, 8, 1298. [Google Scholar] [CrossRef]
- Semenov, A.P.; Mendgaziev, R.I.; Stoporev, A.S.; Istomin, V.A.; Sergeeva, D.V.; Ogienko, A.G.; Vinokurov, V.A. The pursuit of a more powerful thermodynamic hydrate inhibitor than methanol. Dimethyl sulfoxide as a case study. Chem. Eng. J. 2021, 423, 130227. [Google Scholar] [CrossRef]
- Gambelli, A.M. Analyses on CH4 and CO2 hydrate formation to define the optimal pressure for CO2 injection to maximize the replacement efficiency into natural gas hydrate in presence of a silica-based natural porous medium, via depressurization techniques. Chem. Eng. Process. 2021, 167, 108512. [Google Scholar] [CrossRef]
- Rossi, F.; Gambelli, A.M.; Sharma, D.K.; Castellani, B.; Nicolini, A.; Castaldi, M.J. Experiments on methane hydrates formation in seabed deposits and gas recovery adopting carbon dioxide replacement strategies. Appl. Therm. Eng. 2019, 148, 371–381. [Google Scholar] [CrossRef]
- Yan, C.; Li, Y.; Cheng, Y.; Wei, J.; Tian, W.; Li, S.; Wang, Z. Multifield coupling mechanism in formations around wellbore during the exploitation of methane hydrate with CO2 replacement. Energy 2022, 245, 123283. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, X.S.; Wang, Y.; Liu, J.W.; Hu, H.Q. The optimization mechanism for gas hydrate dissociation by depressurization in the sediment with different water saturations and different particle sizes. Energy 2021, 215, 119129. [Google Scholar] [CrossRef]
- Gambelli, A.M. Variations in terms of CO2 capture and CH4 recovery during replacement processes in gas hydrate reservoirs, associated to the “memory effect”. J. Clean. Prod. 2022, 360, 132154. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Lou, W.; Deng, X.; Zhong, J.; Sun, X.; Yin, B.; Sun, B. Research on methane hydrate formation in porous media with gas-water two-phase flow. Gas Sci. Eng. 2023, 110, 204898. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, N.; Sun, X.; Zhong, J.; Wang, Z.; Hou, L.; Li, S.; Sun, B. Pore-scale investigation on methane hydrate formation and plugging under gas-water flow conditions in a micromodel. Fuel 2023, 333, 123612. [Google Scholar] [CrossRef]
- Sun, L.; Sun, H.; Yuan, C.; Zhang, L.; Yang, L.; Ling, Z.; Zhao, J.; Song, Y. Enhanced clathrate hydrate formation at ambient temperatures (287.2 K) and near atmospheric pressure (0.1 MPa): Application to solidified natural gas technology. Chem. Eng. J. 2023, 454, 140325. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F.; Gigliotti, G. Methane replacement by using CO2/C3H8 mixtures for carbon storage and enhanced methane recovery in gas hydrates. Gas Sci. Eng. 2023, 115, 205028. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Partoon, B.; Lal, B.; Keong, L.K. Methane hydrate-liquid-vapor-equilibrium phase condition measurements in the presence of natural amino acids. J. Nat. Gas Sci. Eng. 2017, 37, 425–434. [Google Scholar] [CrossRef]
- Bottger, A.; Kamps, A.P.S.; Maurer, G. An experimental investigation on the phase equilibrium of the binary system (methane+water) at low temperatures: Solubility of methane in water and three-phase (vapour+liquid+hydrate) equilibrium. Fluid Phase Equilibr. 2016, 407, 209–216. [Google Scholar] [CrossRef]
- Kassim, Z.; Khan, M.S.; Lal, B. Thermodynamic modelling on methane hydrate equilibrium condition in the presence of electrolyte inhibitor. Mater. Today Proceed. 2019, 19, 1395–1402. [Google Scholar] [CrossRef]
- Nema, Y.; Ohmura, R.; Senaha, I.; Yasuda, K. Quadruple point determination in carbon dioxide hydrate forming system. Fluid Phase Equilibr. 2017, 441, 49–53. [Google Scholar] [CrossRef]
- Yu, S.H.; Zhou, S.D.; Li, X.S.; Wang, S.L. Effect of graphite nanoparticles on CO2 hydrate phase equilibrium. Fluid Phase Equilibr. 2016, 414, 23–28. [Google Scholar] [CrossRef]
- Jarrahian, A.; Nakaee, A. Hydrate-liquid-vapor equilibrium condition for N2+CO2+H2O system: Measurement and modelling. Fuel 2019, 237, 769–774. [Google Scholar] [CrossRef]
- Kyung, D.; Lee, K.; Kim, H.; Lee, W. Effect of marine environmental factors on the phase equilibrium of CO2 hydrate. Int. J. Greenh. Gas Control 2014, 20, 285–292. [Google Scholar] [CrossRef]
- Seo, Y.T.; Lee, H. Multiple-phase hydrate equilibria of the ternary carbon dioxide, methane, and water mixtures. J. Phys. Chem. B 2001, 105, 10084–10090. [Google Scholar] [CrossRef]
- Sundramoorthy, J.D.; Hammonds, P.; Lal, B.; Philips, G. Gas hydrate equilibrium measurement and observation of gas hydrate dissociation with/without a KHI. Procedia Eng. 2016, 148, 870–877. [Google Scholar] [CrossRef]
- Moojer-van den Heuvel, M.M.; Peters, C.J.; de Swaan Arons, J. Gas hydrate phase equilibria for propane in the presence of additive components. Fluid Phase Equilibr. 2002, 193, 245–249. [Google Scholar] [CrossRef]
- Giovannetti, R.; Gambelli, A.M.; Rossi, A.; Castellani, B.; Minicucci, M.; Zannotti, M.; Nicolini, A.; Rossi, F. Thermodynamic assessment and microscale Raman spectroscopy of binary CO2/CH4 hydrates produced during replacement applications in natural reservoirs. J. Mol. Liq. 2022, 368, 120739. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Alavi, S. Some current challenges in clathrate hydrate science: Nucleation, decomposition and the memory effect. Curr. Opin. Solid State Mater. Sci. 2016, 20, 344–351. [Google Scholar] [CrossRef]
- Wilson, P.W.; Haymet, A.D.J. Hydrate formation and re-formation in nucleating THF/water mixtures show no evidence to support a “memory” effect. Chem. Eng. J. 2010, 161, 146–150. [Google Scholar] [CrossRef]
- Roozeboom, H.W.B. Sur l’hydrate de l’acide sulfureux. Recl. Trav. Chim. Pays.-Bas. 1884, 3, 29–58. [Google Scholar] [CrossRef]
- Takeya, S.; Kida, M.; Minami, H.; Sakagami, H.; Hachikubo, A.; Takahashi, N.; Shoji, H.; Soloviev, V.; Wallmann, K.; Biebow, N.; et al. Structure and thermal expansion of natural gas clathrate hydrates. Chem. Eng. Sci. 2006, 61, 2670–2674. [Google Scholar] [CrossRef]
- Aregba, A.G. Gas Hydrate—Properties, Formation and Benefits. Open J. Yangtze Gas Oil 2017, 2, 27–44. [Google Scholar] [CrossRef]
- Davidson, D.W.; Garg, S.K.; Gough, S.R.; Handa, Y.P.; Ratcliffe, C.I.; Ripmeester, J.A.; Tse, J.S.; Lawson, W.F. Laboratory analysis of a naturally occurring gas hydrate from sediment of the Gulf of Mexico. Geochim. Cosmochim. Acta 1986, 50, 619–623. [Google Scholar] [CrossRef]
- Muller-Bongartz, B.; Wildeman, T.R.; Sloan, E.D. A hypothesis for hydrate nucleation phenomena. In Proceedings of the Second International Offshore and Polar Engineering Conference, San Francisco, CA, USA, 14–19 June 1992. [Google Scholar]
- Gambelli, A.M.; Presciutti, A.; Rossi, F. Kinetic considerations and formation rate for carbon dioxide hydrate, formed in presence of a natural silica-based porous medium: How initial thermodynamic conditions may modify the process kinetic. Thermochim. Acta 2021, 705, 179039. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Experimental characterization of the difference in induction period between CH4 and CO2 hydrates: Motivations and possible consequences on the replacement process. J. Nat. Gas Sci. Eng. 2022, 108, 104848. [Google Scholar] [CrossRef]
- Long, J. Gas Hydrate Formation Mechanism and Kinetic Inhibition. PhD Thesis, Colorado School of Mines, Golden, CO, USA, 1994. [Google Scholar]
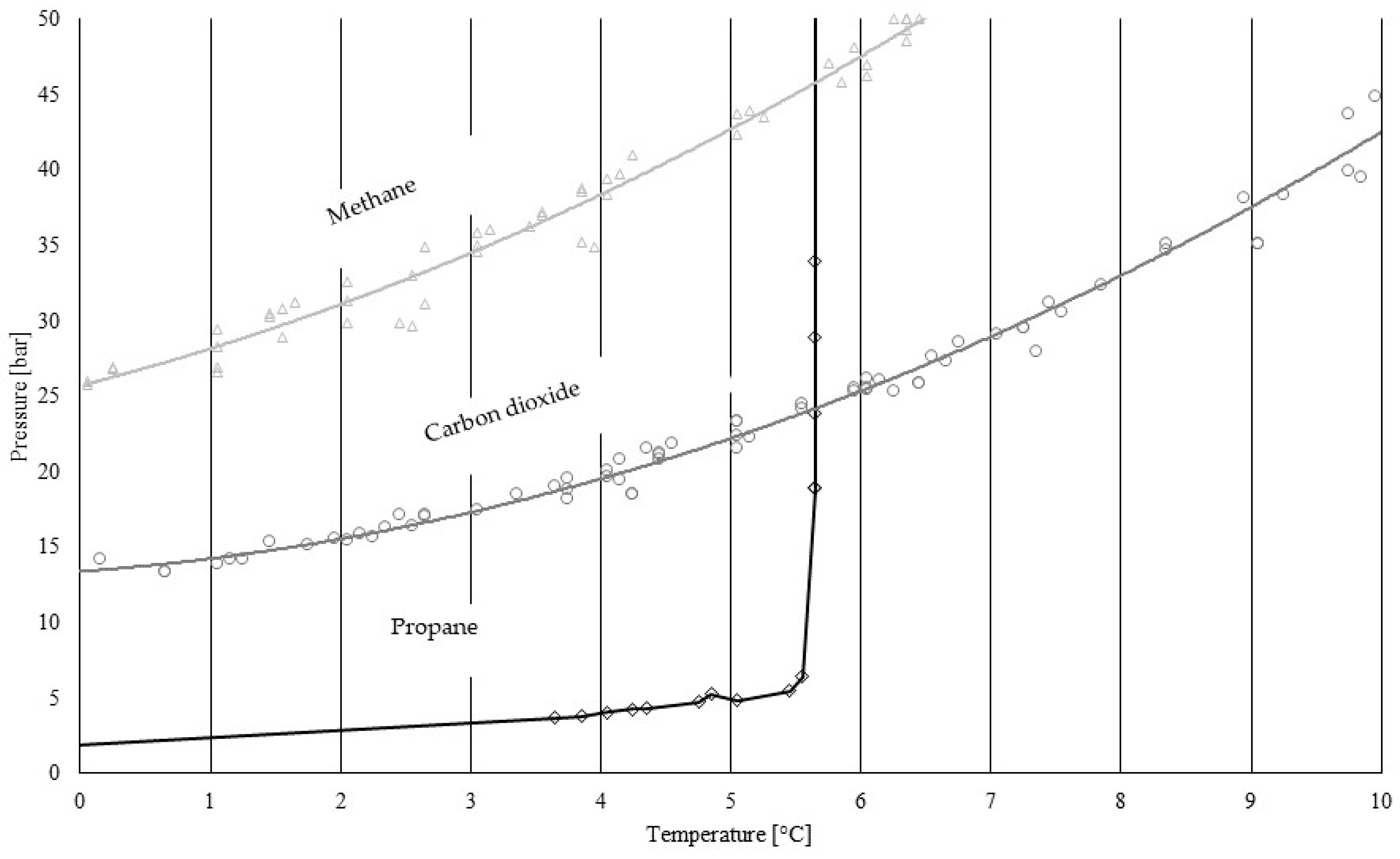
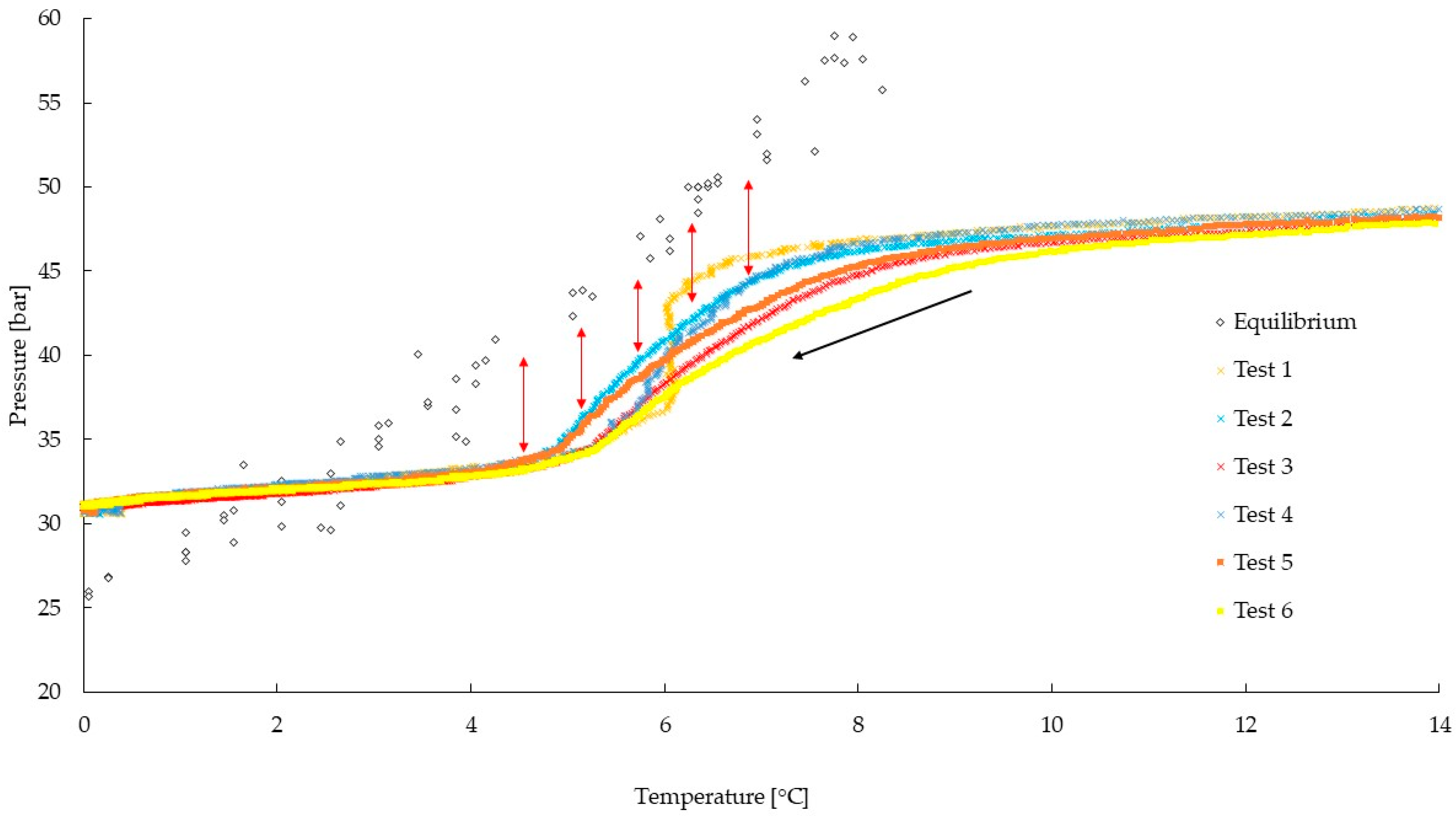

| Temperature [°C] | Equilibrium Pressure [bar] | Experimental Pressure [bar] | Promotion Measured [bar] |
|---|---|---|---|
| 4.9 | 40.81 | 33.78 | 7.03 |
| 5.2 | 43.47 | 34.27 | 9.20 |
| 5.5 | 45.23 | 35.36 | 9.87 |
| 5.8 | 45.82 | 36.86 | 8.96 |
| 6.1 | 46.90 | 37.95 | 8.95 |
| 6.4 | 48.87 | 39.08 | 9.79 |
| 6.7 | 51.33 | 40.21 | 11.12 |
| 7.0 | 51.69 | 40.96 | 10.73 |
| 7.3 | 55.74 | 41.78 | 13.96 |
| 7.6 | 57.59 | 42.45 | 15.14 |
| 7.9 | 57.83 | 43.16 | 14.67 |
| 8.2 | 59.38 | 43.84 | 15.54 |
| 8.5 | 60.72 | 44.52 | 16.20 |
| 8.8 | 61.30 | 44.92 | 16.38 |
| 9.1 | 64.23 | 45.33 | 18.90 |
| 9.4 | 65.11 | 45.69 | 19.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambelli, A.M.; Gigliotti, G.; Rossi, F. Production of CH4/C3H8 (85/15 vol%) Hydrate in a Lab-Scale Unstirred Reactor: Quantification of the Promoting Effect Due to the Addition of Propane to the Gas Mixture. Energies 2024, 17, 1104. https://doi.org/10.3390/en17051104
Gambelli AM, Gigliotti G, Rossi F. Production of CH4/C3H8 (85/15 vol%) Hydrate in a Lab-Scale Unstirred Reactor: Quantification of the Promoting Effect Due to the Addition of Propane to the Gas Mixture. Energies. 2024; 17(5):1104. https://doi.org/10.3390/en17051104
Chicago/Turabian StyleGambelli, Alberto Maria, Giovanni Gigliotti, and Federico Rossi. 2024. "Production of CH4/C3H8 (85/15 vol%) Hydrate in a Lab-Scale Unstirred Reactor: Quantification of the Promoting Effect Due to the Addition of Propane to the Gas Mixture" Energies 17, no. 5: 1104. https://doi.org/10.3390/en17051104
APA StyleGambelli, A. M., Gigliotti, G., & Rossi, F. (2024). Production of CH4/C3H8 (85/15 vol%) Hydrate in a Lab-Scale Unstirred Reactor: Quantification of the Promoting Effect Due to the Addition of Propane to the Gas Mixture. Energies, 17(5), 1104. https://doi.org/10.3390/en17051104







